Abstract
Objective
Evaluation of traditionally used royal jelly (RJ) for the management of hepato-renal damage and gastrointestinal ulcerations caused by diclofenac.
Methods
Forty adult male Wistar rats were allocated into four groups. Rats of the 1st group received only saline and served as normal group. The remaining 3 groups received diclofenac (50 mg/kg/day, I.P.) for 7 days. Group 2 served as diclofenac-control group. Groups 3 and 4 received RJ (150 and 300 mg/kg/day, P.O.) respectively for 30 days. Twenty-four hours after the last treatment, blood samples were collected, rats were sacrificed, and livers, kidneys, stomachs & intestines were harvested. Stomachs and intestines were tested for ulcer counts. Serum levels of AST, ALT, creatinine and urea were investigated. Hepatic, renal, gastric and intestinal tissue contents of myeloperoxidase (MPO) and prostaglandin-E2 (PGE2) were measured. Histopathological examinations were also performed followed by immunohistochemical determination of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) expression.
Results
Diclofenac administration caused significant deterioration of all the above mentioned parameters. RJ improved hepatic and renal functions. Gastric and intestinal ulcer counts were significantly ameliorated. Hepatic, renal, gastric and intestinal tissue PGE-2 contents and COX-2 expression were significantly elevated. RJ also significantly reduced MPO content and iNOS expression as compared to diclofenac-control group. Improvements of the histopathological pictures of hepatic, renal, gastric and intestinal tissues were also apparent.
Conclusion
The study demonstrates promising protective effects of RJ against diclofenac-induced hepato-renal damage and gastrointestinal ulceration in rats.
Keywords: Royal jelly, Diclofenac, Hepatic, Renal, Gastrointestinal, Inflammation, Rats, Pharmaceutical science, Biochemistry, Molecular biology, Gastrointestinal system, Renal system, Pathology, Pharmacology, Toxicology
Royal jelly; Diclofenac; Hepatic; Renal; Gastrointestinal; Inflammation; Rats; Pharmaceutical science; Biochemistry; Molecular biology; Gastrointestinal system; Renal system; Pathology; Pharmacology; Toxicology
1. Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used drugs for treatment of rheumatoid and osteoarthritis as they relief pain and inflammation (Blanca-Lopez et al., 2018). It has been reported that NSAIDs inhibit the activity of cyclooxygenase-1 and cyclooxygenase-2 enzymes and thereby suppress the formation of thromboxane and prostaglandin (Bernardi et al., 2009; Bevaart et al., 2010). Chronic intake of NSAIDs leads to undesirable side effects such as gastrointestinal injury, renal and cardiovascular damages (Bjarnason et al., 2017; El-Yazbi et al., 2018; Baker, 2018).
Diclofenac is a widely used NSAID that is usually prescribed for its analgesic, anti-inflammatory, and anti-pyretic activities (Gan, 2010). Diclofenac causes hepatotoxicity. The mechanism of Diclofenac-induced liver toxicity in humans is idiosyncratic. Diclofenac is metabolized in hepatocyte by multiple cytochrome P-450 enzymes resulting in formation of drug-protein adducts, glutathione (GSH) conjugation, and mitochondrial dysfunction and organ damage (Huang et al., 2017). However, it has been documented that diclofenac rather than its metabolite is responsible for its toxicity (Masubuchi et al., 2002). The kidney is also affected by diclofenac as it contributes to nephritis and nephrotoxicity (Prince, 2018). Moreover, diclofenac administration results in gastrointestinal tract problems such as gastric mucosa bleeding, deficiency in gastric blood flow and apoptosis (Ilic et al., 2011).
Natural products are used as therapeutic agents for the treatment of various diseases due to their minimal side effects (Khan, 2018). Royal jelly (RJ) is a natural honeybee product produced from the hypopharyngeal gland of young worker honey bees (Apis mellifera Linne). RJ is a mixture of free amino acids, proteins, sugars, lipids, vitamins, and minerals (Ahmed et al., 2014). RJ exhibits anti-oxidant (Abdel-Hafez et al., 2017), anti-inflammatory (Karaca et al., 2012), immunomodulatory (El-Nekeety et al., 2007), anti-tumor (Zhang et al., 2017), anti-hypercholesterolemic (Ibrahim, 2014), anti-microbial (Chan et al., 2009) and anti-allergic (Oka et al., 2001) activities. Moreover, RJ has shown neuroprotective effects in stress-induced animal models (Teixeira et al., 2017), aging (Pyrzanowska et al., 2014), and drug-induced neurotoxicity (Mohamed et al., 2015).
Although it has been reported that RJ exhibited protective effect against carbon tetrachloride-induced hepatotoxicity (Cemek et al., 2010) and cisplatin-induced nephrotoxicity (Silici et al., 2011), its effect on diclofenac induced hepato-renal damage and gastrointestinal ulcerations in rats has not been yet investigated.
Therefore this study aims to evaluate the possible ameliorating effect of RJ on biochemical, histopathological, and immunohistochemical changes in diclofenac induced hepato-renal damage and gastrointestinal ulcerations in rats.
2. Materials and methods
2.1. Animals
Forty adult male Wistar rats weighing 180–200 g were used in the current study. Standard food pellets and tap water were supplied ad libitum. Animals and food pellets were obtained from the animal house colony of the National Research Center (NRC, Egypt). The study was conducted in accordance with the National Research Centre–Medical Research Ethics Committee (NRC-MREC) for the use of animal subjects and following the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
2.2. Drugs and chemicals
Diclofenac sodium (Voltarene®, 50 mg coated tablets; Novartis Egypt) and Royal Jelly (Royal Jelly®, 1000 mg soft gelatin capsules; Pharco pharmaceuticals, Egypt) were used throughout the study. All other chemicals were of highest analytical grade available.
2.3. Experimental design and treatment protocol
Animals were randomly allocated into four groups (10 rats each). Rats of the 1st group received only saline intraperitoneally and served as normal control group. Group 2 received diclofenac (50 mg/kg/day, I.P.) for 7 days and served as diclofenac-control group. Groups 3 and 4 received diclofenac (50 mg/kg/day, I.P.) for 7 days and royal jelly (RJ; 150 and 300 mg/kg/day, P.O.) respectively for 30 days. All animals were sacrificed 24 h after the last treatment after overnight fasting.
3. Methods
3.1. Serum biochemical analysis
Twenty-four hours after the last drug dose, rats were anaesthetized with diethyl ether and blood samples were withdrawn from the retro-orbital venous plexus. Collected blood samples were allowed to stand for 10 min at room temperature then centrifuged at 4 °C using cooling centrifuge (Laborezentrifugen, 2k15, Sigma, Germany) at 3000 r.p.m for 10 min and sera were separated for the assessment of levels of urea, creatinine as well as aspartate aminotransferase (AST), alanine aminotransferase (ALT) using commercially available kits (Biodiagnostic, Egypt).
3.2. Tissue biochemical analysis
Immediately after blood sampling, animals were sacrificed by cervical dislocation under ether anesthesia. The two kidneys, the livers, stomachs and intestines were harvested from each rat. All organs were immediately dissected out, and rinsed with PBS to remove excess blood. Stomachs and intestines were examined for ulcer counts and severity. Parts from kidneys, livers, stomachs & intestines were homogenized (MPW-120 homogenizer, Med instruments, Poland) to obtain 20% homogenate that was stored overnight at –20 °C. The homogenates were centrifuged for 5 min at 5000 x g using a cooling centrifuge (Sigma and laborzentrifugen, 2k15, Germany).
Tissue contents of prostaglandin-E2 (PGE2) and myeloperoxidase (MPO) were assessed in hepatic, renal, gastric and intestinal tissues using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions.
3.3. Gastric and intestinal ulcer count and score
Stomachs and intestines were tested for ulcer counts. The stomachs were opened along the greater curvature; the mucosa of each rat was examined for macroscopic mucosal lesions. Gastric mucosal lesions were evaluated by visual scores and results expressed as the number of lesions/rat (Mózsik et al., 1982).
The small intestine was also removed and opened on the side opposite the mesentery and the lesions were assessed as described above for gastric ulcers (Beck et al., 1990).
3.4. Histopathological examination
Other parts of the kidneys, livers, stomachs and intestines were fixed in 10% neutral buffered formalin and embedded in paraffin wax. 4μm thick sections were stained with Hematoxylin and Eosin (H&E) and examined using binocular Olympus CX31 microscope (Bancroft et al., 1996).
3.5. Immunohistochemical determination of cyclooxygenase-2 (COX-2)
Immunohistochemical staining of anti-cyclooxygenase-2 antibodies was performed on polylysine-coated slides of the selected organs. The organs were fixed in neutral buffered formalin and then embedded in paraffin. Following deparaffinization, sections were exposed to 3% hydrogen peroxide for 10 min, followed by rinsing three times in Tris buffer (pH 7.4) for 10 min. Sections were incubated under humid conditions using an anti-COX-2 antibody (1:100). The specimens were counterstained with Hematoxylin & Eosin. Negative controls were prepared by substituting normal mouse serum for each primary antibody (Mansour et al., 2017).
3.6. Immunohistochemical determination of inducible nitric oxide synthase (iNOS)
Immunohistochemical staining of anti- inducible nitric oxide synthase antibodies was performed by streptoavidin–biotin. Four- micrometer–thick sections were deparaffinized and incubated with fresh 0.3% hydrogen peroxide in methanol for 30 min at room temperature. The specimens were then incubated with anti- iNOS antibody as the primer antibody at a 1:100 dilution. The specimens were counterstained with Hematoxylin & Eosin. Negative controls were prepared by substituting normal mouse serum for each primary antibody.
3.7. Statistical analysis
All the values are presented as means ± standard error of the means (SEM). Comparisons between different groups were carried out using one way analysis of variance (ANOVA) followed by Tukey's multiple comparison post hoc test. Difference was considered significant when p ˂0.05. GraphPad prism® software (version 6 for Windows, San Diego, California, USA) were used to carry out these statistical tests.
4. Results
4.1. Effects of royal jelly on serum hepatic (aspartate transaminase; AST & alanine transaminase; ALT) and renal (creatinine & urea) functions in diclofenac-induced hepato-renal damage in rats
Diclofenac (50 mg/kg, I.P.) resulted in hepatic damage in rats as evidenced by the significant elevation of ALT and AST to 220% and 119% respectively as compared to the normal control group. Royal jelly (150 & 300 mg/kg/day, P.O.) significantly decreased the elevated serum ALT to 61% and 42% and decreased the elevated serum AST to 97% and 49% respectively as compared to the diclofenac-control group.
Diclofenac also resulted in renal damage in rats as evidenced by the significant elevation of serum creatinine and urea to 272% and 217% respectively as compared to the normal control group. Royal jelly (150 & 300 mg/kg/day, P.O.) significantly decreased the elevated serum creatinine to 65% and 48% and decreased the elevated serum urea to 80% and 56% respectively as compared to the diclofenac-control group (Table 1).
Table 1.
Effects of royal jelly on serum hepatic (aspartate transaminase; AST & alanine transaminase; ALT) and renal (creatinine & urea) functions in diclofenac-induced hepato-renal damage in rats.
| Groups | Hepatic function test |
Renal function test |
||
|---|---|---|---|---|
| ALT (U/L) | AST (U/L) | Creatinine (mg/dl) | Urea (mg/dl) | |
| Normal control | 45.56 ± 2.58 | 40.23 ± 3.56 | 2.81 ± 0.24 | 47.21 ± 3.95 |
| Diclofenac-control (50 mg/kg, I.P.) | 105.83a ± 5.69 | 96.01a ± 4.22 | 7.65a ± 0.82 | 102.35a ± 2.87 |
| Diclofenac + RJ (150 mg/kg, P.O.) | 86.41a,b ± 3.49 | 78.63a,b ± 4.08 | 5.00a,b ± 0.51 | 81.54a,b ±7.39 |
| Diclofenac + RJ (300 mg/kg, P.O.) | 68.35a,b ± 2.41 | 60.40a,b ± 2.07 | 3.71a,b ± 0.31 | 57.80b ± 3.39 |
Rats of the normal control group received I.P. injections of saline. Hepato-renal damage was induced in the remaining 3 groups by single intraperitoneal injection of diclofenac (50 mg/kg) for 7 days. Group 2 received only distilled water orally for 30 days and served as diclofenac-control group. Groups 3 & 4 received royal jelly (150 & 300 mg/kg/day, P.O.) respectively for 30 days. All animals were sacrificed 24 h after the last treatment after overnight fasting. Blood samples were collected and sera were separated.
Data is presented as mean ± SEM (n = 10).
aSignificantly different from Normal control groupat p < 0.05 (Tukey's post hoc test).
bSignificantly different from Diclofenac-control group at p < 0.05 (Tukey's post hoc test).
4.2. Effects of royal jelly on gastric and intestinal ulcer count in diclofenac-induced gastrointestinal ulcerations in rats
Diclofenac (50 mg/kg, I.P.) resulted in severe gastric and intestinal ulceration in rats as evidenced by the visual inspection of ulcer count in both gastric and intestinal tissues. Gastric and intestinal ulcers were elevated to 317% and 260% respectively as compared to the normal control group. Royal jelly (150 mg/kg/day, P.O.) significantly decreased the number of gastric ulcers to 25% as compared to the diclofenac-control group. Royal jelly (150 & 300 mg/kg/day, P.O.) totally inhibited the gastric and intestinal ulcerations and normalized both gastric and intestinal mucosal tissues as compared to the diclofenac-control group (Table 2).
Table 2.
Effects of royal jelly on gastric and intestinal ulcer count in diclofenac-induced gastrointestinal ulcerations in rats.
| Groups | Gastric Ulcer count | Intestinal Ulcer count |
|---|---|---|
| Normal control | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Diclofenac-control (50 mg/kg, I.P.) | 3.17a ± 0.6 | 2.60a ± 0.62 |
| Diclofenac + RJ (150 mg/kg, P.O.) | 0.80a,b ± 0.34 | 0.0b ± 0.0 |
| Diclofenac + RJ (300 mg/kg, P.O.) | 0.0b ± 0.0 | 0.0b ± 0.0 |
Rats of the normal control group received I.P. injections of saline. Gastrointestinal ulcerations were induced in the remaining 3 groups by single intraperitoneal injection of diclofenac (50 mg/kg) for 7 days. Group 2 received only distilled water orally for 30 days and served as diclofenac-control group. Groups 3 & 4 received royal jelly (150 & 300 mg/kg/day, P.O.) respectively for 30 days. All animals were sacrificed 24 h after the last treatment after overnight fasting. All animals were sacrificed 24 h after the last treatment after overnight fasting. The stomachs and intestines were removed and inspected for the ulcer count.
Data is presented as mean ± SEM (n = 10).
aSignificantly different from Normal control groupat p < 0.05 (Tukey's post hoc test).
bSignificantly different from Diclofenac-control group at p < 0.05 (Tukey's post hoc test).
4.3. Effects of royal jelly on hepatic, renal, gastric and intestinal tissue concentration of prostaglandin E2 (PGE2) in diclofenac-induced hepato-renal damage and gastrointestinal ulcerations in rats
Diclofenac (50 mg/kg, I.P.) resulted in hepato-renal damage and gastrointestinal ulcerations in rats as evidenced by the significant reduction of hepatic, renal, gastric and intestinal tissue concentrations of PGE2 to 77%, 82%, 72% and 85% respectively as compared to the normal control group. Royal jelly (150 mg/kg/day, P.O.) significantly elevated the reduced hepatic, renal, gastric and intestinal tissue concentrations of PGE2 to 86%, 89%, 94% and 87% respectively as compared to the diclofenac-control group.
Royal jelly (300 mg/kg/day, P.O.) significantly elevated the reduced hepatic, renal, gastric and intestinal tissue concentrations of PGE2 to 88%, 91%, 95% and 90% respectively as compared to the diclofenac-control group (Table 3).
Table 3.
Effects of royal jelly on hepatic, renal, gastric and intestinal tissue concentrations of prostaglandin E2 (PGE2) in diclofenac-induced hepato-renal damage and gastrointestinal ulcerations in rats.
| Groups | PGE2 (ng/g tissue) |
|||
|---|---|---|---|---|
| Hepatic | Renal | Gastric | Intestinal | |
| Normal control | 10.59 ± 0.33 | 11.27 ± 0.05 | 9.62 ± 0.05 | 10.78 ± 0.06 |
| Diclofenac-control (50 mg/kg, I.P.) | 8.17a ± 0.02 | 9.23a ± 0.05 | 6.95a ± 0.12 | 8.05a ± 0.08 |
| Diclofenac + RJ (150 mg/kg, P.O.) | 9.06a,b ± 0.02 | 9.96a,b ± 0.62 | 9.00a,b ± 0.01 | 9.37a,b ± 0.04 |
| Diclofenac + RJ (300 mg/kg, P.O.) | 9.32a,b ± 0.18 | 10.20a,b ± 0.01 | 9.13a,b ± 0.10 | 9.73a,b ± 0.05 |
Rats of the normal control group received I.P. injections of saline. Hepato-renal damage and gastrointestinal ulcerations were induced in the remaining 3 groups by single intraperitoneal injection of diclofenac (50 mg/kg) for 7 days. Group 2 received only distilled water orally for 30 days and served as diclofenac-control group. Groups 3 & 4 received royal jelly (150 & 300 mg/kg/day, P.O.) respectively for 30 days. All animals were sacrificed 24 h after the last treatment after overnight fasting. The liver, kidneys, stomachs and intestines were removed, homogenized and the homogenate was obtained.
Data is presented as mean ± SEM (n = 10).
aSignificantly different from Normal control groupat p < 0.05 (Tukey's post hoc test).
bSignificantly different from Diclofenac-control group at p < 0.05 (Tukey's post hoc test).
4.4. Effects of royal jelly on hepatic, renal, gastric and intestinal tissue concentrations of myeloperoxidase (MPO) in diclofenac-induced hepato-renal damage and gastrointestinal ulcerations in rats
Diclofenac (50 mg/kg, I.P.) resulted in hepato-renal damage and gastrointestinal ulcerations in rats as evidenced by the significant elevation of hepatic, renal, gastric and intestinal tissue concentrations of MPO to 258%, 160%, 202% and 160% respectively as compared to the normal control group. Royal jelly (150 mg/kg/day, P.O.) significantly decreased the elevated hepatic, renal, gastric and intestinal tissue concentrations of MPO to 55%, 81%, 73% and 80% respectively as compared to the diclofenac-control group.
Royal jelly (300 mg/kg/day, P.O.) significantly decreased the elevated hepatic, renal, gastric and intestinal tissue concentrations of MPO to 43%, 64%, 53% and 73% respectively as compared to the diclofenac-control group (Table 4).
Table 4.
Effects of royal jelly on hepatic, renal, gastric and intestinal tissue concentration of myeloperoxidase (MPO) in diclofenac-induced hepato-renal damage and gastrointestinal ulcerations in rats.
| Groups | MPO (ng/g tissue) |
|||
|---|---|---|---|---|
| Hepatic | Renal | Gastric | Intestinal | |
| Normal control | 14.42 ± 0.73 | 17.63 ± 0.55 | 12.39 ± 0.64 | 11.40 ± 0.17 |
| Diclofenac-control (50 mg/kg, I.P.) | 37.25a ± 2.10 | 28.20a ± 0.91 | 24.99a ± 2.01 | 18.25a ± 0.19 |
| Diclofenac + RJ (150 mg/kg, P.O.) | 20.62a,b ± 1.09 | 22.83a,b ± 1.39 | 18.28a,b ±0.97 | 14.59a,b ±0.10 |
| Diclofenac + RJ (300 mg/kg, P.O.) | 16.08b ± 0.95 | 18.15b ± 0.17 | 13.23b ± 0.27 | 13.31a,b ±0.23 |
Rats of the normal control group received I.P. injections of saline. Hepato-renal damage and gastrointestinal ulcerations were induced in the remaining 3 groups by single intraperitoneal injection of diclofenac (50 mg/kg) for 7 days. Group 2 received only distilled water orally for 30 days and served as diclofenac-control group. Groups 3 & 4 received royal jelly (150 & 300 mg/kg/day, P.O.) respectively for 30 days. All animals were sacrificed 24 h after the last treatment after overnight fasting. The liver, kidneys, stomachs and intestines were removed, homogenized and the homogenate was obtained.
Data is presented as mean ± SEM (n = 10).
aSignificantly different from Normal control groupat p < 0.05 (Tukey's post hoc test).
bSignificantly different from Diclofenac-control group at p < 0.05 (Tukey's post hoc test).
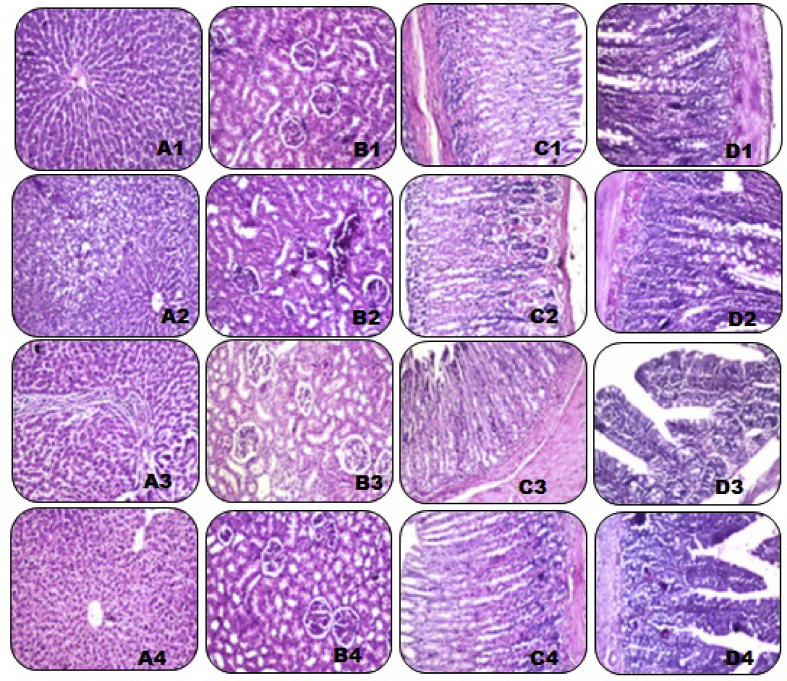
4.5. Histopathological examination of hepatic, renal, gastric and intestinal tissues
Hepatic section (Figure 1 A1), renal section (Figure 1 B1), gastric section (Figure 1 C1) and intestinal section (Figure 1 D1) from a normal control rat shows normal histopathological picture.
Figure 1.
Histopathological examination of hepatic, renal, gastric and intestinal tissues. Photomicrograph of a hepatic (A1), and renal (B1), gastric (C1) and intestinal (D1) sections of a rat from a normal control group (H&E X 100). Photomicrograph of a hepatic (A2), and renal (B2), gastric (C2) and intestinal (D2) sections prepared from a diclofenac-control rat (H&E X 100). Photomicrograph of a hepatic (A3), and renal (B3), gastric (C3) and intestinal (D3) sections prepared from a rat treated with royal jelly (150 mg/kg/day, P.O.) for 30 days along with diclofenac treatment (H&E X 100). Photomicrograph of a hepatic (A4), and renal (B4), gastric (C4) and intestinal (D4) sections prepared from a rat treated with royal jelly (300 mg/kg/day, P.O.) for 30 days along with diclofenac treatment (H&E X 100).
Hepatic section from a diclofenac-control rat shows massive histopathological distortions with fatty changes and cytoplasmic vacuolation detected in the hepatocytes at the most of the peripheral zone of the hepatic lobules (Figure 1 A2).
Renal section from a diclofenac-control rat shows massive areas of fibrosis and ongoing cell apoptosis. Glomerular affection in the form of thickening of glomerular membrane whereas tubules are not much affected (Figure 1 B2).
Gastric section from a diclofenac-control rat shows abnormal histological structure of the mucosa. The nuclei of the glandular lining epithelium showed nuclear pyknosis. Sloughing of superficial mucosa secreting cells is apparent with necrosis of the functioning cells of gastric glands along with inflammatory cellular infiltrate in the submucosa (Figure 1 C2).
Intestinal section from a diclofenac-control rat shows distorted histological structure of the villi with lining mucosal epithelium and serosa (Figure 1 D2).
Hepatic section from a rat treated with royal jelly (150 mg/kg/day, P.O.) for 30 days along with diclofenac treatment shows moderate improvement of the overall histopathological picture. Few inflammatory cells of infiltration were observed in the portal area (Figure 1 A3).
Renal section from a rat treated with royal jelly (150 mg/kg/day, P.O.) for 30 days along with diclofenac treatment shows moderate improvement of the overall histopathological picture. Few fat globules are distributed all over the section (Figure 1 B3).
Gastric section from a rat treated with royal jelly (150 mg/kg/day, P.O.) for 30 days along with diclofenac treatment shows moderate improvement of the overall histopathological picture. Widening of gastric pits can be observed due to slight atrophy of the mucous secreting cells and preserved peptic and partial cells (Figure 1 C3).
Intestinal section from a rat treated with royal jelly (150 mg/kg/day, P.O.) for 30 days along with diclofenac treatment shows huge improvement of the overall histopathological picture (Figure 1 D3).
Hepatic section (Figure 1 A4), renal section (Figure 1 B4), gastric section (Figure 1 C4) and intestinal section (Figure 1 D4) from a rat treated with royal jelly (300 mg/kg/day, P.O.) for 30 days along with diclofenac treatment shows huge improvement of the overall histopathological picture. No histopathological alterations could be seen.
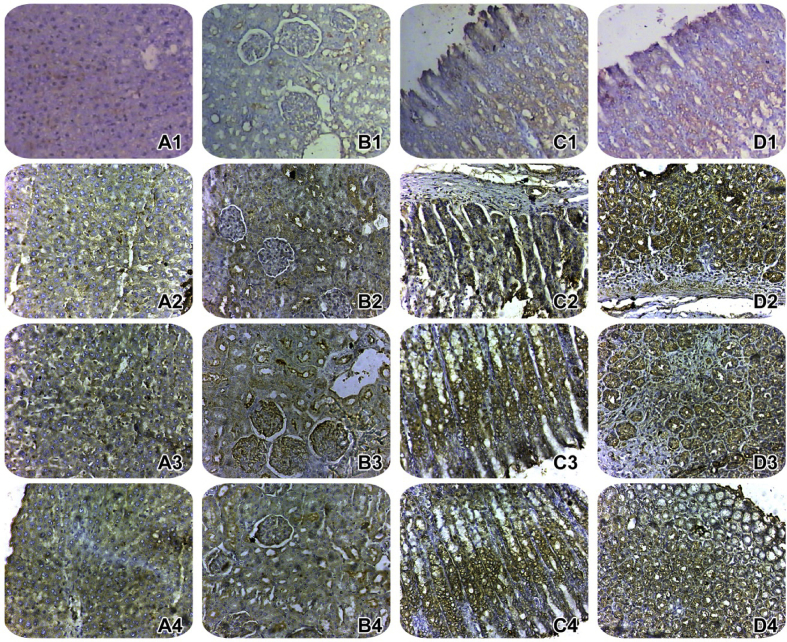
4.6. Immunohistochemical determination of cyclooxygenase-2 (COX-2) in hepatic, renal, gastric and intestinal tissues
Hepatic section (Figure 2 A1), renal section (Figure 2 B1), gastric section (Figure 2 C1) and intestinal section (Figure 2 D1) from a normal control rat shows mild positivity for COX-2 immunostaining.
Figure 2.
Immunohistochemical determination of cyclooxygenase-2 (COX-2) in hepatic, renal, gastric and intestinal tissues. Photomicrograph of a hepatic (A1), and renal (B1), gastric (C1) and intestinal (D1) sections of a rat from a normal control group. Photomicrograph of a hepatic (A2), and renal (B2), gastric (C2) and intestinal (D2) sections prepared from a diclofenac-control rat. Photomicrograph of a hepatic (A3), and renal (B3), gastric (C3) and intestinal (D3) sections prepared from a rat treated with royal jelly (150 mg/kg/day, P.O.) for 30 days along with diclofenac treatment. Photomicrograph of a hepatic (A4), and renal (B4), gastric (C4) and intestinal (D4) sections prepared from a rat treated with royal jelly (300 mg/kg/day, P.O.) for 30 days along with diclofenac treatment (COX-2 immunohistochemistry, H&E X 100).
Hepatic section (Figure 2 A2), renal section (Figure 2 B2), gastric section (Figure 2 C2) and intestinal section (Figure 2 D2) from a diclofenac-control rat shows negative COX-2 immunostaining.
Hepatic section (Figure 2 A3), renal section (Figure 2 B3), gastric section (Figure 2 C3) and intestinal section (Figure 2 D3) from a rat treated with royal jelly (150 mg/kg/day, P.O.) for 30 days along with diclofenac treatment shows moderate COX-2 immunostaining.
Hepatic section (Figure 2 A4), renal section (Figure 2 B4), gastric section (Figure 2 C4) and intestinal section (Figure 2 D4) from a rat treated with royal jelly (300 mg/kg/day, P.O.) for 30 days along with diclofenac treatment shows normalization and positivity for COX-2 immunostaining.
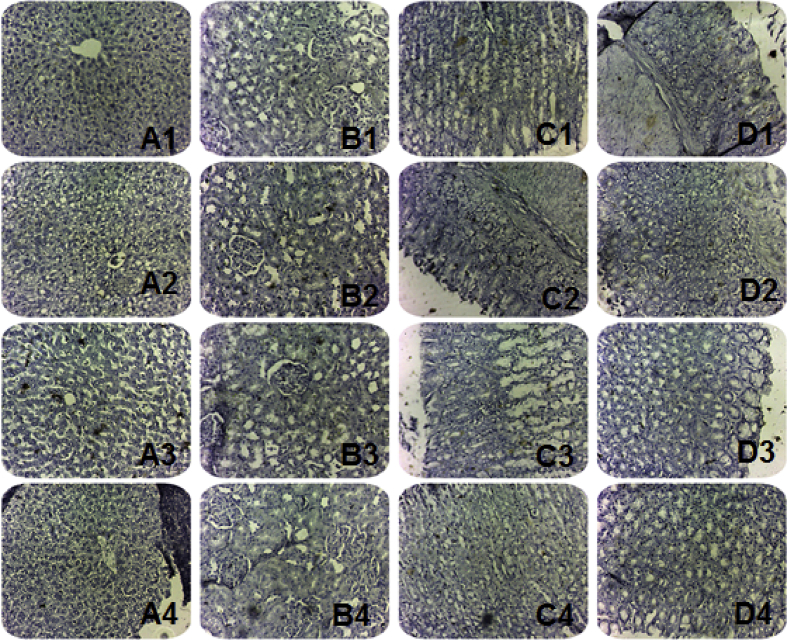
4.7. Immunohistochemical determination of inducible nitric oxide synthase (iNOS) in hepatic, renal, gastric and intestinal tissues
Hepatic section (Figure 3 A1), renal section (Figure 3 B1), gastric section (Figure 3 C1) and intestinal section (Figure 3 D1) from a normal control rat shows no positivity for iNOS immunostaining.
Figure 3.
Immunohistochemical determination of inducible nitric oxide synthase (iNOS) in hepatic, renal, gastric and intestinal tissues. Photomicrograph of a hepatic (A1), and renal (B1), gastric (C1) and intestinal (D1) sections of a rat from a normal control group. Photomicrograph of a hepatic (A2), and renal (B2), gastric (C2) and intestinal (D2) sections prepared from a diclofenac-control rat. Photomicrograph of a hepatic (A3), and renal (B3), gastric (C3) and intestinal (D3) sections prepared from a rat treated with royal jelly (150 mg/kg/day, P.O.) for 30 days along with diclofenac treatment. Photomicrograph of a hepatic (A4), and renal (B4), gastric (C4) and intestinal (D4) sections prepared from a rat treated with royal jelly (300 mg/kg/day, P.O.) for 30 days along with diclofenac treatment (iNOS immunohistochemistry, H&E X 100).
Hepatic section (Figure 3 A2), renal section (Figure 3 B2), and intestinal section (Figure 3 D2) from a diclofenac-control rat shows scattered positive iNOS immunostaining. While, gastric section (Figure 3 C2) from a diclofenac-control rat shows severe significant positive iNOS immunostaining.
Hepatic section (Figure 3 A3), renal section (Figure 3 B3), and intestinal section (Figure 3 D3) from a rat treated with royal jelly (150 mg/kg/day, P.O.) for 30 days along with diclofenac treatment shows minimal iNOS immunostaining. While, iNOS immunostaining for gastric section (Figure 3 C3) from a rat treated with royal jelly (150 mg/kg/day, P.O.) for 30 days along with diclofenac treatment shows persistent scattered positivity.
Hepatic section (Figure 3 A4), renal section (Figure 3 B4), and intestinal section (Figure 3 D4) from a rat treated with royal jelly (300 mg/kg/day, P.O.) for 30 days along with diclofenac treatment shows negative iNOS immunostaining. While, gastric section (Figure 3 C4) from a rat treated with royal jelly (300 mg/kg/day, P.O.) for 30 days along with diclofenac treatment shows very minimal iNOS immunostaining.
5. Discussion
The current study adds new information regarding the potential protective effect of royal jelly (RJ; 150, 300 mg/kg) against diclofenac-induced damage in rats. This study is the first to assess the role of COX-2, prostaglandin and inflammation in the protective effects of RJ against diclofenac-induced hepato-renal damage and gastrointestinal ulcerations in rats.
Diclofenac is commonly prescribed for its analgesic, anti-inflammatory, and anti-pyretic activities (Gan, 2010). Despite the overspread use of diclofenac, hepato-renal and gastrointestinal injuries have been reported post its administration (Ilic et al., 2011; Huang et al., 2017; Prince, 2018).
Elevation of hepatic function enzymes reflects severe hepatocellular injury with subsequent leakage of these cytoplasmic enzymes into the circulation (Mostafa, 2018). In this study, diclofenac administration (50 mg/kg/day, I.P.) for 7 days resulted in significant elevation of hepatic liver enzymes namely; ALT and AST. This finding indicates that diclofenac administration resulted in severe damage of the hepatic membrane. This result is in harmony with prior studies (Giridharan et al., 2017; Adeyemi and Olayaki, 2018).
It is well established that diclofenac has a selective inhibitory effect on COX-2 than COX-1 (Erdal and Sefa, 2017). Prostaglandins derived from COX-2 has protective effect against injury-induced by hepatotoxins such as lipopolysaccharide, acetaminophen, and carbon tetrachloride (Ramm and Mally, 2013). In line with this notion, diclofenac-injected rats in this study significantly exhibited reduced COX-2 protein expression and hepatic PGE2 content. In agreement with the prior study of Gan; diclofenac-induced hepatotoxicity may have resulted from reduction of PGE-2 content which originally resulted from reduction of COX-2 protein expression (Gan, 2010).
MPO is a vital marker of neutrophil function and when neutrophils are activated in response to inflammation, MPO is released from cells into extra-cellular space (Bradley et al., 1982; Sullivan et al., 2000).
MPO exhibits a crucial mechanistic junction between oxidation and inflammation (Mostafa et al., 2018). Inducible nitric oxide synthase (iNos) is a calcium independent enzyme that is prominently induced in case of inflammation leading to generation of large amount of NO. Such events eventually result in massive tissue damage (Nathan, 1997; Lanas, 2008).
The current study revealed that diclofenac significantly elevated hepatic MPO content and iNOS protein expression, revealing that inflammation mediated diclofenac-induced hepatotoxicity. In line with this data, Nouri et al. showed that diclofenac elevated the expression of hepatic pro-inflammatory cytokines in rats (Nouri et al., 2017).
Moreover, the current study stated that diclofenac administration resulted in deterioration of the overall histopathological picture of the hepatic tissue of diclofenac-control group. The histopathological findings are in line with biochemical results.
In line with this data, El-Maddawy et al. stated that diclofenac administration resulted in hepatocellular necrosis, non-fatty degeneration and vacuolation (El-Maddawy and El-Ashmawy, 2013).
Royal jelly (RJ) is a natural honeybee product of young worker bees (Apis mellifera Linne) (Ahmed et al., 2014). RJ has been shown to exhibit promising anti-oxidant (Abdel-Hafez et al., 2017) and anti-inflammatory properties (Karaca et al., 2012).
In the current work, oral administration of RJ (150, 300 mg/kg) significantly reduced serum ALT and AST levels in diclofenac injected rats. These results reveal that RJ prevented hepatic enzyme leakage into the circulation, maintained hepatocyte membrane integrity and significantly provided hepatoprotective effects. Previous studies stated that RJ showed noticeable protective effect on hepatic tissue (Kanbur et al., 2009; Cemek et al., 2010).
Moreover, oral administration of RJ protected against diclofenac-induced hepatic injury via significant elevation of COX-2 protein expression and hepatic PGE-2 content. Oka et al. also described the protective effect of RJ against experimentally-induced immunosupression by elevating PGE2 levels (Oka et al., 2001).
According to the present study, RJ significantly reduced hepatic MPO content and iNOS protein expression revealing that it ameliorated diclofenac-induced hepatotoxicity via reducing hepatic tissue inflammation. This finding may be attributed to the anti-inflammatory activities of RJ which was described in previous studies (Kohno et al., 2004; Karaca et al., 2012). Additionally, it has been stated that tualang honey repressed UVB-induced iNOS protein expression, PGE2 production as well as COX-2 expression (Ahmad et al., 2012).
Oral administration of RJ in the selected doses resulted in significant improvement of the hepatic tissue histopathological picture as compared to diclofenac-control group. This data is in line with the aforementioned biochemical and immunohistochemical findings.
Diclofenac injection resulted in significant impairment of renal function as measured by elevated serum urea and creatinine compared to normal rats. This can be attributed to absence of elimination of metabolized products from the body resulting in severe disruption of renal tubular function. This finding is in accordance with that of Prince (2018). Giridharan et al. also described significant deterioration in renal function as well as oxidative markers post diclofenac injection (Giridharan et al., 2017).
Diclofenac injection significantly reduced renal COX-2 protein expression and thereby renal PGE-2 content. Therefore, it can be postulated that diclofenac-induced nephrotoxicity may have resulted from reduction of PGE-2 production due to COX-2 inhibition. Our results are in agreement with previous studies that stated that inhibition of prostaglandin production results in disruption of glomerular filtration rate as well as destruction of renal tubular function and renal metabolism (Yasmeen et al., 2007; Ratliff et al., 2016). Moreover, diclofenac-control rats showed significant elevation in renal MPO content and iNOS protein expression showing that diclofenc-induced renal toxicity might be mediated through inflammation of renal tissue. Moreover, the histopathological picture of renal tissue of diclofenac-control rats showed severe deterioration as was manifested by massive areas of fibrosis, glomerular affection and ongoing cell apoptosis. These results are in agreement with those of El-Maddawy et al., who stated that diclofenac administration in rats resulted in severe renal tissue inflammation that was manifested by glomerulo-nephritis along with interstitial inflammation and renal tissue necrosis epithelium (El-Maddawy and El-Ashmawy, 2013). Additionally, Giridharan et al. demonstrated severe renal histopathological changes following diclofenac injection (Giridharan et al., 2017).
In the current work, oral administration of RJ significantly reduced serum urea and creatinine as compared to diclofenac-control rats; thereby it improved and almost normalized renal function. Other studies demonstrated that RJ successfully restored renal function in cisplatin-induced nephrotoxicity (Karadeniz et al., 2011; Ibrahim et al., 2016).
Administration of RJ in the selected dose levels showed a significant increase in renal COX-2 protein expression and PGE-2 renal content leading eventually to significant protection against diclofenac toxicity. Moreover, RJ significantly reduced renal MPO content and iNOS protein expression. These data implicates that RJ reduced renal toxicity via inhibiting the inflammatory and pro-inflammatory responses. Prior studies described anti-inflammatory effects of RJ against ethylene glycol-induced renal inflammation in rats (Aslan and Aksoy, 2015).
In harmony with the aforementioned biochemical and immunohistochemical findings, diclofenac-induced renal histopathological damage was reversed by RJ treatment. This data is in line with previous studies that demonstrated the improvement of renal histological changes upon RJ treatment in diabetic rats (Ghanbari et al., 2015).
Diclofenac injected rats showed a significant increase in gastrointestinal ulcer count, indicating severe gastrointestinal damage as evidenced by histopathological changes. Diclofenac-control group showed abnormal histological structure of the gastric mucosa as was manifested by sloughing of superficial secreting cells along with inflammation and necrosis of the functioning cells of gastric glands along with inflammatory cellular infiltrate in the submucosa. Intestinal sections showed distorted histological structure of the villi with lining mucosal epithelium and serosa. This finding is similar to that demonstrated by Devi who described significant elevation in gastric ulcer lesion index in diclofenac-treated rats (Devi et al., 2007). Giridharan et al. also described increasing gastrointestinal ulcer score in diclofenac-treated rats (Giridharan et al., 2017). Khan et al. also described severe deterioration of the overall gastric histopathological pictures of diclofenac-injected rats as compared to normal rats (Khan and Khan, 2013).
Furthermore, diclofenac-control rats exhibited significant reduction in COX-2 protein expression and PGE-2 content in the gastrointestinal tissue. Although COX-1 plays a vital role in maintaining the integrity of gastrointestinal mucosa, it has been demonstrated that COX-1 inhibition up-regulates COX-2 derived PGE-2 and thereby plays a compensatory role in maintaining the gastrointestinal functional properties. It has been reported that both COX-1 and COX-2 inhibition is required for gastrointestinal ulcerative properties of NSAIDS (Takeuchi et al., 2010). Therefore, diclofenac-induced gastrointestinal injury might be attributed to reduction of COX-1 and COX-2 derived PGE-2.
Additionally, diclofenac treated rats showed a significant increase in gastrointestinal MPO content and iNOS protein expression as compared to normal control rats. In line with previous studies, elevated MPO and iNOS activities result from COX-1 and COX-2 inhibition eventually leading to gastrointestinal damage (Takeuchi et al., 2010).
Administration of RJ in the selected dose levels significantly reduced gastrointestinal ulcer counts and attenuated histopathological changes. This data indicates its protective role against diclofenac-induced gastrointestinal damage in rats. These results are in line with those of Karaca et al. who demonstrated that RJ protected the mucosa of the colon against acetic acid-induced injury (Karaca et al., 2010). Moreover, RJ significantly elevated COX-2 protein expression and PGE-2 in the gastrointestinal tissue. RJ also significantly reduced MPO gastrointestinal content and iNOS protein expression, indicating it's anti-inflammatory effect. Kaynar et al. described the efficacy of RJ against methotrexate-induced small intestine damage in rats (Kaynar et al., 2012). Prior studies also stated that RJ significantly improved colon histopathological pictures and protected colon mucosa against 2,4,6 trinitrobenzene sulfonic acid-induced colitis. These effects were mainly attributed to its mainly anti-inflammatory as well as antioxidant properties (Karaca et al., 2015).
6. Conclusion
In the light of all findings, the current study suggests that royal jelly protects against diclofenac induced hepato-renal and gastrointestinal damage by elevating PGE-2 production and COX-2 protein expression as well as down-regulation of iNOS protein expression, eventually leading to reduction of the inflammatory response. The biochemical data is in parallel with amelioration of the hepatic, renal, gastric and intestinal tissues histopathological changes. Royal jelly could be a promising supplement to protect against diclofenac induced multiorgan damage. Further studies are needed to study the molecular mechanisms underlying this protective role.
Declarations
Author contribution statement
R. Mostafa: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
S. El-Marasy: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
G. A. Jaleel: Performed the experiments; Contributed reagents, materials, analysis tools or data R. Bakeer: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abdel-Hafez S.M.N., Rifaai R.A., Abdelzaher W.Y. Possible protective effect of royal jelly against cyclophosphamide induced prostatic damage in male albino rats; a biochemical, histological and immuno-histo-chemical study. Biomed. Pharmacother. Biomedecine pharmacotherapie. 2017;90:15–23. doi: 10.1016/j.biopha.2017.03.020. [DOI] [PubMed] [Google Scholar]
- Adeyemi W.J., Olayaki L.A. Diclofenac–induced hepatotoxicity: low dose of omega-3 fatty acids have more protective effects. Toxicol. Rep. 2018;5:90–95. doi: 10.1016/j.toxrep.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad I., Jimenez H., Yaacob N.S., Yusuf N. Tualang honey protects keratinocytes from ultraviolet radiation-induced inflammation and DNA damage. Photochem. Photobiol. 2012;88(5):1198–1204. doi: 10.1111/j.1751-1097.2012.01100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W.M., Khalaf A.A., Moselhy W.A., Safwat G.M. Royal jelly attenuates azathioprine induced toxicity in rats. Environ. Toxicol. Pharmacol. 2014;37(1):431–437. doi: 10.1016/j.etap.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Aslan Z., Aksoy L. Anti-inflammatory effects of royal jelly on ethylene glycol induced renal inflammation in rats. Int. Braz J. Urol. – Offic. J. Braz. Soc. Urol. 2015;41(5):1008–1013. doi: 10.1590/S1677-5538.IBJU.2014.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker W.L. 13.17 - NSAIDs and cardiovascular toxicity. In: Caplan M., McQueen C., editors. Comprehensive Toxicology. third ed. Elsevier; Oxford: 2018. pp. 341–355. [Google Scholar]
- Bancroft J., Stevens A., Turner D. Churchill Livingstone; New York: 1996. Theory and Practice of Histological Techniques; p. 766. [Google Scholar]
- Beck W., Schneider H., Dietzel K., Nuernberg B., Brune K. Gastrointestinal ulcerations induced by anti-inflammatory drugs in rats. Arch. Toxicol. 1990;64(3):210–217. doi: 10.1007/BF02010727. [DOI] [PubMed] [Google Scholar]
- Bernardi A., Zilberstein A.C., Jager E., Campos M.M., Morrone F.B., Calixto J.B. Effects of indomethacin-loaded nanocapsules in experimental models of inflammation in rats. Br. J. Pharmacol. 2009;158(4):1104–1111. doi: 10.1111/j.1476-5381.2009.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevaart L., Vervoordeldonk M.J., Tak P.P. Evaluation of therapeutic targets in animal models of arthritis: how does it relate to rheumatoid arthritis? Arthritis Rheum. 2010;62(8):2192–2205. doi: 10.1002/art.27503. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Scarpignato C., Holmgren E., Olszewski M., Rainsford K.D., Lanas A. Mechanisms of damage to the gastrointestinal tract from nonsteroidal anti-inflammatory drugs. Gastroenterology. 2017;154(3):500–514. doi: 10.1053/j.gastro.2017.10.049. [DOI] [PubMed] [Google Scholar]
- Blanca-Lopez N., Canto M., Blanca M. Other NSAIDs reactions. In: Khan D., Banerji A., editors. Drug Allergy Testing. Elsevier Health Sciences; 2018. pp. 177–196. [Google Scholar]
- Bradley P.P., Priebat D.A., Christensen R.D., Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 1982;78(3):206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Cemek M., Aymelek F., Buyukokuroglu M.E., Karaca T., Buyukben A., Yilmaz F. Protective potential of Royal Jelly against carbon tetrachloride induced-toxicity and changes in the serum sialic acid levels. Food Chem. Toxicol. – Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2010;48(10):2827–2832. doi: 10.1016/j.fct.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Chan Q., Melathopoulos A., Pernal S., Foster L. Theinnate immune and systemic response in honey bees to abacterial pathogen, Paenibacillus larvae. BMC Genom. 2009;10:387. doi: 10.1186/1471-2164-10-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi R.S., Narayan S., Vani G., Devi C.S.S. Gastroprotective effect of Terminalia arjuna bark on diclofenac sodium induced gastric ulcer. Chem. Biol. Interact. 2007;167(1):71–83. doi: 10.1016/j.cbi.2007.01.011. [DOI] [PubMed] [Google Scholar]
- El-Maddawy Z.K., El-Ashmawy I.M. Hepato-renal and hematological effects of diclofenac sodium in rats. Global J. Pharmacol. 2013;7(2):123–132. [Google Scholar]
- El-Nekeety A.A., El-Kholy W., Abbas N.F., Ebaid A., Amra H.A., Abdel-Wahhab M.A. Efficacy of royal jelly against the oxidative stress of fumonisin in rats. Toxicon – Offic. J. Int. Soc. Toxinol. 2007;50(2):256–269. doi: 10.1016/j.toxicon.2007.03.017. [DOI] [PubMed] [Google Scholar]
- El-Yazbi A., Eida A., El-Masb M. Cardiovascular and renal interactions between cyclosporine andNSAIDs: underlying mechanisms and clinical relevance. Pharmacol. Res. 2018;129:251–261. doi: 10.1016/j.phrs.2017.11.029. inpress. [DOI] [PubMed] [Google Scholar]
- Erdal T., Sefa L. Investigation of possible cardiac side effects of diclofenac in exercise-treated rats. Biomed. Res. 2017;28(17):7675–7678. [Google Scholar]
- Gan T.J. Diclofenac: an update on its mechanism of action and safety profile. Curr. Med. Res. Opin. 2010;26(7):1715–1731. doi: 10.1185/03007995.2010.486301. [DOI] [PubMed] [Google Scholar]
- Ghanbari E., Nejati V., Azadbakht M. Protective effect of royal jelly against renal damage in streptozotocin induced diabetic rats. Iran. J. Toxicol. 2015;9(28):1258–1263. [Google Scholar]
- Giridharan R., Lavinya U., Sabina E.P. Suppressive effect of Spirulina fusiformis on diclofenac-induced hepato-renal injury and gastrointestinal ulcer in Wistar albino rats: a biochemical and histological approach. Biomed. Pharmacother. 2017;88:11–18. doi: 10.1016/j.biopha.2017.01.032. [DOI] [PubMed] [Google Scholar]
- Huang T., Zhang G., Chong S., Liu Y., Zhang N., Fang S. Effects and mechanism of diclofenac degradation in aqueous solution by US/Zn0. Ultrason. Sonochem. 2017;37:676–685. doi: 10.1016/j.ultsonch.2017.02.032. [DOI] [PubMed] [Google Scholar]
- Ibrahim A. Immunomodulatory effects of royal jelly on aorta CD3, CD68 and eNOS expression in hypercholesterolaemic rats. J. Basic Appl. Zool. 2014;67:140–148. [Google Scholar]
- Ibrahim A., Eldaim M.A., Abdel-Daim M.M. Nephroprotective effect of bee honey and royal jelly against subchronic cisplatin toxicity in rats. Cytotechnology. 2016;68(4):1039–1048. doi: 10.1007/s10616-015-9860-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic S., Drmic D., Franjic S., Kolenc D., Coric M., Brcic L. Pentadecapeptide BPC 157 and its effects on a NSAID toxicity model: diclofenac-induced gastrointestinal, liver, and encephalopathy lesions. Life Sci. 2011;88(11–12):535–542. doi: 10.1016/j.lfs.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Kanbur M., Eraslan G., Beyaz L., Silici S., Liman B.C., Altınordulu Ş. The effects of royal jelly on liver damage induced by paracetamol in mice. Exp. Toxicol. Pathol. 2009;61(2):123–132. doi: 10.1016/j.etp.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Karaca T., Bayiroglu F., Yoruk M., Kaya M., Uslu S., Comba B. Effect of royal jelly on experimental colitis induced by acetic acid and alteration of mast cell distribution in the colon of rats. Eur. J. Histochem. EJH. 2010;54(4) doi: 10.4081/ejh.2010.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaca T., Simsek N., Uslu S., Kalkan Y., Can I., Kara A. The effect of royal jelly on CD3(+), CD5(+), CD45(+) T-cell and CD68(+) cell distribution in the colon of rats with acetic acid-induced colitis. Allergol. Immunopathol. 2012;40(6):357–361. doi: 10.1016/j.aller.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Karaca T., Uz Y.H., Demirtas S., Karaboga I., Can G. Protective effect of royal jelly in 2,4,6 trinitrobenzene sulfonic acid-induced colitis in rats. Iran. J. Basic Med. Sci. 2015;18(4):370–379. [PMC free article] [PubMed] [Google Scholar]
- Karadeniz A., Simsek N., Karakus E., Yildirim S., Kara A., Can I. Royal jelly modulates oxidative stress and apoptosis in liver and kidneys of rats treated with cisplatin. Oxidative Med. Cell. Longev. 2011;2011 doi: 10.1155/2011/981793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaynar L., Cetin A., Hacioglu S.K., Eser B., Kocyigit I., Canoz O. Efficacy of royal jelly on methotrexate-induced systemic oxidative stress and damage to small intestine in rats. Afr. J. Tradit. Compl. Altern. Med. AJTCAM. 2012;9(3):412–417. doi: 10.4314/ajtcam.v9i3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.I., Khan M.R. Gastroprotective potential of Dalbergia sissoo roxb. stem bark against diclofenac-induced gastric damage in rats. Osong Public Health Res. Perspect. 2013;4(5):271–277. doi: 10.1016/j.phrp.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R.A. Natural products chemistry: the emerging trends and prospective goals. Saudi Pharmaceut. J. 2018 doi: 10.1016/j.jsps.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno K., Okamoto I., Sano O., Arai N., Iwaki K., Ikeda M. Royal jelly inhibits the production of proinflammatory cytokines by activated macrophages. Biosc. Biotech. Biochem. 2004;68(1):138–145. doi: 10.1271/bbb.68.138. [DOI] [PubMed] [Google Scholar]
- Lanas A. Role of nitric oxide in the gastrointestinal tract. Arthritis Res. Ther. 2008;10(Suppl 2):S4. doi: 10.1186/ar2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour D.F., Saleh D.O., Mostafa R.E. Genistein ameliorates cyclophosphamide-induced hepatotoxicity by modulation of oxidative stress and inflammatory mediators. Open Access Maced. J. Med. Sci. 2017;5(7):836. doi: 10.3889/oamjms.2017.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masubuchi Y., Nakayama S., Horie T. Role of mitochondrial permeability transition in diclofenac-induced hepatocyte injury in rats. Hepatology. 2002;35(3):544–551. doi: 10.1053/jhep.2002.31871. [DOI] [PubMed] [Google Scholar]
- Mohamed A.A., Galal A.A., Elewa Y.H. Comparative protective effects of royal jelly and cod liver oil against neurotoxic impact of tartrazine on male rat pups brain. Acta Histochem. 2015;117(7):649–658. doi: 10.1016/j.acthis.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Mostafa R.E. Potential anti-oxidant and anti-inflammatory effects of losartan against thioacetamide-induced hepatic damage in rats. Lat. Am. J. Pharm. 2018;37(6):1089–1096. [Google Scholar]
- Mostafa R.E., Dalia O., Dina F. Cisplatin-induced nephrotoxicity in rats: modulatory role of simvastatin and rosuvastatin against apoptosis and inflammation. J. Appl. Pharmaceut. Sci. 2018;8(4):43–50. [Google Scholar]
- Mózsik G., Moron F., Jávor T. Cellular mechanisms of the development of gastric mucosal damage and of gastrocytoprotection induced by prostacyclin in rats. A pharmacological study. Prostaglandins Leukot. Med. 1982;9(1):71–84. doi: 10.1016/0262-1746(82)90074-9. [DOI] [PubMed] [Google Scholar]
- Nathan C. Inducible nitric oxide synthase: what difference does it make? J. Clin. Invest. 1997;100(10):2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouri A., Heidarian E., Nikoukar M. Effects of N-acetyl cysteine on oxidative stress and TNF-alpha gene expression in diclofenac-induced hepatotoxicity in rats. Toxicol. Mech. Methods. 2017;27(8):561–567. doi: 10.1080/15376516.2017.1334732. [DOI] [PubMed] [Google Scholar]
- Oka H., Emori Y., Kobayashi N., Hayashi Y., Nomoto K. Suppression of allergic reactions by royal jelly in association with the restoration of macrophage function and the improvement of Th1/Th2 cell responses. Int. Immunopharm. 2001;1(3):521–532. doi: 10.1016/s1567-5769(00)00007-2. [DOI] [PubMed] [Google Scholar]
- Prince S.E. Diclofenac-induced renal toxicity in female Wistar albino rats is protected by the pre-treatment of aqueous leaves extract of Madhuca longifolia through suppression of inflammation, oxidative stress and cytokine formation. Biomed. Pharmacother. 2018;98:45–51. doi: 10.1016/j.biopha.2017.12.028. [DOI] [PubMed] [Google Scholar]
- Pyrzanowska J., Piechal A., Blecharz-Klin K., Joniec-Maciejak I., Graikou K., Chinou I. Long-term administration of Greek Royal Jelly improves spatial memory and influences the concentration of brain neurotransmitters in naturally aged Wistar male rats. J. Ethnopharmacol. 2014;155(1):343–351. doi: 10.1016/j.jep.2014.05.032. [DOI] [PubMed] [Google Scholar]
- Ramm S., Mally A. Role of drug-independent stress factors in liver injury associated with diclofenac intake. Toxicology. 2013;312:83–96. doi: 10.1016/j.tox.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Ratliff B.B., Abdulmahdi W., Pawar R., Wolin M.S. Oxidant mechanisms in renal injury and disease. Antioxidants Redox Signal. 2016;25(3):119–146. doi: 10.1089/ars.2016.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silici S., Ekmekcioglu O., Kanbur M., Deniz K. The protective effect of royal jelly against cisplatin-induced renal oxidative stress in rats. World J. Urol. 2011;29(1):127–132. doi: 10.1007/s00345-010-0543-5. [DOI] [PubMed] [Google Scholar]
- Sullivan G.W., Sarembock I.J., Linden J. The role of inflammation in vascular diseases. J. Leukoc. Biol. 2000;67(5):591–602. doi: 10.1002/jlb.67.5.591. [DOI] [PubMed] [Google Scholar]
- Takeuchi K., Tanaka A., Kato S., Amagase K., Satoh H. Roles of COX inhibition in pathogenesis of NSAID-induced small intestinal damage. Clin. Chim. Acta Int. J. Clin. Chem. 2010;411(7–8):459–466. doi: 10.1016/j.cca.2009.12.026. [DOI] [PubMed] [Google Scholar]
- Teixeira R.R., de Souza A.V., Peixoto L.G., Machado H.L., Caixeta D.C., Vilela D.D. Royal jelly decreases corticosterone levels and improves the brain antioxidant system in restraint and cold stressed rats. Neurosci. Lett. 2017;655:179–185. doi: 10.1016/j.neulet.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Yasmeen T., Qureshi G.S., Perveen S. Adverse effects of diclofenac sodium on renal parenchyma of adult albino rats. JPMA J. Pakistan Med. Assoc. 2007;57(7):349–351. [PubMed] [Google Scholar]
- Zhang S., Nie H., Shao Q., Hassanyar A., Su S. RNA-Seq analysis on effects of royal jelly on tumour growth in 4T1-bearing mice. J. Funct. Foods. 2017;36:459–466. [Google Scholar]