Abstract
Alzheimer's disease (AD) is a progressive neurodegenerative disease and its incidence will increase with age and is aggravating. The senile plaques (SPs) are one of three main pathological features in AD patients, which are formed by amyloid β-protein (Aβ) over-accumulation. β-amyloid precursor protein (APP), β-site APP cleavage (BACE1), and insulin degrading enzyme (IDE) proteins participate in the process of Aβ production and degradation. At present, the pathogenesis of AD is not yet clear and the current treatment methods can only relief the related symptoms of AD. The electro-acupuncture (EA) is a traditional Chinese medicine treatment combined the acupuncture and electrical stimulation and the treatment effect can also be controlled by transform the electrical frequency. Thus, in this experiment, we carried out behavioral test, immunohistochemistry (IHC), and Western Blot (WB) after different period treatments to the model mice by electro-acupuncturing “Baihui” and “Shenshu” acupoints in APP+/PS1+ double transgenic mice. It was found that the EA therapy can improve the ability of learning, memory and spatial exploration, and reduce the deposition of SPs in brain of AD model mice, and reduce the expressions of APP and BACE1, increase the expression of IDE protein. These results prompt that EA can effectively alleviate the pathological process of AD. We speculate that EA may play a comprehensive role in preventing the development of AD, considering the previous data.
Key words: Alzheimer's disease, Amyloid β-protein, Electro-acupuncture, Senile plaques, Transgenic mice
Abbreviations: AD, Alzheimer's disease; Aβ, amyloid β-protein; SPs, senile plaques; APP, β-; amyloid precursor protein, BACE1; β-, site APP cleavage; IDE, insulin degrading enzyme; EA, electro-acupuncture; IHC, immunohistochemistry; SOD, superoxide dismutase; Ach, aetylcholine; ChAT, aetylcholine tansferase; AchE, cholinesterase
Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disease with progressive memory deficits and cognitive impairments. It is estimated to have more than 30 million AD patients worldwide in 2010 and expected 106 million by 2050.1 In China, the incidence of AD is 2.57% for people over 55 years old and 4.6% for over 65 years old.2 The incidence of AD will increase with age and is aggravating, brings heavy burden to the family and society.3 The current physical and medical treatment methods which can only relieve the related symptoms to a certain degree have the corresponding side effects at the same time.4 Although the study of AD has made great progress, however, there is not an absolutely effective treatment of AD so far. Thus, the identification of effective and safe treatments with clear functional mechanisms is urgently needed.
The electro-acupuncture (EA) application is a traditional Chinese medicine treatment way combined the acupuncture and electrical stimulation and the treatment effect can also be controlled by transform the electrical frequency.5, 6 Some previous literature reported that manual acupuncturing or electro-acupuncturing some acupoints could improve the cognitive symptoms of AD model animals which were mainly made by injected intraperitoneally d-galactose or AlCl3.7, 8 Additionally, several studies have found that the effect of EA for improving cognitive symptoms of AD animals is associated with a lot of pathways. For example, EA at the “Baihui” and “Shenshu” acupoints improves learning and memory function through downregulating Notch signaling pathway or increasing PPAR-γ expression and reducing p-p38MAPK expression and attenuating Aβ in the AD rat model.9, 10
As is known, the main pathological features of AD patients are the senile plaques (SP), neurofibrillary tangles and loss of neurons. Potential mechanisms causing the pathology has not been elucidated, and many hypotheses about, including gene mutations, tau hyperphosphorylation, cholinergic neuronal damage.7 The Aβ cascade hypothesis is recognized as the cause of the generation of SP in AD patients.11 Therefore, many studies focused on it. A previous study has demonstrated that EA at “Baihui” acupoint ameliorated learning and memory deficits and reduced Aβ42 deposit in APP+/PS1+ mice and propose that the promotion of neurogenesis in the hippocampus and cortex would be the underlying mechanism of EA.12 Anyway, the therapeutic effect of EA treatment for AD has shown in many works. However, it is seldom reported that EA stimulation regulates the number of SPs and the expression of its related proteins in the APP+/PS1+ model mice.
In view of the above, according to the theory of Traditional Chinese Medicine, acupuncture points of “Baihui” and “Shenshu” were applied in this experiment.13 We evaluated the effect of EA on the formation mechanism of SPs in the APP+/PS1+ double transgenic mice. Therefore, Morris water maze test, IHC and WB technique were used to observe spatial learning and memory ability, the expression of SP and level of the related proteins APP, BACE and IDE of mice.
Materials and methods
Animals and ethics
Male APP+/PS1+ double transgenic mice (APPswe and PS1ΔE) and age-matched wild-type (C57BL/6J) mice were purchased from Beijing Huafukang Biotechnology Co., Ltd. (Beijing, China). Mice were housed in a controlled environment (21–25 °C; 50 ± 10% relative humidity and automatic 12 h light/dark cycle) with access to food and water adlibitum in the Animal Experiment Center of Chongqing Medical University.
Furthermore, all operations were carried out strictly in accordance with international ethical guidelines and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Gene identification
The procedure of DNA identification was carried out to determine the reliability of APP+/PS1+ double transgenic mice. The tails of mice were cut with a length of about 3–5 mm after the mice were fed for about a week. Total DNA was isolated from the mouse by using the DNA extraction kit (Beijing Tiangen Biochemical Technology Co., Ltd.) and was amplified by PCR.
The PCR reaction system was under the following conditions: initial denaturation for 5 min at 94 °C, followed by 35 cycles of denaturation for 30 s at 94 °C, annealing for 40 s at 58 °C, and extension for 40 s at 72 °C followed by a final extension for 10 min at 72 °C. The PCR products were resolved on agarose gels and quantitated using the Bio-Rad gel Imager (90 V constant voltage, continued for 30min). Primers used for PCR in this study are set as following. APP: forword 5′-GAC TGA CCA CTC GAC CAG GTT CTG - 3′ and revise 5′-CTT GTA AGT TGG ATT CTC ATA TCC G - 3′; PS1: forword 5′-AAT AGA GAA CGG CAG GAG CA - 3′ and revise 5′-GCC ATG AGG GCA CTA ATC AT - 3′.
Grouping and method of EA treatment
Mice were randomly divided into four groups: the control group (wild type mouse), the model group (APP+/PS1+ double transgenic mouse), the 2 courses of EA treatment group (APP+/PS1+ double transgenic mouse which were dealt with EA for 2 weeks) and the 3 courses of EA treatment group (APP+/PS1+ double transgenic mouse which were dealt with EA for 3 weeks), six mice in each group. The control group was consisted of the wild type mice, the other three groups were consisted of APP+/PS1+ double transgenic mice.
According to Experimental Acupuncture, the point of “Baihui" (DU 20)and “Shenshu" (BL 23)of the mouse could be located precisely. The EA needle (diameter, 0.18 mm, Beijing Zhongyantaihe Medical Devices Co., Ltd., Beijing China) was inserted at a depth of 2 mm into the DU20 acupoint, which is located at the intersection of the sagittal midline and the line linking the two ears. The BL23 acupoint was inserted at a depth of 4 mm, which is located 1 mm next to the spinous process of the second vertebral body. Stimulation was generated using EA apparatus (model 6805-AⅡ; Shantou Medical Equipment Factory, Guangdong, China) and the stimulation parameters were set as disperse waves of 2 Hz and electric current of 2 mA for 15 min once a day, and 7 days/one course, 1 day rest between two courses.
Morris water maze assay
After the EA treatment, cognitive function was tested by the Morris water maze apparatus, which was a stainless steel circular tank with a diameter of 120 cm and a height of 50 cm. The tank was filled with water (21–23 °C) to the depth of 40 cm and divided into four equal quadrants.
Visual platform experiment: On the first day, a platform (diameter, 6 cm) was placed in the third quadrant and above the surface of the water about 1 cm. Each group of mice was placed in water from four quadrants in turn according to the group number. We can stop the experiment if the mouse find the platform in 60 s. If a mouse did not find the platform within 60 s, it would be removed from the water and placed on the platform for 10 s by researchers. Record the escape latency and the search path of the mouse to find the platform.
Covert platform experiment: From the 2nd day to the 6th day, a platform was submerged 0.5 cm below the surface of the water. Mice were allowed to swim in pool during a period of 60 s to find the hidden platform. Recorded the escape latency and the search path of the mouse to find the platform.
Space exploration experiment: On the 7th day, the platform was removed and each mouse was allowed to swim freely for 60 s. Recorded the times of passing the hidden platform within 60 s to assess spatial reference learning and memory.
Tissue preparation
After the completion of the water maze experiment, animals were terminally anesthetized with 3.5% chloral hydrate (10 mL/kg) by intraperitoneal injection and transcardially perfused with 0.9% sodium chloride solution. Brains were immediately dissected. For Immunohistochemistry (IHC) assay, the brain tissues for each animal were postfixed for 24–48 h in the 4% paraformaldehyde solution, and then dehydrated with gradient alcohol and wrapped in paraffin. Finally, serial coronal cryosections (5 μm thick) were cut on a paraffin slicer (Leica RM2235). For Western blot analysis, the fresh tissues of cortex and hippocampus of each animal were separated and stored at −80 °C independently.
Immunohistochemistry assay
IHC was performed on the 5 μm-thick coronal paraffin sections. The number of SPs was examined with DAB kits according to the manufacturer's protocols. Dissolved the insoluble Aβ in senile plaques for 10 min with 88% formic acid solution. The sections were incubated in 3% hydrogen peroxide at 37 °C for 5 min to block the non-specific protein binding. Blocked with 5% BSA blocking solution at 37 °C for 30 min. Sections were incubated with primary anti-4G8 antibody (1: 250; mouse, monoclonal; Convance Inc, USA) at 4 °C overnight and subsequently incubated with secondary antibody at 37 °C for 30 min. SPs were stained brown by the DAB staining liquid and hematoxylin was used to visualize the nuclei of all cells. Images of SP deposition in the hippocampus were captured using an optic microscope (Leica DM2000, Germany). All experiments were repeated three times independently.
Western Blot assay
The cortex and hippocampal samples were homogenized and centrifuged, then the supernatant was separated. BCA method was used to detect the concentration of protein. The extracted proteins were inactivated by protein loading buffer.
A total of 50 μg protein was separated by electrophoresis (80 V for 120 min) on 10% SDS-PAGE gels (Nanjing Kingsley Biological Technology Co., Ltd. China.). Proteins were transferred onto PVDF membranes (Millipore, USA). The membranes were blocked for 2 h with blocking solution at 37 °C and incubated with antibodies against APP (1: 1000; rabbit, monoclonal; Abcam, USA), BACE1 (1: 500; rabbit, monoclonal; Cell Signaling Technology, USA), IDE (1: 1000; rabbit, monoclonal; Abcam, USA), β-actin (1: 1000; Nanjing Zhongding Biological Technology Co., Ltd.) at 4 °C overnight and subsequently incubated with secondary antibody (1:10000; goat anti-rabbit) for 30 min. The protein bands were visualized with enhanced chemiluminescence luminescence and imaged with the Bio-Image Analysis system. All experiments were repeated three times independently. The ratio of the protein band intensities to the β-actin was determined as the statistical data for analysis.
Statistical analysis
All data were presented as means ± SD. All data were analyzed by the SPSS statistical and Graphpad software. Independent sample t test was used for the comparison between the control group and the model group, one-way ANOVA was used for the comparison between the other three groups. The difference was statistically significant with p < 0.05.
Results
Gene identification
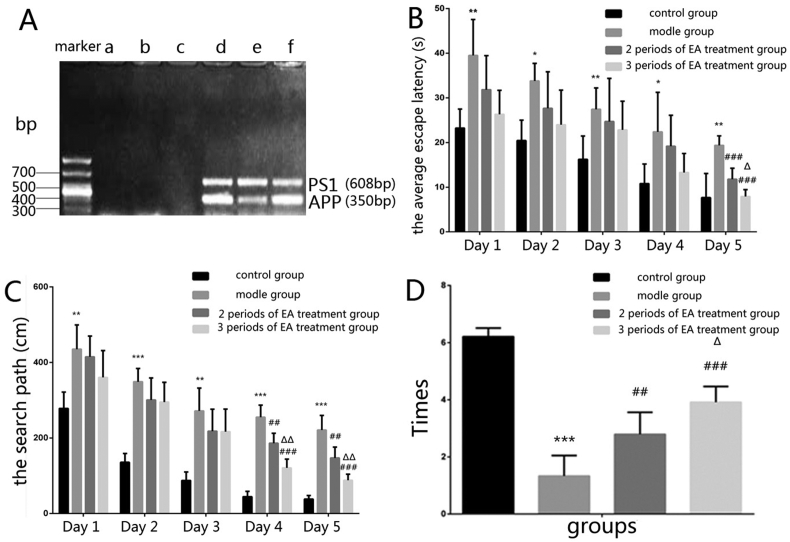
The samples from the APP+/PS1+ double transgenic mice had APP and PS1 expression bands at the same time, while the samples from the WT mice were negative in APP and PS1 bands. The results showed that all the mice that we used in this study were credible (Fig. 1A).
Figure 1.
A Gene identification of experimental model mice. a,b,c were wild-type C57BL/6J mice, d,e,f were APP+/PS1+ double transgenic mice. B The comparison of the average escape latency time (s) by each group of mice in the covert platform experiment. *p < 0.05 and **p < 0.01 versus the control group, ###p < 0.001 versus the model group, ΔP < 0.05 versus the 2 periods of EA treatment group. C The comparison of the search path (cm) by each group of mice in the covert platform experiment. **p < 0.01 and ***p < 0.001 versus the control group, ##p < 0.01 and ###p < 0.001 versus the model group, ΔΔP < 0.01 versus the 2 periods of EA treatment group. D The comparison of the times of passing the hidden platform in the space exploration experiment. ***p < 0.001 versus the control group, ##p < 0.01 and ###p < 0.001 versus the model group, ΔP < 0.05 versus the 2 periods of EA treatment group (n = 6 in each group).
The effect of the learning, memory and spatial exploration ability by EA treatment
In the visual platform experiment, the escape latency and the search path between each group mice had no statistical significance. This result showed that the vision between the four groups mice has no significant difference.
In covert platform experiment, the escape latency and the search path of the model group mice were increased compared with the control group (p < 0.05). The two treatment groups mice were decreased compare with the model group (p < 0.01). It changed obviously with the 3 periods of EA treatment group mice than that of the 2 periods of EA treatment group (p < 0.05).
In space exploration experiment, the model group mice had less the times of passing the hidden platform than the control group (p < 0.001). The treatment group mice had more passing times than the model group (p < 0.01, p < 0.001). The passing times of the 3 periods of EA treatment group mice were increased than that of the 2 periods of EA treatment group (p < 0.05) (Fig. 1 B–D).
These results suggested that EA treatment markedly improved the earning, memory and spatial exploration ability.
The effect of the SP deposition by EA treatment
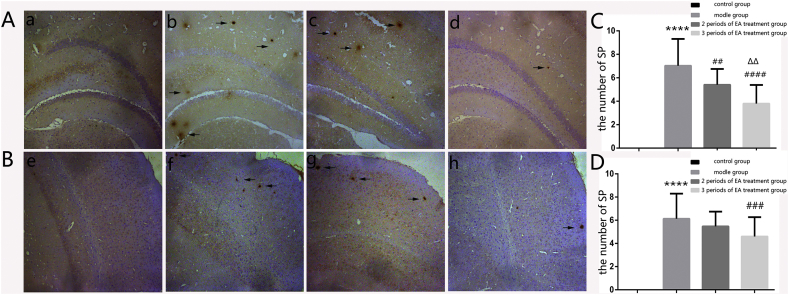
The model group had more SPs both in the hippocampus (p < 0.0001) and the cortex (p < 0.0001) than the control group. The amount of SPs of the two treatment groups mice were distinctly reduced in the hippocampus (p < 0.01, p < 0.0001) and the cortex (p < 0.001) than that of the model group. The 3 periods of EA treatment group mice were obviously reduced in the hippocampus (p < 0.01) compared with the 2 periods of EA treatment group. There were no statistically significant differences between the 2 periods of EA treatment group and not only the model group but also the 3 periods of EA treatment group in the cortex (Fig. 2).
Figure 2.
A The changes of senile plaques (SPs) in the hippocampus. B The changes of SPs in the mice cortex. The stained SPs were indicated by the black arrow, a,e: the control group, b,f: the model group, c,g: the 2 periods of EA treatment group, d,h: the 3 periods of EA treatment group. C The number of staining for SPs in the hippocampus of each group. ****p < 0.0001 versus the control group, ##p < 0.01 and ####p < 0.0001 versus the model group, ΔΔP < 0.01 versus the 2 periods of EA treatment group. D The number of staining for SPs in the cortex of each group. ****p < 0.0001 versus the control group, ###p < 0.001 versus the model group (n = 6 in each group).
This result suggested that EA treatment markedly decreased the SP deposition of the hippocampus and cortex in APP+/PS1+ double transgenic mice.
The effect of the expression of the SP-related proteins by EA treatment
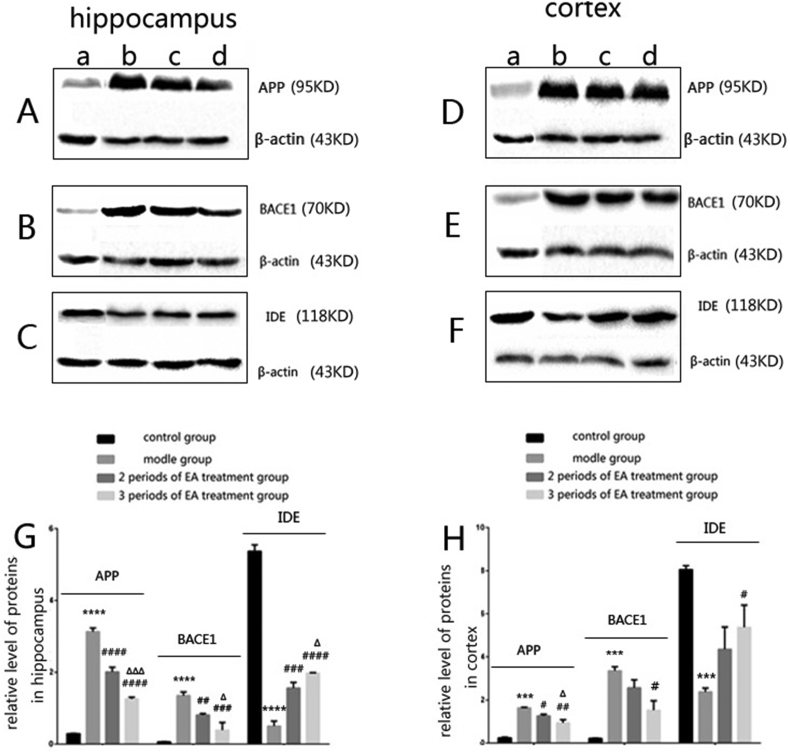
The expression of APP in the hippocampus (p < 0.0001) and cortex (p < 0.001) had increased compared with the control group. The expression of APP in the two treatment group mice were significantly decreased both in the hippocampus (p < 0.0001) and the cortex (p < 0.05, p < 0.01) than that of the model group. The 3 periods of EA treatment group mice had less expression of APP in the hippocampus (p < 0.001) and cortex (p < 0.05) than the 2 periods of EA treatment group mice (Fig. 3 A and D).
Figure 3.
A、B、C The expression of APP 、BACE1、IDE in the hippocampus of each group of mice. D, E, F The expression of APP, BACE1, DE in the cortex of each group of mice. a: the control group. b: the model group. c: the 2 periods of EA treatment group. d: the 3 periods of EA treatment group. G The level of APP, BACE1,IDE in the hippocampus was indicated by column chart of each group of mice. APP: ****p < 0.0001 versus the control group, ####p < 0.0001 versus the model group, ΔΔΔP < 0.001 versus the 2 periods of EA treatment group. BACE1: ****p < 0.0001 versus the control group, ##p < 0.01 and ###p < 0.001 versus the model group, ΔP < 0.05 versus the 2 periods of EA treatment group. IDE: ****p < 0.0001 versus the control group, ###p < 0.001 and ####P < 0.0001 versus the model group, ΔP < 0.05 versus the 2 periods of EA treatment group. H The level of APP 、BACE1、IDE in the cortex was indicated by column chart of each group of mice. APP: ***p < 0.001 versus the control group, #p < 0.05 and ##p < 0.01 versus the model group, ΔP < 0.05 versus the 2 periods of EA treatment group. BACE1: ***p < 0.001 versus the control group, #p < 0.05 versus the model group. IDE: ***p < 0.001 versus the control group, #p < 0.05 versus the model group, (n = 6 in each group).
The expression of BACE1 in the model group mice were significantly increased in the hippocampus (p < 0.0001) and the cortex (p < 0.001) compared with the control group. The expression of BACE1 in the treatment groups mice were decreased both in the hippocampus (p < 0.01, p < 0.001) and cortex (p < 0.05) compared with the model group. The 3 periods of EA treatment group mice had less expression of BACE1 in the hippocampus (p < 0.05) than the 2 periods of EA treatment group (Fig. 3 B and E).
The expression of IDE in model group mice were significantly decreased compared with the control group in the hippocampus (p < 0.0001) and cortex (p < 0.001). The expression of IDE in the two treatment groups mice were distinctly increased both in the hippocampus (p < 0.001, p < 0.0001) and cortex (p < 0.05) compared with the model group. It was obviously increased in the hippocampus (p < 0.05) of the 3 periods of EA treatment group mice compared with that of the 2 periods of EA treatment group (Fig. 3 C and F).
There were no statistically significant differences between the 2 periods of EA treatment group and not only the model group but also the 3 periods of EA treatment group about the expression of BACE1 and IDE in the cortex (Fig. 3 E and F).
These results suggested that EA treatment markedly decreased the expression of APP and BACE1 and increased the IDE of the hippocampus and cortex in APP+/PS1+ double transgenic mice (Fig. 3 G and H).
Discussion
AD is a degenerative disease of the central nervous system. The Aβ cascade hypothesis is the most accepted one in many hypotheses of AD. This hypothesis agrees that the SPs in AD patients’ brain is formed by the accumulation of Aβ fragments produced by the abnormal processing of APP.14 The APP+/PS1+ double transgenic mouse is characterized by the progressive increase in SPs deposition as the month grows. It expresses a mutated allele of the human APP and PS1 genes.15 The deposition of SPs is the main pathological features of AD patients, and also most likely the direct cause of AD. So, it is the ideal animal model of AD to simulate the pathological features of AD patients in all aspects.11 Therefore, in this study, the wild-type mice and APP+/PS1+ double transgenic AD model mice were selected as the experimental animals.
The SP which is formed by Aβ over-accumulation is one of the typical pathological features of the AD. Aβ is produced by the cutting of APP through the β- and γ-secretase. There are two forms of Aβ: Aβ40 and Aβ42. Aβ40/Aβ42 is equal to 10:1 under normal circumstance, too much Aβ42 tends to form SPs in the brain.15 The concentration of Aβ in the body is mainly regulated by the process of its formation and degradation. BACE1 is a kind of β-secretase and is the key rate-limiting enzyme in the Aβ production process, which increases its concentration, and IDE is an important hydrolase that catalyzes the degradation of Aβ, which reduces its concentration.16, 17, 18 In conclusion, the level of APP, BACE1 and IDE were considered to be important factors affecting the deposition of SPs.
In previous numerous animal studies, EA and manual acupuncture have demonstrated therapeutic utility for the treatment of AD.19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 In this work, the results of Morris water maze experiment suggest that the EA treatment can significantly improve the learning, memory and spatial exploration ability of APP+/PS1+ double transgenic mice. This result is consistent with the existing research conclusion. Previous studies reported that acupuncture has positive effects on cognition in AD and dementia by modulating neuronal signaling pathways. These pathways, which are suggested to mediate the beneficial effects of acupuncture on cognitive and physiological functions in animal models, involve those related to cell survival, apoptosis, and glucose metabolism.12, 19, 23, 25, 27, 29, 30 However, little studies show the effect of EA on the mechanism about deposition of SPs and its related proteins in the APP+/PS1+ model mice. We observed that EA treatment cut down the level of SP, APP and BACE1, and also enhance the level of IDE in the hippocampus and cortex of the APP+/PS1+ model mice, which suggest that the EA treatment can effectively lessen the deposition of SPs by reducing the expression of APP and BACE1 and increasing the expression of the IDE in the APP+/PS1+ model mice.
The earliest expression of AD is the loss of memory caused by the hippocampal neuron lesions, then there is cerebral cortex lesions, eventually lead to memory loss and cognitive functions like language and space decreased.31 As was reported, there is fiber projection between the hippocampus and the cortex to connect each other.32 Thus, the neurons of both districts play a critical role in the development of AD. The results in this study revealed that, in the cortex, there are no significantly effects after 2 periods of EA treatment about the amount of SPs and the expression of BACE1 and IDE, but the EA treatment of 3 periods has a significant effect. Nevertheless, SP and these related proteins in hippocampus have already changed in 2 periods of EA treatment. The insufficient treatment time may be the cause of the phenomenon, this prompts us. At the same time, given the different manifestations of the cortex and hippocampus, we speculate that it may rescue the hippocampus neurons at first by EA treatment, and the effects of EA on the cortex may be produced by the recovering of the fiber projection from hippocampus to cortex, and this recovering duration takes time. Therefore, we can extend the treatment time to assess the relationship between the time and efficacy of the EA treatment.
As is reported, from the neurotransmitter point of view, it has been found that the EA was given “Baihui”, “Shenshu”, “Zusanli” can improve the activity of the aetylcholine tansferase (ChAT) and inhibit the cholinesterase (AchE) in cortex of rat. Thus, it increases the content of aetylcholine (Ach) in brain tissue and reverses the decline of its memory.33 In terms of Anti-oxidize effect, some researchers believe that the EA was given “Baihui”, “Dazhui”, “Shenshu”, “Taixi”, “Zusanli” can improve the activity of free radical scavenging enzymes-superoxide dismutase (SOD) in the brain and thus improve the learning and memory abilities of AD model rats.34 In the aspect of brain morphology and histopathology, researchers found that cells in the hippocampus of AD model rats are arranged more neatly after EA treatment of “Shenshu”, “Neiguan”, “Dazhui”.35 These above studies of AD model animals for EA treatment and the results in this work together suggest that EA treatment may exert a comprehensive effect in preventing the development of AD. However, some problems are present in these studies, such as, there is no uniform standard in aspect of acupoint option. Moreover, the therapeutic mechanism of EA simulation was explored in different studies through using various acupoints. But, we have not yet known whether there has the definite link between the different acupoints and its corresponding treatment mechanisms. In addition, the pathogenesis of AD is also associated with tau hyperphosphorylation, estrogen and insulin, etc. How EA affects those factors and their relationship, we don't know yet. So, we will explore them in the further studies.
In summary, the EA treatment can reduce the formation of SP on the AD patients. The results of these studies provide evidences for the widespread application of the EA in the AD clinical treatment.
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Acknowledgments
The present study was supported by the National Natural Science Foundation of China (NO. 81273870), Chongqing Science and Technology Commission Basic and Frontier Project (NO. cstc2014jcyjA10028) and Chongqing Yuzhong Technology Project (NO. 20150122).
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Liu W.L., Zhuo P.Y., Tao J. Activation of brain glucose metabolism ameliorating cognitive impairment in APP/PS1 transgenic mice by electroacupuncture. Free Radic Biol Med. 2017;112:174–190. doi: 10.1016/j.freeradbiomed.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Song X., Hong Y.R., Hu Q.Y. The research progress of the causes and mechanisms of the Alzheimer's disease. J Clin Exp Med. 2015;14:871–873. [Google Scholar]
- 3.He G.N. China aging trend and Alzheimer's disease and prevention. J Clin Med. 2016;3:8083–8084. [Google Scholar]
- 4.Yin Y.F., Tong Y.G., Qing H. Progress in the treatment of Alzheimer's disease. Lett Biotechnol. 2013;24:276–279. [Google Scholar]
- 5.Tang Q. Hubei: Hubei university of traditional Chinese medicine; 2014. The Influence of Acupuncture on Netrins and the Protein of Axonal Growth Inhibition MAG in AD Model Rats. [Google Scholar]
- 6.Han X.B., Jiang J., Li Z.G. The effects of electro-acupuncture on the PS1 protein expression in the hippocampus of mice SAMP8. Prog Mod Biomed. 2015;15:4225–4229. [Google Scholar]
- 7.Lin Y.M. Guangzhou University of Chinese Medicine; Guangzhou: 2014. The Action Mechanism of Zhi San Zhen in Treating AD Mode Rats. [Google Scholar]
- 8.Wang S.J., Kang S.B., Li A.Y. Effect of acupuncture on free radical system and cholinergic system in the brain of Alzheimer's disease rats. Acupunct Res. 2004;29:102–107. [Google Scholar]
- 9.Guo H.-D., Tian J.-X., Zhu J. Electroacupuncture suppressed neuronal apoptosis and improved cognitive impairment in the ad model rats possibly via downregulation of notch signaling pathway. Evid base Compl Alternative Med. 2015 doi: 10.1155/2015/393569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang M., Xv G.-H., Wang W.-X., Meng D.-J., Ji Y. Electroacupuncture improves cognitive deficits and activates PPAR-γ in a rat model of Alzheimer's disease. Acupunct Med. 2017;35:44–51. doi: 10.1136/acupmed-2015-010972. [DOI] [PubMed] [Google Scholar]
- 11.Long Z.M., Zhao L., He G.Q. The effects of valproic acid on senile plaques and neuron in the brain of APP/PS1 double transgenic AD modle mouse. J Chin Neuropsychiatric Dis. 2011;37:477–481. [Google Scholar]
- 12.Li X., Guo F., Zhang Q. Electroacupuncture decreases cognitive impairment and promotes neurogenesis in the APP/PS1 transgenic mice. BMC Complementary and Alternative Medicine. 2014;14 doi: 10.1186/1472-6882-14-37. Article 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X.J. Hebei Medical University; Hebei: 2014. The Experimental Study on the Effect of Electroacupuncture on Single Amine Neurotransmitter and Oxygen Free Radicals on Mice with Vascular Dementia. [Google Scholar]
- 14.Cheng F. Anhui University; Anhui: 2017. Impaired in Vitro Neuronal Differentiation Capability of Mouse APP/PS1ΔE9 Transgenic Embryonic Stem Cells. [Google Scholar]
- 15.Liu Y., He G.Q., Qin W.X. Expression of myeloid beta peptide and its related matebolic enzymes in the brain of model mice with Alzheimer's disease and type 2 diabetes mellitus. Basic & Clin Med. 2017;37:653–657. [Google Scholar]
- 16.Chen T., Long Z.M., He G.Q. Effect of valproic acid on autonomous behaviors and cerebral morphology and structure in APP/PS1 double transgenic mice. J Third Mil Med Univ. 2012;34:870–873. [Google Scholar]
- 17.Llovera R.E., de Tullio M., Alonso L.G. The catalytic domain of insulin -degrading enzyme forms a denaturantresistant complex with amyloid b peptide: implications for Alzheimer's disease pathogenesis. J Biol Chem. 2008;283:17039–17048. doi: 10.1074/jbc.M706316200. [DOI] [PubMed] [Google Scholar]
- 18.Haque R., Nazir A. Identification and functional characterization of a putative IDE, C28F5.4(ceIDE-1), in caenorhabditis elegans: implications for Alzheimer's disease. Biochim Biophys Acta. 2016;1860:2454–2462. doi: 10.1016/j.bbagen.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Cheng H., Yu J., Jiang Z. Acupuncture improves cognitive deficits and regulates the brain cell proliferation of SAMP8 mice. Neurosci Lett. 2008;432:111–116. doi: 10.1016/j.neulet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Dong W.G., Guo W.Q., Zheng X.H. Electroacupuncture improves cognitive deficits associated with AMPK activation in SAMP8 mice. Metab Brain Dis. 2015;30:777–784. doi: 10.1007/s11011-014-9641-1. [DOI] [PubMed] [Google Scholar]
- 21.Dong W., Guo W., Wang F. Electroacupuncture upregulates SIRT1-dependent PGC-1αexpression in SAMP8 mice. Med Sci Mon Int Med J Exp Clin Res. 2015;21:3356–3362. doi: 10.12659/MSM.894864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong W.G., Wang F., Chen Y. Electroacupuncture reduces Aβ production and bace1 expression in SAMP8 mice. Frontiers in Aging Neuroscience. 2015;7(148) doi: 10.3389/fnagi.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo H.D., Tian J.X., Zhu J. Electroacupuncture suppressed neuronal apoptosis and improved cognitive impairment in the ad model rats possibly via downregulation of notch signaling pathway. Evid base Compl Alternative Med. 2015;2015:393569. doi: 10.1155/2015/393569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang J., Gao K., Zhou Y. Electroacupuncture treatment improves learning-memory ability and brain glucose metabolism in a mouse model of Alzheimer's disease: using Morris water maze and micro-PET. Evid base Compl Alternative Med. 2015;2015:142129,. doi: 10.1155/2015/142129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee B., Sur B., Shim J. Acupuncture stimulation improves scopolamine-induced cognitive impairment via activation of cholinergic system and regulation of BDNF and CREB expressions in rats. BMC Compl Alternative Med. 2014;14(1) doi: 10.1186/1472-6882-14-338. Article 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin R., Chen J., Li X. Electroacupuncture at the Baihui acupoint alleviates cognitive impairment and exerts neuroprotective effects by modulating the expression and processing of brain-derived neurotrophic' factor in APP/PS1 transgenic mice. Mol Med Rep. 2016;13:1611–1617. doi: 10.3892/mmr.2015.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu Y., Huang Y., Tang C. Brain areas involved in the acupuncture treatment of AD model rats: a PET study. BMC Complementary and Alternative Medicine. 2014;14(1) doi: 10.1186/1472-6882-14-178. Article 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang F., Zhong H., Li X. Electroacupuncture attenuates reference memory impairment associated with astrocytic NDRG2 suppression in APP/PS1 transgenic mice. Mol Neurobiol. 2014;50:305–313. doi: 10.1007/s12035-013-8609-1. [DOI] [PubMed] [Google Scholar]
- 29.Zhang M., Xv G.H., Wang W.X. Electroacupuncture improves cognitive deficits and activates PPAR-γ in a rat model of Alzheimer's disease. Acupunct Med: J Br Med Acupunct Soc. 2017;35:44–51. doi: 10.1136/acupmed-2015-010972. [DOI] [PubMed] [Google Scholar]
- 30.Park Sunjung, Lee Jun-Hwan, Yang Eun Jin. Effects of acupuncture on Alzheimer's disease in animal-based research. Evid base Compl Alternative Med. 2017;2017:6512520,. doi: 10.1155/2017/6512520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W., Liu H., Ren X. The effects of helium-neon laser acupoints on the cortical and hippocampal Bcl 1 expression in the rat model of Alzheimer's disease model. Chin J Phys Med Rehabil. 2007;29:373–374. [Google Scholar]
- 32.Song Y.T., Wang S.K., Guo L.K. Cortical afferent projections of infralimbic cortex in rat. Chin J Anat. 1997;20:36–40. [Google Scholar]
- 33.Wang L.S., Zhou L.S. Effect of electro-acupuncture on Ach, ChAT and AchE in Alzheimer's disease rats. J Acupunct Clin. 2009;25:40–42. 54. [Google Scholar]
- 34.Zhang P., Guan S.S., Jiang G.H. Effects of electroacupuncture on expression of Aβ positive cells of the hippocampus and SOD activity in rats with streptozocin-Alzheimer's disease. Chin Acupunct Moxibustion. 2010;30:1007–1010. [PubMed] [Google Scholar]
- 35.Shen F., Sun G.J., Du Y.J. Effect of electro-acupuncture on pathomorphology of hippocampus with Alzheimer's disease in Rats. Chin Med Emerg. 2012;21:722–723. 739. [Google Scholar]