Abstract
Argininosuccinate lyase (ASL) plays an important role in the hepatic urea cycle, and can catalyze the reversible reaction of argininosuccinate to arginine and fumarate. However, the function of ASL in hepatocellular carcinoma (HCC) is not fully understood. In this study, we found that ASL expression was frequently upregulated in HCC tissues and HCC cell lines. Knock down of ASL inhibited cell proliferation and induced apoptosis in HCC cells. Mechanistic studies revealed the BCL2-associated X protein (Bax) signaling pathway which determines cancer cell apoptosis was regulated by ASL. Moreover, the depletion of Bax restored the inhibition of cell growth and reduced apoptosis initiated by ASL silencing. Together, the study demonstrated that ASL regulated HCC cell growth and apoptosis by modulating Bax signaling. Thus, the therapeutic targeting of ASL may offer options for HCC treatment.
KEYWORDS: Apoptosis, ASL, Bax, HCC, Proliferation
Introduction
Hepatocellular carcinoma (HCC) is the second-leading cause of cancer-related death worldwide,1 which accounts for roughly 90% of all primary liver neoplasms.2 It is the third and the fifth leading most common cancer in men and women, respectively,3 with more than 700,000 new cases of HCC being diagnosed and over 600,000 deaths yearly worldwide.4 Hepatitis B Virus (HBV) infection, Hepatitis C Virus (HCV) infection and other inflammatory liver diseases such as steatohepatitis are the most important risk factors for HCC.5 Despite the improvements have been made in HCC diagnosis and treatment, HCC still has a poor prognosis which closely related to the aggressive and highly proliferative capacity of HCC cells. Moreover, the dysregulation of cellular proliferation and apoptosis are the most frequently biological heterogeneous in HCC.6 Therefore, a better understanding of how HCC cells evade death stimuli and maintain growth is the imperative requirement for the development of new therapeutic targets for HCC therapy.
The metabolic differences are very common between normal cells and tumor cells which provide opportunities for developing novel approaches for the diagnosis and treatment of cancer.7, 8, 9 Urea circulation is an important metabolic pathway that coordinates function of six enzymes and two mitochondrial transporters to convert toxic ammonia into urea.10 Argininosuccinate lyase (ASL) is one of the six enzymes in the urea cycle, which catalyzes the reversible hydrolytic cleavage of argininosuccinate into arginine and fumarate.11 Arginine is a semi-essential amino acid and essential for rapidly proliferating cells.12, 13 However, owing to lack of argininosuccinate synthetase (ASS), a key enzyme which converts citrulline to arginine, some tumors can't synthesize arginine from urea cycle including melanoma, hepatocellular carcinoma, some mesotheliomas and some renal cell cancers.12, 14 Additionally, ASL is likely to play a key role in maintaining arginine homeostasis at the tissue level in arginine-deficient states.15 Therefore, ASL is closely related to the development of many tumors. It has been reported that the expression of ASL in colon cancer tissues was enhanced, which was highly associated with poorer survival rates, and the loss of ASL inhibited the proliferation of colon cancer cells.16 Moreover, ASL was significantly upregulated in breast cancer tissues and downregulation of ASL inhibited the growth of breast cancer in vitro and in vivo.17 In addition, epigenetic status of ASS and ASL was changed in arginine deprivation, and the epigenetic modification regulated autophagy and cell death in glioblastoma.18 Interestingly, a study showed that arginase, an arginine-depleting enzyme, could inhibit proliferation of HCC by inducing cell cycle arrest,19 suggesting arginine may play an important role in HCC progression. However, the functions and mechanisms of ASL in the development of HCC have not been clearly elucidated.
In this study, we found that ASL expression is elevated in a subset of HCC tissues and cell lines. Furthermore, we discovered depletion of ASL inhibited cell growth and induced apoptosis in HCC cells by regulating Bax signaling pathway. This work demonstrates ASL is a novel therapeutic target for HCC.
Materials and methods
Plasmids and antibodies
The lentivirus expressing shASL was obtained from Genechem (China). Anti-ASL (AV41666) was purchased from Sigma–Aldrich (USA). Anti-PARP (#9542), anti-Bax (#5023S), anti-caspase 3 (#9665), anti-caspase 9 (#9502), anti-Bcl-2 (#2872), anti-Smac (#2954S) and anti-Cytochrome C (#4280S) were obtained from Cell Signaling Technology (USA). Anti-GAPDH (sc-365062) and Anti-β-actin (sc-47778) were purchased from Santa Cruz Biotechnology (USA).
Cell culture
PLC/PRF/5, Hep3B, SK-Hep-1 and HepG2 cells were obtained from the American Type Culture Collection. Huh-7 cell line was acquired from the Health Science Research Resource Bank. MIHA cell line was obtained from Professor Ben C.B. Ko (The Hong Kong Polytechnic University). Primary human hepatocyte (PHH) was purchased from Sciencell Research Laboratories. PLC/PRF/5, Hep3B, SK-Hep-1, HepG2 and MIHA cells were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS). PHH were cultured in hepatocyte medium (HM). All cells were cultured in a humidified incubator at 37 °C with 5% CO2.
HCC specimens
All HCC tissues and the adjacent non-tumorous liver tissues were collected from the First Affiliated Hospital of Chongqing Medical University. Collection of patient samples with informed consent to an established protocol and all study procedures were approved by the Research Ethics Committee of Chongqing Medical University. Total proteins and RNA were extracted from these specimens.
Cell proliferation assay
The equal number of PLC/PRF/5 and Hep3B cells were seeded into 24-well plates and infected with lentivirus expressing ASL-targeting shRNA (shASL) or control shRNA (shCont). At indicated time, the number of cells was counted using a trypan blue exclusion assay (Thermo Fisher Scientific, USA).
Colony formation assay
PLC/PRF/5 and Hep3B cells were infected with lentivirus expressing shASL or shCont and cultured for 72 h. The cells were re-plated in 6-well plates at the density of 1000 per well and maintained in medium containing 0.25 μg/mL puromycin for two weeks. The colonies were fixed with methanol and stained with 0.5% crystal violet for 15 min. The numbers of colonies were counted.
Soft agar assay
The PLC/PRF/5 and Hep3B cells infected indicated lentivirus were re-plated in 6-well plates at the density of 1000 per well. The cells were cultured in a bottom layer of 1 mL of 0.6% agar in DMEM supplemented with 10% FBS with a top layer of 1 mL of 0.35% agar in DMEM with 10% FBS. Then cells were incubated for 3 weeks in a 5% CO2 incubator at 37 °C. Colonies were stained with 0.005% crystal violet, and the number of clones was counted.
Western blotting assay
The cells were lysed with RIPA lysis buffer containing an inhibitor cocktail (Roche Diagnostics). The protein concentration was measured by BCA method. Then 30 μg protein sample solution was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and the separated proteins were transferred to a polyvinylidene difluoride membrane. After blocking with 5% nonfat milk, the membrane was immunoblotted with relevant primary antibodies overnight at 4 °C. The immunoblotted membrane was then incubated with horseradish peroxidase-conjugated secondary antibody. The bands were visualized using an ECL Western blotting reagents (Millipore). The densities of protein bands were quantified by ImageJ Java 1.8.0_112 software.
Quantitative reverse transcription PCR (qRT-PCR)
Total RNA was extracted from tissues and cells with TRIzol Reagent (Life Technologies, USA). cDNA was synthesized from 1 μg of total extracted RNA using an iScript cDNA Synthesis kit (Bio-Rad). Relative quantification of gene expression was performed by using SYBR green (Roche, Germany) with β-actin mRNA as an internal control. Specific primers used for qRT-PCR assays were designed by our group and are listed in Supplementary Table S1.
Apoptosis analysis
Apoptosis analysis was carried out by using cell apoptosis kit (Invitrogen) as described in the manufacturer's protocol. Briefly, 2 × 105 cells were resuspended in 200 μl binding buffer, followed by incubation with 6 μl Annexin V diluted 20 times and 4 μl PI for 15 min. The stained cells were analyzed by flow cytometry.
Statistical analysis
Data were represented as means ± SEM of three independent experiments. Significant differences between two groups were determined by the t test. Significant differences among multiple groups were determined by the one way ANOVA. A value of P < 0.05 was considered statistically significant. All the statistical analyses were performed by GraphPad Prism 5.
Results
ASL was frequently upregulated in HCC
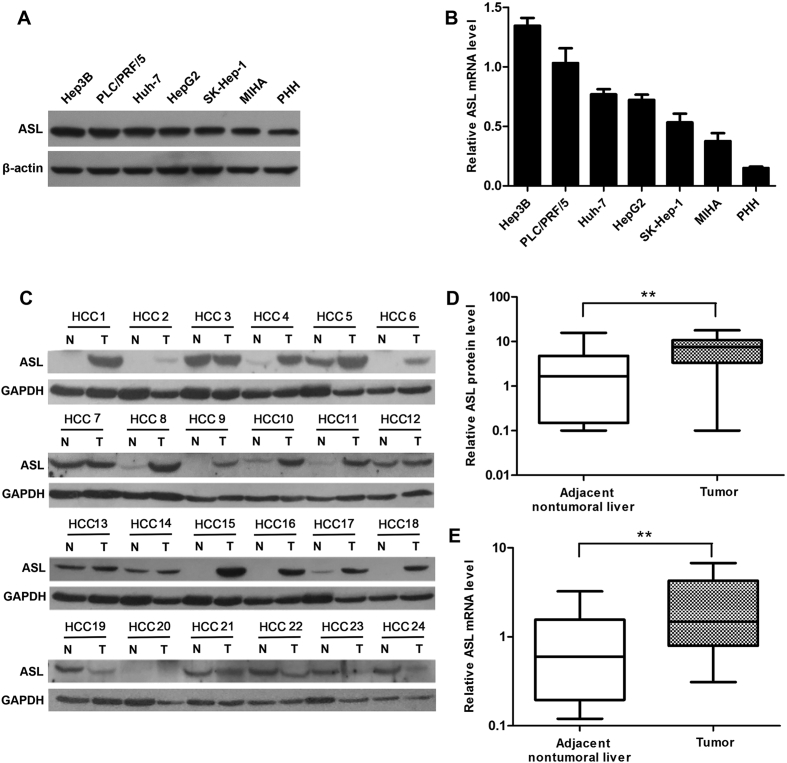
First, to study whether ASL involved in the development of HCC, we analyzed ASL expression in human liver cancer cell lines (Huh-7, HepG2, Hep3B, PLC/PRF/5, and SK-Hep-1), immortalized normal hepatic liver cells (MIHA) and primary hepatocyte (PHH) by using western blot and qRT-PCR. The results showed that among all cell lines, the protein and mRNA expression of ASL in liver cancer cell lines were both at a high level compared with the other cell lines (Fig. 1A,B). Next, we further confirmed ASL expression in 24 pairs of HCC tissues and adjacent non-tumoral liver tissues by western blot. Compared with their adjacent non-tumoral liver tissues, the ASL expression in 67% HCC tissues was up-regulated (Fig. 1C). Moreover, the average protein level of ASL in HCC tissues was significantly higher than that in adjacent non-tumoral tissues (Fig. 1D). Additionally, ASL mRNA levels in these samples were also detected by qRT-PCR. Consistently, ASL mRNA levels were significantly up-regulated in HCC tissues compared with adjacent nontumoral liver tissues (Fig. 1E). These results suggest that ASL might play a role in HCC tumorigenesis.
Figure 1.
ASL expression in HCC. (A and B) ASL protein and mRNA expression in five liver cancer cell lines, the immortalized liver cell line (MIHA), and PHH were measured by western blot and qRT-PCR, respectively. β-actin was used as a loading control for Western blot and as a reference gene for qRT-PCR. (C) Western blot analysis of ASL protein levels in 24 paired frozen HCC tissues (T) and adjacent nontumoral liver tissues (N). GAPDH was used as a loading control. (D) Quantitative analysis of ASL protein levels in 24 paired HCC tissues. **P < 0.01. (E) qRT-PCR analysis of ASL mRNA levels in 24 paired HCC tissues and adjacent nontumoral tissues. GAPDH was used as a reference gene. **P < 0.01.
ASL knockdown inhibited HCC cell proliferation
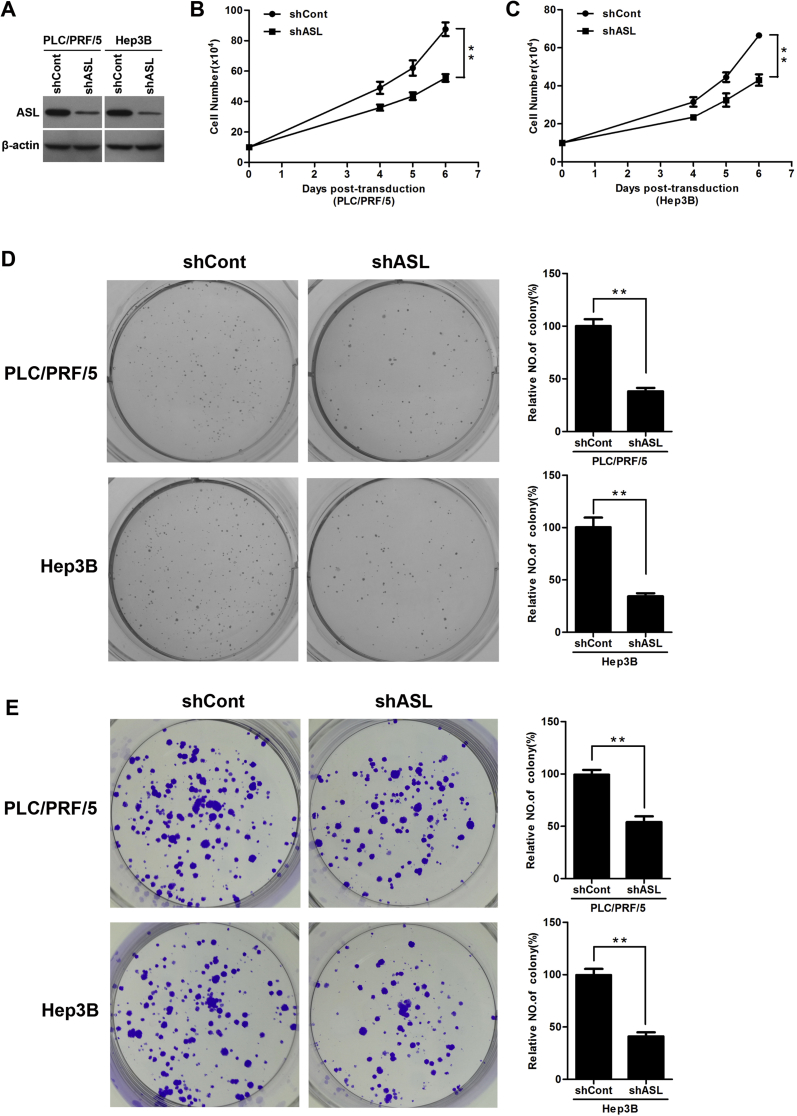
To elucidate the biological function of ASL in HCC development, The HCC cells PLC/PRF/5 and Hep3B were infected with lentivirus expressing ASL-targeting shRNA (shASL) or control shRNA (shCont). ASL was markedly silenced in both cell lines (Fig. 2A). Knock down of ASL significantly decreased the growth of PLC/PRF/5 and Hep3B cells over the course of 6 days (Fig. 2B,C). Moreover, the ASL knockdown could reduce anchorage-dependent growth of PLC/PRF/5 and Hep3B cell as determined by soft agar assays (Fig. 2D). Furthermore, the colony-formation assay showed that ASL silencing also decreased the number of PLC/PRF/5 and Hep3B cell colonies (Fig. 2E). These observations suggest that ASL exert a significant role in HCC cell growth.
Figure 2.
ASL knockdown inhibited HCC cell proliferation. (A) PLC/PRF/5 and Hep3B cells were infected with lentivirus expressing ASL-targeting shRNA (shASL) or control shRNA (shCont) for 3 days. ASL protein level was detected by western blot. β-actin was used as a loading control. (B and C) ASL knockdown significantly inhibited the proliferation of PLC/PRF/5 and Hep3B cells at indicated time points. Cell numbers were counted using a trypan blue exclusion assay. **P < 0.01. (D) Soft agar assay. PLC/PRF/5 and Hep3B cells were infected with lentiviruses as indicated and were grown for 3 weeks under antibiotic selection. The quantification of colonies was performed by counting visible colonies in each well and expressing then as a percentage relative to control cells. Columns show the mean values of triplicate experiments. **P < 0.01. (E) Colony-formation assay. PLC/PRF/5 and Hep3B cells infected with lentiviruses as indicated were cultured for 2 weeks and stained with crystal violet. All histograms show mean values from three independent experiments. **P < 0.01.
ASL knockdown induced apoptosis in HCC cells
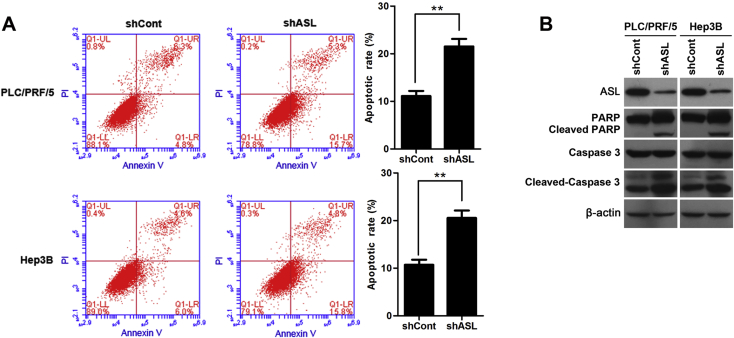
We next investigated the apoptosis-inducing ability of ASL depletion in HCC cells. It was indicated that the loss of ASL in PLC/PRF/5 and Hep3B cell significantly increased the percentage of total apoptotic cells as determined by flow cytometry using Annexin V/PI double staining (Fig. 3A). Additionally, the effect of ASL silencing on PARP cleavage and caspase 3 cleavage were analyzed by western blot. As shown in Fig. 3B, knock down of ASL significantly induced a specific cleavage of PARP and caspase 3 (Fig. 3B). These data confirm that ASL silencing could induce apoptosis in HCC cells.
Figure 3.
ASL knockdown induced apoptosis in HCC cells. (A) ASL depletion induced apoptosis in PLC/PRF/5 and Hep3B cells was analyzed by flow cytometry with Annexin V/PI. Columns show the mean values of triplicate experiments. **P < 0.01. (B) Apoptosis in PLC/PRF/5 and Hep3B cells infected with shASL or shCont was analyzed by PARP cleavage and caspase 3 cleavage analysis. β-actin was used as a loading control.
ASL regulated Bax signaling in HCC cells
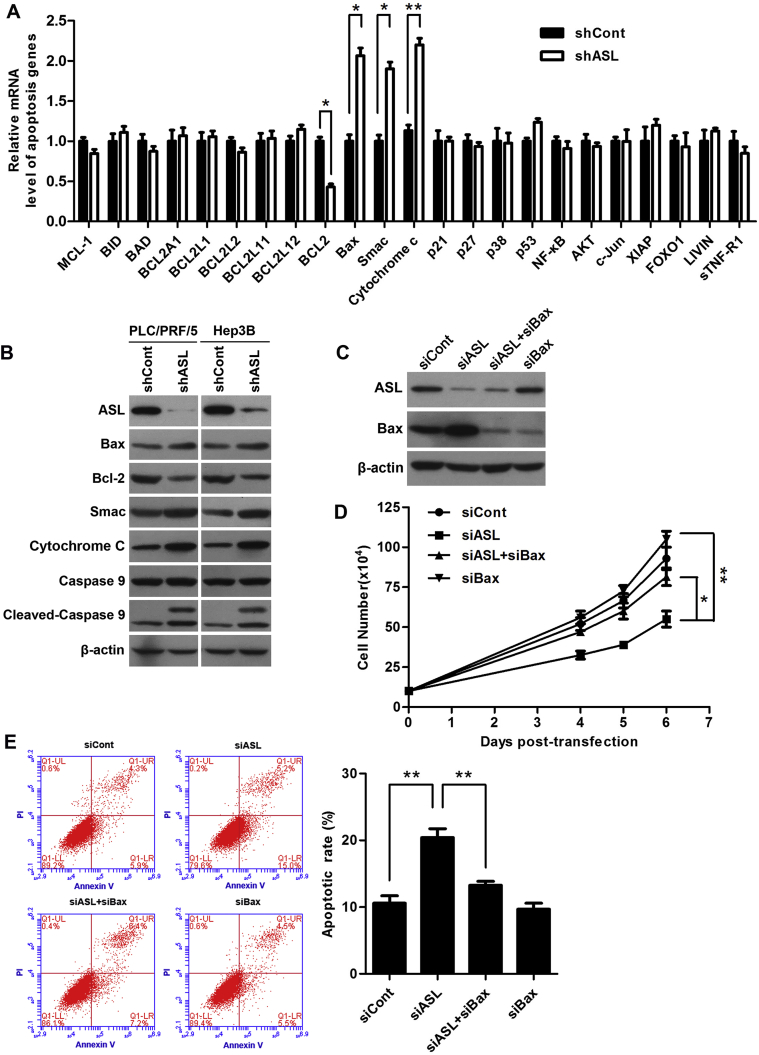
To investigate the underlying mechanisms of apoptosis induced by ASL in HCC cells, we determined the effect of ASL silencing on apoptosis signal transduction by qRT-PCR. Interestingly, the results showed the mRNA levels of Bax, Smac, Cytochrome C were increased and the mRNA level of Bcl-2 was decreased in ASL silenced cells (Fig. 4A). Moreover, western blot also validate the loss of ASL could increase the protein levels of Bax, Smac, Cytochrome C and reduce the protein level of Bcl-2 as well as enhance the cleavage of caspase 9 (Fig. 4B). These findings suggest the pro-apoptotic activity of ASL silencing was associated with Bax signaling.
Figure 4.
ASL regulated Bax signaling in HCC cells. (A) PLC/PRF/5 cells were infected with lentiviruses as indicated for 3 days. mRNA microarray analysis indicated alternations in several key regulators in the Bax-mediated apoptotic pathway. β-actin mRNA expression was used as an internal control. *P < 0.05. **P < 0.01. (B) Western blot analysis of several components of the Bax-mediated apoptotic pathway in PLC/PRF/5 and Hep3B cells transduced with shASL or shCont. β-actin was used as a loading control. (C) PLC/PRF/5 cells were transfected with indicated siRNA for 3 days. The ASL and Bax protein levels were determined by western blot. β-actin was used as a loading control. (D) Gene silencing of Bax rescued the growth inhibition induced by ASL knockdown in PLC/PRF/5 cells. Cell numbers were counted using a trypan blue exclusion assay at indicated time points. *P < 0.05. **P < 0.01. (E) Bax knockdown abolished the induction of apoptosis in ASL-silenced PLC/PRF/5 cells. Apoptosis rate was analyzed by flow cytometry with Annexin V/PI. Columns show the mean values of triplicate experiments. **P < 0.01.
For further test the possibility, we silenced the Bax expression in PLC/PRF/5 cells that were overexpressed ASL (Fig. 4C). The growth – inhibition of HCC cells induced by ASL overexpression was substantially restored by knock down of Bax protein (Fig. 4D). Importantly, the results of flow cytometry indicated that Bax silencing markedly deceased apoptosis that was induced by ASL overexpression (Fig. 4E). These data further confirm that ASL regulates HCC cell proliferation and apoptosis by Bax signaling.
Discussion
Due to the important role of arginine in maintaining cancer homeostasis, ASL which is a crucial enzyme of arginine biosynthesis involves in multiple cancer progression. In breast cancer, downregulation of ASL induced autophagy and inhibited the growth of breast cancer accompany with a delay in G2/M transition.17 Furthermore, in ASL depleted colon cancer cells, cyclin A2 degradation was induced which resulted in a G2/M arrest and the inhibition of cell proliferation, and autophagosomes were also increased.16 In addition, the role of ASL in HCC cells growth was also initially confirmed. The study suggested ASL could interact with cyclin A2 and maintain cyclin A2 protein expression as well as the process of cell cycle, which subsequently promoted HCC formation.20, 21 However, the relationship between ASL and apoptosis in HCC cells is unclear. In the present study, we demonstrated the mRNA and protein levels of ASL in the majority of HCC tissues were significantly elevated compared with adjacent nontumoral liver tissues, and the ASL mRNA and protein levels of HCC cell lines were also higher than that of immortalized normal hepatic liver cells and primary hepatocyte. ASL knockdown suppressed cell proliferation and anchorage-dependent growth of HCC cells. Moreover, the apoptosis was also induced by the silence of ASL. These data support the notion that ASL is a putative tumor promoter during HCC carcinogenesis.
To investigate the molecule mechanism of the apoptosis induced by ASL depletion, the change of apoptosis signal transduction was detected in ASL silenced cells. The results confirmed that the loss of ASL contributes to the apoptosis process in hepatoma cells partially via Bax regulation. Consistently, a study showed that arginine depletion could induce Bax conformation changes and mitochondrial inner membrane depolarization in ASS (−) cells which leads to apoptosis.22 Bax signaling pathway is a central cell death regulator and an indispensable gateway to mitochondrial dysfunction in cancer cells.23, 24 In response to death stimuli, Bax is activated and translocated to mitochondria,25 where it facilitates mitochondrial membrane permeabilization. Subsequently, the release of cytochrome c and Smac/DIABLO from the intermembrane space into cytosol is increased.26, 27, 28 The released cytochrome c binds apoptotic protease activating factor 1 (Apaf-1), procaspase-9 and dATP forms a complex “apoptosome”, which activates caspase 9 followed by downstream activation of other executioner caspases and ultimately results in cell death.28 Therefore, we further revealed ASL silencing induced the increase of cytochrome c and Smac as well as the activation of caspase 9, which are downstream effectors of Bax activation. Importantly, silencing Bax in ASL depletion HCC cells partially abolished the pro-apoptotic effect of ASL knockdown. These findings suggest the activation of Bax signaling pathway may be the molecular mechanism of the apoptosis increase induced by ASL silencing in HCC cells.
In summary, this study demonstrated that ASL acts as a carcinogene in the progression of HCC by regulating Bax signaling pathway. Therefore we propose that ASL may be a potential therapeutic target for HCC treatment.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This research is founded by National Natural Science Foundation of China (81672012) and Chongqing Yuzhong District science and technology planning project (20170102).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2018.11.003.
Contributor Information
Juan Chen, Email: chenjuan2014@cqmu.edu.cn.
JiHua Ren, Email: renjihua2016@cqmu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ploeger C., Waldburger N., Fraas A. Chromosome 8p tumor suppressor genes SH2D4A and SORBS3 cooperate to inhibit interleukin-6 signaling in hepatocellular carcinoma. Hepatology (Baltimore Md.) 2016;64(3):828–842. doi: 10.1002/hep.28684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peng L., Yuan X.Q., Zhang C.Y. The emergence of long non-coding RNAs in hepatocellular carcinoma: an update. J Cancer. 2018;9(14):2549–2558. doi: 10.7150/jca.24560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrizzo A., Mauriello A., Tornesello M.L., Buonaguro F.M., Tagliamonte M., Buonaguro L. Cellular prognostic markers in hepatitis-related hepatocellular carcinoma. Infect Agent Cancer. 2018;13:10. doi: 10.1186/s13027-018-0183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petruzziello A. Epidemiology of hepatitis B Virus (HBV) and hepatitis C Virus (HCV) related hepatocellular carcinoma. Open Virol J. 2018;12:26–32. doi: 10.2174/1874357901812010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budny A., Kozlowski P., Kaminska M. Epidemiology and risk factors of hepatocellular carcinoma. Polski merkuriusz lekarski – Organ Polskiego Towarzystwa Lekarskiego. 2017;43(255):133–139. [PubMed] [Google Scholar]
- 6.Schattenberg J.M., Schuchmann M., Galle P.R. Cell death and hepatocarcinogenesis: dysregulation of apoptosis signaling pathways. J Gastroenterol Hepatol. 2011;26(suppl 1):213–219. doi: 10.1111/j.1440-1746.2010.06582.x. [DOI] [PubMed] [Google Scholar]
- 7.Kuo M.T., Savaraj N., Feun L.G. Targeted cellular metabolism for cancer chemotherapy with recombinant arginine-degrading enzymes. Oncotarget. 2010;1(4):246–251. doi: 10.18632/oncotarget.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh A.K., Kumar R., Pandey A.K. Hepatocellular carcinoma: causes, mechanism of progression and biomarkers. Curr Chem Genomics Transl Med. 2018;12:9–26. doi: 10.2174/2213988501812010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giri B., Dey S., Das T., Sarkar M., Banerjee J., Dash S.K. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: an update on glucose toxicity. Biomed Pharmacother Biomed Pharmacother. 2018;107:306–328. doi: 10.1016/j.biopha.2018.07.157. [DOI] [PubMed] [Google Scholar]
- 10.Helman G., Pacheco-Colon I., Gropman A.L. The urea cycle disorders. Semin Neurol. 2014;34(3):341–349. doi: 10.1055/s-0034-1386771. [DOI] [PubMed] [Google Scholar]
- 11.Hu L., Pandey A.V., Eggimann S. Understanding the role of argininosuccinate lyase transcript variants in the clinical and biochemical variability of the urea cycle disorder argininosuccinic aciduria. J Biol Chem. 2013;288(48):34599–34611. doi: 10.1074/jbc.M113.503128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feun L., You M., Wu C.J. Arginine deprivation as a targeted therapy for cancer. Curr Pharmaceut Des. 2008;14(11):1049–1057. doi: 10.2174/138161208784246199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paul N.E., Losel R., Hemmrich K., Goy D., Pallua N., Klee D. L-arginine and arginine ethyl ester enhance proliferation of endothelial cells and preadipocytes - how an arginine ethyl ester-releasing biomaterial could support endothelial cell growth in tissue engineering. Bio Med Mater Eng. 2015;25(3):289–297. doi: 10.3233/BME-151275. [DOI] [PubMed] [Google Scholar]
- 14.Yoon J.K., Frankel A.E., Feun L.G., Ekmekcioglu S., Kim K.B. Arginine deprivation therapy for malignant melanoma. Clin Pharmacol Adv Appl. 2013;5:11–19. doi: 10.2147/CPAA.S37350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stettner N., Rosen C., Bernshtein B. Induction of nitric-oxide metabolism in enterocytes alleviates colitis and inflammation-associated colon cancer. Cell Rep. 2018;23(7):1962–1976. doi: 10.1016/j.celrep.2018.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang H.L., Chen W.C., Hsu H.P. Silencing of argininosuccinate lyase inhibits colorectal cancer formation. Oncol Rep. 2017;37(1):163–170. doi: 10.3892/or.2016.5221. [DOI] [PubMed] [Google Scholar]
- 17.Huang H.L., Chen W.C., Hsu H.P. Argininosuccinate lyase is a potential therapeutic target in breast cancer. Oncol Rep. 2015;34(6):3131–3139. doi: 10.3892/or.2015.4280. [DOI] [PubMed] [Google Scholar]
- 18.Syed N., Langer J., Janczar K. Epigenetic status of argininosuccinate synthetase and argininosuccinate lyase modulates autophagy and cell death in glioblastoma. Cell Death Dis. 2013;4:e458. doi: 10.1038/cddis.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam T.L., Wong G.K., Chong H.C. Recombinant human arginase inhibits proliferation of human hepatocellular carcinoma by inducing cell cycle arrest. Cancer Lett. 2009;277(1):91–100. doi: 10.1016/j.canlet.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 20.Huang H.L., Hsu H.P., Shieh S.C. Attenuation of argininosuccinate lyase inhibits cancer growth via cyclin A2 and nitric oxide. Mol Cancer Therapeut. 2013;12(11):2505–2516. doi: 10.1158/1535-7163.MCT-12-0863. [DOI] [PubMed] [Google Scholar]
- 21.Hung Y.H., Huang H.L., Chen W.C. Argininosuccinate lyase interacts with cyclin A2 in cytoplasm and modulates growth of liver tumor cells. Oncol Rep. 2017;37(2):969–978. doi: 10.3892/or.2016.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szlosarek P.W., Klabatsa A., Pallaska A. In vivo loss of expression of argininosuccinate synthetase in malignant pleural mesothelioma is a biomarker for susceptibility to arginine depletion. Clin Cancer Res – Offic J Am Assoc Cancer Res. 2006;12(23):7126–7131. doi: 10.1158/1078-0432.CCR-06-1101. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z., Ding Y., Ye N., Wild C., Chen H., Zhou J. Direct activation of Bax protein for cancer therapy. Med Res Rev. 2016;36(2):313–341. doi: 10.1002/med.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfeffer C.M., Singh A.T.K. Apoptosis: a target for anticancer therapy. Int J Mol Sci. 2018;19(2) doi: 10.3390/ijms19020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song C.L., Tang H., Ran L.K. Sirtuin 3 inhibits hepatocellular carcinoma growth through the glycogen synthase kinase-3beta/BCL2-associated X protein-dependent apoptotic pathway. Oncogene. 2016;35(5):631–641. doi: 10.1038/onc.2015.121. [DOI] [PubMed] [Google Scholar]
- 26.Jurgensmeier J.M., Xie Z., Deveraux Q., Ellerby L., Bredesen D., Reed J.C. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci U S A. 1998;95(9):4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eskes R., Desagher S., Antonsson B., Martinou J.C. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20(3):929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li P., Nijhawan D., Budihardjo I. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91(4):479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.