Abstract
Chronic Hepatitis C Viral (HCV) infection is a leading health problem worldwide and resulted in fibrotic scar formation, and finally liver-cirrhosis. Although contemporary therapies can partially reverse this destructive process, the rehabilitation is too slow and unsuitable for all chronic infections. The current study elucidates the mechanism of disease progression from early (F1) to moderate (F2, F3), and to severe fibrosis (F4)/cirrhosis in HCV genotype 3a infected patients to find out new candidates as potential disease progression markers and antiviral therapeutic agents. A total of 550 genes were found differentially regulated in the four fibrosis stages and grouped in 22 classes according to their biological functions. Gene set enrichment (GSEA) and Ingenuity pathway analysis (IPA) were used to identify the regulation of crucial biological functions and pathways involved in HCV progression. HCV differentially regulated the expression of genes involved in apoptosis, cell structure, signal transduction, proliferation, metabolism, cytokine signaling, immune response, cell adhesion and maintenance, and post translational modifications by pathway analysis. There was an increasing trend of proliferative and cell growth related genes and shutting down of immune response as the disease progress mild to moderate to advanced stage cirrhosis. The myriad of changes in gene expression showed more chances of developing liver cancer in patients infected with HCV genotype 3a in a systematic manner. The identified gene set can act as disease markers for prediction, whether the fibrosis lead to cirrhosis and its association with end stage liver disease development.
Keywords: Gene expression profiling, Hepatitis C, Liver cirrhosis, Liver fibrosis, Viral infection
Introduction
Hepatitis C virus (HCV) is one of the major causes of prolonged liver fibrosis; cirrhosis and end stage hepatocellular carcinoma (HCC).1, 2 The typical development of severe to chronic liver infection takes 10–30 years following serious HCV infection.3 Currently, 3% of the world population is chronically infected with this deadly virus and genotype 3a abundant in Pakistan.4, 5, 6, 7 Although, recently a combination drug daclatasvir and sofosbuvir have been found to produce a sustained biological response in HCV chronically infected patients,8 efforts in the development of more effective drugs are in progress.
For the antiviral drugs either against HCV genes or the host genes involved in HCV replication, better understanding of the disease pathogenesis from early to prolonged infection is required. The whole pathogenesis picture of HCV from acute to chronic infection is still unclear. Similarly, HCV genotype specific mechanism of disease progression from early moderate to end stage cirrhosis is currently unknown. Yet many important clues regarding cell differentiation, cell cycle, apoptosis and growth factor-related genes have made able to unravel this complex process.9 Better understandings of mechanisms behind the changes implied by viral–host cell interactions are critical for developing new strategies to cope with the disease.
HCV infection causes liver damage, resulting in the wound healing process known as fibrosis leads to excessive matrix deposition. Activated hepatic stellate cells infected with HCV produce many growth factors and cytokines that help in the fibrotic process.10, 11 This distortion of hepatic architecture results in ultimate progression to liver cirrhosis, liver failure or hepatocellular carcinoma.12 The recently developed high-throughput microarray technique has allowed the analysis of genome wide changes associated to particular conditions or disease like HCV and cancer. Limited studies are available on early to moderate to end stage liver cirrhosis associated with HCV genotype 3a, these studies carried out usually only in genotype 1 or 2.6, 7, 13, 14 Researchers have identified that there is increased expression of pro-apoptotic genes like FAS in HCV liver injury.15 Similarly, increased level of other growth factors like.
CTGF, TGF and up-regulation of metal-proteinases is also observed in HCV infected liver samples.16, 17, 18
In the present study, to get better understanding behind the chronic HCV infection mechanism at the molecular stage; we compared the genetic profile of HCV human liver fibrosis stages F1, F2, F3 and F4 (cirrhosis). HCV gene expression and gene network analysis, in particular, state of the disease may be helpful in finding of new biomarkers which will benefit as an efficient surveillance protocol to differentiate the disease progression non-invasively and seek new potential therapies.
Materials and methods
Patients
HCV RNA-positive patients were identified among HCV antibody (anti-HCV)-positive patients. Patients who had received a previous course of interferon (INF) or immunosuppressive therapy, or who had clinical evidence of hepatitis B virus (HBV) or human immunodeficiency virus infection (HIV), and any other types of liver cancer were excluded from the study. Patients who refused to have a liver biopsy or for whom it was contraindicated, i.e., because of a low platelet count, prolonged prothrombin time or decompensated cirrhosis were also excluded from the study. The liver biopsy procedure, its advantages and promising adverse effects were explained to the patients. Informed consent was obtained from patients contained information about demographic data, possible transmission route of HCV infection, clinical, biological and biochemical data. The study was approved by the institutional ethical committee. The study included 157 patients (Male/Female 114/43; mean age 38.1 ± 10.2, age range 19–58 years) admitted to Jinnah Hospital (Table S1).
Evaluation of liver biopsy
The histological evaluation of formalin fixed paraffin-embedded (FFPE) liver specimens was done at Pathology Department, Jinnah Hospital, Lahore according to METAVIR scoring system.19 Liver biopsies were evaluated by two independent pathologists without prior information to patient's history. Histological staging based upon the degree of fibrosis were divided as: F0 (no fibrosis), F1 (mild fibrosis without septa), F2 (moderate fibrosis with few septa), F3 (severe fibrosis with numerous septa without cirrhosis) and F4 (cirrhosis).
Total RNA isolation
Total RNA from liver biopsy samples was isolated using Qiagen RNeasy kit (Qiagen, Maryland, USA) according to manufacturer's protocol. The isolated RNA was quantified on nanodrop-ND1000 (Thermoscientific, Wilmington DE, USA) and the quality of the RNA was checked on 1% formaldehyde denaturating agarose gel (Aranda et al, 2012). The best-quality RNA samples were further processed for antisense RNA (aRNA or cRNA) amplification and dye labeling.
aRNA preparation, and dye labeling for microarray experiments
Total RNA was amplified into aRNA by using Ambion Messag Amp II kit (Ambion, New York, USA). The amplified patients and normal aRNA samples were labeled with Cy3, and Cy5 dyes respectively, using Kreatech RNA labeling module (Kreatech, Amsterdam, Netherland). A detailed protocol describing each step from start to microarray hybridization can be downloaded from (http://www.operon.com/products/microarrays/OpArray%20Protocol.pdf).
Array hybridization and scanning
Six individual liver biopsy samples for each fibrosis stage (F1, F2, F3 and F4, n = 24 total) and normal (n = 6) were analyzed on cDNA microarrays (Eurofins Genomics, Alabama, USA) that contain >22,000 named genes with 37,584 spots. Equal amounts of Cy3 and Cy5 (100 pmol each) labeled targets were mixed with 45 μl of Opera Hub Buffer (Eurofins Genomics, Alabama, USA). Pre-washing, array hybridization and post-washing of microarray labeled slides was performed according to the manufacturer protocols at 42 °C for 18 h on fully automated workstation “GeneTAC TM HybStation” (Cambridgeshire, UK).
Microarray data analysis
Post hybridization processing, slides were immediately scanned on GeneTAC TM UC 4 × 4 scanner at 10 μm resolution for both Cy3 and Cy5 channels. GeneTAC Integrator 4 .0 software was used for initial analysis, which generated output as ‘csv’ file format containing all necessary information. For the normalization, the ‘csv’ file was converted into ‘me’ format using ‘ExpressConverter’ (http://www.tm4.org/utilities.html) software. Normalization of the data was carried out using MIDAS (Microarray Data Analysis System) software (http://www.tm4.org/midas.html). A rank-based permutation method Statistical Analysis of Microarrays (SAM) was used to identify significantly expressed genes among fibrosis stages (http://www-stat.stanford.edu/∼tibs/SAM/). Gene expression patterns through k-means clustering were produced and viewed using CLUSTER 3.0 (http://rana.lbl.gov/EisenSoftware.htm) and Tree View 1.45 (http://rana.lbl.gov/downloads/TreeView/) programs, respectively. To identify biological themes among gene expression profiles, the Expression Analysis Systematic Explorer (EASE) was used (http://david.abcc.ncifcrf.gov/content.jsp?file=/ease/ease1.htm&type=1).20 The microarray data is available on Gene Expression Omnibus (GEO), accession number GSE33258 https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE33258.
The analysis of significantly enriched genes (GSEA)
SAM is able to isolate significantly expressing gene data however, to determine regulation of biological events during HCV fibrosis progression; we used the approach of Gene Set Enrichment Analysis (GSEA).21 The identified gene sets through EASE with less than 5 genes were excluded. The t-statistic mean of the identified genes was computed using 1000 permutations with the significance p value of 0.05. We compared fibrosis stage F1 vs F2/F3, F1 vs F4, and F2/F3 vs F4.
Construction of interaction network and functional pathway analysis
Gene's interaction networks were analyzed using the Ingenuity pathway analysis (IPA) tools (http://www.ingenuity.com). List of significant genes in each group generated by pair wise analysis using the SAM analysis tool was uploaded to the IPA software.
Potential pathways involved in initial and moderate fibrosis to end stage liver cirrhosis were investigated using ingenuity software. The gene list contained information about gene symbols. Entrez gene IDs, functions, Pubmed IDs and fold changes were mapped in the Ingenuity software knowledge base. The up and down regulated genes in each group were searched for their disease relevance and canonical pathway analysis. This software analyzes molecular data within the context of known biological response and regulatory networks as well as other higher-order response pathways. Ingenuity functional analysis identified biological functions and/or diseases that were most important enriched and generated p-value to determine the probability that each biological function assigned to that data set was due to chance alone. Enrichment p-values of <0.05 were considered statistically significant.
Real-time PCR analysis
Genes with known function and significantly up-regulated or down-regulated (≥2.0-fold change among fibrosis stages) were analyzed by real-time reverse transcription-PCR using similar RNA used for microarray analysis. Total RNA was reverse transcribed to cDNA using MmLV (Moloney murine leukemia virus). Selected and tested oligonucleotide primer pairs for their specificity (Table S2) were used for Real Time-PCR using an ABI 7500 Real Time PCR system (Applied Biosystems Inc, NY, USA) and Syber green chemistry (Fermentas International Inc, Canada). Data was normalized using GAPDH as endogenous control and each experiment was run in triplicate. Each gene was quantified relative to the calibrator. Applied Biosystem Sequence Detection Software Version (1.3.1) and calculations were made by instrument using the equation 2−ΔΔCT.
Results
Patient's characteristics
Among the 157 HCV genotype 3a infected biopsy samples six best RNA samples with precise biopsy stage evaluation from each F1, F2, F3 and F4 group were further processed for microarray analysis compared to pool of six normal liver biopsy samples. The detailed parameters and demographic of the patients are mentioned in Table S1. Briefly, serum Hb, bilirubin, viral loads were significantly low as the fibrosis progress to cirrhosis, whereas serum ALP and AST levels were high in moderate and end stage cirrhosis compared to F1 and F2 (Table S1).
Gene's ontology and pathway analysis
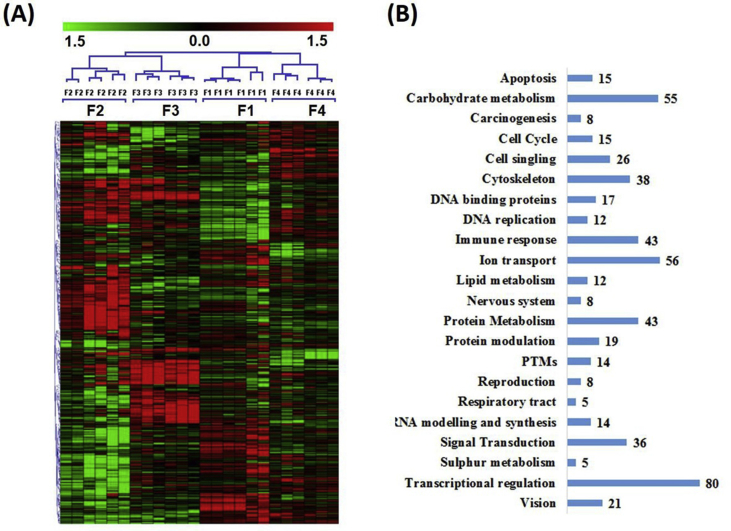
We found differentially regulated genes in all four groups (F1, F2, F3 and F4) when compared to F0 (normal/-vet control) patients. A total of 1019 significantly differentiated genes were observed in HCV infected liver biopsy samples (n = 24) compared to normal liver biopsy samples (n = 6). The false discovery rate (FDR) was set to 0.03%. Unsupervised hierarchical clustering analysis was performed for the grouping of these genes with closely related functions (Fig. 1A). These genes were grouped according to their biological functions. The uncharacterized and unnamed genes (259 genes) were removed from further functional analysis. From remaining 760 genes, 554 genes with known ontologies were considered for gene set enrichment analysis and classified in 22 groups according to their biological functions (Fig. 1B). Genes related to immune response, signal transduction, protein synthesis, protein modulation, ion transport, cytoskeleton, transcriptional regulations, cell signaling were differentially regulated in all the four fibrosis stages.
Figure 1.
(A) Clustering results for differentially expressed genes between HCV infected patients with different fibrosis stages. Clustering was done by using Cluster 3.0 software. The fold changes in mRNA expression are represented with green and red squares showing down and up regulation if genes in liver biopsy samples, respectively. Each vertical column represents an independent experiment, while color scale represents the fold change magnitude. (B) Distribution of genes according to their functions. Genes were grouped in 22 different classes.
The 554 genes identified in the second step were further scrutinized based on t-Est among individual groups (F1 vs F2, F1 vs F3, F1 vs F4, F2 vs F3, F2 vs F4, and F3 vs F4) and 441 genes were identified whose levels significantly differed between F1 vs F2, F1 vs F3, F1 vs F4, F2 vs F3, F2 vs F4, and F3 vs F4 fibrosis stages. We identified 215,162, 120, 214, 236, and 138 genes (Table 1, Table 2) with fold induction >2.0 that can be discriminate between F1 vs F2, F1 vs F3, F1 vs F4, F2 vs F3, F2 vs F4, and F3 vs F4, respectively. Furthermore, levels of these transcripts in each stage were completely separated by unsupervised hierarchical clustering, and no liver sample was misclassified. Venn diagrams were drawn to find out overlapping genes, singular gene representing each group and common genes in three groups. We found 35 communal genes (Fig. 2) that can differentiate different fibrosis stages.
Table 1.
Significant genes involved in F1 vs F2, F1 vs F3 and F1 vs F4 comparison analysis.
| Names | Total | Gene IDs |
|---|---|---|
| F2 vs F3 F2 vs F4 F3 vs F4 |
35 | KIF1A SOCS2 FAM62B AMICA1 MUC2 UROD THRAP3 CCR1 SLIT3 TF2H4 IMP3 NFXL1 PTP4A1 MRPL46 PACS1 GON4L FAM22F KRTAP19-5 CCNG1 RNF34 USP53 KPNB1 CAPN7 Q96NT9 PRMT2 CKM HOXA7 K1024 PSMD4 ZBED4 SMAP1 SHF DYRK1B YIF1A HP |
| F2 vs F3 F2 vs F4 |
89 | CLCA1 WNT3A D106A PHACTR4 CTR9 SOS1 GIMAP2 GAL3ST3 ANR38 SENP1 MRPS35 MARVELD1 NLGN4X SLC33A1 CLGN UBE2M TMEM155 COL23A1 LRRC52 PDCD1 BAK1 LMNB1 NR4A2 ARF6 ITGB6 PRMT8 MADCAM1 CACNB4 KCNK17 CCNL1 K1196 SLC22A18 ASPHD2 C17orf25 SPATA6 FOXK2 ARHGAP25 MAGEB18 OTUD7A GTF2A2 LGALS4 PLEKHA7 STX7 LCT BTBD12 UPK2 UBE1L SOCS6 POLE2 CLCN1 SERPINB6 SCRN2 NAT13 JMJD1B TDRD9 KIAA1143 TRPM1 SLC30A3 C10orf68 CAPN11 TNKS1BP1 CORO1A KLF17 RABL5 ZBTB11 CALCRL SENP7 FBXO32 PTPRB MYO18B DEFB114 KRT6A Q8N9G6 HYLS1 SLC35A5 RNPEP ZBTB6 MYBBP1A NPAS3 FAM125A AGPS Q96RF1 TNFRSF14 UBE1 CASP8AP2 SIX3 PRKCB1 GPR133 NDE1 |
| F2 vs F3 F3 vs F4 |
55 | DSG4 CREB3L1 MT-ATP8 LRRC41 SYDE1 PIK3R4 FOXD4L3 SLC4A5 GZMM GSTO2 PSMC4 UNC5D KIAA1199 NOS2C TIMM13 PCDHA8 MARVELD3 ADCY2 LYG2 MAP6D1 PTGS2 SAT2 CACNB3 ANKRD17 NINJ2 TNPO3 IK CSK2B MTIF2 TSPAN4 Q9BVX4 APOOL CPSF6 MYST2 CCDC63 C17orf79 SAA3P MDS2 POR SLC25A11 BCL2L1 PIGR DDX3X FCRL3 KRTAP13-1 RP9 ADA ARFGAP1 DPYSL3 VHL LSS KNG1 TMEM100 VPS13B NOL5A |
| F2 vs F4 F3 vs F4 |
25 | DZIP1L COQ5 CNGA2 FCER2 MT1E DDX19B EEF1AL3 DGAT1 ACSBG2 ACO1 CLDND2 SLC25A16 GRK7 CLSTN3 SSU_rRNA_5 C7 ZBP1 FAM119B TMOD3 Q96HF5 ANTXR2 WRN HAT1 DARS2 KRTAP13-2 |
| F2 vs F3 | 35 | YBX1 BCL2L12 CENPJ PRDM5 ZFYVE21 FRMD6 MSH3 ASCL2 SIM2 KIAA1919 MLZE SMAD3 PAX3P LAT IQCA SRI FAS CEP55 ATE1 KCNK5 FNDC3A MRPL48 ZNF14 AHCTF1 NUP62 OFD1 POU4F1 MUCL KIR2DL1 GLT6D1 GCP4 LYSMD3 VEGFB FLRT1 BBX |
| F2 vs F4 | 87 | ENO3 KIFC3 ZNF554 MPL WDR5 BRMS1L IL13RA2 NUTF2 WISP2 SH3BGRL3 DDX11 IQCK DOCK9 CRYBB3 SF3A2 NAT10 Q96IN8 LOC283116 NEUROD2 RHPN2 GTF2E2 TJP2 INTU MOBKL2A TTLL2 EXOSC2 PLGLB2 MAPKBP1 HEXB PLTP NOBOX SNRPB2 ITM2C TXNL1 DCN C8G VDAC3 ZNF306 ATP6V0C MLF1 PRKG2 CTCFL ZNF460 MEF2A GNB2L1 F10 G8 PPAPDC3 SNRPA CDA BMP1 SP2 O75863 HMGCL CD300LB CHKA DEGS2 OGDHL ANKRD9 PCMTD1 CUGBP2 ATG16L2 FLNA AKAP4 DSCR1L2 NDUFC1 USH1G ZC3H12B IFNA21 PKD2L2 COMMD5 CDCA5 ADAR PDE10A Q5R387 XCL2 GATA1 SNX17 RUNX2 POLR2A GDF8 IFIT3 USP54 TEX12 SRR MYOD1 IFITM2 |
| F3 vs F4 | 23 | CCRL1 SET TMEM180 UBADC1 MFSD5 LRRTM2 ROPN1B PCDHB5 SLC7A11 WRB WHDC1 CDY2B SLC35C2 CPSF7 RUFY2 ENAM USP37 MAGEA4 WFS1 TUBA6 NR1I3 SNF8 KLRF1 |
Table 2.
Significant genes involved in F2 vs F3, F2 vs F4 and F3 vs F4 comparison analysis.
| Names | total | elements |
|---|---|---|
| F1 vs F2 F1 vs F3 F1 vs F4 |
35 | KIF1A SOCS2 FAM62B AMICA1 MUC2 UROD THRAP3 CCR1 SLIT3 TF2H4 IMP3 NFXL1 PTP4A1 MRPL46 PACS1 GON4L FAM22F KRTAP19-5 CCNG1 RNF34 USP53 KPNB1 CAPN7 Q96NT9 PRMT2 CKM HOXA7 K1024 PSMD4 ZBED4 SMAP1 SHF DYRK1B YIF1A HP |
| F1 vs F2 F1 vs F3 |
33 | ENO3 Q5VTU8 CLPB IL13RA2 NUTF2 SH3BGRL3 DIRAS1 BAK1 FCER2 Q96IN8 PPP5C DDX19B LOC283116 GTF2E2 MRPS25 PCSK5 IL8RB ELL2 HOMER1 PRRG2 GRK7 CSDE1 WHDC1 BMP1 HMGCL NPAS3 Q96HF5 EMR1 POLR2A TUBA6 GRIN3A MYOD1 DARS2 |
| F1 vs F2 F1 vs F4 |
27 | GOT2 NANOGP8 ZNF236 SLC10A5 PCDHB2 UBADC1 IQCA KIF17 Q5VYG3 SRI NUP160 IQCH Q9BVX4 CPT1A FOLR2 ELL PSMD11 SNF1LK2 CPSF7 SNF1LK PKHD1 CRLF3 PAX8 ADH1A ARHGAP5 LYSMD3 DDX24 |
| F1 vs F3 F1 vs F4 |
16 | ANKRD45 BCL2L12 PHACTR4 MRPS35 FRMD6 PDCD1 KIAA1199 ATAD2 OTUD7A BCL2L13 TCF1 ATG16L2 GTF2B MUCL Q8N9G6 AGPS |
| F1 vs F2 | 120 | WNT3A D106A SOS1 GIMAP2 WISP2 COL6A2 DDX11 MARVELD1 CLGN UBE2M ZFYVE21 TMEM155 COL23A1 LRRC52 U34 ARF6 PSMC4 PRMT8 RTN4IP1 KCNK17 MT1E ASCL2 NGRN SIM2 K1196 SLC22A18 KIAA1919 RHPN2 ASPHD2 MLZE SPATA6 TJP2 GTF2A2 PAX3 PLAT PLEKHA7 CACNB3 LCT TTLL2 EXOSC2 PLGLB2 FGF17 HEXB TMEM46 PLTP UBE1L SNRPB2 SOCS6 POLE2 BTBD14A HMGCS1 CLCN1 TXNL1 SERPINB6 C8G SCRN2 CEP55 ZNF306 TDRD9 CRIP3 ATP6V0C KIAA1143 MLF1 WFDC13 SLC30A3 CAPN11 MEF2A ZNF14 AHCTF1 ABO F10 GNB2L1 CALCRL G8 RPS4Y1 LAGE3 SPINT1 ZNF444 SNRPA SENP7 FBXO32 O75863 PLEKHG6 PTPRB DEGS2 DCP2 AMELY PCMTD1 IGSF2 CUGBP2 RBM5 FLNA AKAP4 PDYN GLT6D1 SLC35A5 KIAA1344 ZBTB6 MYBBP1A GCP4 FAM125A SYCP1 PRDM7 PKD2L2 TNFRSF14 CDCA5 ADAR PDE10A SIX3 PRKCB1 XCL2 MYST1 FLRT1 IFIT3 LLGL1 NOL5A GPR133 KCTD14 DDX17 NDE1 |
| F1 vs F3 | 78 | DSG4 SCG3 SCN7A MT-ATP8 LRTM1 PIK3R4 CST4 FOXD4L3 GZMM GSTO2 DZIP1L RCOR1 EBI3 CASP9 UNC5D CCRL1 NOS2C SET FAHD2B PTPN22 TMEM180 TIMM13 TOP3B MARVELD3 KRT25 FOXK2 LYG2 MAP6D1 PTGS2 SAT2 ANKRD17 MOBKL2A CDKL5 NINJ2 ACO1 TNPO3 CLDND2 MFSD5 ZNF484 SLC4A2 FAS CSK2B LRRTM2 TSPAN4 DNAL1 PCDHB5 APOOL CLSTN3 MYST2 ATE1 CCDC63 AMH C17orf79 SAA3P MDS2 POR KLF17 SLC25A11 SLC7A11 TBX6 FOXI1 CDY2B ZBP1 OFD1 POU4F1 PPP2R1B RP9 SOCS1 ENAM ARFGAP1 USP37 VHL LANCL1 VPS13B KRTAP13-2 WFS1 NR1I3 KLRF1 |
| F1 vs F4 | 42 | KIFC3 GPR98 CTR9 RBM41 SLC4A5 IQCK DOCK9 COQ5 CNGA2 CACNB4 CCNL1 DGAT1 ACSBG2 MYCL1 MAPKBP1 NAT13 JMJD1B PRKG2 ZNF460 RABL5 WRB BCL2L1 SLC35C2 SP2 C7 CD300LB CHKA OGDHL DSCR1L2 KLK3 USH1G RUFY2 UBE1 DPYSL3 CASP8AP2 WRN Q5R387 GATA1 BBX MAGEA4 GDF8 SNF8 |
Figure 2.
(A) Venn diagram showing unique and overlapped genes interactions across F1 vs F2, F1 vs F3, and F1 vs F4. (B) Venn diagram showing unique and overlapped genes interactions across F2 vs F3, F2 vs F4, and F3 vs F4. (C) Heat map showing the expression of 35 overlapped genes representing and differentiating all stages of fibrosis (p < 0.005).
Biological functions and network analysis of differentially regulated genes
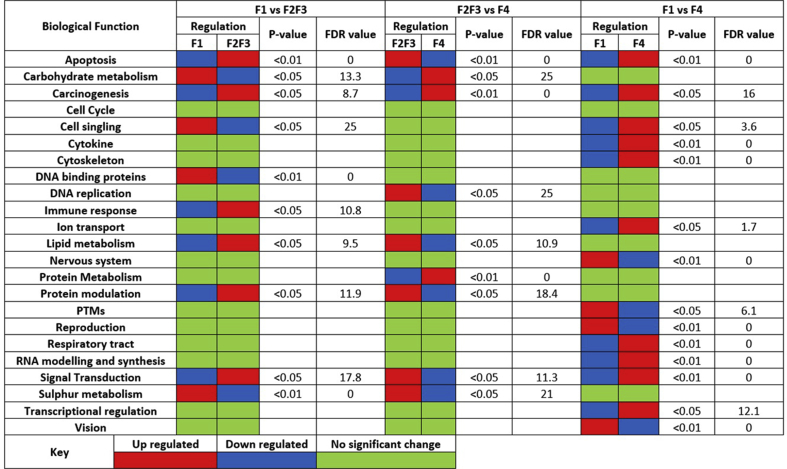
GSEA was used to compare and sort out differentially regulated genes according to their biological functions. Network analysis of the differentially expressed genes was performed using IPA. The comparative analyses of top five canonical pathways that represent differentially regulated genes in the four different stages of liver fibrosis (fibrosis 1st to 4th) were evaluated. The leading five recognized pathways which were studied are related to cell death and survival, ubiquitination pathway, cancer, organ morphology and transcription regulation.
Differential expression of genes in F1
The GSEA results analysis showed that genes related to carbohydrate, cell signaling, DNA binding protein and Sulphur metabolism were high in F1 in comparison to F2/F3. Whereas, apoptosis, signal transduction immune responsive, lipid metabolism and protein modulation related genes were high in F2/F3. Whereas the genes related to vision, reproduction and PTMs were up-regulated in F1 in comparison to F4 stage (Fig. 3).
Figure 3.
Summary of gene set enrichment analysis (GSEA). GSEA was performed on the genes according to their significance in all fibrosis groups. 22 gene sets with a p-value of <0.05 and FDR value < 25 were considered significant. Differential regulation of entire gene set in different fibrosis stages.
The top significant canonical pathways regulated in F1 stage and important molecular and cellular function associated with these genes are liver damage (p = 2.80E−03–4.71E−01), pro-apoptosis (2.84E−02), Cell growth and proliferation (p = 7.68E−04–2.84E−02). The top canonical pathway included protein Kinase A signaling (p = 8.25E−03), Lactose degradation III (p = 1.25E−02), interferon signaling (p = 1.91E−02) (Fig. 4).
Figure 4.
Differentially expressed genes in F1 compare to F2, F3 and F4 HCV liver fibrosis stage. (A) The 5 top scoring networks up-regulated in HCV F1 stage was plotted for their interaction. (B) The top down regulated gene network in F1 stage. Ingenuity network diagram showing the interaction of genes associated with ubiquitin pathway, HNF4A signaling, apoptosis related, immune responsive genes. Solid lines show known interaction between proteins. Solid lines show direct while dashed lines represent indirect interaction.
Differential expression of genes in F2 and F3
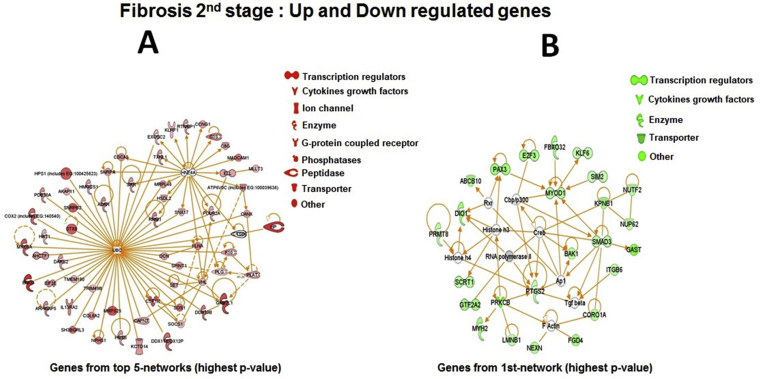
GSEA results show that immune response, apoptosis, signal transduction, protein metabolism, lipid metabolism become highly regulated in F2 and F3 fibrosis in comparison to both F1 and F4 stage (Fig. 3). IPA analysis of genes further showed that genes up-regulated in mild/advanced fibrosis are mainly linked to two different pathways one ubiquitin (UBC) related pathway and second is a key hepatic transcription factor HNF4 (hepatocyte nuclear factor 4). These are regulating many genes required to limit the liver injury. Although the genes are mainly found to be linked with transcriptional regulators HNF4A and UBC, but these genes have no predictive values in disease's progression from F1, F2/F3 and F4. We found 5 top expressing networks scored ≥25. The top molecular and cellular functions were classified into cell death and survival (score = 45), cellular movement and disease morphology (score = 40), cell cycle, infectious diseases and post translational modifications (score = 25). The molecular and cellular functions associated with genes, gene expression range, post translational modifications (p = 9.76E−05–1.53E−02), protein modification (p = 9.76E−05–1.09E−02), cell death and survival (2.76E−04–2.64E−02) and cell cycle (p = 6.90E−04–2.72E−02) were more relevant. A number of genes found highly expressed in F2 stage compared to other fibrosis stages (Fig. 5, Table S3).
Figure 5.
Network analysis of significantly deregulated genes in HCV liver fibrosis F2 stage compared to normal. (A) The up-regulated network includes genes related to liver specific gene, transcription, cell adhesion, cell cycle, cytoskeleton, protein metabolism, ion transport and signal transduction. (B) The down-regulated network includes transcription factor, immune responsive, metabolism and transduction. PRKCB, GAST and PAX3 are darker in green color showing more significantly down-regulated compared to other genes in network. The grey outlined modules in the network are the linker genes, which are not altered in the study.
Up regulated genes in F2 were mainly related to the immune response and increasing cell growth taking it to carcinoma. The IPA software was used to redefine the biological interaction and significance of the genes. The top five genetic pathways observed were with score ≥26. The top networks with significant values were associated with connective tissue disorder (score = 31), cellular assembly and organization (score = 31), organizational assembly and abnormalities (score = 31), development and function (score = 29) and cell to cell signaling and interaction (score = 26). The biological functions with highest p values were related to cell death and survival (p = 1.96E−04–4.00E−02), cellular compromise (p = 3.29E−04–3.22E−02), drug metabolism (p = 3.90E−04–3.22E−02), cellular assembly and organization (p = 3.90E−04–3.30E−02). The top canonical pathways associated with F3 liver fibrosis were P53 signaling (p = 4.24E−02), increase transmembrane potential of mitochondria (p = 6.25E−02), hepatocellular hypertrophy (p = 6.33E−02) and pro-apoptosis (4.58E−02), enols signaling (p = 4.06E−03) and lateral sclerosis signaling (p = 8.09E−03). The genes (UQCC, SOCS2, VHL, CTSD, TNPO3, ZNF692, GON4L and HOXA7) are known in liver cancer. GSTO2 gene is involved in carcinogenesis and xenobiotic (Fig. 6).
Figure 6.
Ingenuity network diagram showing significantly up-regulated and down-regulated genes in HCV fibrosis stage F3. (A) Up-regulated genes network (B) down-regulated genes network. Direct interaction of the genes is shown with solid arrows while indirect relation with dashed lines. Network was mainly representing the top scored genes regulated in the infection and not all gene interaction from different pathways. Genes with fold change ≥2 were considered significant.
Differential expression of genes in F4
The expression of cell signaling, cytoskeleton, cytokine, ion transport and RNA modeling and synthesis related genes were up regulated in F4 stage compared to other stages (Fig. 7). The pathways associated with F4 are cell death and survival (score = 33), cellular assembly and response (score = 30), lipid metabolism and repair (score = 22). The molecular and cellular functions associated with these genes were: the inflammatory response (p = 3.33E−04–2.67E−02), injury and abnormalities (p = 4.67E−05–2.48E−02), cell death and survival (p = 5.60E−05–2.72E−02), gene expression (p = 4.32E−04–1.59E−02) and cellular development (p = 8.33E−04–2.75E−02).
Figure 7.
Differentially expressed genes network in liver fibrosis stage F4. (A) Up-regulated genes network (B) Down regulated genes network showing the interaction and link with different pathways. Node color is scaled to gene expression fold changes between four stages of liver fibrosis. Green and Red shading indicates relative Down and Up-regulation of gene expression in each individual group. In the functional networks, genes are represented as respective shapes based on functional property (as indicated inside the figure), and the biological relationship (direct and indirect) between two genes is represented as an edge (line). All edges are supported by at least one published reference or from canonical information stored in the Ingenuity Pathways Knowledge Base.
The host immune regulatory mechanisms like apoptosis, complement, etc. was regulated by down regulating the HP, C7, Glutathione peroxidase, PRMT2 and RCAN3 genes. In our study, we found the expression of HP gene highly up-regulated in 2nd stage of Fibrosis while going down at the 4th stage of fibrosis; probably caused by the damaged liver at the end stage of fibrosis.
Real-time PCR for microarray outcome
Real-time PCR was used to analyze the expression of randomly picked significant differentially expressed genes obtained from microarray analysis. The expression of SLIT3, IMP3, RNF34, HP, GON4L, SOCS2, HOXA7, KPNB1 and TF2H4 genes was tested (Fig. 8). The Real-time PCR results complement the microarray results showing the possible reproducibility in future studies.
Figure 8.
Validation of microarray results on Real Time PCR. The top scored up and down-regulated genes were picked from each group and subjected to Real Time PCR using Syber green PCR. All experiments were performed in triplicate and normalized with GAPDH used as internal control.
Discussion
HCV acute to chronic infection is a complex process which involve both viral and host genes interaction, and modulation of host gene expression by the virus for its self-replication. The whole mechanism of this liver damage is still need to be explored to find the early stage liver fibrosis markers and targets to limit the damage to end stage liver cancer. Few studies are available in context of HCV induced initial, moderate to severe fibrosis progression based on genotypes.22, 23 The aim of the present study is to elucidate the mechanism of disease's progression from initial (F1) to mild/moderate (F2/F3), to advance fibrosis or cirrhosis stage (F4) in patient's liver biopsy samples infected with HCV genotype 3a using microarray. HCV genotype 3a is a more common genotype in Asia as well as in Pakistan and known to have effect on disease severity and antiviral treatment outcome.24, 25, 26
Many studies have compared cirrhosis to HCC or cirrhosis induced by HBV or HCV from patients of alcoholic or chronic cholestasis.27, 28 They usually compared the diseased tissue and normal tissue from the same individual.27, 29 We, however, compared the diseased samples with the control taken from normal subjects. This helped us to compare the normal samples from all the four fibrosis stages and to get the better picture of onset of disease. The genes were found to be regulated in systematic way and affecting important cellular functions. The pattern we found in the expression of these genes was comparable to previously reported studies.18, 30, 31 Nevertheless, we also found some novel set of genes too, involved in post translational modification, protein degradation, cellular trafficking, etc.
In this study, most genes related to cellular immune response and defense were found differentially regulated in all the four fibrosis stages (Table S3). The apoptotic genes (Bak1, Casp9 and ST13) we found up-regulated in HCV F1 stage. BAK1 a pro-apoptotic gene involved in mitochondria dependent apoptosis. It releases apoptosis regulatory protein Casp9.32 A precise mechanism exists between apoptosis and anti-apoptosis for normal functioning and survival. Apoptosis is induced by both intrinsic and extrinsic pathways.33 High expression of apoptosis-related genes in advance stage of liver fibrosis as compared to F1 stage is in accordance with the previous studies. Whereas successive reduction in tumor suppressor gene ST13 was observed. High expression of ST13 gene decreased the cell proliferation and down regulation of this gene re-initiates the cell growth, as it has been observed in colorectal cancer.34 HCV supports the immune-mediated apoptosis and plays a role in pathogenesis. Host immune system triggers to limit the damage by induction of apoptosis and many other pathways. There are studies showing an increasing trend of HCC from liver cirrhosis and steatosis in patients infected with HCV genotype 3a.35 In our study IMP3 (insulin-like growth factor like growth factor II binding protein 3) gene was found up regulated in early fibrosis stage, and significantly down regulated in latter stages. IMP3 belongs to the insulin-like growth factor II (IGFII) and was found up-regulated in cancer.36 Despite recent studies reporting its high expression in cancer, its function is largely unknown. Jing et al, 2008,37 reported that depleting the expression of IMP3 using siRNA, decreased the cell motility and invasion. IMP3 depletion is associated with down regulation of many genes involved in cancer progression. High expression of this gene in our study at initial stage of fibrosis may be due to the virus-host immune response for its sustained replication and survival.
Oxidative stress remained an attractive cause for inducing HCV-associated pathogenesis and other metabolic diseases.38, 39, 40 HP a liver specific gene is known as acute phase protein and has a role in suppression of oxidative species and enhancement in angiogenesis. We found HP expression significantly expressed in mild/moderate fibrosis compared to other stages where it was negatively regulated. Differential expression of an important gene and transcription factor HNF4A involved in lipid homeostasis and liver specific gene expression has also been reported. It regulates expression of many genes involved in drug metabolism, inflammation and glucose homeostasis.31 Down regulation of this gene is associated with hepatocyte proliferation and cancer development.32 In the present study, we found HNF4A as a major regulatory pathway in F1, F2 and F3 with no predictive value. This suggests that HNF4A is not only controlling the expression of liver specific genes but also has tumor suppressor activity. The expression of HNFA pathway-related genes were not observed in F4 (cirrhotic) patient samples. This may be because there is increasing trend of HCC in HCV 3a genotype infected patients. Bonze et al,41 has observed increased hepatocyte development in HNF4A knockout mouse. We observed in our study the ubiquitin linked regulation of genes in all the four stage, regulating both positively and negatively of the genes. Ubiquitination of genes either results in proteasome degradation or alter their expression without degradation.42
The genes involved in cellular metabolism were also found regulated by HCV genotype 3a. HCV has not only modulated the expression of genes related to lipid metabolism but also related to protein biosynthesis and degradation, post translational modifications, cellular trafficking, carbohydrate and energy metabolism. Lipids have an important role in the replication and vesicle formation and virus assembly.43 The lipid metabolism-related genes found deregulated in the present study are DEGS, PPAPDC3, OSBPL2, PCDHB5 and BCAR1. Consistent with these genes involved in lipid synthesis, sphingolipid formation related genes were found significantly regulated by the virus. The expression of DEGS (sphingolipid) which is a member of a de novo dihydroceramide pathway was induced in mild/moderate fibrosis whereas it is down regulated in other fibrosis stages. This gene was shown to be stimulated in hypoxic condition and having a role both in autophagy and also in cell proliferation. Over expression of DEGS1/2 is found to be linked with increased in proliferation during hypoxia.44 Previously, its expression was found high in human colon cancer cells.45 The expression of DEGS2 is first time found elevated in our study predict important association between HCV infection and carcinogenesis.
HCV also regulate the expression of several genes involved in cell differentiation and cellular processes like HOX7, a member of homeobox family.46 We observed the expression of this gene significantly induced at F3 stage compared to other stages where it is negatively regulated. Cello et al, 2011 observed high expression of HOX family in HCC samples compared to normal.47 The other gene with significantly high expression in F3 is GSTO2 (Glutathione S transferase). It has a role in cell signaling control and the over expression of this gene may facilitate apoptosis.48 Previously, polymorphism of this gene with N14D in many cancers like gastric, leukemia including HCC has been reported.49 Reduced expression in our study from F3 to F4 may be that virus has overcome the deleterious effect of this gene on cellular control for its own replication.
Another important viral induced gene expression is related to suppression of cytokine signaling (SOCS), which has been recently identified as a group of host genes manipulated by the viruses for their own survival. In our study, we have observed high expression of SOCS1 gene in mild/moderate fibrosis and down regulation of SOCS3 in severe fibrosis. SOCS genes inhibit the antiviral signaling pathway like JAK/STAT pathway to help the virus to evade the immune system. These proteins also help in the control of viral trafficking by ubiquitination of the viral genes. Increased expression of SOCS1 abrogate the propagation of IFN α and γ-production, while decreased expression permanently activates JAK-STAT pathway and is consider as treatment failure in chronic HCV infected patients.50, 51 SOCS3 but not SOCS2 has the capability in altering and inhibiting both IFN- α and γ-pathways. The role of SOCS2 is largely unknown. There are both types of studies available one in which researchers have found the induced expression of SOCS genes and in other they found decreased expression of these genes in HCV infection.52, 53 We in our study found elevated expression of SOCS1 and low expression of SOCS3 with lofty expression of anti-apoptotic genes, which promote the cancer development.
Another set of genes which were found dysregulated are WRN (Werner syndrome), KPNB1 (Karyopherin beta 1) and MAPKB1 (mitogen activated protein kinase binding protein 1) were significantly up regulated whereas RCNA3 (regulator of calcineurin 3), MEF2A (myocyte enhancer factor 2), HP (haptoglobin), C7 and SCL4A were down regulated in liver cirrhotic patients. KPNB1 is a nuclear transport protein help to transport the signal, cell cycle proteins and maintain cell homeostasis. Many studies have reported its high expression in cancers like, breast cancer,54 liver cancer,55 ovarian cancer56 and melanomas.57 KPBN1 has been proposed as a new cancer marker. Another down regulated gene RCAN3 in HCV F4 stage, is a recently identified gene, its function was not studied previously in HCV associated liver diseases. However, can aider et al,58 has reported modulation of proliferation in human umbilical vein endothelial cells. They observed that inhibiting the expression of RCAN3 using siRNA significantly increased the growth of endothelial cells. This is in accordance to our result that may be the virus is shutting down the expression of this gene and helping in the proliferation of the cells, irrespective of the angiogenic and inflammatory process.
Unfortunately, few studies are available in which host gene expression is studied based on HCV genotypes. Recently, Goolsby et al (2017) reported that liver disease progression varies with genotype whereas patients infected with HCV genotype 3 have more severe disease as compared to 1 and 2.59 Some studies linked HCV genotype to varied gene expression while in others genotypes were linked to IFN therapy response only. Earlier, Bigger et al (2004), compared the gene expression in HCV infected Chimpanzee with three different genotypes (1a, 1b and 3a).60 They found high expression of stearoyl coenzyme A desaturase 4 (SCD4) and CDP-diacylglycerol synthase (which play role in steatosis development) and low expression of ISG related genes in genotype 3a infected animal as compared to genotype 1a and 1b. Many genes in our study were found to be differentially regulated like SOCS family which was found to be downregulated as the liver fibrosis progress to F4 stage. This may show a trend of HCC development in genotype 3a patients as previously observed by Meng et al.61 Increased expression of SOCS1 and SOCS3 was also observed by Collins et al, 2014 in HCV genotype 1a infected patients, however liver biopsy stage/grade was not mention.62 Meanwhile, we found lower expression of SOCS2 gene as the disease progress to higher fibrosis stage that is F4 stage.
It has been found that inhibiting the expression of SOCS2 gene in Huh-7.5 replicon cells results in increased viral replication (Lee et al, 2012).63 These results showed that different genotypes could results in different end results. We also observed induced IMP3 expression that has not been previously reported and linked to HCV. However, IMP3 expression is found to be higher in cancer cells related to intra hepatic cholangiocarcinoma while aberrant expression of IMP3 results in aggressiveness of the tumor (Gao et al, 2014).64 In our study, higher expression of IMP3 in early fibrosis stages as compared to F3 and F4 could lead to carcinoma in 3a infected patients if not detected early.
Many transporter related genes have been linked to HCV life cycle. Ng et al (2007) reported that inhibiting the expression of SLC12A4 and SLC12A5 using siRNA inhibited more than 90% viral RNA of HCV 1 genotype in replicon cells.65 We in our study also observed differential expression of transporter genes like SLC33A1, SLC22A18, SLC30A3, SLC35A5, SLC4A5 and SLC25A11 in HCV-3a infected patient's samples. These genes could be explored for their therapeutic potential. Nanda et al (2008) studied the HCV infection mechanism in relation to clearance of the virus in infected chimpanzees with 1b genotype.66 They found KLF4, SOCS3 and NUP1530 genes induced in the animal in self-limiting the infection. We in our study also found KLF17, SOCS2 and SOCS6 differentially expressed I different liver stages showing the involvement in persistent infection. Conclusively, these observations showed that HCV genotypes could affect disease progression and pathogenesis. This could result in varied results of medicines given to cop with the HCV infection.
Conclusions
In the present study, we have highlighted a key subset of the differentially regulated genes among the liver fibrosis stages. The trend of gene regulations during HCV disease progression gives us an early insight of mechanism involved behind severity of infection. Overall, our study suggests a mechanistic shut down of immune response that resulted in severe fibrosis and cirrhosis. Early diagnosis of selective gene response will enable the clinicians to better evaluate the patient's condition and dose requirements. We also identified genes like DEGS2, RCAN3, GSTO2, SCL4A, GON4L and N14D which were not previously reported to be associated with HCV infection and carcinogenesis. All of these genes may be potential targets for future therapeutic interventions and it could represent a panel to determine the level of liver damage that occurred due to chronic HCV infection.
Compliance with ethical standards
Funding
The authors are thankful for Higher Education Commission (HEC grant# NRPU-863), Pakistan for the funding.
Conflict of interests
All authors declare that they have no competing interests.
Ethical approval
Informed consent: Informed consent was obtained from all individual participants included in the study. This study was approved by the office of human research protection, Government of Pakistan with Federal-wide assurance # FWA00001758 with CEMB Ref# IRB-HCV-06.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2019.04.007.
Contributor Information
Bushra Ijaz, Email: bijaz_009@yahoo.com.
Waqar Ahmad, Email: waqar.ahmad@uqconnect.edu.au, waqar.ahmad@uaeu.ae.ac.
Trina Das, Email: trinad6@uw.edu.
Khadija Shabbiri, Email: k.shabbiri@uq.edu.au, k_shabbiri@yahoo.com.
Tayyab Husnain, Email: tayyabhusnain@gmail.com.
Sajida Hassan, Email: sajihassan2004@yahoo.com.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Lavanya D. The global burden of hepatitis C. Liver Int. 2009;29(Suppl. 1):74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 2.Milliman A.J., Nelson N.P., Vellozzi C. Hepatitis C: review of the epidemiology, clinical care, and continued challenges in the direct acting antiviral era. Curr Epidemiol Rep. 2017;4(2):174–185. doi: 10.1007/s40471-017-0108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alazawi W., Cunningham M., Dearden J., Foster G.R. Systematic review: outcome of compensated cirrhosis due to chronic hepatitis C infection. Aliment Pharmacol Ther. 2010;32(3):344–355. doi: 10.1111/j.1365-2036.2010.04370.x. [DOI] [PubMed] [Google Scholar]
- 4.Memon M.I., Memon M.A. Hepatitis C: an epidemiological review. J Viral Hepat. 2002;9(2):84–100. doi: 10.1046/j.1365-2893.2002.00329.x. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad W., Ijaz B., Javed F.T. HCV genotype distribution and possible transmission risks in Lahore, Pakistan. World J Gastroenterol. 2010;16(34):4321–4328. doi: 10.3748/wjg.v16.i34.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad W., Ijaz B., Javed F.T. HCV genotype-specific correlation with serum markers: higher predictability for genotype 4a. Virol J. 2011;8:293. doi: 10.1186/1743-422X-8-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ijaz B., Ahmad W., Javed F.T. Association of laboratory parameters with viral factors in patients with hepatitis C. Virol J. 2011;8:361. doi: 10.1186/1743-422X-8-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poynard T., Yuen M.F., Ratziu V., Lai C.L. Viral hepatitis C. Lancet. 2003;362(9401):2095–2100. doi: 10.1016/s0140-6736(03)15109-4. [DOI] [PubMed] [Google Scholar]
- 9.Levrero M. Viral hepatitis and liver cancer: the case of hepatitis C. Oncogene. 2006;25(27):3834–3847. doi: 10.1038/sj.onc.1209562. [DOI] [PubMed] [Google Scholar]
- 10.Gressner A.M., Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10(1):76–99. doi: 10.1111/j.1582-4934.2006.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmad W., Ijaz B., Gull S. A brief review on molecular, genetic and imaging techniques for HCV fibrosis evaluation. Virol J. 2011;8:53. doi: 10.1186/1743-422X-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bataller R., Brenner D.A. Liver fibrosis. J Clin Invest. 2005;115(2):209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caillot F., Hiron M., Goria O. Novel serum markers of fibrosis progression for the follow-up of hepatitis C virus-infected patients. Am J Pathol. 2009;175(1):46–53. doi: 10.2353/ajpath.2009.080850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao R.X., Hoshida Y., Otsuka M. Hepatic gene expression profiles associated with fibrosis progression and hepatocarcinogenesis in hepatitis C patients. World J Gastroenterol. 2005;11(13):1995–1999. doi: 10.3748/wjg.v11.i13.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macías J., Japón M.A., Sáez C. Increased hepatocyte fas expression and apoptosis in HIV and hepatitis C virus coinfection. J Infect Dis. 2005;192(9):1566–1576. doi: 10.1086/491736. [DOI] [PubMed] [Google Scholar]
- 16.Levy M.T., McCaughan G.W., Abbott C.A. Fibroblast activation protein: a cell surface dipeptidyl peptidase and gelatinase expressed by stellate cells at the tissue remodelling interface in human cirrhosis. Hepatology. 1999;29(6):1768–1778. doi: 10.1002/hep.510290631. [DOI] [PubMed] [Google Scholar]
- 17.El-Bassiouny A.E., Zoheiry M.M., Nosseir M.M., El-Ahwany E.G., Ibrahim R.A., El Bassiouni N.E. Expression of cyclooxygenase-2 and transforming growth factor-beta1 in HCV induce chronic liver disease and hepatocellular carcinoma. Medscape Gen Med. 2007;9(3):45. [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad W., Ijaz B., Hassan S. Gene expression profiling of HCV genotype 3a initial liver fibrosis and cirrhosis patients using microarray. J Transl Med. 2012;10:41. doi: 10.1186/1479-5876-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmad W., Ijaz B., Javed F.T. A comparison of four fibrosis indexes in chronic HCV: development of new fibrosis-cirrhosis index (FCI) BMC Gastroenterol. 2011;11:44. doi: 10.1186/1471-230X-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosack D.A., Dennis G., Jr., Sherman B.T., Lane H.C., Lempicki R.A. Identifying biological themes within lists of genes with EASE. Genome Biol. 2003;4(10) doi: 10.1186/gb-2003-4-10-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramanian A., Tamayo P., Mootha V.K. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zekri A.-R.N., Hafez M.M., Bahnassy A.A. Genetic profile of Egyptian hepatocellular-carcinoma associated with hepatitis C virus genotype 4 by 15K cDNA microarray: preliminary study. BMC Res Notes. 2008;1(1):106. doi: 10.1186/1756-0500-1-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blackham S., Baillie A., Al-Hababi F. Gene expression profiling indicates the roles of host oxidative stress, apoptosis, lipid metabolism, and intracellular transport genes in the replication of hepatitis C virus. J Virol. 2010;84(10):5404–5414. doi: 10.1128/JVI.02529-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zein N.N. Clinical significance of hepatitis C virus genotypes. Clin Microbiol Rev. 2000;13(2):223–235. doi: 10.1128/cmr.13.2.223-235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bochud P.-Y., Cai T., Overbeck K. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J Hepatol. 2009;51(4):655–666. doi: 10.1016/j.jhep.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Adinolfi L.E., Gambardella M., Andreana A., Tripodi M.f., Utili R., Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33(6):1358–1364. doi: 10.1053/jhep.2001.24432. [DOI] [PubMed] [Google Scholar]
- 27.Okabe H., Satoh S., Kato T. Genome-wide analysis of gene expression in human hepatocellular carcinomas using cDNA microarray identification of genes involved in viral carcinogenesis and tumor progression. Cancer Res. 2001;61(5):2129–2137. [PubMed] [Google Scholar]
- 28.Lee J.S., Chu I.S., Heo J. Classification and prediction of survival in hepatocellular carcinoma by gene expression profiling. Hepatology. 2004;40(3):667–676. doi: 10.1002/hep.20375. [DOI] [PubMed] [Google Scholar]
- 29.Smith M.W., Yue Z.N., Korth M.J. Hepatitis C virus and liver disease: global transcriptional profiling and identification of potential markers. Hepatology. 2003;38(6):1458–1467. doi: 10.1016/j.hep.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 30.Wurmbach E., Chen Y.b., Khitrov G. Genome-wide molecular profiles of HCV-induced dysplasia and hepatocellular carcinoma. Hepatology. 2007;45(4):938–947. doi: 10.1002/hep.21622. [DOI] [PubMed] [Google Scholar]
- 31.Mas V.R., Maluf D.G., Archer K.J. Genes involved in viral carcinogenesis and tumor initiation in hepatitis C virus-induced hepatocellular carcinoma. Mol Med. 2009;15(3–4):85. doi: 10.2119/molmed.2008.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brenner C., Grimm S. The permeability transition pore complex in cancer cell death. Oncogene. 2006;25(34):4744–4756. doi: 10.1038/sj.onc.1209609. [DOI] [PubMed] [Google Scholar]
- 33.Hengartner M., Bryant J. Apoptotic cell death: from worms to wombats... but what about the weeds? Symp Soc Exp Biol. 2000:1. [PubMed] [Google Scholar]
- 34.Yu D.B., Zhong S.Y., Yang M. Potent antitumor activity of double-regulated oncolytic adenovirus-mediated ST13 for colorectal cancer. Cancer Sci. 2009;100(4):678–683. doi: 10.1111/j.1349-7006.2009.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jahan S., Ashfaq U.A., Qasim M., Khaliq S., Saleem M.J., Afzal N. Hepatitis C virus to hepatocellular carcinoma. Infect Agent Cancer. 2012;7(1):2. doi: 10.1186/1750-9378-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nielsen F.C., Nielsen Jacob, Christiansen Jan. A family of IGF-II mRNA binding proteins (IMP) involved in RNA trafficking. Scand J Clin Lab Invest. 2001;61(234):93–99. [PubMed] [Google Scholar]
- 37.Jeng Y.M., Chang C.C., Hu F.C. RNA binding protein insulin-like growth factor II mRNA-binding protein 3 expression promotes tumor invasion and predicts early recurrence and poor prognosis in hepatocellular carcinoma. Hepatology. 2008;48(4):1118–1127. doi: 10.1002/hep.22459. [DOI] [PubMed] [Google Scholar]
- 38.Machida K., Cheng K.T.-H., Lai C.-K., Jeng K.-S., Sung V.M.-H., Lai M.M. Hepatitis C virus triggers mitochondrial permeability transition with production of reactive oxygen species, leading to DNA damage and STAT3 activation. J Virol. 2006;80(14):7199–7207. doi: 10.1128/JVI.00321-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang T., Weinman S.A. Causes and consequences of mitochondrial reactive oxygen species generation in hepatitis C. J Gastroenterol Hepatol. 2006;21(s3):S34–S37. doi: 10.1111/j.1440-1746.2006.04591.x. [DOI] [PubMed] [Google Scholar]
- 40.Ahmad W., Ijaz B., Shabbiri K., Ahmed F., Rehman S. Oxidative toxicity in diabetes and Alzheimer's disease: mechanisms behind ROS/RNS generation. J Biomed Sci. 2017;24(1):76. doi: 10.1186/s12929-017-0379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonzo J.A., Ferry C.H., Matsubara T., Kim J.H., Gonzalez F.J. Suppression of hepatocyte proliferation by hepatocyte nuclear factor 4 alpha in adult mice. J Biol Chem. 2012;287(10):7345–7356. doi: 10.1074/jbc.M111.334599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shoji I. Roles of the two distinct proteasome pathways in hepatitis C virus infection. World J Virol. 2012;1(2):44. doi: 10.5501/wjv.v1.i2.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Syed G.H., Amako Y., Siddiqui A. Hepatitis C virus hijacks host lipid metabolism. Trends Endocrinol Metab. 2010;21(1):33–40. doi: 10.1016/j.tem.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Devlin C.M., Lahm T., Hubbard W.C. Dihydroceramide-based response to hypoxia. J Biol Chem. 2011;286(44):38069–38078. doi: 10.1074/jbc.M111.297994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Signorelli P., Munoz-Olaya J.M., Gagliostro V., Casas J., Ghidoni R., Fabrias G. Dihydroceramide intracellular increase in response to resveratrol treatment mediates autophagy in gastric cancer cells. Cancer Lett. 2009;282(2):238–243. doi: 10.1016/j.canlet.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 46.Cillo C., Cantile M., Faiella A., Boncinelli E. Homeobox genes in normal and malignant cells. J Cell Physiol. 2001;188(2):161–169. doi: 10.1002/jcp.1115. [DOI] [PubMed] [Google Scholar]
- 47.Cillo C., Schiavo G., Cantile M. The HOX gene network in hepatocellular carcinoma. Int J Cancer. 2011;129(11):2577–2587. doi: 10.1002/ijc.25941. [DOI] [PubMed] [Google Scholar]
- 48.Wang L., Xu J., Ji C. Cloning, expression and characterization of human glutathione S-transferase Omega 2. Int J Mol Med. 2005;16(1):19–27. [PubMed] [Google Scholar]
- 49.Masoudi M., Saadat I., Omidvari S., Saadat M. Genetic polymorphisms of GSTO2, GSTM1, and GSTT1 and risk of gastric cancer. Mol Biol Rep. 2009;36(4):781–784. doi: 10.1007/s11033-008-9245-0. [DOI] [PubMed] [Google Scholar]
- 50.Brand S., Zitzmann K., Dambacher J. SOCS-1 inhibits expression of the antiviral proteins 2',5' OAS and MxA induced by the novel interferon-lambdas IL-28A and IL-29. Biochem Biophys Res Commun. 2005;331(2):543–548. doi: 10.1016/j.bbrc.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Fenner I.E., Starr R., Cornish A.L. Suppressor of cytokine signaling 1 regulates the immune response to infection by a unique inhibition of type I interferon activity. Nat Immunol. 2006;7(1):33–39. doi: 10.1038/ni1287. [DOI] [PubMed] [Google Scholar]
- 52.Shao R.-X., Zhang L., Peng L.F. Suppressor of cytokine signaling 3 suppresses hepatitis C virus replication in an mTOR-dependent manner. J Virol. 2010;84(12):6060–6069. doi: 10.1128/JVI.02484-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El-Saadany S., Ziada D., El Bassat H. The role of hepatic expression of STAT1, SOCS3 and PIAS1 in the response of chronic hepatitis C patients to therapy. Can J Gastroenterol. 2013;27(2):e13–e17. doi: 10.1155/2013/562765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gluz O., Wild P., Meiler R. Nuclear karyopherin alpha2 expression predicts poor survival in patients with advanced breast cancer irrespective of treatment intensity. Int J Cancer. 2008;123(6):1433–1438. doi: 10.1002/ijc.23628. [DOI] [PubMed] [Google Scholar]
- 55.Yoshitake I., Shinji Tanaka M., Murakata A., Matsumura S., Mitsunori Y. Importin-α1 as a novel prognostic target for hepatocellular carcinoma. Ann Surg Oncol. 2011;18(7):2093–2103. doi: 10.1245/s10434-011-1569-7. [DOI] [PubMed] [Google Scholar]
- 56.Zheng I., Tang L., Huang L. Overexpression of karyopherin-2 in epithelial ovarian cancer and correlation with poor prognosis. Obstet Gynecol. 2010;116(4):884–891. doi: 10.1097/AOG.0b013e3181f104ce. [DOI] [PubMed] [Google Scholar]
- 57.Winnepenninckx V., Lazar V., Michiels S., Melanoma Group of the European Organization for Research and Treatment of Cancer Gene expression profiling of primary cutaneous melanoma and clinical outcome. J Natl Cancer Inst. 2006;98(7):472–482. doi: 10.1093/jnci/djj103. [DOI] [PubMed] [Google Scholar]
- 58.Canaider S., Vettraino M., Norling L.V. Human RCAN3 gene expression and cell growth in endothelial cells. Int J Mol Med. 2010;26(6):913–918. doi: 10.3892/ijmm_00000542. [DOI] [PubMed] [Google Scholar]
- 59.Goolsby H.A., Rosenblatt L., Patel C., Blauer-Peterson C., Anduze-Faris B. Clinical characteristics, healthcare costs, and resource utilization in hepatitis C vary by genotype. Curr Med Res Opin. 2017;33(5):829–836. doi: 10.1080/03007995.2017.1288613. [DOI] [PubMed] [Google Scholar]
- 60.Bigger C.B.1, Guerra B., Brasky K.M. Intrahepatic gene expression during chronic hepatitis C virus infection in chimpanzees. J Virol. 2004:1377–13792. doi: 10.1128/JVI.78.24.13779-13792.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meng C., Shen X., Jiang W. Potential biomarkers of HCC based on gene expression and DNA methylation profiles. Oncol Lett. 2018;16:3183–3192. doi: 10.3892/ol.2018.9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Collins A.S., Ahmed S., Napoletano S. Hepatitis C virus (HCV)-induced suppressor of cytokine signaling (SOCS) 3 regulates proinflammatory TNF-responses. J Leukoc Biol. 2014;96(2):255–263. doi: 10.1189/jlb.2A1211-608RRRR. [DOI] [PubMed] [Google Scholar]
- 63.J1 Lee, Lim S., Kang S.M. Saponin inhibits hepatitis C virus propagation by up-regulating suppressor of cytokine signaling 2. PLoS One. 2012;7(6):e39366. doi: 10.1371/journal.pone.0039366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao Y., Yang M., Jiang Z. IMP3 expression is associated with poor outcome and epigenetic deregulation in intrahepatic cholangiocarcinoma. Human Pathol. 2014;45:1184–1191. doi: 10.1016/j.humpath.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 65.Ng T.I., Mo H., Pilot-Matias T. Identification of host genes involved in hepatitis C virus replication by small interfering RNA technology. Hepatology. 2007;45:1413–1421. doi: 10.1002/hep.21608. [DOI] [PubMed] [Google Scholar]
- 66.Nanda S., Havert M.B., Calderon G.M. Hepatic transcriptome analysis of hepatitis C virus infection in chimpanzees defines unique gene expression patterns associated with viral clearance. PLoS One. 2008;3(10):e3442. doi: 10.1371/journal.pone.0003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.