Abstract
Although bone morphogenetic proteins (BMPs) initially showed effective induction of ectopic bone growth in muscle, it has since been determined that these proteins, as members of the TGF-β superfamily, play a diverse and critical array of biological roles. These roles include regulating skeletal and bone formation, angiogenesis, and development and homeostasis of multiple organ systems. Disruptions of the members of the TGF-β/BMP superfamily result in severe skeletal and extra-skeletal irregularities, suggesting high therapeutic potential from understanding this family of BMP proteins. Although it was once one of the least characterized BMPs, BMP9 has revealed itself to have the highest osteogenic potential across numerous experiments both in vitro and in vivo, with recent studies suggesting that the exceptional potency of BMP9 may result from unique signaling pathways that differentiate it from other BMPs. The effectiveness of BMP9 in inducing bone formation was recently revealed in promising experiments that demonstrated efficacy in the repair of critical sized cranial defects as well as compatibility with bone-inducing bio-implants, revealing the great translational promise of BMP9. Furthermore, emerging evidence indicates that, besides its osteogenic activity, BMP9 exerts a broad range of biological functions, including stem cell differentiation, angiogenesis, neurogenesis, tumorigenesis, and metabolism. This review aims to summarize our current understanding of BMP9 across biology and the body.
Keywords: Adipogenesis, Bone morphogenetic proteins (BMPs), BMP9/GDF2, Mesenchymal stem cells (MSCs), Metabolism, Osteogenesis, Regenerative medicine, Tumorigenesis
Introduction and historical overview
Bone defects are a complicated clinical problem. The great range of pathologies, as well as the variation in size, shape, and type of a bony defect can make the restoration of missing or damaged tissues exceedingly difficult. In addition to limitations caused by the complexity of the defects, more common issues such as fracture non-unions and delayed fracture healing have an immense burden on both the financial wellbeing and quality of life of patients, as well as on the healthcare system as a whole.1 Reconstruction of bone defects therefore has vast implications for both physicians and patients.
Treatment of these complications requires a solution that can address both the variety and complexity of defects, including having the desirable osteoconductive, osteoinductive, and osteogenic properties necessary to form robust, mature bone.2 The current treatment of choice for treating impaired bone regeneration is an autologous bone graft,3 but this treatment suffers from limitations such as donor site morbidity, limited material from donor site, and the varied ability of the bone to conduct healing of the defect.4, 5, 6
One of the most promising strategies to address the aforementioned issues is the use of bone morphogenic proteins (BMPs), a group of signaling proteins belonging to the TGF-β superfamily that have immense potential in the differentiation of mesenchymal stem cells (MSCs). BMPs were first discovered by the late Dr. Marshall Urist, an orthopedic surgeon who stumbled upon an unknown growth factor with the ability to induce bone growth in muscle.7, 8, 9, 10, 11, 12, 13, 14, 15 BMPs have been shown to induce differentiation of MSCs to bone and cartilage, as well as playing a role in adipogenesis,10, 12, 16 angiogenesis and lymphatic vessel formation,17, 18, 19, 20, 21 stimulation of hepatocyte proliferation, several critical roles in various forms of metabolism, and numerous other indispensable processes.13, 22 Of special interest is BMP9; which has proved to have the most osteogenic potential of any of the BMPs both in vitro and in vivo.14, 15, 22, 23, 24, 25, 26, 27, 28 BMP9 is also unique amongst the BMP family in its resistance to the inhibitors of BMP signaling noggin and BMP3.26, 60 It also may play a unique role in the production of dentin in teeth.63 The potent osteogenic potential of BMP9 is due to its strong activation of specific downstream mediators of osteogenic signaling12, 22, 23, 24, 25, 26, 27, 29, 30 as well as through a vast network of crosstalk with other major signaling pathways.28, 31, 32, 33, 34, 35, 36, 37 We will first explore these various signaling pathways relevant to BMP9, and afterwards present current clinical ramifications of BMP9.
Molecular basis of BMP9 signaling through Tgf-β/Bmp receptors
BMPs transduce signals through a number of canonical and non-canonical pathways. The BMP family is involved in a myriad of physiologic processes; from its role in the creation of bone to oncogenic or tumor suppressing roles in various cancers. To unravel all the ways in which BMP is active in the body, we must first examine the different signaling mechanisms these proteins use to exert their various effects. BMP9 acts by activation of the SMAD signaling pathway. Numerous other factors modulate this activation: the type I TGF-β receptors ALK1 and ALK2 are receptors that are necessary for SMAD activation, while the type II TGF-β receptors DN-BMPRII, DN-ActRII, and DN-ActRIIB inhibit SMAD activation and thus BMP activity. SMAD phosphorylation is further influenced by the MAPK family of proteins, wherein p38 increases SMAD phosphorylation to increase BMP-related effects. This is then balanced by ERK1/2 driven inhibition of SMAD phosphorylation.
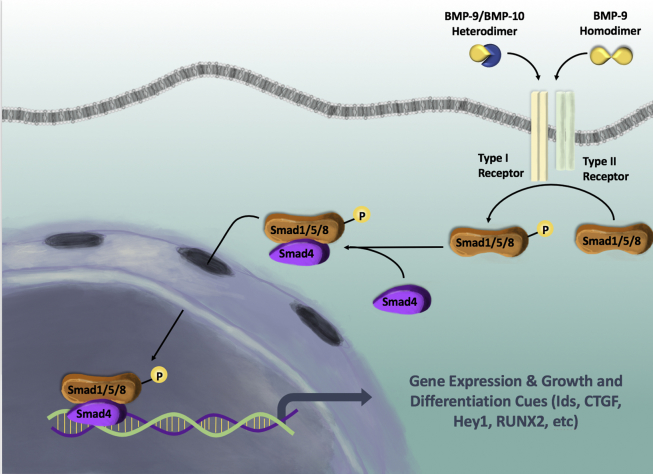
To this day, BMPs 2/4/6/7/9 have been shown to have osteogenic potential, with BMP9 having the strongest.14, 16, 22, 23 Several studies have detailed the signaling pathways necessary for osteoinduction mediated by BMPs, outlined in this section. BMP signaling occurs when the BMP binds to a dimer of the heterodimeric complex of type I and type II BMPR.38, 39 This interaction facilitates phosphorylation and thus activation of the type I receptor by the constitutively active type II receptor at the glycine-serine rich motif, also known as the GS domain.40 This in turn stimulates cytoplasmic Smad1/5/8 which can form a heterodimeric complex with Smad4 within the nucleus, activating the transcription of target genes40 (Fig. 1). ALK1 is an endothelial-specific type I receptor for BMP9, and BMPR-II is a type II receptor for the family of BMP ligands.38
Figure 1.
BMP9/Smad signaling. BMP ligands bind to and subsequently activate receptor kinases, which eventually phosphorylate and promote heterodimeric formation of Smad proteins. These Smads proteins can then interact with Smad4 for proper nuclear localization, which is necessary for proper BMP9-induced osteogenic differentiation. Experiments have demonstrated that RNAi-mediated knockdown of Smad4 reduced Smad heterodimer formation and nuclear localization; thus decreased osteogenic differentiation of mesenchymal stem cells.
Luo et al had previously shown that out of the potential type I receptors ALK1, ALK5, and endoglin, ALK1 and ALK2 are necessary for BMP9-mediated osteogenesis.41 They confirmed this by functionally analyzing the type I receptors via dominant negative and RNAi silencing experiments. Dominant-negative mutants of ALK1 and ALK2 inhibited BMP-induced ectopic bone formation and expression of Smad6 and Smad7. These results suggest that ALK1 and ALK2 are the type I TGF-β receptors involved in BMP9 osteogenic signaling.
Several studies by Yamashita et al and Dijke et al have identified that type II TGF-β receptor is responsible for the osteogenic activity of BMPs.39, 42 Wu et al further investigated the specific type of TGF-β receptor specifically required for the BMP9 induced osteogenic differentiation of MSCs.43 They did this by introducing a series of dominant negative type II TGF-β receptors (DNIIRs) into C3H10T1/2 stem cells and assessed their functionality in the BMP9 induced osteogenesis both in vivo and in vitro. Of the four types of type II TGF-β receptors (TGFβRII, BMPRII, ActRII, and ActRIIB), the study found that DN-BMPRII, DN-ActRII, and DN-ActRIIB attenuated BMP9-Smad specific luciferase reporter activity and reduced the expression levels of Smad6 and Smad7. DN-TGFβRII had no effect on attenuating Smad activity, strongly suggesting that DN-BMPII, DN-ActRII, and DN-ActRIIB resulted in inhibited BMP9 activity by reducing the transcriptional activity and signaling of Smad. DN-BMPRII, DN-ActRII, and DN-ActRIIB also inhibited ALP activity and matrix mineralization in vitro, and showed decreased formation of ectopic bony masses in vivo. Furthermore, the use of RNAi established that BMPRII and ActRII knockdown caused reduced ALP activity. This demonstrates that BMPRII and ActRII may play a functional role in BMP9 induced osteogenic differentiation of C3H10T1/2 cells.
BMP signaling through the canonical BMPR-Smad-dependent pathway
The Smad family of proteins is essential to the signal transduction of the TGF-B superfamily, including the BMPs. BMP ligands bind to and subsequently activate receptor kinases, which eventually phosphorylate and promote heterodimeric formation of Smad proteins. Xu et al showed that BMP9 activates Smad1/5/8 in C3H10T1/2 cells.44 These Smads then interact with Smad4 for proper nuclear localization, which is necessary for proper BMP9-induced osteogenic differentiation. Western blot showed simultaneously upregulated levels of Smad1/5/8 upon BMP9 treatment. Consistently, RNAi-mediated knockdown of Smad4 reduced Smad heterodimer formation and nuclear localization; this decreased osteogenic differentiation of C3H10T/12 cells. Since Smad signaling is upregulated following BMP9 treatment and blockade of Smad signaling prevents BMP9 from inducing osteogenic change, Smad signal transduction is necessary for BMP9-induced osteogenic differentiation of MSCs.
Mitrofan et al showed that Smad1 and Smad5 also play an important role in mediating BMP9 induced surface expression of adhesion molecules on vascular endothelial cells.45 Specifically, Smad1/5 mediates the response of BMP9-induced expression of adhesion molecules E-selectin, VCAM-1, and ICAM-1 in TNF-α-stimulated human amniotic epithelial cells. This effect was reduced upon siRNA-mediated knockdown of Smad1/5. Scharpfenecker et al found that BMP9-mediated phosphorylation of Smad1/5 in endothelial cells stimulates ALK1 activation, and expression of ID1 protein and endoglin mRNA.46
BMP signaling through the non-canonical BMPR-Smad-independent pathway: the involvement of mitogen activated protein kinases (MAPKs)
The mitogen activated protein kinases (MAPKs) family includes the extracellular signal-related kinases ERK1/2, ERK5, Jun amino-terminal kinases (JNKs) and p38 MAPKs.47 These MAPKs are protein Ser/Thr kinases that compose an ancient signaling pathway that is highly conserved throughout evolution.47, 48 These MAPKs regulate a plethora of vital functions, including gene expression, mitosis, metabolism and innumerable other functions.47
It is no surprise, then, that MAPKs activated by BMPs regulate a diverse array of cellular responses49, 50, 51, 52 including osteogenic differentiation. The role of the MAPKs p38 and ERK1/2 on BMP action has been elucidated by selective inhibition.44 C3H10T1/2 stem cells were exposed to BMP9 in the presence of SB203580 (a selective inhibitor for p38) and PD98059 (a selective inhibitor for ERK1/2). SB203580 (p38 inhibitor) inhibited BMP9 mediated ALP activity in a dose dependent manner, while PD98059 (ERK1/2 inhibitor) enhanced BMP9 induced ALP activity in a mostly dose-dependent manner.44 They demonstrated that SB203580 (p38 inhibition) reduced BMP9-induced calcium deposition, while PD98059 (ERK1/2 inhibition) led to increased calcium deposition. Mechanistically, it was found that p38 inhibition led to diminished BMP9-induced phosphorylation of Smad1/5/8 and its translocation to the nucleus. Inhibition of ERK1/2 has the opposite effect, promoting the phosphorylation of Smad1/5/8 and enhanced nuclear translocation and transcription.44 These results suggest that p38 and ERK1/2 work in opposition to regulate BMP9 induced osteogenic differentiation of MSCs via their interactions on Smad signaling.
In a follow-up study, Zhao et al expanded on the oppositional roles of p38 and ERK1/2 in BMP9-induced osteogenic differentiation of MSCs.53 Inhibiting p38 and ERK1/2 using RNAi, SB203580 and PD98059, they found that p38 inhibition decreased both the early marker ALP as well as later markers of osteogenic differentiation, osteocalcin, and matrix mineralization suggesting that p38 had pro-osteogenic properties. Inhibition of ERK1/2 showed opposing results, leading to increased ALP activity and upregulation of the later markers osteocalcin and matrix mineralization, suggesting that ERK1/2 had anti-osteogenic properties. Smad1/5/8 signaling and nuclear translocation was also found to be reduced with inhibition of p38 and enhanced while inhibiting ERK1/2. Runx2, a transcription factor, was also found to follow the same pattern: p38 inhibition reduced BMP9-induced Runx2 activity, while Runx2 activity was enhanced with ERK1/2 inhibition at both the gene and protein level. The study further examined the effects of these MAPKs in vivo, demonstrating that in mouse calvarial tissue inhibition of p38 activity led to only focal ossification and thinner trabeculae. ERK1/2 knockdown increased bony masses in BMP9-transduced cells with thicker trabeculae and greater quantities of bone. These results corroborate the molecular findings in the previously cited study by Xu et al44 regarding the opposing roles of p38 and ERK1/2 on BMP9-induced osteogenic differentiation of mesenchymal progenitor cells.
BMP9 and noggin resistance: a unique feature within the BMP family
In a comprehensive analysis of both the in vitro and in vivo osteogenic activity of 14 different BMPs conducted by Kang et al, BMP9 was shown to have the most potent osteogenic potential.16, 22 Multiple other studies utilizing adenoviral-mediated overexpression of BMP9 have shown that BMP9 upregulates alkaline phosphatase activity (an early osteogenic marker), as well as increasing matrix mineralization and later osteoblastic markers such as osteocalcin.23, 44 Unlike other BMPs, it has also been shown that BMP9 is not susceptible to inhibition by BMP3 or noggin,22, 54 findings suggestive of a unique mechanism of BMP9 signaling.
The superior osteogenic properties of BMP9 in comparison to other BMPs may be due to its resistance to several BMP antagonists. Past studies have found that unlike other osteogenic BMPs, such as BMP2, BMP4, BMP6, and BMP7, the bone formation induced by BMP9 is not inhibited by BMP3.22 This led to further investigations of the BMP inhibitor noggin, a glycoprotein that binds BMPs selectively and antagonizes their actions. Transgenic mice overexpressing noggin have increased long bone fractures in the first month of life, as well as reduced bone mineral density and impaired osteoblastic function.55 Interestingly, the osteogenic properties of BMP9 seem to be resistant to the inhibitory effects of noggin, unlike all other osteogenic BMPs.56 Wang et al was able to demonstrate that in pre-osteoblastic cell lines, activity of the early osteogenic marker ALP was significantly inhibited in BMP2, BMP4, BMP6, and BMP7 transduced cells, but ALP activity remained high in the BMP9-stimulated cells in multiple different cell lines.56 Using immunohistochemical staining with antibodies against osteopontin and osteocalcin, they were further able to demonstrate that BMP9-induced cell lines were also resistant to the inhibitory effects of noggin on these later osteogenic markers. Wang et al also showed that BMP9 is resistant to noggin-mediated inhibition of ectopic bone formation unlike all other osteogenic BMPs.56
While the in vivo functions of BMP9 remain to be fully investigated, a recent study has indicated that BMP9 may play an important role on tooth development.57 The investigators found that BMP9 was widely expressed in odontoblasts, ameloblasts, dental pulp cells, and osteoblasts in alveolar bones.57 The deletion of Bmp9 in mice exhibited that the first molars of the Bmp9-null mice exhibited a reduced thickness dentin, enlarged pulp canals, and shortened roots, resembling the phenotypes of the common hereditary dental disease dentinogenesis imperfecta.57 Furthermore, the alveolar bone of the Bmp9-KO mutants was found to be shorter and had a decreased mineral density, trabecular thickness, and bone volume, compared with that of the wild-type control.57 Nonetheless, the mice with global deletion of Bmp9 were viable and fertile without apparent bone and skeletal abnormalities, suggesting that Bmp9's biological functions may be well compensated by other yet-to-be-identified mechanisms.
Mediators of BMP9-Induced osteogenic signaling
As discussed in the previous section, BMP9 signaling is primarily through activation of the SMAD transcription factor. In the following sections, we will elaborate on the various proteins and growth factors that are targeted by SMAD activation, allowing the bone morphogenic proteins to carry out their various physiologic functions.
RUNX transcription factors
The runt-related (RUNX) family of evolutionary conserved proteins act as transcription factors that perform a number of crucial functions pertaining to cellular differentiation, embryonic and organ development as well as osteogenesis.58, 59, 60, 61 Generally, RUNX2 has been recognized for its necessity in bone formation, as deletion of the gene in mice results in maturational arrest of chondrocytes and osteoblasts, leading to complete absence of intramembranous and endochondral bone.59, 62, 63, 64
Related to the interplay of RUNX with osteogenesis are microRNAs (miRNAs), which are non-coding RNAs that act as negative regulators at the post-transcriptional level by degrading targeted mRNA and may play significant role in bone formation.65, 66 Chen et al investigated the possible role of microRNAs (miRNAs) inhibition on BMP9-induced osteogenic differentiation by targeting RUNX2.66 The study found that expression levels of miR-23b, a specific miRNA previously identified as being expressed during the early stages of MSC osteogenesis, were reduced in the early stages of BMP9-induced osteoblastic differentiation of MSC, C2C12 cells. The study also concluded that overexpression of miR-23b was able to blunt the response of BMP9-induced osteogenesis, while downregulation of miR-23b showed enhanced osteoblastic differentiation when induced by BMP9. The study further investigated the mechanism behind their findings, using a bioinformatics database to identify the Runx2 gene as a potential target of miR-23b. RT-qPCR analysis revealed that Runx2 mRNA levels were not altered when cells were transfected with miR-23b mimics or inhibitors. However RUNX2 protein levels were repressed by miR-23b mimics and promoted by the miR-23b inhibitor when compared with controls. Upon examining the binding sites, it was found that co-transfection of miR-23b mimics and the Runx2-3′-UTR-wild-type plasmid reduced luciferase expression level compared with the control group. These results suggest that Runx2 mRNA is a direct target of miR-23b. The study also found that Runx2 knockdown reduced the effect of miR-23b. These observations suggest that miR-23b acts through RUNX2 to serve an important role in BMP9-mediated osteogenic signaling through negative regulation, opening up the possibility of mi-R23b inhibitors as a novel therapeutic strategy in bone fractures.
The previously discussed study66 implicating RUNX2 in BMP9-induced osteogenic differentiation made RUNX1 of interest, as it shares a similar structure with RUNX2 and is co-expressed in many skeletal elements. Ji et al investigated the interplay of RUNX1 with BMP9 induced stem cells, finding that BMP9 effectively increased the mRNA and protein levels of RUNX1 in varying stem cell lines.58 The study also found that RUNX1 also promoted early and late markers of BMP9-induced osteogenic differentiation and matrix mineralization, while showing that knockdown of RUNX1 through small interference RNA (siRNA) had the opposite effect. Through ChIP analysis, this study revealed that RUNX1 was likely a direct target of the BMP9-specific Smad1/5/8. Overexpression of Runx1 promoted BMP9-induced activation of Smad1/5/8, while siRNA against Runx1 inhibited this effect. The BMP9-induced phosphorylation of p38 and ERK1/2 was not influenced by overexpression or knockdown of RUNX1, indicating that RUNX1 exerts its influences on BMP9-induced osteogenesis through canonical Smad signaling. The studies by Ji et al58 and Chen et al66 have shown that both RUNX1 and RUNX2 are important players in osteogenesis and are affected by BMP9 signaling.
Inhibitors of differentiation (Ids) HLH factors
Like the RUNX proteins, Id genes code for ubiquitously expressed proteins that have been shown to be important downstream targets of TGFβ and BMP signaling.67 Id genes form a group of ubiquitously expressed proteins that serve as negative regulators of basic helix-loop-helix (bHLH) proteins, a group of transcription factors involved in cell type specific transcription and cell lineage commitment.68, 69, 70, 71 Id proteins dimerize with bHLH proteins, resulting in a defunct heterodimer that is unable to bind DNA and modulate transcription.67, 68, 70 Upon finding that Id-1, Id-2, and Id-3 were among the most significantly upregulated genes by the osteogenic BMPs,24 Peng et al sought to further elucidate the interaction with Id protein involvement in BMP-induced osteoblastic differentiation.25 They were able to demonstrate that Id-1, Id-2, and Id-3 genes were highly induced upon BMP9 stimulation. The most surprising result of the study is that they found that they were able to disrupt BMP9 induced osteogenic differentiation by using RNAi-mediated depletion as well as overexpression of the 3 Id genes. This seems to suggest a highly regulated balance of Id expression early on, with downregulation during terminal differentiation to the osteoblastic lineage.
Hairy/enhancer of split-related repressor protein 1 (Hey1) bHLH transcriptional factor
Many transcriptional processes that are critical to development are implicated in the BMP9 pathway, and the Hairy/Enhancer of split-related proteins (HERP) subfamily of basic helix-loop-helix (bHLH) proteins are no exception. These proteins normally function as DNA-binding transcriptional repressors in direct opposition to bHLH transcriptional activator proteins, such as proneural and myogenic proteins.12, 72 Hey1, a protein of the HERP family, has been found to be heavily expressed during embryologic development of the nervous system, somites, the heart, and the craniofacial region.73 Sharff et al was able to use gene expression-profiling analysis to demonstrate that Hey1 was one of the most significantly up-regulated targets in early BMP9-stimulated osteogenic differentiation of MSCs.27 Chromatic immunoprecipitation (ChIP) analysis revealed Hey1 as a direct target of the BMP9-induced Smad signaling pathway. Constitutive Hey 1 expression promoted BMP9-mediated osteogenic differentiation in vitro and matrix mineralization in vivo. Silencing of Hey1 expression via siRNA led to diminished osteogenic differentiation both in vivo and in vitro and led to chondrogenic differentiation. This Hey1 knockdown phenotype demonstrated significant downregulation of RunX2 as determined by qPCR and was partially rescued by RunX2 overexpression in MSCs both in vitro and in vivo. As discussed above, Runx2 is a vital mediator is bone differentiation. These results suggest that Hey1 may operate upstream of RunX2 during BMP9-mediated osteogenesis, and may be key to promoting osteogenic differentiation and preventing chondrogenic differentiation.
Connective tissue growth factor (CTGF)
Just as in the previous examples, it is no surprise that the crucial role of connective tissue growth factor (CTGF, CCN2) in bone formation, chondrocyte maturation, embryogenesis, and fibrotic disease74, 75, 76, 77, 78 implicates it in the BMP9 signaling pathway. Luo et al demonstrated that CTGF is one of the most significantly up-regulated genes by BMP9 stimulation in C3H10T1/2 MSCs.26 CTGF expression was induced in the early stages of BMP9 stimulation as confirmed by microarray and qPCR, returning to basal levels after 5 days. The early stage upregulation is significant, as CTGF expression was shown to be downregulated in later stages as pre-osteoblasts become committed to the osteogenic lineage. Using RNAi, the study found that siRNA targeting CTGF resulted in a significant decrease in BMP9-induced ALP activity. Interestingly, adenovirus mediated overexpression of CTGF also inhibited BMP9-mediated osteogenesis, suggesting that tight regulation of CTGF expression was necessary for BMP9 induced osteoblastic differentiation of MSCs. Exogenous expression of CTGF demonstrated enhanced migration and recruitment of MSCs in several in vitro assays. These results together suggest a model in which early stage CTGF upregulation promotes early stage BMP9 induced osteogenesis and MSC recruitment and migration.
Crosstalk between BMP9 and other major signaling pathways during osteogenesis
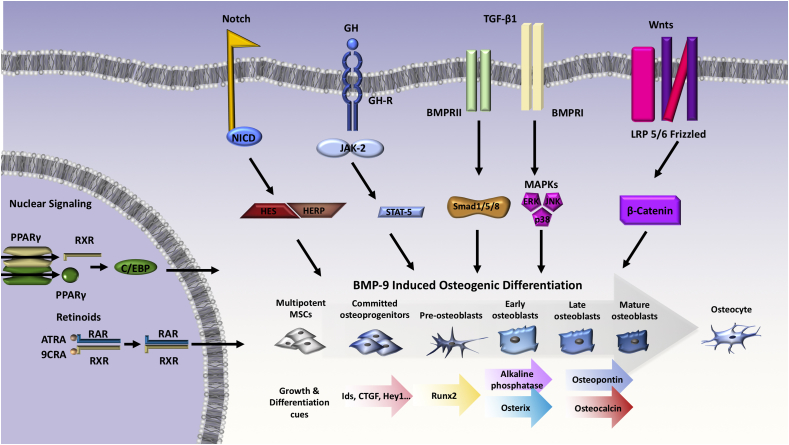
Several other signaling pathways occur in the cell also play roles in BMP9-mediated osteogenesis or stem cell differentiation (Fig. 3). Here, we summarize findings of crosstalk occurring between BMP9 signaling and other key pathways necessary for proper cell function and development. Integrating these studies will broaden our mechanistic understanding of BMP9-mediated osteogenesis.
Figure 3.
BMP9 signaling in endothelial cells and angiogenesis. The most current evidence supports the theory that a heterodimer formed by BMP9 and BMP10 provides most BMP biological activity in plasma, and acts as a key regulator of blood vessel homeostasis. ENG and ALK1 are necessary mediators of angiogenesis, with evidence suggesting that BMP9 and closely related BMP10 are the key cytokines upstream of ALK1/pSMAD1/5/8 signaling in endothelial cells.
TGF-β1 signaling pathway
Transforming growth factor-β (TGF-β) and bone morphogenetic protein (BMP) are both growth factors that play crucial roles in embryonic skeletal development and postnatal bone homeostasis. TGF-β1 is a polypeptide member of the TGF-β superfamily of cytokines, and it regulates many biological functions including immune homeostasis, cell proliferation, differentiation, development, apoptosis, and migration.10, 11, 79, 80, 81, 82 TGF-β1 also regulates bone formation and growth during mammalian development, and is important for MSC maintenance.10, 11, 79, 83 TGF-βs, like BMPs, bind to a tetrameric receptor complex to transduce signals to the Smad-dependent signaling pathway and the Smad-independent signaling pathway (i.e., p38 mitogen-activated protein kinase/p38 MAPK) to regulate MSC differentiation during bone development, formation, and homeostasis.84 Smad2/3 are phosphorylated and complex with Smad4 in order to localize to the nucleus to affect transcription factor activity.85 Both BMPs and TGF-βs signal through Smad4. Embryonic deletion of Smad4 in pre-osteoblast causes stunted growth.86 Unlike BMPs, TGF-β1 is unable to induce osteogenesis in mesenchymal pluripotent cells,87 but can direct committed osteoprogenitors toward osteogenic differentiation and bone remodeling.88 Li et al found that TGFβ1 appears to exert a biphasic effect on BMP9-induced osteogenic differentiation of mesenchymal stem cells89; specifically, low TGF-β1 leads to greater expression of later osteogenic markers, whereas higher levels show decreased levels of osteopontin and osteocalcin. This study also showed that the addition of TGF-β to MSC treated with high phosphate abolished BMP pathway activation.90 Taken together, this evidence has shown that TGF-β1 has a complementary role with BMPs and aids in production of mature bone.
Wnt/β-catenin signaling pathway
Just as important as growth factors such as TGF- β1, Wnt genes transcribe critical protein products in skeletal development and osteoblastic differentiation.11, 91, 92, 93, 94 Deletions of Wnt in mice have revealed intriguing phenotypes, such as deletion of Wnt-7A resulting in biventral limbs with footpads on both sides.95, 96 Wnts bind to the frizzled (Fz) and LRP-5/6 co-receptors to activate specific signaling pathways including the canonical β-catenin pathway.28 Tang et al established the importance of the canonical Wnt/β-catenin in the BMP9-mediated osteogenic differentiation of MSCs.28 This study demonstrated that Wnt3A and BMP9 acted complementarily to induce ALP activity in MSCs. This effect was inhibited by the Wnt signaling inhibitor, FrzB. The importance of β-catenin signaling was demonstrated upon RNAi-mediated silencing of β-catenin, as this diminished the early stage of BMP9-induced osteogenic differentiation. β-catenin was also shown to enhance BMP9 or RUNX2-induced expression of the osteocalcin promoter activity, while silencing β-catenin led to decreased BMP9 induced osteocalcin reporter activity, and decreased expression of osteopontin and osteocalcin. ChIP analysis confirmed these findings, showing that BMP9 induced the recruitment of both RUNX2 and β-catenin to the osteocalcin promoter. In vivo studies demonstrated that both FrzB overexpression and β-catenin knockdown resulted in significantly inhibited BMP9-induced ectopic bone formation and mineralization, and instead produced a chondrogenic phenotype. These results suggest that Wnt signaling, possibly through β-catenin interaction with RUNX2, is critical in BMP9 mediated osteogenic differentiation of MSCs.
Notch pathway
Elaborating on the themes discussed in the preceding sections, Notch is a transmembrane protein that has been recognized as an important regulator of bone formation,97, 98 in addition to promoting osteogenesis and angiogenesis in bone.99, 100 Earlier work investigating the Notch pathway has found that activation of Notch signaling augments BMP-induced ALP activity and calcified nodule formation in vitro.12 This combined with studies demonstrating that Notch inhibition causes decreased ALP activity and promoter activity of BMP target genes12 makes it a worthwhile subject of future research. More recent research points to the interconnectivity of Notch signaling and BMP9, as it has been found that BMP9 increases expression of Notch target Hey1 in MSCs,27 in addition to the finding that Notch signaling inhibition blunts BMP9-mediated tumor growth in osteogenic defective human osteosarcoma cells.37
Liao et al were able to demonstrate that BMP9 upregulates the expression of Notch receptors and ligands at the intermediate stage of osteogenic differentiation.101 The use of a constitutively activated form of Notch1 (NICD1) caused significant enhancement of BMP9-induced osteogenic differentiation both in vitro and in vivo. Their findings were validated by finding that dominant-negative Notch1 (dnNotch1) significantly blunted BMP9-induced osteogenic differentiation. The study then investigated the effects of BMP9 and NICD1 induction of immortalized murine adipocytes (iMADs) seeded within PPCNg, a biodegradable, thermoresponsive, citrate-based scaffold.102, 103, 104 They ultimately found extensive angiogenesis and vascularization when MSCs were stimulated by both BMP9 and NICD1 in the PPCNg scaffold. These results combined with the finding that BMP9 and NICD1 upregulated the expression of iMAD cells in vitro and Vegfa in ectopic bone tissues point to evidence that concurrent activation of BMP9 and NICD1 effectively couples osteogenic and angiogenic processes of MSCs in a 3D scaffold environment, pointing to great potential for application in bone tissue engineering.
Epidermal growth factor (EGF)
Studies have indicated that epidermal growth factor (EGF) signaling may play an important role in endochondral bone development and bone remodeling,105, 106, 107 as well as earlier studies demonstrating that EGF administration at physiological doses induces distinct effects on endosteal and periosteal bone formation with both time and dose dependency.108, 109 Liu et al investigated the role of cross-talk between EGF signaling and BMP9, ultimately demonstrating that EGF amplified BMP9-induced early and late osteogenic markers in MSCs in vitro.33 Conversely, EGFR inhibitors Gefitinib and Erlotinib and other protein kinase inhibitors are able to blunt these BMP9-induced markers in a dose-dependent manner. In addition, EGF was found to enhance BMP9-induced endochondral bone formation in cultured mouse fetal limb explants, an effect that was also blunted with EGFR inhibitors.33 The in vivo stem cell implantation experiments conducted by Liu et al also showed that exogenous expression of EGF enhanced BMP9-induced ectopic bone formation, resulting in higher yields of mature trabecular bone. Experimental results demonstrate a synergistic role for epidermal growth factor and the bone forming properties of BMP9.
Hedgehog signaling
Although the Hedgehog (Hh) family of proteins are now known to be extensively involved in the control of cell growth, survival, and patterning of the vertebrate body plan,110, 111 more recent evidence indicates that Hh signaling may act as a key modulator in bone homeostasis112, 113, 114, 115 including the regulation of osteoblast and osteoclast differentiation with BMP2, BMP4, and BMP7.116, 117 Naturally, these findings led Li et al to investigate the possible involvement of Hh signaling in the BMP9-induced osteogenic differentiation of MSCs.118 The study first determined that BMP9 exerts an effect on Hh signaling molecules by using RT-PCR to follow the expression levels of crucial Hh signaling molecules including Smo, Hhip, Ptch1, Gli1, Gli2, Dhh, Ihh, and Shh and observing altered expression after expression of BMP9. In their other experiments, cyclopamine (Cy) was used to block the activity of Hh signaling while purmorphamine (Pur) was used to activate Hh signaling. They found that cyclopamine inhibited the BMP9-induced ALP activity in a dose dependent manner, while purmorphamine enhanced the BMP9-induced ALP activity. Similar results were obtained with later osteogenic markers, finding that cyclopamine decreased both matrix mineralization, as well as osteopontin and osteocalcin, while purmorphamine markedly increased all of these markers as measured by RT-PCR and Western blot. To gain further insight into the mechanism of Hh signaling interplay with BMP9, the group used the BMP responsive Smad1/5/8 reporter p12xSBE-Luc. They found that transcriptional activity of Smad1/5/8 was augmented by purmorphamine, and inhibited by cyclopamine without influencing the phosphorylation of Smad1/5/8, ERK1/2, and p38. These results suggest that Hh signaling plays a pivotal role in both the early and late stages of BMP-induced osteogenic differentiation, and partially works by affecting the transcriptional activity of canonical Smad1/5/8 without altering its phosphorylation status.
Retinoid signaling
Retinoids are the active metabolites of vitamin A and regulate a complex network of genes involved in growth, cellular differentiation, tissue homeostasis and vertebrate morphogenesis.119, 120 Retinoic acids (RAs) act as ligands for two classes of receptors, including the RA receptors (RAR) that bind all-trans-retinoic acid (ATRA) as well as the Retinoid X Receptors (RXR) that are able to bind 9-cis-retinoic acid (9CRA)120, 121, 122, 123 (Fig. 3). Upon binding RAs, these receptors regulate gene expression by acting as heterodimers (RAR/RXR) or homodimers (RXR/RXR) that can bind to retinoic acid response elements (RARE) in target genes to stimulate their transcription.123 Because of the conflicting data regarding the role of retinoic acids influencing osteogenic differentiation,124, 125, 126, 127, 128, 129, 130 Zhang et al sought to clarify the effects of RA signaling on BMP9-induced osteogenic differentiation.31 Both 9CRA and ATRA were found to induce the early osteogenic marker ALP as well as later markers osteopontin and osteocalcin in mesenchymal progenitor cells as well as replicated in vivo with enhanced osteogenic differentiation and mineralization.
In vivo studies were conducted by using mouse perinatal limb explants, showing that BMP9 and RAs collectively promote the development of the hypertrophic chondrocyte zone at the growth plate. Histologic examination of stem cell implantation studies in athymic nude mice demonstrated that co-expression of BMP9 and RXRα or RARα in MEFs significantly increased trabecular bone formation and osteoid matrix deposition. Mechanistically, they found that retinoic acids were able to significantly up-regulate BMP9 expression in addition to finding that 9CRA and ATRA were able to enhance the BMPR Smad-mediated transcriptional activity. This suggests that crosstalk exists between the signaling pathways involved in BMP9-mediated osteogenesis and retinoic acids, and the interactions between these pathways synergistically enhance osteogenic differentiation.
Insulin-like growth factors (IGFs)
Insulin-like growth factors are critical growth promoting peptides that play a role in the regulation of CNS development, wound healing, macronutrient metabolism, aging, and numerous other functions.131, 132, 133, 134, 135 Of particular interest to the field of regenerative medicine may be the interaction of IGF-2 in combination with BMP9 to synergistically potentiate BMP9 mediated osteogenic differentiation.32 IGF-2 is a member of the IGF signaling system, which activates the mitogen activated protein kinase (MAPK) or phosphatidylinositol-3-kinase (PI3K)/AKT pathway.32, 136 Chen et al were able to demonstrate that exogenous expression of IGF-2 could potentiate the effects of BMP9 induced early osteogenic marker alkaline phosphatase (ALP) activity, as well as later osteogenic markers osteopontin (OPN) and osteocalcin (OC).32 In vivo, transduced C3H10T1/2 stem cells were implanted in athymic nude mice, demonstrating that while IGF-2 was unable to form any detectable bony masses on its own, IGF-2 synergistically enhanced the effects of BMP9 in the formation of bony masses to a greater degree than the BMP9 transduced cells alone. The synergistic effects of IGF-2 on BMP9 mediated osteogenesis were inhibited upon the use of IGF binding proteins (IGFBP3 and IGFBP3), confirming the role of IGF-2 in potentiating the effects of BMP9.
Mechanistically, it was found that IGF-2 was able to augment BMP9 induced BMPR-Smad reporter activity while also increasing nuclear translocation of Smad1/5/8. As IGF-2 signals though activation of the PI3K/AKT pathway, the group was able to demonstrate that phosphorylated AKT1/2/3 was most pronounced in cells stimulated by IGF-2 and BMP9 when compared to controls, suggesting crosstalk between IGF-2 and BMP9 pathways. As AKT is activated by PI3K, use of the PI3K inhibitor LY294002 inhibited the potentiation of IGF-2 on BMP9 mediated osteogenesis, suggesting that PI3K may cross-regulate BMP9 mediated osteogenic signaling.
Growth hormone (GH)
Growth hormone (GH) maintains a crucial and extensive role in the endocrine system, contributing to a wide variety of processes including growth and development, metabolism, bone mineral density, and numerous other functions.137, 138, 139, 140, 141, 142, 143 When bound by GH, the GH receptor dimerizes and results in activation of the JAK-STAT pathway, the main effector pathway for GH effects on gene transcription.144 Huang et al found that GH is a direct target of the BMP9/Smad pathway, resulting in strong upregulation of the GH transcript.30 While GH expression alone failed to induce detectable osteopontin or osteocalcin expression, exogenous GH potentiated BMP9-induced early osteogenic marker alkaline phosphatase (ALP) in MMCs, as well as later osteogenic markers osteopontin and osteocalcin to a greater extent than BMP9 alone. C3H10T1/2 cells co-expressing GH and BMP9 showed more robust mineralization than BMP9 expression alone, reaffirming the synergistic nature of GH-BMP9 induced late osteogenic differentiation. The study was also able to show that BMP9 and GH co-expression caused substantial mature ectopic bone masses, in addition to significant expansion of the growth plate of long-bone explants. The synergistic nature of BMP9 and GH was found to be considerably blunted by JAK/STAT inhibitors, leading to decreased GH-regulated IGF1 expression in MMCs. These findings support the theory that GH plays an important role in regulating and potentiating the effects of BMP9 in osteogenesis by activating JAK/STAT/IGF1 signaling pathways in MMCs, suggesting that this axis may be utilized for novel therapeutic targets in regenerative medicine.
Hypoxia inducible factor 1 alpha (HIF1α)
The pathway for hypoxia inducible factor 1 alpha (HIF1α) is a well-established regulator of the angiogenic cascade and many growth-related processes including skeletal formation and maturation.145, 146 The fact that osteogenic and angiogenic pathways are tightly coordinated in parallel during bone formation145 inspired further investigation of the role of (HIF1α) in relation to BMP9. Hu et al investigated the role of hypoxia-inducible factor 1α (HIF1α)-mediated angiogenic signaling in the BMP9 regulated osteogenic differentiation of MSCs.34 This study found that HIFα was upregulated in MSCs at 48 h after BMP9 transduction and also demonstrated that BMP9 upregulated HIFα expression in MSCs through Smad1/5/8 signaling. Exogenous overexpression of HIFα was found to have a profound synergistic effect on BMP9-induced ALP activity in MSCs, in addition to augmenting late osteogenic markers osteopontin (OPN) and osteocalcin (OCN). This ultimately provided strong evidence of the ability of HIFα to enhance BMP9-induced osteogenesis and terminal differentiation of MSCs in vitro. In vivo, HIFα augmented BMP9-induced bony mass formation in athymic nude mice, as confirmed through gross size difference with 3D analysis of μCT imaging data, increased mineral density, in addition to increased average trabecular thickness, even when controlled for trabecular area over total area. The study lastly strengthened the evidence behind HIFα in BMP9 signaling by using CAY10585, a siRNA-mediated inhibitor of HIFα, and found it to significantly blunt BMP9-induced osteogenic signaling in MSCs. Overall, this study provided strong evidence highlighting the importance of HIFα induced angiogenic signaling in BMP9-initiated differentiation of MSCs.
Peroxisome proliferator-activated receptor γ (PPAR-γ)
PPAR-γ specifically has been found to be a critical regulator of adipogenesis and osteogenesis, making it an interesting potential target in skeletal diseases.147 Relevant to this discussion, PPARs have been found to exert a role in bone metabolism and osteoclast formation.148, 149, 150 In bone marrow, mesenchymal stem cells differentiate into adipocytes or osteoblasts via a competitively balanced mechanism in which complex cross talk between various signaling pathways (including BMPs and PPARs) influence the fate of mesenchymal stem cells151 (Fig. 3). Kang et al investigated the involvement of BMPs and PPAR-γ2 regulating multilineage commitment of MSCs.16 They found that PPAR-γ2 overexpression not only promoted adipogenic differentiation, but also enhanced osteogenic differentiation upon BMP2, BMP6, and BMP9 stimulation. Conversely, knockdown or deletion of PPAR-γ2 inhibited adipogenic differentiation, in addition to diminishing BMP9 induced ossification.16 These findings implicate PPAR-γ2 as an important contributor to BMP-induced osteogenic and adipogenic differentiation. The study also found that BMP-regulated osteogenic and adipogenic lineage commitment of MSCs is mutually exclusive, as C3H10T1/2 cells stimulated with BMP either exhibited high ALP activity indicating early osteogenic activity or lipid accumulation suggesting adipogenic commitment. No cells were observed exhibiting both high ALP activity and lipid accumulation.
NELL-1 cross-talk with BMP9
NELL-1 is a potent osteoinducer first discovered by Ting et al when they identified and isolated it as an upregulated cDNA fragment in the premature fusing and fused coronal sutures.152, 153 It has been proven both in vitro and in vivo as a potent osteo-inductive factor, while repressing adipogenic differentiation and inflammation.153, 154, 155 Given its osteogenic potential, Wang et al conducted a study investigating the interaction of NELL-1 with BMP9.156 Using an adenoviral vector to induce BMP9 expression in MSCs, NELL1 expression was significantly upregulated. They also found they were able to silence this effect by using recombinant adenovirus-mediated RNAi. NELL1 overexpression was unable to induce any detectable ALP activity, and NELL1 transduced MSCs expressed reduced ALP activity upon BMP9 stimulation when compared with that of the MSCs stimulated with BMP9 alone. Silencing NELL1 expression resulted in increased BMP9-induced ALP activity in an adenovirus titer-dependent manner. While the early marker ALP was inhibited by NELL1, late stage mineral nodule formation was significantly enhanced in vitro by overexpression of NELL1 in BMP9-induced cells. Silencing NELL1 expression did not significantly affect BMP9-induced mineralization. These results together suggest that NELL1 overexpression may accelerate osteogenic differentiation of BMP9-induced committed osteoblastic precursor cells. Overexpression of NELL1 alone also slightly induced RUNX2 and Osterix expression but did not significantly alter the expression of late osteogenic markers osteocalcin and osteopontin. However, BMP9 induced expression of RUNX2, Osterix, osteopontin and osteocalcin was shown to be increased by NELL1 overexpression. The effect of NELL1 overexpression on chondrogenic markers was less remarkable, although NELL1 overexpression was able to potentiate the BMP9-induced expression of SOX9, COL2A1, and COL9A1 at early time points.
The ability of BMP9 to induce adipogenesis has been well established,16, 157, 158 and may eventually prove problematic to its implementation clinically. Perhaps the most clinically relevant finding of Wang et al is that NELL1 overexpression was able to inhibit BMP9 induced adipogenesis, while silencing NELL1 did not have significant effects on BMP9-induced adipogenesis. This was further confirmed by analyzing the expression of regulators and markers of adipogenesis in BMP9-stimulated MSCs, finding that BMP9 up-regulated the expression of Pparγ2, C/ebpα, C/ebpβ, Pgc1 and Ap2/Fabp4, all of which were inhibited by forced NELL1 expression. To confirm the in vitro results, the team conducted in vivo experiments, finding that 4 weeks after subcutaneous implantation of MSCs transduced with BMP9 into athymic nude mice, NELL1 overexpression resulted in higher average bone mineral density, while silencing NELL1 reduced the mineral density of BMP9-induced bone masses when compared to the BMP9-transduced MSCs alone. Histologic evaluation demonstrated that while BMP9 alone was able to induce robust bone formation and adipogenesis, NELL1 overexpression promoted the formation of more mature and trabecular bone-like structures in addition to the NELL1 & BMP9 co-expression group having the highest level of bone mineralization. Silencing NELL1 expression in MSCs inhibited BMP9-induced ossification and enhanced chondrogenesis, and also led to the formation of less mineralized chondroid matrix. These results together suggest that NELL1 may serve as a useful co-osteogenic factor to enhance BMP9-induced bone formation while inhibiting adipogenesis in regenerative medicine.
BMP9 signaling in various physiologic and pathological processes
Given the variety of signaling mechanisms through which BMP9 exerts effects, it could be hypothesized that it likely fulfills a number of physiological roles as well. This hypothesis would be correct. BMP9 signaling has been shown to be involved in such processes as angiogenesis, glucose metabolism, neurogenesis and tumorigenesis. In the next section, we will investigate its part in regulation of these systems, the potential cellular mediators for its actions, and potential therapeutic applications of BMP9 throughout the various organ systems.
BMP9 signaling in angiogenesis and endothelial cell biology
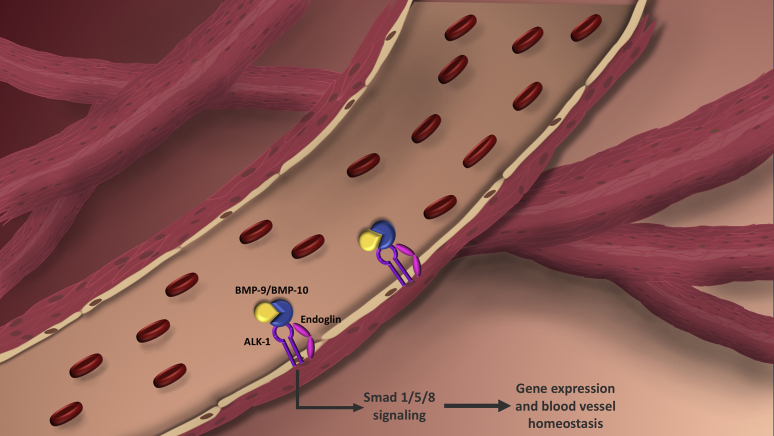
It has long been recognized that osteogenic and angiogenic processes are coupled and coordinated during bone formation145 but the exact role of BMP9 has yet to be fully elucidated. However, there is a collecting body of research demonstrating the importance of BMP9 in regulating angiogenic processes159 via its interaction with endoglin160, 161, 162 (Fig. 2), vascular remodeling and homeostasis,163 the pathogenesis of diseases such as hereditary hemorrhagic telangiectasia,164 and proper closure of the ductus arteriosus.165
Figure 2.
Signaling crosstalk with BMP9. Several major signaling events must take place in the BMP9-induced osteogenic differentiation of mesenchymal stem cells. The action of BMP9 results in a complicated albeit well-coordinated signaling cascade that results in gene transcription at precise steps of osteogenic differentiation. Cross talk between with BMP9 and other pathways such as the Wnt, Notch, and the other pathways shown results in augmentation of BMP9 signaling.
It has recently been revealed in a study conducted by Tillet et al that a heterodimer formed by BMP9 and BMP10 provides most BMP biological activity in plasma166 (Fig. 2). BMP9 was co-transfected with BMP10 and was shown to form a disulfide-bonded heterodimer in vitro that is able to function by interacting with endothelial cells via ALK1. They then developed an ELISA that could recognize the BMP9/10 heterodimer and found it present in both human and mouse plasma. To further investigate the activity of this heterodimer, they generated either BMP9 or BMP10 knockout mice, finding that plasma from either group was completely unable to activate ALK1-transfected 3T3 cells or phospho-Smad 1-5 on endothelial cells. This strongly indicates that circulating BMP activity is due to the BMP9/10 heterodimeric form and is likely a key regulator of blood vessel homeostasis. The heterodimer of BMP9/10 suggests a common production site of both BMPs. Analysis of mRNA expression revealed that BMP9, and to a lesser extent BMP10, mRNAs are present in hepatic stellate cells (HSCs), providing a possible cellular source for this heterodimer.
Endoglin (ENG) is a co-receptor for several TGFβ-family cytokines that are expressed in hypoxic and replicating endothelial cells. The angiogenic function of endoglin is closely tied to the function of the ALK1 receptor, the gene product of the Activin-like Receptor Kinase 1 gene (ACVRL1).162 ENG and ALK1 are necessary mediators of angiogenesis, with evidence suggesting that BMP9 and closely related BMP10 are the key cytokines upstream of ALK1/pSMAD1/5/8 signaling in endothelial cells46, 162, 167 (Fig. 2). Acting as ligands for ALK1, BMP9 and BMP10 have been found to strongly inhibit microvascular endothelial cell migration and growth.167 David et al was able to demonstrate that human serum induced Smad1/5 phosphorylation, but not in the presence of anti-BMP9 antibodies. This suggests that BMP9 is circulating in a biologically active form, and likely plays a critical role in the regulation of vascular tone adult blood vessel quiescence.17, 167
Regulation of ENG and ACVRL1 signaling pathways is of critical significance, as mutations in either ENG or ACVRL1 lead to Hereditary Hemorrhagic Telangiectasia (HHT), an autosomal dominant disease characterized by severe arteriovenous malformations. Currently working models suggest that HHT pathogenesis involves the ALK1/endoglin/Smad signaling pathway, with BMP9 and BMP10 present in blood binding to ALK1 and endoglin on endothelial cells, inducing vascular quiescence.168 GDF2 mutations (encoding BMP9) have been shown to result in a vascular defect syndrome with similarities to HHT.19 These findings are of great clinical significance, as future therapeutic approaches for HHT may be able to stimulate this deficient inhibitory pathway via BMP9 or BMP10 ligands, mimicking peptides, or increasing bioavailable forms of these BMPs.168
BMP9 signaling in glucose metabolism and insulin resistance
BMP9 has been shown to regulate a variety of enzymes involved in glucose homeostasis,169, 170, 171 opening up the possibility of therapeutic value in this arena. BMP9 has been shown to reduce blood glucose levels in diabetic mice with maximal reduction in around 24–30 h after injection.169 Caperuto et al was able to demonstrate that BMP9 mRNA was decreased in multiple distinct paradigms of insulin resistance, as well as prove that anti-BMP9 antibody delivered to fasted rats induced glucose intolerance and insulin resistance.170 Kuo et al showed that MB109, a recombinant BMP9 derivative, enhanced brown adipogenesis of human adipose tissue derived stem cells.158 They also showed that systemic intraperitoneal injection of MB109 resulted in suppressed weight gain in high fat diet-induced obese mice, reducing the size of white adipocytes and decreased 16 h fasting blood glucose level, without effecting food consumption or behavior. The effects of recombinant BMP9 may result from preferential browning of subcutaneous white adipose tissue and upregulating fatty acid synthase (FAS) expression in the liver. These results are significant as brown adipose tissue has been associated with improved glucose homeostasis and insulin sensitivity.172, 173
Interestingly, the relation of BMP9 to metabolic syndrome and insulin resistance has also been demonstrated in humans. Xu et al was able to demonstrate that in patients with metabolic syndrome, circulating BMP9 levels were significantly lower in metabolic syndrome patients compared to those of healthy controls.174 In analysis of the subgroups of the study, subjects with central obesity had significantly lower circulating BMP9 levels when compared to lean individuals (P < 0.05). Lower BMP9 levels were also found in individuals with dyslipidemia or hypertension (P < 0.01). In subjects with more than 3 components of metabolic syndrome, circulating BMP9 circulating concentrations were found to be significantly reduced. Lastly, plasma BMP9 concentrations were found to be significantly associated with metabolic syndrome even after controlling for anthropometric variables, lipid profiles, and hormone levels. The high degree of negative associations of low circulating BMP9 levels with key factors of metabolic syndrome points to its potential role in the pathophysiology underlying this disease.
Luo et al conducted a study further investigating the role of BMP9 in Type 2 diabetes mellitus (T2DM) and insulin resistance (IR).175 They found that circulating BMP9 levels were substantially lower in study subjects with T2DM than in normal subjects (P < 0.01) with the difference in levels remaining significant after adjustment for BMI, sex, and age. Obese subjects with IR (HOMA-IR >3.8) were found to have lower BMP9 levels than obese subjects without IR (HOMA-IR <3.8, P < 0.01). Of relevance, however, is that BMP9 concentrations were no different in overweight and obese subjects when compared to normal-weight subjects. In all study populations, a positive relationship was found between BMP9 levels and homoeostasis model assessment of insulin secretion (HOMO-β) and non-esterified fatty acids, but found an inverse relationship between BMP9 and HBA1c, FBG, 2-hour oral glucose tolerance test, AUCglucose and HOMA-IR.
The study went further to demonstrate that circulating BMP9 levels were correlated with newly diagnosed T2DM after controlling for anthropometric variables, age, gender, % body fat and lipid profile. These results were confirmed by finding that BMP9 mRNA and protein expression were decreased in T2DM patients compared to healthy subjects. They went on to show that short term hyperinsulinemia led to a gradual reduction of circulating BMP9 levels during an Euglycemia Hyperinsulinemic Clamp (EHC)176 study. The study further investigated the effects of induced insulin resistance via lipid infusion,175, 177 finding that intralipid/heparin infusion combined with EHCs in healthy subjects produced a greater decline in circulating BMP9 levels than in EHCs alone. Using a GLP-1 agonist on patients with T2DM, they found that after 24 weeks, patients experienced an increase in insulin sensitivity and decrease in weight, however no change in circulating BMP9 levels was found. In placebo-controlled patients, circulating BMP9 levels were found to be significantly decreased, but this decline was halted in the GLP-1 agonist group. The aforementioned studies, as well as new evidence that demonstrates decreased circulating BMP9 levels with significantly high risk of hypertension and/or coronary heart disease178 show promising results of the potential therapeutic value of BMP9 outside of osteogenesis.
BMP9 signaling in neurogenesis
During neural development, BMP ligands and receptors can be found within specific zones of the embryonic brain as well as in neural crest derived tissues to regulate gastrulation, and influence neuronal linage commitment in the peripheral nervous system.179 Lopez-Coviella et al investigated the role of BMP9 in neural development, finding that BMP9 holds a role in regulating the cholinergic phenotype.180 Their study first examined the expression of BMP9 in the CNS during mouse development, finding that BMP9 was most highly expressed in the septum and spinal cord of embryonic day 14 (E14) mice, resembling the expression pattern of mature cholinergic neurons. They then took these cells derived from the E14 mice and treated them with recombinant BMP9, finding that after a lag period of 24 h, the BMP9 increased acetylcholine (ACh) levels in the cultures in a concentration and time-dependent manner up to 72 h later, while controls showed low levels of ACh throughout the study. Other members of the BMP family were able to induce ACh levels to a variable extent compared to control levels, however the 20-fold ACh content induced by BMP9 was unmatched. This effect of BMPs was not shared by TGF-β1, which was unable to significantly affect ACh levels.
BMP9 was also found to alter the morphology of neural cultures, causing them to take on a characteristic round cluster shape in contrast to the uniform monolayers formed by control groups. In addition, these BMP9 treated cells highly expressed the early neuronal marker βIII-tubulin, as well as choline acetyltransferase (ChAT) which was found to be almost completely absent in controls. BMP9 was found to be specific to the cholinergic phenotype, as it did not induce markers characteristic of catecholaminergic and γ-aminobutyric acid–ergic neurons. In response to septal cultures obtained between E11 and E18, BMP9 was found to have the greatest effect in cells from E14 embryos, coinciding with the apex of cholinergic differentiation. In the absence of BMP9, mRNA levels of ChAT and the vesicular acetylcholine transporter (VAChT, another cholinergic marker) were found to be undetectable in E14 derived cells. Adding BMP9 induced ChAT and VAChT expression, indicating that it can induce the cholinergic gene locus. They also establish that in primary brain cultures BMP9 acts directly on cholinergic precursor cells. BMP9 was also found to be necessary for maintenance of the cholinergic phenotype, as ACh levels were reduced by 80% in cells cultured without BMP9. The study further investigated the interaction of BMP9 with basic fibroblast growth factor (bFGF), finding that in the presence of bFGF, the effect of BMP9 on cholinergic differentiation was dependent on the concentration of bFGF. These results together suggest that BMP9 acts directly and specifically to induce expression of the cholinergic gene locus, resulting in the induction and maintenance of the cholinergic phenotype in embryonic development.
BMP9 signaling and tumorigenesis
In addition to the relevance to bone, recent research has shown that BMP9 and other BMPs regulate tumor processes in cancer such as development, progression, bony metastasis of tumors,181 as well as a number of cellular processes such as proliferation and apoptosis.182 This has spurred research in BMPs role in different cancer cells in hopes of finding a novel therapeutic target in cancer treatment. Interestingly, BMP9 has been shown to have different effects on different cancers; for example BMP9 has been shown to promote the proliferation of liver183, 184 and ovarian cancer cells,185 while inhibiting the growth of osteosarcoma186 and breast cancer cells.187 This section will briefly cover the existing research of BMP9's proposed action and mechanism of action in these cancers.
Osteosarcoma
Osteosarcoma is the most common primary malignancy of bone in children and young adults and has a poor prognosis because it has high rates of metastasis and chemo-resistance.188, 189 BMP9 has been shown in many studies to play a key role in promoting osteosarcoma growth.27, 190 But other studies have shown that BMP9 overexpression in osteosarcoma leads to reduced invasion and migration.188 BMP9's role in osteosarcoma has not been fully explored yet, and it could be that BMP9 plays overlapping roles in the regulation of osteosarcoma cells.
As discussed previously the Notch pathway plays a central role in mediating BMP9s many effects, although the exact interaction remains unclear.191 The Hey1 gene is a highly conserved target of the Notch signaling pathway that increases during BMP9 induced differentiation of mesenchymal stem cells.27 Hey1 also seems to be a target of BMP9-Smad signaling and the silencing of the Hey1 gene diminishes BMP9 induced osteogenic differentiation both in vitro and in vivo.27 The Notch-BMP9 studies189 showed that cell proliferation and migration in and OS cell line (MG63) was more intense in cells with adenoviral transfection of BMP9, and that this increase was dampened by the expression of AdR-dnNotch1, a dominant negative form of the Notch1 protein, and compound E, a known inhibitor of Notch signaling.
In contrast to the above experiment, Xie et al192 showed that increased OS growth was correlated to decreased levels of BMP9. The researchers established that in patient OS tumor cells there was an increase of the levels of a microRNA, miR-149, and that the level of miR-149 had a negative correlation with the level of BMP9. They then showed in vitro that increased expression of miR-149 lead to increased cell proliferation and decreased apoptosis in an OS cell line (MG63), by leading to a decrease in Bcl-2 expression and increased cleavage of caspase-3. They also demonstrated that miR-149 directly targets the BMP9 gene, and that the miR-149 inhibitory effects on OS progression are mediated by BMP9, by revealing that BMP9 knockdown cells did not have an increase in cleaved caspase-3 or a decrease of Bcl-2 in the presence of miR-149. In summary, these experiments showed that increased miR-149 lead to decreased BMP9 levels which promoted OS cells proliferation,192 although these results did not appear in BMP9 knockdown cells.
BMP9 most likely plays overlapping and complex roles in OS, and the specific interactions of the gene regulatory pathways are not yet clear. More research is needed for a better understanding of the natural history of the disease and may potentially lead to development of novel therapies.
Liver cancer
As well as contributing to liver homeostasis, BMPs and BMP9 in particular, have been found to be tumorigenic in the liver.184 Studies have shown that BMP9 is overexpressed in a subset of HCC (Hepatocellular carcinoma) tissues,193 and that it promotes the epithelial to mesenchymal transition, an important step in the cancer progression and metastasis.193
Researchers from the Complutense University of Madrid have done multiple studies showing the proliferative effects that BMP9 has on HCC cells, and proposed a possible mechanism of action.183, 184 Their experiments showed that human HCC cells incubated with BMP9 show a significant increase in cell number compared to controls after 4 days, but immortalized hepatocytes did not show the same response.184 The researchers theorized that these differences resulted from differing receptor levels in the cells. They provided further evidence that BMP9 signaling in the HepG2 HCC cells most likely works through the type I receptor ALK2. TGF-B inhibition has also been shown to reduce proliferation of HCC cells194 and BMP autocrine loops have been previously described in cancer models.185, 195 This supports the possibility that HCC cells that acquire BMP9 autocrine signaling promote proliferation and cell survival.184 They further showed that BMP9 has a cell survival effect as well as a proliferation effect. Previous studies have shown that serum deprived HCC cells undergo apoptosis that can be blocked by IGF and other growth factors.196, 197 BMP9 showed similar action to the other growth factors, with HepG2 cells showing little to no apoptosis when grown in serum deprived medium for 4 days.184
In more recent experiments, it was shown that BMP9's pro-survival effects in serum deprived medium require p38MAPK activity that does not seem to be dependent on the canonical Smad signaling.183 BMP9 has been shown to induce p38MAPK activation in other cells, such as mesenchymal progenitor cells and osteosarcoma cells. Although p38MAPK is classically associated with the stress response, recent studies have shown that p38MAPK can also activate protective pathways in certain environments such as neurogenesis, colorectal cancer, and DNA damaged fibroblasts.183 Together, these experiments demonstrate the important role of BMP9 in hepatocellular tumorigenesis and provide a target for future treatments.
Breast cancer
As in osteosarcoma, BMP9 inhibits the growth, proliferation, and metastases of breast cancer cells. In a study of breast cancer tissue taken from 23 patients with breast cancer, BMP9 levels were elevated in normal tissue adjacent to the cancerous tissue.198 In addition, an in vitro assay demonstrated that overexpression of BMP9 inhibited proliferation and invasion of cancer cells while also having pro-apoptotic effects.198 Interestingly, an in vivo study using BMP9 or BMP10 knockout mice found that only BMP9 has an inhibitory role in breast cancer.199 As previously discussed in this review, BMP9 and BMP10 have many of their pro-osteogenic effects through binding to the shared receptor ALK1. This study suggests that the effects of BMP9 on breast cancer must be through a different signaling pathway.
In fact, BMP9 appears to modulate oncogenic cells in breast cancer through a myriad of different mechanisms. One of these mechanisms seems to be via downregulation of the PI3/Akt signaling pathway. Although BMP9 and PI3/Akt have a synergistic role in promoting bone growth, BMP9 overexpression in MDA-MB-231 tumor cells significantly decreased levels of p-Akt and tumor volumes in vitro and in vivo.200 In HER2 positive breast cancer cells, increased BMP9 expression decreased HER2 expression.201 BMP9 may also have some of its effects through alteration of long-non-coding RNA (lncRNA), or RNA of more than 200 base pairs that do not code for proteins. Different lncRNAs have previously been shown to influence breast cancer metastasis.202 Liu et al used gene chip and qPCR analysis to demonstrate that BMP9 significantly decreased expression of the lncRNA ITGB2-AS1.203 Higher levels of ITGB2-AS1 expression increased breast cancer cell migration and invasion.203
BMP9 may also inhibit metastases of breast cancer. Nearly all advanced stage breast cancer includes a metastatic component to bone, which causes significant pain, risk of fracture, and hypercalcemia.204 MDA-MB-231 breast cancer cells co-cultured with bone-marrow derived stem cells (BMSC) +/− exogenously added BMP9 demonstrated that BMP9 also increased breast cancer apoptosis and impaired proliferation without damaging BMSCs.205 The mechanism by which this occurs may be through decreased RANKL expression on BMSCs, which blocks the AKT signaling pathway; these findings again suggest the importance of BMP9 regulation on the AKT signaling pathway for breast cancer metastasis.206
Obesity has been shown to be a risk factor for breast cancer by inducing the epithelial-mesenchymal transition.207, 208 Co-culture of MDA-MB-231 breast cancer cells with adipocytes increased proliferation and migration of cancer cells, but addition of BMP9 to this co-culture effectively reduced cancer cell proliferation by blocking leptin and Ob-R signaling.187
In summary, the inhibitory role of BMP9 in breast cancer has been demonstrated in many different studies.187, 198, 199, 200, 201, 202, 205, 206 Though the mechanisms are still not fully understood, the major inhibitory effects on migration and proliferation appear be mediated through downregulation of the PI3/AKT signaling pathway. Finally, increased expression of BMP9 may serve as a chemotherapeutic tool against HER2+ cancers and as a means of decreasing bone metastasis in late stage breast cancer.
Ovarian cancer
The BMP family plays an important role in ovarian function, including regulation of oocyte development and granulosa cell proliferation.209, 210 The general BMP family target genes ID1 and ID2 have been shown to be upregulated in ovarian cancer.211 A 2006 study demonstrated that BMP2 expression was upregulated in ovarian cancer cells as compared to normal epithelial ovarian cells, suggesting a possible role for the BMP family in ovarian malignancy.212
BMP9 may also play a complex role in the pathogenesis of ovarian cancer. Previous studies have suggested an anti-oncogenic role for BMP9 through inhibition of endothelial cell proliferation and angiogenesis via an ALK1 mediated mechanism.46 In contrast to this, BMP9 seems to promote epithelial ovarian cancer cells through an ALK2 mediated mechanism.185 Furthermore, ovarian cancer cells express significantly more BMP9 than normal epithelial ovarian cells, and tumor proliferation is significantly decreased with downregulation of BMP9.185 Though the role of BMP9 in ovarian cancer has not been explored as thoroughly as in other cancers, the research does seem to suggest some pro-oncogenic role.
BMP9 signaling in other human diseases
BMP9 signaling in bronchopulmonary dysplasia (BPD) and pulmonary arterial hypertension
Of the various complications that may afflict a survivor of premature birth, bronchopulmonary dysplasia (BPD) is the most common complication of births at <30 weeks gestation.213 The hallmark of BPD is alveolar enlargement, caused by aberrant alveolar and vascular development with no effective treatments other than supportive care. Chen et al investigated BMP9 treatment of rat pups with experimental BPD, finding that BMP9 was able to improve aberrant alveolar development as demonstrated by reduced alveolar enlargement and septal thickness. BMP 9 treatment also reduced pulmonary inflammation and extravascular collagen deposition without negative effects on normal neonatal lung development.214 The in vitro data showed reduced pulmonary influx of macrophages and neutrophils, less fibrosis, and suppression of the expression of pro-inflammatory cytokines IL-6 and MCP1.
Nikolic and colleagues recently published their work on BMP9 and its role in portopulmonary hypertension (PoPH), which could signify a fundamental advance in the understanding of the disease process.215, 216 To date, the etiology of PoPH is not clearly understood but due to previous studies suggesting the importance of BMP9 in pulmonary arterial hypertension,162, 167, 215 the authors hypothesized that altered expression of BMP9 may serve as a sensitive mechanistic biomarker of pulmonary arterial hypertension (PAH) associated with liver disease. In two independent groups with PAH as a result of diverse etiologies, they found staggeringly diminished circulating BMP9 levels amongst patients with PoPH. BMP9 was found to be an important biomarker that distinguishes PoPH from other types of PAH, and may represent a risk for PoPH that is not currently represented by standard indices of liver disease. This is of great clinical significance and interest, as BMP9 was found to predict several important clinical parameters. Interestingly, BMP9 was able to predict transplant-free survival in patients with group 1 PAH. This predictive value remained independent of parameters indicative of RV function, suggesting that the predictive value of BMP9 is not dependent on RV function.215 As PoPH carries a worse prognosis than other forms of PAH,215 this work is critical to further understanding of the loss of BMP9 as a potential risk factor for PoPH.
The most concerning adverse effect of BMP9 therapy is aberrant osteogenesis, however, these effects were not observed in rat pups treated with BMP9 for 10 days, nor were they observed in adult rats that were treated with BMP9 for pulmonary arterial hypertension with the same concentration of BMP9 for 3 weeks.21, 214 The role of BMP9 in neonatal chronic lung disease is still unclear, although it is hypothesized that hyperoxia-induced neonatal lung disease may result when the balance between BMP and TGF-β dependent signaling is disturbed.217, 218 Exogenous BMP9 treatment has promising therapeutic potential, as restoring the balance via decreased TGF-β/BMP-dependent signaling may attenuate the development and severity of bronchopulmonary dysplasia.214
BMP9 signaling in liver fibrosis and regeneration
Although much of the research on BMP9 has been on skeletal elements, it is becoming increasingly relevant to understanding the health and disease of hepatic function. Liver fibrosis is a pathological process in which excessive extracellular matrix deposition leads to progressive liver dysfunction.219 Breitkopf-Heinlein et al investigated the role of BMP9 in hepatocyte function,220 revealing that cell lines from healthy male mice hepatic stellate cells (HSC) produced the highest amount of BMP9 mRNA. BMP9 stimulation of cultured hepatocytes acted to inhibit proliferation, stabilize healthy hepatocytes including maintenance of cell polarization, also preserved the expression of important metabolic enzymes such as cytochrome P450. In addition, Breitkopf-Heinlein et al found that low levels of BMP9 promoted hepatic wound healing and regeneration in mouse models of acute liver damage. In vivo experiments demonstrated that the absence or inhibition of BMP9 during chronic liver damage in mice was found to significantly ameliorate fibrogenesis. They further investigated these findings in the context of human liver fibrosis: through analyzing publicly available array data with human liver samples, they found that BMP9 was significantly upregulated in samples that came from patients with acute liver failure, but not significantly changed with increasing fibrosis stages. Expression of the BMP9 receptor ALK1 was significantly upregulated in both cohorts of acute liver failure as well as in various stages of fibrosis, indicating that under conditions of damage the number of ALK-1 positive cells increases. This implies that under conditions of damage the sensitivity and responsiveness to BMP9 stimulation increases, demonstrating that local elevations of BMP9 may directly affect cellular crosstalk. This evidence signifies the great potential of BMP9 inhibition as a therapeutic, as in combination with existing therapies, it could significantly improve hepatic regeneration and wound healing capacity in the setting of both acute and chronic liver injury.
Potential applications of BMP9 in regenerative medicine
The powerful osteoinductive activity of BMP9 has also been demonstrated via non-adenoviral delivery. Sheyn et al was successful in demonstrating that non-viral, ultrasound based, osteogenic gene delivery of naked DNA encoding BMP9 was able to induce bone formation in vivo.221 Direct sonoporation of rhBMP9 into mouse quadriceps muscle was shown to effectively induce ectopic bone formation confirmed with multiple modalities. Success with other methods using electropermeabilization,222 peptides derived from BMP9,54 and even BMP9 gene delivery via lipid-nucleic acid nanoparticles have been demonstrated as effective BMP-9 delivery methods.223, 224 More recently, Khorsand et al investigated the use of chemically modified ribonucleic acid (cmRNA) encoding BMP2and BMP9, finding that at 3 days post transfection, ALP expression in the BMP9-cmRNA was significantly higher than the BMP2-cmRNA group. This was further confirmed by showing enhanced bone matrix production using Alizarin red staining and atomic absorption spectroscopy. In vivo studies demonstrated increased bone formation in calvarial defects treated with BMP9-cmRNA and BMP2-cmRNA collagen scaffold in comparison with empty defects, with the BMP9-cmRNA group showing twice the connectivity density of the regenerated bone compared to the BMP2-cmRNA group.
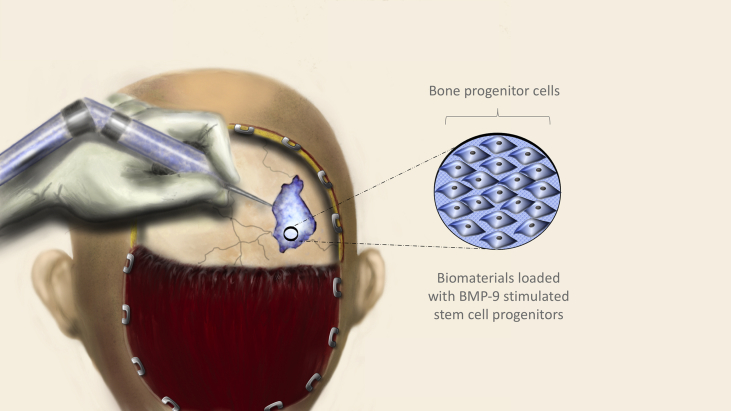
One of the most interesting and clinically applicable studies demonstrating the potency of BMP9 was in the repair of critical sized cranial defects in mice by Dumanian et al.225 Using an adenoviral vector, BMP9 was transduced into immortalized murine calvarial mesenchymal progenitor cells (iCALs). Poly (polyethylene glycol citrate-co-N-isopropylacrylamide) (PPCN) is a thermoresponsive, biomacromolecule that can undergo temperature dependent liquid–solid phase change, and has been demonstrated to provide a viable and supportive environment to cells in vivo and in vitro.103, 104, 226 In this experiment, the BMP9-transduced iCALs as well as GFP-transduced controls were suspended within PPCN-g and introduced within surgically induced calvarial defects. MicroCT imaging revealed that defects treated with PPCN-g scaffold alone did not statistically change in size at 8 weeks postoperatively, while both animal groups with iCALs showed statistically significant reductions in defect size at 12 weeks compared to initial measurements. At 12 weeks postoperatively, significantly greater bone regeneration was evident in the BMP9 treatment group in comparison with controls. While the microCT data was able to quantify residual defect volume and ossification, H & E and trichrome staining verified that PPCN-g/AdGFP showed incomplete healing with defects primarily bridged by fibrous tissue and cartilage. In contrast, robust, mature bone was shown to have completely filled in some of the defects of the PPCN-g/AdBMP9 group. The BMP9 induction and cell delivery using PPCN in addition to new studies using carbon nanotube based scaffolds227 and Matrigel scaffolding228 demonstrates the powerful potential of this method as a therapeutic strategy that may one day be extremely useful in the repair of complex bone defects (Fig. 4).
Figure 4.
Potential use of BMP9 in regenerative medicine. The numerous pathologies that lead to bone defects can result in complex shapes, loss of bone, and a myriad of other complications. BMP9 expressing stem cells in conjunction with a biodegradable, thermoresponsive scaffold such as Poly (polyethylene glycol citrate-co-N-isopropylacrylamide) (PPCN) may one day provide a solution to many of the issues encountered in complex bone defects.
Due to high availability and easy of harvest, adipose tissue makes for an ideal source for progenitor stem cells in regenerative medicine. From this concept, the same group from the aforementioned study225 conducted further experiments to test the utility of BMP9 transduced immortalized murine adipocyte (iMAD) progenitor cells in the PPCN scaffold in a surgical defect model.229 Creation of a critical-sized cranial defect using craniectomy was performed on 12 mice, followed by iMAD cell/PPCN-gelatin scaffold mixture injected into the defects. Both groups were followed using MicroCT at 2-week intervals to evaluate defect healing. While both the BMP9 and control group had similar initial defect sizes, the BMP9 group demonstrated smaller defect size, higher percentage of total defect healed, and a larger percent of defect change over time at each time point over the 12-week study. At completion of the study, the BMP9 group showed a statistically significant mean-defect closure of 27.39% while the control group only showed a 9.89% defect closure. This not only provides further promise of the clinical application of BMP9, but also the feasibility of using adipocyte derived progenitor cells.
In a similar study, Nakamura et al investigated the comparison of BMP2 to BMP9 on bone formation in rat calvarial critical-size defects.230 Wistar rats were given surgically induced defects in the parietal bone bilaterally, then allocated into four groups including absorbable collagen sponge (ACS) alone, recombinant human (rh) BMP2 with ACS, rhBMP9-ACS, and sham surgery (control). Bone volume was analyzed using 3D μ-CT, finding that after 2 weeks the rhBMP2 produced the highest bone volume, but at 8 weeks the rhBMP2 and rhBMP9 groups had created significantly more bone volume than controls, and had statistically similar bone volumes. Histologic photomicrographs revealed that the control groups defect sites had minimal bone formation, with the defect mostly composed to connective tissue. The ACS group had very limited bone formation, although somewhat greater bone formation compared with sites that received the sham surgery. Most importantly, the histologic analysis revealed that while both the rhBMP2/ACS group and rhBMP9/ACs group formed robust, highly mineralized, bone, the rhBMP2/ACS group was afflicted by large, fatty marrow spaces while the rhBMP9/ACS group formed more dense bone, with less fatty marrow spaces and more osteocytes. These findings suggest that rhBMP9/ACS is comparable to rhBMP2/ACS in the generation of bone, and that BMP9 has greater potential to induce bone more similar to the native host bone.
Another compelling study demonstrating the clinical applicability of BMP9 investigated the ability of bio-implants composed of BMP9 transduced rat dental follicle stem cells (rDFCs) in a coralline hydroxyapatite (CHA) scaffold to repair alveolar bone defects. Nie et al found that adenoviral transfection of rDFCs with BMP9 was able to induce osteogenic differentiation and discovered some osteogenic promoting activity of the CHA scaffold itself in comparison to the control group.231 To investigate the BMP9-treated rDFCs in vivo, the group set up several experiments including CHA, rDCFs/CHA, GFP-rDCFs/CHA, and BMP9-rDCFs/CHA implants. After 6 weeks the various controls of this experiment presented with very little bone formation. They did, however, find that the BMP9-rDFCs/CHA group had significantly more bone formation than all other groups (P < 0.01) as well as enhanced blood vessel formation in the area of the bone defect. MicroCT data was also used to analyze the bone formation in each group, finding that the bone defect was still visible in the blank group, while defects treated with CHA, rDFCs/CHA, and BMP9-rDFCs/CHA implants were found to have newly produced bone and CHA scaffolds. Consistent with prior results, the best result of bone regeneration was found in the BMP9-rDFCs/CHA implant group. Lastly, the study found that by using the Smad1/5/8 inhibitor Compound C, they could inhibit the BMP9 mediated osteogenic differentiation of rDFCs, concluding that Smad1/5/8 signaling is essential to the BMP9-induced osteogenesis of rDFCs. Taken together, this study was able to demonstrate that the novel implant composed of BMP9-rDFCs/CHA was able to effectively regenerate bone in an alveolar defect, and BMP9 can induce osteogenic differentiation in rDFCs by affecting Smad1/5/8 signaling.
A major goal of regenerative medicine seeks to target tissue regeneration after traumatic injury, a domain in which mammals tend to recover poorly232,. In previous experiments, it has been found that transient treatment of an amputation wound with BMP2 induces a regeneration response that can elongate the stump bone, but fails to regenerate distal structures such as the joint.234 This led to the hypothesis that since Noggin deficiency causes joint formation to fail, and BMP9 is resistant to Noggin inhibition, BMP9 expression may act as a regenerative agent to joint formation.233 Using MicroCT in a mouse model, Yu et al found that BMP9 treatment generates new distal skeletal elements that appear to articulate with stump bone, a finding not replicated in the control group (P < 0.0001). Using histologic analysis, they also discovered that the regenerated skeletal element is not ectopic, but also forms a joint-like articulation with a distinct cavity associated with a chondrogenic layer of cells in 61% of the digits. This layer later differentiated into articular cartilage that histologically resembles undamaged articular cartilage. They also demonstrated that BMP9-treated digits result in a chondrogenic response, but it is necessary to regenerate a cavity in order to form a joint-like structure, suggesting that the cavitation response is pivotal to joint regeneration. They found that Prg4 expression (which is associated with synovial cavity formation during development) is also required for BMP9 induced joint cavitation and formation. In adult mice, synergistic effects were demonstrated in joint regeneration by using both BMP2 and BMP9. They carried out studies in which amputation of the wound was first treated with BMP2 to induce proliferating chondrocytes and secondary with BMP9 to stimulate joint regeneration. Using sequential treatments BMP2-BMP9 in 3- or 7-day intervals, they found that the 7-day interval stimulated regeneration at a significantly higher frequency (56/80 (70%), P = 0.0135) than in the 3-day interval or BMP9 alone (57/95 (58%)). In both series, histologic analysis demonstrated regeneration of a complete joint structure including a layer of articular cartilage lining the stump. This study provides exciting evidence that the cells of a non-regenerative mammalian amputation wound retain the positional information necessary to regenerate after wound amputation, and that the wound environment can be manipulated to induce regeneration.
Concluding remarks and future directions
The research to date has clearly demonstrated the critical importance of BMP9 in both osteogenic processes and a wide array of other biological functions (Fig. 5). The growing evidence demonstrating the powerful effect of BMP9 over other BMPs support the theory that BMP9 may have the most promise for delivering an effective clinical solution for the augmentation of bone regeneration and healing. This notion is further supported by studies demonstrating that BMP9 mediated osteogenesis parallels the physiologic phases of bone healing during fracture repair. In addition to the powerful osteoinductive importance of BMP9, it is becoming increasingly apparent that BMP9 may also have tremendous implications for processes involving angiogenesis, the development of malignancy, glucose and insulin homeostasis, and a wide variety of other functions not yet discovered. It is of vital importance that the complex interplay of BMP9 with osteogenesis and its many other functions continue to be elucidated to access the vast untapped potential of BMP9 in the arena of research and translation into clinical therapeutics.
Figure 5.
A schematic summary of currently known functions of BMP9 signaling.
Conflict of interest
The authors declare that they do not have any competing interest.
Acknowledgments
The reported work was supported in part by research grants from the National Institutes of Health (CA226303, DE020140 to TCH and RRR), the U.S. Department of Defense (OR130096 to JMW), the Scoliosis Research Society (TCH and MJL), the Scoliosis Research Society (TCH and MJL), and the National Key Research and Development Program of China (2016YFC1000803 and 2011CB707906). This project was also supported in part by The University of Chicago Cancer Center Support Grant (P30CA014599) and the National Center for Advancing Translational Sciences of the National Institutes of Health through Grant Number UL1 TR000430. SM and MP were supported by the Summer Research Program of The University of Chicago Pritzker School of Medicine. TCH was also supported by the Mabel Green Myers Research Endowment Fund and The University of Chicago Orthopaedic Alumni Fund. Funding sources were not involved in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Tong-Chuan He, Email: tche@uchicago.edu.
Russell R. Reid, Email: rreid@surgery.bsd.uchicago.edu.
References
- 1.Sprague S., Bhandari M. An economic evaluation of early versus delayed operative treatment in patients with closed tibial shaft fractures. Arch Orthop Trauma Surg. 2002;122(6):315–323. doi: 10.1007/s00402-001-0358-3. [DOI] [PubMed] [Google Scholar]
- 2.Campana V., Milano G., Pagano E. Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci Mater Med. 2014;25(10):2445–2461. doi: 10.1007/s10856-014-5240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emara K.M., Diab R.A., Emara A.K. Recent biological trends in management of fracture non-union. World J Orthop. 2015;6(8):623–628. doi: 10.5312/wjo.v6.i8.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flierl M.A., Smith W.R., Mauffrey C. Outcomes and complication rates of different bone grafting modalities in long bone fracture nonunions: a retrospective cohort study in 182 patients. J Orthop Surg Res. 2013;8:33. doi: 10.1186/1749-799X-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu C.C. Bone grafting techniques in treating fracture nonunion. Chang Gung Med J. 2000;23(6):319–330. [PubMed] [Google Scholar]
- 6.Sen M.K., Miclau T. Autologous iliac crest bone graft: should it still be the gold standard for treating nonunions? Injury. 2007;38(Suppl. 1):S75–S80. doi: 10.1016/j.injury.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Wagner D.O., Sieber C., Bhushan R., Börgermann J.H., Graf D., Knaus P. BMPs: from bone to body morphogenetic proteins. Sci Signal. 2010;3(107):mr1. doi: 10.1126/scisignal.3107mr1. [DOI] [PubMed] [Google Scholar]
- 8.Urist M.R. Bone: formation by autoinduction. Science. 1965;150(3698):893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 9.Brand R.A. Marshall R. Urist, 1914–2001. Clin Orthop Relat Res. 2009;467(12):3049–3050. doi: 10.1007/s11999-009-1067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang R.N., Green J., Wang Z. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1(1):87–105. doi: 10.1016/j.gendis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng Z.L., Sharff K.A., Tang N. Regulation of osteogenic differentiation during skeletal development. Front Biosci. 2008;13:2001–2021. doi: 10.2741/2819. [DOI] [PubMed] [Google Scholar]
- 12.Lamplot J.D., Qin J., Nan G. BMP9 signaling in stem cell differentiation and osteogenesis. Am J Stem Cells. 2013;2(1):1–21. [PMC free article] [PubMed] [Google Scholar]
- 13.Luther G., Wagner E.R., Zhu G. BMP-9 induced osteogenic differentiation of mesenchymal stem cells: molecular mechanism and therapeutic potential. Curr Gene Ther. 2011;11(3):229–240. doi: 10.2174/156652311795684777. [DOI] [PubMed] [Google Scholar]
- 14.Luu H.H., Song W.X., Luo X. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res. 2007;25(5):665–677. doi: 10.1002/jor.20359. [DOI] [PubMed] [Google Scholar]
- 15.Luo J., Sun M.H., Kang Q. Gene therapy for bone regeneration. Curr Gene Ther. 2005;5(2):167–179. doi: 10.2174/1566523053544218. [DOI] [PubMed] [Google Scholar]
- 16.Kang Q., Song W.X., Luo Q. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev. 2009;18(4):545–559. doi: 10.1089/scd.2008.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.David L., Mallet C., Keramidas M. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ Res. 2008;102(8):914–922. doi: 10.1161/CIRCRESAHA.107.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshimatsu Y., Lee Y.G., Akatsu Y. Bone morphogenetic protein-9 inhibits lymphatic vessel formation via activin receptor-like kinase 1 during development and cancer progression. Proc Natl Acad Sci U S A. 2013;110(47):18940–18945. doi: 10.1073/pnas.1310479110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wooderchak-Donahue W.L., McDonald J., O'Fallon B. BMP9 mutations cause a vascular-anomaly syndrome with phenotypic overlap with hereditary hemorrhagic telangiectasia. Am J Hum Genet. 2013;93(3):530–537. doi: 10.1016/j.ajhg.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levet S., Ciais D., Merdzhanova G. Bone morphogenetic protein 9 (BMP9) controls lymphatic vessel maturation and valve formation. Blood. 2013;122(4):598–607. doi: 10.1182/blood-2012-12-472142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long L., Ormiston M.L., Yang X. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med. 2015;21(7):777–785. doi: 10.1038/nm.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang Q., Sun M.H., Cheng H. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004;11(17):1312–1320. doi: 10.1038/sj.gt.3302298. [DOI] [PubMed] [Google Scholar]
- 23.Cheng H., Jiang W., Phillips F.M. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85-A(8):1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Peng Y., Kang Q., Cheng H. Transcriptional characterization of bone morphogenetic proteins (BMPs)-mediated osteogenic signaling. J Cell Biochem. 2003;90(6):1149–1165. doi: 10.1002/jcb.10744. [DOI] [PubMed] [Google Scholar]
- 25.Peng Y., Kang Q., Luo Q. Inhibitor of DNA binding/differentiation helix-loop-helix proteins mediate bone morphogenetic protein-induced osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279(31):32941–32949. doi: 10.1074/jbc.M403344200. [DOI] [PubMed] [Google Scholar]
- 26.Luo Q., Kang Q., Si W. Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279(53):55958–55968. doi: 10.1074/jbc.M407810200. [DOI] [PubMed] [Google Scholar]
- 27.Sharff K.A., Song W.X., Luo X. Hey1 basic helix-loop-helix protein plays an important role in mediating BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. J Biol Chem. 2009;284(1):649–659. doi: 10.1074/jbc.M806389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang N., Song W.X., Luo J. BMP-9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/beta-catenin signalling. J Cell Mol Med. 2009;13(8B):2448–2464. doi: 10.1111/j.1582-4934.2008.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J., Weng Y., Liu X. Endoplasmic reticulum (ER) stress inducible factor cysteine-rich with EGF-like domains 2 (Creld2) is an important mediator of BMP9-regulated osteogenic differentiation of mesenchymal stem cells. PLoS One. 2013;8(9):e73086. doi: 10.1371/journal.pone.0073086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang E., Zhu G., Jiang W. Growth hormone synergizes with BMP9 in osteogenic differentiation by activating the JAK/STAT/IGF1 pathway in murine multilineage cells. J Bone Miner Res. 2012;27(7):1566–1575. doi: 10.1002/jbmr.1622. [DOI] [PubMed] [Google Scholar]
- 31.Zhang W., Deng Z.L., Chen L. Retinoic acids potentiate BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. PLoS One. 2010;5(7):e11917. doi: 10.1371/journal.pone.0011917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L., Jiang W., Huang J. Insulin-like growth factor 2 (IGF-2) potentiates BMP-9-induced osteogenic differentiation and bone formation. J Bone Miner Res. 2010;25(11):2447–2459. doi: 10.1002/jbmr.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X., Qin J., Luo Q. Cross-talk between EGF and BMP9 signalling pathways regulates the osteogenic differentiation of mesenchymal stem cells. J Cell Mol Med. 2013;17(9):1160–1172. doi: 10.1111/jcmm.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu N., Jiang D., Huang E. BMP9-regulated angiogenic signaling plays an important role in the osteogenic differentiation of mesenchymal progenitor cells. J Cell Sci. 2013;126(Pt 2):532–541. doi: 10.1242/jcs.114231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H., Li L., Dong Q. Activation of PKA/CREB signaling is involved in BMP9-induced osteogenic differentiation of mesenchymal stem cells. Cell Physiol Biochem. 2015;37(2):548–562. doi: 10.1159/000430376. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H., Wang J., Deng F. Canonical Wnt signaling acts synergistically on BMP9-induced osteo/odontoblastic differentiation of stem cells of dental apical papilla (SCAPs) Biomaterials. 2015;39:145–154. doi: 10.1016/j.biomaterials.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li R., Zhang W., Cui J. Targeting BMP9-promoted human osteosarcoma growth by inactivation of notch signaling. Curr Cancer Drug Targets. 2014;14(3):274–285. doi: 10.2174/1568009614666140305105805. [DOI] [PubMed] [Google Scholar]
- 38.Wei Z., Salmon R.M., Upton P.D., Morrell N.W., Li W. Regulation of bone morphogenetic protein 9 (BMP9) by redox-dependent proteolysis. J Biol Chem. 2014;289(45):31150–31159. doi: 10.1074/jbc.M114.579771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamashita H., Miyazono K. Bone morphogenetic protein (BMP) receptors and signal transduction. Nihon Rinsho. 1999;57(1):220–226. [PubMed] [Google Scholar]
- 40.Massagué J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 41.Luo J., Tang M., Huang J. TGFbeta/BMP type I receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic signaling in mesenchymal stem cells. J Biol Chem. 2010;285(38):29588–29598. doi: 10.1074/jbc.M110.130518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ten Dijke P., Korchynskyi O., Valdimarsdottir G., Goumans M.J. Controlling cell fate by bone morphogenetic protein receptors. Mol Cell Endocrinol. 2003;211(1–2):105–113. doi: 10.1016/j.mce.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 43.Wu N., Zhao Y., Yin Y., Zhang Y., Luo J. Identification and analysis of type II TGF-β receptors in BMP-9-induced osteogenic differentiation of C3H10T1/2 mesenchymal stem cells. Acta Biochim Biophys Sin (Shanghai) 2010;42(10):699–708. doi: 10.1093/abbs/gmq075. [DOI] [PubMed] [Google Scholar]
- 44.Xu D.J., Zhao Y.Z., Wang J., He J.W., Weng Y.G., Luo J.Y. Smads, p38 and ERK1/2 are involved in BMP9-induced osteogenic differentiation of C3H10T1/2 mesenchymal stem cells. BMB Rep. 2012;45(4):247–252. doi: 10.5483/bmbrep.2012.45.4.247. [DOI] [PubMed] [Google Scholar]
- 45.Mitrofan C.G., Appleby S.L., Nash G.B. Bone morphogenetic protein 9 (BMP9) and BMP10 enhance tumor necrosis factor-alpha-induced monocyte recruitment to the vascular endothelium mainly via activin receptor-like kinase 2. J Biol Chem. 2017;292(33):13714–13726. doi: 10.1074/jbc.M117.778506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scharpfenecker M., van Dinther M., Liu Z. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci. 2007;120(Pt 6):964–972. doi: 10.1242/jcs.002949. [DOI] [PubMed] [Google Scholar]
- 47.Cargnello M., Roux P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75(1):50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Widmann C., Gibson S., Jarpe M.B., Johnson G.L. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79(1):143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 49.Gallea S., Lallemand F., Atfi A. Activation of mitogen-activated protein kinase cascades is involved in regulation of bone morphogenetic protein-2-induced osteoblast differentiation in pluripotent C2C12 cells. Bone. 2001;28(5):491–498. doi: 10.1016/s8756-3282(01)00415-x. [DOI] [PubMed] [Google Scholar]
- 50.Nöth U., Tuli R., Seghatoleslami R. Activation of p38 and Smads mediates BMP-2 effects on human trabecular bone-derived osteoblasts. Exp Cell Res. 2003;291(1):201–211. doi: 10.1016/s0014-4827(03)00386-0. [DOI] [PubMed] [Google Scholar]
- 51.Miyazono K., Kamiya Y., Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147(1):35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 52.Verheyen E.M. Opposing effects of Wnt and MAPK on BMP/Smad signal duration. Dev Cell. 2007;13(6):755–756. doi: 10.1016/j.devcel.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 53.Zhao Y., Song T., Wang W. P38 and ERK1/2 MAPKs act in opposition to regulate BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. PLoS One. 2012;7(8):e43383. doi: 10.1371/journal.pone.0043383. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Bergeron E., Senta H., Mailloux A., Park H., Lord E., Faucheux N. Murine preosteoblast differentiation induced by a peptide derived from bone morphogenetic proteins-9. Tissue Eng A. 2009;15(11):3341–3349. doi: 10.1089/ten.TEA.2009.0189. [DOI] [PubMed] [Google Scholar]
- 55.Devlin R.D., Du Z., Pereira R.C. Skeletal overexpression of noggin results in osteopenia and reduced bone formation. Endocrinology. 2003;144(5):1972–1978. doi: 10.1210/en.2002-220918. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y., Hong S., Li M. Noggin resistance contributes to the potent osteogenic capability of BMP9 in mesenchymal stem cells. J Orthop Res. 2013;31(11):1796–1803. doi: 10.1002/jor.22427. [DOI] [PubMed] [Google Scholar]
- 57.Huang X., Wang F., Zhao C. Dentinogenesis and tooth-alveolar bone complex defects in BMP9/GDF2 knockout mice. Stem Cells Dev. 2019;28(10):683–694. doi: 10.1089/scd.2018.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ji C., Liu X., Xu L., Yu T., Dong C., Luo J. RUNX1 plays an important role in mediating BMP9-induced osteogenic differentiation of mesenchymal stem cells line C3H10T1/2, murine multi-lineage cells lines C2C12 and MEFs. Int J Mol Sci. 2017;18(7) doi: 10.3390/ijms18071348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi J.Y., Pratap J., Javed A. Subnuclear targeting of Runx/Cbfa/AML factors is essential for tissue-specific differentiation during embryonic development. Proc Natl Acad Sci U S A. 2001;98(15):8650–8655. doi: 10.1073/pnas.151236498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Speck N.A., Stacy T., Wang Q. Core-binding factor: a central player in hematopoiesis and leukemia. Cancer Res. 1999;59(Suppl. 7):1789s–1793s. [PubMed] [Google Scholar]
- 61.Westendorf J.J., Hiebert S.W. Mammalian runt-domain proteins and their roles in hematopoiesis, osteogenesis, and leukemia. J Cell Biochem. 1999;(Suppl. 32–33):51–58. doi: 10.1002/(sici)1097-4644(1999)75:32+<51::aid-jcb7>3.3.co;2-j. [DOI] [PubMed] [Google Scholar]
- 62.Komori T., Yagi H., Nomura S. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89(5):755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 63.Kim I.S., Otto F., Zabel B., Mundlos S. Regulation of chondrocyte differentiation by Cbfa1. Mech Dev. 1999;80(2):159–170. doi: 10.1016/s0925-4773(98)00210-x. [DOI] [PubMed] [Google Scholar]
- 64.Inada M., Yasui T., Nomura S. Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev Dynam. 1999;214(4):279–290. doi: 10.1002/(SICI)1097-0177(199904)214:4<279::AID-AJA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 65.Papaioannou G., Mirzamohammadi F., Kobayashi T. MicroRNAs involved in bone formation. Cell Mol Life Sci. 2014;71(24):4747–4761. doi: 10.1007/s00018-014-1700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen C., Tang Z., Song Q., Yang M., Shi Q., Weng Y. Downregulated microRNA-23b promotes BMP9-mediated osteogenesis in C2C12 myoblast cells by targeting Runx2. Mol Med Rep. 2016;13(3):2492–2498. doi: 10.3892/mmr.2016.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ruzinova M.B., Benezra R. Id proteins in development, cell cycle and cancer. Trends Cell Biol. 2003;13(8):410–418. doi: 10.1016/s0962-8924(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 68.Kreider B.L., Benezra R., Rovera G., Kadesch T. Inhibition of myeloid differentiation by the helix-loop-helix protein Id. Science. 1992;255(5052):1700–1702. doi: 10.1126/science.1372755. [DOI] [PubMed] [Google Scholar]
- 69.Tang J., Gordon G.M., Nickoloff B.J., Foreman K.E. 2002. The Helix–Loop–Helix Protein Id-1 Delays Onset of Replicative Senescence in Human Endothelial Cells. [DOI] [PubMed] [Google Scholar]
- 70.Norton J.D. ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J Cell Sci. 2000;113(Pt 22):3897–3905. doi: 10.1242/jcs.113.22.3897. [DOI] [PubMed] [Google Scholar]
- 71.Benezra R., Davis R.L., Lockshon D., Turner D.L., Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61(1):49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- 72.Fisher A., Caudy M. The function of hairy-related bHLH repressor proteins in cell fate decisions. Bioessays. 1998;20(4):298–306. doi: 10.1002/(SICI)1521-1878(199804)20:4<298::AID-BIES6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 73.Leimeister C., Externbrink A., Klamt B., Gessler M. Hey genes: a novel subfamily of hairy- and Enhancer of split related genes specifically expressed during mouse embryogenesis. Mech Dev. 1999;85(1–2):173–177. doi: 10.1016/s0925-4773(99)00080-5. [DOI] [PubMed] [Google Scholar]
- 74.Ivkovic S., Yoon B.S., Popoff S.N. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130(12):2779–2791. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blom I.E., van Dijk A.J., Wieten L. In vitro evidence for differential involvement of CTGF, TGFbeta, and PDGF-BB in mesangial response to injury. Nephrol Dial Transplant. 2001;16(6):1139–1148. doi: 10.1093/ndt/16.6.1139. [DOI] [PubMed] [Google Scholar]
- 76.Dammeier J., Brauchle M., Falk W., Grotendorst G.R., Werner S. Connective tissue growth factor: a novel regulator of mucosal repair and fibrosis in inflammatory bowel disease? Int J Biochem Cell Biol. 1998;30(8):909–922. doi: 10.1016/s1357-2725(98)00046-6. [DOI] [PubMed] [Google Scholar]
- 77.Frazier K., Williams S., Kothapalli D., Klapper H., Grotendorst G.R. Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J Investig Dermatol. 1996;107(3):404–411. doi: 10.1111/1523-1747.ep12363389. [DOI] [PubMed] [Google Scholar]
- 78.Lasky J.A., Ortiz L.A., Tonthat B. Connective tissue growth factor mRNA expression is upregulated in bleomycin-induced lung fibrosis. Am J Physiol. 1998;275(2 Pt 1):L365–L371. doi: 10.1152/ajplung.1998.275.2.L365. [DOI] [PubMed] [Google Scholar]
- 79.Massague J., Chen Y.G. Controlling TGF-beta signaling. Genes Dev. 2000;14(6):627–644. [PubMed] [Google Scholar]
- 80.Hadaschik E.N., Enk A.H. TGF-beta1-induced regulatory T cells. Hum Immunol. 2015;76(8):561–564. doi: 10.1016/j.humimm.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 81.Derynck R., Zhang Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425(6958):577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 82.Wrana J.L. Signaling by the TGFbeta superfamily. Cold Spring Harb Perspect Biol. 2013;5(10):a011197. doi: 10.1101/cshperspect.a011197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen G., Deng C., Li Y.P. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8(2):272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tan Y., Xu Q., Li Y., Mao X., Zhang K. Crosstalk between the p38 and TGF-beta signaling pathways through TbetaRI, TbetaRII and Smad3 expression in plancental choriocarcinoma JEG-3 cells. Oncol Lett. 2014;8(3):1307–1311. doi: 10.3892/ol.2014.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zi Z., Chapnick D.A., Liu X. Dynamics of TGF-beta/Smad signaling. FEBS Lett. 2012;586(14):1921–1928. doi: 10.1016/j.febslet.2012.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu M., Chen G., Li Y.P. TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4:16009. doi: 10.1038/boneres.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Janssens K., ten Dijke P., Janssens S., Van Hul W. Transforming growth factor-beta1 to the bone. Endocr Rev. 2005;26(6):743–774. doi: 10.1210/er.2004-0001. [DOI] [PubMed] [Google Scholar]
- 88.Yamada T., Kamiya N., Harada D., Takagi M. Effects of transforming growth factor-beta1 on the gene expression of decorin, biglycan, and alkaline phosphatase in osteoblast precursor cells and more differentiated osteoblast cells. Histochem J. 1999;31(10):687–694. doi: 10.1023/a:1003855922395. [DOI] [PubMed] [Google Scholar]
- 89.Li R.D., Deng Z.L., Hu N. Biphasic effects of TGFbeta1 on BMP9-induced osteogenic differentiation of mesenchymal stem cells. BMB Rep. 2012;45(9):509–514. doi: 10.5483/bmbrep.2012.45.9.053. [DOI] [PubMed] [Google Scholar]
- 90.Guerrero F., Herencia C., Almaden Y. TGF-beta prevents phosphate-induced osteogenesis through inhibition of BMP and Wnt/beta-catenin pathways. PLoS One. 2014;9(2):e89179. doi: 10.1371/journal.pone.0089179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nusse R., Varmus H.E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 92.Rijsewijk F., Schuermann M., Wagenaar E., Parren P., Weigel D., Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987;50(4):649–657. doi: 10.1016/0092-8674(87)90038-9. [DOI] [PubMed] [Google Scholar]
- 93.Cabrera C.V., Alonso M.C., Johnston P., Phillips R.G., Lawrence P.A. Phenocopies induced with antisense RNA identify the wingless gene. Cell. 1987;50(4):659–663. doi: 10.1016/0092-8674(87)90039-0. [DOI] [PubMed] [Google Scholar]
- 94.Cadigan K.M., Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11(24):3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 95.Parr B.A., McMahon A.P. Dorsalizing signal Wnt-7a required for normal polarity of D-V and A-P axes of mouse limb. Nature. 1995;374(6520):350–353. doi: 10.1038/374350a0. [DOI] [PubMed] [Google Scholar]
- 96.Woods C.G., Stricker S., Seemann P. Mutations in WNT7A cause a range of limb malformations, including Fuhrmann syndrome and Al-Awadi/Raas-Rothschild/Schinzel phocomelia syndrome. Am J Hum Genet. 2006;79(2):402–408. doi: 10.1086/506332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yavropoulou M.P., Yovos J.G. The role of Notch signaling in bone development and disease. Hormones (Athens) 2014;13(1):24–37. doi: 10.1007/BF03401318. [DOI] [PubMed] [Google Scholar]
- 98.Zanotti S., Canalis E. Notch signaling and the skeleton. Endocr Rev. 2016;37(3):223–253. doi: 10.1210/er.2016-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kusumbe A.P., Ramasamy S.K., Adams R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492):323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ramasamy S.K., Kusumbe A.P., Wang L., Adams R.H. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507(7492):376–380. doi: 10.1038/nature13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liao J., Wei Q., Zou Y. Notch signaling augments BMP9-induced bone formation by promoting the osteogenesis-angiogenesis coupling process in mesenchymal stem cells (MSCs) Cell Physiol Biochem. 2017;41(5):1905–1923. doi: 10.1159/000471945. [DOI] [PubMed] [Google Scholar]
- 102.Lu S., Wang J., Ye J. Bone morphogenetic protein 9 (BMP9) induces effective bone formation from reversibly immortalized multipotent adipose-derived (iMAD) mesenchymal stem cells. Am J Transl Res. 2016;8(9):3710–3730. [PMC free article] [PubMed] [Google Scholar]
- 103.Ye J., Wang J., Zhu Y. A thermoresponsive polydiolcitrate-gelatin scaffold and delivery system mediates effective bone formation from BMP9-transduced mesenchymal stem cells. Biomed Mater. 2016;11(2):025021. doi: 10.1088/1748-6041/11/2/025021. [DOI] [PubMed] [Google Scholar]
- 104.Yang J., van Lith R., Baler K., Hoshi R.A., Ameer G.A. A thermoresponsive biodegradable polymer with intrinsic antioxidant properties. Biomacromolecules. 2014;15(11):3942–3952. doi: 10.1021/bm5010004. [DOI] [PubMed] [Google Scholar]
- 105.Fisher M.C., Clinton G.M., Maihle N.J., Dealy C.N. Requirement for ErbB2/ErbB signaling in developing cartilage and bone. Dev Growth Differ. 2007;49(6):503–513. doi: 10.1111/j.1440-169X.2007.00941.x. [DOI] [PubMed] [Google Scholar]
- 106.Krampera M., Pasini A., Rigo A. HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: inducing cell expansion and reversibly preventing multilineage differentiation. Blood. 2005;106(1):59–66. doi: 10.1182/blood-2004-09-3645. [DOI] [PubMed] [Google Scholar]
- 107.Schneider M.R., Sibilia M., Erben R.G. The EGFR network in bone biology and pathology. Trends Endocrinol Metab. 2009;20(10):517–524. doi: 10.1016/j.tem.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 108.Canalis E., Raisz L.G. Effect of epidermal growth factor on bone formation in vitro. Endocrinology. 1979;104(4):862–869. doi: 10.1210/endo-104-4-862. [DOI] [PubMed] [Google Scholar]
- 109.Marie P.J., Hott M., Perheentupa J. Effects of epidermal growth factor on bone formation and resorption in vivo. Am J Physiol. 1990;258(2 Pt 1):E275–E281. doi: 10.1152/ajpendo.1990.258.2.E275. [DOI] [PubMed] [Google Scholar]
- 110.Varjosalo M., Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22(18):2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 111.Nüsslein-Volhard C., Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 112.Mundy G.R., Yang X. Hedgehog coordination of postnatal osteoclast and osteoblast activities. Dev Cell. 2008;14(5):637–638. doi: 10.1016/j.devcel.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 113.Ohba S., Kawaguchi H., Kugimiya F. Patched1 haploinsufficiency increases adult bone mass and modulates Gli3 repressor activity. Dev Cell. 2008;14(5):689–699. doi: 10.1016/j.devcel.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 114.Wu X., Walker J., Zhang J., Ding S., Schultz P.G. Purmorphamine induces osteogenesis by activation of the hedgehog signaling pathway. Chem Biol. 2004;11(9):1229–1238. doi: 10.1016/j.chembiol.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 115.Mak K.K., Bi Y., Wan C. Hedgehog signaling in mature osteoblasts regulates bone formation and resorption by controlling PTHrP and RANKL expression. Dev Cell. 2008;14(5):674–688. doi: 10.1016/j.devcel.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 116.Zhang Y., Zhang Z., Zhao X. A new function of BMP4: dual role for BMP4 in regulation of Sonic hedgehog expression in the mouse tooth germ. Development. 2000;127(7):1431–1443. doi: 10.1242/dev.127.7.1431. [DOI] [PubMed] [Google Scholar]
- 117.Yuasa T., Kataoka H., Kinto N. Sonic hedgehog is involved in osteoblast differentiation by cooperating with BMP-2. J Cell Physiol. 2002;193(2):225–232. doi: 10.1002/jcp.10166. [DOI] [PubMed] [Google Scholar]
- 118.Li L., Dong Q., Wang Y. Hedgehog signaling is involved in the BMP9-induced osteogenic differentiation of mesenchymal stem cells. Int J Mol Med. 2015;35(6):1641–1650. doi: 10.3892/ijmm.2015.2172. [DOI] [PubMed] [Google Scholar]
- 119.Mark M., Ghyselinck N.B., Chambon P. Function of retinoic acid receptors during embryonic development. Nucl Recept Signal. 2009;7:e002. doi: 10.1621/nrs.07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mark M., Ghyselinck N.B., Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451–480. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- 121.Chawla A., Repa J.J., Evans R.M., Mangelsdorf D.J. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294(5548):1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 122.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10(9):940–954. [PubMed] [Google Scholar]
- 123.Barber T., Esteban-Pretel G., Marín M.P., Timoneda J. Vitamin a deficiency and alterations in the extracellular matrix. Nutrients. 2014;6(11):4984–5017. doi: 10.3390/nu6114984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang A., Ding X., Sheng S., Yao Z. Retinoic acid inhibits osteogenic differentiation of rat bone marrow stromal cells. Biochem Biophys Res Commun. 2008;375(3):435–439. doi: 10.1016/j.bbrc.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 125.Wan D.C., Shi Y.Y., Nacamuli R.P., Quarto N., Lyons K.M., Longaker M.T. Osteogenic differentiation of mouse adipose-derived adult stromal cells requires retinoic acid and bone morphogenetic protein receptor type IB signaling. Proc Natl Acad Sci U S A. 2006;103(33):12335–12340. doi: 10.1073/pnas.0604849103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hoffman L.M., Garcha K., Karamboulas K. BMP action in skeletogenesis involves attenuation of retinoid signaling. J Cell Biol. 2006;174(1):101–113. doi: 10.1083/jcb.200604150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Drissi M.H., Li X., Sheu T.J. Runx2/Cbfa1 stimulation by retinoic acid is potentiated by BMP2 signaling through interaction with Smad1 on the collagen X promoter in chondrocytes. J Cell Biochem. 2003;90(6):1287–1298. doi: 10.1002/jcb.10677. [DOI] [PubMed] [Google Scholar]
- 128.Skillington J., Choy L., Derynck R. Bone morphogenetic protein and retinoic acid signaling cooperate to induce osteoblast differentiation of preadipocytes. J Cell Biol. 2002;159(1):135–146. doi: 10.1083/jcb.200204060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Weston A.D., Rosen V., Chandraratna R.A., Underhill T.M. Regulation of skeletal progenitor differentiation by the BMP and retinoid signaling pathways. J Cell Biol. 2000;148(4):679–690. doi: 10.1083/jcb.148.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cowan C.M., Aalami O.O., Shi Y.Y. Bone morphogenetic protein 2 and retinoic acid accelerate in vivo bone formation, osteoclast recruitment, and bone turnover. Tissue Eng. 2005;11(3–4):645–658. doi: 10.1089/ten.2005.11.645. [DOI] [PubMed] [Google Scholar]
- 131.Allard J.B., Duan C. IGF-binding proteins: why do they exist and why are there so many? Front Endocrinol (Lausanne) 2018;9:117. doi: 10.3389/fendo.2018.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ohlsson C., Mohan S., Sjögren K. The role of liver-derived insulin-like growth factor-I. Endocr Rev. 2009;30(5):494–535. doi: 10.1210/er.2009-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Emmerson E., Campbell L., Davies F.C. Insulin-like growth factor-1 promotes wound healing in estrogen-deprived mice: new insights into cutaneous IGF-1R/ERα cross talk. J Investig Dermatol. 2012;132(12):2838–2848. doi: 10.1038/jid.2012.228. [DOI] [PubMed] [Google Scholar]
- 134.Taguchi A., White M.F. Insulin-like signaling, nutrient homeostasis, and life span. Annu Rev Physiol. 2008;70:191–212. doi: 10.1146/annurev.physiol.70.113006.100533. [DOI] [PubMed] [Google Scholar]
- 135.Rajpathak S.N., Gunter M.J., Wylie-Rosett J. The role of insulin-like growth factor-I and its binding proteins in glucose homeostasis and type 2 diabetes. Diabetes Metab Res Rev. 2009;25(1):3–12. doi: 10.1002/dmrr.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zheng W.H., Kar S., Quirion R. Insulin-like growth factor-1-induced phosphorylation of transcription factor FKHRL1 is mediated by phosphatidylinositol 3-kinase/Akt kinase and role of this pathway in insulin-like growth factor-1-induced survival of cultured hippocampal neurons. Mol Pharmacol. 2002;62(2):225–233. doi: 10.1124/mol.62.2.225. [DOI] [PubMed] [Google Scholar]
- 137.Blair J.C., Savage M.O. The GH-IGF-I axis in children with idiopathic short stature. Trends Endocrinol Metab. 2002;13(8):325–330. doi: 10.1016/s1043-2760(02)00631-8. [DOI] [PubMed] [Google Scholar]
- 138.Brooks A.J., Waters M.J. The growth hormone receptor: mechanism of activation and clinical implications. Nat Rev Endocrinol. 2010;6(9):515–525. doi: 10.1038/nrendo.2010.123. [DOI] [PubMed] [Google Scholar]
- 139.Hull K.L., Harvey S. Growth hormone: roles in male reproduction. Endocrine. 2000;13(3):243–250. doi: 10.1385/ENDO:13:3:243. [DOI] [PubMed] [Google Scholar]
- 140.Hull K.L., Harvey S. Growth hormone: a reproductive endocrine-paracrine regulator? Rev Reprod. 2000;5(3):175–182. doi: 10.1530/ror.0.0050175. [DOI] [PubMed] [Google Scholar]
- 141.Park P., Cohen P. Insulin-like growth factor I (IGF-I) measurements in growth hormone (GH) therapy of idiopathic short stature (ISS) Growth Hormone IGF Res. 2005;15(Suppl. A):S13–S20. doi: 10.1016/j.ghir.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 142.Tanaka H. Growth hormone and bone diseases. Endocr J. 1998;45(Suppl):S47–S52. doi: 10.1507/endocrj.45.suppl_s47. [DOI] [PubMed] [Google Scholar]
- 143.Thomas M.J. The molecular basis of growth hormone action. Growth Hormone IGF Res. 1998;8(1):3–11. doi: 10.1016/s1096-6374(98)80316-x. [DOI] [PubMed] [Google Scholar]
- 144.Postel-Vinay M.C., Kelly P.A. Growth hormone receptor signalling. Bailliere Clin Endocrinol Metabol. 1996;10(3):323–336. doi: 10.1016/s0950-351x(96)80455-1. [DOI] [PubMed] [Google Scholar]
- 145.Wan C., Shao J., Gilbert S.R. Role of HIF-1alpha in skeletal development. Ann N Y Acad Sci. 2010;1192:322–326. doi: 10.1111/j.1749-6632.2009.05238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Majmundar A.J., Wong W.J., Simon M.C. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40(2):294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tontonoz P., Spiegelman B.M. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 148.Giaginis C., Tsantili-Kakoulidou A., Theocharis S. Peroxisome proliferator-activated receptors (PPARs) in the control of bone metabolism. Fundam Clin Pharmacol. 2007;21(3):231–244. doi: 10.1111/j.1472-8206.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- 149.Chan B.Y., Gartland A., Wilson P.J. PPAR agonists modulate human osteoclast formation and activity in vitro. Bone. 2007;40(1):149–159. doi: 10.1016/j.bone.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 150.Mbalaviele G., Abu-Amer Y., Meng A. Activation of peroxisome proliferator-activated receptor-gamma pathway inhibits osteoclast differentiation. J Biol Chem. 2000;275(19):14388–14393. doi: 10.1074/jbc.275.19.14388. [DOI] [PubMed] [Google Scholar]
- 151.Muruganandan S., Roman A.A., Sinal C.J. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci. 2009;66(2):236–253. doi: 10.1007/s00018-008-8429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ting K., Vastardis H., Mulliken J.B. Human NELL-1 expressed in unilateral coronal synostosis. J Bone Miner Res. 1999;14(1):80–89. doi: 10.1359/jbmr.1999.14.1.80. [DOI] [PubMed] [Google Scholar]
- 153.Pakvasa M., Alverdy A., Mostafa S. Neural EGF-like protein 1 (NELL-1): signaling crosstalk in mesenchymal stem cells and applications in regenerative medicine. Genes Dis. 2017;4(3):127–137. doi: 10.1016/j.gendis.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lu S.S., Zhang X., Soo C. The osteoinductive properties of Nell-1 in a rat spinal fusion model. Spine J. 2007;7(1):50–60. doi: 10.1016/j.spinee.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 155.James A.W., Pan A., Chiang M. A new function of Nell-1 protein in repressing adipogenic differentiation. Biochem Biophys Res Commun. 2011;411(1):126–131. doi: 10.1016/j.bbrc.2011.06.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang J., Liao J., Zhang F. NEL-like molecule-1 (Nell1) is regulated by bone morphogenetic protein 9 (BMP9) and potentiates BMP9-induced osteogenic differentiation at the expense of adipogenesis in mesenchymal stem cells. Cell Physiol Biochem. 2017;41(2):484–500. doi: 10.1159/000456885. [DOI] [PubMed] [Google Scholar]
- 157.Schulz T.J., Tseng Y.H. Emerging role of bone morphogenetic proteins in adipogenesis and energy metabolism. Cytokine Growth Factor Rev. 2009;20(5–6):523–531. doi: 10.1016/j.cytogfr.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kuo M.M., Kim S., Tseng C.Y., Jeon Y.H., Choe S., Lee D.K. BMP-9 as a potent brown adipogenic inducer with anti-obesity capacity. Biomaterials. 2014;35(10):3172–3179. doi: 10.1016/j.biomaterials.2013.12.063. [DOI] [PubMed] [Google Scholar]
- 159.Kim J., Kim M., Jeong Y. BMP9 induces cord blood-derived endothelial progenitor cell differentiation and ischemic neovascularization via ALK1. Arterioscler Thromb Vasc Biol. 2015;35(9):2020–2031. doi: 10.1161/ATVBAHA.115.306142. [DOI] [PubMed] [Google Scholar]
- 160.Redgrave R.E., Tual-Chalot S., Davison B.J. Cardiosphere-derived cells require endoglin for paracrine-mediated angiogenesis. Stem Cell Rep. 2017;8(5):1287–1298. doi: 10.1016/j.stemcr.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Saito T., Bokhove M., Croci R. Structural basis of the human endoglin-BMP9 interaction: insights into BMP signaling and HHT1. Cell Rep. 2017;19(9):1917–1928. doi: 10.1016/j.celrep.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nolan-Stevaux O., Zhong W., Culp S. Endoglin requirement for BMP9 signaling in endothelial cells reveals new mechanism of action for selective anti-endoglin antibodies. PLoS One. 2012;7(12):e50920. doi: 10.1371/journal.pone.0050920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Franco C.A., Gerhardt H. Blood flow boosts BMP signaling to keep vessels in shape. J Cell Biol. 2016;214(7):793–795. doi: 10.1083/jcb.201609038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Baeyens N., Larrivée B., Ola R. Defective fluid shear stress mechanotransduction mediates hereditary hemorrhagic telangiectasia. J Cell Biol. 2016;214(7):807–816. doi: 10.1083/jcb.201603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Levet S., Ouarné M., Ciais D. BMP9 and BMP10 are necessary for proper closure of the ductus arteriosus. Proc Natl Acad Sci U S A. 2015;112(25):E3207–E3215. doi: 10.1073/pnas.1508386112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tillet E., Ouarné M., Desroches-Castan A. A heterodimer formed by bone morphogenetic protein 9 (BMP9) and BMP10 provides most BMP biological activity in plasma. J Biol Chem. 2018;293(28):10963–10974. doi: 10.1074/jbc.RA118.002968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.David L., Mallet C., Mazerbourg S., Feige J.J., Bailly S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood. 2007;109(5):1953–1961. doi: 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- 168.Tillet E., Bailly S. Emerging roles of BMP9 and BMP10 in hereditary hemorrhagic telangiectasia. Front Genet. 2014;5:456. doi: 10.3389/fgene.2014.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen C., Grzegorzewski K.J., Barash S. An integrated functional genomics screening program reveals a role for BMP-9 in glucose homeostasis. Nat Biotechnol. 2003;21(3):294–301. doi: 10.1038/nbt795. [DOI] [PubMed] [Google Scholar]
- 170.Caperuto L.C., Anhê G.F., Cambiaghi T.D. Modulation of bone morphogenetic protein-9 expression and processing by insulin, glucose, and glucocorticoids: possible candidate for hepatic insulin-sensitizing substance. Endocrinology. 2008;149(12):6326–6335. doi: 10.1210/en.2008-0655. [DOI] [PubMed] [Google Scholar]
- 171.Miller A.F., Harvey S.A., Thies R.S., Olson M.S. Bone morphogenetic protein-9. An autocrine/paracrine cytokine in the liver. J Biol Chem. 2000;275(24):17937–17945. doi: 10.1074/jbc.275.24.17937. [DOI] [PubMed] [Google Scholar]
- 172.Stanford K.I., Middelbeek R.J., Townsend K.L. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Investig. 2013;123(1):215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gunawardana S.C., Piston D.W. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes. 2012;61(3):674–682. doi: 10.2337/db11-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xu X., Li X., Yang G. Circulating bone morphogenetic protein-9 in relation to metabolic syndrome and insulin resistance. Sci Rep. 2017;7(1):17529. doi: 10.1038/s41598-017-17807-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Luo Y., Li L., Xu X. Decreased circulating BMP-9 levels in patients with Type 2 diabetes is a signature of insulin resistance. Clin Sci (Lond) 2017;131(3):239–246. doi: 10.1042/CS20160543. [DOI] [PubMed] [Google Scholar]
- 176.Picchini U., De Gaetano A., Panunzi S., Ditlevsen S., Mingrone G. A mathematical model of the euglycemic hyperinsulinemic clamp. Theor Biol Med Model. 2005;2:44. doi: 10.1186/1742-4682-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kershaw E.E., Flier J.S. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6):2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 178.Liu R., Hu W., Li X. Association of circulating BMP9 with coronary heart disease and hypertension in Chinese populations. BMC Cardiovasc Disord. 2019;19(1):131. doi: 10.1186/s12872-019-1095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mehler M.F., Mabie P.C., Zhang D., Kessler J.A. Bone morphogenetic proteins in the nervous system. Trends Neurosci. 1997;20(7):309–317. doi: 10.1016/s0166-2236(96)01046-6. [DOI] [PubMed] [Google Scholar]
- 180.López-Coviella I., Berse B., Krauss R., Thies R.S., Blusztajn J.K. Induction and maintenance of the neuronal cholinergic phenotype in the central nervous system by BMP-9. Science. 2000;289(5477):313–316. doi: 10.1126/science.289.5477.313. [DOI] [PubMed] [Google Scholar]
- 181.Ye L., Bokobza S.M., Jiang W.G. Bone morphogenetic proteins in development and progression of breast cancer and therapeutic potential (review) Int J Mol Med. 2009;24(5):591–597. doi: 10.3892/ijmm_00000269. [DOI] [PubMed] [Google Scholar]
- 182.Reddi A.H., Reddi A. Bone morphogenetic proteins (BMPs): from morphogens to metabologens. Cytokine Growth Factor Rev. 2009;20(5–6):341–342. doi: 10.1016/j.cytogfr.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 183.García-Álvaro M., Addante A., Roncero C. BMP9-Induced survival effect in liver tumor cells requires p38MAPK activation. Int J Mol Sci. 2015;16(9):20431–20448. doi: 10.3390/ijms160920431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Herrera B., García-Álvaro M., Cruz S. BMP9 is a proliferative and survival factor for human hepatocellular carcinoma cells. PLoS One. 2013;8(7):e69535. doi: 10.1371/journal.pone.0069535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Herrera B., van Dinther M., Ten Dijke P., Inman G.J. Autocrine bone morphogenetic protein-9 signals through activin receptor-like kinase-2/Smad1/Smad4 to promote ovarian cancer cell proliferation. Cancer Res. 2009;69(24):9254–9262. doi: 10.1158/0008-5472.CAN-09-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lv Z., Wang C., Yuan T. Bone morphogenetic protein 9 regulates tumor growth of osteosarcoma cells through the Wnt/β-catenin pathway. Oncol Rep. 2014;31(2):989–994. doi: 10.3892/or.2013.2931. [DOI] [PubMed] [Google Scholar]
- 187.Wang T., Zhang Z., Wang K. Inhibitory effects of BMP9 on breast cancer cells by regulating their interaction with pre-adipocytes/adipocytes. Oncotarget. 2017;8(22):35890–35901. doi: 10.18632/oncotarget.16271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lv Z., Yang D., Li J. Bone morphogenetic protein 9 overexpression reduces osteosarcoma cell migration and invasion. Mol Cells. 2013;36(2):119–126. doi: 10.1007/s10059-013-0043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liu P., Man Y., Wang Y., Bao Y. Mechanism of BMP9 promotes growth of osteosarcoma mediated by the Notch signaling pathway. Oncol Lett. 2016;11(2):1367–1370. doi: 10.3892/ol.2015.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tang N., Song W.X., Luo J., Haydon R.C., He T.C. Osteosarcoma development and stem cell differentiation. Clin Orthop Relat Res. 2008;466(9):2114–2130. doi: 10.1007/s11999-008-0335-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Andersson E.R., Sandberg R., Lendahl U. Notch signaling: simplicity in design, versatility in function. Development. 2011;138(17):3593–3612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- 192.Xie Z., Xu J., Peng L., Gao Y., Zhao H., Qu Y. miR-149 promotes human osteocarcinoma progression via targeting bone morphogenetic protein 9 (BMP9) Biotechnol Lett. 2018;40(1):47–55. doi: 10.1007/s10529-017-2445-8. [DOI] [PubMed] [Google Scholar]
- 193.Li Q., Gu X., Weng H. Bone morphogenetic protein-9 induces epithelial to mesenchymal transition in hepatocellular carcinoma cells. Cancer Sci. 2013;104(3):398–408. doi: 10.1111/cas.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fransvea E., Angelotti U., Antonaci S., Giannelli G. Blocking transforming growth factor-beta up-regulates E-cadherin and reduces migration and invasion of hepatocellular carcinoma cells. Hepatology. 2008;47(5):1557–1566. doi: 10.1002/hep.22201. [DOI] [PubMed] [Google Scholar]
- 195.Thawani J.P., Wang A.C., Than K.D., Lin C.Y., La Marca F., Park P. Bone morphogenetic proteins and cancer: review of the literature. Neurosurgery. 2010;66(2):233–246. doi: 10.1227/01.NEU.0000363722.42097.C2. discussion 246. [DOI] [PubMed] [Google Scholar]
- 196.Wang Y.D., Yang F., Chen W.D. Farnesoid X receptor protects liver cells from apoptosis induced by serum deprivation in vitro and fasting in vivo. Mol Endocrinol. 2008;22(7):1622–1632. doi: 10.1210/me.2007-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kang S., Song J., Kang H., Kim S., Lee Y., Park D. Insulin can block apoptosis by decreasing oxidative stress via phosphatidylinositol 3-kinase- and extracellular signal-regulated protein kinase-dependent signaling pathways in HepG2 cells. Eur J Endocrinol. 2003;148(1):147–155. doi: 10.1530/eje.0.1480147. [DOI] [PubMed] [Google Scholar]
- 198.Wang K., Feng H., Ren W. BMP9 inhibits the proliferation and invasiveness of breast cancer cells MDA-MB-231. J Cancer Res Clin Oncol. 2011;137(11):1687–1696. doi: 10.1007/s00432-011-1047-4. [DOI] [PubMed] [Google Scholar]
- 199.Ouarné M., Bouvard C., Boneva G. BMP9, but not BMP10, acts as a quiescence factor on tumor growth, vessel normalization and metastasis in a mouse model of breast cancer. J Exp Clin Cancer Res. 2018;37(1):209. doi: 10.1186/s13046-018-0885-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li S., Dai H., He Y. BMP9 inhibits the growth of breast cancer cells by downregulation of the PI3K/Akt signaling pathway. Oncol Rep. 2018;40(3):1743–1751. doi: 10.3892/or.2018.6572. [DOI] [PubMed] [Google Scholar]
- 201.Ren W., Liu Y., Wan S. BMP9 inhibits proliferation and metastasis of HER2-positive SK-BR-3 breast cancer cells through ERK1/2 and PI3K/AKT pathways. PLoS One. 2014;9(5):e96816. doi: 10.1371/journal.pone.0096816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Liu B., Sun L., Liu Q. A cytoplasmic NF-κB interacting long noncoding RNA blocks IκB phosphorylation and suppresses breast cancer metastasis. Cancer Cell. 2015;27(3):370–381. doi: 10.1016/j.ccell.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 203.Liu M., Gou L., Xia J. LncRNA ITGB2-AS1 could promote the migration and invasion of breast cancer cells through up-regulating ITGB2. Int J Mol Sci. 2018;19(7) doi: 10.3390/ijms19071866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Coleman R.E. Skeletal complications of malignancy. Cancer. 1997;80(Suppl. 8):1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 205.Wang W., Weng Y., Ren W. Biological roles of human bone morphogenetic protein 9 in the bone microenvironment of human breast cancer MDA-MB-231 cells. Am J Transl Res. 2015;7(9):1660–1674. [PMC free article] [PubMed] [Google Scholar]
- 206.Wan S., Liu Y., Weng Y. BMP9 regulates cross-talk between breast cancer cells and bone marrow-derived mesenchymal stem cells. Cell Oncol (Dordr) 2014;37(5):363–375. doi: 10.1007/s13402-014-0197-1. [DOI] [PubMed] [Google Scholar]
- 207.Renehan A.G., Tyson M., Egger M., Heller R.F., Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 208.Lee Y., Jung W.H., Koo J.S. Adipocytes can induce epithelial-mesenchymal transition in breast cancer cells. Breast Canc Res Treat. 2015;153(2):323–335. doi: 10.1007/s10549-015-3550-9. [DOI] [PubMed] [Google Scholar]
- 209.Shimasaki S., Moore R.K., Erickson G.F., Otsuka F. The role of bone morphogenetic proteins in ovarian function. Reprod Suppl. 2003;61:323–337. [PubMed] [Google Scholar]
- 210.Gordon K.J., Blobe G.C. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim Biophys Acta. 2008;1782(4):197–228. doi: 10.1016/j.bbadis.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 211.Shepherd T.G., Nachtigal M.W. Identification of a putative autocrine bone morphogenetic protein-signaling pathway in human ovarian surface epithelium and ovarian cancer cells. Endocrinology. 2003;144(8):3306–3314. doi: 10.1210/en.2003-0185. [DOI] [PubMed] [Google Scholar]
- 212.Le Page C., Ouellet V., Madore J. Gene expression profiling of primary cultures of ovarian epithelial cells identifies novel molecular classifiers of ovarian cancer. Br J Canc. 2006;94(3):436–445. doi: 10.1038/sj.bjc.6602933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Baraldi E., Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357(19):1946–1955. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 214.Chen X., Orriols M., Walther F.J. Bone morphogenetic protein 9 protects against neonatal hyperoxia-induced impairment of alveolarization and pulmonary inflammation. Front Physiol. 2017;8:486. doi: 10.3389/fphys.2017.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Nikolic I., Yung L.M., Yang P. Bone morphogenetic protein 9 is a mechanistic biomarker of portopulmonary hypertension. Am J Respir Crit Care Med. 2019;199(7):891–902. doi: 10.1164/rccm.201807-1236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Toshner M. BMP9 morphs into a potential player in portopulmonary hypertension. Am J Respir Crit Care Med. 2019;199(7):819–821. doi: 10.1164/rccm.201810-1886ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Alejandre-Alcázar M.A., Kwapiszewska G., Reiss I. Hyperoxia modulates TGF-beta/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2007;292(2):L537–L549. doi: 10.1152/ajplung.00050.2006. [DOI] [PubMed] [Google Scholar]
- 218.Hilgendorff A., Reiss I., Ehrhardt H., Eickelberg O., Alvira C.M. Chronic lung disease in the preterm infant. Lessons learned from animal models. Am J Respir Cell Mol Biol. 2014;50(2):233–245. doi: 10.1165/rcmb.2013-0014TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bi J., Ge S. Potential roles of BMP9 in liver fibrosis. Int J Mol Sci. 2014;15(11):20656–20667. doi: 10.3390/ijms151120656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Breitkopf-Heinlein K., Meyer C., König C. BMP-9 interferes with liver regeneration and promotes liver fibrosis. Gut. 2017;66(5):939–954. doi: 10.1136/gutjnl-2016-313314. [DOI] [PubMed] [Google Scholar]
- 221.Sheyn D., Kimelman-Bleich N., Pelled G., Zilberman Y., Gazit D., Gazit Z. Ultrasound-based nonviral gene delivery induces bone formation in vivo. Gene Ther. 2008;15(4):257–266. doi: 10.1038/sj.gt.3303070. [DOI] [PubMed] [Google Scholar]
- 222.Aslan H., Zilberman Y., Arbeli V. Nucleofection-based ex vivo nonviral gene delivery to human stem cells as a platform for tissue regeneration. Tissue Eng. 2006;12(4):877–889. doi: 10.1089/ten.2006.12.877. [DOI] [PubMed] [Google Scholar]
- 223.Bergeron E., Leblanc E., Drevelle O. The evaluation of ectopic bone formation induced by delivery systems for bone morphogenetic protein-9 or its derived peptide. Tissue Eng A. 2012;18(3–4):342–352. doi: 10.1089/ten.TEA.2011.0008. [DOI] [PubMed] [Google Scholar]
- 224.Vhora I., Lalani R., Bhatt P., Patil S., Misra A. Lipid-nucleic acid nanoparticles of novel ionizable lipids for systemic BMP-9 gene delivery to bone-marrow mesenchymal stem cells for osteoinduction. Int J Pharm. 2019;563:324–336. doi: 10.1016/j.ijpharm.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 225.Dumanian Z.P., Tollemar V., Ye J. Repair of critical sized cranial defects with BMP9-transduced calvarial cells delivered in a thermoresponsive scaffold. PLoS One. 2017;12(3):e0172327. doi: 10.1371/journal.pone.0172327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Cellesi F. Thermoresponsive hydrogels for cellular delivery. Ther Deliv. 2012;3(12):1395–1407. doi: 10.4155/tde.12.114. [DOI] [PubMed] [Google Scholar]
- 227.Zhang R., Li X., Liu Y., Gao X., Zhu T., Lu L. Acceleration of bone regeneration in critical-size defect using BMP-9-loaded nHA/ColI/MWCNTs scaffolds seeded with bone marrow mesenchymal stem cells. BioMed Res Int. 2019;2019:7343957. doi: 10.1155/2019/7343957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Fu T., Liang P., Song J. Matrigel scaffolding enhances BMP9-induced bone formation in dental follicle stem/precursor cells. Int J Med Sci. 2019;16(4):567–575. doi: 10.7150/ijms.30801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lee C.S., Bishop E.S., Dumanian Z. Bone morphogenetic protein-9-stimulated adipocyte-derived mesenchymal progenitors entrapped in a thermoresponsive nanocomposite scaffold facilitate cranial defect repair. J Craniofac Surg. 2019 doi: 10.1097/SCS.0000000000005465. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Nakamura T., Shirakata Y., Shinohara Y. Comparison of the effects of recombinant human bone morphogenetic protein-2 and -9 on bone formation in rat calvarial critical-size defects. Clin Oral Investig. 2017;21(9):2671–2679. doi: 10.1007/s00784-017-2069-3. [DOI] [PubMed] [Google Scholar]
- 231.Nie L., Yang X., Duan L. The healing of alveolar bone defects with novel bio-implants composed of Ad-BMP9-transfected rDFCs and CHA scaffolds. Sci Rep. 2017;7(1):6373. doi: 10.1038/s41598-017-06548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Dolan C.P., Dawson L.A., Muneoka K. Digit Tip regeneration: merging regeneration biology with regenerative medicine. Stem Cells Transl Med. 2018;7(3):262–270. doi: 10.1002/sctm.17-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yu L., Dawson L.A., Yan M. BMP9 stimulates joint regeneration at digit amputation wounds in mice. Nat Commun. 2019;10(1):424. doi: 10.1038/s41467-018-08278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dawson L.A., Yu L., Yan M. The periosteal requirement and temporal dynamics of BMP2-induced middle phalanx regeneration in the adult mouse. Regeneration (Oxf) 2017;4(3):140–150. doi: 10.1002/reg2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]