Abstract
Phospholipases A2 (PLA2s) catalyze hydrolysis of the sn-2 substituent from glycerophospholipids to yield a free fatty acid (i.e., arachidonic acid), which can be metabolized to pro- or anti-inflammatory eicosanoids. Macrophages modulate inflammatory responses and are affected by Ca2+-independent phospholipase A2 (PLA2)β (iPLA2β). Here, we assessed the link between iPLA2β-derived lipids (iDLs) and macrophage polarization. Macrophages from WT and KO (iPLA2β−/−) mice were classically M1 pro-inflammatory phenotype activated or alternatively M2 anti-inflammatory phenotype activated, and eicosanoid production was determined by ultra-performance LC ESI-MS/MS. As a genotypic control, we performed similar analyses on macrophages from RIP.iPLA2β.Tg mice with selective iPLA2β overexpression in β-cells. Compared with WT, generation of select pro-inflammatory prostaglandins (PGs) was lower in iPLA2β−/−, and that of a specialized pro-resolving lipid mediator (SPM), resolvin D2, was higher; both changes are consistent with the M2 phenotype. Conversely, macrophages from RIP.iPLA2β.Tg mice exhibited an opposite landscape, one associated with the M1 phenotype: namely, increased production of pro-inflammatory eicosanoids (6-keto PGF1α, PGE2, leukotriene B4) and decreased ability to generate resolvin D2. These changes were not linked with secretory PLA2 or cytosolic PLA2α or with leakage of the transgene. Thus, we report previously unidentified links between select iPLA2β-derived eicosanoids, an SPM, and macrophage polarization. Importantly, our findings reveal for the first time that β-cell iPLA2β-derived signaling can predispose macrophage responses. These findings suggest that iDLs play critical roles in macrophage polarization, and we posit that they could be targeted therapeutically to counter inflammation-based disorders.
Keywords: inflammation, macrophage phenotype, lipidomics, eicosanoids, resolvin D2, intercellular signaling
T1D is a consequence of autoimmune destruction of β-cells, involving activation of cellular immunity and inflammation that lead to infiltration of islets by leukocytes (1). Lipid signaling is recognized as a key modulator of inflammation and immune responses (2). For instance, arachidonic acid (AA) and oxidized lipids [i.e., prostaglandins (PGs) and leukotrienes (LTs)] (3) generated via its metabolism can trigger immune responses leading to β-cell death (4, 5). They are generated via cyclooxygenases (COXs) and lipoxygenases (LOs), subsequent to phospholipase A2 (PLA2)-mediated hydrolysis of AA (6–12) from membrane glycerophospholipids.
Among the family of PLA2s is the Ca2+-independent phospholipase A2β (iPLA2β), and its activity promotes deleterious outcomes in experimental and clinical diabetes (13–15). Immune cells express iPLA2β (16–20) and inhibition of iPLA2β reduces generation of reactive oxygen species (18) and antibody production from B-cells and TNFα from CD4+ T-cells (20) and macrophages (21). TNFα acts as a powerful chemoattractant (22) and is produced by CD4+ T-cells within inflamed islets during diabetes development (23). TNFα overexpression exacerbates insulitis while the opposite occurs in TNFα-receptor-null mice (24). In this regard, lipids derived from iPLA2β activation, but not other PLA2s, promote monocyte chemotaxis (18, 21, 25, 26) and provide migratory directionality to monocytes to inflamed sites (27). Inhibition of iPLA2β has shown to be effective against diseases related to autoimmunity (28) and inflammation (29–32).
Macrophages are important for innate and adaptive immunity and participate in autoimmune-mediated destruction of β-cells and T1D. In diabetes-prone individuals, immune cells, including macrophages, migrate to pancreatic islets and secrete pro-inflammatory cytokines and reactive oxygen species that result in β-cell death (33). Two different activation states of macrophages have been described; M1 pro-inflammatory macrophages (34), which are classically activated by interferon-γ (IFNγ), lipopolysaccharide (LPS), or TNFα, and M2 macrophages, which are alternatively activated by interleukin (IL)-4 or IL-10 (35). Whereas M1 macrophages are recognized causative factors in T1D development (36), M2 macrophages protect against T1D (37). Pro-inflammatory eicosanoids have been linked to macrophage phagocytosis, adhesion, and apoptosis, and amplifying macrophage-derived eicosanoid release (38–41).
Macrophages express iPLA2β (18, 42), and our recent studies reveal that activation of iPLA2β promotes macrophage polarization toward the M1 inflammatory phenotype (42). In contrast, iPLA2β deficiency favors macrophage polarization toward the M2 anti-inflammatory phenotype. Further, inhibitors of iPLA2β, COX, and 12-LO reduce M1 inflammatory markers, recapitulating the iPLA2β −/− macrophage phenotype. Collectively, these findings suggest that iPLA2β-derived lipids (iDLs) modulate immune responses.
Given the importance of macrophages to T1D development and evidence for a role of iPLA2β in modulating macrophage polarization and function, we sought to identify iDLs generated by activated macrophages, which could be targeted to counter autoimmune/inflammatory-based disorders. To facilitate our assessments, we utilized targeted/quantitative MS analyses of eicosanoids produced by macrophages isolated from WT (MΦWT) and iPLA2β−/− (KO, MΦKO) mice. As a control for the genotype, we performed similar analyses with macrophages isolated from RIP.iPLA2β.Tg [transgenic (Tg), MΦTg] mice, which selectively overexpress iPLA2β in β-cells (43, 44).
We found that iPLA2β deficiency led to an attenuated pro-inflammatory and amplified anti-inflammatory lipid profile that was consistent with a macrophage M2 phenotype. In contrast, MΦTg were found to exhibit an opposite landscape, encompassing an exaggerated pro-inflammatory and attenuated anti-inflammatory lipid profile that was associated with a macrophage M1 phenotype. These findings suggest that iPLA2β modulates the inflammatory lipid profile and raises the intriguing possibility that increased expression of iPLA2β in the β-cells can confer increased susceptibility of macrophages to activation.
EXPERIMENTAL PROCEDURES
Animals
Mouse breeders obtained from Dr. John Turk (Washington University School of Medicine, St. Louis, MO) were used to generate colonies of WT, RIP.iPLA2β.Tg (selectively overexpressing iPLA2β in β-cells only), and global iPLA2β-KO mice at the University of Alabama at Birmingham. Tg founders (TG1 line) were mated with WT C57BL/6J mice (Jackson Laboratory) to generate RIP.iPLA2β.Tg (Tg) mice and WT mice, and male and female iPLA2β +/− pairings were used to generate iPLA2β −/− (KO) and iPLA2β +/+ (WT) mice, as previously described (43, 45). Prior to experimentation, the mice were genotyped, as described (44), utilizing primers described in Table 1. Animal experiments were conducted according to approved IACUC guidelines at the University of Alabama at Birmingham. As our earlier studies suggested differences between the WT generated by the two schemes, data from the KO and Tg genotypes were compared against their corresponding WT littermates.
TABLE 1.
Genotyping primers
| Name | Sequence (5′ to 3′) | Target | Product Size (bp) |
| WT-PLA2G6_F | AGCTTCAGGATCTCATGCCCATC | WT (KO) iPLA2β | 1,400 |
| WT-PLA2G6_R | CTCCGCTTCTCGTCCCTCATGGA | ||
| KO-Neo_F | TCGCCTTCTATCGCCTTCTTGAC | KO-iPLA2β | 400 |
| KO-Ex_R | GGGGCCTCAGACTGGGAATC | ||
| WT-PLA2G6_F | CCTCCGGAGAGCAGCGATTAAAAGTGTCAG | WT (Tg) iPLA2β | 450 |
| WT-PLA2G6_R | TAGAGCTTTGCCACATCACAGGTCATTCAG | ||
| Tg-PLA2G6_F | CTAGGCTCAGACATCATGCTGGACGAGGT | RIP.iPLA2β.Tg | 200 |
| Tg-PLA2G6_R | AAGATCTCAGTGGTATTTGTGAGCCAGGG |
Isolation and culture of peritoneal macrophages
Mice (7–8 weeks of age) were euthanized by CO2 inhalation and cervical dislocation. Peritoneal macrophages were obtained by filling the peritoneal cavity with 5 ml of cold PBS containing 2% FBS, massaging gently, and withdrawing the cell-containing solution. Cells were pelleted at 300 g for 5 min and resuspended in growth medium [Eagle’s minimum essential medium (Sigma-Aldrich; M0894), 2.0 mg/ml sodium bicarbonate (Fisher Scientific; BP328-500), 2 mM l-glutamine (Life Technologies; 25030-081), 100 U/ml penicillin-100 μg/ml streptomycin [Life Technologies; 15140-122), and 10% heat-inactivated FBS (Life Technologies; 16000044)] supplemented with 10% L929 cell-conditioned medium (source of M-CSF). Macrophages from a single collection were sufficient to seed six 60 mm nontreated culture dishes. Adherent macrophages appeared after 16 h of culture. All experiments were performed with expanded freshly isolated peritoneal macrophages under classical and alternate activation conditions, as described below. Macrophages isolated from WT, iPLA2β−/− (KO), and RIP.iPLA2β.Tg (Tg) mice are designated as MΦWT, MΦKO, and MΦTg, respectively.
Macrophage activation
Macrophage activation was accomplished according to previously published methods (42). For classical activation, macrophages were treated with 15 ng/ml recombinant IFNγ (R&D Systems; 485-MI-100) for 8 h in growth medium followed by addition of 10 ng/ml ultrapure LPS (InvivoGen; tlrl-3pelps) and incubated for 16 h at 37°C. For alternative activation, macrophages were treated with 8 ng/ml recombinant IL-4 (R&D Systems; 404-ML-010) in growth medium for 16 h. Naïve macrophages, which received no activation stimuli, were maintained in growth medium with no additional treatment. In some experiments, the macrophages were pretreated for 1 h with either a secretory phospholipase A2 (sPLA2) (LY315920, 10 μM; Cayman Chemical) or cytosolic (c)PLA2α (CAY 10502, 50 nM; Cayman Chemical) inhibitor prior to activation. The inhibitors were present during the entire activation period.
Macrophage mRNA target analyses
Macrophages cultured in 60 mm non-tissue culture-treated dishes were lysed in 1 ml of TRIzol (Life Technologies; 15596-026). Total RNA was prepared and purified using RNeasy mini kits (QIAGEN; 74104), and 1 μg RNA was converted to cDNA using the Superscript III first strand synthesis system (Life Technologies; 18080-051), according to manufacturer’s instructions. The cDNA was diluted 10-fold and used as template in conventional or real-time quantitative PCR (qPCR). cDNA transcripts were amplified with primers (Table 2) designed using NCBI Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). Real-time qPCR was carried out using SYBR Select Mastermix (Life Technologies; 4472908) according to the manufacturer’s instructions. Relative gene expression levels were determined using the 2−ΔΔCt method.
TABLE 2.
Real-time qPCR primers
| Name | Sequence (5′ to 3′) | Tm (salt) | Target |
| msMRC1.F | GTCAGAACAGACTGCGTGGA | 60.0 | Mrc1 |
| msMRC1.R | AGGGATCGCCTGTTTTCCAG | 60.0 | |
| msARG2.F | GCAAATTCCTTGCGTCCTGA | 60.0 | Arg2 |
| msARG2.R | AGGCCCACTGAACGAGGATA | 60.0 | |
| msPtgs2.F3 | TGAGTGGGGTGATGAGCAAC | 60.0 | PTGS2 |
| msPtgs2.R3 | TTCAGAGGCAATGCGGTTCT | 60.0 | |
| ALOX12.F | GGCTATCCAGATTCAGCCCC | 60.0 | ALOX-12 |
| ALOX12.R | CCGGCTTCGCGTGTTAATTT | 57.1 | |
| miPLA2.F | TATGCGTGGTGTGATCTTCCG | 57.1 | iPLA2β |
| miPLA2.R | CATGGAGCTCAGGATGAACGC | 60.0 | |
| msPLA2 GV.F (101) | AGCCTCGATCATGGCCTTT | 56.8 | GV sPLA2 |
| msPLA2 GV.R (101) | GCCGAATCATTTCCCCAAA | 56.8 | |
| mcPLA2.F | CCCTGAGTAGTTTGAAGGAAAAGG | 55.4 | cPLA2 |
| mcPLA2.R | ACACGTGAAGAGAGGCAAAGG | 55.4 |
Eicosanoid preparation
Eicosanoids were extracted using a modified extraction process, as previously described (46, 47). Medium from cells (2 ml) was combined with an internal standard mixture comprised of 10% total volume methanol (200 μl) and glacial acetic acid (10 μl) before spiking with internal standard (20 μl) containing the following deuterated eicosanoids (2 pmol/μl, 40 pmol total) (all standards purchased from Cayman Chemicals): (d4) 6-keto-PGF1α, (d4) PGF2α, (d4) PGE2, (d4) PGD2, (d8) 5-HETE, (d8) 12-HETE, (d8) 15-HETE, (d6) 20-HETE, (d11) 8,9-epoxyeicosatrienoic acid (EET), (d8) 14,15-EET, (d8) AA, (d5) eicosapentaenoic acid, (d5) DHA, (d4) PGA2, (d4) LTB4, (d4) LTC4, (d4) LTD4, (d4) LTE4, (d5) 5(S),6(R)-lipoxin A4, (d11) 5-iPF2α-VI, (d4) 8-iso PGF2α, (d11) (±)14,15-dihydroxyeicosatrienoic acid (DHET), (d11) (±)8,9-DHET, (d11) (±)11,12-DHET, (d4) PGE1, (d4) thromboxane B2 (TXB2), (d6) dihomo-γ linoleic acid, (d5) resolvin D2, (d5) resolvin D1, (d5) maresin2, and (d5) resolvin D3. Samples and vial rinses (5% methanol; 2 ml) were applied to Strata-X SPE columns (Phenomenex) previously washed with methanol (2 ml) and then dH2O (2 ml). Eicosanoids eluted with isopropanol (2 ml), were dried in vacuo and reconstituted in ethanol:dH2O (50:50;100 μl) prior to ultra-performance (UP)LC ESI-MS/MS analysis.
Analysis of eicosanoids by UPLC ESI-MS/MS
Eicosanoids were separated using a Shimadzu Nexera X2 LC-30AD coupled to a SIL-30AC auto injector, coupled to a DGU-20A5R degassing unit in the following way [as previously described by us (47)]. A 14 min reversed phase LC method utilizing an Acentis Express C18 column (150 × 2.1 mm, 2.7 μm) was used to separate the eicosanoids at a 0.5 ml/min flow rate at 40°C as we previously described (47). The column was equilibrated with 100% solvent A [acetonitrile:water:formic acid (20:80:0.02, v/v/v)] for 5 min and then 10 μl of sample were injected. Solvent A (100%) was used for the first 2 min of elution. Solvent B [acetonitrile:isopropanol:formic acid (20:80:0.02, v/v/v)] was increased in a linear gradient to 25% solvent B at 3 min, to 30% at 6 min, to 55% at 6.1 min, to 70% at 10 min, and to 100% at 10.10 min. Solvent B (100%) was held constant until 13.0 min, where it was decreased to 0% solvent B and 100% solvent A from 13.0 min to 13.1 min. From 13.1 min to 14.0 min, solvent A was held constant at 100%. All solvents were purchased from Fischer Scientific.
Eicosanoids were analyzed via MS using an AB Sciex Triple Quad 5500 mass spectrometer, as previously described by us (47). Q1 and Q3 were set to detect distinctive precursor and product ion pairs. Ions were fragmented in Q2 using N2 gas for collisionally induced dissociation. Analysis used multiple-reaction monitoring in negative-ion mode. Eicosanoids were monitored using precursor → product MRM pairs. The mass spectrometer parameters used were: curtain gas, 20 psi; CAD, medium; ion spray voltage, −4,500 V; temperature, 300°C; gas 1, 40 psi; gas 2, 60 psi (declustering potential, entrance potential, collision energy, and cell exit potential varied per transition). MRM transitions with corresponding declustering potentials, collision energies, entrance potentials, and collision cell exit potentials are shown in supplemental Table S1.
Selectivity of transgene function
As described (43), to generate Tg mice with selective overexpression of iPLA2β in β-cells only, rat iPLA2β cDNA was inserted downstream of the rat insulin promoter (RIP) at a site within the rabbit globin gene sequence. As such, transcription of the sequence encoding iPLA2β is under control of RIP, and transgenic overexpression of iPLA2β is expected only in cells that express insulin, i.e., pancreatic islet β-cells, but not in other cells (i.e., macrophages). To verify that induction of the transgene was specific to β-cells, PCR analyses were performed using islet and macrophage cDNA as a template with two pairs of primers. One pair amplified the sequence in the internal control fatty acid-binding protein, intestinal (Fabpi) gene, and the primer sequences were (Fabpi 5′) cctccggagagcagcgattaaaagtgtcag and (Fabpi 3′) tagagctttgccacatcacaggtcattcag (expected product size, 450 bp). The other primer pair amplified a sequence that spanned the junction of iPLA2β and globin cDNA in the TG construct. The primer sequences were (TG 5′) ctaggctcagacatcatgctggacgaggt and (TG 3′) aagatctcagtggtatttgtgagccaggg (expected product size, 200 bp). Subsequently, the islets and macrophages were processed for insulin content and iPLA2β immunoblotting (1° antibodies: iPLA2β, E-8, sc-166616; tubulin, TU-02, sc-8035) analyses, as described (48, 49).
Statistical analysis
Lipidomics samples were analyzed via a multivariate approach using SPSS. In experiments with more than two conditions, samples were analyzed by ANOVA on the R platform with a Tukey’s post hoc test. In experiments using two conditions, samples were analyzed using a Student’s t-test. Presented data are mean ± SEM and, where appropriate, as fold-change relative to control (vehicle). Values of P < 0.05 were considered significant.
RESULTS
iPLA2β−/− model verification
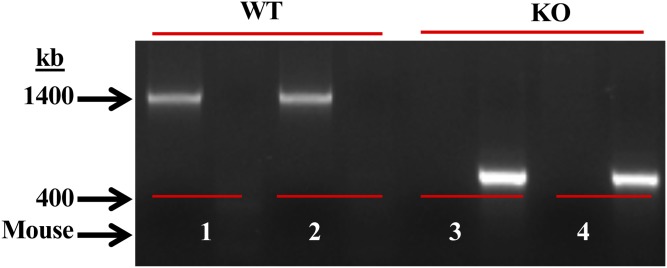
As previously reported (43, 45), DNA was generated from tail clips and progeny were genotyped by PCR analyses. The primers used for the WT or for the disrupted iPLA2β−/− (KO) sequence were expected to yield product sizes of 1,400 or 400 bp, respectively. Consistently, only a 1,400 bp product in WT (lanes 1 and 2) and a 400 bp product in KO (lanes 3 and 4) were evident (Fig. 1). The iPLA2β−/− group is referred to as KO and macrophages isolated from these mice as MΦKO, and macrophages from WT, MΦWT.
Fig. 1.
Genotyping and verification of the iPLA2β−/− model. DNA was generated from tail clips and progeny were genotyped by PCR analyses. Reactions were performed in the presence of primers for the WT sequence (sets 1 and 2) or for the disrupted KO sequence (sets 3 and 4) for each mouse. The expected bands for WT (1,400 bp) and KO (400 bp) in two mice each are presented.
Comparison of basal lipid species in WT and KO
The MS protocol identified several lipid species (Table 3), including pro-inflammatory and anti-inflammatory PGs, LTs, HETEs, DHETs, EETs, and specialized pro-resolving lipid mediators (SPMs). Analyses of lipid production under basal conditions revealed no significant differences in the abundances of detected lipids between WT and KO (supplemental Table S2). These findings suggest an overall similar profile in MΦWT and MΦKO production of lipids.
TABLE 3.
Macrophage lipids identified by UPLC ESI-MS/MS analyses
| Lipid Species | |
| Pro-inflammatory | |
| PGs | 6-keto PGF1α, TXB2, PGF2α, 8-iso PGF2α, 5-iPF2α-VI, PGE2, PGD2, PGA2, 15-deoxy-Δ12,14-PGJ2 |
| LTs | LTB4, LTC4, LTD4, LTE4 |
| Dihydoxyeicosatrienoic acids | (±)11,12-DHET, (±)14,15-DHET, (±)8,9-DHET |
| Hydoxyeicosatrienoic acids | 20-HETE, 15-HETE, 12-HETE, 5-HETE |
| Anti-inflammatory | |
| PGs | PGE1 |
| SPMs | Resolvin D2, resolvin D1, lipoxin A4 |
| EETs | (±)11,12-EET, (±)14,15-EET, (±)8,9-EET |
Comparison of pro-inflammatory lipid production from activated MΦWT and MΦKO
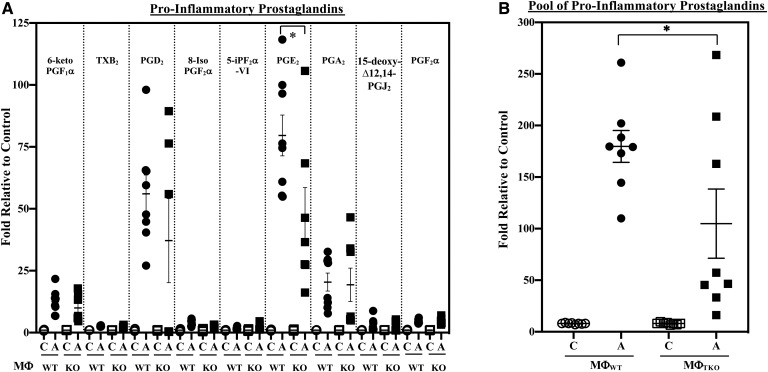
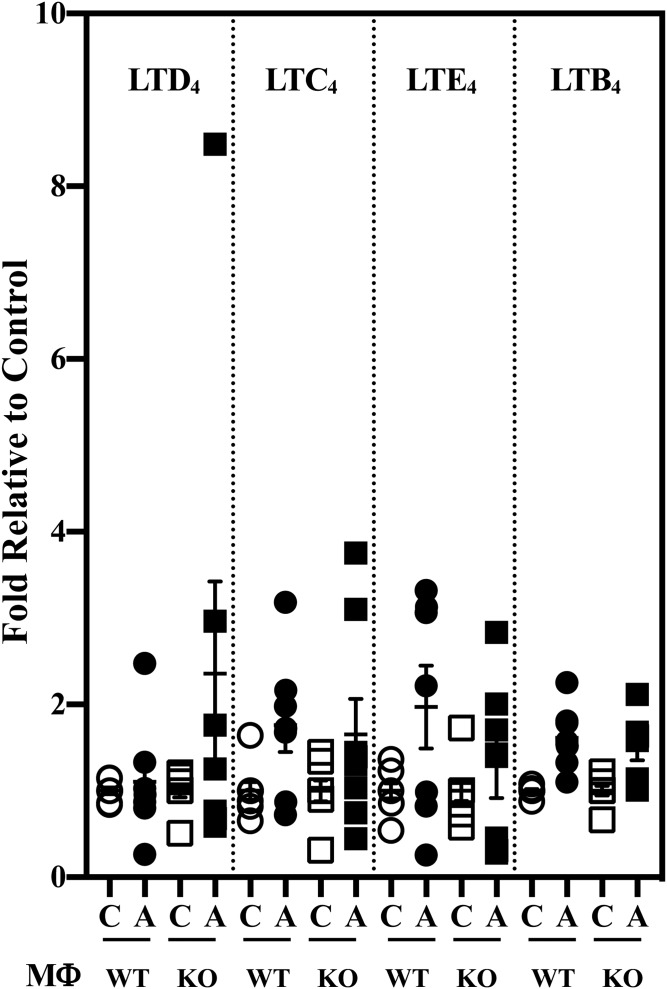
AA, subsequent to its release from membrane glycerophospholipids, can be metabolized by COXs and LOs to generate oxidized bioactive products, designated eicosanoids. Some of these lipids are considered to be pro-inflammatory. They include several PGs, LTs, and HETEs (Table 3). Further, epoxidation of AA by cytochrome P450 epoxygenases leads to the generation of EETs, which are then hydrolyzed by soluble epoxide hydrolases to pro-inflammatory DHETs. Comparison of macrophage pro-inflammatory lipid production in response to classical activation revealed an increase in pro-inflammatory PGs generated by both MΦWT and MΦKO in response to IFNγ + LPS (Fig. 2A). However, the increase in PGE2 in the KO group was of lower magnitude relative to the WT group. Overall, a nearly 40% lower production of pro-inflammatory PGs (pool of PGs presented in Fig. 2A) by MΦKO relative to MΦWT was evident (Fig. 2B). However, production of other pro-inflammatory lipid species, including LTs (Fig. 3), HETEs (supplemental Fig. S1A), or DHETs (±14,15 and ±11,12; supplemental Fig. S2A), by MΦKO was similar to MΦWT, and the production of (±)8,9-DHET by MΦKO was higher relative to MΦWT. These observations suggest that iPLA2β promotes production of select pro-inflammatory PGs in macrophages.
Fig. 2.
Pro-inflammatory PG production by MΦWT and MΦKO. Peritoneal macrophages isolated from 8-week-old WT and KO mice were treated with vehicle [control (C)] or IFNγ + LPS and the media collected for lipidomics analyses. The data represent activated (A) fold-changes in lipids relative to C. A: Individual PGs. Control (pmol lipid/1e+06) MΦWT and MΦKO: 6-keto PGF1α, 2.512 ± 0.302 and 3.818 ± 0.564; TXB2, 4.563 ± 0.585 and 4.491 ± 0.538; PGD2, 0.568 ± 0.083 and 0.881 ± 0.128; 8-Iso PGF2α, 0.056 ± 0.008 and 0.059 ± 0.013; 5-IPF2α-VI, 0.263 ± 0.033 and 0.301 ± 0.068; PGE2, 1.213 ± 0.313 and 1.690 ± 0.349; PGA2, 0.472 ± 0.035 and 0.525 ± 0.050; 15-deoxyΔ12,14-PGJ2, 0.253 ± 0.087 and 0.240 ± 0.072; PGF2α, 0.646 ± 0.055 and 0.591 ± 0.032. B: PG pool. Control (pmol lipid/1e+06) MΦWT and MΦKO: 3.804 ± 0.253 and 4.564 ± 0.443. Data are mean ± SEM determined from seven to nine independent experiments. *MΦKO significantly different from MΦWT, P < 0.05).
Fig. 3.
LT production by MΦWT and MΦKO. Peritoneal macrophages isolated from 8-week-old WT and KO mice were treated with vehicle [control (C)] or IFNγ + LPS and the media collected for lipidomics analyses. The data represent activated (A) fold-changes in lipids relative to C. Control (pmol lipid/1e+06) MΦWT and MΦKO: LTD4, 0.072 ± 0.028 and 0.072 ± 0.027; LTC4, 0.022 ± 0.005 and 0.019 ± 0.005; LTE4, 0.101 ± 0.016 and 0.158 ± 0.038; LTB4, 0.429 ± 0.074 and 0.573 ± 0.118. Data are mean ± SEM determined from seven to nine independent experiments.
Comparison of anti-inflammatory lipid production from activated WT and MΦKO
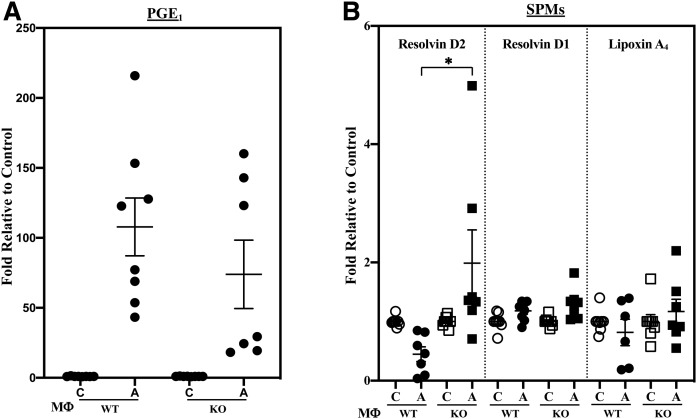
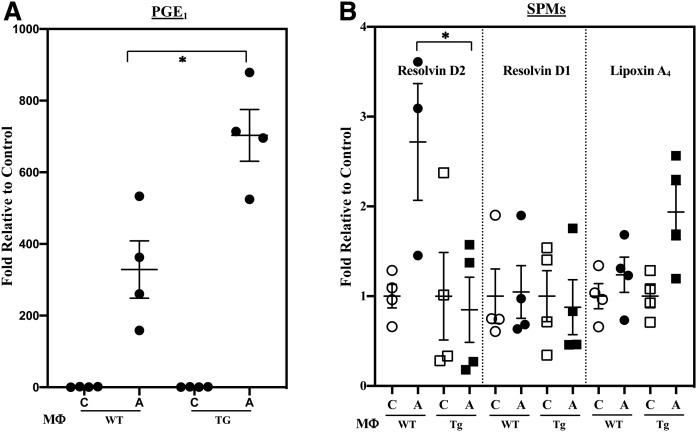
In addition to generation of pro-inflammatory lipids, metabolism of AA can generate anti-inflammatory lipids (Table 3), such as EETs, which have been reported to manifest anti-inflammatory properties (50). Further, dihomo-γ-linolenic acid (DHGLA) can be converted to PGE1, and SPMs are generated via metabolism of AA (lipoxin A4) or DHA (resolvins D1 and D2). With classical activation, anti-inflammatory PGs increased similarly in the WT and KO (Fig. 4A). In contrast, production of resolvin D2 by MΦKO was significantly higher (Fig. 4B) relative to MΦWT; whereas, production of EETs was similar between WT and KO (supplemental Fig. S2B). Further, alternative activation did not promote differential changes in the production of SPMs (data not shown). These observations suggest that iPLA2β can modulate macrophage production of select SPMs.
Fig. 4.
Production of anti-inflammatory PGE1 and SPMs by MΦWT and MΦKO. Peritoneal macrophages isolated from 8-week-old WT and KO mice were treated with vehicle [control (C)] or IFNγ + LPS and the media collected for lipidomics analyses. The data represent activated (A) fold-changes in lipids relative to C. A: PGE1 ± IFNγ + LPS. Control (pmol lipid/1e+06) MΦWT and MΦKO: 0.162 ± 0.020 and 0.240 ± 0.021. B: SPMs. Control (pmol lipid/1e+06) MΦWT and MΦKO: resolvin D2, 0.110 ± 0.029 and 0.100 ± 0.032; resolvin D1, 0.100 ± 0.013 and 0.115 ± 0.019; lipoxin A4, 0.235 ± 0.065 and 0.288 ± 0.109. Data are mean ± SEM determined from seven to nine independent experiments. *MΦKO significantly different from MΦWT, P < 0.05.
iPLA2β−/− (KO) macrophage phenotype
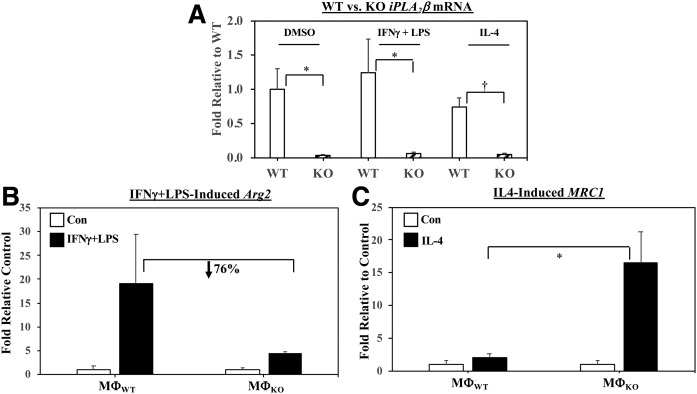
As expected, real-time qPCR analyses (Fig. 5A) confirmed a relative absence of iPLA2β mRNA in MΦKO under both basal and activated conditions. iPLA2β mRNA in MΦWT was modestly, but not significantly, affected by classical activation (approximately 25% increased) and alternative activation (approximately 25% decreased) relative to basal expression. We previously demonstrated that classical activation of macrophages promotes an M1 inflammatory phenotype, whereas alternative activation favors an M2 anti-inflammatory phenotype (42). To confirm that macrophages used in the present lipid analyses exhibited the expected phenotype, they were stimulated with IFNγ + LPS or IL-4 and the macrophages were assessed for expression of markers for M1 [arginase 2 (Arg2)] and M2 [mannose receptor C type 1 (MRC1)], respectively, by real-time qPCR. Activation induced the corresponding markers in MΦWT and MΦKO; however, relative expression of Arg2 was reduced (Fig. 5B) and MRC1 increased (Fig. 5C) in MΦKO in comparison with MΦWT. These findings are consistent with the reduced pro-inflammatory lipid profile in the KO. However, induction of Arg2 and MRC1 in MΦWT and MΦKO by alternative and classical activation, respectively, was similar (supplemental Fig. S3A, B).
Fig. 5.
Induction of M1 (Arg2) and M2 (MRC1) markers and iPLA2β in MΦWT and MΦKO. Peritoneal macrophages isolated from 8-week-old WT and KO mice were treated with vehicle [control (Con)], IFNγ + LPS, or IL-4. The cells were harvested and processed for real-time qPCR analyses. A: iPLA2β . B: Arg2 (MΦWT and MΦKO Arg2 2−ΔΔCT, 0.095 ± 0.074 and 1.506 ± 0.558.) C: MRC1 (MΦWT and MΦKO MRC1 2−ΔΔCT, 4.273 ± 2.357 and 16.053 ± 10.294). Data are mean ± SEM of fold-change relative to Con determined from four independent experiments. *MΦKO significantly different than MΦWT group, P < 0.05; †MΦKO significantly different than MΦWT group, P < 0.005.
RIP.iPLA2β.Tg (Tg) model verification
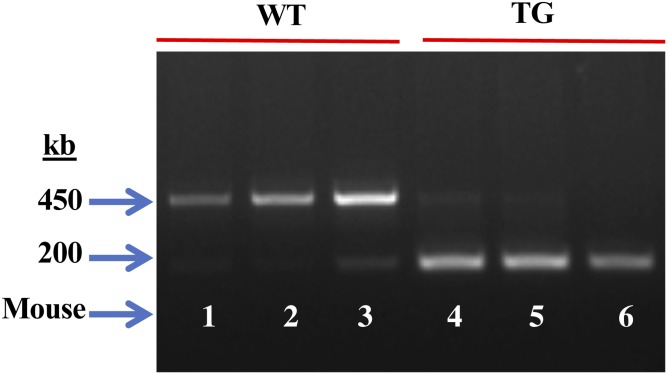
In view of the observed effects on macrophage lipid production associated with iPLA2β-deficiency and literature evidence for intercellular communication between inflamed sites and immune cells, we explored the possibility that signals generated by β-cells could manifest effects in macrophages. To address this, we compared lipid production from MΦTg and MΦWT littermates. We previously demonstrated that the Tg mice overexpress iPLA2β selectively in β-cells (44). To assess the genotype of the RIP.iPLA2β.Tg (Tg), PCR analyses were performed in the presence of two sets of primers expected to yield product sizes of 450 and 200 bp for the WT (Fig. 6, lanes 1–3) and the RIP.iPLA2β.Tg (Fig. 6, lanes 4–6), respectively. Such analyses (Fig. 6) revealed only a 450 bp band in the WT and a 200 bp in the Tg. The RIP.iPLA2β.Tg group is referred to as Tg and macrophages isolated from these mice as MΦTg.
Fig. 6.
Genotyping and verification of the RIP.iPLA2β.Tg model. DNA was generated from tail clips and progeny were genotyped by PCR analyses. Reactions were performed in the presence of two sets of primers and the expected bands for WT (lanes 1–3, 450 bp) and Tg (lanes 4–6, 200 bp) in three mice each are presented.
Comparison of basal lipid species in WT and Tg
Because the KO and Tg mice were derived through different breeding schemes, the Tg mice were compared with their own littermate WT mice. Lipidomics analyses of basal production of lipids revealed no significant differences in the abundances of lipids between MΦWT and MΦTg (supplemental Table S3). These findings suggest that similar to MΦKO, inherent production of lipids by MΦTg is unaffected.
Comparison of pro-inflammatory lipid production from activated WT and MΦTg
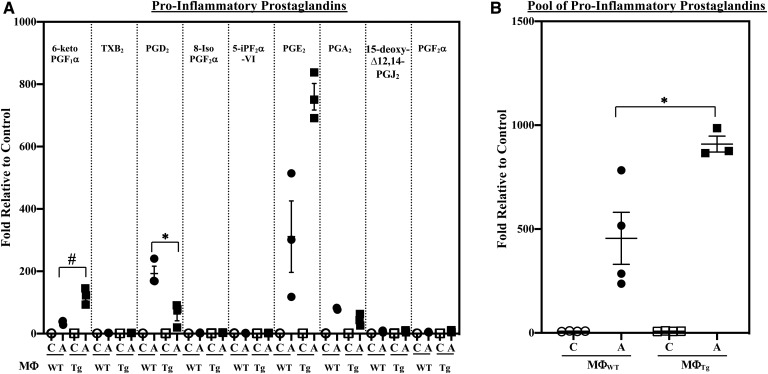
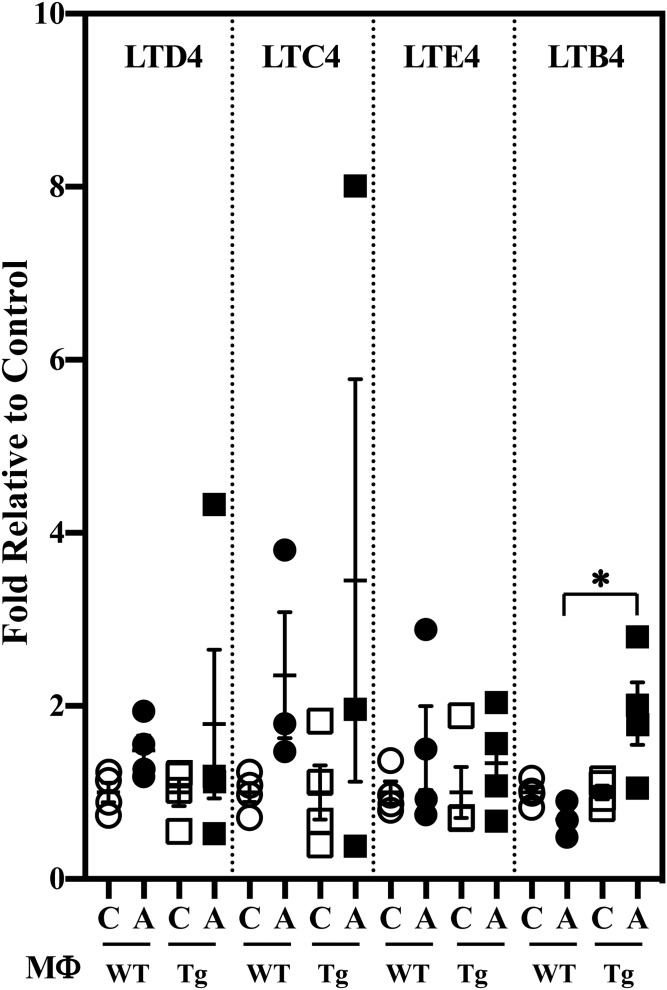
Classical activation of the macrophages promoted higher production of select pro-inflammatory PG lipids (6-ketoPGF1α and PGE2) by MΦTg relative to MΦWT (Fig. 7A). Though PGD2 production by MΦTg was decreased, there was an overall 2-fold higher production of pro-inflammatory PGs (pool of PGs presented in Fig. 7A) by MΦTg relative to MΦWT (Fig. 7B). Further, while LTD4, LTC4, and LTE4 production was similar in MΦWT and MΦTg (Fig. 8), LTB4 was significantly elevated in MΦTg relative to MΦWT. However, production of HETEs (supplemental Fig. S1B) or DHETs (supplemental Fig. S4A) was not significantly different between MΦWT and MΦTg. These observations suggest that MΦTg are primed to respond to classical activation.
Fig. 7.
Pro-inflammatory PG production by MΦWT and MΦTg. Peritoneal macrophages isolated from 8-week-old WT and Tg mice were treated with vehicle [control (C)] or IFNγ + LPS and the media collected for lipidomics analyses. The data represent activated (A) fold-changes in lipids relative to C. Data are mean ± SEM determined from three to four independent experiments. A: Individual PGs. Control (pmol lipid/1e+06) MΦWT and MΦTg: 6-keto PGF1α, 0.502 ± 0.179 and 0.462 ± 0.069; TXB2, 3.725 ± 0.206 and 4.139 ± 0.152; PGD2, 0.036 ± 0.006 and 0.039 ± 0.008; 8-Iso PGF2α, 0.131 ± 0.006 and 0.103 ± 0.039; 5-IPF2α-VI, 0.200 ± 0.082 and 0.162 ± 0.030; PGE2, 0.147 ± 0.044 and 0.083 ± 0.008; PGA2, 0.177 ± 0.068 and 0.139 ± 0.018; 15-deoxyΔ12,14-PGJ2, 0.028 ± 0.006 and 0.037 ± 0.012; PGF2α, 4.587 ± 0.784 and 3.856 ± 0.448. #MΦTg significantly different from MΦWT, P < 0.01; *MΦTg significantly different from MΦWT, P < 0.05. B: PG pool. Control (pmol lipid/1e+06) MΦWT and MΦTg: 9.533 ± 0.153 and 9.019 ± 0.087. *MΦTg significantly different from MΦWT, P < 0.05.
Fig. 8.
LT production by MΦWT and MΦTg. Peritoneal macrophages isolated from 8-week-old WT and Tg mice were treated with vehicle [control (C)] or IFNγ + LPS and the media collected for lipidomics analyses. The data represent activated (A) fold-changes in lipids relative to C. Control (pmol lipid/1e+06) MΦWT and MΦTg: LTD4, 0.0020 ± 0.0001 and 0.0060 ± 0.0001; LTC4, 0.011 ± 0.002 and 0.016 ± 0.005; LTE4, 0.009 ± 0.002 and 0.009 ± 0.002; LTB4, 0.229 ± 0.015 and 0.188 ± 0.015. Data are mean ± SEM determined from four independent experiments. *MΦTg significantly different from MΦWT, P < 0.05.
Comparison of anti-inflammatory lipid production from activated WT and MΦTg
Classical activation increased PGE1 production by MΦTg relative to MΦWT (Fig. 9A), but the production of SPMs by MΦWT and MΦTg was similar (data not shown). Alternative activation promoted a 3-fold increase in resolvin D2 production by MΦWT; however, its production by MΦTg was unchanged from basal production (Fig. 9B). EETs were not significantly different between MΦWT and MΦTg (supplemental Fig. S4B). These observations suggest that MΦTg are predisposed to differential generation of PGE1, derived from DHGLA, and select SPMs.
Fig. 9.
Production of anti-inflammatory PGE1 and SPMs MΦWT and MΦTg. Peritoneal macrophages isolated from 8-week-old WT and Tg mice were treated with vehicle [control (C)], IFNγ + LPS, or IL-4 and the media collected for lipidomics analyses. The data represent activated (A) fold-changes in lipids relative to C. Data are mean ± SEM determined from four independent experiments. A: PGE1 ± IFNγ + LPS. Control (pmol lipid/1e+06) MΦWT and MΦTg: 0.033 ± 0.011 and 0.017 ± 0.003. B: SPMs ± IL-4. Control (pmol lipid/1e+06) MΦWT and MΦTg: resolvin D2, 0.561 ± 0.073 and 1.074 ± 0.524; resolvin D1, 0.134 ± 0.042 and 0.136 ± 0.039; lipoxin A4, 0.050 ± 0.008 and 0.050 ± 0.006. *MΦTg significantly different from MΦWT, P < 0.05.
Impact of sPLA2 and cPLA2α on macrophage lipid profiles
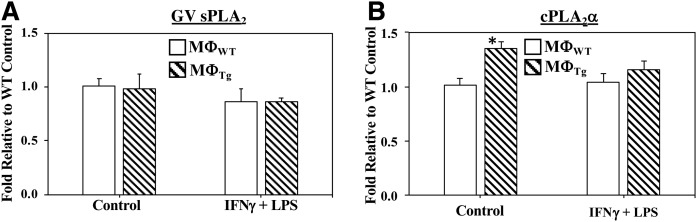
Noting that other PLA2s may also contribute to the altered lipid profile, we sought to determine whether sPLA2s or cPLA2αs contribute to the observed changes in eicosanoid production by MΦTg. The C57BL/J6 macrophages are reported to express GV and GX sPLA2 (51, 52) and cPLA2α (16), but not GIIA sPLA2 (51, 53–56). mRNA analyses revealed that sPLA2 mRNA in MΦTg was not altered under basal or activated conditions, whereas cPLA2 mRNA was higher under basal but similar under activated conditions relative to MΦWT (Fig. 10). We next determine whether the lipid profiles of MΦWT and MΦTg were modulated by inhibitors of sPLA2 [LY315920 (57), which inhibits several sPLA2s including GIIA, GV, and GX] or cPLA2α [CAY 10502 (58)]. Lipidomics analyses revealed that neither basal (Table 4) nor activated (Table 5) production of lipids by MΦWT and MΦTg was affected by LY315920 or CAY 10502. Comparisons between MΦWT and MΦTg exposed to LY315920 revealed a higher basal production of several pro-inflammatory PGs and LTE4 by MΦTg relative to MΦWT (Table 4). In contrast, basal production of lipids by MΦWT and MΦTg exposed to CAY 10502 was unaffected. Classical activation resulted in increases in lipid production by both MΦWT and MΦTg (Table 5), with production by MΦTg significantly higher relative to MΦWT. Such increases were maintained in the presence of LY315920 or CAY 10502. These findings suggest that GV and GX sPLA2s and cPLA2α are not likely contributors to the inflammatory lipid profile in activated MΦTg.
Fig. 10.
Comparison of GV sPLA2 and cPLA2α mRNA in MΦWT and MΦTg. Peritoneal macrophages isolated from 8-week-old WT and Tg mice were treated with vehicle (Control) or IFNγ + LPS. The cells were harvested and processed for real-time qPCR analyses. A: sPLA2 GV (Control MΦWT 2−ΔΔCT, 1.01 ± 0.06). B: cPLA2α (Control MΦWT 2−ΔΔCT, 1.01 ± 0.07). Data are mean ± SEM of fold-change relative to control determined from four independent experiments. *MΦTg significantly different from MΦWT, P < 0.01.)
TABLE 4.
Effects of sPLA2 and cPLA2 inhibition on basal eicosanoid production from MΦWT and MΦTg
| Lipid | MΦWT (n = 4) (pmol lipid/1e+06) | MΦTg (n = 3) (pmol lipid/1e+06) | ||||
| DMSO | +sPLA2 Inhibitor | +cPLA2 Inhibitor | DMSO | +sPLA2 Inhibitor | +cPLA2 Inhibitor | |
| 6-Keto PGF1α | 5.555 ± 0.585 | 4.775 ± 0.599 | 5.425 ± 1.082 | 9.418 ± 2.493 | 9.742 ± 1.732a | 9.604 ± 3.708 |
| TXB2 | 4.619 ± 0.173 | 4.575 ± 0.190 | 4.696 ± 0.188 | 4.949 ± 0.108 | 4.892 ± 0.294 | 5.108 ± 0.593 |
| PGE2 | 2.343 ± 0.297 | 2.320 ± 0.406 | 3.944 ± 1.372 | 3.734 ± 1.053 | 5.347 ± 1.074a | 8.549 ± 5.762 |
| PGA2 | 1.674 ± 0.114 | 1.550 ± 0.074 | 1.858 ± 0.207 | 1.850 ± 0.176 | 2.010 ± 0.143a | 2.008 ± 0.322 |
| PGD2 | 0.395 ± 0.058 | 0.330 ± 0.046 | 0.419 ± 0.067 | 0.413 ± 0.027 | 0.507 ± 0.145 | 0.705 ± 0.137 |
| PGF2α | 0.638 ± 0.043 | 0.536 ± 0.022 | 0.579 ± 0.053 | 0.613 ± 0.021 | 0.649 ± 0.032a | 00.685 ± 0.069 |
| PGE1 | 0.332 ± 0.059 | 0.288 ± 0.037 | 0.633 ± 0.250 | 0.532 ± 0.105 | 0.648 ± 0.167 | 1.239 ± 0.864 |
| Resolvin D1 | 0.516 ± 0.012 | 0.509 ± 0.008 | 0.506 ± 0.012 | 0.520 ± 0.017 | 0.514 ± 0.009 | 0.495 ± 0.006 |
| LTE4 | 0.928 ± 0.107 | 0.959 ± 0.151 | 0.762 ± 0.072 | 12.560 ± 1.089 | 1.879 ± 0.285a | 1.411 ± 0.600 |
| (±)14,15-DHET | 0.671 ± 0.018 | 0.639 ± 0.017 | 0.626 ± 0.021 | 0.664 ± 0.100 | 0.643 ± 0.026 | 0.678 ± 0.021 |
| (±)11,12-DHET | 0.761 ± 0.017 | 0.725 ± 0.016 | 0.757 ± 0.047 | 0.745 ± 0.010 | 0.729 ± 0.012 | 0.788 ± 0.027 |
| (±)8,9-DHET | 0.717 ± 0.052 | 0.655 ± 0.015 | 0.692 ± 0.052 | 0.697 ± 0.048 | 0.713 ± 0.021 | 0.881 ± 0.102 |
| (±)14(15)-EET | 0.458 ± 0.028 | 0.403 ± 0.009 | 0.458 ± 0.024 | 0.479 ± 0.044 | 0.432 ± 0.026 | 0.468 ± 0.032 |
| (±)8(9)-EET | 0.044 ± 0.014 | 0.111 ± 0.059 | 0.123 ± 0.077 | 0.182 ± 0.067 | 0.132 ± 0.073 | 0.048 ± 0.007 |
| 20-HETE | 48.113 ± 0.951 | 47.770 ± 1.245 | 46.796 ± 1.049 | 54.631 ± 5.502 | 52.000 ± 3.387 | 51.376 ± 2.587 |
| 15-HETE | 8.658 ± 0.492 | 8.344 ± 0.194 | 9.514 ± 0.749 | 9.049 ± 0.390 | 8.850 ± 0.367 | 10.759 ± 1.188 |
| 12-HETE | 26.115 ± 3.799 | 24.261 ± 1.304 | 30.636 ± 1.518 | 30.433 ± 3.028 | 27.678 ± 0.959 | 40.769 ± 5.260 |
| 5-HETE | 15.490 ± 0.906 | 15.002 ± 0.897 | 16.287 ± 1.389 | 16.100 ± 2.337 | 15.301 ± 1.812 | 17.798 ± 1.123 |
| EPA | 110.120 ± 4.666 | 108.113 ± 5.052 | 108.650 ± 6.474 | 120.756 ± 4.953 | 116.367 ± 3.977 | 123.173 ± 3.299 |
| DHA | 541.748 ± 25.043 | 528.699 ± 24.293 | 531.345 ± 24.191 | 528.597 ± 16.190 | 535.621 ± 26.864 | 539.104 ± 25.425 |
| AA | 1,416.140 ± 136.869 | 1,346.699 ± 115.662 | 1,413.377 ± 126.250 | 1,431.009 ± 165.233 | 1,391.521 ± 170.268 | 1,547.321 ± 140.321 |
| DHGLA | 461.100 ± 37.288 | 438.201 ± 39.349 | 457.010 ± 33.448 | 472.864 ± 54.049 | 427.581 ± 46.155 | 486.472 ± 17.667 |
Media from MΦWT and MΦTg treated with vehicle, sPLA2 inhibitor (LY315920), or cPLA2 inhibitor (CAY 10502) only were processed for lipidomics analyses. The data are mean ± SEM.
Significantly different from WT-sPLA2 inhibitor, P < 0.05.
TABLE 5.
Effects of sPLA2 and cPLA2 inhibition on eicosanoid production from classically activated MΦWT and MΦTg
| Lipid | MΦWT (n = 4) (pmol lipid/1e+06) | MΦTg (n = 3) (pmol lipid/1e+06) | ||||
| IFNγ + LPS alone | +sPLA2 Inhibitor | +cPLA2 Inhibitor | IFNγ + LPS alone | +sPLA2 Inhibitor | +cPLA2 Inhibitor | |
| 6-keto PGF1α | 9.561 ± 0.967 | 9.263 ± 2.300 | 10.615 ± 2.484 | 25.550 ± 5.683a | 24.711 ± 6.504 | 30.651 ± 11.155 |
| TXB2 | 5.483 ± 0.119 | 5.465 ± 0.425 | 5.607 ± 0.793 | 7.213 ± 0.608a | 6.680 ± 0.422 | 7.672 ± 1.082 |
| PGE2 | 12.345 ± 1.661 | 11.542 ± 2.797 | 18.140 ± 5.066 | 29.945 ± 5.518a | 28.940 ± 7.541 | 43.413 ± 17.386 |
| PGA2 | 2.745 ± 0.263 | 2.521 ± 0410 | 3.436 ± 0.707 | 5.029 ± 0.923 | 4.798 ± 1.116 | 6.769 ± 2.360 |
| PGD2 | 0.406 ± 0.013 | 0.372 ± 0.027 | 0.469 ± 0.101 | 0.629 ± 0.177 | 0.688 ± 0.237 | 1.239 ± 0.386 |
| PGF2α | 0.659 ± 0.040 | 0.595 ± 0.060 | 0.665 ± 0.137 | 0.882 ± 0.105 | 0.865 ± 0.123 | 1.002 ± 0.226 |
| PGE1 | 1.862 ± 0.264 | 1.791 ± 0.509 | 3.129 ± 1.055 | 5.253 ± 0.949a | 4.707 ± 1.301 | 7.204 ± 2.719 |
| Resolvin D1 | 0.497 ± 0.020 | 0.497 ± 0.012 | 0.519 ± 0.025 | 0.519 ± 0.016 | 0.493 ± 0.006 | 0.513 ± 0.017 |
| LTE4 | 0.993 ± 0.182 | 0.663 ± 0.170 | 0.631 ± 0.165 | 1.544 ± 0.471 | 1.555 ± 0.135* | 0.776 ± 0.190 |
| (±)14,15-DHET | 0.652 ± 0.022 | 0.668 ± 0.044 | 0.602 ± 0.049 | 0.701 ± 0.033 | 0.700 ± 0.030 | 0.693 ± 0.030 |
| (±)11,12-DHET | 0.783 ± 0.062 | 0.792 ± 0.007 | 0.841 ± 0.057 | 0.823 ± 0.037 | 0.852 ± 0.015 | 0.865 ± 0.037 |
| (±)8,9-DHET | 0.683 ± 0.016 | 0.669 ± 0.049 | 0.833 ± 0.180 | 0.716 ± 0.036 | 0.906 ± 0.132 | 0.934 ± 0.094 |
| (±)14(15)-EET | 0.473 ± 0.012 | 0.440 ± 0.019 | 0.525 ± 0.027 | 0.446 ± 0.019 | 0.464 ± 0.035 | 0.573 ± 0.050 |
| (±)8(9)-EET | 0.127 ± 0.089 | 0.063 ± 0.011 | 0.031 ± 0.009 | 0.217 ± 0.086 | 0.049 ± 0.011 | 0.046 ± 0.009 |
| 20-HETE | 49.441 ± 1.344 | 48.211 ± 0.504 | 51.483 ± 1.518 | 48.650 ± 3.136 | 49.563 ± 1.295 | 53.640 ± 3.342 |
| 15-HETE | 10.040 ± 0.608 | 9.840 ± 0.902 | 10.632 ± 1.832 | 13.091 ± 1.920 | 12.933 ± 1.949 | 14.962 ± 2.682 |
| 12-HETE | 27.123 ± 2.916 | 25.808 ± 1.084 | 30.599 ± 3.665 | 37.284 ± 8.799 | 40.913 ± 6.892 | 49.287 ± 11.085 |
| 5-HETE | 14.827 ± 1.023 | 14.756 ± 0.983 | 15.383 ± 0.506 | 14.036 ± 0.558 | 14.717 ± 1.211 | 17.931 ± 1.451 |
| EPA | 97.624 ± 9.624 | 89.281 ± 6.085 | 87.666 ± 1.709 | 92.912 ± 4.441 | 92.378 ± 5.735 | 96.929 ± 5.827 |
| DHA | 531.386 ± 27.303 | 512.364 ± 27.212 | 497.151 ± 34.632 | 517.238 ± 20.479 | 497.449 ± 34.805 | 519.231 ± 29.269 |
| AA | 1,326.516 ± 36.127 | 1,276.119 ± 58.193 | 1,300.538 ± 27.380 | 1,241.023 ± 39.727 | 1,247.448 ± 62.748 | 1,557.577 ± 174.420 |
| DHGLA | 428.036 ± 32.703 | 395.946 ± 21.708 | 410.002 ± 20.994 | 401.953 ± 14.966 | 392.479 ± 31.565 | 482.797 ± 49.330 |
Media from MΦWT and MΦTg treated with IFNγ + LPS ± sPLA2 (LY315920) or cPLA2 (CAY 10502) inhibitor were processed for lipidomics analyses. The data are mean ± SEM.
Significantly different from WT-FNγ + LPS, P < 0.05.
Phenotype comparison of MΦWT and MΦTg
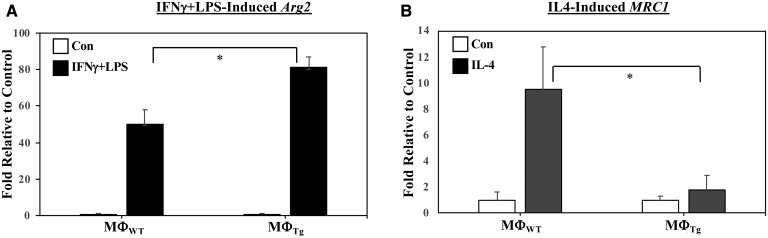
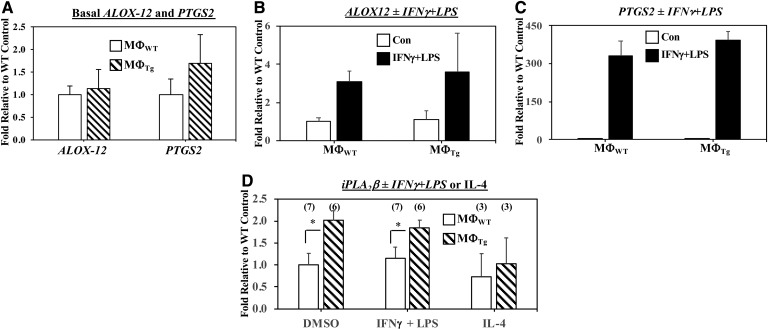
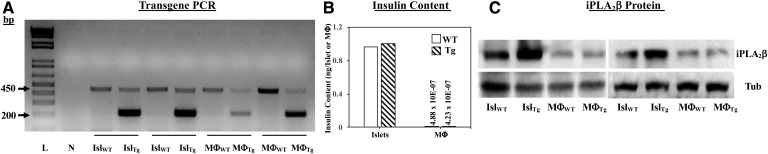
In view of the observations that MΦTg exhibit a higher inflammatory state, we assessed macrophage phenotype with activation. We found that classical activation of MΦTg promoted a greater induction of the M1 marker Arg2 (Fig. 11A), while the M2 marker MRC1 was reduced with alternative activation (Fig. 11B) relative to MΦWT. Whereas, induction of Arg2 in MΦWT and MΦTg by alternative activation was not different, induction of MRC1 by classical activation was reduced in the MΦTg (supplemental Fig. S3C, D). These findings are consistent with a heightened pro-inflammatory and reduced anti-inflammatory lipid profile in MΦTg. No differences were observed in the expression of ALOX-12 or PTGS2 mRNA, which encode 12-LO and COX2, respectively, in MΦWT and MΦTg under basal (Fig. 12A) or activated (Fig. 12B, C) conditions. Under both basal and activated conditions, iPLA2β mRNA was modestly and similarly increased in MΦTg (Fig. 12D) relative to MΦWT. To determine whether the transgene is induced in MΦTg, PCR, insulin content, and iPLA2β immunoblotting analyses were performed in islets and macrophages isolated from WT and Tg mice. As expected, transgene expression was evident in both islets and macrophages from Tg mice but not from WT mice (Fig. 13A). However, as islet β-cells but not macrophages produce insulin (Fig. 13B), overexpression of iPLA2β protein was only detected in the islets and not in the MΦTg relative to MΦWT (Fig. 13C). These findings suggest that signals generated by iPLA2β-overexpressing β-cells, upon appropriate stimulation, prime macrophages to adopt an M1 phenotype, independent of transgene leakage.
Fig. 11.
Induction of M1 (Arg2) and M2 (MRC1) markers in MΦWT and MΦTg. Peritoneal macrophages isolated from 8-week-old WT and Tg mice were treated with vehicle [control (Con)], IFNγ + LPS, or IL-4. The cells were harvested and processed for real-time qPCR analyses. A: Arg2 (Control MΦWT and MΦTg 2−ΔΔCT, 2.21 ± 0.86 and 2.41 ± 0.39). B: MRC1 (Control MΦWT and MΦTg 2−ΔΔCT, 4.81 ± 2.91 and 15.38 ± 4.28). Data are mean ± SEM of fold-change relative to Con determined from four independent experiments. *MΦTg significantly different from MΦWT, P < 0.05.
Fig. 12.
Comparisons of PLA2, ALOX-12, and PTGS2 mRNA in MΦWT and MΦTg. Peritoneal macrophages isolated from 8-week-old WT and Tg mice were treated with vehicle (Control) or activated with IFNγ + LPS or IL-4. The cells were harvested and processed for real-time qPCR analyses for ALOX-12 (A, B) and PGST2 (A, C) ± IFNγ + LPS, and iPLA2β ± IFNγ + LPS or IL-4 (D). A: Control ALOX-12 and PTGS2 2−ΔΔCT, 6.46 ± 1.17 and 2.98 ± 1.04. B: Control MΦWT 2−ΔΔCT, 6.46 ± 1.17. C: Control MΦWT 2−ΔΔCT, 2.98 ± 1.04. D: Control MΦWT 2−ΔΔCT, 1.01 ± 0.084. Data are mean ± SEM of fold-change relative to control determined from four independent experiments. *MΦTg significantly different from MΦWT, P < 0.05.
Fig. 13.
Transgene verification. Pancreatic islets and peritoneal macrophages were isolated from 8-week-old WT and Tg mice for the following analyses: A: Transgene PCR. Analyses were performed using cDNA from WT or RIP.iPLA2β.TG (Tg) mice, and primers that amplify sequence that either spans junction between iPLA2β and globin. cDNA (200 bp product, lower band) or is within internal control Fabpi (450 bp product, upper band). Lane L, molecular weight ladder; lane N, control reaction without template. B: Insulin content was determined by ELISA using acid-extracted samples. C: iPLA2β and tubulin (loading control) immunoblotting were performed using 30 μg protein. The data were obtained from two independent replicates.
DISCUSSION
Inflammatory processes play critical roles in promoting autoimmune-mediated β-cell death leading to T1D; however, the underlying mechanisms promoting inflammation are not well understood. Eicosanoids are recognized contributors to inflammatory responses (4, 59, 60) and these bioactive lipids are generated via metabolism of AA, following its release from membrane glycerophospholipids by a PLA2-mediated mechanism. We reported that activation of iPLA2β participates in β-cell death due to ER stress and pro-inflammatory cytokines (44, 61, 62), which are key contributors to β-cell demise leading to T1D. We also observed that activation of iPLA2β promotes an M1 inflammatory macrophage phenotype, whereas iPLA2β deficiency favors an M2 anti-inflammatory phenotype (42). Consistent with these reports, selective inhibition of iPLA2β was found to markedly reduce insulitis, CD4+ T-cell and B-cell responses, and diabetes incidence in the nonobese diabetic (NOD) mouse model, a spontaneous autoimmune diabetes-prone mouse model of T1D (20). Collectively, these findings suggested a prominent role for iDLs in promoting inflammatory responses.
Eicosanoids generated via metabolism of AA by COX and 12-LO are recognized to play significant roles in a variety of inflammatory diseases, including diabetes, cancer, atherosclerosis, osteoporosis, and EAE (3, 5, 41, 60, 63–66). The most potent of the inflammatory lipids include PGs, LTs, HETEs, and DHETs. Roles ascribed to them include promoting CD4 Th1/Th17 differentiation (67), inhibition of Tr1 differentiation (68), inducing NF-κB (69), inhibiting Treg cell differentiation (70), modulating local activation of T-cells (71), inducing NO (72), participating in oxidative stress pathways (73), increasing expression of cytokine genes and monocyte chemoattractant protein 1 (74, 75), and reducing inflammation-resolving processes (76–79). In contrast, other PGs (i.e., PGE1), SPMs, and EETs are involved in resolving inflammation (50, 80–82).
Our collection of earlier findings suggests that iDLs are key contributors to inflammation, β-cell death, and subsequent development of T1D. While eicosanoids have been linked with inflammation development (46, 83), to date, very little is known about the eicosanoid profile of activated macrophages and whether iDLs contribute to an inflammatory state. Therefore, in view of our findings of a link between iPLA2β and macrophage polarization and responses, we utilized a targeted lipidomics approach with commercially available eicosanoid standards and WT, iPLA2β−/− (KO), and RIP.iPLA2β.Tg (Tg) mouse models to identify iDLs generated by activated macrophages. Our studies revealed two salient and previously undescribed features: 1) the predominant anti-inflammatory M2 phenotype due to iPLA2β-deficiency is associated with reduced generation of select pro-inflammatory eicosanoids and increased production of the SPM, resolvin D2; and 2) greater production of select pro-inflammatory eicosanoids and reduced resolvin D2 from MΦTg is associated with macrophage polarization toward the inflammatory M1 phenotype. These findings, for the first time, reveal an association between selective changes in eicosanoids and SPMs with macrophage polarization and, further, that the relevant lipid species are modulated by iPLA2β activity. Most importantly, our findings unveil the possibility that events triggered in β-cells can modulate macrophage responses. This is supported by observations in the RIP.iPLA2β.Tg model with a select overexpression of iPLA2β in β-cells; yet, macrophages from these mice exhibit an opposite spectrum relative to MΦKO. To our knowledge, this is the first demonstration of intercellular signaling, in particular iPLA2β-derived intercellular signaling, originating from β-cells affecting macrophage function.
iPLA2β deficiency, verified by genotype and real-time qPCR analyses, was associated with a significant reduction in the pool of pro-inflammatory PGs with a selective decrease in the abundance of PGE2. Other recognized inflammatory lipids, including LTs and DHETs, were not significantly affected by iPLA2β deficiency. While there were no significant differences in the induction of anti-inflammatory PGs by MΦWT and MΦKO, production of resolvin D2 by MΦKO in response to classical activation was significantly higher relative to MΦWT. These lipid changes are associated with decreased Arg2 (M1 marker) and increased MRC1 (M2 marker) in MΦKO. Resolvin D2 arises from the metabolism of DHA, and lipids containing DHA are substrates for iPLA2β (84). However, sPLA2s exhibit greater activity toward lipids containing DHA than does iPLA2β (84), and the group V and IIa sPLA2s are activated during inflammation (85, 86). Consistent with this premise, it has been suggested that at the onset of inflammation, iPLA2β activity predominates and leads to increased production of inflammatory lipids (2). However, a subsequent resolution phase is unmasked during which groups IIA and V sPLA2s are activated. Taken together with our present findings, it is likely that iPLA2β deficiency is permissive for sPLA2 activation to affect resolution through generation of resolvin D2. This latter phase appears to occur in the absence of increased expression of sPLA2s, at least at the time point studied. Acknowledging that in vitro systems are artificial and limited, we speculate that the in vivo inflammatory landscape gives rise to a more dramatic lipid profile.
Intriguingly, we found that the production of pro-inflammatory lipids by MΦTg was increased. These included 6-keto PGF1α, PGE2, and LTB4. Concurrently, there was increased production of anti-inflammatory PGE1 but decreased production of PGD2, suggesting differential regulation of pathways leading to generation of these PGs during inflammation. The increase in PGE1 most likely reflects an attempt at resolution (87). Interestingly, in contrast to MΦKO, resolvin D2 production by MΦTg was not altered with classical activation, but was significantly reduced under alternative activation. This suggests a decrease in the metabolism of DHA, which has been reported to increase susceptibility to inflammation (88, 89). In our earlier study, we observed that the polarization toward M2 macrophage phenotype with iPLA2β deficiency was not a direct effect on iDL production, but was most likely due to the overall decrease in an inflammatory state, which is permissive for the evolution of anti-inflammatory processes (42). The differential production of resolvin D2 by MΦKO and MΦTg supports this possibility; in the absence of iPLA2β, there is a reduced inflammatory state, which unmasks pathways leading to production of resolvin D2; whereas a heightened inflammatory status in MΦTg mitigates such processes. Overall, our lipidomics analyses suggest a pro-inflammatory status in MΦTg and, consistently, polarization of MΦTg favored an M1 over M2 phenotype.
Examination of potential involvement of other PLA2s expressed in macrophages revealed an absence in induction of sPLA2 GV or cPLA2α in activated MΦTg. Because MΦTg exhibited more profound lipid changes with classical activation, we tested the impact of inhibiting sPLA2s or cPLA2α on the lipid profile under this condition. Production of eicosanoids by MΦWT or MΦTg under basal or activated conditions were found to be similar following exposure to CAY 10502, which inhibits cPLA2α, or LY315920, which inhibits mGIB, mGIIA, mGIID, mGIIE, mGV, and mGX sPLA2 (personal communication). In the presence of LY315920, basal production of pro-inflammatory PGs by MΦTg was found to be higher relative to MΦWT, most likely a reflection of modest decreases in their abundances in the WT group. Nevertheless, the inability of either CAY 10502 or LY315920 to mitigate the select lipid changes in MΦTg relative to MΦWT suggests that neither sPLA2s nor cPLA2α contribute to the pro-inflammatory lipid profile in MΦTg during the activated state during the time course of our studies. Additionally, expression of ALOX-12 and PTGS2 mRNA, which encode 12-LO and COX2 proteins, respectively, were similar in the MΦWT and MΦTg, suggesting that they likely are not responsible for the differences between the two groups.
These studies add to a growing body of literature indicating a role for iPLA2β in inflammatory disease, particularly in T1D. We previously reported that glucose-stimulated insulin secretion (GSIS) is accompanied by iPLA2β-mediated hydrolysis of AA from β-cell membrane glycerophospholipids and that overexpression of iPLA2β leads to higher GSIS (6, 7, 9). As reported in those studies, the RIP.iPLA2β.Tg mice exhibited lower circulating glucose levels and amplified GSIS insulin (43). Further, whereas survival of β-cells (INS-1 or in RIP.iPLA2β.Tg islets) was unaffected by iPLA2β overexpression under basal conditions, ER stress and pro-inflammatory cytokines amplified apoptosis of the β-cells (44, 48, 61, 90–92). It is important to note that the impact of iPLA2β on insulin secretion is evident within 1 h of glucose stimulation and is not associated with an increase in iPLA2β expression. In contrast, its effect on β-cell survival is manifested after several hours of exposure to ER stressors or pro-inflammatory cytokines and is associated with induction of iPLA2β (44, 48, 61, 62, 90, 92, 93).
In this regard, it was intriguing to find that iPLA2β mRNA was modestly higher in MΦTg relative to MΦWT. However, this did not translate to greater protein expression or increased production of lipids by resting MΦTg. In contrast, classical activation led to significantly higher production of the select lipids by MΦTg, and this may be a consequence of increased availability of substrates derived through iPLA2β-mediated hydrolysis of sn-2 fatty acyl substituents for metabolism by downstream enzymes. This is analogous to previous reports of minimal effects of higher iPLA2β expression in β-cells under basal conditions, but amplified in response to stress (43, 44), suggesting that the iPLA2β overexpressing β-cells are sensitized to respond to stimulation. Our current observations raise the possibility that there may be cross-talk between β-cells and macrophages in vivo, to the extent that MΦTg are predisposed to exhibiting enhanced responses to classical activation leading to an inflammatory landscape. While transfer of stress-induced signals between cells of different types has been suggested (60, 94, 95), to our knowledge, this is the first demonstration of lipid signaling generated by β-cells having an impact on an immune cell that elicits inflammatory consequences.
While the present study did not discern the intercellular signaling molecules in RIP.iPLA2β.Tg mice, it is likely that they are derived directly or indirectly through an increase in iDLs, as a consequence of increased iPLA2β expression. In support of this, recent studies reveal that inhibition of iPLA2β activity mitigates inflammatory processes in different cell systems (18, 78, 96–98), raising the possibility that bioactive lipids generated through β-cell-iPLA2β activity or factors arising from the effects of such lipids may serve as potential candidate intercellular signals. For instance, insulin secretion from β-cells is accompanied by a parallel iPLA2β-mediated hydrolysis of AA from β-cell membranes and generation of PGE2, which is mitigated by selective inhibition of iPLA2β (7, 99). Mice with selective overexpression of iPLA2β in β- cells exhibit lower basal blood glucose (43). Further β-cells in these mice express higher pPERK and generate more ceramides under basal conditions (44). This leads to the possibility that circulating insulin, PGE2 or other iDLs, ER stress factors, or ceramides originating from β-cells could prime macrophages to respond more robustly to classical activation. This is supported by the greater induction of Arg2 and lower induction of MRC1 in MΦTg, which sets the table for the onset and progression of an inflammatory state. Further detailed studies are needed to identify the specific signals from β-cells that affect macrophages and they are currently underway.
The importance of intercellular signaling originating from β-cells is realized in view of findings that β-cells in the diabetes-prone NOD mice express higher iPLA2β during the prediabetic phase relative to β-cells in spontaneous diabetes-resistant models (20). This early phase encompasses spontaneous evolution of inflammatory signaling leading to the onset of insulitis. Our findings offer the possibility that higher activation of iPLA2β in the β-cells of NOD mice during this phase contributes signals that can be transmitted to infiltrating leukocytes to initiate immune responses that work in concert with events in the β-cells to amplify the onset and progression of inflammation that subsequently induces β-cell death and T1D. As discussed in a recent review (100), inflammation and β-cell dysfunction are also associated with the development of insulin resistance, and iPLA2β has been implicated in this process as well, suggesting that signaling between β-cells and immune cells must also be considered as a contributor to the development of T2D.
In summary, our findings reveal that iPLA2β modulates macrophage production of select lipids, which collectively are recognized to be associated with an enhanced inflammatory state. The selective nature of the lipid changes suggests that the impact of iPLA2β is not broad but is specific to certain pathways that lead to the generation of such lipids. We find that induction of an M1 inflammatory macrophage phenotype is associated with increased production of iPLA2β-derived pro-inflammatory lipids and that responses in macrophages can be modulated by iPLA2β-derived signals of β-cell origin. These findings raise the importance of assessing more carefully the mechanisms governing modulation of the function of immune cells constituting an inflammatory landscape.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Jason W. Ashley and Dr. Robert N. Bone for their advice on macrophage assays and Dr. Gerard Lambeau for his advice on sPLA2 inhibitors.
Footnotes
Abbreviations:
- AA
- arachidonic acid
- Arg2
- arginase 2
- COX
- cyclooxygenase
- cPLA2
- cytosolic phospholipase A2
- DHET
- dihydroxyeicosatrienoic acid
- DHGLA
- dihomo-γ-linolenic acid
- EET
- epoxyeicosatrienoic acid
- Fabpi
- fatty acid-binding protein, intestinal
- GSIS
- glucose-stimulated insulin secretion
- iDL
- Ca2+-independent phospholipase A2β-derived lipid
- IFNγ
- interferon-γ
- IL
- interleukin
- iPLA2β
- Ca2+-independent phospholipase A2β
- LO
- lipoxygenase
- LPS
- lipopolysaccharide
- LT
- leukotriene
- Mrc1
- mannose receptor C type 1
- MΦKO
- macrophages isolated from KO (iPLA2β−/−) mice
- MΦTg
- macrophages isolated from transgenic (RIP.iPLA2β.Tg) mice
- MΦWT
- macrophages isolated from WT mice
- NOD
- nonobese diabetic
- PLA2
- phospholipase A2
- PG
- prostaglandin
- RIP
- rat insulin promoter
- qPCR
- quantitative PCR
- sPLA2
- secretory phospholipase A2
- SPM
- specialized pro-resolving lipid mediator
- Tg
- transgenic
- TXB2
- thromboxane B2
- UPLC ESI-MS/MS
- ultra-performance LC ESI-MS/MS
This work was supported by funding from: the University of Alabama at Birmingham Department of Cell, Developmental, and Integrative Biology; the Iacocca Family Foundation, the King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia (A.A.); Veterans Administration Grants BX001792 (C.E.C.) and 13F-RCS-002 (C.E.C.); National Institutes of Health Grants R01 DK69455 (S.R.), R01 DK110292 (S.R.), Diversity Research Supplement (T.D.W.), R01 HL125353 (C.E.C.), U01 HD087198 (C.E.C.), RR031535 (C.E.C.), and R01 AI139072 (C.E.C.); and University of South Florida funds [initiative 0131845 (M.A.P.)]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare that they have no conflicts of interest with the contents of this article.
The online version of this article (available at https://www.jlr.org) contains a supplement.
REFERENCES
- 1.Atkinson M. A., and Maclaren N. K.. 1994. The pathogenesis of insulin-dependent diabetes mellitus. N. Engl. J. Med. 331: 1428–1436. [DOI] [PubMed] [Google Scholar]
- 2.Gilroy D. W., Newson J., Sawmynaden P., Willoughby D. A., and Croxtall J. D.. 2004. A novel role for phospholipase A2 isoforms in the checkpoint control of acute inflammation. FASEB J. 18: 489–498. [DOI] [PubMed] [Google Scholar]
- 3.Khanapure S. P., Garvey D. S., Janero D. R., and Letts L. G.. 2007. Eicosanoids in inflammation: biosynthesis, pharmacology, and therapeutic frontiers. Curr. Top. Med. Chem. 7: 311–340. [DOI] [PubMed] [Google Scholar]
- 4.Basu S., Larsson A., Vessby J., Vessby B., and Berne C.. 2005. Type 1 diabetes is associated with increased cyclooxygenase- and cytokine-mediated inflammation. Diabetes Care. 28: 1371–1375. [DOI] [PubMed] [Google Scholar]
- 5.Luo P., and Wang M. H.. 2011. Eicosanoids, beta-cell function, and diabetes. Prostaglandins Other Lipid Mediat. 95: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramanadham S., Gross R. W., Han X., and Turk J.. 1993. Inhibition of arachidonate release by secretagogue-stimulated pancreatic islets suppresses both insulin secretion and the rise in β-cell cytosolic calcium ion concentration. Biochemistry. 32: 337–346. [DOI] [PubMed] [Google Scholar]
- 7.Ramanadham S., Bohrer A., Mueller M., Jett P., Gross R. W., and Turk J.. 1993. Mass spectrometric identification and quantitation of arachidonate-containing phospholipids in pancreatic islets: prominence of plasmenylethanolamine molecular species. Biochemistry. 32: 5339–5351. [DOI] [PubMed] [Google Scholar]
- 8.Ramanadham S., Gross R., and Turk J.. 1992. Arachidonic acid induces an increase in the cytosolic calcium concentration in single pancreatic islet beta cells. Biochem. Biophys. Res. Commun. 184: 647–653. [DOI] [PubMed] [Google Scholar]
- 9.Ramanadham S., Hsu F-F., Bohrer A., Ma Z., and Turk J.. 1999. Studies of the role of group VI phospholipase A2 in fatty acid incorporation, phospholipid remodeling, lysophosphatidylcholine generation, and secretagogue-induced arachidonic acid release in pancreatic islets and insulinoma cells. J. Biol. Chem. 274: 13915–13927. [DOI] [PubMed] [Google Scholar]
- 10.Nowatzke W., Ramanadham S., Ma Z., Hsu F. F., Bohrer A., and Turk J.. 1998. Mass spectrometric evidence that agents that cause loss of Ca2+ from intracellular compartments induce hydrolysis of arachidonic acid from pancreatic islet membrane phospholipids by a mechanism that does not require a rise in cytosolic Ca2+ concentration. Endocrinology. 139: 4073–4085. [DOI] [PubMed] [Google Scholar]
- 11.Ramanadham S., Song H., Hsu F. F., Zhang S., Crankshaw M., Grant G. A., Newgard C. B., Bao S., Ma Z., and Turk J.. 2003. Pancreatic islets and insulinoma cells express a novel isoform of group VIA phospholipase A2 (iPLA2β) that participates in glucose-stimulated insulin secretion and is not produced by alternate splicing of the iPLA2β transcript. Biochemistry. 42: 13929–13940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramanadham S., Wolf M. J., Jett P. A., Gross R. W., and Turk J.. 1994. Characterization of an ATP-stimulatable Ca2+-independent phospholipase A2 from clonal insulin-secreting HIT cells and rat pancreatic islets: a possible molecular component of the beta-cell fuel sensor. Biochemistry. 33: 7442–7452. [DOI] [PubMed] [Google Scholar]
- 13.Xie L., Zhu X., Hu Y., Li T., Gao Y., Shi Y., and Tang S.. 2008. Mitochondrial DNA oxidative damage triggering mitochondrial dysfunction and apoptosis in high glucose-induced HRECs. Invest. Ophthalmol. Vis. Sci. 49: 4203–4209. [DOI] [PubMed] [Google Scholar]
- 14.Rahnema P., Shimoni Y., and Nygren A.. 2011. Reduced conduction reserve in the diabetic rat heart: role of iPLA2 activation in the response to ischemia. Am. J. Physiol. Heart Circ. Physiol. 300: H326–H334. [DOI] [PubMed] [Google Scholar]
- 15.Ayilavarapu S., Kantarci A., Fredman G., Turkoglu O., Omori K., Liu H., Iwata T., Yagi M., Hasturk H., and Van Dyke T. E.. 2010. Diabetes-Induced oxidative stress is mediated by Ca2+-independent phospholipase A2 in neutrophils. J. Immunol. 184: 1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gil-de-Gómez L., Astudillo A. M., Guijas C., Magrioti V., Kokotos G., Balboa M. A., and Balsinde J.. 2014. Cytosolic group IVA and calcium-independent group VIA phospholipase A2s act on distinct phospholipid pools in zymosan-stimulated mouse peritoneal macrophages. J. Immunol. 192: 752–762. [DOI] [PubMed] [Google Scholar]
- 17.Lee S. H., Park D. W., Park S. C., Park Y. K., Hong S. Y., Kim J. R., Lee C. H., and Baek S. H.. 2009. Calcium-independent phospholipase A2beta-Akt signaling is involved in lipopolysaccharide-induced NADPH oxidase 1 expression and foam cell formation. J. Immunol. 183: 7497–7504. [DOI] [PubMed] [Google Scholar]
- 18.Tan C., Day R., Bao S., Turk J., and Zhao Q. D.. 2014. Group VIA phospholipase A2 mediates enhanced macrophage migration in diabetes mellitus by increasing expression of nicotinamide adenine dinucleotide phosphate oxidase 4. Arterioscler. Thromb. Vasc. Biol. 34: 768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bao S., Li Y., Lei X., Wohltmann M., Jin W., Bohrer A., Semenkovich C. F., Ramanadham S., Tabas I., and Turk J.. 2007. Attenuated free cholesterol loading-induced apoptosis but preserved phospholipid composition of peritoneal macrophages from mice that do not express group VIA phospholipase A2. J. Biol. Chem. 282: 27100–27114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bone R. N., Gai Y., Magrioti V., Kokotou M. G., Ali T., Lei X., Tse H. M., Kokotos G., and Ramanadham S.. 2015. Inhibition of Ca2+-independent phospholipase A2β (iPLA2β) ameliorates islet infiltration and incidence of diabetes in NOD mice. Diabetes. 64: 541–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S., Xie Z., Zhao Q., Pang H., Turk J., Calderon L., Su W., Zhao G., Xu H., Gong M. C., et al. . 2012. Smooth muscle-specific expression of calcium-independent phospholipase A2β (iPLA2β) participates in the initiation and early progression of vascular inflammation and neointima formation. J. Biol. Chem. 287: 24739–24753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ming W. J., Bersani L., and Mantovani A.. 1987. Tumor necrosis factor is chemotactic for monocytes and polymorphonuclear leukocytes. J. Immunol. 138: 1469–1474. [PubMed] [Google Scholar]
- 23.Held W., MacDonald H. R., Weissman I. L., Hess M. W., and Mueller C.. 1990. Genes encoding tumor necrosis factor alpha and granzyme A are expressed during development of autoimmune diabetes. Proc. Natl. Acad. Sci. USA. 87: 2239–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrera P. L., Harlan D. M., and Vassalli P.. 2000. A mouse CD8 T cell-mediated acute autoimmune diabetes independent of the perforin and Fas cytotoxic pathways: possible role of membrane TNF. Proc. Natl. Acad. Sci. USA. 97: 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lauber K., Bohn E., Krober S. M., Xiao Y. J., Blumenthal S. G., Lindemann R. K., Marini P., Wiedig C., Zobywalski A., Baksh S., et al. . 2003. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 113: 717–730. [DOI] [PubMed] [Google Scholar]
- 26.Zhao X., Wang D., Zhao Z., Xiao Y., Sengupta S., Xiao Y., Zhang R., Lauber K., Wesselborg S., Feng L., et al. . 2006. Caspase-3-dependent activation of calcium-independent phospholipase A2 enhances cell migration in non-apoptotic ovarian cancer cells. J. Biol. Chem. 281: 29357–29368. [DOI] [PubMed] [Google Scholar]
- 27.Mishra R. S., Carnevale K. A., and Cathcart M. K.. 2008. iPLA2beta: front and center in human monocyte chemotaxis to MCP-1. J. Exp. Med. 205: 347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalyvas A., Baskakis C., Magrioti V., Constantinou-Kokotou V., Stephens D., Lopez-Vales R., Lu J. Q., Yong V. W., Dennis E. A., Kokotos G., et al. . 2009. Differing roles for members of the phospholipase A2 superfamily in experimental autoimmune encephalomyelitis. Brain. 132: 1221–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H., Zhao Z., Antalis C., Zhao Z., Emerson R., Wei G., Zhang S., Zhang Z. Y., and Xu Y.. 2011. Combination therapy of an inhibitor of group VIA phospholipase A2 with paclitaxel is highly effective in blocking ovarian cancer development. Am. J. Pathol. 179: 452–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McHowat J., Gullickson G., Hoover R. G., Sharma J., Turk J., and Kornbluth J.. 2011. Platelet-activating factor and metastasis: calcium-independent phospholipase A2beta deficiency protects against breast cancer metastasis to the lung. Am. J. Physiol. Cell Physiol. 300: C825–C832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicotera T. M., Schuster D. P., Bourhim M., Chadha K., Klaich G., and Corral D. A.. 2009. Regulation of PSA secretion and survival signaling by calcium-independent phopholipase A2β in prostate cancer cells. Prostate. 69: 1270–1280. [DOI] [PubMed] [Google Scholar]
- 32.Scuderi M. R., Anfuso C. D., Lupo G., Motta C., Romeo L., Guerra L., Cappellani A., Ragusa N., Cantarella G., and Alberghina M.. 2008. Expression of Ca2+-independent and Ca2+-dependent phospholipases A2 and cyclooxygenases in human melanocytes and malignant melanoma cell lines. Biochim. Biophys. Acta. 1781: 635–642. [DOI] [PubMed] [Google Scholar]
- 33.Padgett L. E., Broniowska K. A., Hansen P. A., Corbett J. A., and Tse H. M.. 2013. The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Ann. N. Y. Acad. Sci. 1281: 16–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., and Locati M.. 2004. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25: 677–686. [DOI] [PubMed] [Google Scholar]
- 35.Martinez F. O., and Gordon S.. 2014. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calderon B., Suri A., and Unanue E. R.. 2006. In CD4+ T-cell-induced diabetes, macrophages are the final effector cells that mediate islet beta-cell killing: studies from an acute model. Am. J. Pathol. 169: 2137–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parsa R., Andresen P., Gillett A., Mia S., Zhang X. M., Mayans S., Holmberg D., and Harris R. A.. 2012. Adoptive transfer of immunomodulatory M2 macrophages prevents type 1 diabetes in NOD mice. Diabetes. 61: 2881–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guimaraes J. P. T., Filgueiras L. R., Martins J. O., and Jancar S.. 2019. Leukotriene involvement in the insulin receptor pathway and macrophage profiles in muscles from type 1 diabetic mice. Mediators Inflamm. 2019: 4596127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duan L., Gan H., Arm J., and Remold H. G.. 2001. Cytosolic phospholipase A2 participates with TNF-alpha in the induction of apoptosis of human macrophages infected with Mycobacterium tuberculosis H37Ra. J. Immunol. 166: 7469–7476. [DOI] [PubMed] [Google Scholar]
- 40.Nikolic D. M., Gong M. C., Turk J., and Post S. R.. 2007. Class A scavenger receptor-mediated macrophage adhesion requires coupling of calcium-independent phospholipase A2 and 12/15-lipoxygenase to Rac and Cdc42 activation. J. Biol. Chem. 282: 33405–33411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaitseva L., Vaisburd M., Shaposhnikova G., and Mysyakin E.. 2000. Role of eicosanoids in regulation of macrophage phagocytic functions by platelet-activating factor during endotoxic shock. Bull. Exp. Biol. Med. 130: 879–881. [DOI] [PubMed] [Google Scholar]
- 42.Ashley J. W., Hancock W. D., Nelson A. J., Bone R. N., Tse H. M., Wohltmann M., Turk J., and Ramanadham S.. 2016. Polarization of macrophages toward M2 phenotype is favored by reduction in iPLA2β (Group VIA Phospholipase A2). J. Biol. Chem. 291: 23268–23281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bao S., Jacobson D. A., Wohltmann M., Bohrer A., Jin W., Philipson L. H., and Turk J.. 2008. Glucose homeostasis, insulin secretion, and islet phospholipids in mice that overexpress iPLA2β in pancreatic β-cells and in iPLA2beta-null mice. Am. J. Physiol. Endocrinol. Metab. 294: E217–E229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lei X., Bone R. N., Ali T., Wohltmann M., Gai Y., Goodwin K. J., Bohrer A. E., Turk J., and Ramanadham S.. 2013. Genetic modulation of islet β-cell iPLA2β expression provides evidence for its impact on β-cell apoptosis and autophagy. Islets. 5: 29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bao S., Miller D. J., Ma Z., Wohltmann M., Eng G., Ramanadham S., Moley K., and Turk J.. 2004. Male mice that do not express group VIA phospholipase A2 produce spermatozoa with impaired motility and have greatly reduced fertility. J. Biol. Chem. 279: 38194–38200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blaho V. A., Buczynski M. W., Brown C. R., and Dennis E. A.. 2009. Lipidomic analysis of dynamic eicosanoid responses during the induction and resolution of Lyme arthritis. J. Biol. Chem. 284: 21599–21612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simanshu D. K., Kamlekar R. K., Wijesinghe D. S., Zou X., Zhai X., Mishra S. K., Molotkovsky J. G., Malinina L., Hinchcliffe E. H., Chalfant C. E., et al. . 2013. Non-vesicular trafficking by a ceramide-1-phosphate transfer protein regulates eicosanoids. Nature. 500: 463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lei X., Bone R. N., Ali T., Zhang S., Bohrer A., Tse H. M., Bidasee K. R., and Ramanadham S.. 2014. Evidence of contribution of iPLA2β-mediated events during islet beta-cell apoptosis due to proinflammatory cytokines suggests a role for iPLA2β in T1D development. Endocrinology. 155: 3352–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramanadham S., Hsu F. F., Bohrer A., Nowatzke W., Ma Z., and Turk J.. 1998. Electrospray ionization mass spectrometric analyses of phospholipids from rat and human pancreatic islets and subcellular membranes: comparison to other tissues and implications for membrane fusion in insulin exocytosis. Biochemistry. 37: 4553–4567. [DOI] [PubMed] [Google Scholar]
- 50.Zhang G., Kodani S., and Hammock B. D.. 2014. Stabilized epoxygenated fatty acids regulate inflammation, pain, angiogenesis and cancer. Prog. Lipid Res. 53: 108–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balboa M. A., Balsinde J., Winstead M. V., Tischfield J. A., and Dennis E. A.. 1996. Novel group V phospholipase A2 involved in arachidonic acid mobilization in murine P388D1 macrophages. J. Biol. Chem. 271: 32381–32384. [DOI] [PubMed] [Google Scholar]
- 52.Morioka Y., Saiga A., Yokota Y., Suzuki N., Ikeda M., Ono T., Nakano K., Fujii N., Ishizaki J., Arita H., et al. . 2000. Mouse group X secretory phospholipase A2 induces a potent release of arachidonic acid from spleen cells and acts as a ligand for the phospholipase A2 receptor. Arch. Biochem. Biophys. 381: 31–42. [DOI] [PubMed] [Google Scholar]
- 53.Koratkar R., Pequignot E., Hauck W. W., and Siracusa L. D.. 2002. The CAST/Ei strain confers significant protection against Apc(Min) intestinal polyps, independent of the resistant modifier of Min 1 (Mom1) locus. Cancer Res. 62: 5413–5417. [PubMed] [Google Scholar]
- 54.MacPhee M., Chepenik K. P., Liddell R. A., Nelson K. K., Siracusa L. D., and Buchberg A. M.. 1995. The secretory phospholipase A2 gene is a candidate for the Mom1 locus, a major modifier of ApcMin-induced intestinal neoplasia. Cell. 81: 957–966. [DOI] [PubMed] [Google Scholar]
- 55.Markova M., Koratkar R. A., Silverman K. A., Sollars V. E., MacPhee-Pellini M., Walters R., Palazzo J. P., Buchberg A. M., Siracusa L. D., and Farber S. A.. 2005. Diversity in secreted PLA2-IIA activity among inbred mouse strains that are resistant or susceptible to Apc Min/+ tumorigenesis. Oncogene. 24: 6450–6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Triggiani M., Granata F., Giannattasio G., and Marone G.. 2005. Secretory phospholipases A2 in inflammatory and allergic diseases: not just enzymes. J. Allergy Clin. Immunol. 116: 1000–1006. [DOI] [PubMed] [Google Scholar]
- 57.Smart B. P., Oslund R. C., Walsh L. A., and Gelb M. H.. 2006. The first potent inhibitor of mammalian group X secreted phospholipase A2: elucidation of sites for enhanced binding. J. Med. Chem. 49: 2858–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barnett J. M., McCollum G. W., and Penn J. S.. 2010. Role of cytosolic phospholipase A2 in retinal neovascularization. Invest. Ophthalmol. Vis. Sci. 51: 1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanna V. S., and Hafez E. A. A.. 2018. Synopsis of arachidonic acid metabolism: A review. J. Adv. Res. 11: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Umamaheswaran S., Dasari S. K., Yang P., Lutgendorf S. K., and Sood A. K.. 2018. Stress, inflammation, and eicosanoids: an emerging perspective. Cancer Metastasis Rev. 37: 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lei X., Zhang S., Barbour S. E., Bohrer A., Ford E. L., Koizumi A., Papa F. R., and Ramanadham S.. 2010. Spontaneous development of endoplasmic reticulum stress that can lead to diabetes mellitus is associated with higher calcium-independent phospholipase A2 expression: a role for regulation by SREBP-1. J. Biol. Chem. 285: 6693–6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lei X., Zhang S., Bohrer A., Barbour S. E., and Ramanadham S.. 2012. Role of calcium-independent phospholipase A2beta in human pancreatic islet beta-cell apoptosis. Am. J. Physiol. Endocrinol. Metab. 303: E1386–E1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abdulkhaleq L. A., Assi M. A., Abdullah R., Zamri-Saad M., Taufiq-Yap Y. H., and Hezmee M. N. M.. 2018. The crucial roles of inflammatory mediators in inflammation: a review. Vet. World. 11: 627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tessaro F. H., Ayala T. S., and Martins J. O.. 2015. Lipid mediators are critical in resolving inflammation: a review of the emerging roles of eicosanoids in diabetes mellitus. BioMed Res. Int. 2015: 568408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kühn H., and O’Donnell V. B.. 2006. Inflammation and immune regulation by 12/15-lipoxygenases. Prog. Lipid Res. 45: 334–356. [DOI] [PubMed] [Google Scholar]
- 66.Issan Y., Hochhauser E., Guo A., Gotlinger K. H., Kornowski R., Leshem-Lev D., Lev E., Porat E., Snir E., Thompson C. I., et al. . 2013. Elevated level of pro-inflammatory eicosanoids and EPC dysfunction in diabetic patients with cardiac ischemia. Prostaglandins Other Lipid Mediat. 100–101: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hooper K. M., Kong W., and Ganea D.. 2017. Prostaglandin E2 inhibits Tr1 cell differentiation through suppression of c-Maf. PLoS One. 12: e0179184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hooper K. M., Yen J. H., Kong W., Rahbari K. M., Kuo P. C., Gamero A. M., and Ganea D.. 2017. Prostaglandin E2 Inhibition of IL-27 production in murine dendritic cells: a novel mechanism that involves IRF1. J. Immunol. 198: 1521–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aoki T., Frosen J., Fukuda M., Bando K., Shioi G., Tsuji K., Ollikainen E., Nozaki K., Laakkonen J., and Narumiya S.. 2017. Prostaglandin E2-EP2-NF-kappaB signaling in macrophages as a potential therapeutic target for intracranial aneurysms. Sci. Signal. 10: eaah6037. [DOI] [PubMed] [Google Scholar]
- 70.Li H., Chen H. Y., Liu W. X., Jia X. X., Zhang J. G., Ma C. L., Zhang X. J., Yu F., and Cong B.. 2017. Prostaglandin E2 restrains human Treg cell differentiation via E prostanoid receptor 2-protein kinase A signaling. Immunol. Lett. 191: 63–72. [DOI] [PubMed] [Google Scholar]
- 71.Ganapathy V., Gurlo T., Jarstadmarken H. O., and von Grafenstein H.. 2000. Regulation of TCR-induced IFN-gamma release from islet-reactive non-obese diabetic CD8+ T cells by prostaglandin E2 receptor signaling. Int. Immunol. 12: 851–860. [DOI] [PubMed] [Google Scholar]
- 72.Ling J. J., Sun Y. J., Zhu D. Y., Chen Q., and Han X.. 2005. Potential role of NO in modulation of COX-2 expression and PGE2 production in pancreatic beta-cells. Acta Biochim. Biophys. Sin. (Shanghai). 37: 139–146. [PubMed] [Google Scholar]
- 73.Boizel R., Bruttmann G., Benhamou P. Y., Halimi S., and Stanke-Labesque F.. 2010. Regulation of oxidative stress and inflammation by glycaemic control: evidence for reversible activation of the 5-lipoxygenase pathway in type 1, but not in type 2 diabetes. Diabetologia. 53: 2068–2070. [DOI] [PubMed] [Google Scholar]
- 74.Chakrabarti S. K., Cole B. K., Wen Y., Keller S. R., and Nadler J. L.. 2009. 12/15-lipoxygenase products induce inflammation and impair insulin signaling in 3T3-L1 adipocytes. Obesity (Silver Spring). 17: 1657–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Imai Y., Dobrian A. D., Morris M. A., Taylor-Fishwick D. A., and Nadler J. L.. 2016. Lipids and immunoinflammatory pathways of beta cell destruction. Diabetologia. 59: 673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang H., Weng J., and Wang M. H.. 2016. EETs/sEH in diabetes and obesity-induced cardiovascular diseases. Prostaglandins Other Lipid Mediat. 125: 80–89. [DOI] [PubMed] [Google Scholar]
- 77.Jouihan S. A., Zuloaga K. L., Zhang W., Shangraw R. E., Krasnow S. M., Marks D. L., and Alkayed N. J.. 2013. Role of soluble epoxide hydrolase in exacerbation of stroke by streptozotocin-induced type 1 diabetes mellitus. J. Cereb. Blood Flow Metab. 33: 1650–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luther J. M., and Brown N. J.. 2016. Epoxyeicosatrienoic acids and glucose homeostasis in mice and men. Prostaglandins Other Lipid Mediat. 125: 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodriguez M., and Clare-Salzler M.. 2006. Eicosanoid imbalance in the NOD mouse is related to a dysregulation in soluble epoxide hydrolase and 15-PGDH expression. Ann. N. Y. Acad. Sci. 1079: 130–134. [DOI] [PubMed] [Google Scholar]
- 80.Gezginci-Oktayoglu S., Orhan N., and Bolkent S.. 2016. Prostaglandin-E1 has a protective effect on renal ischemia/reperfusion-induced oxidative stress and inflammation mediated gastric damage in rats. Int. Immunopharmacol. 36: 142–150. [DOI] [PubMed] [Google Scholar]
- 81.Gundala N. K. V., Naidu V. G. M., and Das U. N.. 2018. Amelioration of streptozotocin-induced type 2 diabetes mellitus in Wistar rats by arachidonic acid. Biochem. Biophys. Res. Commun. 496: 105–113. [DOI] [PubMed] [Google Scholar]
- 82.Serhan C. N., and Levy B. D.. 2018. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J. Clin. Invest. 128: 2657–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Norris P. C., and Dennis E. A.. 2014. A lipidomic perspective on inflammatory macrophage eicosanoid signaling. Adv. Biol. Regul. 54: 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mouchlis V. D., Chen Y., McCammon J. A., and Dennis E. A.. 2018. Membrane allostery and unique hydrophobic sites promote enzyme substrate specificity. J. Am. Chem. Soc. 140: 3285–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Triggiani M., Granata F., Oriente A., De Marino V., Gentile M., Calabrese C., Palumbo C., and Marone G.. 2000. Secretory phospholipases A2 induce beta-glucuronidase release and IL-6 production from human lung macrophages. J. Immunol. 164: 4908–4915. [DOI] [PubMed] [Google Scholar]
- 86.Ruipérez V., Casas J., Balboa M. A., and Balsinde J.. 2007. Group V phospholipase A2-derived lysophosphatidylcholine mediates cyclooxygenase-2 induction in lipopolysaccharide-stimulated macrophages. J. Immunol. 179: 631–638. [DOI] [PubMed] [Google Scholar]
- 87.Fantone J. C., Marasco W. A., Elgas L. J., and Ward P. A.. 1983. Anti-inflammatory effects of prostaglandin E1: in vivo modulation of the formyl peptide chemotactic receptor on the rat neutrophil. J. Immunol. 130: 1495–1497. [PubMed] [Google Scholar]
- 88.Basselin M., Rosa A. O., Ramadan E., Cheon Y., Chang L., Chen M., Greenstein D., Wohltmann M., Turk J., and Rapoport S. I.. 2010. Imaging decreased brain docosahexaenoic acid metabolism and signaling in iPLA2beta (VIA)-deficient mice. J. Lipid Res. 51: 3166–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheon Y., Kim H. W., Igarashi M., Modi H. R., Chang L., Ma K., Greenstein D., Wohltmann M., Turk J., Rapoport S. I., et al. . 2012. Disturbed brain phospholipid and docosahexaenoic acid metabolism in calcium-independent phospholipase A2-VIA (iPLA2β)-knockout mice. Biochim. Biophys. Acta. 1821: 1278–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lei X., Zhang S., Bohrer A., and Ramanadham S.. 2008. Calcium-independent phospholipase A2 (iPLA2β)-mediated ceramide generation plays a key role in the cross-talk between the endoplasmic reticulum (ER) and mitochondria during ER stress-induced insulin-secreting cell apoptosis. J. Biol. Chem. 283: 34819–34832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lei X., Zhang S., Emani B., Barbour S. E., and Ramanadham S.. 2010. A link between endoplasmic reticulum stress-induced beta-cell apoptosis and the group VIA Ca2+-independent phospholipase A2 (iPLA2β). Diabetes Obes. Metab. 12 (Suppl. 2): 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ramanadham S., Hsu F. F., Zhang S., Jin C., Bohrer A., Song H., Bao S., Ma Z., and Turk J.. 2004. Apoptosis of insulin-secreting cells induced by endoplasmic reticulum stress is amplified by overexpression of group VIA calcium-independent phospholipase A2 (iPLA2β) and suppressed by inhibition of iPLA2β. Biochemistry. 43: 918–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lei X., Zhang S., Bohrer A., Bao S., Song H., and Ramanadham S.. 2007. The group VIA calcium-independent phospholipase A2 participates in ER stress-induced INS-1 insulinoma cell apoptosis by promoting ceramide generation via hydrolysis of sphingomyelins by neutral sphingomyelinase. Biochemistry. 46: 10170–10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Papa F. R. 2012. Endoplasmic reticulum stress, pancreatic beta-cell degeneration, and diabetes. Cold Spring Harb. Perspect. Med. 2: a007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shore G. C., Papa F. R., and Oakes S. A.. 2011. Signaling cell death from the endoplasmic reticulum stress response. Curr. Opin. Cell Biol. 23: 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Amati A. L., Zakrzewicz A., Siebers R., Wilker S., Heldmann S., Zakrzewicz D., Hecker A., McIntosh J. M., Padberg W., and Grau V.. 2017. Chemokines (CCL3, CCL4, and CCL5) inhibit ATP-induced release of IL-1beta by monocytic cells. Mediators Inflamm. 2017: 1434872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee J. G., Lim E. J., Park D. W., Lee S. H., Kim J. R., and Baek S. H.. 2008. A combination of Lox-1 and Nox1 regulates TLR9-mediated foam cell formation. Cell. Signal. 20: 2266–2275. [DOI] [PubMed] [Google Scholar]
- 98.Schenten V., Brechard S., Plancon S., Melchior C., Frippiat J. P., and Tschirhart E. J.. 2010. iPLA2, a novel determinant in Ca2+- and phosphorylation-dependent S100A8/A9 regulated NOX2 activity. Biochim. Biophys. Acta. 1803: 840–847. [DOI] [PubMed] [Google Scholar]
- 99.Ramanadham S., Bohrer A., Gross R. W., and Turk J.. 1993. Mass spectrometric characterization of arachidonate-containing plasmalogens in human pancreatic islets and in rat islet β-cells and subcellular membranes. Biochemistry. 32: 13499–13509. [DOI] [PubMed] [Google Scholar]
- 100.Turk J., White T. D., Nelson A. J., Lei X., and Ramanadham S.. 2019. iPLA2β and its role in male fertility, neurological disorders, metabolic disorders, and inflammation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 1864: 846–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eerola L. I., Surrel F., Nevalainen T. J., Gelb M. H., Lambeau G., and Laine V. J.. 2006. Analysis of expression of secreted phospholipases A2 in mouse tissues at protein and mRNA levels. Biochim. Biophys. Acta. 1761: 745–756. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.