Abstract
GPR120 is implicated as a lipid receptor in the oro-sensory detection of dietary fatty acids. However, the effects of GPR120 activation on dietary fat intake or obesity are not clearly understood. We investigated to determine whether the binding of TUG891, a novel GPR120 agonist, to lingual GPR120 modulates fat preference in mice. We explored the effects of TUG891 on obesity-related hormones and conducted behavioral choice tests on mice to better understand the physiologic relevance of the action of TUG891. In cultured mouse and human taste bud cells (TBCs), TUG891 induced a rapid increase in Ca2+ by acting on GPR120. A long-chain dietary fatty acid, linoleic acid (LA), also recruited Ca2+ via GPR120 in human and mouse TBCs. Both TUG891 and LA induced ERK1/2 phosphorylation and enhanced in vitro release of glucagon-like peptide-1 from cultured human and mouse TBCs. In situ application of TUG891 onto the tongue of anesthetized mice triggered the secretion of pancreatobiliary juice, probably via the tongue-brain-gut axis. Furthermore, lingual application of TUG891 altered circulating concentrations of cholecystokinin and adipokines, associated with decreased circulating LDL, in conscious mice. In behavioral tests, mice exhibited a spontaneous preference for solutions containing either TUG891 or LA instead of a control. However, addition of TUG891 to a solution containing LA significantly curtailed fatty acid preference. Our study demonstrates that TUG891 binds to lingual GPR120 receptors, activates the tongue-brain-gut axis, and modulates fat preference. These findings may support the development of new fat taste analogs that can change the approach to obesity prevention and treatment.
Keywords: linoleic acid, extracellular signal-regulated kinase 1/2, glucagon-like peptide-1, obesity
In the recent years, it has been proposed that there might exist a sixth fat taste modality in rodents and humans, besides the well-characterized five basic taste qualities (1, 2). Two lipid sensors, CD36 and GPR120, have been shown to be essential for the oro-sensory detection of fat taste (3), and their downstream signaling mechanisms are under vigorous investigation (1, 4–6). CD36 and GPR120 are known to play complementary functions in the mediation of fat preference (7). Cartoni et al. (8) have shown that GPR120 was involved in mediating fat taste perception, and GPR120 KO mice showed a diminished preference for fatty acids. In fact, fat taste sensitivity is closely linked to the development of obesity in animals and humans (9–13). For instance, Stewart et al. (14) have shown that human subjects hypersensitive to fatty acids had lower energy, fat intake, and BMI. Ichimura et al. (10) have demonstrated that GPR120 dysfunction was associated with the development of obesity in both mice and human subjects. Hence, transgenic mice knocked out for GPR120 developed obesity, increased inflammation, and insulin resistance (15). Tanaka et al. (16) have further demonstrated that free fatty acids induced the secretion of cholecystokinin (CCK), an anorexigenic peptide, through GPR120.
Sayed et al. (17) have shown that individuals less sensitive to oleic acid were also less responsive to changes in the lipid content of food, consumed significantly more energy, and had greater BMI compared with highly sensitive healthy subjects. Hence, it becomes obvious to think of developing and testing lipid taste modifiers that can bind to fat taste receptors like CD36 and GPR120 on taste buds and bring fewer calories, as a novel way of modulating obesity and related disorders.
The potential of noncaloric GPR120 activators as fat taste agonists was investigated for the first time by Godinot et al. (18), who tested several GPR40 and GPR120 agonists as candidate fat taste analogs. Along the same lines, we employed TUG891, a more selective and potent agonist for GPR120 than linolenic acid (19). Previous studies have indicated that this agent possesses anti-obesity properties (20). We hypothesized that TUG891 may exert anti-obesity effects, in part, through the modulation of preference for dietary fat. Hence, the current experiments were designed to investigate the role of TUG891 in GPR120-triggered Ca2+ signaling in the taste bud cells (TBCs) of mice (mTBCs) and humans (hTBCs) and its role in fat-eating behavior.
MATERIALS AND METHODS
Materials
TUG891 was synthesized as previously described (18). Fura-2/AM was purchased from Life Technologies (France). Trypsin was purchased from Gibco. RPMI 1640 medium, FCS, and l-glutamine were bought from Lonza Verviers SPRL (Verviers, Belgium). AH7614 was purchased from Cayman Chemicals. ELISA kits for CCK, PYY, glucagon-like peptide (GLP)-1, and GLP-2 were purchased from Cloud-Clone Corporation, while ELISA kits for ghrelin, adiponectin, and leptin were from Bertin Pharma (France). For Western blotting, Phospho-p44/42 (Erk1/2) (#4370) and p44/42 MAPK (Erk1/2) (#9102) antibodies were purchased from Cell Signaling Technology. Unless mentioned otherwise, all other chemicals were purchased from Sigma-Aldrich. Once thawed/prepared, the linoleic acid (LA) solution was kept in the airtight tubes capped under a stream of nitrogen.
Mice and diets
Two-month-old male wild-type C57BL/6J mice were purchased from Janvier Labs (France). Mice were maintained in the animal facility of the Université de Bourgogne-Franche-Comté (France) under standard laboratory conditions, i.e., constant temperature (25°C) and humidity (60 ± 5%) with a 12 h light/dark cycle and provided with standard diet and water ad libitum. The general guidelines for the care and use of laboratory animals, recommended by the council of European Economic Communities, were followed and the protocol was approved by the Regional Ethical Committees (protocol #16158).
Culture of mTBCs and hTBCs
The previously reported procedures for the isolation of type II TBCs were used (21, 22). Briefly, mice were euthanized by cervical dislocation and the tongues were removed for the isolation of TBCs. The lingual epithelium containing circumvallate papillae was separated and peeled off from connective tissue by enzymatic dissociation (elastase and dispase mixture, 2 mg/ml each in Tyrode buffer: 140 mM NaCl, 5 mM KCl, 10 mM HEPES, 1 mM CaCl2, 10 mM glucose, 1 mM MgCl2, 10 mM Na pyruvate, pH 7.4) under a microscope. The peeled epithelium was further cut into pieces in an enzyme mixture (2 mM EDTA, 1.2 mg/ml elastase, 0.6 mg/ml collagenase (type I), and 0.6 mg/ml trypsin inhibitor to release the TBCs. The supernatant, containing released cells, was collected and centrifuged (600 g for 10 min) and further resuspended in RPMI 1640 complete medium supplemented with 10% fetal bovine serum. Isolated mTBCs were seeded onto the WillCo-dish wells at least overnight before proceeding to calcium signaling or other experiments. The hTBC lines were purified and maintained according to our previously published work (3, 23).
Measurement of calcium signaling
The increase in intracellular free calcium concentrations, [Ca2+]i, was measured according to our protocols described elsewhere (3). Briefly, the TBCs seeded onto the WillCo-dish wells were washed and then incubated for 30 min with 1 μM Fura-2/AM prior to the experiments in loading buffer containing: 110 mM, NaCl; 5.5 mM, KCl; 25 mM, NaHCO3; 0.8 mM, MgCl2; 0.4 mM, KH2PO4; 0.33 mM, Na2HPO4; 20 mM, HEPES; 1.2 mM, CaCl2, and the pH was adjusted to 7.4. Cells were again washed and further incubated in the dark for 15–20 min to ensure complete de-esterification of Fura-2/AM. The changes in [Ca2+]i, were monitored using a Nikon microscope (TiU) equipped with an EM-CCD (Luca-S) camera for real-time recording of digital images and an S-fluor 40× oil immersion objective (Nikon, Tokyo, Japan). Different test and control solutions were added alone or in combinations to the WillCo-dish wells. Images were taken at Z intervals of 0.3 mm and were analyzed using NIS-Elements software (Nikon, Tokyo, Japan). The changes in [Ca2+]i were expressed as Δ ratio, calculated as the difference between the peak F340/F380. All test molecules were added in small volumes with no interruption in recordings. The concentration of LA that was used was based on our previous work showing that LA at 50 μM exerts its action via GPR120 (3).
Western blot analysis
TBCs were homogenized in the lysis buffer containing: 20 mM HEPES, pH 7.3; 1 mM EDTA; 1 mM EGTA; 0.15 mM NaCl; 1% Triton X-100; 10% glycerol; 1 mM phenylmethylsulfonyl fluoride; 2 mM sodium orthovanadate, and 2 μl/ml anti-protease cocktail. The samples were then centrifuged (13,000 g for 10 min) and after denaturation were separated on SDS-PAGE (10%). Separated proteins were transferred to PVDF membranes and probed with primary (1:1,000; v/v dilution) and then secondary (1:2,000; v/v dilution) antibodies. Blots were developed using the enhanced chemiluminescence method and reagents according to the manufacturer’s protocol (Bio-Rad, UK).
Lingual application of TUG891 and LA in mice
The mice were used after an overnight fasting. Mice were operated under anesthesia by using isoflurane (2%). To avoid ingestion of any droplets of the lingual applied fatty acid deposition, the esophagus was ligated. To recuperate the pancreato-biliary secretion, a catheter (27 gauge) was introduced into the bile duct at the junction of the duodenum as described elsewhere (20). After an observation of basal secretion of pancreato-biliary juice, the fatty acid solution (20 μl of 7.1 mM LA in 0.1% DMSO) or TUG891 (100 μM in 0.1% DMSO) was applied onto the tongues with the help of a paint brush, covering the region of circumvallate and fungiform papillae as previously described (24). Care was taken to keep the mice alive during the experiments and the flow rate (microliters per minute) of pancreato-biliary secretions was measured following the application of each agent.
We conducted another set of similar experiments, as above, but without catheter or cannulation, to assess the levels of circulating hormones/peptides. The mice were acutely anesthetized for just 5 min, and after the application of test agents (LA or TUG891), alone or in combination, on the tongue, the animals were returned to their cages. After 30 min, they were bled from the tail for the determination of blood parameters. The plasma was removed by spinning the collection tubes, containing EDTA as an anticoagulant, in a centrifuge (5,000 g for 10 min at 4°C). Plasma samples were stored at −20°C until use.
Measurement of GLP-1 release from cultured TBCs
Mouse and human TBCs were incubated at 37°C in an oxygenized medium containing LA or TUG891, added alone, together, or after AH7614 pretreatment. After 2 h of incubation, the supernatant was collected, and the active GLP-1 release was measured by ELISA. Dipeptidyl peptidase 4 inhibitor (0.1%; Millipore) was added to the medium to prevent GLP-1 degradation (25).
Determination of lipid and other physiological parameters
The blood concentrations of various proteins including CCK, PYY, GLP-1, GLP-2, adiponectin, and leptin were determined by utilizing ELISA kits according to manufacturer’s guidelines for each product. Other parameters including LDL, triglycerides, HDL cholesterol, and glucose were determined by using KONELAB SYSTEM (Thermo Fisher Scientific, Finland) according to manufacturer’s guidelines.
Licking and two bottle preference test
The licking test consisted of subjecting a mouse to different solutions to determine the number of licks given on a feeding bottle by using a contact lickometer (Med Associates, St. Albans, VT) (26). Mice were deprived of food and water for 6 h before the test, which took place 6 h after beginning of the dark period. After a training period, which was required to learn the procedure, mice were randomly subjected to a bottle that contained either control, TUG891 (100 μM), LA (0.2%), or both for 15 min. In this experiment, data were analyzed for only 1 and 5 min from first lick.
The experiments on the spontaneous preference for lipid-enriched solutions were performed by a two-bottle preference test according to previously described procedures (3). The mice were deprived of water for 6 h before the start of the experiment. Later on, the mice were subjected to two bottles: one containing test solution and the other containing control solution over a period of 12 h overnight. Hence, they had to choose between a control solution and the test solution. The control solution contained water with 0.3% xanthan gum (w/v), and the test solution contained 0.2% LA (7.1 mM) emulsified in 0.3% xanthan gum (w/v) in water. While we did experiments on TUG891, the mice were similarly provided two choices, i.e., control and TUG891 solution (100 μM). Due to a solubility problem, the TUG891 was dissolved in a small quantity of ethanol (50 μl) and added directly to thr final quantity of water with constant stirring. Ethanol was similarly added to control bottles containing water as a control. The intake was determined by weighing the feeders before and at the end of the 12 h period.
Statistical analysis
The results are shown as mean ± SEM for a given number of experiments (n). Data were analyzed by using Prism (GraphPad Software). For more than two samples, the significance of differences between mean values was determined by one-way ANOVA, followed by Fisher’s least significant difference (LSD) test. Differences with P < 0.05 were considered to be significant.
RESULTS
TUG891 induces increases in [Ca2+]i in mouse and human TBCs through GPR120
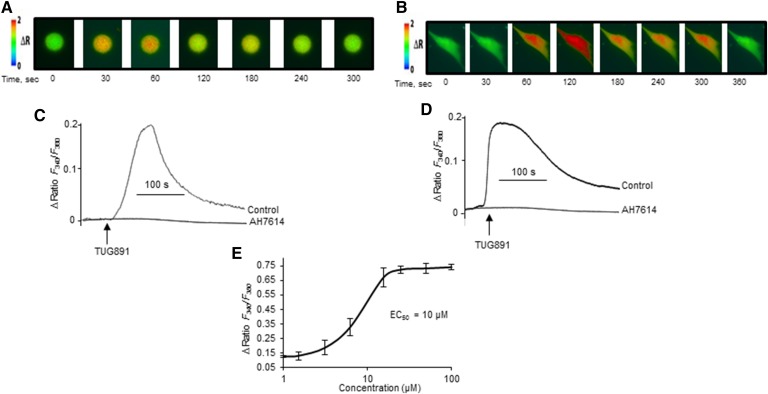
Calcium (Ca2+) signaling is an event that occurs very early during taste perception. Figure 1 shows that TUG891 triggered increases in [Ca2+]i in mTBCs (Fig. 1A, C). Similar increases in [Ca2+]i were observed following the addition of TUG891 to hTBCs (Fig. 1B, D). Interestingly, TUG891-induced increases in [Ca2+]i were completely abolished in TBCs that were preincubated with AH7614, a selective blocker of GPR120 (Fig. 1C, D). This implies that TUG891 recruits Ca2+ only via GPR120 receptors in mouse and human TBCs. Figure 1E shows the dose-response curve of TUG891 with an EC50 = 10 μM in mTBCs, and the same was observed in hTBCs (not shown).
Fig. 1.
Effect of TUG891 on Ca2+ signaling in TBCs. The cultured TBCs were loaded with Fura-2/AM, and the changes in [Ca2+]i were monitored as described in the Materials and Methods section. A, B: The pseudo-colored images compare the changes in [Ca2+]i evoked by TUG891 (10 μM) in mouse and human TBCs, respectively. C, D: Shown is the effect of AH7614 on TUG891-triggered increase in [Ca2+]i in mouse and human TBCs, respectively. E: Shown is the dose-response curve of TUG891 on Ca2+ signaling in mTBCs. For this purpose, TBCs were preincubated for 15 min in the presence of 100 μM of AH7614 followed by addition of TUG891. The arrows indicate the time of addition of TUG891 without interruption in the recording. The traces show the identical images reproduced independently (n = 5).
TUG891 and LA recruit Ca2+ from the same pool
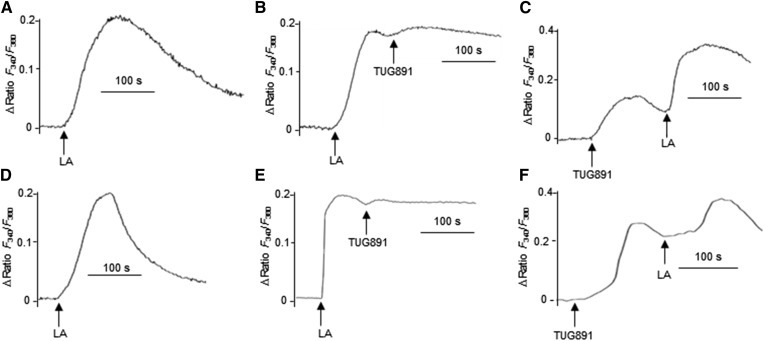
LA is a long-chain fatty acid and has been employed for the study of fat taste perception in various reports. LA triggered an increase in [Ca2+]i in mTBCs (Fig. 2A). However, no additive increases in LA-triggered rise in [Ca2+]i were observed after the addition of TUG891 (Fig. 2B). Interestingly, addition of LA to TUG891-stimulated mTBCs resulted in additive increases in [Ca2+]i (Fig. 2C). Similar findings were observed during experiments on hTBCs (Fig. 2D–F).
Fig. 2.
TUG891 recruits [Ca2+]i mobilized by LA. The cultured mouse (A–C) and human (D–F) TBCs were loaded with Fura-2/AM, and the changes in [Ca2+]i were monitored as described in the Materials and Methods section. TBCs were stimulated by LA (50 μM) alone (A, D) and before or after TUG891 (10 μM) (B, C, E, F), and changes in [Ca2+]i were recorded. The arrows indicate the time of addition of LA or TUG891 without interruption in the recording. The traces show the identical images reproduced independently (n = 5).
TUG891 binds to GPR120 and induces MAPK phosphorylation in TBCs
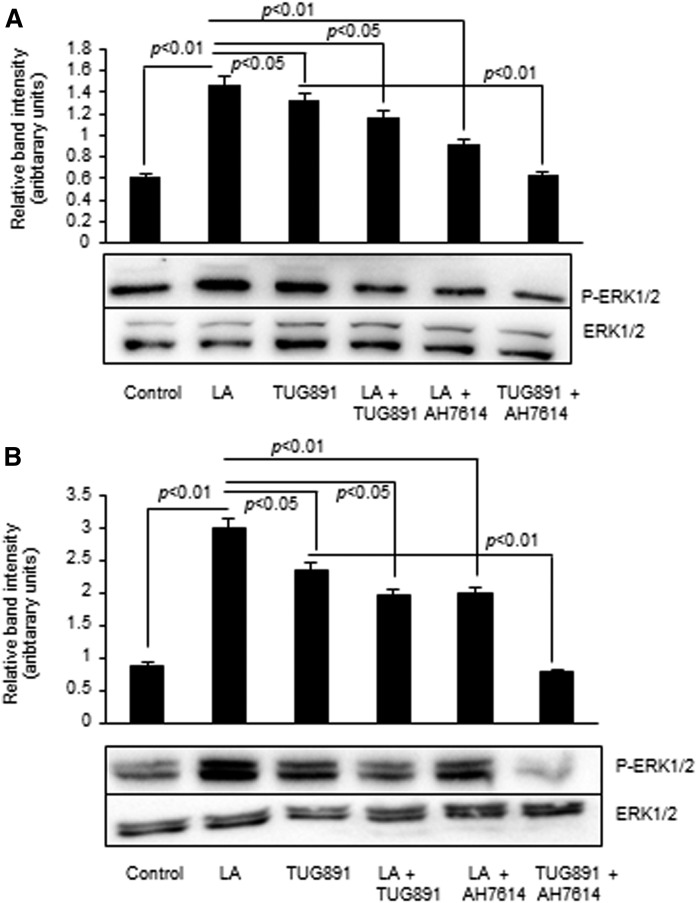
Both TUG891 and LA brought about the phosphorylation of ERK1/ERK2 as compared with control in mouse and human TBCs, respectively (Fig. 3A, B). Interestingly, the ERK1/ERK2 phosphorylation was decreased after cotreatment of cultured TBCs with LA and TUG891 (Fig. 3A, B). Moreover, LA- and TUG891-induced ERK1/ERK2 phosphorylation was inhibited partially or totally in cells pretreated with AH7614 (Fig. 3A, B).
Fig. 3.
Effect of TUG891 and LA on ERK1/2 phosphorylation in cultured TBCs. Mouse (A) or human (B) TBCs were untreated (control) or treated with LA (50 μM) or TUG891 (10 μM) in combination and in the presence or absence of AH7614 followed by Western blot analysis for the determination of phosphorylated protein expression, as described in the Materials and Methods. The histograms show the results of independent experiments (n = 3). The difference between values was determined by one-way ANOVA, followed by LSD test. P < 0.05 was considered as statistically significant.
TUG891 and LA induce GLP-1 release in cultured mouse and human TBCs
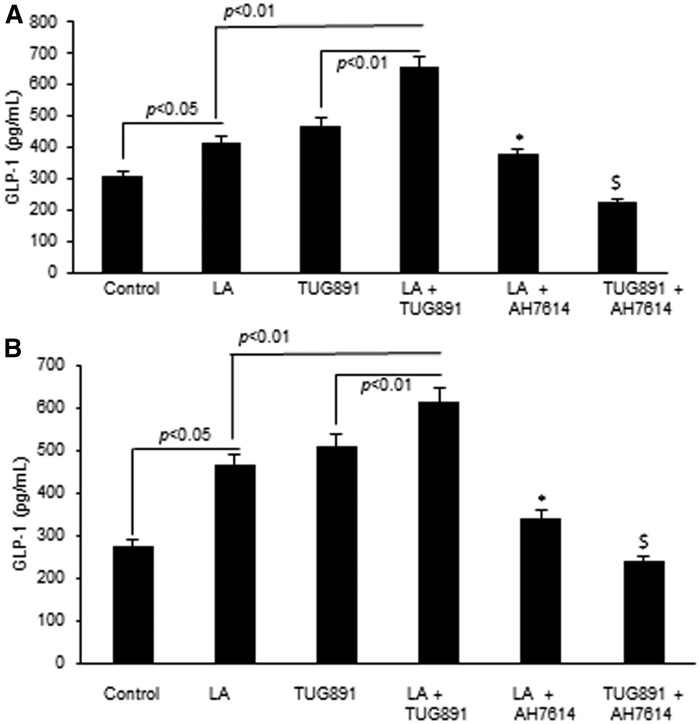
It has been shown previously that binding of fatty acids to mouse and human taste buds induces the release of GLP-1. We observed that both TUG891 and LA resulted in higher release of GLP-1 from mouse TBCs than controls (Fig. 4A). However, cotreatment of mTBCs by TUG891 and LA resulted in a significantly higher increase in the release of GLP-1 from mTBCs than other treatments (Fig. 4A). These results were reproduced in cultured hTBCs (Fig. 4B). As expected, AH7614, a GPR120 inhibitor, curtailed the release of GLP-1 from both mouse and human TBCs.
Fig. 4.
Effect of TUG891 and LA on GLP-1 release from TBCs. Cultured mouse (A) and human (B) TBCs were untreated (control) or treated with TUG891 (10 μM) or LA (50 μM) in combination or after preincubation with AH7614 (100 μM). GLP-1 release from TBCs was determined by ELISA, as mentioned in the Material and Methods section. The histograms represent the results of independent experiments (n = 3). The difference between values was determined by one-way ANOVA, followed by LSD test. P < 0.05 was considered as statistically significant. *In comparison to LA; $In comparison to TUG891.
Lingual application of TUG891 triggers pancreatobiliary secretions and alters the concentrations of circulating hormones
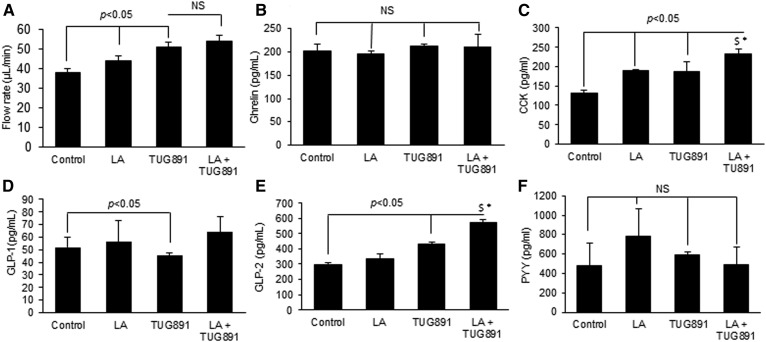
To understand the implication of TUG891 on the tongue-gut-brain axis, this agent was applied onto the tongue epithelium covering fungiform and circumvallate papillae in anesthetized mice and its effect on pancreatobiliary secretions was investigated. We observed that both TUG891 and LA resulted in a significantly increased rate of pancreatobiliary secretions than the control (Fig. 5A). Plasma concentrations of ghrelin were not affected by TUG891 or LA (Fig. 5B). The concentrations of CCK, which is widely known for its hunger-reducing properties, were significantly increased following lingual application of both agents (Fig. 5C). TUG891 slightly, but significantly, potentiated the LA-induced CCK release. Circulating concentrations of GLP-1 were decreased in the plasma of mice (Fig. 5D), while those of GLP-2 were significantly increased after the application of TUG891, and in the presence of LA, this agent exerted further increased response on GLP-2 concentrations (Fig. 5E). Interestingly, plasma concentrations of GLP-1 and GLP-2 remained unaltered after lingual application of LA (Fig. 5D, E). Plasma concentrations of PYY, another anorectic agent, were not significantly altered following the lingual application of LA or TUG891 (Fig. 5F).
Fig. 5.
Effect of lingual application of TUG891 on the activation of the tongue-brain-gut axis. Experiments were conducted as described in the Materials and Methods section, and the concentrations of different peptides were measured in plasma samples by ELISA kits. A: The histogram expresses the flow rate of hepato-biliary secretions after lingual application of LA or TUG891. The other histograms show plasma concentrations of different hormones: ghrelin (B), CCK (C), GLP-1 (D), GLP-2 (E), and PYY (F). The histograms show the results of independent experiments (n = 3). The difference between values was determined by one-way ANOVA, followed LSD test. P < 0.05 was considered as statistically significant. *In comparison to LA; $In comparison to TUG891.
Lingual application of TUG891 alters the concentrations of adipokines and inflammatory cytokines
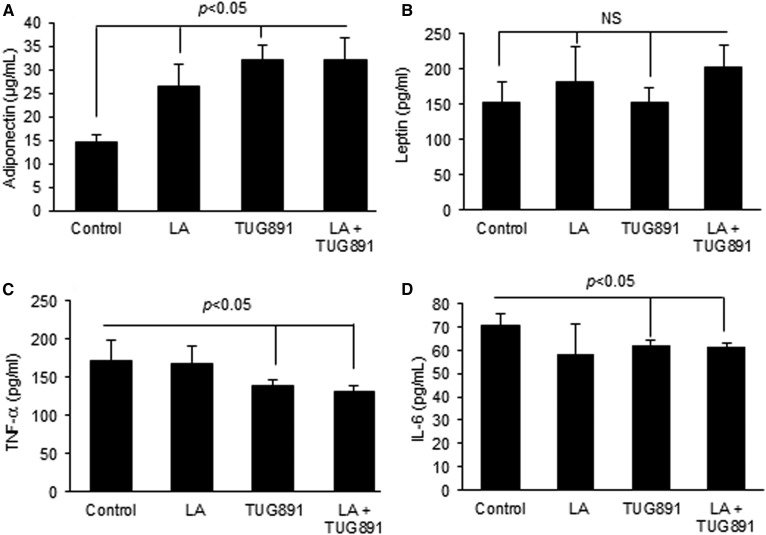
Plasma concentrations of adiponectin, an anti-inflammatory adipokine, were significantly increased over the control after lingual application of TUG891 and LA (Fig. 6A). However, the plasma concentrations of leptin, another adipokine, remained unaffected after lingual application of TUG891 (Fig. 6B). Plasma concentrations of TNF-α and IL-6 were curtailed by LA and TUG891 (Fig. 6C, D). Additive alterations in the circulating blood concentrations of adiponectin, leptin, TNF-α, or IL-6 were not observed after co-administration of LA and TUG891 onto the lingual epithelium (Fig. 6 A–D).
Fig. 6.
Effect of lingual application of TUG891 on adipokines and pro-inflammatory cytokines in mice. Different control and test agents were applied on tongues of anesthetized mice followed by euthanization. The histograms indicate the circulating concentrations of adiponectin (A), leptin (B), TNF-α (C), and IL-6 (D). Analyses were performed by using suitable ELISA kits as described in the Materials and Methods section. The histograms show the results of independent experiments (n = 3). The difference between values was determined by one-way ANOVA, followed by LSD test. P < 0.05 was considered as statistically significant. *In comparison to LA; $In comparison to TUG891.
Plasma concentrations of LDL are reduced following the lingual application of TUG891 and LA
Both TUG891 and LA resulted in reduced concentrations of LDL in mice following TUG891 application (control 0.33 ± 0.02 g/l, LA 0.185 ± 0.01 g/l, TUG891 0.22 ± 0.01 g/l, P < 0.05). However, the plasma concentrations of HDL, triglycerides, cholesterol, and glucose remained unaffected following lingual application of TUG891 or LA in mice.
TUG891 modulates fatty acid preference in mice
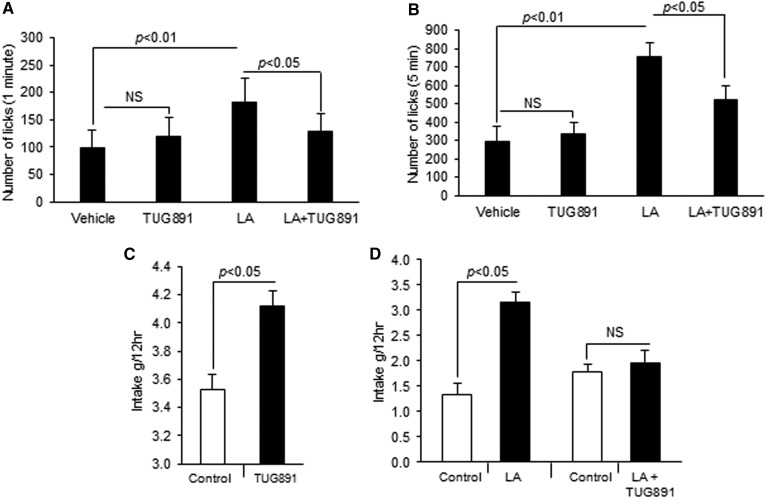
In order to assess whether TUG891-induced calcium and MAPK signaling in mTBCs are also associated with its oro-sensory detection in mice, behavioral tests were conducted. To avoid postingestive cues, we used a short-term licking test. Figure 7A and B indicate that TUG891 was not preferred by mice over the control, unlike LA, during licking for 1 or 5 min. Surprisingly, numbers of licks in mice were significantly decreased after TUG891 was added to the bottles containing LA solution (Fig. 7A, B). During double-choice experiments, mice preferred both drinking solutions containing TUG891 or fatty acid over control (Fig. 7C, D). Interestingly, the preference for the fatty acid-containing solution was abolished after the addition of TUG891 to the LA solution (Fig. 7D).
Fig. 7.
Effect of TUG891 on fat preference in mice. In the first set of experiments involving a lickometer, each mouse was offered bottles containing different solutions and the number of licks was recorded for 1 (A) and 5 min (B). The control solution consisted of 0.3% xanthan gum, while the test solution contained 0.2% LA in 0.3% xanthan gum solution or TUG891 (100 μM). Individually caged mice were also subjected to a two-bottle preference test with a choice between control solution (water) and TUG891 (100 μM) (C). In other double-choice experiments, mice were offered the control (0.3% xanthan gum) and LA (0.2% in 0.3% xanthan gum) or control and LA solution containing TUG891 (100 μM) (D). Histograms show the results of independent experiments (n = 3). The difference between values was determined by one-way ANOVA, followed by LSD test. P < 0.05 was considered as statistically significant.
DISCUSSION
Previous reports have indicated that TUG891 exerted significantly beneficial effects on weight, food intake, and inflammation in different models of obesity. For instance, Gozal et al. (27) have shown that TUG891 was able to normalize food intake and decrease body weight in mice undergoing sleep defragmentation paradigm, a highly reproducible model of metabolic dysfunction. Schilperoort et al. (20) have reported that this agonist was able to decrease fat mass, body weight, food intake, and increase fat oxidation and mitochondrial respiration in brown adipose tissue in mice. Because this agent appears to act on multiple targets, we were intrigued to find out whether the anti-obesity effects of TUG891 also involved the modulation of fat taste preference via its action on lingual GPR120, and whether TUG891 could trigger gut activation through tongue-brain-axis. Hence, we conducted three sets of experiments: 1) in vitro studies on cultured human and mouse TBCs in order to characterize the mechanism of action of TUG891 (Ca2+ signaling, MAPK activation, GLP-1 release); 2) determination of in vivo physiological consequences of lingual application of TUG891 (quantification of obesity-related peptides/hormones in blood); and 3) interaction of TUG891 with the preference for a dietary fatty acid, i.e., LA (licking and two-bottle preference tests).
We observed that TUG891 and LA triggered increases in [Ca2+]i in both mouse and human TBCs. Interestingly, LA induced an additive increase in [Ca2+]i after the addition of TUG891, in accordance with Ozdener et al. (3) who have shown that the addition of LA after grifolic acid, a GPR120 agonist, resulted in an additive increase in [Ca2+]i in cultured hTBCs. LA is known to activate both GPR120 and CD36 receptors. Hence, the fatty acid-induced additive increases might be the result of the activation of CD36, in addition to GPR120 in TBCs. TUG891 and LA also induced the phosphorylation of MAPK (ERK1/2) via GPR120 in both cultured TBC lines. However, the two agents (LA and TUG891) antagonized the effects of one another on MAPK phosphorylation as reported previously wherein grifolic acid (a GPR120 agonist) inhibited the LA-induced ERK responses in GPR120-expressing cells (28). Nonetheless, TUG891-activated MAPK might be involved in fat taste perception as the inactivation of lingual MAPK cascade both in mice and humans has been reported to decrease oro-sensory detection of fatty acids (26).
We have documented that the GLP-1 secretion from cultured human TBCs is associated with increased levels of GPR120 in raft signaling domains during oro-sensory detection of dietary fatty acids (3). TUG891 via GPR120 has been reported to induce GLP-1 secretion in different cell types including STC-1 and GLUTag enteroendocrine cells (29). The GLP-1 release from both mouse and human TBCs by LA and TUG891 further confirms that GLP-1 might be a regulatory factor in GPR120-mediated fat taste perception.
As mentioned here before, we applied TUG891 onto the lingual papillae to assess whether this agent might activate the tongue-brain-gut loop. In a recent study, Yasumatsu et al. (30) have demonstrated the existence of F-type fibers in the chorda tympani nerve in the mouse. These fibers were named so because they showed a maximum response to fatty acids among various taste stimuli and provide evidence for the existence of taste quality coding neurons in the brain. Interestingly, the response of F-type fibers to fat application was suppressed by a GPR120 antagonist (AH7614) and in GPR120 KO mice, further strengthening the role of GPR120 in fat sensing (30). As expected, acute (nearly 5 min) lingual application of TUG891 or LA triggered pancreatobiliary secretions as reported previously for a dietary fatty acid (21). The lingual application of TUG891 also induced the secretion of gut-derived hormones in blood circulation. Notably, the release of CCK was upregulated by lingual application of both LA and TUG891, showing that these two agents will trigger satiation during eating behavior, though TUG891 will be more potent than LA as the former also induced the release of GLP-2 (probably from gut-entroendocrine cells). CCK, leptin, GLP-1, and GLP-2 are known to induce satiation and reduce the food intake (31). Interestingly, plasma levels of GLP-1 were reduced while those of ghrelin, PYY, and leptin remained unaffected following lingual application of TUG891. Similarly, plasma GLP-1 or GLP-2 levels remained unchanged after lingual application of LA in mice. Hence, it is possible that TUG891 might be a more potent agonist than LA as far as gut physiology is concerned.
Furthermore, lingual application of TUG891 and LA decreased the circulating level of LDL in mice. This observation is in accordance with previous findings, which have shown that LA lowered LDL levels in hypercholesterolemic subjects (32). However, in our study, other lipid parameters remained unaffected. Nonetheless, our results on TUG891 are in agreement with the observations of Yamada et al. (33) who have shown that the activation of GPR120 exerts anti-inflammatory action and lowers blood LDL levels in obese mice. Interestingly, the lingual application of TUG891 exhibited an anti-inflammatory profile, marked by increased plasma adiponectin and low IL-6 and TNF-α levels in mice. Adiponectin is a fat-derived hormone that is an important physiological regulator of insulin sensitivity, glucose, and lipid metabolism (34). Our results are in corroboration of the previous reports, in which, it has been shown that adiponectin suppressed the release of pro-inflammatory peptides, including IL-6 and TNF-α, and the production of reactive oxygen species (35). Anti-inflammatory effects of TUG891 have also been demonstrated in previous studies. Indeed, Hudson et al. (29) have shown that LPS-stimulated TNF-α release from RAW 264.7 cells was significantly reduced after treatment with TUG891. Anbazhagan et al. (36) have also recently reported the anti-inflammatory effects of this agonist on intestinal epithelial cells. Our findings further corroborate the results of Walenta et al. (37) who have shown that another GPR120 agonist, compound A, exerted potent anti-inflammatory effects on macrophage cells in vitro and in obese mice in vivo.
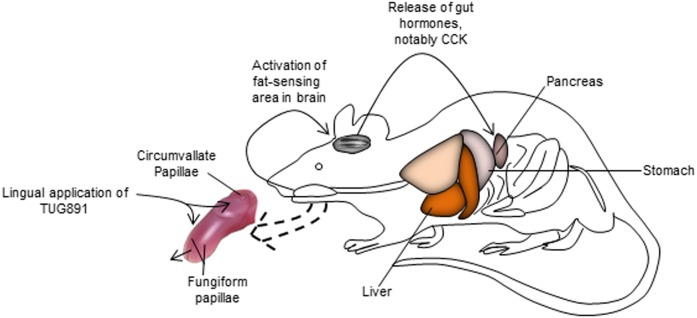
Another important finding of our study is that mice exhibited a spontaneous preference for LA both in short-term (1 and 5 min) and long-term (12 h) assays, whereas TUG891 was preferred by mice only in long-term two-bottle preference assays. However, TUG891 significantly curtailed the preference for a solution containing a long-chain fatty acid both in short-term and long-term experiments. The exact mechanism of this diminished fat taste perception is not well understood. We have presented a schematic representation of the probable mechanism of action of TUG891 in Fig. 8. We can assume that TUG891, being an exogenous chemical compound and by binding to lingual GPR120, will occupy all the receptor sites and, consequently, will decrease the binding of a dietary fatty acid and its gustatory signaling toward afferent pathways. Indeed, TUG891 is more potent than LA for binding to GPR120 receptors (19). However, we have used the fixed concentration of TUG891 in our study and lower concentrations of TUG891 may not be able to curtail the taste signals for fat preference. Hence, it would be interesting to undertake future studies using variable concentrations of TUG891. It is noteworthy that TUG891 is a selective agonist of GPR120 receptors and, during our study, we have observed that calcium signaling evoked by TUG891 was completely abolished after preincubation with AH7614, a GPR120 blocker. Therefore, the biological effects of TUG891 involved only GPR120 and not CD36 receptor. We have mentioned that TUG891 blocked the fatty-acid lingual MAPK activation via GPR120. Hence, the inhibition of MAPK by TUG891 may be one of the mechanisms of decreased fat taste perception. Currently, there is no study available that justifies the safe use of TUG891 in human subjects; hence, we were unable to confirm our findings in healthy volunteers. However, such studies are highly recommended in future to facilitate the investigation of the role of TUG891 in the modulation of fat taste in humans, as there is an increased need of fat taste modifiers that might mimic fat-like taste and decrease fat eating behavior in obesity. Nonetheless, the present study demonstrates, for the first time, that activation of GPR120 in the tongue upregulated the release of CCK, GLP-2, and adiponectin, and caused a decrease in LDL concentrations.
Fig. 8.
TUG891 will bind to lingual GPR120 in the TBCs and trigger a cascade of signalization (Ca2+ signaling and MAPK phosphorylation) leading to afferent signaling toward gustatory nerves that will bring the message to the brain. The central nervous system via vagus nerve will activate the gut axis and trigger release of gut hormone and other obesity-related mediators.
Acknowledgments
to the authors thank Aurelie Dastugue for her technical assistance in lickometer experiments (INSERM 1231, Dijon, France). Thanks are due to the Higher Education Commission (HEC) Pakistan that sanctioned the PhD scholarship to one of the authors (B.M.).
Footnotes
Abbreviations:
- [Ca2+]i
- intracellular free calcium concentrations
- CCK
- cholecystokinin
- GLP
- glucagon-like peptide
- hTBC
- human taste bud cell
- LA
- linoleic acid
- LSD
- least significant difference
- mTBC
- mouse taste bud cell
- TBC
- taste bud cell
This work was supported financially by Labex LIPSTIC (ANR-11-LABEX-002-01)/Region Bourgogne-Franche Comté, France. The authors declare that they have no conflicts of interest with the contents of this article.
REFERENCES
- 1.Besnard P., Passilly-Degrace P., and Khan N. A.. 2016. Taste of fat: a sixth taste modality? Physiol. Rev. 96: 151–176. [DOI] [PubMed] [Google Scholar]
- 2.Keast R. S. J., and Costanzo A.. 2014. Is fat the sixth taste primary? Evidence and implications. Flavour. 4: 1–7. [Google Scholar]
- 3.Ozdener M. H., Subramaniam S., Sundaresan S., Sery O., Hashimoto T., Asakawa Y., Besnard P., Abumrad N. A., and Khan N. A.. 2014. CD36- and GPR120-mediated Ca2+ signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology. 146: 995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dramane G., Abdoul-Azize S., Hichami A., Vögtle T., Akpona S., Chouabe C., Sadou H., Nieswandt B., Besnard P., and Khan N. A.. 2012. STIM1 regulates calcium signaling in taste bud cells and preference for fat in mice. J. Clin. Invest. 122: 2267–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbertson T. A., and Khan N. A.. 2014. Cell signaling mechanisms of oro-gustatory detection of dietary fat: advances and challenges. Prog. Lipid Res. 53: 82–92. [DOI] [PubMed] [Google Scholar]
- 6.Khan A. S., Subramaniam S., Dramane G., Khelifi D., and Khan N. A.. 2017. ERK1 and ERK2 activation modulates diet-induced obesity in mice. Biochimie. 137: 78–87. [DOI] [PubMed] [Google Scholar]
- 7.Ancel D., Bernard A., Subramaniam S., Hirasawa A., Tsujimoto G., Hashimoto T., Passilly-Degrace P., Khan N. A., and Besnard P.. 2015. The oral lipid sensor GPR120 is not indispensable for the orosensory detection of dietary lipids in mice. J. Lipid Res. 56: 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cartoni C., Yasumatsu K., Ohkuri T., Shigemura N., Yoshida R., Godinot N., Le Coutre J., Ninomiya Y., and Damak S.. 2010. Taste preference for fatty acids is mediated by GPR40 and GPR120. J. Neurosci. 30: 8376–8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X. J., Zhou L-H., Ban X., Liu D. X., Jiang W., and Liu X. M.. 2011. Decreased expression of CD36 in circumvallate taste buds of high-fat diet induced obese rats. Acta Histochem. 113: 663–667. [DOI] [PubMed] [Google Scholar]
- 10.Ichimura A., Hirasawa A., Poulain-Godefroy O., Bonnefond A., Hara T., Yengo L., Kimura I., Leloire A., Liu N., Iida K., et al. . 2012. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 483: 350–354. [DOI] [PubMed] [Google Scholar]
- 11.Naville D., Duchampt A., Vigier M., Oursel D., Lessire R., Poirier H., Niot I., Bégeot M., Besnard P., and Mithieux G.. 2012. Link between intestinal CD36 ligand binding and satiety induced by a high protein diet in mice. PLoS One. 7: e30686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart J. E., and Keast R. S. J.. 2012. Recent fat intake modulates fat taste sensitivity in lean and overweight subjects. Int. J. Obes. (Lond.). 36: 834–842. [DOI] [PubMed] [Google Scholar]
- 13.Mrizak I., Šerý O., Plesnik J., Arfa A., Fekih M., Bouslema A., Zaouali M., Tabka Z., and Khan N. A.. 2015. The A allele of cluster of differentiation 36 (CD36) SNP 1761667 associates with decreased lipid taste perception in obese Tunisian women. Br. J. Nutr. 113: 1330–1337. [DOI] [PubMed] [Google Scholar]
- 14.Stewart J. E., Feinle-Bisset C., Golding M., Delahunty C., Clifton P. M., and Keast R. S.. 2010. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br. J. Nutr. 104: 145–152. [DOI] [PubMed] [Google Scholar]
- 15.Oh D. Y., and Olefsky J. M.. 2012. Omega 3 fatty acids and GPR120. Cell Metab. 15: 564–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka T., Katsuma S., Adachi T., Koshimizu T. A., Hirasawa A., and Tsujimoto G.. 2008. Free fatty acids induce cholecystokinin secretion through GPR120. Naunyn Schmiedebergs Arch. Pharmacol. 377: 523–527. [DOI] [PubMed] [Google Scholar]
- 17.Sayed A., Šerý O., Plesnik J., Daoudi H., Rouabah A., Rouabah L., and Khan N. A.. 2015. CD36 AA genotype is associated with decreased lipid taste perception in young obese, but not lean, children. Int. J. Obes. (Lond.). 39: 920–924. [DOI] [PubMed] [Google Scholar]
- 18.Godinot N., Yasumatsu K., Barcos M. E., Pineau N., Ledda M., Viton F., Ninomiya Y., le Coutre J., and Damak S.. 2013. Activation of tongue-expressed GPR40 and GPR120 by non caloric agonists is not sufficient to drive preference in mice. Neuroscience. 250: 20–30. [DOI] [PubMed] [Google Scholar]
- 19.Shimpukade B., Hudson B. D., Hovgaard C. K., Milligan G., and Ulven T.. 2012. Discovery of a potent and selective GPR120 agonist. J. Med. Chem. 55: 4511–4515. [DOI] [PubMed] [Google Scholar]
- 20.Schilperoort M., Van Dam A. D., Hoeke G., Shabalina I. G., Okolo A., Hanyaloglu A. C., Dib L. H., Mol I. M., Caengprasath N., Chan Y., et al. . 2018. The GPR120 agonist TUG-891 promotes metabolic health by stimulating mitochondrial respiration in brown fat. EMBO Mol. Med. 10: e8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laugerette F., Passilly-Degrace P., Patris B., Niot I., Febbraio M., Montmayeur J. P., and Besnard P.. 2005. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J. Clin. Invest. 115: 3177–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Yassimi A., Hichami A., Besnard P., and Khan N. A.. 2008. Linoleic acid induces calcium signaling, Src kinase phosphorylation, and neurotransmitter release in mouse CD36-positive gustatory cells. J. Biol. Chem. 283: 12949–12959. [DOI] [PubMed] [Google Scholar]
- 23.Ozdener M. H., Brand J. G., Spielman A. I., Lischka F. W., Teeter J. H., Breslin P. A. S., and Rawson N. E.. 2011. Characterization of human fungiform papillae cells in culture. Chem. Senses. 36: 601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterschmitt Y., Abdoul-Azize S., Murtaza B., Barbier M., Khan A., Millot J-L., and Khan N.. 2018. Fatty acid lingual application activates gustatory and reward brain circuits in the mouse. Nutrients. 10: E1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin C., Passilly-Degrace P., Chevrot M., Ancel D., Sparks S. M., Drucker D. J., and Besnard P.. 2012. Lipid-mediated release of GLP-1 by mouse taste buds from circumvallate papillae: putative involvement of GPR120 and impact on taste sensitivity. J. Lipid Res. 53: 2256–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subramaniam S., Ozdener M. H., Abdoul-Azize S., Saito K., Malik B., Maquart G., Hashimoto T., Marambaud P., Aribi M., Tordoff M. G., et al. . 2016. ERK1/2 activation in human taste bud cells regulates fatty acid signaling and gustatory perception of fat in mice and humans. FASEB J. 30: 3489–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gozal D., Qiao Z., Almendros I., Zheng J., Khalyfa A., Shimpukade B., and Ulven T.. 2016. Treatment with TUG891, a free fatty acid receptor 4 agonist, restores adipose tissue metabolic dysfunction following chronic sleep fragmentation in mice. Int. J. Obes. (Lond.). 40: 1143–1149. [DOI] [PubMed] [Google Scholar]
- 28.Hara T., Hirasawa A., Sun Q., Sadakane K., Itsubo C., Iga T., Adachi T., Koshimizu T., Hashimoto T., Asakawa Y., et al. . 2009. Novel selective ligands for free fatty acid receptors GPR120 and GPR40. Naunyn Schmiedebergs Arch. Pharmacol. 380: 247–255. [DOI] [PubMed] [Google Scholar]
- 29.Hudson B. D., Shimpukade B., Mackenzie A. E., Butcher A. J., Pediani J. D., Christiansen E., Heathcote H., Tobin A. B., Ulven T., and Milligan G.. 2013. The pharmacology of a potent and selective agonist, TUG-891, demonstrates both potential opportunity and possible challenges to therapeutic agonism of FFA4 (GPR120). Mol. Pharmacol. 84: 710–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasumatsu K., Iwata S., Inoue M., and Ninomiya Y.. 2019. Fatty acid taste quality information via GPR120 in the anterior tongue of mice. Acta Physiol. (Oxf.). 226: e13215. [DOI] [PubMed] [Google Scholar]
- 31.Perry B., and Wang Y.. 2012. Appetite regulation and weight control: the role of gut hormones. Nutr. Diabetes. 2: e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rassias G., Kestin M., and Nestel P. J.. 1991. Linoleic acid lowers LDL cholesterol without a proportionate displacement of saturated fatty acid. Eur. J. Clin. Nutr. 45: 315–320. [PubMed] [Google Scholar]
- 33.Yamada H., Umemoto T., Kakei M., Momomura S., Kawakami M., Ishikawa S., and Hara K.. 2017. Eicosapentaenoic acid shows anti-inflammatory effect via GPR120 in 3T3–L1 adipocytes and attenuates adipose tissue inflammation in diet-induced obese mice. Nutr. Metab. (Lond.). 14: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Achari A. E., and Jain S. K.. 2017. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int. J. Mol. Sci. 18: E1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouchi N., and Walsh K.. 2007. Adiponectin as an anti-inflammatory factor. Clin. Chim. Acta. 380: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anbazhagan A. N., Priyamvada S., Gujral T., Bhattacharyya S., Alrefai W. A., Dudeja P. K., and Borthakur A.. 2016. A novel anti-inflammatory role of GPR120 in intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 310: C612–C621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh D. Y., Walenta E., Akiyama T. E., Lagakos W. S., Lackey D., Pessentheiner A. R., Sasik R., Hah N., Chi T. J., Cox J. M., et al. . 2014. A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat. Med. 20: 942–947. [DOI] [PMC free article] [PubMed] [Google Scholar]