Abstract
EZH2 is a component of the polycomb repressive complex 2 (PRC2), which is a highly conserved histone methyltransferase that methylates lysine 27 of histone 3. EZH2 mutations are associated with oncogenesis and progression of cancers. However, the relationship between the clinical outcome of patients with myeloid malignancies and EZH2 mutations is controversial. Therefore, we performed a meta-analysis of 8 studies (n = 2243 patients) that evaluates the correlation between EZH2 mutations and overall survival (OS) in patients with myeloid neoplasms. EZH2 mutations were associated with significantly worse OS (hazard ratio [HR] = 2.37, 95% confidential interval (CI), 1.48–3.79). In a word, EZH2 mutations indicate a poor prognosis for patients with myeloid neoplasms.
Keywords: EZH2, Meta-analysis, Mutations, Myeloid neoplasms, Prognostic
Introduction
Enhancer of zeste homolog 2 (EZH2), a functional enzymatic component of the polycomb repressive complex 2 (PRC2), participates in histone methylation and gene silencing by posttranslational histone modifications.1 Moreover, EZH2 is involved with cell proliferation, differentiation, invasion, as well as metastasis.2 EZH2 localizes to the long arm of chromosome 7 at position 7q35.3 Emerging data have shown that EZH2 is abnormally expressed in different human malignancies including breast cancer,4 prostate cancer,5 gastric cancer,6 colorectal cancer7 and lung cancer. Nevertheless, the prognostic role of EZH2 is unknown in myeloid neoplasms.
Myeloid malignancies are defined as clonal hematopoietic disorders originating from myeloid stem/progenitor cells, including acute myeloid leukemia (AML), myelodysplastic syndromes (MDSs), myeloproliferative neoplasms (MPNs), and myelodysplastic/myeloproliferative neoplasms (MDSs/MPNs). Altering different hematopoietic cell lineages at different stages of maturation leads to various clinical and prognostic features. With the advent of next-generation sequencing (NGS) in the last several years, certain recurrently mutated genes, such as SF3B1,8 TP53,9 ASXL110 and so on, have been revealed to provide prognostic information. Although numerous studies have demonstrated that EZH2 is tightly connected with all kinds of human malignancies, the prognostic roles of EZH2 in myeloid neoplasms are still inconclusive and unclear. The aim of this study is to explore the association between EZH2 and prognosis in patients with myeloid neoplasms.
Materials and methods
Literature search
Relevant articles from PubMed, the Cochrane Library, Embase and Medline were included in this meta-analysis. An upper date limit of Dec 01, 2018 was applied, and there was no lower date limit. The keywords included the following terms: EZH2 or Enhancer of zeste homolog 2; acute myeloid leukemia or myelodysplastic syndromes or myeloproliferative neoplasms. Subsequently, we glanced over titles and abstracts to select available studies.
Literature selection
Studies were considered eligible if they met the following criteria: (1) the diagnosis was made according to the World Health Organization (WHO) 2008 criteria or French-American-British (FAB) criteria; (2) The correlation between EZH2 mutations and clinical outcome was analyzed; (3) Detailed survival information of patients with EZH2 mutations was provided, such as overall survival (OS) with a hazard ratio (HR) and 95% confidence interval (CI); (4) The studies were published in English. The articles which did not directly offer HR and 95%CI were also kept if we could rebuild them using the P values and other data reported. OS was measured from the date of the first sample collection to the time of death from any cause, or the time at last follow-up.
Data extraction
Data retrieved from the reports included the name of the first author, publication year, region, subtype of myeloid neoplasms, sample size, number of EZH2 mutations, age and gender distribution of patients (Table 1). HR and 95%CI for OS were also extracted from the selected articles.
Table 1.
Characteristics of the included studies.
| First Author | Year | Region | Subtype | Number | Sex (M/F) | Age (range) | EZH2 mutations |
|---|---|---|---|---|---|---|---|
| Grossmann V | 2011 | Germany | AML | 81 | 57/24 | 72.8 (40–85.5) | 9 (11.1%) |
| Saygin C | 2018 | America | AML | 100 | 52/48 | 58.5 (24–75) | 5 (5.0%) |
| Wang XL | 2013 | China | MDS | 714 | 396/318 | 43 (8–83) | 13 (1.8%) |
| Wang JY | 2013 | China | MDS | 153 | 106/47 | 51 (16–81) | 8 (5.2%) |
| Bejar R | 2012 | America | MDS | 288 | 203/85 | 69 (15–90) | 23 (8.0%) |
| Tobiasson M | 2016 | Sweden and UK | MDS | 134 | Unknown | 70.5 (35–88) | 12 (9.0%) |
| SN Khan | 2013 | America | MDS, MDS/MPN, MPN, AML | 469 | Unknown | Unknown | 38 (8.0%) |
| Wu LY | 2015 | China | MDS | 304 | 162/142 | 57 (11–89) | 11 (3.6%) |
Statistical methods
HR and 95%CI for OS were used to assess the association between EZH2 mutations and the survival outcomes for patients with myeloid neoplasms. If HRs and 95%CIs were not directly offered, we calculated them on the basis of the reported Kaplan-Meier, P-value, or other statistical parameters given in the text using the method proposed by Parmar et al.11 and Hotta et al.12 By convention, an observed HR > 1 indicates worse survival for the group with EZH2 mutations. The impact of EZH2 on survival was considered to be statistically significant if the 95%CI did not overlap with 1. In the figures, 95%CIs are represented with horizontal lines. Each box indicates the HR point estimate and its area is proportional to the weight of the study. The overall summary estimate is represented by the diamond, with CI represented by its width. The unbroken vertical line is set at the null value (HR = 1.0).
We assessed the heterogeneity among the studies based on the Q value and I2 statistical value (25%, 50%, and 75% correspond to the cut-off points for low, moderate, and high degrees of heterogeneity, respectively). Heterogeneity was considered to be statistically significant if I2 was greater than 50%; otherwise, no significant heterogeneity was observed. When the heterogeneity across studies was identified as greater than 50%, the random effects model (the DerSimonian and Laird method) was used; otherwise, the fixed-effect model (the Mantel-Haenszel method)was used. Eventually, we adopted the random-effects model. To find the causes of heterogeneity, we performed subgroup analyses according to the region, sample size, and sequencing method. In addition, we conducted a sensitivity analysis in order to assess the impact of each individual study on the strength and stability of the results. Evidence of publication bias was sought using the methods of Begg et al13 and Egger et al.14 In this study, all calculations were performed by Stata version 12.0 (College Station, TX, USA). A two-tailed P-value of P < 0.05 was considered statistically significant.
Results
Characteristics of the selected studies
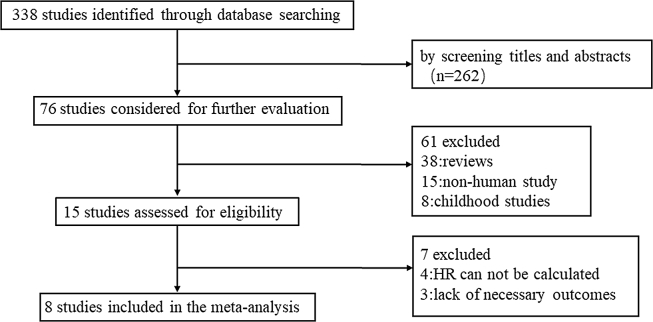
Eight studies published between 2011 and 2018, covering a total of 2243 patients were included in this meta-analysis (Fig. 1, Table 1). The main characteristics of the studies and patients are summarized in Table 1. Detailed information of the patients in the study published by Khan21 are not available. The sample size ranged from 81 to 714, and the frequency of EZH2 mutated cases from eight studies varied between 1.8% and 11.1%. Among the 2243 patients, 1040 patients had MDS, 942 had AML, 195 had MDS/MPN, and 66 had MPN. EZH2 mutations were frequently observed in MDS (2.59%), AML (0.94%)and MDS/MPN (0.80%), but were rare in the patients with MPN (0.09%). In total, 119 of 2243 patients were found to harbor EZH2 mutations.
Figure 1.
Flowchart of study search and selection strategy.
Prognostic influence of EZH2 mutations in patients with myeloid neoplasms
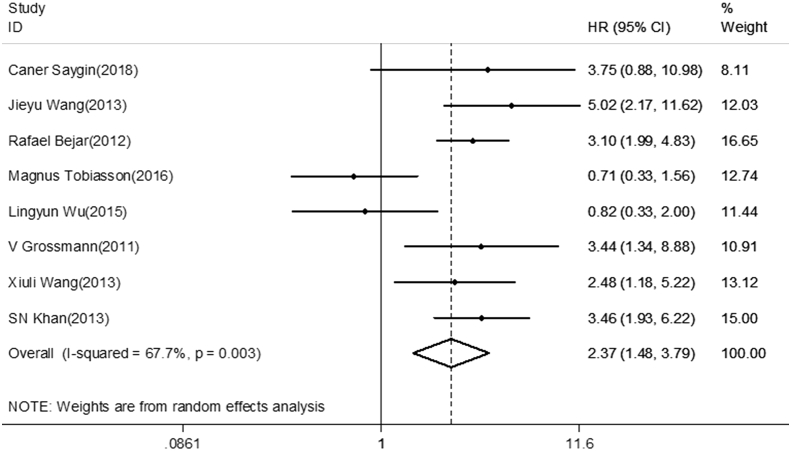
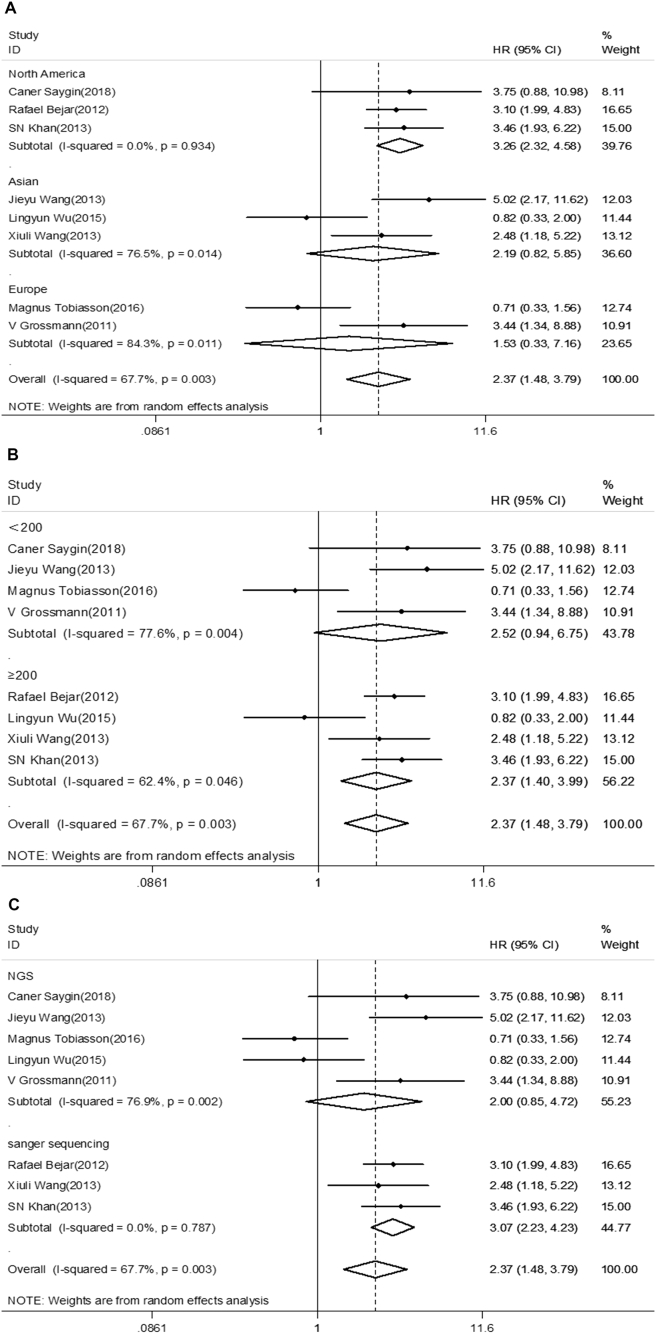
After evaluating all available studies, the overall HR for the OS was 2.37 (95%CI 1.48–3.79), revealing that EZH2 mutations are associated with poor prognosis for myeloid neoplasms (Fig. 2). Furthermore, subgroup analysis was adopted in order to find the causes of heterogeneity in the analysis of OS. A subgroup analysis for region revealed the pooled HR for North America was 3.26 (95%CI 2.32–4.58, I2 = 0.0%, P < 0.001). However, no significant relationship was found for Europe or Asia (HR = 1.53, 95%CI 0.33–7.16, I2 = 84.3%, P = 0.592; HR = 2.19, 95%CI 0.82–5.85, I2 = 76.5%, P = 0.117, respectively) (Fig. 3). The pooled HR for sample size less than 200 was 2.52 (95%CI 0.94–6.75, I2 = 77.6%, P = 0.066), whereas the HR when more than 200 cases were enrolled in studies was 2.37 (95%CI 1.40–3.99, I2 = 62.4%, P = 0.001). For studies evaluating OS by using different methods for EZH2 mutations detection, the results indicate the pooled HR for Sanger sequence was 3.07 (95%CI 2.23–4.23, I2 = 0.0%, P < 0.001). There was no statistically significant association observed between detection by next-generation sequence (NGS) and OS (HR = 2.00, 95%CI 0.85–4.72, I2 = 76.9%, P = 0.114) (Fig. 3).
Figure 2.
Forest plots of pooled HRs and 95% CI of association between EZH2 mutations and overall survival in the entire cohort of patients with myeloid neoplasms. The size of the blocks or diamonds represent the weight of the random-effect model in the meta-analysis.
Figure 3.
Forest plot of a different region, sample size, and mutation detection method included in the subgroup analysis for OS. (A) Subgroup analysis of OS by region. (B) Subgroup analysis of OS by sample size. (C)Subgroup analysis of OS by the mutation detection method.
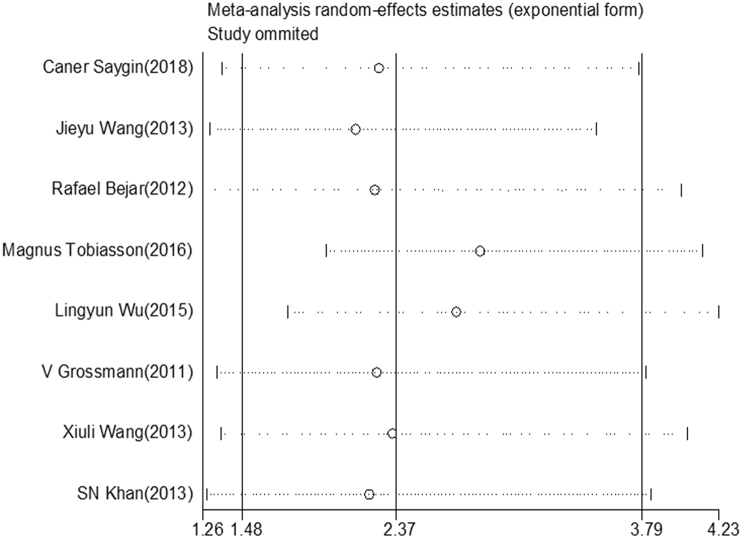
Sensitivity test and publication bias
A sensitivity analysis was conducted by calculating pooled HR again when omitting one study each time. As shown in Fig. 4, there is no obvious difference when any study was omitted, which implies that each individual study did not affect the stability of the association between EZH2 mutations and OS in patients with myeloid neoplasms.
Figure 4.
Sensitivity analysis. The middle vertical axis represents the pooled HR, and the two vertical axes indicate the corresponding 95% CI. Each hollow circle represents the pooled HR when the left study was omitted in this meta-analysis, and the two ends of every broken line indicate the 95% CI.
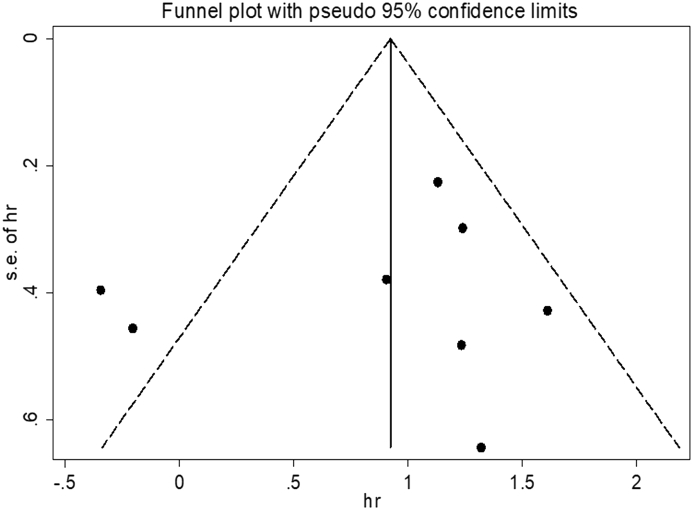
The funnel plot and Egger's test were performed to detect the existence of publication bias, and the resulting figure indicates asymmetry (Fig. 5). Nevertheless, the P value for Egger's test was 0.595, which demonstrates that there is no publication bias.
Figure 5.
Funnel plot for publication bias in terms of the association of EZH2 mutation with OS.
Discussion
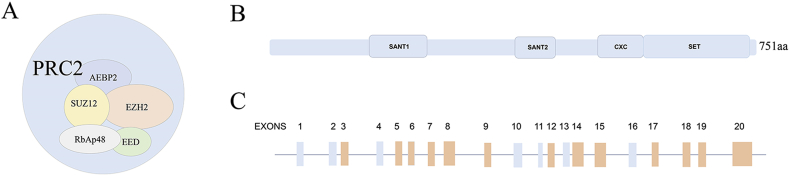
Polycomb group (PcG) proteins have two main families that are polycomb repressive complexes 1(PRC1), and PRC2.1 The human PRC2 complex is composed of EZH2, EED, SUZ12, RbAp46/48 and AEBP2 (Fig. 6A).24 The EZH2 gene is a catalytic subunit of the PRC2, and its C-terminal SET domain has methyltransferase activity (Fig. 6B). EZH2 spans about 40 kb and is composed of 20 exons.3 Furthermore, EZH2 has been reported to harbor mutations in some exons (Fig. 6C).25, 26, 27, 28, 29 EZH2 plays an important role in oncogenesis and cancer progression by epigenetic gene silencing and chromatin remodeling.5 Moreover, cancer progression may be affected by EZH2 mutations. For instance, the mutation of tyrosine 641(Y641) with the C-terminal catalytic SET domain of EZH2 increases the level of trimethylated H3K27 (H3K27me3) and thus represses the expression of Polycomb targets.23 Although the prognostic function of EZH2 mutations in myeloid neoplasms have been assessed by several studies, the results are disputable.15, 16, 17, 18, 19 Therefore, we performed a meta-analysis of published studies to explore the prognostic impact of EZH2 mutations in patients with myeloid neoplasms. This is the first time that a review of relevant literature has been performed to evaluate the correlation between EZH2 mutations and overall survival in patients with myeloid neoplasms.
Figure 6.
Schematic representation of Polycomb complex PRC2, EZH2 and distribution of EZH2 mutations. (A)The five core subunits of human PRC2. (B) The domain architecture of human EZH2. (C) Localization of EZH2 mutations. Orange bars correspond to mutations that have been reported before.
Our meta-analysis combined 8 different publications, and included 2243 patients with myeloid malignancies. Combined hazard ratios suggest that the HR for the OS was 2.37 (95%CI = 1.48–3.79), which indicates that patients carrying an EZH2 mutation have shorter OS in myeloid neoplasms. These results were consistent with most studies previously done.15, 16, 19, 20, 21, 22 Although the heterogeneity was large (I2 = 67.6%), the sensitivity analysis demonstrates that the results of this meta-analysis were stable and reliable. Subgroup analyses showed that North American cohorts (HR = 3.26, 95%CI 2.32–4.58), Sanger sequencing (HR = 3.07, 95%CI 2.23–4.23), and sample size over 200 individuals (HR = 2.37, 95%CI 1.40–3.99) were significantly associated with OS, suggesting the influence of ethnic variations on EZH2 mutations.
Our meta-analysis has its own limitations. First, it is inevitable that relevant studies are left out, in spite of using a comprehensive search strategy. In particular studies published in languages other than English have been omitted. In addition, the first-hand data of a few studies could not be acquired, and they therefore were not included in the analysis. Second, all the enrolled studies were retrospective studies rather than prospective studies. Finally, this analysis covers only a small subset of patients with myeloid neoplasms.
In conclusion, this meta-analysis showed that EZH2 mutations have significant influence in OS for myeloid neoplasms, which suggests that EZH2 mutations may be useful in predicting prognosis and guiding treatment in clinical practice. At the same time, prospective randomized controlled studies with large numbers and different types of myeloid neoplasms are needed.
Conflicts of interest
All authors have none to declare.
Acknowledgments
This work is supported in part by The National Natural Science Foundation of China, China (81770172, 81270613); Key Research & Technology Projects in Jiangsu Province, China (BE2017747); Milstein Medical Asian American Partnership (MMAAP) Foundation Research Project Award, USA in Hematology (2017).
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Margueron R., Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469(7330):343–349. doi: 10.1038/nature09784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu C., Han H.D., Mangala L.S. Regulation of tumor angiogenesis by EZH2. Cancer Cell. 2010;18(2):185–197. doi: 10.1016/j.ccr.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardoso C., Mignon C., Hetet G., Grandchamps B., Fontes M., Colleaux L. The human EZH2 gene: genomic organisation and revised mapping in 7q35 within the critical region for malignant myeloid disorders. Eur J Hum Genet. 2000;8(3):174–180. doi: 10.1038/sj.ejhg.5200439. [DOI] [PubMed] [Google Scholar]
- 4.Kleer C.G., Cao Q., Varambally S. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci USA. 2003;100(20):11606–11611. doi: 10.1073/pnas.1933744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varambally S., Dhanasekaran S.M., Zhou M. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419(6907):624–629. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 6.Matsukawa Y., Semba S., Kato H., Ito A., Yanagihara K., Yokozaki H. Expression of the enhancer of zeste homolog 2 is correlated with poor prognosis in human gastric cancer. Cancer Sci. 2006;97(6):484–491. doi: 10.1111/j.1349-7006.2006.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C.G., Ye Y.J., Yuan J., Liu F.F., Zhang H., Wang S. EZH2 and STAT6 expression profiles are correlated with colorectal cancer stage and prognosis. World J Gastroenterol. 2010;16(19):2421–2427. doi: 10.3748/wjg.v16.i19.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshida K., Sanada M., Shiraishi Y. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature. 2011;478(7367):64–69. doi: 10.1038/nature10496. [DOI] [PubMed] [Google Scholar]
- 9.Rücker F.G., Schlenk R.F., Bullinger L. TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood. 2012;119(9):2114–2121. doi: 10.1182/blood-2011-08-375758. [DOI] [PubMed] [Google Scholar]
- 10.Metzeler K.H., Becker H., Maharry K. ASXL1 mutations identify a high-risk subgroup of older patients with primary cytogenetically normal AML within the ELN Favorable genetic category. Blood. 2011;118(26):6920–6929. doi: 10.1182/blood-2011-08-368225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parmar M.K., Torri V., Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Hotta K., Matsuo K., Ueoka H., Kiura K., Tabata M., Tanimoto M. Meta-analysis of randomized clinical trials comparing cisplatin to carboplatin in patients with advanced non–small-cell lung cancer. J Clin Oncol. 2004;22(19):3852–3859. doi: 10.1200/JCO.2004.02.109. [DOI] [PubMed] [Google Scholar]
- 13.Begg C.B.1, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 14.Egger M., Davey S.G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grossmann V., Kohlmann A., Eder C. Molecular profiling of chronic myelomonocytic leukemia reveals diverse mutations in >80% of patients with TET2 and EZH2 being of high prognostic relevance. Leukemia. 2011;25(5):877–879. doi: 10.1038/leu.2011.10. [DOI] [PubMed] [Google Scholar]
- 16.Saygin C., Hirsch C., Przychodzen B. Mutations in DNMT3A, U2AF1, and EZH2 identify intermediate-risk acute myeloid leukemia patients with poor outcome after CR1. Blood Canc J. 2018;8(1) doi: 10.1038/s41408-017-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X., Dai H., Wang Q. EZH2 mutations are related to low blast percentage in bone marrow and -7/del(7q) in de novo acute myeloid leukemia. PLoS One. 2013;8(4):e61341. doi: 10.1371/journal.pone.0061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J., Ai X., Gale R.P. TET2, ASXL1 and EZH2 mutations in Chinese with myelodysplastic syndromes. Leuk Res. 2013;37(3):305–311. doi: 10.1016/j.leukres.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Bejar R., Stevenson K.E., Caughey B.A. Validation of a prognostic model and the impact of mutations in patients with lower-risk myelodysplastic syndromes. J Clin Oncol. 2012;30(27):3376–3382. doi: 10.1200/JCO.2011.40.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tobiasson M., McLornan D.P., Karimi M. Mutations in histone modulators are associated with prolonged survival during azacitidine therapy. Oncotarget. 2016;7(16):22103–22115. doi: 10.18632/oncotarget.7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan S.N., Jankowska A.M., Mahfouz R. Multiple mechanisms deregulate EZH2 and histone H3 lysine 27 epigenetic changes in myeloid malignancies. Leukemia. 2013;27(6):1301–1309. doi: 10.1038/leu.2013.80. [DOI] [PubMed] [Google Scholar]
- 22.Wu L., Song L., Xu L. Genetic landscape of recurrent ASXL1, U2AF1, SF3B1, SRSF2, and EZH2 mutations in 304 Chinese patients with myelodysplastic syndromes. Tumor Biol. 2016;37(4):4633–4640. doi: 10.1007/s13277-015-4305-2. [DOI] [PubMed] [Google Scholar]
- 23.Chang W.S., Liao C.H., Tsai C.W. Association of enhancer of zeste 2 (EZH2) genotypes with bladder cancer risk in Taiwan. Anticancer Res. 2016;36(9):4509–4514. doi: 10.21873/anticanres.10997. [DOI] [PubMed] [Google Scholar]
- 24.Tan J.Z., Yan Y., Wang X.X., Jiang Y., Xu H.E. EZH2: biology, disease, and structure-based drug discovery. Acta Pharmacol Sin. 2014;35(2):161–174. doi: 10.1038/aps.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morin R.D., Johnson N.A., Severson T.M. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet. 2010;42(2):181–185. doi: 10.1038/ng.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ernst T., Pflug A., Rinke J. A somatic EZH2 mutation in childhood acute myeloid leukemia. Leukemia. 2012;26(7):1701–1703. doi: 10.1038/leu.2012.16. [DOI] [PubMed] [Google Scholar]
- 27.Grossmann V., Bacher U., Kohlmann A. EZH2 mutations and their association with PICALM-MLLT10 positive acute leukemia. Br J Haematol. 2012;157(3):387–390. doi: 10.1111/j.1365-2141.2011.08986.x. [DOI] [PubMed] [Google Scholar]
- 28.Guglielmelli P., Biamonte F., Score J. EZH2 mutational status predicts poor survival in myelofibrosis. Blood. 2011;118(19):5227–5234. doi: 10.1182/blood-2011-06-363424. [DOI] [PubMed] [Google Scholar]
- 29.Nikoloski G., Langemeijer S.M.C., Kuiper R.P. Somatic mutations of the histone methyltransferase gene EZH2 in myelodysplastic syndromes. Nat Genet. 2010;42(8):665–667. doi: 10.1038/ng.620. [DOI] [PubMed] [Google Scholar]