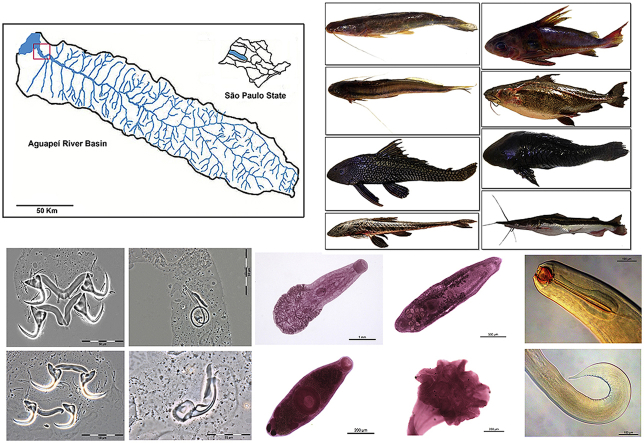
Abstract
Fishes of the order Siluriformes, known as catfishes, have a global distribution with more than 3,600 valid species of which 2,087 occur in the Neotropical region. Despite being highly diverse, abundant, and of economic importance as food and ornamental fishes, knowledge about the diversity and distribution of their helminth parasites is fragmentary and scarce. Eight species of catfishes (Pterodoras granulosus, Trachydoras paraguayensis, Pimelodella avanhandavae, Loricariichthys platymetopon, Pterygoplichthys ambrosettii, Rhinelepis aspera, Hemisorubim platyrhynchos, and Sorubim lima) from the Aguapeí River, Upper Paraná River basin, municipality of Castilho, São Paulo State, Brazil, were surveyed for helminth parasites.
Collected fishes were weighed, measured, and examined for helminth parasites following standard methodology. Fifty helminth parasite taxa (23 monogeneans, 13 digeneans, 11 nematodes, and three cestodes) were found from a total of 405 fishes screened. The helminth taxon that showed the highest mean intensity of infection and mean abundance was the nematode Rondonia rondoni from P. granulosus, followed by the nematode Parasynodontisia petterae from R. aspera. The ecological analyses were carried out at the component community level and at the infracommunity level. Trachydoras paraguayensis had the richest helminth component community. Pterygoplichthys ambrosettii had the most diverse helminth component community and R. aspera had the lowest. Both hosts are loricariids and have similar diet. However, the high parasite diversity of P. ambrosettii is due to the number of dactylogyrids species found (10), which are directly transmitted, whereas only three dactylogyrid species were found in R. aspera. At infracommunity level, the nematode species R. rondoni and P. petterae dominated the parasite communities. This study presents 38 new host records, contributing considerably to increase the diffuse knowledge of helminth parasites of Neotropical siluriforms.
Keywords: Neotropical region, Catfishes, Parasite diversity, Helminthological survey
Graphical abstract
Highlights
-
•
Fifty helminth parasite taxa were recovered from 405 catfishes surveyed.
-
•
Monogenea was the most diverse group, representing 46% of the helminth taxa found.
-
•
Thirty-eight new host records are presented.
1. Introduction
With the global increase in research on the biodiversity of freshwater environments, it is becoming more apparent that freshwater parasites are a fundamental part of this diversity. To date, parasites constitute one of the least studied groups in most biodiversity studies, even though they play a key role in the functioning of ecosystems as an integral component of trophic webs (Marcogliese and Cone, 1997; Lafferty et al., 2008; Adlard et al., 2015). Parasites are indicators of several biological aspects of their hosts and can be used in the assessment of environmental quality and richness (Sures et al., 2017). An adequate understanding of the dynamics of parasite communities in natural ecosystems, as well as the identification of spots of high parasite diversity, is important to fully understand how the biosphere works (Lafferty, 1997; Luque and Poulin, 2007). Consequently, knowledge of the diversity of parasites is crucial for any ecological and evolutionary studies as parasitism plays an important role in ecosystems by regulating the abundance or density of the host populations, establishing food chains, and structuring animal communities (Lafferty and Kuris, 2005; Thomas et al., 2005).
The Neotropical region contains the largest number of freshwater fish species, in which Characiformes, Siluriformes, and Gymnotiformes represent approximately 77% of the species (Albert and Reis, 2011). The order Siluriformes comprises more than 3,600 valid species of which 2,087 occur in the Neotropics distributed amongst 16 families. However, the real number of species and geographic distribution of Neotropical siluriforms is unknown, with 35% of the species remaining to be described (Ota et al., 2015 and references therein). Siluriformes constitutes a monophyletic group whose species are keys to historical biogeography due to their global distribution and primarily freshwater habitat (Sullivan et al., 2006). Even though they are highly diverse, abundant, and of economic importance as food and ornamental fishes, there is a paucity of knowledge about their helminth parasites (Mendoza-Palmeiro et al., 2012; de Chambrier et al., 2015a).
Monogeneans represent the most diverse group of fish parasites in South America (Luque et al., 2016). From the vast global diversity of monogenean dactylogyrids from catfishes, 17 genera and more than 90 species are described originally from the Neotropical region with a recent increase in species described by means of integrative taxonomy (Cohen et al., 2013; Acosta et al., 2017a, Acosta et al., 2017b; Aguiar et al., 2017; Acosta et al. 2018, 2019; Franceschini et al., 2018; Yamada et al., 2018; Mendoza-Palmero et al., 2019; Franceschini et al., 2020). Moreover, Mendoza-Palmeiro et al. (2012) listed about 60 undescribed species from catfishes from the Peruvian Amazonia, which shows that the current number of known species of Neotropical dactylogyrids represents a small portion of the real diversity (Mendoza-Palmero et al., 2015). Trematoda is the second-richest group of fish helminth parasites in South America (Luque et al., 2016). However, it is estimated that less than 5% of the fish fauna in South America have been studied for digenean parasites (Choudhury et al., 2016). Kohn et al. (2007) presented the only exhaustive checklist of digenean parasites of fishes of South America, with 111 records of digeneans from 55 catfish species. However, the actual number of host-parasite associations for digeneans of siluriforms in South America has certainly increased over the last 12 years. Cestoda is the third species-rich group of helminths parasites of fishes in South America (Luque et al., 2016). Recently, Alves et al. (2017) published a checklist of fish cestodes from South America, presenting 238 records of cestodes from 77 siluriform species. Nematoda represents the fourth species-rich group of helminth fish parasites in South America (Luque et al., 2016). The most recent checklist of nematodes from Brazil listed 253 records in 77 siluriform fish species (Luque et al., 2011). Acanthocephalans are the least representative group of fish helminth parasites in South America (Luque et al., 2016). The latest checklist of acanthocephalans associated with fishes from Brazil presents only 21 records in 19 siluriform species (Santos et al., 2008). A list presenting previous records of helminths from the same catfish species sampled for this study is provided as Supplementary data.
Brazil is considered a hotspot for biodiversity and holds the highest number of freshwater fish species in the Neotropical region (Heywood, 1995; Luque and Poulin, 2007). The Upper Paraná river basin harbors approximately 211 fish species in ten orders, 41 families and 126 genera, of which 37% belong to Siluriformes (Ota et al., 2018). Ichthyofaunistic surveys of the Aguapeí River (Upper Paraná River basin, municipality of Castilho, São Paulo State, Brazil) in 2011 and 2012 revealed that Siluriformes constituted the largest portion (45.2%) of the fish orders present (Vilela et al., 2013). Prior to the present study, Yamada et al. (2017) was the first and only study on the ecology of helminth communities of siluriform hosts [Auchenipterus osteomystax (Miranda, 1918) and Trachelyopterus galeatus (Linnaeus, 1766)] from the same portion of the Aguapeí River. Therefore, the aim of this study was to characterize the helminth fauna of eight of the most abundant siluriform species from the Aguapeí River.
2. Material and methods
2.1. Sampling of hosts and helminth parasites
This study was conducted in one portion (approximately 3 km of sampling area) of the Aguapeí River [21°03′36″ S, 51°45′39″ W (Fig. 1)], a tributary of the Paraná River, municipality of Castilho, São Paulo State, Brazil. The sampling area is located inside the conservation unit “Reserva Particular do Patrimônio Nacional (RPPN) Foz do Rio Aguapeí”.
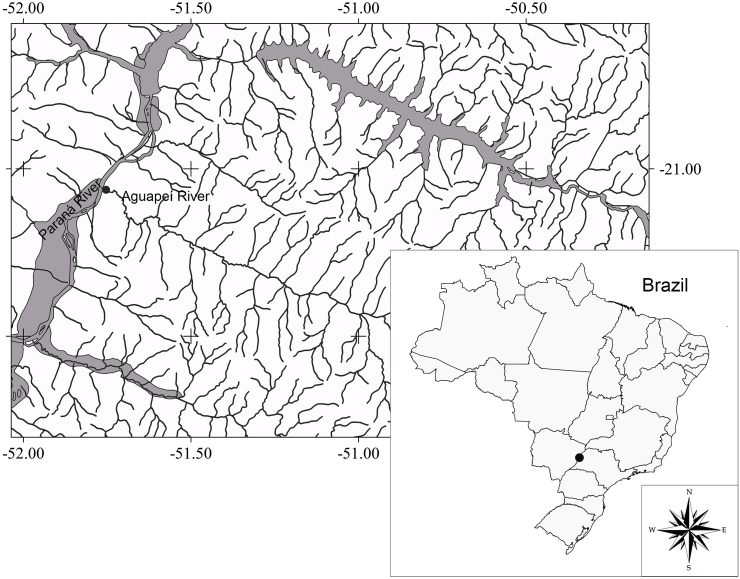
Fig. 1.
Map of the study area. Black dots represent the sampling location in the mouth of the Aguapeí River, Upper Paraná River basin, at the border of São Paulo and Mato Grosso do Sul States, Brazil.
Catfishes belonging to eight species were collected from August 2013 to November 2014 by angling, gill nets, and multifilament cast nets (Table 1). The fishes were collected under the permit license number 577/2015 IBAMA (Brazilian Institute of Environment and Renewable Natural Resources). Hosts were euthanized via spinal cord severance and most of the fish specimens were placed individually in plastic bags, frozen, and transported to the Department of Parasitology, Institute of Bioscience, São Paulo State University (UNESP), municipality of Botucatu, São Paulo State, Brazil. Some hosts were examined on site. In the laboratory, they were defrosted, weighed, measured, and examined for helminth parasites. Classification and nomenclature of hosts followed Froese and Pauly (2018).
Table 1.
Number of fish hosts collected and sampling periods in the Aguapeí River, Upper Paraná River basin, municipality of Castilho, São Paulo State, Brazil.
| Host species | Aug/13 | Jan/14 | Jun/14 | Nov/14 | Total |
|---|---|---|---|---|---|
| Doradidae | |||||
| Pterodoras granulosus | 19 | 20 | – | 20 | 59 |
| Trachydoras paraguayensis | 20 | 20 | 20 | 10 | 70 |
| Heptapteridae | |||||
| Pimelodella avanhandavae | 20 | – | – | 20 | 40 |
| Loricariidae | |||||
| Loricariichthys platymetopon | 20 | 20 | 20 | 20 | 80 |
| Pterygoplichthys ambrosettii | 20 | 20 | 20 | 20 | 80 |
| Rhinelepis aspera | 3 | 3 | 5 | 19 | 30 |
| Pimelodidae | |||||
| Hemisorubim platyrhynchos | 4 | 8 | 8 | 1 | 21 |
|
Sorubim lima |
7 |
8 |
4 |
6 |
25 |
| Total | 113 | 99 | 77 | 116 | 405 |
The body, fins, nasal cavity, gills, eyes, and inner face of the operculum of the fish hosts were examined for ectoparasites. Following external examination, a longitudinal incision in the ventral surface was made and all inner organs were removed and separated. The visceral cavity and all organs were examined using a stereomicroscope. The helminths collected were preserved in 70% ethanol and some were preserved in 96% ethanol for molecular analyses. For species identification, the monogeneans were mounted on slides with Hoyer's or Grey and Wess' medium, and/or a mixture of glycerin-ammonium picrate (GAP) to study sclerotized structures (Humason, 1979; Kritsky et al., 1986a; Ergens, 1969), while some specimens were stained with Gomori's trichrome to study internal organs. Digeneans and cestodes were stained with carmine and cleared with clove oil, while the nematodes were cleared with lactophenol (Eiras et al., 2006). The parasites were identified and analyzed using an Olympus BX51 microscope equipped with DIC and phase contrast optics and the Qwin Lite 3.1 (Leica) computerized system for image analysis with differential interference contrast (DIC). The methodology described in Dias et al. (2004) was followed to estimate the number of specimens of the nematode Rondonia rondoni Travassos, 1920 from Pterodoras granulosus (Valenciennes, 1821).
Voucher specimens are deposited in the Helminthological Collection of the Instituto de Biociências de Botucatu (CHIBB), São Paulo State, Brazil (deposit numbers provided in Table 2).
Table 2.
Number of specimens (N); Prevalence (P); mean intensity of infection (MII); mean abundance (MA); and infestation/infection site (IS) of the helminths found in the eight fish species from the Aguapeí River, Upper Paraná River basin, municipality of Castilho, São Paulo State, Brazil. MII and MA are showed as mean ± standard error (minimum–maximum). Number of voucher specimens deposited at the Helminthological Collection of the Instituto de Biociências de Botucatu (CHIBB), São Paulo State, Brazil, are provided below the helminth taxa name.
| Siluriform hosts and helminths | N | P (%) | MII | MA | IS |
|---|---|---|---|---|---|
| Doradidae | |||||
| Pterodoras granulosus | |||||
| Monogenea | |||||
| Cosmetocleithrum bulbocirrus (CHIBB 461–64L) | 8,820 | 98 | 152 ± 21.5 (9–862) | 149.5 ± 21.3 (0–862) | G |
| Vancleavus janauacaensis (CHIBB 457–60L) | 10,659 | 98 | 180 ± 29.7 (2–1225) | 176.8 ± 29.4 (0–1225) | G |
| Digenea | |||||
| Dadaytrema oxycephalum (CHIBB 8729–30) | 638 | 66 | 16.3 ± 6.3 (1–246) | 10.8 ± 4.2 (0–246) | I |
| Type 1 metacercariae of Diplostomidaea (CHIBB 8731) | 1 | 1 | – | 0.01 ± 0.01 (0–1) | C |
| Cestoda | |||||
| Proteocephalidae gen. sp. (CHIBB 8734) | 3 | 5 | 1 (1–1) | 0.05 ± 0.02 (0–1) | I |
| Nematoda | |||||
| Procamallanus (Spirocamallanus) inopinatus (CHIBB 8732) | 2 | 1 | – | 0.03 ± 0.03 (0–2) | I |
| Rondonia rondoni (CHIBB 8733) | 464,343 | 93 | 8442.6 ± 1340.7 (1–42,560) | 7870.2 ± 1279.7 (0–42,560) | I |
| Trachydoras paraguayensis | |||||
| Monogenea | |||||
| Ameloblastella sp. 1a (CHIBB 471–73L) | 67 | 48 | 2 ± 0.2 (1–5) | 0.9 ± 0.2 (0–5) | G |
| bParacosmetocleithrum trachydorasi (CHIBB 465–70L) | 413 | 70 | 8.4 ± 2.8 (1–127) | 6 ± 2 (0–127) | G |
| Pavanelliella pavanelliia (CHIBB 474–77L) | 17 | 11 | 2.1 ± 0.5 (1–5) | 0.2 ± 0.09 (0–5) | NC |
| Digenea | |||||
| Austrodiplostomum sp. metacercariaea (CHIBB 8735) | 3 | 3 | 1.5 ± 0.5 (1–2) | 0.04 ± 0.03 (0–2) | E |
| Type 1 metacercariae of Diplostomidaea (CHIBB 8736) | 1 | 1 | – | 0.02 ± 0.02 (0–1) | C |
| Type 2 metacercariae of Diplostomidaea (CHIBB 8737) | 22 | 7 | 4.4 ± 1.8 (1–11) | 0.3 ± 0.2 (0–11) | Go |
| Type 3 metacercariae of Diplostomidaea (CHIBB 8738) | 32 | 8 | 4.8 ± 2.6 (1–18) | 0.4 ± 0.3 (0–18) | C |
| Cestoda | |||||
| Proteocephalidae gen. sp. (CHIBB 8748) | 1 | 1 | – | 0.01 ± 0.01 (0–1) | I |
| Nematoda | |||||
| Ichthyouris laterifilamenta (CHIBB 8739–40) | 1,051 | 47 | 31.9 ± 6.9 (1–137) | 15 ± 3.8 (0–137) | I |
| Neoparaseuratum travassosi (CHIBB 8741–42) | 122 | 64 | 2.7 ± 0.2 (1–8) | 1.7 ± 0.2 (0–8) | I |
| Procamallanus (Spirocamallanus) inopinatus (CHIBB 8743–44) | 125 | 94 | 1.8 ± 0.09 (1–6) | 1.7 ± 0.1 (0–6) | I |
| Procamallanus (Spirocamallanus) inopinatus fourth stage larvaea (CHIBB 8745) | 145 | 27 | 7.6 ± 2.9 (1–50) | 2 ± 0.8 (0–50) | I |
| Contracaecum sp. larvaea (CHIBB 8746–47) | 293 | 53 | 7.9 ± 1.7 (1–52) | 4.2 ± 1.04 (0–52) | SB |
| Heptapteridae | |||||
| Pimelodella avanhandavae | |||||
| Monogenea | |||||
| Ameloblastella formatriuma (CHIBB 478L) | 1 | 2 | – | 0.02 ± 0.02 (0–1) | G |
| cAphanoblastella magna (CHIBB 479–83L) | 339 | 87 | 9.7 ± 1.8 (1–48) | 8.5 ± 1.7 (0–48) | G |
| Digenea | |||||
| Austrodiplostomum sp. metacercariaea (CHIBB 8749) | 18 | 20 | 2.2 ± 0.5 (1–5) | 0.4 ± 0.2 (0–5) | E |
| Dadaytrema sp.a (CHIBB 8750) | 10 | 7 | 3.3 ± 0.3 (3–4) | 0.2 ± 0.1 (0–4) | I |
| Genarchella sp.a (CHIBB 8751–52) | 23 | 35 | 1.6 ± 0.2 (1–3) | 0.6 ± 0.1 (0–3) | S |
| Phyllodistomum sp.a (CHIBB 8753) | 8 | 10 | 2 ± 0.7 (1–4) | 0.2 ± 0.1 (0–4) | Go |
| Type 1 digeneana (CHIBB 8754–55) | 35 | 5 | 17.5 ± 16.5 (1–34) | 0.8 ± 0.8 (0–34) | I |
| Unidentified metacercariaea (CHIBB 8756) | 66 | 7 | 22 ± 16.1 (2–54) | 1.6 ± 1.3 (0–54) | C |
| Nematoda | |||||
| Brasilnema pimellodellaea (CHIBB 8757–58) | 313 | 57 | 13 ± 3.8 (1–75) | 7.8 ± 2.5 (0–75) | I |
| Rondonia rondonia (CHIBB 8759) | 2,934 | 17 | 419 ± 280 (15–2014) | 73.3 ± 52.6 (0–2014) | I |
| dSpinitectus aguapeiensis (CHIBB 8760–61) | 97 | 50 | 4.85 ± 1 (1–21) | 2.4 ± 0.6 (0–21) | I |
| Contracaecum sp. larvaea (CHIBB 8762) | 12 | 15 | 2 ± 0.8 (1–6) | 0.3 ± 0.1 (0–6) | SB |
| Loricariidae | |||||
| Loricariichthys platymetopon | |||||
| Monogenea | |||||
| Gyrodactylidae gen. sp. 1a (CHIBB 484–85L) | 8 | 5 | 2 ± 0.4 (1–3) | 0.1 ± 0.05 (0–3) | G |
| Digenea | |||||
| Austrodiplostomum sp. metacercariaea (CHIBB 8763) | 1 | 1 | – | 0.01 ± 0.01 (0–1) | E |
| Clinostomum sp. metacercariae (CHIBB 8764–65) | 29 | 5 | 7.2 ± 3.0 (1–13) | 0.4 ± 0.2 (0–13) | Su, F |
| Type 1 metacercariae of Diplostomidaea (CHIBB 8766–69) | 1,034 | 85 | 15.2 ± 2.3 (1–90) | 13 ± 2 (0–90) | C |
| Type 4 metacercariae of Diplostomidaea (CHIBB 8770) | 27 | 1 | – | 0.3 ± 0.3 (0–27) | L |
| Nematoda | |||||
| Raphidascaris (Sprentascaris) mahnerti (CHIBB 8772–74) | 5,620 | 94 | 74.9 ± 7 (3–358) | 70.2 ± 7 (0–358) | I |
| Pterygoplychthys ambrosettii | |||||
| Monogenea | |||||
| Heteropriapulus anchoradiatus (CHIBB 495–98L) | 95 | 44 | 2.7 ± 0.7 (1–20) | 1.2 ± 0.3 (0–20) | G |
| Heteropriapulus bitomus (CHIBB 499–502L) | 34 | 1.3 ± 0.1 (1–3) | 0.4 ± 0.08 (0–3) | G | |
| Heteropriapulus heterotylus (CHIBB 487–90L) | 653 | 66 | 12.3 ± 2.4 (1–83) | 8.2 ± 1.7 (0–83) | G |
| Heteropriapulus microcleithrus (CHIBB 503–06L) | 171 | 60 | 3.5 ± 0.5 (1–20) | 2.1 ± 0.4 (0–20) | G |
| Heteropriapulus pterygoplichtyi (CHIBB 507–09L) | 55 | 2.6 ± 0.6 (1–13) | 0.7 ± 0.2 (0–13) | G | |
| Heteropriapulus simplex (CHIBB 491–94L) | 566 | 62 | 11.3 ± 1.9 (1–69) | 7 ± 1.4 (0–69) | G |
| Telethecium nasalisa (CHIBB 510–13L) | 144 | 44 | 4.1 ± 0.7 (1–17) | 1.8 ± 0.4 (0–17) | NC |
| eTrinigyrus peregrinus | 294 | 12 | 29.4 ± 24 (1–246) | 14.7 ± 12.2 (0–246) | G |
| Unilatus brittania (CHIBB 514–17L) | 14 | 12 | 1.4 ± 0.1 (1–2) | 0.2 ± 0.05 (0–2) | G |
| Unilatus unilatusa (CHIBB 518–21L) | 24 | 10 | 1.4 ± 0.1 (1–9) | 0.3 ± 0.1 (0–9) | G |
| Digenea | |||||
| Austrodiplostomum sp. metacercariaea (CHIBB 8775–76) | 67 | 9 | 9.6 ± 6.2 (1–45) | 0.9 ± 0.6 (0–45) | E |
| Rhinelepis aspera | |||||
| Monogenea | |||||
| bDemidospermus rhinelepisi (CHIBB 522–25L) | 332 | 100 | 11.7 ± 3.3 (1–70) | 11.7 ± 3.3 (0–70) | G |
| fHeteropriapulus semitortus | 20 | 23 | 2.9 ± 1 (1–8) | 0.7 ± 0.3 (0–8) | G |
| Telethecium nasalisa (CHIBB 526–28L) | 5 | 13 | 1.2 ± 0.2 (1–2) | 0.2 ± 0.08 (0–2) | NC |
| Digenea | |||||
| Austrodiplostomum sp. metacercariaea (CHIBB 8777) | 11 | 33 | 1.1 ± 0.1 (1–2) | 0.4 ± 0.1 (0–2) | E |
| Type 1 metacercariae of Diplostomidaea (CHIBB 8778) | 46 | 13 | 11.5 ± 10.5 (1–43) | 1.53 ± 1.43 (0–43) | C |
| Nematoda | |||||
| Parasynodontisia petterae (CHIBB 8779–80) | 11,676 | 87 | 449 ± 88.6 (4–1432) | 389.2 ± 81.6 (0–1432) | I |
| Pimelodidae | |||||
| Hemisorubim platyrhynchos | |||||
| Monogenea | |||||
| Ameloblastella formatriuma (CHIBB 536–39L) | 99 | 10 | 49 ± 23 (26–72) | 4.7 ± 3.6 (0–72) | G |
| Nanayella amplofalcis (CHIBB 529–32L) | 1,031 | 86 | 57.3 ± 22.3 (1–390) | 49 ± 19.6 (0–390) | G |
| Nanayella processusclavis (CHIBB 533–35L) | 74 | 47 | 7.4 ± 1.4 (2–17) | 3.5 ± 1 (0–17) | G |
| Digenea | |||||
| Austrodiplostomum sp. metacercariaea (CHIBB 8781) | 16 | 33 | 2.3 ± 1 (1–8) | 0.8 ± 0.4 (0–8) | E |
| Type 1 metacercariae of Diplostomidaea (CHIBB 8782) | 3 | 3 | – | 0.1 ± 0.1 (0–3) | C |
| Type 1 metacercariaea (CHIBB 8783–84) | 29 | 38 | 3.6 ± 1.3 (1–12) | 1.4 ± 0.6 (0–12) | C |
| Nematoda | |||||
| Nematode larva (CHIBB 8785) | 1 | 3 | – | 0.04 ± 0.04 (0–1) | C |
| Sorubim lima | |||||
| Monogenea | |||||
| Nanayella fluctuatrium (CHIBB 540–41) | 1,102 | 92 | 47.9 ± 12.5 (3–223) | 44 ± 11.8 (0–223) | G |
| Digenea | |||||
| Austrodiplostomum sp. metacercariaea (CHIBB 8786–87) | 27 | 52 | 2.1 ± 0.6 (1–8) | 1.1 ± 0.4 (0–8) | E |
| Genarchella sp. (CHIBB 8788) | 2 | 4 | – | 0.08 ± 0.08 (0–2) | S |
| Type 1 metacercariae of Diplostomidaea (CHIBB 8789–90) | 25 | 20 | 5 ± 2.2 (1–13) | 1 ± 0.6 (0–13) | C |
| Cestoda | |||||
| Manaosia bracodemoca (CHIBB 8793–94) | 24 | 24 | 4 ± 0.8 (2–8) | 1 ± 0.4 (0–8) | I |
| Spatulifer maringaensis (CHIBB 8796–97) | 655 | 88 | 29.8 ± 9.5 (1–194) | 26.2 ± 8.6 (0–194) | I |
| Nematoda | |||||
| Contracaecum sp. larvaea (CHIBB 8791–92) | 1,113 | 60 | 74.2 ± 33.8 (2–460) | 44.5 ± 21.3 (0–460) | SB |
Infection/infestation sites: Su – surface; NC – nasal cavity; G – gills; E − eyes; Go – gonads; SB – swim bladder; L – liver; S – stomach; I - Intestine; F – flesh; C – cavity.
New host record.
Data on P, MII, and MA have first been published in Acosta et al. (2018).
Data on P, MII, and MA have first been published in Yamada et al. (2018).
Data on P, MII, and MA have first been published in Acosta et al. (2017b).
Data on P, MII, and MA and deposit number (CHIBB) have first been published in Franceschini et al., 2020.
Deposit numbers (CHIBB) have first been published in Acosta et al. (2017b).
2.2. Statistical analyses
The prevalence, infection/infestation mean intensity, and mean abundance of the parasites were determined following Bush et al. (1997). Analyses were carried out at component community level (all helminths in all hosts) and at infracommunity level (all helminths in each individual) (Holmes and Price, 1986).
To determine the ecological attributes of the helminth communities, the following indices were calculated: (a) Shannon-Wiener diversity index that was calculated at the component community level. This index was used to measure the order or disorder in a system, by attributing greater weight to rare species, and was relatively independent of sample size (Krebs, 1989; Begon et al., 2007); (b) Simpson index to analyze the diversity of parasites, which was calculated at component community level ranging from 0 (low diversity) to 1 (high diversity); (c) Berger-Parker index was used to demonstrate the dominance of parasite species, which was calculated at the infracommunity level (Krebs, 1989); and (d) Discrepancy index (D), described by Poulin (1993) that was used to evaluate the spatial distribution of parasites based on their abundance. This index ranges from 0 to 1, interpreted as: D = 0, all hosts harboring a similar number of parasites; D = 1, all parasites found in a single host. This analysis was calculated using the Quantitative Parasitology 3.0 software (Rózsa et al., 2000). Shannon-Wiener, Simpson, and Berger-Parker indices were calculated using the software PAST (version 4.3) (Hammer et al., 2001).
3. Results
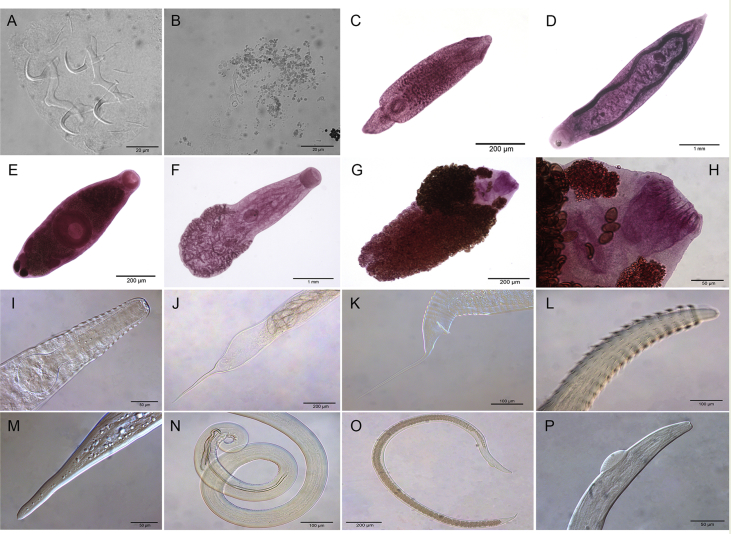
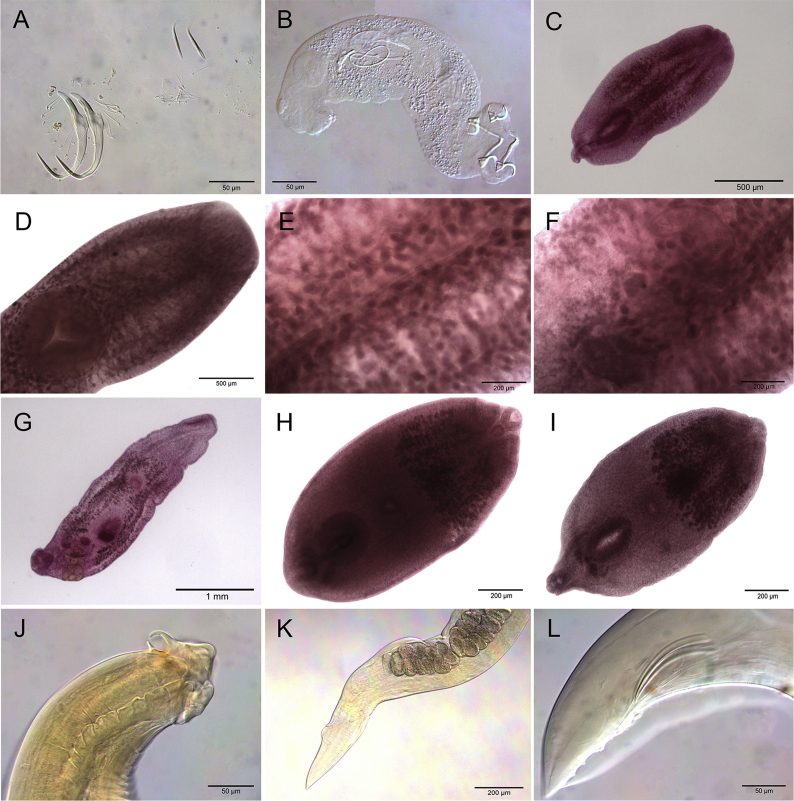
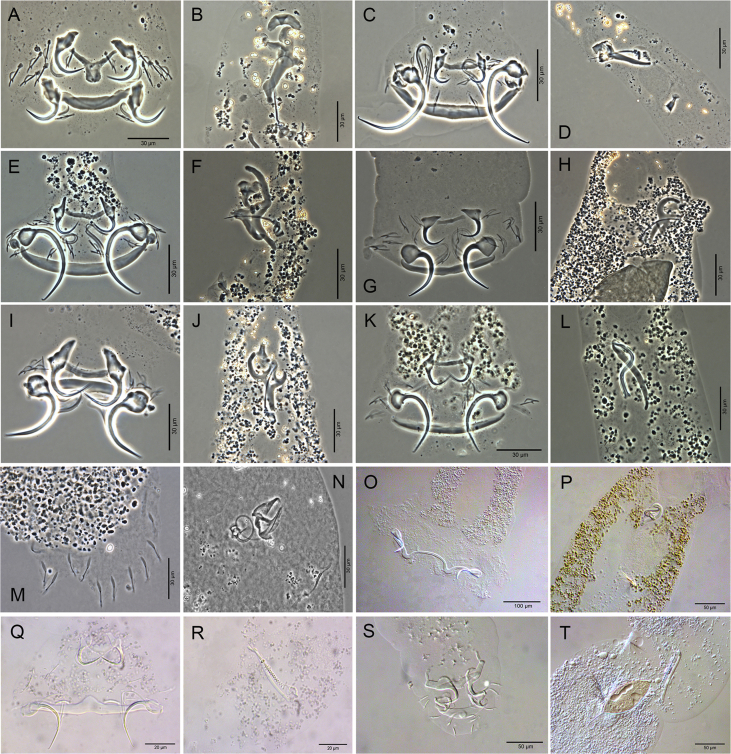
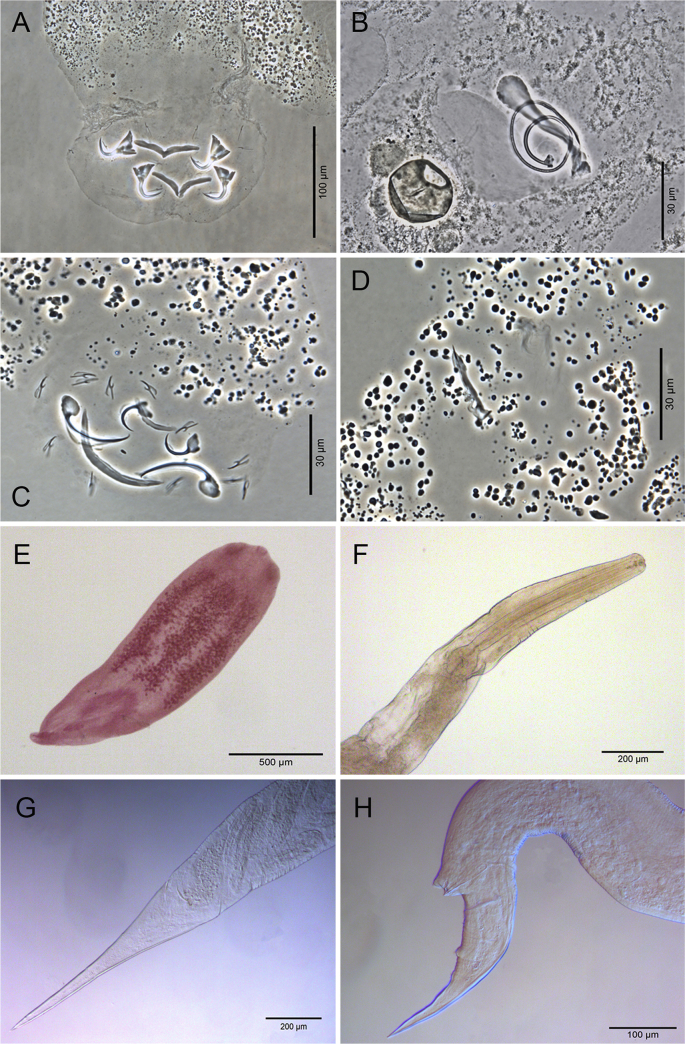
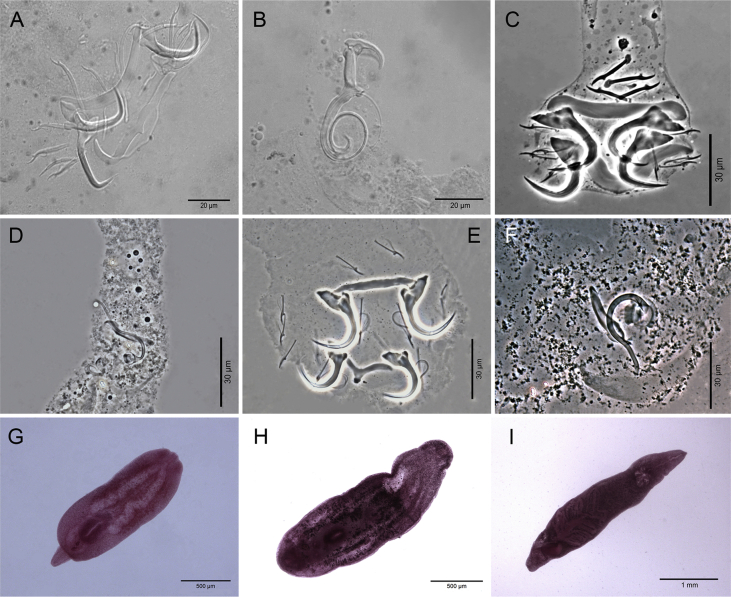
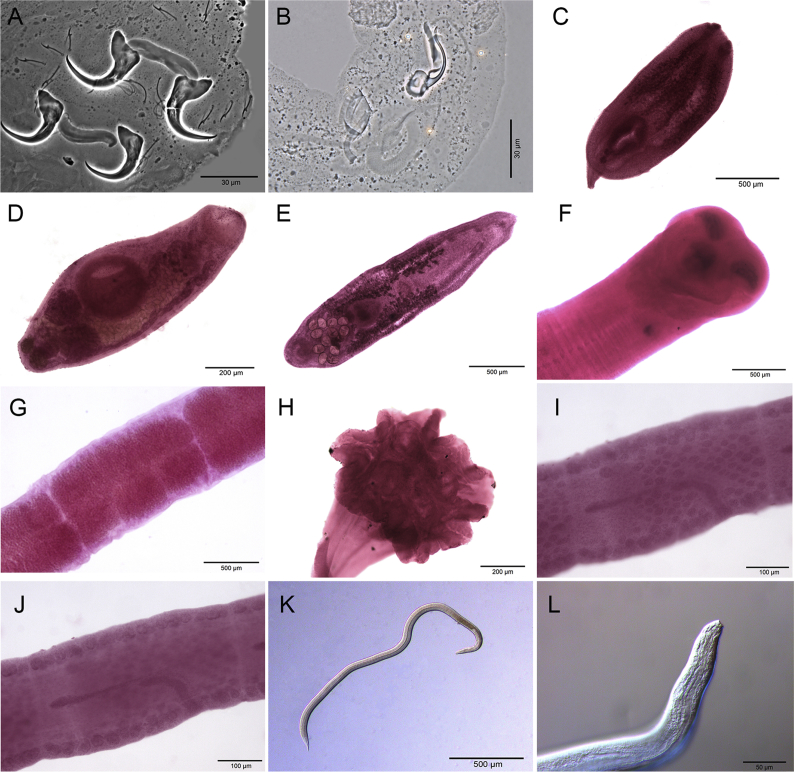
A total of 405 host specimens were screened for ecto- and endohelminth parasites. Data on the number of helminth specimens collected by species, prevalence, mean intensity of infection, mean abundance, and infection/infestation sites for all helminths found, and whether each helminth represents a new host record are presented in Table 2. Photomicrographs of the helminths collected are available as Supplementary data (Fig. S1–S8).
Four hundred host specimens (99%) were infected with at least one helminth taxon. A total of 517,775 helminth specimens were recovered in this study, with a mean of 1,278.45 parasites per individual fish. Fifty helminth parasite taxa were found: 23 monogeneans, 13 digeneans, 11 nematodes, and three cestodes. Of the 38 adult helminth taxa found in this study, only five occurred in more than one host: the monogeneans Ameloblastella formatrium Mendoza-Franco, Mendoza-Palmero et Scholz, 2016 and Telethecium nasalis Kristky, Van Every et Boeger, 1996; the digenean Genarchella sp.; and the nematodes Procamallanus (Spirocamallanus) inopinatus Travassos, Artigas et Pereira, 1928, and R. rondoni. Of the 12 metacercarial and/or larval stage found in this study, only 3 occurred in more than one host: Austrodiplostomum sp. metacercariae and Type 1 metacercariae of Diplostomidae, and the nematode Contracaecum sp. larvae (see Table 2 for host species).
Metacercariae of Austrodiplostomum sp. were the most widely distributed helminth, occurring in seven fish species (except in P. granulosus). Type 1 metacercariae of Diplostomidae also showed wide distribution, occurring in six host species (see Table 2 for host species). The helminth taxon that showed the highest mean intensity of infection [8,442.6 ± 1,340.7 (1–42,560)] and mean abundance [7,870.2 ± 1,279.7 (0–42,560)] was the nematode R. rondoni from P. granulosus, followed by the nematode Parasynodontisia petterae Moravec, Kohn et Fernandes, 1992 [mean intensity of infection 449 ± 88.6 (4–1,432); mean abundance 389.2 ± 81.6 (0–1,432)] from Rhinelepis aspera Spix et Agassiz, 1829. The helminth taxon with the greatest number of specimens recovered was R. rondoni from P. granulosus (n = 464,343) followed by P. petterae (n = 11,676) from R. aspera. Table 3 summarizes data on overall prevalence, number of taxa, number of specimens, mean per individual host, richness, and mean richness of the helminths from the eight siluriform species, along with Simpson, Shannon-Wiener, and Discrepancy indices of the helminth communities of each host species. It was not possible to calculate the discrepancy index for P. granulosus because the maximum intensity was too high. Table 3 shows the Berger-Parker index of each helminth taxa recovered in this study, indicating their dominance rank.
Table 3.
Overall prevalence (P%), number of taxa (NT), number of specimens (N), mean of parasite specimens per host specimen (MH), richness (R), and mean richness (MR) of the helminths from the eight siluriform species from the Aguapeí River, Upper Paraná River basin, municipality of Castilho, São Paulo State, Brazil, along with Simpson (SI), Shannon-Wiener (SWI), and Discrepancy (DI) indices of the helminth communities from the same eight siluriform species.
| Host | P (%) | NT | N | MH | R | MR | SI | SWI | DI |
|---|---|---|---|---|---|---|---|---|---|
| Doradidae | |||||||||
| Pterodoras granulosus | 100 | 6 | 484,466 | 8,211.28 | 1–4 | 3.67 | 0.08 | 0.21 | – |
| Trachydoras paraguayensis | 100 | 14 | 2,293 | 32.75 | 1–8 | 4.4 | 0.73 | 1.68 | 0.54 |
| Heptapteridae | |||||||||
| Pimelodella avanhandavae | 100 | 12 | 3,856 | 96.4 | 1–5 | 3.2 | 0.41 | 0.93 | 0.78 |
| Loricariidae | |||||||||
| Loricariichthys platymetopon | 100 | 8 | 8,753 | 109.4 | 1–4 | 2.8 | 0.52 | 0.93 | 0.41 |
| Pterygoplichthys ambrosettii | 96 | 11 | 2,115 | 26.4 | 1–8 | 3.56 | 0.8 | 1.87 | 0.6 |
| Rhinelepis aspera | 100 | 6 | 12,090 | 403 | 2–4 | 2.53 | 0.07 | 0.17 | 0.5 |
| Pimelodidae | |||||||||
| Hemisorubim platyrhynchos | 95 | 7 | 1,253 | 59.7 | 1–4 | 2.34 | 0.31 | 0.69 | 0.6 |
| Sorubim lima | 100 | 7 | 2,948 | 117.9 | 1–5 | 3.3 | 0.67 | 1.20 | 0.52 |
The helminth component community of P. granulosus presented low diversity (Table 3). The only new host record in P. granulosus in this study is Type 1 metacercariae of Diplostomidae. The helminth community of Trachydoras paraguayensis (Eigenmann et Ward, 1907) is the second most diverse, according to the diversity indices values (Table 3). The monogeneans Ameloblastella sp. 1 and Pavanelliella pavanelli Kritsky and Boeger (1998); the digeneans Austrodiplostomum sp. metacercariae, and Types 1, 2, and 3 metacercariae of Diplostomidae; the cestode Proteocephalidae gen. sp.; and the nematodes Procamallanus (Spirocamallanus) inopinatus 4th stage larvae Travassos, Artigas et Pereira, 1928, and Contracaecum sp. larvae represent new host records for T. paraguayensis. For Pimelodella avanhandavae Eigenmann, 1917, 12 helminth taxa were recovered, also considered diverse, and showed an aggregated pattern (Table 3). The monogenean A. formatrium; the digeneans Austrodiplostomum sp. metacercariae, Dadaytrema sp., Genarchella sp., Phyllodistomum sp., Type 1 digenean, and unindentified metacercariae; and the nematodes Brasilnema pimellodellae Moravec, Kohn et Fernandes, 1992, R. rondoni, and Contracaecum sp. larvae are new host records for P. avanhandavae. The helminth component community of Loricariichthys platymetopon Isbrücker et Nijssen, 1979 is also diverse, which was shown by the diversity indices values. The monogenean species Gyrodactylidae gen. sp. 1; and the digeneans Austrodiplostomum sp. metacercariae, Types 1, 4, and 5 metacercariae of Diplostomidae represent new host records for L. platymetopon. The host species Pterygoplichthys ambrosettii (Holmberg, 1893) presented the most diverse helminth component community of this study, according to the diversity indices (Table 3). The helminth component community of P. ambrosettii also tends to show an aggregated pattern (Table 3). For dactylogyrids, the host species P. ambrosettii presented an unexpected diversity, harboring nine different species on the gills and one in the nasal cavity (see Table 2). The monogeneans T. nasalis, Unilatus brittani Mizelle, Kritsky et Crane, 1968, and Unilatus unilatus Mizelle et Kritsky, 1967; and the digenean Austrodiplostomum sp. metacercariae are new host records for P. ambrosettii. The diversity of the helminth component community of R. aspera was the lowest among all host species analyzed in this study, which is shown by the diversity indices values. The monogenean T. nasalis; and the digeneans Austrodiplostomum sp. metacercariae, and Type 1 metacercariae of Diplostomidae represent new host records for R. aspera. The helminth component community of Hemisorubim platyrhynchos (Valenciennes, 1840) is not very diverse, as shown by the diversity indices values, and it tends to show an aggregated pattern. The monogenean A. formatrium; and the digeneans Austrodiplostomum sp. metacercariae, Type 1 metacercariae of Diplostomidae, and Type 1 metacercariae are new host records for H. platyrhynchos. For Sorubim lima (Bloch et Schneider, 1801), the helminth component community can be considered diverse, which is shown by the diversity indices. The digeneans Austrodiplostomum sp. metacercariae, and Type 1 metacercariae of Diplostomidae; and the nematode Contracaecum sp. larvae represent new host records for S. lima.
Even though monogenean was the most diverse group, Berger-Parker index showed dominance by nematodes in six host species [Contracaecum sp. larvae in S. lima, P. petterae in R. aspera, R. rondoni in P. granulosus and P. avanhandavae, Ichthyouris laterifilamenta Moravec, Kohn et Fernandes, 1992 in T. paraguayensis, and Raphidascaris (Sprentascaris) mahnerti (Petter and Cassone, 1984) in L. platymetopon]. The monogeneans Nanayella amplofalcis Acosta, Mendoza-Palmero, Silva et Scholz, 2019 and Heteropriapulus heterotylus (Jogunoori, Kritsky et Venkatanarasaah, 2004) were the dominant species in the hosts H. platyrhynchos and P. ambrosettii, respectively (Table 4).
Table 4.
Berger-Parker index (B–P) for the helminth species of the eight siluriform species from the Aguapeí River, Upper Paraná River basin, municipality of Castilho, São Paulo State, Brazil. Abbreviations: met. – metacercariae; P. trachydorasi – Paracosmetocleithrum trachydorasi; Procamallanus (S.) inopinatus – Procamallanus (Spirocamallanus) inopinatus; P. (S.) inopinatus 4th stage larv. - Procamallanus (Spirocamallanus) inopinatus 4th stage larvae; Raphidascaris (S.) mahnerti – Raphidascaris (Sprentascaris) mahnerti.
| Hemisorubim platyrhynchos | B–P | Sorubim lima | B–P | Rhinelepis aspera | B–P | Pterodoras granulosus | B–P |
|---|---|---|---|---|---|---|---|
| Nanayella amplofalcis | 0.822 | Contracaecum sp. larvae | 0.378 | Parasynodontisia petterae | 0.966 | Rondonia rondoni | 0.958 |
| Ameloblastella formatrium | 0.079 | Nanayella fluctuatrium | 0.374 | Demidospermus rhinelepisi | 0.027 | Vancleavus januacaensis | 0.022 |
| Nanayella processusclavis | 0.059 | Spatulifer maringaensis | 0.222 | Type 1 met. of Diplostomidae | 0.004 | Cosmetocleithrum bulbocirrus | 0.018 |
| Type 1 metacercariae | 0.023 | Austrodiplostomum sp. met. | 0.009 | Heteropriapulus semitortus | 0.002 | Dadaytrema oxycephalum | 0.001 |
| Austrodiplostomum sp. met. | 0.013 | Type 1 met. of Diplostomidae | 0.008 | Austrodiplostomum sp. met. | 0.001 | ||
| Type 1 met. of Diplostomidae | 0.002 | Manaosia bracodemoca | 0.008 | ||||
| Nematode larva |
0.001 |
Genarchella sp. |
0.001 |
||||
|
Pimelodella avanhandavae |
B–P |
Pterygoplychthys ambrosettii |
B–P |
Trachydoras paraguayensis |
B–P |
Loricariichthys platymetopon |
B–P |
| Rondonia rondoni | 0.761 | Heteropriapulus heterotylus | 0.309 | Ichthyouris laterifilamenta | 0.458 | Raphidascaris (S.) mahnerti | 0.642 |
| Aphanoblastella magna | 0.088 | Heteropriapulus simplex | 0.268 | P. trachydorasi | 0.180 | Diplostomidae met. Type 1 | 0.118 |
| Brasilnema pimellodellae | 0.081 | Trinigyrus peregrinus | 0.139 | Contracaecum sp. larvae | 0.128 | Clinostomum sp. metacercariae | 0.003 |
| Spinitectus aguapeiensis | 0.025 | Heteropriapulus microcleithrus | 0.081 | P. (S.) inopinatus 4th stage larv. | 0.063 | Diplostomidae met. Type 4 | 0.003 |
| Unidentified metacercariae | 0.017 | Telethecium nasalis | 0.068 | Procamallanus (S.) inopinatus | 0.055 | Diplostomidae met. Type 5 | 0.002 |
| Type 1 digenean | 0.009 | Heteropriapulus anchoradiatus | 0.045 | Neoparaseuratum travassosi | 0.053 | Gyrodactylidae gen. sp. 1 | 0.001 |
| Genarchella sp. | 0.006 | Austrodiplostomum sp. met. | 0.032 | Ameloblastella sp. 1 | 0.029 | ||
| Austrodiplostomum sp. met. | 0.005 | Heteropriapulus pterygoplichthyi | 0.026 | Type 3 met. of Diplostomidae | 0.014 | ||
| Contracaecum sp. larvae | 0.003 | Heteropriapulus bitomus | 0.016 | Type 2 met. of Diplostomidae | 0.010 | ||
| Dadaytrema sp. | 0.003 | Unilatus unilatus | 0.011 | Pavanelliella pavanellii | 0.007 | ||
| Phyllodistomum sp. | 0.002 | Unilatus brittani | 0.007 | Austrodiplostomum sp. met. | 0.001 |
4. Discussion
Monogenea was the most diverse parasite group collected in this study, representing 46% of the helminth taxa found. Monogenea represents a diverse group of parasites regarding numbers of species, as well as their morphology and ecology. Moreover, these parasites have a phylogeny that is well resolved at least to the family level (Poulin, 2002). Monogeneans tend to be host-specific with most species infecting only one or very few host species, which makes the parasites of this group suitable to investigate their diversification in the past and their diversity in the present (Poulin, 1992, 2002; Sasal et al., 1998). From the 23 monogenean species found in this study, only two species, A. formatrium and T. nasalis, were found in more than one host species. The dactylogyrid A. formatrium is recorded for the first time in a member of Heptapteridae (P. avanhandavae) since it is only known record in the literature is from the type-host Hypophthalmus edentatus Spix et Agassiz, 1829 (Mendoza-Franco et al., 2016) and now in the present study from H. platyrhynchos, but both belong to Pimelodidae. However, only a single specimen of A. formatrium was found in P. avanhandavae in the present study, which could be an accidental infection. Both host species recorded in this study for T. nasalis belong to Loricariidae (P. ambrosettii and R. aspera). Telethecium nasalis was described from the nasal cavity of Osteoglossum bicirrhosum (Cuvier, 1829) (Osteoglossiformes: Osteoglossidae) from the Amazon in Brazil (Kritsky et al., 1996) and was reported only once more by Lemos et al. (2012) in the same host in the Brazilian Amazon. Every gill dactylogyrid species found in this study is a parasite of Neotropical siluriform hosts, presenting host-specificity at family level (See host-parasite list of Supplementary data), with the exception of the occasional record of A. formatrium aforementioned. Furthermore, 11 gill dactylogyrid species were recently described for some of the hosts from this study (Acosta et al., 2017a, 2018; 2019; Yamada et al., 2018), and such species have not yet been recorded in other siluriform host species or geographical locations. The host-parasite associations for dactylogyrids found in this study are similar to Braga et al. (2014), which revealed, by analyses of network interaction, that each freshwater fish order has a unique composition of genera of gill monogeneans in the Neotropics.
Digenea was the second most diverse group in this study (26% of the helminth taxa found). Five adult digenean species were found: Dadaytrema oxycephalum (Diesing, 1836), Dadaytrema sp., and Type 1 digenean from the intestine; Genarchella sp. from the stomach; and Phyllodistomum sp. from the gonads (Table 2). Species of Dadaytrema are generalist, being reported in several different host species in Brazil, Argentina, and Venezuela (Pantoja et al., 2019 and references therein). Species of Genarchella are widely distributed in characids and siluriforms in the Neotropical region, with several records in Mexico, Central America, Venezuela, Brazil, Argentina, and Uruguay (Scholz et al., 1995; Kohn et al., 2007). Species of Phillodistomum usually inhabit the urinary bladder and/or ureters of marine and freshwater fishes globally (Stunzenas et al., 2017; Cutmore and Cribb, 2018). Five species of Phillodistomum are recorded in eight marine and freshwater fishes in South America (Kohn et al., 2007), and two species were recently described in the Neotropical region (Razo-Mendivil et al., 2013; Pérez-Ponce de León et al., 2015). The Phillodistomum specimens from this study were found in the gonads of P. avanhandavae, however, the parasites might have moved from the urinary bladder. The findings of adult digeneans in fishes indicate that the studied environment is in suitable ecological conditions to enable these parasites to complete their life cycle, which is complex and requires the presence of invertebrates: mollusks as the first host, and some invertebrates (copepods and aquatic arthropods) as the second host.
Eight digenean taxa occurred as metacercariae in the hosts analyzed in this study (Table 2). Metacercariae of Austrodiplostomum sp. are widely distributed in the Neotropical Region in a broad variety of freshwater host families (Ramos et al., 2013). These metacercariae are usually found in the eyes (as in this study), which may lead to host's blindness and even mortality (Locke et al., 2015). Five morphotypes of diplostomid metacercariae were found in this study. A considerable number of Type 1 metacercariae of Diplostomidae found in the body cavity of some host specimens was progenetic (Fig. S4G and Fig. S8E of Supplementary data). Metacercariae of Clinostomum sp. were found only in L. platymetopon (Table 2) in this study. However, metacercariae of Clinostomum have been reported in many different fish species in Brazil and all over the world, showing no host-specificity (Locke et al., 2014; Acosta et al., 2016a). The presence of metacercariae in fishes from the studied area indicates that this ecosystem possesses all the required hosts for digeneans with birds, reptiles, and even mammals as their final host, to complete their life cycle. Furthermore, it can be suggested that every host species from this study plays an important role as an intermediate host of these digeneans, being used as a food resource by their final host.
Nematoda was the third most diverse group in this study (22% of the helminth taxa found). Eight nematode species were found as adults, and three in the larval stage (Table 2). The hosts T. paraguayensis and P. avanhandavae had the most diverse nematode communities [3 adult species and 2 species in larval stage; and 3 adult species and 1 species in the larval stage, respectively (see Table 2)]. Both host species are demersal (Froese and Pauly, 2019). T. paraguayensis is considered a bottom-feeder species, feeding on organisms from the substrate, with additional records of arthropods and debris (Agostinho et al., 1997; Peretti and Andrian, 2004), whereas P. avanhandavae feed mainly on insect larvae (Hahn et al., 2004). Host diet and habitat are important factors in helminth acquisition (Dogiel, 1970; Guidelli et al., 2003). Nematodes have indirect life cycles, in which fish can act as both final and intermediate hosts (Moravec, 1998). In life cycles with fish as final hosts, the eggs hatch in the water and are ingested by an invertebrate, which is then preyed upon by the final host (Eiras et al., 2010). Therefore, the feeding habits and the demersal habitat of T. paraguayensis and P. avanhandavae might expose them to a broader variety of nematode parasites than other potential hosts. The presence of nematode larvae in some fish species (T. paraguayensis, P. avanhandavae, H. platyrhynchos, and S. lima) in this study indicate that the environment is suitable for the completion of their life cycle, since fish-eating birds and mammals are abundant in the studied area (Matsunaka et al., 2014). Additionally, these fish species play an important role as intermediate hosts in the life cycle of these nematodes. The presence of anisakid larvae (Contracaecum sp.) in S. lima draws attention to the risk of human infection since this fish species is valued for consumption. Even though Contracaecum sp. larvae encyst in the viscera of fishes (Levsen et al., 2008), inappropriate evisceration and cooking of fish may result in human infection.
Cestoda was the least representative helminth parasite group in this study (6% of the taxa found). Only S. lima had a high infection with Spatulifer maringaensis Pavanelli and Rego (1989) (prevalence of 88%), and a moderate infection with Manaosia bracodemoca Woodland (1935) (prevalence of 24%). These two proteocephalids are known to parasitize the sorubimine catfishes H. platyrhynchos and S. lima in South America (Alves et al., 2017). However, no infection with protecephalids was recorded in this study for H. platyrhynchos.
Pterygoplichthys ambrosettii had the most diverse helminth community of this study, whereas R. aspera had the lowest (Table 3). Both hosts belong to Loricariidae, make use of a demersal habitat and have similar diet, feeding on fine particulate organic matter (mud), algae, and benthonic debris (Delariva and Agostinho, 2001; Orr and Fisher, 2009; Froese and Pauly, 2019). However, the high parasite diversity of P. ambrosettii is due to the number of dactylogyrids species (10) that is directly transmitted and not through the consumption of an intermediate host, whereas only three dactylogyrid species were found in R. aspera. Trachydoras paraguayensis had the second most diverse helminth component community, with a higher variety of parasite groups compared to P. ambrosettii (3 species of monogeneans, 4 digenenas, 1 cestode, and 5 nematodes versus 10 species of monogeneans and 1 digenean). The feeding habits of T. paraguayensis expose this host species to a broader variety of parasites, as discussed before, contributing to its helminth diversity.
To date, there have been only two other studies published on the helminth fauna (endoparasites) for two of the host species analyzed in this study. Guidelli et al. (2003) analyzed the endoparasite infracommunities of H. platyrhynchos from the Baía River, Upper Paraná River floodplain, Brazil, and showed the digenean Crocodilicola pseudostoma (Willemoes-Suhm, 1870) as the most prevalent helminth taxon. However, this digenean species was not recorded in the present study. Ferrari-Hoeinghaus et al. (2007) presented a survey on digeneans from L. platymetopon from the Upper Paraná River floodplain, Brazil, and showed metacercariae Clinostomum complanatum (Rudolphi, 1819) as the most prevalent. Metacercariae of Clinostomum were also found in L. platymetopon from this study, but showed low prevalence (5%). As for the other hosts, the data available in the literature are related to helminth species descriptions or host records (see host-parasite list in Supplementary material).
The present study is the first complete survey on the diversity of helminths (ecto- and endoparasites) of the eight catfish hosts analyzed. With 38 new host records (see Table 2), this study contributes considerably to increasing the diffuse knowledge of helminth parasites of Neotropical siluriforms. Additionally, data on species diversity at component community and infracommunity levels of the parasites found will assist future comparative studies on the helminth fauna of the same host species in other areas of the Neotropical region. More studies on helminth diversity are still necessary for the Aguapeí River since this study represents a small portion of the river (3 km of sampled area in a 420 km river). Therefore, the true diversity of parasites, including undiscovered species, is yet to be known.
Declaration of competing interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Acknowledgments
Two anonymous reviewers are acknowledged for valuable comments and suggestions. The authors thank Companhia Energética de São Paulo (CESP) for providing the facilities and logistic support for field expeditions. Aline A. Acosta was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP (2012/22895–7; 2015/22382–8), and Reinaldo J. Silva was supported by FAPESP #2016/50377-1; and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq#309125/2017-0; CNPq-PROTAX #440496/2015-2). We also thank André B. Nobile for assistance with Fig. 1, Aline G. S. Lins for helping with the figures in Supplementary data, and Ryan Corso for reviewing the English.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2020.01.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Image.
Image.
Image.
Image.
Image.
Image.
Image.
Image.
References
- Acosta A.A., Caffara M., Fioravanti M.L., Utsunomia R., Zago A.C., Franceschini L., Silva R.J. Morphological and molecular characterization of Clinostomum detruncatum (Trematoda: Clinostomidae) metacercariae infecting Synbranchus marmoratus. J. Parasitol. 2016;102(1):151–156. doi: 10.1645/15-773. [DOI] [PubMed] [Google Scholar]
- Acosta A.A., Franceschini L., Zago A.C., Scholz T., Silva R.J. Six new species of Heteropriapulus (Monogenea: Dactylogyridae) from South American fishes with an amended diagnosis to the genus. Zootaxa. 2017;4290(3):459–482. [Google Scholar]
- Acosta A.A., González-Sólis, Silva R.J. Spinitectus aguapeiensis n. sp. (Nematoda: Cystidicolidae) from Pimelodella avanhandavae Eigenmann (Siluriformes: Heptapteridae) in the river Aguapeí, upper Paraná river basin, Brazil. Syst. Parasitol. 2017;94:649–656. doi: 10.1007/s11230-017-9729-1. [DOI] [PubMed] [Google Scholar]
- Acosta A.A., Scholz T., Blasco-Costa I., Alves P.V., Silva R.J. A new genus and two new species of dactylogyrid monogeneans from gills of Neotropical catfishes (Siluriformes: Doradidae and Loricariidae) Parasitol. Int. 2018;67:4–12. doi: 10.1016/j.parint.2017.09.012. [DOI] [PubMed] [Google Scholar]
- Acosta A.A., Mendoza-Palmero C.A., Silva R.J., Scholz T. A new genus and four new species of dactylogyrids (Monogenea), gill parasites of pimelodid catfishes (Siluriformes: Pimelodidae) in South America and the reassignment of Urocleidoides megorchis Mizelle et Kritsky, 1969. Folia Parasitol. 2019;66 doi: 10.14411/fp.2019.004. 004. [DOI] [PubMed] [Google Scholar]
- Adlard R.D., Miller T.L., Smit N.J. The butterfly effect: parasite diversity, environment, and emerging disease in aquatic wildlife. Trends Parasitol. 2015;31(4):160–166. doi: 10.1016/j.pt.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Agostinho A.A., Hahn N.S., Gomes L.C., Bini L.M. Estrutura trófica. In: Vazzoler A.E.A.M., Agostinho A.A., Hahn N.S., editors. A planície de inundação do alto Rio Paraná: aspectos físicos, biológicos e socioeconômicos. EDUEM, Nupélia; Maringá: 1997. pp. 229–248. [Google Scholar]
- Aguiar J.C., Maia A.A.M., Silva M.R.M., Ceccarelli P.S., Domingues M.V., Adriano E.A. An integrative taxonomic study of Pavanelliella spp. (Monogenoidea, Dactylogyridae) with the description of a new species from the nasal cavities of an Amazon pimelodid catfish. Parasitol. Int. 2017;66:777–788. doi: 10.1016/j.parint.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Albert J.S., Reis R.E. Introduction to neotropical freshwater. In: Albert J.S., Reis R.E., editors. Historical Biogeography of Neotropical Freshwater Fishes. University of California Press; California: 2011. pp. 3–19. [Google Scholar]
- Alves P.V., de Chambrier A., Scholz T., Luque J.L. Annotated checklist of fish cestodes from South America. ZooKeys. 2017;650:1–205. doi: 10.3897/zookeys.650.10982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begon M., Townsend C.R., Harper J.L. fourth ed. Artmed; Porto Alegre: 2007. Ecologia de Indivíduos a Ecossistemas. [Google Scholar]
- Braga M.P., Araújo S.B.L., Boeger W.A. Patterns of interaction between Neotropical freshwater fishes and their gill Monogenoidea (Platyhelminthes) Parasitol. Res. 2014;113:481–490. doi: 10.1007/s00436-013-3677-8. [DOI] [PubMed] [Google Scholar]
- Bush A.O., Lafferty K.D., Lotz J.M., Shostak A.W. Parasitology meets ecology on its own terms: margolis et al. revisited. J. Parasitol. 1997;83(4):575–583. [PubMed] [Google Scholar]
- Choudhury A., Aguirre-Macedo M.L., Curran S.S., Ostrowski de Núñez M., Overstreet R.M., Pérez-Ponce de León G., Santos C.P. Trematode diversity in freshwater fishes of the globe II: ‘new world’. Syst. Parasitol. 2016;93:271–282. doi: 10.1007/s11230-016-9632-1. [DOI] [PubMed] [Google Scholar]
- Cohen S.C., Justo M.C.N., Kohn A. Oficina de Livros; Rio de Janeiro: 2013. South American Monogenoidea Parasites of Fishes, Amphibians and Reptiles. Fundação Oswaldo Cruz – FIOCRUZ and Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq. [Google Scholar]
- Cutmore S.C., Cribb T.H. Two species of Phillodistomum Braun, 1899 (Trematoda: gorgoderidae) from moreton Bay, Australia. Syst. Parasitol. 2018;95:325–336. doi: 10.1007/s11230-018-9784-2. [DOI] [PubMed] [Google Scholar]
- Delariva R.L., Agostinho A.A. Relationship between morphology and diets of six neotropical loricariids. J. Fish Biol. 2001;58:832–847. [Google Scholar]
- de Chambrier A., Kuchta R., Scholz T. Tapeworms (Cestoda: Proteocephalidea) of teleost fishes from the Amazon River in Peru: additional records as an evidence of unexplored species diversity. Rev. Suisse Zool. 2015;122:149–163. [Google Scholar]
- Dias P.G., Furuya W.M., Pavanelli G.C., Machado M.H., Takemoto R.M. Carga parasitária de Rondonia rondoni, Travassos, 1920 (Nematoda, Atrictidae) e fator de condição do armado, Pterodoras granulosus, Valenciennes, 1833 (Pisces, Doradidae) Acta Sci. Biol. Sci. 2004;26(2):151–156. [Google Scholar]
- Dogiel V.A. Ecology of the parasites of freshwater fishes. In: Dogiel V.A., Petrushevski G.K., Polyansky Y.I., editors. Parasitology of Fishes. Olivier & Boyd; London: 1970. pp. 1–47. [Google Scholar]
- Eiras J.C., Takemoto R.M., Pavanelli G.C. second ed. Eduem; Maringá: 2006. Métodos de estudo e técnicas laboratoriais em parasitologia de peixes. [Google Scholar]
- Eiras J.C., Takemoto R.M., Pavanelli G.C. Clichetec; Maringá: 2010. Diversidade de peixes de água doce do Brasil. [Google Scholar]
- Ergens R. The suitability of ammonium picrate-glycerin in preparing slides of lower Monogenoidea. Folia Parasitol. 1969;16:320. [Google Scholar]
- Ferrari-Hoeinghaus A.P., Takemoto R.M., Pavanelli G.C. Digenetic trematode parasites of Loricariichthys platymetopon (Loricariidae: Siluriformes) of the upper Paraná river floodplain, Brazil. Acta Sci. Biol. Sci. 2007;29(3):327–329. [Google Scholar]
- Franceschini L., Acosta A.A., Zago A.C., Müller M.I., Silva R.J. Trinigyrus spp. (Monogenea: Dactylogyridae) from Brazilian catfishes: new species, molecular data and new morphological contributions to the genus. J. Helminthol. 2020 doi: 10.1017/S0022149X20000097. (in press) [DOI] [PubMed] [Google Scholar]
- Franceschini L., Zago A.C., Müller M.I., Francisco C.J., Takemoto R.M., Silva R.J. Morphology and molecular characterization of Demidospermus spirophallus n. sp., D. prolixus n. sp. (Monogenea: Dactylogyridae) and a redescription of D. anus in siluriform catfish from Brazil. J. Helminthol. 2018;92:228–243. doi: 10.1017/S0022149X17000256. [DOI] [PubMed] [Google Scholar]
- Froese R., Pauly D. World Wide Web electronic publication; 2018. FishBase.www.fishbase.org 6/2018, 10/2019. [Google Scholar]
- Guidelli G.M., Isaac A., Takemoto R.M., Pavanelli G.C. Endoparasites infracommunities of Hemisorubim platyrhyncos (Valenciennes, 1840) (Pisces: Pimelodidae) of the Baía River, upper Paraná river floodplain, Brazil: specific composition and ecological aspects. Braz. J. Biol. 2003;63(2):261–268. doi: 10.1590/s1519-69842003000200011. [DOI] [PubMed] [Google Scholar]
- Hahn N.S., Fugi R., Adrian I.F. Trophic ecology of the fish assemblages. In: Thomaz S.M., Agostinho A.A., Hahn N.S., editors. The Upper Paraná River and its Floodplain: Physical Aspects, Ecology and Conservation. Backhuys Publishers; Leiden: 2004. pp. 247–269. [Google Scholar]
- Hammer Ø., Harper D.A., Ryan P.D. PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001;4(1):9. [Google Scholar]
- Heywood V.H. Cambridge University Press; Cambridge: 1995. Global Biodiversity Assessment. United Nations Environment Programme. [Google Scholar]
- Holmes J.C., Price P.W. Communities of parasites. In: Anderson D.J., editor. Kikkawa J. Community Ecology: Patterns and Process. Blackwell Scientific Publications; Oxford, U.K: 1986. pp. 187–213. [Google Scholar]
- Humason G.L. W.H. Freeman Co.; San Francisco, California: 1979. Animal Tissue Techniques. [Google Scholar]
- Kohn A., Fernandes B.M.M., Cohen S.C. Editora Imprinta; Rio de Janeiro: 2007. South American Trematodes Parasites of Fishes. [Google Scholar]
- Krebs C.J. Harper & Row; New York: 1989. Ecological Methodology. [Google Scholar]
- Kritsky D.C., Boeger W.A., Thatcher V.E. Neotropical monogenea. 9. Status of Trinigyrus hanek, Molnar & Fernando, 1974 (Dactylogyridae) with descriptions of two new species from loricariid catfishes from the Brazilian Amazon. Proc. Biol. Soc. Wash. 1986;99:392–398. [Google Scholar]
- Kritsky D.C., Van Every L.R., Boeger W.A. Neotropical monogenoidea. 27. Two new species of Telethecium gen. sp. from the nasal cavities of Central amazonian fishes and a redescription of Kritskyia moraveci Kohn, 1990 (Dactylogyridae, ancyrocephalinae) J. Helminthol. Soc. Wash. 1996;63(1):35–41. [Google Scholar]
- Kritsky D.C., Boeger W.A. Neotropical Monogenoidea. 35. Pavanelliella pavanellii, a new genus and species (Dactylogyridae, Ancyrocephalinae) from the nasal cavities of siluriform fishes in Brazil. J. Helminthol. Soc. Wash. 1998;65(2):160–163. [Google Scholar]
- Lafferty K.D. Environmental parasitology: what can parasites tell us about human impacts on the environment? Parasitol. Today. 1997;13:251–255. doi: 10.1016/s0169-4758(97)01072-7. [DOI] [PubMed] [Google Scholar]
- Lafferty K.D., Kuris A.M. Parasitism and environmental disturbances. In: Thomas F., Renaud F., Guégan J.F., editors. Parasitism & Ecosystems. Oxford University Press; 2005. [Google Scholar]
- Lafferty K.D., Allesina S., Arim M., Briggs J., de Leo G., Dobson A.P., Dunne J.A., Johnson P.T.J., Kuris A.M., Marcogliese D.J., Martinez N.D., Memmott J., Marquet P.A., McLaughlin J.P., Mordecai E.A., Pascual M., Poulin R., Thieltges D.W. Parasites in food webs: the ultimate missing links. Ecol. Lett. 2008;11:533–546. doi: 10.1111/j.1461-0248.2008.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos J.R.G., Santos M.Q.C., Araújo C.S.O., Andrade S.M.S., Viana G.M. Parasitological evaluation and body indices of Osteoglossum bicirrhosum (Vandelli, 1829) traded in a fair of Manaus, Amazonas, Brazil. J. Fish. Sci. 2012;6:263–270. [Google Scholar]
- Levsen A., Lunestad B.T., Berland B. Parasites in farmed fish and fishery products. In: Øyvind L., editor. Improving Farmed Fish Quality and Safety. Woodehead Publishing; Sawston, UK, Cambridge: 2008. pp. 428–445. [Google Scholar]
- Locke S.A., Caffara M., Marcogliese D.J., Fioravanti M.L. A large-scale molecular survey of Clinostomum (Digenea, Clinostomidae) Zool. Scr. 2014;44:203–217. [Google Scholar]
- Locke S.A., Al-Nasiri F.S., Caffara M., Drago F., Kalbe M., Lapierre A.R., McLaughlin J.D., Nie P., Overstreet R.M., Souza G.T.R., Takemoto R.M., Marcogliese D.J. Diversity, specificity and speciation in larval Diplostomidae (Platyhelminthes: Digenea) in the eyes of freshwater fish, as revealed by DNA barcodes. Int. J. Parasitol. 2015;45:841–855. doi: 10.1016/j.ijpara.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Luque J.L., Poulin R. Metazoan parasite species richness in Neotropical fishes: hotspots and the geography of biodiversity. Parasitology. 2007;134:865–878. doi: 10.1017/S0031182007002272. [DOI] [PubMed] [Google Scholar]
- Luque J.L., Aguiar J.C., Vieira F.M., Gibson D.I., Santos C.P. Checklist of Nematoda associated with the fishes of Brazil. Zootaxa. 2011;3082:1–88. [Google Scholar]
- Luque J.L., Pereira F.B., Alves P.V., Oliva M.E., Timi J.T. Helminth parasites of South American fishes: current status and characterization as a model for studies of biodiversity. J. Helminthol. 2016;91(2):150–164. doi: 10.1017/S0022149X16000717. [DOI] [PubMed] [Google Scholar]
- Matsunaka A.M., Santos R.M., Posso S.R. Abundância e riqueza da avifauna nos buritizais da RPPN Foz do Rio Aguapeí, Paulicéia/SP. Fórum Ambient. Alta Paul. 2014;10(3):42–55. [Google Scholar]
- Marcogliese D.J., Cone D.K. Food webs: a plea for parasites. Trends Ecol. Evol. 1997;12:320–325. doi: 10.1016/S0169-5347(97)01080-X. [DOI] [PubMed] [Google Scholar]
- Mendoza-Franco E.F., Mendoza-Palmero C.A., Scholz T. New species of Ameloblastella Kritsky, mendoza-franco & Scholz, 2000 and Cosmetocleithrum Kritsky, Thatcher & Boeger, 1986 (monogenea: Dactylogyridae) infecting the gills of catfishes (Siluriformes) from the Peruvian Amazonia. Syst. Parasitol. 2016;93:847–862. doi: 10.1007/s11230-016-9671-7. [DOI] [PubMed] [Google Scholar]
- Mendoza-Palmeiro C.A., Scholz T., Mendonza-Franco E.F., Kuchta R. New species and geographical records of dactylogyrids (monogenea) of catfish (Siluriformes) from the Peruvian Amazonia. J. Parasitol. 2012;98(3):484–497. doi: 10.1645/GE-2941.1. [DOI] [PubMed] [Google Scholar]
- Mendoza-Palmero C.A., Blasco-Costa I., Scholz T. Molecular phylogeny of neotropical monogeneans (Platyhelminthes: monogenea) from catfishes (Siluriformes) Parasites Vectors. 2015;8:164. doi: 10.1186/s13071-015-0767-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Palmero C.A., Mendoza-Franco E.F., Acosta A.A., Scholz T. Walteriella n. g. (Monogenoidea: Dactylogyridae) from the gills of pimelodid catfishes (Siluriformes: Pimelodidae) from the Peruvian Amazonia based on morphological and molecular data. Syst. Parasitol. 2019;96:441–452. doi: 10.1007/s11230-019-09866-8. [DOI] [PubMed] [Google Scholar]
- Moravec F. Academia Praha; Czech Republic: 1998. Nematodes of Freshwater Fishes of the Neotropical Region. [Google Scholar]
- Orr R., Fisher J.P. Trinational risk assessment guidelines for aquatic alien invasive species. In: Orr R., Fisher J.P., editors. Trinational Risk Assessment Guidelines for Aquatic Alien Invasive Species, Commission for Environmental Cooperation Project Report. 2009. pp. 9–15. Montreal, Canada. [Google Scholar]
- Ota R.R., Message H.J., Graça W.J., Pavanelli C.S. Neotropical Siluriformes as a model for insights on determining biodiversity of animal groups. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0132913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota R.R., Deprá G.C., Graça W.J., Pavanelli C.S. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes: revised, annotated and updated. Neotrop. Ichthyol. 2018;16(2) [Google Scholar]
- Pantoja C., Scholz T., Luque J.L., Jones A. First molecular assessment of the interrelationships of cladorchiid digeneans (Digenea: Paramphistomoidea), parasites of Neotropical fishes, including descriptions of three new species and new host and geographical records. Folia Parasitol. 2019;66 doi: 10.14411/fp.2019.011. [DOI] [PubMed] [Google Scholar]
- Pavanelli G.C., Rego A.A. Novas espécies de proteocefalídeos (Cestoda) de Hemisorubim platyrhynchos (Pisces: Pimelodidae) do Estado do Paraná. Rev. Bras. Biol. 1989;49:381–386. [Google Scholar]
- Peretti D., Andrian I.F. Trophic structure of fish assemblages in five permanent lagoons of the high Paraná River floodplain, Brazil. Environ. Biol. Fish. 2004;71:95–103. [Google Scholar]
- Pérez-Ponce de León G., Pinacho-Pinacho C.D., Mendoza-Garfias M., García-Varela M. Phyllodistomum spinopapillatum sp. nov. (Digenea: gorgoderidae), from the Oaxaca killifish Profundulus balsanus (Osteichthyes: Profundulidae) in Mexico, with new host and locality records of P. inecoli: morphology, ultrastructure and molecular evidence. Acta Parasitol. 2015;60:298–307. doi: 10.1515/ap-2015-0042. [DOI] [PubMed] [Google Scholar]
- Petter A.J., Cassone J. Nematodes de Poisson du Paraguay; I. Ascaridoidea: Sprentascaris, n. gen. Rev. Suisse Zool. 1984;91:617–634. [Google Scholar]
- Poulin R. Determinants of host-specificity in parasites of freshwater fishes. Int. J. Parasitol. 1992;22:753–758. doi: 10.1016/0020-7519(92)90124-4. [DOI] [PubMed] [Google Scholar]
- Poulin R. The disparity between observed and uniform distributions – a new look at parasite aggregation. Int. J. Parasitol. 1993;23(7):937–944. doi: 10.1016/0020-7519(93)90060-c. [DOI] [PubMed] [Google Scholar]
- Poulin R. The evolution of monogenean diversity. Int. J. Parasitol. 2002;32:245–254. doi: 10.1016/s0020-7519(01)00329-0. [DOI] [PubMed] [Google Scholar]
- Ramos I.P., Franceschini L., Zago A.C., Zica E.O.P., Wunderlich A.C., Carvalho E.D., Silva R.J. New host records and a checklist of fishes infected with Austrodiplostomum compactum (Digenea: Diplostomidae) in Brazil. Rev. Bras. Parasitol. Vet. 2013;22(4):511–518. doi: 10.1590/S1984-29612013000400010. [DOI] [PubMed] [Google Scholar]
- Razo-Mendivil U., Pérez Ponce de León G., Rubio-Godoy M. Integrative taxonomy identifies a new species of Phyllodistomum (Digenea: gorgoderidae) from the twospot livebearer, Heterandria bimaculata (Teleostei: Poeciliidae), in Central Veracruz, Mexico. Parasitol. Res. 2013;112:4137–4150. doi: 10.1007/s00436-013-3605-y. [DOI] [PubMed] [Google Scholar]
- Rózsa L., Reiczigel J., Majoros G. Quantifying parasites in samples of hosts. J. Parasitol. 2000;86(2):228–232. doi: 10.1645/0022-3395(2000)086[0228:QPISOH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Santos C.P., Gibson D.I., Tavares L.E.R., Luque J.L. Checklist of Acanthocephala associated with the fishes of Brazil. Zootaxa. 2008;1938:1–22. [Google Scholar]
- Sasal P., Desdevises Y., Morand S. Host-specialization and species diversity in fish parasites: phylogenetic conservatism? Ecography. 1998;21:639–643. [Google Scholar]
- Scholz T., Vargas-Vazquez J., Salgado-Maldonado G. Revision of Genarchella species (Digenea: Derogenidae) parasitizing freshwater fishes in Mexico and Central America. J. Nat. Hist. 1995;29:1403–1417. [Google Scholar]
- Stunzenas V., Petkeviciute R., Poddubnaya L.G., Staneviciute G., Zhokhov A.E. Host specificity, molecular phylogeny and morphological differences of Phyllodistomum pseudofolium Nybelin, 1926 and Phyllodistomum angulatum Linstow, 1907 (Trematoda: gorgoderidae) with notes on Eurasian ruffe as final host for Phyllodistomum spp. Parasit. Vectors. 2017;10:286. doi: 10.1186/s13071-017-2210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J.P., Lundberg J.G., Hardman M. A phylogenetic analysis of the major groups of catfshes (Teleostei: Siluriformes) using rag 1 and rag 2 nuclear gene sequences. Mol. Phylogenetics Evol. 2006;41:636–662. doi: 10.1016/j.ympev.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Sures B., Nachev M., SElbach C., Marcogliese D.J. Parasite responses to pollution: what we know and where we go in ‘Environmental Parasitology’. Parasites Vectors. 2017;10:65. doi: 10.1186/s13071-017-2001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas F., Bonsall M.B., Dobson A. Parasitism, biodiversity and conservation. In: Thomas F., Renaud F., Guégan J.F., editors. Parasitism & Ecosystems. Oxford. 2005. [Google Scholar]
- Vilela M.J., Dutra M.A., Marques H., Dias J.H.P., Langeani F., Carvalho F.R., Martins F.O. Bacia do Alto Paraná. XX Encontro Brasileiro de Ictiologia; Maringá: 2013. Resultados parciais do inventário da ictiofauna do rio Aguapeí, na área da RPPN Foz do Aguapeí, São Paulo. [Google Scholar]
- Woodland W.N.F. Some more remarkable cestodes from Amazon siluroid fish. Parasitology. 1935;27:207–225. [Google Scholar]
- Yamada P.O.F., Yamada F.H., Silva R.J., Anjos L.A. Ecological implications of floods on the parasite communities of two freshwater catfishes in a Neotropical floodplain. Acta Parasitol. 2017;62(2):312–318. doi: 10.1515/ap-2017-0039. [DOI] [PubMed] [Google Scholar]
- Yamada F.H., Acosta A.A., Yamada P.O.F., Scholz T., Silva R.J. A new species of aphanoblastella Kritsky, mendoza-franco and Scholz, 2000 (monogenea, Dactylogyridae) parasitic on heptapterid catfish (Siluriformes) in the neotropical region. Acta Parasitol. 2018;63(4):772–780. doi: 10.1515/ap-2018-0092. 2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.