Graphical abstract
Keywords: TBARS, Allium cepa, Artemia salina, Free radicals
Highlights
-
•
The antioxidant effects in vitro were evaluated, as well as the toxicological and mutagenic effects of ODG.
-
•
ODG reduced the levels of hydroxyl radicals, nitric oxide, and TBARS in vitro and was nontoxic at low concentrations.
-
•
ODG was able to inhibit the OH• radical by 36 % in the highest concentration tested.
-
•
The ODG substance was able to reduce lipid peroxidation by almost 65 % in the highest concentration tested.
Abstract
2-oleyl-1,3-dipalmitoyl-glycerol (ODG) was obtained from Platonia insignis (bacurizeiro) seeds. There are no studies on its toxicity and protective activities against oxidative stress. This study was aimed to evaluate antioxidant effects in vitro, as well as to evaluate the toxicological and mutagenic effects of the ODG. ODG showed a median lethal dose (LD50) greater than 1200 μg mL−1 in A. salina. In the assay of A. cepa (0.2–0.002 mg mL−1) the ODG compound at the highest concentration was slightly cytotoxic with decrease in the size of roots and mitotic indexes, but did not induce chromosomal alterations. ODG (8.75–140.00 μg mL−1) was found to reduce nitric oxide production by 41.6 %, while the antioxidant standard ascorbic acid (AA) reduced 54.14 %. ODG (15.625–250.00 μg mL−1) promoted removal of the hydroxyl radical by 35.69 % at the highest concentration and was able to prevent lipid peroxidation induced by 2,2'-azobis-2-amidinopropane (AAPH), inhibiting the amount of TBARS formed, up to 35.69 %, a result close to that obtained with AA. Thus, ODG moderately reduced the levels of hydroxyl radicals, nitric oxide, and TBARS in vitro and was nontoxic at low concentrations.
1. Introduction
Plants are a rich source of therapeutic agents for the prevention and treatment of numerous diseases comprising about 60 % of pharmaceuticals [1]. The incessant search for new drugs from plants led to the discovery of many metabolites with potential for drug development through the isolation, elucidation of structure, composition and evaluation of bioactivity based on phytochemical composition and uses in traditional medicine [1,2].
In this context, the plants belonging to the family Clusiaceae have potential medicinal value and are widely used in popular medicine mainly in Brazil. Several studies involving the biological-pharmacological properties of this family can be observed in the literature where the majority of these studies concentrate, mainly, in the vegetal extracts [3]. This family includes trees, shrubs, lianas and herbs of economic interest for the production of edible fruits, woods, chemicals of pharmaceutical interest and paints [4]. Related studies on the chemical composition of the plants of this family indicate that they are rich in xanthones and polyisoprenylated benzophenones that were isolated not only from the resins, but also in other parts of the plants [5,6]. The known biological activities include antidepressive, antioxidant, antifungal, anti-HIV and antibacterial activity.
P. insignis is a species belonging to the family Clusiaceae occurs in the Amazon to Piauí, where its fruit (bacuri) is very much consumed in natural or processed forms [7]. The greases obtained from bacuri seeds are commonly used as cicatrizantes and anti-inflammatory agents [8]. There are reports in the literature where the extracts of the seeds, fractions and isolated compounds were tested in several biological activities and presented promising results as, reduction of oxidative stress [[9], [10], [11]], elimination of parasites [12,13] and enzymatic inhibition of α-glucosidase and acetylcholinesterase [14,15]. Immuno-modulatory effects and low in vivo toxicity have also been reported for extracts obtained from seeds [4].
P. insignis seeds are rich in fatty acids, triacylglycerols and metabolites with potential therapeutic activity, such as xanthones that exhibit antiepileptic and antiparasitic effects [6,16] and polyisoprenylated benzophenones, with vasorelaxant effect in animal models [17]. A triacylglycerol isolated from the hexane extract of its seeds, 1,3-distearyl-2-oleylglycerol (TG1), in formulations, was effective in healing wounds in rats [18].
Free Radical is defined as any atom or molecule that has one or more unpaired electrons being commonly formed in biological processes and can be eliminated by antioxidant defenses [19]. Antioxidants can act as biologically important defenses against reactive species derived from oxygen (EROS) and nitrogen (ERNS) by preventing or reducing oxidative damage to human tissues produced by these reactive species.
The process of development of new drugs with antioxidant action has become an important object of research since the current pharmacological therapy for the treatment of various neurodegenerative diseases such as Alzheimer's, Parkinson's, multiple sclerosis, Huntington's disease, as well as epilepsy and some types of neoplasms can be performed due to the antioxidant properties of many natural and/or synthetic compounds [20]. In addition, compounds with antioxidant action may delay aging [21]. Thus, the search for new antioxidant agents is extremely necessary and currently it is an object of interest in new research by the pharmaceutical industry.
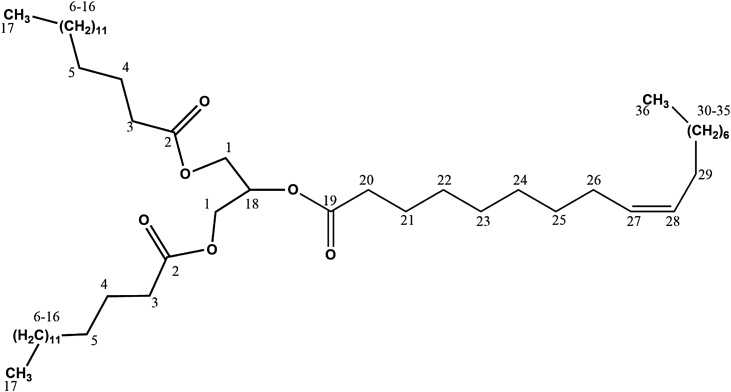
In this scenario, the compound 2-oleyl-1,3-dipalmitoyl-glycerol (ODG) (Fig. 1) is a triacylglycerol obtained from P. insignis seeds, for which there are no reports in the literature about their toxicity and protective activities against oxidative stress (OS), thus justifying the need to carry out this study. Considering the medicinal potential of the compounds obtained from P. insignis, this study was aimed to evaluate antioxidant effects in vitro, as well as to evaluate the toxicological and mutagenic effects of the 2-oleyl-1,3-dipalmitoyl-glycerol (ODG) compound, isolated from the dichloromethane fraction of the hexanic extract of the bacuri seeds.
Fig. 1.
Chemical structure of 2-oleil-1,3-dipalmitoil-glicerol (ODG).
2. Materials and methods
2.1. Reagents and chemicals
Potassium dichromate (K2Cr2O7) and copper sulphate (CuSO4.5H2O), FeSO4, Griess modified reagent, 2-deoxyribose, 2-thio-barbituric acid, (2,20-azobis(2-amidinopropan) dihydrochloride [AAPH]); sodium nitroprusside (SNP), trichloroacetic acid, hydrogen peroxide, ascorbic acid, acetic acid, and polyoxyethylenesorbitan monolate (Tween 80); dimethyl sulfoxide (DMSO) were purchased from Sigma Co. All other reagents were of analytical grade.
2.2. Preparation of the samples
The ODG compound was isolated from the dichloromethane fraction of the hexanic extract obtained from P. insignis seeds according to the methodology described by Cavalcante et al. (2019), [14]. The structure was elucidated by spectroscopic techniques (1H NMR, 13C NMR and FT-IR shown in supplementary material). The ODG compound and ascorbic acid (AA) were solubilized in 0.05 % (v/v) Tween 80 and saline solution with NaCl 0.9 % (w/v).
2.3. Toxicity tests
2.3.1. Artemia salina lethality test
Toxicity to Artemia salina was performed according to the assay proposed by Mclaughlin et al. [22]. Solutions with ODG (75–1200 μg mL−1) were prepared in triplicate and a negative control was prepared with saline solution alone. Subsequently, 10 nauplio in each tube were added to these solutions. After 24 h counting the survivors were performed, the procedure was repeated at intermediate concentrations to determine the median lethal dose (LD50).
2.3.2. Allium cepa toxigenic assay
The cytotoxicity and mutagenicity of the ODG compound in Allium cepa was conducted according to Guerra and Souza (2002) [23]. When roots of A. cepa with approximately 1.0 cm were placed in the solutions with ODG (0.002–0.2 mg mL−1), in triplicate and observed at different exposure times (24, 48 and 72 h). Root size (RS) was used as an evaluation of toxicity. The mutagenic potential was evaluated by analyzing the total chromosomal alterations (TCA) and micronuclei in the meristematic cells of A. cepa, observing 1000 cells per bulb. The cytotoxic potential was determined by the mitotic index (MI). The positive control (PC) was the diluted 6 × 10−4 mg mL−1 copper sulphate and the negative control (NC) was distilled water only.
2.4. Antioxidant activity
2.4.1. Inhibition test of nitric oxide radicals (NO•)
The radical nitric oxide (NO·) was generated from the spontaneous decomposition of sodium nitroprusside (SNP) in phosphate buffer 20 mmol L−1 (pH 7.4). After generation, NO• interacts with oxygen to produce nitrite ions, which was measured by Griess reaction [24]. The 200 μL of solutions in triplicate of the ODG compound of P. insignis seeds at 5 concentrations: 8.75 μg mL−1; 17.5 μg mL−1; 35 μg mL−1; 70 μg mL−1 and 140 μg mL−1 and ascorbic acid (AA) were added 1.0 mL of the 10 mmol L−1 SNP reaction medium in 20 mmol L−1 sodium phosphate buffer in pH 7.4. Subsequently, the tubes were placed in a water bath at 37 °C for 1 h. The 500 μL of Griess reagent was added until complete homogenization. After 10 min the absorbances were measured at 540 nm (AJX-3000PC UV–vis spectrophotometer).
The percentage reduction in nitrite production (%) was calculated from the formula % = SNP - C, where SNP corresponds to sodium nitroprusside, which is equivalent to the maximum production percentage (100 %) of the nitrite radical, and C corresponds to production of nitrite in the presence of ODG in the concentrations (8.75–140 μg mL−1). The results were expressed as percentage of nitrite formed by SNP alone [25].
2.4.2. Inhibition test of hydroxyl radicals (OH•)
Hydroxyl radicals were generated by the Fe3+-ascorbate-EDTA-H2O2 system. This assay was performed by a standard method [26]. The 200 μL of triplicate solutions of the compound ODG in 5 concentrations: 15.625 μg mL−1; 31.25 μg mL−1; 62.5 μg mL−1; 125 μg mL−1 and 250 μg mL−1 and from the positive control AA at the same concentrations were added 480 μL of the reaction medium and 20 μL of FeSO4 solution 6 mmol L−1. After 15 min at room temperature 500 μL of phosphoric acid 4 % was added, followed by the addition of 500 μL thiobarbituric acid – TBA 1 %. The solutions were heated in a water bath for 1 h at 37 °C and then cooled to room temperature for 10 min. The absorbances were measured at 532 nm (AJX-3000PC UV–vis spectrophotometer). The percentages of reduction in the production of malonaldehyde (%MDA) were calculated from the formula %MDA = System - C where, the System corresponds to a mixture of FeSO4, 2-deoxyribose, H2O2 and phosphate buffer equivalent at the maximum production percentage (100 %) of malonaldehyde, and C corresponds to the percentage of malonaldehyde production in the presence of ODG and AA in various concentrations (15.625–250 μg mL−1) [25].
2.4.3. Lipid peroxidation assay (TBARS)
In this essay egg yolk was used as a homogenate medium rich in lipids, according to the methodology proposed [27,28]. The 200 μL of the solutions of the compound ODG in 5 concentrations: 15.625 μg mL−1; 31.25 μg mL−1; 62.5 μg mL−1; 125 μg mL−1 and 250 μg mL−1 and the AA control at the same concentrations were transferred in triplicate to tubes with 500 μL of the egg yolk homogenate in 50 mmol L−1 phosphate buffer (pH 7.4). Then about 100 μL to 0.12 mol L−1 of 2,2'-azobis-2-methylpropinamide dihydrochloride (AAPH) were added to induce lipid peroxidation. Soon after, the system was kept under stirring for 0.5 h at 37 °C. After cooling, 500 μL of trichloroacetic acid was added and the system was centrifuged at 100 rpm for 10 min. To 500 μL of the supernatant was added 500 μL of thiobarbituric acid. The reaction was kept under heating at 95 °C for 0.5 h. Absorbances were measured at 532 nm (AJX-3000PC UV–vis spectrophotometer). The results were expressed as percentage of TBARS formed by AAPH alone (control induced during lipid peroxidation). The percentage of the production of TBARS (%TBARS) was calculated by the formula: %TBARS = [(Abscontrol(AAPH) - Abs reaction mixture)] x 100/Abscontrol (AAPH).
The antioxidant activities were expressed as EC50, defined as the effective concentration of the ODG compound required to reduce the initial concentration of the generated radicals by 50 %.
2.5. Statistical analyzes
The results are presented as mean ± standard deviation (SD). The data were analyzed by means of analysis of variance (ANOVA) followed by One-way, and Bonferroni’s tests by using GraphPad Prism (version 6.0). For statistical significance of the antioxidant assays, a unidirectional analysis of the variance followed by the multiple comparison of Holm-Šídák was used. Statistical significance was defined as p ≤ 0.05 in relation to control, at confidence level of 95 %. EC50 values were calculated by linear regression analysis between percent inhibition against compound concentrations.
3. Results and discussion
3.1. Toxicity against Artemia salina of the compound 2-oleyl-1,3-dimalpitoyl-glycerol
Toxicity tests are essential for the development of synthetic or natural medicines. Preclinical analyzes in vitro, in silico and in biological systems reveal specific toxic effects and safe doses that will guide future research [29].
The bioassay with A. salina is considered useful for preliminary evaluations of general toxicity, being widely used due to its low cost and rapidity [30]. The LD50 of the ODG, a lethal dose capable of killing 50 % of nauplii, was considered to be greater than the highest tested concentration, 1200 μg mL−1 in A. salina. Therefore, the ODG substance can be considered to have low toxicity, as proposed by Mclaughlin et al. (1998) [31] and when compared to the dichloromethane and ethyl acetate fractions of the ethanolic extract of the bacuri seeds, which had a mean toxicity of 24.89 and 129.0 μg mL−1, respectively [16]. The low cytotoxicity presented by the ODG corroborates with results obtained with another triglyceride isolated from the bacuri seeds, TG1 in cancer cells [32].
The results of this assay are shown in Table 1. There were no nauplii deaths at the five concentrations tested as well as the negative control.
Table 1.
Results of the toxicity test against Artemia salina. n = 6 in triplicate.
| Groups | Number of nauplii (t =0 h) | Number of nauplii (t =24 h) |
|---|---|---|
| NC | 10 | 10 |
| 1200 μg mL−1 | 10 | 10 |
| 600 μg mL−1 | 10 | 10 |
| 300 μg mL−1 | 10 | 10 |
| 150 μg mL−1 | 10 | 10 |
| 75 μg mL−1 | 10 | 10 |
NC: negative control artificial saline solution.
The ODG compound showed no toxicity to A. salina, since the LD50 > 1200 μg mL−1. According to the World Health Organization (WHO), substances with LD50 values below 1000 ppm in A. salina are considered to be non-toxic [33].
3.2. Cytotoxicity tests against Allium cepa of the compound 2-oleyl-1,3-dimalpitoyl-glycerol
The toxicogenic characterization of ODG was evaluated by macroscopic and cytogenetic parameters: root size; mitotic index and total chromosome alterations. From Table 2, its evident that in the lowest concentrations of the isolated compound ODG (0.002 and 0.02 mg mL−1) the results regarding toxicity, measured by root size (RS) and cytotoxicity, as measured by the mitotic index, showed statistically significant nontoxic effects. However, in the highest concentration tested, it was slightly toxic and cytotoxic as compared to the negative control (NC) at the exposure time, evaluated at 72 h, where the RS decreased from 206.20 ± 9.87 to 127.84 ± 8.78 the MI had a reduction from 53.80 ± 4.84 to 29.89 ± 4.12. Regarding the mutagenic evaluation, as measured by total chromosome alterations, it can be observed that none of the concentrations tested showed a statistically significant genotoxic effect as shown in Table 2.
Table 2.
Evaluation of toxic, cytotoxic and mutagenic effect of ODG in meristematic cells of Allium cepa.
| Treatment | Toxicity | Cytotoxicity | Mutagenicity |
|---|---|---|---|
| RS (mm) | MI | TCA | |
| NC | 206.20 ± 9.87b | 53.80 ± 4.84b | 2.80 ± 0.83b |
| PC | 71.40 ± 7.73ª | 11.36 ± 0.59a | 29.20 ± 2.95ª |
| 0.002 mg mL−1 | 244.66 ± 11.23b | 49.20 ± 2.37b | 6.00 ± 2.34b |
| 0.02 mg mL−1 | 164.64 ± 10.94b | 45.45 ± 4.67b | 3.80 ± 2.49b |
| 0.2 mg mL−1 | 127.84 ± 8.78ª | 29.89 ± 4.12ab | 6.90 ± 2.58b |
RS: root size; MI: mitotic index; TCA: total chromosomal alterations; NC: negative control (distilled water); PC: positive control (aqueous solution of copper sulphate at 6 × 10−4 mg mL−1). ANOVA-One-way and Bonferroni post-test. n = 5. Significant values (MD ± SD) of p < 0.05 for b compared to the untreated group, a to PC.
Another toxic and cytotoxic test used was Allium cepa. This assay is considered to be one of the best in vivo cytogenetic evaluation systems due to its high sensitivity, good correlation with other test systems, in addition to the convenience of large numbers of chromosomes (2n = 16), results can be extrapolated to other animals and plants [34,35].
Macroscopic analysis (root growth) of A. cepa, and MI, which is calculated by dividing the number of cells divided by the total number of cells observed, showed that ODG had a significant toxic effect on the test concentration (0.2 μg mL−1), compared to the negative control (p < 0.001). A reduction in MI can be interpreted as cell death resulting from abnormal cell conditions caused by the treatment, ranging from the normal course of the cell cycle, DNA breakage, inhibition of microtubule formation, to the reduction of ATP levels, important in the dynamics of cell division [36]. However, mutagenicity was not significant when compared to the control. This result shows that the compound has low and selective toxicity and is dependent on high concentrations, similar to another compound isolated from bacuri seeds, Garcinielliptona FC (GFC), which in mice presented low frequency of damage to the bone marrow, liver and central nervous system [13].
3.3. Inhibition test of nitric oxide radicals (NO•)
The antioxidant assays of medicinal plants are performed to evaluate their contribution in minimizing the effects of oxidative stress on pathophysiological processes, as occurs in neurodegenerative diseases [37]. In Alzheimer's disease (AD) oxidative stress contributes to accelerate pathological processes related to pathology, such as memory impairment and amyloid plaque deposition [38]. Although the body provides endogenous antioxidant defenses, excess oxidative stress causes an imbalance, which can be counterbalanced by supplementation with natural antioxidants [39]. Thus, substances obtained from natural products could be an alternative for the prevention and control of oxidative stress, and consequently, modulate the underlying neurodegenerative mechanisms [40], as the natural substance ODG.
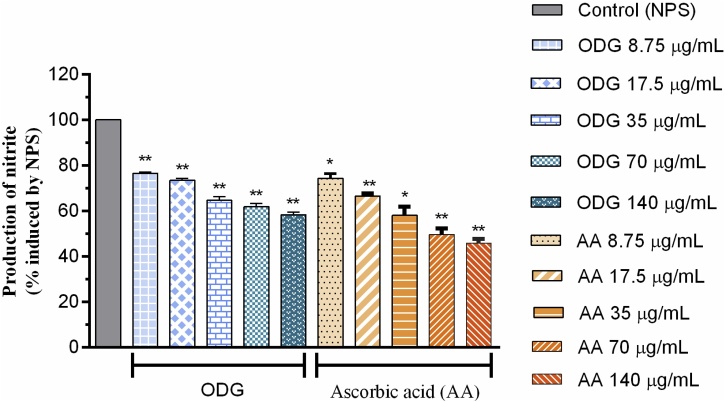
The results shown in Fig. 2 demonstrate that the ODG compound was able to decrease the nitrite content from sodium nitroprusside in a moderate manner, and in a concentration dependent manner, when compared to the AA control. The concentrations used (8.75, 17.50, 35.00, 70.00 and 140 μg mL−1) of ODG and the AA control reduced nitrite production in (23.44, 26.58; 35.35, 38.19 and 41.6 %) and (25.66, 33.47, 41.96, 50.32 and 54.14 %), respectively. The concentration of ODG that reduced 50 % of the nitrite radical (EC50) content was approximately 29.92 μg mL−1 while that of the EC50 ascorbic acid was 29.77 μg mL−1 values as can be seen in Table 3.
Fig. 2.
Antioxidant capacity of the substance ODG and the positive control ascorbic acid, in different concentrations (8.75–140 μg/mL), against the production of the nitrite radical.
Table 3.
EC50 of in vitro antioxidant potential of ODG and AA in TBARS production, (OH)• hydroxyl radical content and nitrite formation.
| Test in vitro | ODG |
AA |
||
|---|---|---|---|---|
| EC50 (μg mL−1) | ||||
| EC50 | 95% | EC50 | 95% | |
| NO· | 29.92 | 24.97–35.86 | 29.77 | 23.50–37.70 |
| OH· | 118.90 | 88.06–158.6 | 85.57 | 72.79–100.6 |
| TBARS | 69.39 | 45.57–105.7 | 26.80 | 14.02–51.22 |
EC50: Efetive concentration 50 % in μg mL−1; 95 %: Confidence Interval in μg mL−1.
ODG effects against the production of the metabolite nitrite by decomposition of sodium nitroprusside (SNP). Values represent the mean ± E.P.M. of the values of inhibition in vitro, n = 5 experiments in triplicate. Statistically significant "p" is considered when ** p ≤ 0.01; * p ≤ 0.05 when compared to control. Unidirectional analysis of variance followed by multiple comparison of Holm-Šídák.
Nitric oxide (NO)• is an essential bioregulatory molecule with many physiological functions. However, the chronic expression of the nitric oxide radical is associated with several carcinomas and inflammatory conditions [41]. Sustained levels of production of this radical are directly toxic to the tissues and contribute to vascular collapse, cerebral hypoperfusion with consequent neural degeneration as occurs in AD [42,43]. The toxicity of NO· increases greatly when it reacts with the superoxide radical and forms peroxynitrite (ONOO−) highly reactive to cell membranes [44]. Antioxidant substances act by competing with nitric oxide for oxygen and can thus inhibit the production of nitrite ions and reduce membrane [45,46]. The ODG compound moderately reduces the nitrite content formed by the Griess reaction at concentrations close to ascorbic acid, so its use in the prevention of the lesions produced by nitric oxide to the cell membranes in vivo methodologies is stimulated.
The ODG substance was able to sequester the NO• radical and consequently decrease the nitrite content formed significantly by up to 41.6 % at the highest concentration tested, and that the ascorbic acid used as a positive control at the same concentration reduced the formation of the nitrite in 54.14 % (Fig. 2). Better results than those obtained by Moukett et al. (2015) when he used the ethanolic and hydroethanolic extracts of the Piper guineense stem at a concentration of 150 μg mL−1 [47]. The effective concentration (EC50) of ODG reducing the nitrite radical content by 50 % was approximately 29.92 μg mL−1, varying from 24.97 to 35.86 μg mL−1 with a 95 % confidence interval and R2 of 0.9260. The EC50 of the positive control AA was 29.77 μg mL−1 ranging from 23.50 to 37.70 μg mL−1 with a 95 % confidence interval and R2 of 0.8890 (Table 3) with those similar values it can be said that the ODG substance is natural antioxidant as good as the synthetic antioxidant ascorbic acid.
The results obtained from the EC50 of ODG were significantly better than those found by Moukett et al. [47] in ethanol extracts, leaves (EC50 = 126.51 μg mL−1) and stem (EC50 = 538.83 μg mL−1), as well as in hydroethanolic extracts, leaves (EC50 = 83.39 μg mL−1) and stem (EC50 = 433.17 μg mL−1) of P. guineense.
Values close to that found were determined in experiments performed by Sannigrahi et al. [48], where the ethyl acetate fraction of Enhydra fluctuans Lour's methanolic extract, widely used by indigenous medicine for different purposes, also moderately inhibited nitric oxide in dose dependent manner with an average effective concentration (EC50) of 26.1 μg mL−1. In tests with Rhododendron anthopogonoides Maxim, a traditional Tibetan medicinal species, an EC50 of 183.83 ± 2.34 μg mL−1 [46] was obtained, much higher values compared to ODG.
3.4. Inhibition test of hydroxyl radicals (OH•)
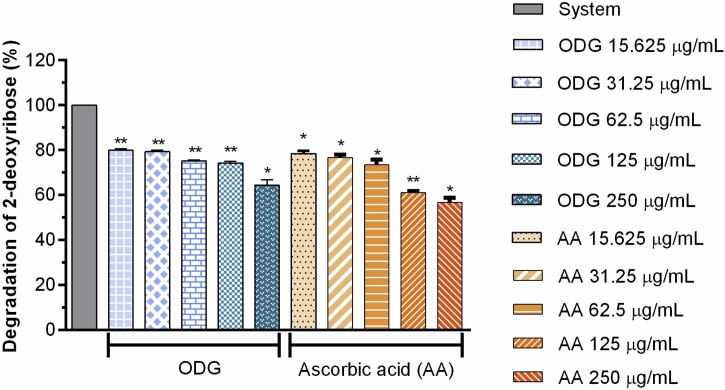
Hydroxyl radical scavenging activity was quantified using oxidative degradation of 2-deoxyribose in vitro, which produces malonaldehyde by condensation with 2-thiobarbituric acid, forming a pink chromogen [49,50]. At the concentrations tested, ODG was able to reduce the hydroxyl radical moderately and in a concentration dependent manner when compared to the AA control. The concentrations used (15.625–250 μg mL−1) of ODG and the AA control reduced hydroxyl production in (19.92, 20.66, 24.87, 25.79 and 35.69 %) and (21.51, 23.38, 26.51, 38.88 and 43.23 %), respectively (Fig. 3). The average effective concentration (EC50) of ODG for inhibition of hydroxyl radical formation was approximately 118.9 μg mL−1, in its turn, the EC50 value of ascorbic acid was approximately 85.57 μg mL−1 as shown in Table 3.
Fig. 3.
Antioxidant capacity of the substance ODG and the positive control ascorbic acid, in different concentrations (15.625–250 μg/mL), against the hydroxyl radical formation.
The results obtained from the EC50 of the ODG were significantly better than those found by Moukett et al. [47] in the ethanolic extracts of the stem (EC50 = 211.37 μg mL−1) and hydroethanolic extracts of the stem (EC50 = 173.79 μg mL−1), however, when compared to the ethanolic extracts of the leaves (EC50 = 82.94 μg mL−1) and hydroethanolic (EC50 = 53.56 μg mL−1) of P. guineense, the values of the substance ODG were less effective.
ODG effects on the removal of hydroxyl radical. Values represent the mean ± E.P.M. of the values of inhibition in vitro, n = 5 experiments in triplicate. Statistically significant "p" is considered when ** p ≤ 0.01; * p ≤ 0.05 when compared to control. Unidirectional analysis of variance followed by multiple comparison of Holm-Šídák.
Hydroxyl radicals are also involved in the pathogenesis of many diseases. They are generated under physiological and pathological conditions, and are the main reactive species of oxygen, causing lipid peroxidation and enormous biological damages [41,51]. When these radicals react with polyunsaturated fatty acids of cell membrane phospholipids hydroxyl radicals produce lipid hydroperoxides that can be decomposed by numerous carbonyl products, such as malondialdehyde [52]. Carbonyl products are responsible for damage to DNA and proteins, culminating in cancer and aging-related diseases. Thus, the decrease in MDA concentration indicates the role of the compounds tested as antioxidant [53]. In Alzheimer's MDA content is increased as a consequence of the vulnerability to lipid peroxidation of the brain, which occurs due to the presence of a rich content of fatty acids and phospholipids in their membranes and their high oxygen consumption [54].
The compound ODG suppressed degradation of deoxyribose with hydroxyl radical mediated a concentration dependent manner, as the AA. ODG was able to inhibit the OH• radical by 36 % in the highest concentration tested, while ascorbic acid, used as a positive control at this same concentration, reduced hydroxyl radical formation by almost 44 % (Fig. 3). The EC50 of ODG for inhibition of hydroxyl radical formation was approximately 118.9 μg mL−1 ranging from 88.06–158.6 μg mL−1 with 95 % confidence interval and R2 of 0.8177 on the other EC50 of ascorbic acid was approximately 85.57 μg mL−1 ranging from 72.79–100.6 μg mL−1 with a 95 % confidence interval and R2 of 0.9327 as can be observed in Table 3. The EC50 of the ODG was 1.4 higher than that of AA. The results obtained with the compound ODG are lower than those found for the hexanic extract of the bacuri seeds and their inclusion complex, the greatest reduction of which was observed in the MDA content [11]. As with the toxic effect, the antioxidant effects of bacuri seeds extract can also be justified by the presence of other substances, such as phenolic compounds, which act additionally to reduce reactive species [16].
3.5. Lipid peroxidation assay (TBARS)
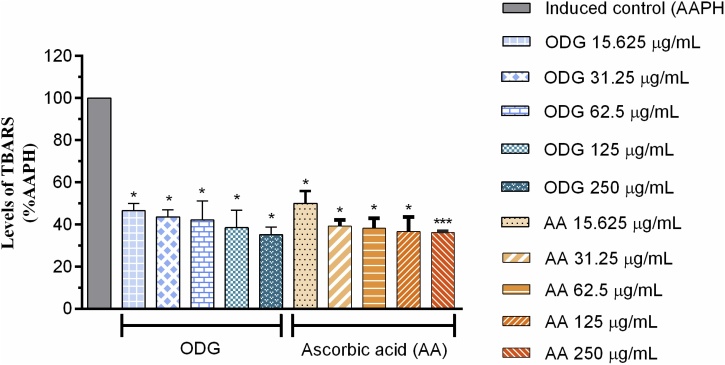
Lipid peroxidation was analyzed by the TBARS quantification method, which is a widely used assay to estimate lipid peroxidation in membranes, as well as in biological systems [52]. The ODG compound at the concentrations tested (15.625–250 μg mL−1) significantly reduced lipid peroxidation in (53.43, 56.33, 57.74, 61.48 and 64.7 %), respectively, in relation to the AAPH group. Ascorbic acid at the same concentrations reduced lipid peroxidation in (49.96, 60.72, 61.74, 63.36 and 63.72 %) as shown in Fig. 4. The EC50 of the ODG presented an approximate value of 69.39 μg mL−1 and a value for AA, under the same conditions, was 26.80 μg mL−1 as shown in Table 3.
Fig. 4.
Antioxidant capacity of the ODG substance against the production of TBARS and the positive ascorbic acid control at different concentrations (15.625–250 μg mL-1) against the production of AAPH.
ODG effects against the production of reactive substances to thiobarbituric acid. Values represent the mean ± E.P.M. of the values of inhibition in vitro, n = 5 experiments in triplicate. Statistically significant "p" is considered when *** p ≤ 0.001; * p ≤ 0.05 when compared to control. Unidirectional analysis of variance followed by multiple comparison of Holm-Šídák.
Lipid peroxidation is a free radical-related process that in biologic systems may occure under enzymatic control, or non-enzymatically. This latter form is associated mostly with cellular damage as a result of oxidative stress. The primary targets for attack by oxygen free radicals are the polyunsaturated fatty acids of membrane phospholipids [55]. Lipid peroxidation can be defined as biological damage caused by free radicals [56]. Numerous extracts of medicinal plants have shown an inhibitory effect against lipid peroxidation as verified by the in vitro TBARS production method [57]. The ODG substance was able to reduce lipid peroxidation by almost 65 % in the highest concentration tested, while ascorbic acid, used as a positive control, decreased lipid peroxidation by almost 64 %, a very considerable value, as shown Fig. 3. ODG decreased levels of lipid peroxidation in vitro and promoted 50 % reduction in the production of reactive substances with thiobarbituric acid (TBARS) with an EC50 value of 69.39 μg mL−1 ranging from 45.57–105.7 μg mL−1 with 95 % confidence interval and R2 0.7248. The EC50 value of AA under the same conditions was 26.80 μg mL−1 ranging from 14.02–51.22 μg mL−1 with a 95 % confidence interval and R2 0.7659 as shown in Table 3. Although the EC50 of the ODG was 3.9 higher than that of the AA control, this substance is considered a good natural antioxidant since it significantly reduced the production of TBARS.
Girish and et al. [58] determined the activities of eliminating DPPH radicals in isolated flavonoid glycosides, vitexin, isovitexin and ascorbic acid, finding EC50 values of 19.2, 21 and 14.2 μM, respectively.
According to Ahmadi et al. [59], the lower the EC50 value, the higher the antioxidant potential of the sample. Although the ODG compound exhibited high effective mean concentrations of inhibition of hydroxyl radicals and TBARS relative to the positive control AA was effective at all concentrations tested in preventing peroxidation of cell membranes, induced by peroxyl radicals generated by AAPH, in a very significant way. This fact can be explained in part, by the interaction of ODG components with intermediates of partial oxygen reduction and nitric oxide metabolism, as well as possible protection against membrane lipid autoxidation, the main factors involved in lipid peroxidation [53]. These results suggest that the compound ODG exerts a protective action on the biomolecules against the peroxidation of phospholipids, triacylglycerols, polyunsaturated fatty acids, and essential for cell membrane function and paracrine signaling [21].
4. Conclusions
The 2-oleyl-1,3-dipalmitoyl-glycerol compound showed no toxicity to A. salina. In relation to the tests against A. cepa the substance showed nontoxic and non-cytotoxic effects at low concentrations, but at higher concentration the ODG substance demonstrated slight toxicity and cytotoxicity in comparison to the negative control. ODG substance had no mutagenic effect. The ODG substance showed no larvicidal activity against A. aegypti larvae at the analyzed concentrations.
The results obtained in the present study demonstrate that the substance ODG produces antioxidant effect by the in vitro methods used. However, further studies are needed to elucidate the possible mechanism of action that measure the antioxidant activity of ODG, especially in vivo to justify its possible use in clinical treatment.
Author’s contribution
All authors contributed to conception, design, acquisition of data, the analysis and writing the manuscript. The authors have seen and approved the manuscript being submitted. The authors affirm that the article is the authors original work and has not received prior publication.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by CNPq and CAPES. The UFPI for administrative and technical support.
References
- 1.Pan S.Y., Zhou S.F., Gao S.H., Yu Z.L., Zhang S.F., Tang M.K., Ko K.M. New perspectives on how to discover drugs from herbal medicines: CAM’s outstanding contribution to modern therapeutics. Evid. Based Complementary Altern. Med. 2013:1–25. doi: 10.1155/2013/627375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dias D.A., Urban S., Roessner U. A historical overview of natural products in drug discovery. Metabolites. 2012;2:303–336. doi: 10.3390/metabo2020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilanda D.C., Dimo T., Djomeni P.D.D., Bella N.M.T., Aboubakar O.B.F., Nguelefack T.B., Tan P.V., Kamtchouing P. Antihypertensive and antioxidant effects of Allanblackia floribunda Oliv. (Clusiaceae) aqueous. J. Ethnopharmacol. 2010;128:634–640. doi: 10.1016/j.jep.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 4.Lustosa A.K.M., Arcanjo D.D., Ribeiro R.G., Rodrigues K.A.F., Passos F.F.B., Piauilino C.A., Carvalho F.A.A. Immunomodulatory and toxicological evaluation of the fruit seeds from Platonia insignis, a native species from Brazilian Amazon Rainforest. Rev. Bras. Farmacogn. 2016;26:77–82. [Google Scholar]
- 5.Piccinelli A.L., Campone L., Piaz F.D., Cuesta-Rubio O., Rastrelli L. Fragmentation pathways of polycyclic polyisoprenylated benzophenones and degradation profile of nemorosone by multiple-stage tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 2009;20:1688–1698. doi: 10.1016/j.jasms.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Costa-Júnior J.S., Almeida A.A.C., Tomé A.D.R., Citó A.M.G.L., Saffi J., Freitas R.M. Evaluation of possible antioxidant and anticonvulsant effects of the ethyl acetate fraction from Platonia insignis Mart. (Bacuri) on epilepsy models. Epilepsy Behav. 2011;22:678–684. doi: 10.1016/j.yebeh.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 7.Aguiar L.P., Figueiredo R.W.D., Alves R.E., Maia G.A., Souza V.A.B.D. Physical and physico-chemical characterization of fruits from different genotypes of bacuri (Platonia insignis Mart.) Food Sci. Technol. 2008;28:423–428. [Google Scholar]
- 8.Santos-Júnior R.Q., Soares L.C., Maia-Filho A.L.M., Araujo K.S., Santos I.M.S.P., Costa-Júnior J.S., Saffi J. Estudo histologico da cicatrização de feridas cutâneas utilizando a banha de bacuri (Platonia insignis Mart.) ConScientiae Saude. 2010;9:1–7. [Google Scholar]
- 9.Costa-Júnior J.S., Freitas R.M., Citó A.M.G.L., Henriques J.A.P., Saffi J. Evaluation of effects of ethanolic extract (EE) from Platonia insignis Mart. on pilocarpine-induced seizures. J. Biol. Sci. 2010;10:747–753. [Google Scholar]
- 10.Rufino M.S.M., Alves R.E., Brito E.S., Jiménez J.P., Saura-Calixto F., Mancini-Filho J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010;121:996–1002. [Google Scholar]
- 11.Nascimento J.L., Coêlho A.G., Barros Y.S.O., Silva O.A., Freitas R.M., Rocha M.S., Citó A.M.G.L. Avaliação da atividade antioxidante in vitro do extrato hexânico da semente do bacuri (Platonia insignis Mart.) e de seu complexo de inclusão com β-ciclodextrina. Boletim Informativo Geum. 2014;2:1–18. [Google Scholar]
- 12.Silva A.P., Silva M.P., Oliveira C.G., Monteiro D.C., Pinto P.L., Mendonça R.Z., Moraes J. Garcinielliptone FC: antiparasitic activity without cytotoxicity to mammalian cells. Toxicol. In Vitro. 2015;29:681–687. doi: 10.1016/j.tiv.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Coelho V.R., Prado L.S., Rossato R.R., Ferraz A.B.F., Vieira C.G., Souza L.P., Pfluger P., Regner G.G., Valle M.T.C., Leal M.B., Dallegrave E., Corrêa D.S., Picada J.N., Pereira P. A 28‐day sub‐acute genotoxic and behavioural assessment of garcinielliptone FC. Basic Clin. Pharmacol. Toxicol. 2018;123:207–212. doi: 10.1111/bcpt.13010. [DOI] [PubMed] [Google Scholar]
- 14.Cavalcante A.C., Feitosa C.M., Santos F.P.S., Sousa A.P.R., Júnior R.S.S., Souza A.A., Pinto B.F., Araújo C.M., Rashed K. Elaboration and characterization of the inclusion complex between β-cyclodextrin and the anticholinesterase 2-oleyl-1,3-dipalmitoyl-glycerol extracted from the seeds of Platonia insignis Mart. J. Mol. Struct. 2019;1177:286–301. [Google Scholar]
- 15.Freitas F.A., Araújo R.C., Soares E.R., Nunomura R.C.S., Silva F.M.A., Silva S.R.S., Souza A.Q.L., Souza A.D.L., Franco-Montalban F., Acho L.D.R., Lima E.S., Bataglion G.A., Koolen H.H.F. Biological evaluation and quantitative analysis of antioxidant compounds in pulps of the Amazonian fruits bacuri (Platonia insignis Mart.), ingá (Inga edulis Mart.), and uchi (Sacoglottis uchi Huber) by UHPLC‐ESI‐MS/MS. J. Food Biochem. 2017;42:1–10. [Google Scholar]
- 16.Costa-Júnior J.S., Ferraz A.B.F., Sousa T.O., Silva R.A.C., Lima S.G., Feitosa C.M., Citó A.M.G.L., Cavalcante A.A.C.M., Freitas R.M.A. Sperotto, Investigation of Biological Activities of Dichloromethane and Ethyl Acetate Fractions of Platonia insignis Mart. seed. Basic Clin. Pharmacol. Toxicol. 2013;112:34–41. doi: 10.1111/j.1742-7843.2012.00924.x. [DOI] [PubMed] [Google Scholar]
- 17.Arcanjo D.D.R., Costa-Júnior J.S., Moura L.H.P., Ferraz A.B.F., Rossatto R.R., David J.M., Quintans-Júnior L.J., Oliveira R.C.M., Citó A.M.G.L., Oliveira A.P., Garcinielliptone F.C. A polyisoprenylated benzophenone from Platonia insignis Mart., promotes vasorelaxant effect on rat mesenteric artery. Nat. Prod. Res. 2014;28:923–927. doi: 10.1080/14786419.2014.889136. [DOI] [PubMed] [Google Scholar]
- 18.Mendes M.C.S., Lacerda J.S., Junior L.M.R., Silva M.L.D.G., Coelho M.L., Tome A.R., Freitas R.M. Evaluation of the cicatrizant activity of a semisolid pharmaceutical formulation obtained from Platonia insignis Mart. Afr. J. Pharm. Pharmacol. 2015;9:154–164. [Google Scholar]
- 19.Gutteridge J.M.C., Halliwell B. Antioxidants: molecules, medicines, and myths. Biochem. Biophys. Res. Commun. 2010;393:561–564. doi: 10.1016/j.bbrc.2010.02.071. [DOI] [PubMed] [Google Scholar]
- 20.Reed T.T. Lipid peroxidation and neurodegenerative disease. Free Radic. Biol. Med. 2011;51:1302–1319. doi: 10.1016/j.freeradbiomed.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 21.Costa D.A., Oliveira G.A.L.D., Sousa D.P.D., Freitas R.M. Avaliação do potencial antioxidante in vitro do composto ciano-carvona. Rev. Ciênc. Farm. Basic. Aplic. 2013;33:567–575. [Google Scholar]
- 22.Mclaughlin J.L., Chang C.J., Smith D.L. Bench-top" bioassays for the discovery of bioactive natural products: an update. Stud. Nat. Prod. Chem. 1991;9:383–409. [Google Scholar]
- 23.Guerra M., Souza M. Como observar os cromossomos: um guia de técnicas em citogenética vegetal, animal e humana, FUNPEC. Ribeirão Preto. 2002;191 [Google Scholar]
- 24.Basu S., Hazra B. Evaluation of nitric oxide scavenging activity, in vitro and ex vivo, of selected medicinal plants traditionally used in inflammatory diseases. Phytother. Res. 2006;20:896–900. doi: 10.1002/ptr.1971. [DOI] [PubMed] [Google Scholar]
- 25.Carvalho A.A., Santos L.R.D., Farias R.R.S.D., Chaves M.H., Feitosa C.M., Vieira-Júnior G.M., Pessoa C.D. Phenolic derivatives and antioxidant activity of polar extracts from Bauhinia pulchella. Quim. Nova. 2018;41:405–411. [Google Scholar]
- 26.Lopes G.K.B., Schulman H.M., Lima M.H. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim. Biophys. Acta Gen. Subj. 1999;1472:142–152. doi: 10.1016/s0304-4165(99)00117-8. [DOI] [PubMed] [Google Scholar]
- 27.Esterbauer H., Cheeseman K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 28.Guimarães A.G., Oliveira G.F., Melo M.S., Cavalcanti S.C., Antoniolli A.R., Bonjardim L.R., Silva F.A., Santos J.P.A., Rocha R., Moreira J.C.F., Araújo A.A., Gelainn D.P., Quintans-Júnior L.J. Bioassay-guided evaluation of antioxidant and antinociceptive activities of carvacrol. Basic Clin. Pharmacol. Toxicol. 2010;107:949–957. doi: 10.1111/j.1742-7843.2010.00609.x. [DOI] [PubMed] [Google Scholar]
- 29.Parasuraman S. Toxicological screening. J. Pharmacol. Pharmacother. 2011;2:74. doi: 10.4103/0976-500X.81895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nunes B.S., Carvalho F.D., Guilhermino L.M., Stappen G.V. Use of the genus Artemia in ecotoxicity testing. Environ. Pollut. 2006;144:453–462. doi: 10.1016/j.envpol.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 31.Mclaughlin J.L., Rogers L.L., Anderson J.E. The use of biological assays to evaluate botanicals. Drug Inf. J. 1998;32:513–524. [Google Scholar]
- 32.Feitosa C.M., Santos P.R.P., Freitas R.M., Rodrigues A.M.X., Costa-Junior J.S., Cavalcante A.N. Pre clinical trials in rats treated with 1, 3-diestearil-2-oleil-glicerol (TG1) constituent isolated from Platonia insignis. ConScientiae Saúde. 2015;14:327–345. [Google Scholar]
- 33.Meyer B.N., Ferrigni N.R., Putnan J.E., Jacobsen L.B., Nichols D.E., Mcl J. Aughlin, Brine shrimp: A convenient general bioassay for active plant constituents. J. Med. Plant Res. 1982;45:31–34. [PubMed] [Google Scholar]
- 34.Bagatine M.D., Silva A.C.F., Tedesco S.B. Uso do sistema teste de Allium cepa como bioindicador de genotoxicidade de infusões de plantas medicinais. Rev. Bras. Farmacogn. 2007;17:444–447. [Google Scholar]
- 35.Islam M.T., Streck L., Alencar M.V.O.B., Silva S.W.C., Machado K.C., Costa-Junior J.S. Evaluation of toxic, cytotoxic and genotoxic effects of phytol and its nanoemulsion. Chemosphere. 2017;177:93–101. doi: 10.1016/j.chemosphere.2017.02.145. [DOI] [PubMed] [Google Scholar]
- 36.Leite A.D.S., Dantas A.F., Oliveira G.L.D.S., Gomes Júnior A.L., Lima S.G., Citó A.M.G.L., Lopes J.A.D. Evaluation of toxic, cytotoxic, mutagenic, and antimutagenic activities of natural and technical cashew nut shell liquids using the Allium cepa and Artemia salina bioassays. Biomed Res. Int. 2015:1–16. doi: 10.1155/2015/626835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kasote D.M., Katyare S.S., Hegde M.V., Bae H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Bio. Sci. 2015;11:982–991. doi: 10.7150/ijbs.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang W.J., Zhang X.I.A., Chen W.W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016;4:519–522. doi: 10.3892/br.2016.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lima L.K.F., Pereira S.K.S., Junior R.S.S., Santos F.P.S., Nascimento A.S., Feitosa C.M., Figuerêdo J.S., Cavalcante A.N., Araújo E.C.C., Rai M. A brief review onthe neuroprotective mechanisms of vitexin. Biomed Res. Int. 2018:1–8. doi: 10.1155/2018/4785089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phaniendra A., Jestadi D.B., Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015;30:11–26. doi: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Titheradge M.A. Nitric oxide in septic shock. Biochim. Biophys. Acta Bioenergy. 1999;1411:437–455. doi: 10.1016/s0005-2728(99)00031-6. [DOI] [PubMed] [Google Scholar]
- 43.Sevidova D., Aliyev A., Rzayev N., Obrenovich M., Lamb B.T., Smith M.A., Aliev G. The role of nitric oxide in the pathogenesis of brain lesions during the development of Alzheimer’s disease. In Vivo. 2004;18:325–334. [PubMed] [Google Scholar]
- 44.Hazra B., Biswas S., Mandal N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement. Altern. Med. 2008;8:327–345. doi: 10.1186/1472-6882-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gangwar M., Gautam M.K., Sharma A.K., Tripathi Y.B., Goel R.K., Nath G. Antioxidant capacity and radical scavenging effect of polyphenol rich Mallotus philippenensis fruit extract on human erythrocytes: an in vitro study. Sci. World J. 2014:1–12. doi: 10.1155/2014/279451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jing L., Ma H., Fan P., Gao R., Jia Z. Antioxidant potential, total phenolic and total flavonoid contents of Rhododendron anthopogonoides and its protective effect on hypoxia-induced injury in PC12 cells. BMC Complement. Altern. Med. 2015;15:1–12. doi: 10.1186/s12906-015-0820-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moukette B.M., Anatole P.C., Biapa C.P.N., Njimou J.R., Ngogang J.Y. Free radicals quenching potential, protective properties against oxidative mediated ion toxicity and HPLC phenolic profile of a Cameroonian spice: Piper guineenses. Toxicol. Rep. 2015;2:792–805. doi: 10.1016/j.toxrep.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sannigrahi S., Kanti M.U., Kumar P.D., Parida S., Jain S. Antioxidant Potential of Crude Extract and Different Fractions of Enhydra fluctuans Lour. Iran. J. Pharm. Res. 2010;9:75–82. [PMC free article] [PubMed] [Google Scholar]
- 49.Lopes G.K.B., Schulman H.M., Lima M.H. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim. Biophys. Acta Gen. Subj. 1999;1472:142–152. doi: 10.1016/s0304-4165(99)00117-8. [DOI] [PubMed] [Google Scholar]
- 50.Halliwell B., Gutteridge J.M.C., Aruoma O.I. The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem. 1987;165:215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- 51.Cheng F.C., Jen J.F., Tsai T.H. Hydroxyl radical in living systems and its separation methods. J. Chromatogr. B. 2002;781:481–496. doi: 10.1016/s1570-0232(02)00620-7. [DOI] [PubMed] [Google Scholar]
- 52.Ayala A., Muñoz M.F., Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxi. Med. Cell. Longev. 2014:1–31. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Repetto M., Semprine J., Boveris A. Lipid peroxidation: chemical mechanism, biological implications and analytical determination. Lipid Peroxidation. 2012;1:680–716. [Google Scholar]
- 54.Coyle J.T., Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 55.Johar D., Maher A., Aboelmagd O., Hammad A., Morsi M., Warda H.F., Awad H.I., Mohamed T.A., Zaky S. Whole-food phytochemicals antioxidative potential in alloxan-diabetic rats. Toxicol. Rep. 2018;5:240–250. doi: 10.1016/j.toxrep.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zin Z.M., Abdul-hamid A., Osman A. Antioxidative activity of extracts from Mengkudu (Morinda citrifolia L.) root, fruit and leaf. Food Chem. 2002;78:227–231. [Google Scholar]
- 57.Manian R., Anusuya N., Siddhuraju P., Manian S. The antioxidant activity and free radical scavenging potential of two different solvent extracts of Camellia sinensis (L.) O. Kuntz, Ficus bengalensis L. and Ficus racemosa L. Food Chem. 2008;107:1000–1007. [Google Scholar]
- 58.Girish T.K., Kumar K.A., Rao U.J.S.P. C-Glycosylated flavonoids from black gram husk: Protection against DNA and erythrocytes from oxidative damage and their cytotoxic effect on HeLa cells. Toxicol. Rep. 2016;3:652–663. doi: 10.1016/j.toxrep.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahmadi F., Sadeghi S., Modarresi M., Abiri R., Mikaeli A. Chemical composition, in vitro anti-microbial, antifungal and antioxidant activities of the essential oil and methanolic extract of Hymenocrater longiflorus Benth. of Iran. Food Chem. Toxicol. 2010;48:1137–1144. doi: 10.1016/j.fct.2010.01.028. [DOI] [PubMed] [Google Scholar]