Highlights
-
•
Yeast species influenced the de novo synthesis of 2-phenylethylacetate.
-
•
Inhibitory compounds showed a strong influence on cell growth and 2-phenylethylacetate production for the evaluated yeasts.
-
•
More than a 50 % reduction in the chemical and biochemical oxygen demand was achieved by yeast fermentation.
Keywords: 2 phenylethanol, 2 phenylethylacetate, Nonconventional yeasts, Tequila vinasses, Metabolic modeling
Abstract
Vinasses from the tequila industry are wastewaters with highly elevated organic loads. Therefore, to obtain value-added products by yeast fermentations, such as 2-phenylethanol (2-PE) and 2-phenylethylacetate (2-PEA), could be interesting for industrial applications from tequila vinasses. In this study, four yeasts species (Wickerhamomyces anomalus, Candida glabrata, Candida utilis, and Candida parapsilosis) were evaluated with two different chemically defined media and tequila vinasses. Differences in the aroma compounds production were observed depending on the medium and yeast species used. In tequila vinasses, the highest concentration (65 mg/L) of 2-PEA was reached by C. glabrata, the inhibitory compounds decreased biomass production and synthesis of 2-PEA, and biochemical and chemical oxygen demands were reduced by more than 50 %. Tequila vinasses were suitable for the production of 2-phenylethylacetate by the shikimate pathway. A metabolic network was developed to obtain a guideline to improve 2-PE and 2-PEA production using flux balance analysis (FBA).
1. Introduction
Tequila is the most recognized Mexican distillate around the world. Production and consumption have grown significantly over the last five years, reaching 309 million liters in 2018 [1]. According to the Tequila Regulatory Council (CRT), this industrial activity generates significant amounts of solid and liquid wastes, such as bagasse and vinasses, which both constitute an environmental problem, but particularly vinasses.
For each liter of tequila produced, 7–10 liters of vinasses are generated, producing approximately 2500–3000 million liters of vinasses each year. In addition, since there is not an updated regulation for disposing of these residues, they are poured into water bodies, which represents a severe environmental issue [2]. Vinasses have characteristics that makes them highly contaminant residues; such as a high biochemical and chemical oxygen demand (BOD and COD respectively), low pH, high temperature and turbidity [[2], [3], [4], [5]].
Non-Saccharomyces yeasts, also known as nonconventional yeasts, have emerged as novel microbial sources for the development of new bioprocesses, and some of them exhibit a higher capacity for aroma metabolite production than Saccharomyces cerevisiae [6,7]. Therefore, these microorganisms could offer an excellent option for the development of bioprocesses to obtain high-value products. Furthermore, the use of tequila vinasses in a biorefinery model is emerging as an alternative for the integral use of byproducts of the tequila industry.
Several studies have been performed to obtain added-value products and to reduce the environmental impact of tequila vinasses, including physicochemical treatments [8] fermentative processes [9,10], biohydrogen production [11], the xylitol process [12], feedstock protein production [13], and fertilizer production [14].
The first study of aroma production from tequila vinasses has recently been addressed by Dos Reis et al. [9], who evaluated Saccharomyces and non-Saccharomyces yeasts in vinasses from cachaça and tequila. Nevertheless, this study focused to characterize a single kind of tequila vinasses and to compare them to cachaça vinasses. They did not carry out a correlation between the vinasses composition and the aroma compound that was produced, or the effect of the presence of inhibitory compounds in the fermentation.
Aroma compounds are important for the food, pharmaceutical, tobacco, and cosmetic industries [[15], [16], [17], [18]]. In the particular case of 2-phenylethanol (2-PE) and its ester, 2-phenylethylacetate (2- PEA), they have industrial applications by providing the characteristic aroma of honey and roses. After vanillin, 2-PE and 2-PEA are the second most used aroma compounds in the industry (10,000 tons per year). 2-PE and 2-PEA can be obtained by chemical synthesis, however, benzene and its derivatives, when used as raw material, are hazardous regulated compounds. Alternatively, the extraction of essential oils from plants presents low yield and generates industrial wastes [19]. Most of the studies of 2-PE synthesis by fermentation, have been carried out from the catabolic breakdown of phenylalanine, which is a more efficient pathway than that of anabolic synthesis [16,[20], [21], [22], [23], [24]]. This is probably due to a more direct set of steps: the first pathway involves a deamination of amino acids to produce the α-keto acid (precursor of higher alcohols); the second pathway involves several steps from sugars and nitrogen sources through the shikimate pathway to synthesis of the α-keto acid [24].
For the production of 2-PE by fermentation, research has focused mainly in the microorganisms S. cerevisiae and Kluyveromyces marxianus [16,17,25,18,24]. It has also been observed that the nature and concentration of carbon and nitrogen sources have an effect on the production of these aromatic compounds. Fabre et al. [26] reported that different carbon sources have an effect on 2-PE production in fermentations with yeast K. marxianus. Martin et al. [27] observed that nitrogen source other than amino acids significantly reduced higher alcohols production with Hanseniaspora vinae yeasts. Hence, culture conditions and yeast species are important factors in the aroma compounds production.
For the improvement of biotechnological production of 2-PE, metabolic models are useful tools in the study of bioprocesses to predict optimal fluxes during growth and metabolite production. To the best of our knowledge, this is the first time that metabolic modeling tools were used in aroma compound production from tequila vinasses as a substrate by using nonconventional yeasts.
A flux balance analysis (FBA) was applied in this study to understand the metabolic interaction between the precursors and products 2-PE and 2-PEA, which considered the carbon and nitrogen fluxes, and characterized the steady-state solution space within the stoichiometric network [28]. The construction of metabolic networks is achieved by an extensive literature review to define the equations of the reactions for the desired system. Nevertheless, the biomass formation equations of a specific microorganism require concrete assumptions for the system, or carbon tracking experiments (which is a difficult and expensive task to accomplish). Therefore, several studies with non-common microorganisms have inferred these biomass formation equations from other model microorganisms, such as S. cerevisiae or E. coli. Even though differences may be observed in the results of this parameter, their validity is accepted [[29], [30], [31], [32], [33]]
These tools have been used to optimize bioprocesses, such as methane production [34], lipids accumulation and citric acid production [31], clavulanic acid production [35], and alcohols [29,32,33].
The aims of the present work were: (i) to evaluate the metabolic capacity of four nonconventional yeasts (Wickerhamomyces anomalus, Candida glabrata, Candida utilis, and Candida parapsilosis) in two different metabolic conditions, (ii) to evaluate the effect of tequila vinasse variability as a substrate for the production of aroma compounds, such as 2-PE and 2-PEA; (iii) to form a deeper characterization of aroma compounds precursors in tequila vinasses, such as amino acids; (iv) to determine the effect of inhibitory compounds present in tequila vinasses fermentations, and (v) to implement metabolic modeling in the carbon and nitrogen fluxes and evaluate the feasibility of different metabolic scenarios for the production of 2-PE and 2-PEA. The production of biomass, 2-PE and its ester (2-PEA) were quantified during fermentation, and the reduction in BOD and COD in tequila vinasses was also evaluated.
2. Materials and methods
2.1. Microorganisms
The strains that were evaluated in this study belong to two different culture collections, yeasts Candida glabrata (119) and Candida parapsilosis (448), which were isolated from coffee fermentation and belong to the Culture Collection of the Agricultural Microbiology Department (CCMA), the Federal University of Lavras, (Brazil); Candida utilis (CUT) and Wickerhamomyces anomalus (DB) were isolated from mezcal fermentation and belong to the Culture Collection of the Industrial Biotechnology Department (CCIB), CIATEJ (Mexico).
2.2. Evaluation of catabolic and de novo synthesis of 2-phenylethanol and 2-phenylethylacetate
The production capacity of 2-PE and 2-PEA by yeasts was evaluated in two different metabolic conditions, either through the Ehrlich catabolic pathway (ECP) or the de novo pathway (DNP). The yeasts were activated (overnight culture) in YPD medium at 30 °C and 250 rpm. The initial inoculum used was at a cellular concentration of 1 × 106 cells/mL in 100 mL of medium in 250 mL Erlenmeyer flasks. ECP evaluation was carried out as reported by Yin et al. [24] by using a specific culture medium composed of 40 g/L sucrose, 0.5 g/L Na2HPO4, 1.8 g/L yeast nitrogen base without amino acids (BD Difco™, NJ, USA) and 7 g/L L-phenylalanine as a nitrogen source (pH 5.0). DNP was evaluated according to [17,18], with a culture medium composed of 20 g/L glucose, 6.7 g/L yeast nitrogen base without amino acids (BD Difco™, NJ, USA) and 0.77 g/L complete supplement mixture without uracil (Adenine 10 mg/L, L-isoleucine 50 mg/L, L-leucine 100 mg/L, L-methionine, L-phenylalanine 50 mg/L, L-threonine 100 mg/L, L-tryptophan 50 mg/L, L-lysine 50 mg/L, L-tyrosine 50 mg/L, L-valine 140 mg/L). Fermentations were carried out at 30 °C and 250 rpm agitation for 96 h for both culture media. Samples were taken at 6, 12, 24, 48, 72 and 96 h to monitor growth by the optical density with absorbance recorded at 600 nm; 2-PE and 2-PEA analyses were performed by gas chromatography. Biomass production was quantified using the dry constant weight method (60 °C). Substrate consumption was measured using spectrophotometric methods, dinitrosalicylic acid reagent for reducing sugars [36] and anthrone for total sugars.
2.3. Gas chromatography
2-PE and 2-PEA quantifications were carried out under the methodology by Arellano et al. [37]. Samples were analyzed using a GC Agilent 7890B system (Agilent Technologies, CA, USA), a 7890A Headspace Sampler, and an FID detector, using an HP-INNOWax (60 m x 250 μm × 0.25 μm) column. Then, 2 mL samples were placed in 20 mL vials, to take them to the 7890A Headspace Sampler with the following conditions: vial temperature: 90 °C, loop temperature: 110 °C, transfer line: 115 °C, vial equilibrium time: 5 min, pressurization time: 2 min, loop filling: 0.2 min, loop equilibrium time: 0.5 min, injection time: 1 min, injection volume: 10 μL. GC Agilent 7890B system conditions were as follows: the initial setup was programmed at 45 °C for 8 min, with increased steps of 2 °C until 80 °C, followed by a 5 °C increase until 160 °C, and finally, an increased step of 25 °C up to 220 °C for 4 min. The detector and injector were set at 250 °C, in a split-less injection mode. The analysis time was 55 min, including the headspace extraction time. Quantification of metabolites was accomplished by using external standards of 2-PE and 2-PEA (Millipore Corporation, MA, USA).
2.4. Physical and chemical characterization of tequila vinasses
Vinasses were collected from ten different tequila factories in two tequila producing regions in the Jalisco state, Mexico. Region “Del Valle” corresponds to the towns of “Tequila”, Amatitán” and “El Arenal”, while the region “Los Altos Sur” includes the towns of “Arandas”, “Tepatitlán de Morelos” and “San Ignacio Cerro Gordo”. Fresh vinasses were collected in plastic containers immediately after distillation and were stored at −20 °C in a freezing chamber prior use. Amino acids were quantified by HPLC (AOAC 982.30-A, modified from WATERS ACCQ TAG ultra (Waters Corporation, MA, USA)) (2007), biochemical oxygen demand (BOD) was determined by a BODTrak II respirometric apparatus (Hach company, CO, USA) and chemical oxygen demand (COD) was determined by the Reactor Digestion Method, using a DRB 200 Reactor (Hach company, CO, USA). Both parameters were calculated from the amount of oxygen that is needed to oxidize the organic and inorganic components, respectively. Total nitrogen and ammonium-nitrogen were estimated by the Kjeldahl method, according to the guidelines of the American Public Health Association [38], photocolorimetric methods were used in the determination of total sugars using the anthrone method at 620 nm; the Miller method (DNS) was used at 540 nm for reducing sugars, and the total solids were calculated by the dry weight method (60 °C). Mineral quantifications of calcium, copper, nickel, magnesium, and iron were determined by atomic absorption spectrometry by the U.S. Environmental Protection Agency method 6010B (EPA, 1996).
2.5. Evaluation of the inhibitory compounds in tequila vinasses
Inhibitory compounds were quantified by a 1220 Infinity LC high-performance liquid chromatography system (Agilent Technologies, CA, USA) equipped with a Zorbax Eclipse Plus C18 (4.6 mm x 250 mm, 5 μm) (Agilent Technologies, CA, USA). The mobile phases were A: (methanol 100 %) and B: formic acid-water (2.5 % v/v); the operating conditions were as follows: flow rate of 0.8 ml/min at 30 °C, with a flow gradient during 55 min from 0 % to 48 % of phase B, a pressure of 1200 ± 100 psi, and detection by a diode array detector (DAD) at wavelength screenings at 262, 275, 295 and 342 nm.
2.6. Nonconventional yeasts fermentations on tequila vinasses for aroma compounds production
Tequila vinasses were pretreated as follows: centrifugation was performed (13,000 rpm, 20 min, 4 °C) to remove insoluble solids. The centrifuged vinasses were added into 250 mL Erlenmeyer flasks (100 mL), with the following formulation: 0.1 % yeast extract w/v, 0.05 % K2HPO4 w/v, 0.2 % glucose w/v, 0.5 % peptone w/v, and fresh vinasses without dilution. Prior to inoculation, these media solutions were sterilized at 121 °C for 15 min. Cultures were incubated for each strain with 1 × 106 cells/mL at 30 °C and 150 rpm agitation for 120 h. Samples were taken every 24 h. The factors that were evaluated were the four different strains and the 10 sampling locations. The response variables were the 2-PE and 2-PEA titers, biomass production, pH measurements, and substrate consumption.
2.7. Evaluation of the chemical and biochemical oxygen demand reduction by yeasts during tequila vinasse fermentations
The reduction in COD and BOD in tequila vinasses after yeast fermentation was evaluated as stated in the section of Physical and chemical characterization of tequila vinasses, as a complement to the production of the aroma compounds. A selection of analyzed vinasses was in accord with the results of characterization, as well as yeasts species from the results of tequila vinasse fermentations. Analyses were performed in triplicate from a single batch of the selected vinasses, which corresponds to the initial value of the COD and BOD parameters. 1 × 106 cells/mL were inoculated in the selected tequila vinasses, and fermentation was performed in 250 ml Erlenmeyer flasks at 30 °C and 150 rpm for 120 h. The cells were removed by centrifugation at 4000 rpm for 15 min at 4 °C prior to analysis.
2.8. Metabolic network modeling
Cellular metabolic pathways from a metabolic network could be described by the set of elementary modes (EMs). The EMs are a set of nondecomposable pathways consisting of a minimal set of reactions that function in steady-state [32,33]. In this work, the constructed network was based on the metabolic conversion of carbon and nitrogen sources as substrates to biomass, 2-PE and 2-PEA as products for yeasts in anaerobic conditions. It consists of 48 reactions, with 58 internal metabolites and 5 main metabolites (glucose, biomass, 2-PE, 2-PEA, maintenance), which includes the glycolysis pathway, pyruvate metabolism, the pentose phosphate pathway, the Krebs cycle, the shikimate and Ehrlich pathways, glutamate and glutamine metabolism, and biomass formation [[31], [32], [33],39]. The amino acid metabolism of phenylalanine is related to the nitrogen uptake that is linked through glutamate metabolism in the constructed metabolic network [40]. Only the cytosol and mitochondria were considered in order to simplify the transport reactions. The computation of elementary modes from the metabolic network was performed through the CellNet Analyzer toolbox [41] in the MATLAB® software (Mathworks Inc., MA, USA). A complete set of reactions and abbreviations can be found as supplementary material in appendix A.
2.9. Statistical analysis
Experiments were performed as a triplicate of independent samples. Data are expressed as the mean ± standard deviation. The results were statistically analyzed using one-way ANOVA (p < 0.05) and comparison among groups was performed using Tukey’s test to observe the differences among the different culture media and strains. Analyses were performed using the Minitab® 17.1.0 software (Minitab Inc., PA, USA)
3. Results and discussion
3.1. Evaluation of 2-phenylethanol and 2-phenylethylacetate production by fermentation under catabolic and de novo synthesis conditions
Production of 2-PE and 2-PEA in the ECP and DNP inductor media are shown in Table 1. Significant differences in the percentage of the consumed sugar can be observed depending on the medium and the yeast strains. Complete depletion of glucose was observed in the DNP medium with all yeasts, while C. glabrata and W. anomalus were not able to achieve total consumption of sucrose in the ECP medium. However, biomass was higher in comparison with C. parapsilosis and C. utilis. Glucose consumption was similar to those studies in which most of the carbon source was completely depleted after 24−28 h, either by yeasts (S. cerevisiae and K. marxianus) [16,23] or bacteria (Enterobacter sp.) [42]. Previous studies in the same DNP medium reported a biomass production for K. marxianus in the range of 2–3 g/L [17,18], which is similar to the one that was obtained with the evaluated yeasts (data not shown).
Table 1.
Evaluation of consumed sugar, biomass, 2-phenylethanol (2-PE) and 2-phenylethylacetate (2-PEA) production by yeasts in different media.
| DNP |
ECP |
|||||||
|---|---|---|---|---|---|---|---|---|
| Yeast | Y x/s(g biomass/ g glucose) | Consumed sugar (%) | 2-PE [mg/L] | 2-PEA [mg/L] | Y x/s(g biomass/ g sucrose) | Consumed sugar (%) | 2-PE [mg/L] | 2-PEA [mg/L] |
| C. glabrata | 0.23 ± 0.06aA | 99.42 ± 0.05aA | ND | 35.08 ± 0.54aA | 0.24 ± 0.01aA | 61.29 ± 1.09aB | ND | 665.33 ± 25.55aB |
| C. parapsilossis | 0.14 ± 0.05aA | 99.11 ± 0.13aA | ND | 28.56 ± 4.91bA | 0.15 ± 0.04aA | 95.51 ± 2.58aB | ND | 357.46 ± 19.32aB |
| W. anomalus | 0.26 ± 0.06bA | 99.51 ± 0.01aA | 2.69 ± 0.57aA | 36.93 ± 0.11aA | 0.53 ± 0.09bA | 44.62 ± 1.23bB | ND | 689.16 ± 66.63aB |
| C. utilis | 0.31 ± 0.08bA | 99.63 ± 0.04aA | 4.47 ± 1.87bA | 29.50 ± 1.97bA | 0.18 ± 0.01aA | 96.47 ± 2.31aB | 242.65 ± 26.9bA | 6.67 ± 0.49bB |
DNP= de novo pathway medium, ECP = Ehrlich catabolic pathway medium. Results are shown as an average of duplicates with standard deviation; lower case letters show comparisons between strains and upper case letters show comparisons between media. Statistical significance given at p < 0.05. ND - Not detected.
2-PE was produced only by C. utilis in the ECP medium, while in the DNP medium it was observed a significant decrease of 98 %. For 2-PEA, the highest concentration was produced by yeasts C. glabrata and W. anomalus in the ECP medium, as shown in Table 1, with 665 and 689 mg/L, respectively. The same behavior was observed for these two yeasts in the DNP medium, with a decrease of 95 %. There are reports that mention that the nitrogen source is of great importance in the synthesis of phenylpropanoids. Martin et al. (2016) observed that the addition of ammonium salts (75 mg/L of yeast assimilable nitrogen) to the culture medium negatively affected the production of these aromatic compounds (reduction from 13 to 2 μg/L approximately) with H. vineae yeast. This result is consistent with the results obtained in this work.
Yin et al., [24] produced a maximum concentration of 800 mg/L of 2-PE with the wild-type S. cerevisiae from the Erhlich pathway with a phenylalanine (7 g/L) based medium and sucrose as carbon source (40 g/L), which was different from the results that were obtained in this study, since metabolite production was observed to be directed towards 2-PEA accumulation, probably because the biosynthesis of 2-PE depends on the capability of each one of the microorganisms in tolerating this compound, as well as the activity of alcohol acetyltransferase that is needed for 2-PEA production [43,17,18] reported a concentration of 200 mg/L of 2-PE in a wild-type strain of K. marxianus from glucose (20 g/L) and ammonium sulfate (5 g/L) as nitrogen source, with a maximum amount of 1 g/L in a recombinant strain. Hence, the capacity for synthesis of these compounds depends on the culture media composition and the inherent metabolic capability of the microorganism using different pathways for the production of these metabolites. For example, Jimenez-Marti and del Olmo, [44] observed that for the same microorganism (S. cerevisiae) in alcohol fermentation conditions, the use of ammonia increased the expression of ARO8 gene (involved in aromatic amino acids synthesis), while the use of amino acids repressed the Sfp1p protein (transcriptional factor of ribosomal protein synthesis). Thus, the nature of nitrogen source is the most important factor to induce different metabolic pathways for the production of aromatic compounds. This could be inferred from the differences observed between ECP and DNP conditions for the four nonconventional yeasts (W. anomalus, C. glabrata, C. utilis, and C. parapsilosis).
It also should be highlighted that in the majority of yeasts, accumulation of 2-PE causes intracellular toxicity, as reported by several authors [16,45,46]. This could lead to the transformation of this higher alcohol to the ester form, affecting the levels of 2-PE accumulation in the culture medium. Even though there is no precise evidence for the biological function of these compounds, it has been suggested that the alcohol acetyltransferase (Atf2p) reaction is related to a detoxification process [47]. It is interesting to point out, however, that this detoxification process may be reverted over time. Wittmann et al. [23] observed in K. marxianus that 2-PEA production is significantly lower than 2-PE, and determined that accumulation of this higher alcohol in the late stages of fermentation is due to 2-PEA cleavage.
3.2. Physical and chemical characterization of tequila vinasses
Results of the physicochemical characterization of vinasses are presented in Table 2. Three different agave cooking processes and two distillation systems were identified in the elaboration process of tequila from the two regions that were sampled. A low level of reducing sugars was observed (1.79–4.5 g/L), which was similar to the findings reported in previous vinasses characterization studies [2,5,9,13]. There were differences in the total nitrogen concentration, vinasse A had the lowest amount at 50 mg/L, while the other evaluated vinasses presented a range between 177−482 mg/L.
Table 2.
Physical and chemical characterization of the different vinasses.
| Region | “Del Valle” |
“Los Altos” |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vinasse | A | B | C | D | E | F | G | H | I | J |
| Parameter | ||||||||||
| Reducing Sugars (g/L) | 4.25 ± 0.5 | 3.62 ± 1.2 | 3.4 ± 1.0 | 1.79 ± 0.1 | 3.31 ± 0.6 | 3.89 ± 0.1 | 2.55 ± 1.6 | 4.34 ± 0.1 | 2.72 ± 1.4 | 3.06 ± 0.6 |
| Total sugars (g/L) | 7.78 ± 2.9 | 9.31 ± 4.5 | 7.17 ± 1.5 | 5.82 ± 0.2 | 7.54 ± 1.5 | 7.19 ± 0.7 | 7.22 ± 1.6 | 17.15 ± 6.7 | 7.29 ± 0.8 | 8.68 ± 0.3 |
| Total nitrogen (mg/L) | 50.4 ± 20.1 | 482.4 ± 57.2 | 232.6 ± 110.0 | 226.5 ± 124.0 | 439.6 ± 46.3 | 341.8 ± 46.5 | 388.5 ± 138.7 | 312.4 ± 25.0 | 177.8 ± 121.9 | 315.9 ± 205.7 |
| NH4+-nitrogen (mg/L) | 8.8 ± 3.9 | 14.9 ± 0.98 | 8.1 ± 3.23 | 11.47 ± 5 | 16.68 ± 0.58 | 11.42 ± 1.87 | 27.03 ± 9.9 | 7.64 ± 1.47 | 8.77 ± 2.65 | 9.09 ± 6.71 |
| Organic nitrogen (mg/L) | 41.63 ± 16.12 | 467.47 ± 56.18 | 224.48 ± 106.8 | 215 ± 117.99 | 346 ± 63.1 | 330.39 ± 44.64 | 361.445 ± 128 | 304.75 ± 26.48 | 169.01 ± 15.66 | 306.79 ± 199.02 |
| Phenylalanine (μg/L) | <0.01* | <0.01* | <0.01* | 0.026 | <0.01* | 0.011 | 0.026 | <0.01* | 0.013 | 0.019 |
| Valine (μg/L) | 0.039 | <0.01* | 0.022 | 0.041 | <0.01* | <0.01* | 0.016 | <0.01* | <0.01* | <0.01* |
| Leucine (μg/L) | 0.02 | <0.01* | <0.01* | 0.036 | <0.01* | 0.011 | 0.017 | 0.01 | <0.01* | <0.01* |
| Isoleucine (μg/L) | <0.01* | <0.01* | <0.01* | 0.01 | <0.01* | <0.01* | <0.01* | <0.01* | <0.01* | <0.01* |
| C/N ratio | 154.0 | 19.0 | 31.0 | 26.0 | 17.0 | 21.0 | 19.0 | 55.0 | 41.0 | 27.0 |
| COD (g/L) | 68.7 ± 15.6 | 55.7 ± 34.8 | 58.8 ± 0.4 | 53.5 ± 13.6 | 50.0 ± 26.7 | 50.6 ± 32.8 | 59.8 ± 17.4 | 66.2 ± 2.0 | 46.9 ± 17.2 | 69.1 ± 2.0 |
| BOD (g/L) | 23.1 ± 0.8 | 22.7 ± 2.1 | 20.6 ± 3.4 | 18.5 ± 1.3 | 22.8 ± 2.1 | 20.7 ± 1.7 | 28.0 ± 8.6 | 28.6 ± 12.9 | 22.5 ± 6.0 | 29.8 ± 12.9 |
| Total Solids (mg/L) | 33.5 ± 23.4 | 23.7 ± 19.0 | 44.3 ± 13.9 | 17.3 ± 0.1 | 26.5 ± 1.4 | 20.7 ± 10.7 | 38.8 ± 6.7 | 66.7 ± 12.8 | 20.7 ± 12.3 | 56.4 ± 16.7 |
Results are shown as an average with a standard deviation of two replicates from vinasses collected in two different regions at different production periods. Region “Del Valle” (A, B, C, D, E), and the region “Los Altos” (F, G, H, I, J). *0.01 Minimum detection limit.
Total nitrogen is given by the sum of organic and inorganic nitrogen. In wastewaters, the latter is constituted by the sum of ammonium, nitrites, and nitrates [48]. Previous studies of tequila vinasses indicated that the main portion of available nitrogen comes from organic nitrogen by the presence of amino acids and proteins from the fermentation previous distillation of the must [49]. The quantity of total amino acids that were found corresponds approximately to the organic nitrogen available in vinasses (data not shown).
Other reports for tequila vinasses found a low content of nitrogen (20−50 mg/L) [2]. However, mezcal vinasses showed a higher concentration of this parameter, with oscillations from 660−5650 mg/L [5], which is more consistent with results that were found by Dos Reis et al. [9] (818 mg/L). Therefore, values that are observed in Table 2 could be associated with differences in the tequila elaboration process. As the C/N ratio with a low nitrogen content has negative effects on the metabolism of microorganisms [50], the C/N ratio that was found for the vinasses samples (12−67 g/g) indicates that there is no surplus that may interfere in the fermentation process, except for vinasse A, which obtained a low nitrogen concentration and a high C/N ratio (154 g/g).
The maximum results of COD and BOD were higher (6.7 g/L and 3.8 g/L, respectively) than the values that were previously reported for mezcal vinasses (6.0 g/L and 2.2 g/L) [5], Brazilian cachaça production vinasses (5.9 g/L and 1.9 g /L) found by Silva et al. [13], and tequila vinasses (4.2 g/L and 1.8 g/L) [9]. This could indicate that tequila vinasses are more recalcitrant wastewaters than other similar beverages, and the amount depends on the origin of the corresponding vinasses. COD and BOD values in vinasses can be affected by the raw material origin, the preparation of the agave must, the alcoholic fermentation system, the types of yeast, the distillation and the separation processes [[2], [3], [4],51,52]. Nevertheless, the results observed in Table 2 did not show correlation between the origin of the vinasses and the process from which they were obtained.
In contrast, differences in the process had an effect on total solids. Total solids and reducing sugars were significantly lower in the vinasses obtained from a diffusor process compared to vinasses from the traditional process. Thus, the extraction of sugars is a key parameter that has an influence in the final composition of tequila vinasses, and therefore could affect the fermentation performance of yeasts using these wastewaters as a substrate.
Aromatic amino acid quantification is also presented in Table 2. It showed that the amount of aromatic amino acids is low (0.01-0.026 μg/L), which is consistent with the results that were obtained by Díaz-Montaño et al. [53], who deduced that the low concentrations of higher alcohols and other byproducts may be linked to the very poor amino acid concentration in agave juice and consequently, in tequila vinasses.
Inhibitory compounds that are present in tequila vinasses normally come from the hydrolysis of agave fructans during the cooking process in the elaboration of tequila, such as the furan hydroxymethylfurfural (HMF) or terpenic compounds that come from the agave plant, such as vanillin [54]. Even though these compounds are presumed not to be metabolized by yeasts, it has been found that they affect the fermentation yields [49]. However, some species are able to tolerate these compounds at a concentration close to 2.5 g/L expressed as total inhibitors [55]. The results of the measured inhibitory compounds are displayed in Table 3. Vinasse H presented the highest measure of these compounds with a total of 455 mg/L, while vinasses B and D obtained the least amount with a total of 85 mg/L and 99 mg/L, respectively. Even though there is no exhaustive information about these compounds in the literature, the results by García et al. [56] in vinasses from sugarcane ethanol production show a similar inhibitory compounds concentration, presented as total phenols (469 mg/L).
Table 3.
Inhibitory compounds present in tequila vinasses.
| Vinasse | A | B | C | D | E | F | G | H | I | J |
|---|---|---|---|---|---|---|---|---|---|---|
| Compound (mg/L) | ||||||||||
| Hydroquinone | 4.29 ± 2.64g | 12.66 ± 0.08f | 4.88 ± 0.18g | 37.42 ± 0.04d | 42.06 ± 1.12c | 43.60 ± 0.01c | 49.07 ± 1.46b | 96.43 ± 0.30a | 1.82 ± 0.05g | 22.74 ± 0.12e |
| Hydroxymethylfurfural | 127.94 ± 3.53c | 18.18 ± 0.32f | 140.25 ± 0.51b | 22.05 ± 0.02f | 126.38 ± 0.06c | 21.79 ± 0.03f | 115.63 ± 0.24d | 227.13 ± 1.09a | 85.91 ± 0.37e | 137.47 ± 0.85b |
| Furfural | 8.17 ± 0.37b | 5.87 ± 0.11d | 4.47 ± 0.01e | 0.18 ± 0.01h | 10.61 ± 0.05a | 7.39 ± 0.01c | 2.71 ± 0.15f | 8.70 ± 0.12b | 2.00 ± 0.02g | 4.95 ± 0.01e |
| 2-Furoic acid | 24.95 ± 7.05b | 20.49 ± 1.00b | 17.07 ± 0.07b | 2.03 ± 0.01e | 11.49 ± 0.01b | 10.43 ± 0.01b | 19.89 ± 0.25b | 38.59 ± 0.37a | 9.93 ± 0.04b | 18.75 ± 0.17b |
| Hydroxybenzoic acid | 5.09 ± 3.14b | 5.06 ± 0.11b | 7.02 ± 0.25b | 3.10 ± 0.05c | 3.70 ± 0.18c | 5.20 ± 1.19b | 5.32 ± 0.30b | 4.71 ± 0.07b | 9.05 ± 0.09a | 8.87 ± 0.07b |
| Hydroxybenzaldehyde | 0.54 ± 0.76g | 0.92 ± 0.04g | 22.17 ± 0.10d | 23.57 ± 0.01d | 19.40 ± 0.32e | 25.07 ± 0.14c | 2.60 ± 0.78f | 49.50 ± 0.19a | 22.69 ± 0.06d | 31.60 ± 0.23b |
| Vanillic acid | 37.78 ± 1.27a | 16.46 ± 0.26c | 4.25 ± 0.02d | 4.27 ± 0.04d | 3.84 ± 0.02d | 4.21 ± 0.001d | 3.56 ± 0.19d | 2.91 ± 0.12d | 26.81 ± 0.19b | 37.38 ± 0.27a |
| Vanillin | 1.57 ± 0.63d | 1.025 ± 0.02d | 4.23 ± 0.05c | 5.86 ± 0.01c | 7.22 ± 0.01c | 6.21 ± 0.03c | 22.63 ± 0.74a | 24.51 ± 1.10a | 1.84 ± 0.47d | 10.57 ± 0.20b |
| Acetovanillone | 0.44 ± 0.62a | 1.45 ± 0.86a | ND | ND | 0.68 ± 0.01a | 0.89 ± 0.01a | 0.86 ± 0.08a | 0.81 ± 0.11a | ND | ND |
| Acetosyringone | 1.51 ± 0.03a | 1.18 ± 0.04c | 1.18 ± 0.02c | 1.19 ± 0.01c | 1.36 ± 0.01b | 1.19 ± 0.002c | ND | 1.41 ± 0.03a | ND | 1.34 ± 0.06b |
| Coniferyl aldehyde | 5.26 ± 0.65a | 2.28 ± 0.01b | ND | ND | ND | ND | ND | ND | ND | ND |
Results are shown as an average of duplicate vinasse samples with standard deviation. Comparisons were made between vinasses for each compound; different lower-case letters show Statistical significance given at p < 0.05. ND - not detected.
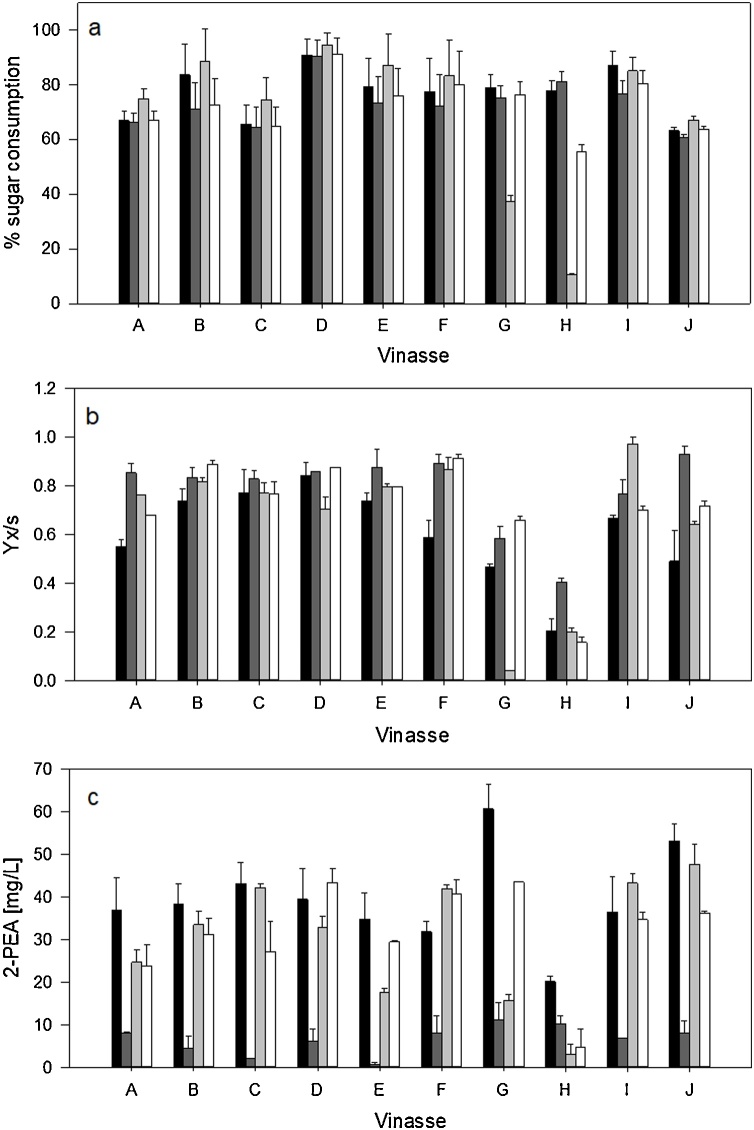
3.3. Nonconventional yeast fermentations on tequila vinasses for 2-phenylethanol and 2-phenylethylacetate production
Fig. 1 shows the fermentation performance of evaluated yeasts with different tequila vinasses. There were no differences in the sugar consumption percentage among strains in almost all tequila vinasses, except in vinasses G and H, where is can be observed that W. anomalus was the yeast that consumed less substrate (Fig. 1a). The biomass for the four yeasts of the evaluated vinasses are shown in Fig. 1b. There was a similar behavior for all yeasts, with the exception of G and H vinasses, wherein the lowest yields of biomass were observed. These two vinasses showed a high inhibitory compounds concentration (in particular vinasse H), where C. parapsilosis was able to produce high biomass, which indicates that it possesses better tolerance and ability to survive under stress conditions, as was observed by Dos Reis et al. [9], with a biomass production of 5 g/L in both cachaça and tequila vinasses fermentations. In contrast, W. anomalus showed the lowest biomass in vinasses G and H. Nevertheless, this yeast produced 4−7 g/L of biomass in tequila vinasses (data not shown), which was similar to the biomass quantity that was produced for this same yeast strain in cachaça vinasses (4−8 g/L) according to Silva et al. [13].
Fig. 1.
Fermentation performances in different tequila vinasses for percentage of sugar consumed (a), Biomass (b) and 2-Phenylethylacetate production (c) of C. glabrata (◼), C. parapsilosis (◼), W. anomalus (◼) and C. utilis (□) yeasts.
Vinasses contain an initial concentration of aroma compounds (ranging from 10 mg/L to 25 mg/L) which were considered and subtracted before performing the calculations of the final yields of 2-PEA (C. glabrata: 32−60 mg/L; W. anomalus: 3−47 mg/L; C. utilis: 4−40 mg/L; C. parapsilosis: 1−11 mg/L). The presence of aroma compounds in the initial composition of the medium is due to the previous fermentation and distillation of the agave must. This finding has been previously reported in another kind of beverages, such as wine and beer [7,57]. Highest 2-PEA production was accomplished by yeast C. glabrata with a concentration of 60 mg/L, followed by W. anomalus with 47 mg/L, which was similar to the amounts reported by Dos Reis et al. [9] (19 mg/L to 68 mg/L). C. parapsilosis showed the lowest amount of 2-PEA accumulation, as it can be seen in Fig. 1c. As it was stated above, the amino acid content in tequila vinasses is not enough for catabolic breakdown pathway production, and therefore, the aromatic compounds were produced through the De novo pathway from glucose.
Several studies have approached the production of these aromatic compound from glucose. For example, overexpression of the ARO10 gene in a S. cerevisiae strain implemented by Shen et al. [22] reported concentrations of 2-PE of less than 10 mg/L for the wild-type strain, and a maximum concentration of 90 mg/L with a transformant strain. Another approach was evaluated by Rollero et al. [58] to test the combined effect of nutrients in aroma compounds production in wine fermentations, wherein they observed by a surface response methodology that, 2-PEA and 2-PE, which can assimilate nitrogen, had a negative quadratic effect in the synthesis and thus, established an adequate concentration of 200 mg/L of nitrogen for a production of 16 mg/L of 2-PE, which is similar to the nitrogen amount in most vinasses. Another example is the study of Etschmann et al. [20], who obtained concentrations of 0.31 g/L for 2-PEA from an optimized molasses medium supplemented with phenylalanine by using a K. marxianus yeast before applying in situ product removal strategies (ISPR), and obtaining final concentrations of 1.27 g/L of 2-PEA.
Regarding 2-PE and 2-PEA production, similar results were found in the previous section. 2-PE is not accumulated in the medium and is likely directly transformed to 2-PEA; this behavior has been reported in fermentations with agave juice to compare the performance of nonconventional yeasts Kloeckera africana and Kloeckera apiculata with that of S. cerevisiae strains, where there was a significantly higher 2-PEA accumulation than the 2-PE, with similar results (51−60 mg/L) [53].
It has been found that aroma compound synthesis is associated with growth [20], which was also observed for biomass and aroma compounds productions in tequila vinasses fermentations. These two parameters decreased as the concentration of the inhibitory compound increased. Thus, the levels of 2-PEA were more affected by the concentration of the inhibitors present in tequila vinasses than by the nutrient concentration (C/N ratio).
3.4. Chemical and biochemical oxygen demand reduction
Vinasse J was selected due to the highest COD and BOD levels (Table 2). This vinasse was used in a fermentation process using the yeasts C. glabrata and W. anomalus. It was found that after 120 h, both microorganisms reduced approximately over 50 % of the COD and BOD. Previous studies have reported similar findings for wastewater treatments; Seluy and Isla [10] found a reduction of 60 % in beer breweries effluents; Pires et al. [59] obtained a removal of COD (39 %–76 %) and BOD (42 %–56 %) from cachaça vinasses (50 % v/v dilution), by mixing inoculum of yeasts and bacteria; and Dos Reis et al. [9] reduced over 80 % for both COD and BOD in tequila vinasses (70 % v/v dilution). It must be pointed out that in the present work, the tequila vinasses were not diluted, which could explain the differences in the obtained COD and BOD reductions.
3.5. Metabolic pathways and elementary modes analysis
Fig. 2 displays the proposed reduced metabolic network of nonconventional yeasts for 2-PE and 2-PEA production. 48 reactions were taken in to account in this network, with 58 internal metabolites and 5 main metabolites (glucose, biomass, 2-PE, 2-PEA, maintenance), which includes the glycolysis pathway, pyruvate metabolism, the pentose phosphate pathway, the Krebs cycle, the shikimate and Ehrlich pathways, glutamate and glutamine metabolism, and biomass formation [[31], [32], [33],39]. The amino acid metabolism of phenylalanine related to the nitrogen uptake, which is linked through glutamate metabolism in the constructed metabolic network [40].
Fig. 2.
Reduced metabolic network constructed for the 2-PE and 2-PEA production by nonconventional yeasts. ( ) biomass components.
) biomass components.
The computational analysis of the network using CellNet Analyzer led to 142 elementary modes (EMs), which were lower than the models constructed by Robles-Rodriguez et al. [31] with 1944 elementary modes, and the one evaluated by [32,33] with 369 computed EMs. These differences are due to the number of metabolites, and reactions involved in the evaluated process, as well as the number of inputs or substrate intakes. Therefore, each system must be delimited by every modeler’s judgment and objectives.
Fig. 3 shows the distribution of the found EMs for the biomass, 2-PE, 2-PEA and ethanol production, where the diagonal depicts the histograms that relate the number of EMs versus metabolite yields. From 142 EMs, 136 included biomass and 139 included ethanol production, while 72 and 62 EMs were involved in 2-PEA and 2-PE production respectively. Therefore, biomass and ethanol productions are coupled with 2-PEA and 2-PE synthesis. The lower diagonal matrix in Fig. 3 shows the yield plots of the metabolites (biomass, ethanol, 2-PE, 2-PEA) in the axes with respect to the consumed carbon source (glucose). It can be observed that the yields found in the convex space hull, biomass and ethanol production are favored in comparison with the ones obtained for 2-PE and 2-PEA.
Fig. 3.
Distribution of elementary modes of the reduced metabolic network. Histograms of elementary modes distribution (diagonal) show the number of solutions found for each product. Yield plots depict the geometry of the found solutions for predicted yields of the metabolites (biomass, ethanol, 2-PE, 2-PEA) in the axes with respect to the consumed carbon source (lower diagonal). Units of yields are given in (mmol/mmol GLUC) except for biomass (g BIOM/mmol GLUC).
This information permits to understand (from a stoichiometric point of view), the importance of each set of reactions in the metabolic network. Therefore, for the synthesis of 2-PE and 2-PEA, the use of low ethanol-producing yeast species may increase the carbon flux to these metabolites. Thus, these nonconventional yeasts are suitable for the production by fermentation in anaerobic conditions of 2-PE and 2-PEA, as they are not considered high ethanol-producing yeasts [60].
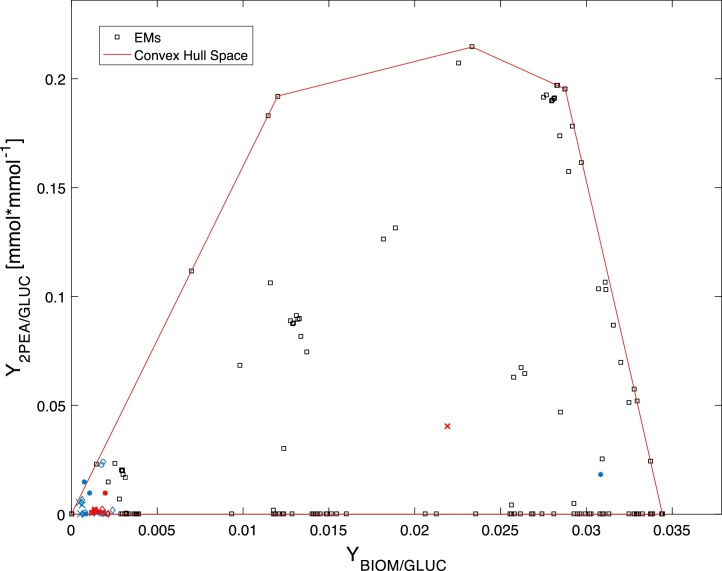
The convex hull space shown in Fig. 4 was used to validate the experimental data of the yields (Y2PEA/YGLUC) obtained in ECP and DNP media for the four nonconventional yeasts. The experimental data of 2-PEA were selected as it was the metabolite with the highest production (Table 1). It can be observed that all of the evaluated data are within the solution space, which corroborates that the constructed metabolic network predicts the metabolism of the studied yeasts.
Fig. 4.
Yield analysis for 2-PEA production. Red symbols represent the De novo pathway (DNP) experimental data, while blue symbols represent the Ehrlich catabolic pathway (EPC) for (*) C. glabrata; (o) C. parapsilosis; (x) W. anomalus; (◊) C. utilis yeasts. Units of yields are given in (mmol/mmol GLUC) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
The differences observed among these yeasts showed that the best 2-PEA producers were W. anomalous and C. glabrata. These strains produce high biomass concentrations, which is correlated with the behavior observed in Fig. 3. The metabolic network also showed that could be theoretically possible to increase the 2-PEA yield for the yeasts evaluated in ECP and DNP, especially in the second one (low phenylalanine concentration). Thus, other factors would be investigated to improve the yield of this metabolite; such as C/N ratio, type of carbon and nitrogen source, 2-PE toxicity, among others.
Analysis of the EMs obtained from this metabolic network in the shikimate pathway (de novo biosynthesis) and phenylalanine catabolic pathway, allowed to observe that they did not coexistence at the same time (Data not shown). This has been stated in other studies [44], where the gene expression of different metabolic conditions was studied. They reported that yeasts cells are capable of selectively use different nitrogen sources as a regulatory mechanism, showing a differential reprogramming of the gene expression depending on the nitrogen source added. They also found that ammonia addition resulted in a higher expression of genes involved in amino acids biosynthesis while amino acid addition prepares the cells for protein biosynthesis.
In the case of tequila vinasses, their composition showed that the aromatic amino acids (Table 2), including phenylalanine, are very low. Thus, the production of 2-PEA in tequila vinasses is carried out by the shikimate pathway. An analysis of EMs (35) was performed taking only into account the shikimate pathway, where it was found that 23 of them were not biologically feasible due to an interruption in the citric acid cycle and a lack of biomass production (even when one of these scenarios was the EM with the highest yield for 2-PE production, thus it was discarded), resulting in 12 biologically possible scenarios for the metabolites of interest (Table 4). The maximum yield values attained for 2-PEA and 2-PE were 0.13 and 0.21 mmol/mmol GLUC respectively, which must be considered as values of reference for their production from tequila vinasses. To improve 2-PE and 2-PEA production, yield analysis will be considered as a first guideline to optimize the process.
Table 4.
Analysis of elementary modes (EMs) in the metabolism of nonconventional yeasts for the shikimate pathway.
| EM | YBIOM/GLUC | Y2-PE/GLUC | Y 2-PEA/GLUC | Y N/GLUC | Y ETH/GLUC |
|---|---|---|---|---|---|
| 18 | 0.003 | 0.0091 | 0 | 0.0221 | 0.7589 |
| 20 | 0.0028 | 0 | 0.0069 | 0.0208 | 0.7597 |
| 26 | 0.0268 | 0.0819 | 0 | 0.1985 | 0.8935 |
| 27 | 0.0264 | 0 | 0.0646 | 0.1958 | 0.84 |
| 53 | 0.0015 | 0 | 0.0232 | 0.0107 | 0.7464 |
| 55 | 0.012 | 0 | 0.192 | 0.089 | 0.7204 |
| 80 | 0.0032 | 0.0005 | 0 | 0.0236 | 0.7291 |
| 82 | 0.0032 | 0 | 0.0005 | 0.0235 | 0.7296 |
| 122 | 0.0021 | 0.0295 | 0 | 0.0157 | 0.7448 |
| 124 | 0.0021 | 0 | 0.0149 | 0.0158 | 0.7532 |
| 128 | 0.0155 | 0.2155 | 0 | 0.1146 | 0.7123 |
| 129 | 0.0189 | 0 | 0.1314 | 0.1398 | 0.7779 |
EMs are expressed as yields with respect to glucose. Units of yields are given in (mmol/mmol GLUC) except for biomass, given in (g BIOM/mmol GLUC).
4. Conclusions
The results indicate that vinasses could be of interest for the production of industrial metabolites, such as 2-phenylethylacetate, through the Shikimate pathway. The yeasts W. anomalus and C. glabrata obtained the best performances for cell growth and aroma compound production and accomplished over 50 % COD and BOD reduction from tequila vinasses. However, the composition of the different vinasses play a major role in the usage of this residue, since the presence of inhibitory compounds negatively affected cell growth and 2-PEA production; thus, these compounds should be monitored prior to vinasse fermentation. Elementary modes and yield analysis obtained from the FBA showed the distribution and the theoretical fluxes for aroma compound production, which offers a guideline and starting point for improvement using tequila vinasses as substrate.
CRediT authorship contribution statement
José de Jesús Rodríguez-Romero: Writing - original draft. César Arturo Aceves-Lara: Data curation, Formal analysis. Cristina Ferreira Silva: Conceptualization. Anne Gschaedler: Investigation, Methodology. Lorena Amaya-Delgado: Funding acquisition. Javier Arrizon: Project administration, Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare no conflict of interests involved in the study.
Acknowledgments
This work was supported by a CONACYT grant, announcement 291137; FSE-CONACYT-SENER248090 and 245750 projects, as well as the “Plan de Movilidad AGARED 2017” program. The authors thank the Tequila factories involved in the study for providing the vinasses used in the tequila vinasses fermentations.
Appendix A
Reactions on the reduced metabolic network of nonconventional yeasts holding different metabolic pathways. Irreversible reactions are described by ‘=>’, whereas reversible reactions are denoted by ‘=’. Biomass equation was modified from the one proposed by Robles-Rodriguez et al. [31], Song and Ramkrishna [32]
| Glycolysis | |
| R1 | GLCx => GLC |
| R2 | GLC + ATP => G6P + ADP |
| R3 | G6P = F6P |
| R4 | F6P + ATP = DHAP + GAP + ADP |
| R5 | DHAP = GAP |
| R6 | DHAP + NADH = GOL + NAD |
| R7 | GOL = GOLx |
| R8 | GAP + NAD + ADP = PG3 + NADH + ATP |
| R9 | PG3 = PEP |
| R10 | PEP + ADP = PYR + ATP |
| Pyruvate metabolism | |
| R11 | PYR => ACD + CO2 |
| R12 | ACD + NADH => ETH + NAD |
| R13 | ACD + NADHm => ETH + NADm |
| R14 | ACD + NADP => ACT + NADPH |
| R15 | ACT => ACTx |
| R16 | ACT + CoA + 2ATP => AcCoA + 2ADP |
| R17 | PYR + ATP + CO2 => OAA + ADP |
| Pentose phosphate pathway | |
| R18 | G6P + 2NADP => Ru5P + CO2 + 2NADPH |
| R19 | Ru5P = X5P |
| R20 | Ru5P = R5P |
| R21 | R5P + X5P = S7P + GAP |
| R22 | S7P + GAP = E4P + F6P |
| R23 | E4P + X5P = F6P + GAP |
| Krebs cycle | |
| R24 | PYR + NADm + CoAm => AcCoAm + CO2 + NADHm |
| R25 | OAA + NADm + NADH = OAAm + NADHm + NAD |
| R26 | OAAm + AcCoAm => ICT + CoAm |
| R27 | ICT + NADm => AKG + CO2 + NADHm |
| R28 | ICT + NADPm => AKG + CO2 + NADPHm |
| R29 | AKG + ADP + NADm => SUC + ATP + CO2 + NADHm |
| R30 | SUC + 0.5 NADm = MAL + 0.5 NADHm |
| R31 | MAL + NADm = OAAm + NADHm |
| Shikimate-Ehrlich pathway | |
| R32 | E4P + PEP => DHA7P |
| R33 | DHA7P + NADH = SHKT + NAD |
| R34 | SHKT + PEP + ATP = CHO + ADP |
| R35 | CHO => PHP + CO2 |
| R36 | PHE + AKG = GLUT + PHP |
| R37 | PHP => PHAC + CO2 |
| R38 | PHAC + NADH => 2_PE + NAD |
| R39 | 2_PE + AcCoA => 2_PEA + CoA |
| Biomass formation | |
| R40 | 1.04 AKG + 0.57 E4P + 0.11 GOL + 2.39 G6P + 1.07 OAA + 0.99 PEP + 0.57 PG3 + 1.15 PYR + 0.74 R5P + 2.36 + AcCoA + 0.31 AcCoAm + 2.68 NAD + 0.53 NADm + 11.55 NADPH + 1.51 NADPHm + 30.48 ATP => 1 g BIOM + 2.36 CoA + 0.31 CoAm + 2.68 NADH + 0.53 NADHm 11.55 NADP + 1.51 NADPm + 30.48 ADP |
| Glutamine, glutamate metabolism | |
| R46 | NH4 => NH3 |
| R47 | NADPH + AKG + NH3 => NADP + GLUT |
| R48 | ATP + GLUT + NH3 => ADP + GLUM |
| Others | |
| R41 | ATP => ADP + MAINT |
| R42 | NADH => NAD |
| R43 | 2_PE => 2_PEx |
| R44 | 2_PEA => 2_PEAx |
| R45 | PHEx => PHE |
Abreviations
AcCoA Acetyl Coenzyme A (cytosol)
AcCoAm Acetyl Coenzyme A (mitochondria)
ACD Acetaldehyde
ACT Acetate
ACTx Acetate (extracellular)
ADP Adenosine Bisphosphate
AKG α-ketoglutarate
ATP Adenosine Triphosphate
BIOM Catalytic Biomass
CHO Chorismate
CoA Coenzyme A
CoAm Coenzyme A (mitochondria)
CO2 Carbon dioxide
DHAP Dihydroxyacetone phosphate
DHA7P 3-Deoxy-D-arabino-heptulosonic acid 7-phosphate
E4P Erythrose 4 phosphate
ETH Ethanol
F6P Fructose 6-phosphate
G6P Glucose 6-phosphate
GAP Glucose 3-phosphate
GLC Glucose
GLCx Glucose (extracellular)
GLUM Glutamine
GLUT Glutamate
GOL Glycerol 3-phosphate
GOLx Glycerol 3-phosphate (extracellular)
ICT Isocitrate
MAINT Maintenance
MAL Malate
NAD Nicotinamide adenine dinucleotide oxidized (cytosol)
NADH Nicotinamide adenine dinucleotide reduced (cytosol)
NADHm Nicotinamide adenine dinucleotide reduced (mitochondria)
NADm Nicotinamide adenine dinucleotide oxidized (mitochondria)
NADP Nicotinamide adenine dinucleotide phosphate oxidized (cytosol)
NADPH Nicotinamide adenine dinucleotide phosphate reduced (cytosol)
NADPHm Nicotinamide adenine dinucleotide phosphate reduced (mitochondria)
NADPm Nicotinamide adenine dinucleotide phosphate oxidized (mitochondria)
NH3 Ammonia
NH4 Ammonium
OAA Oxaloacetate
OAAm Oxaloacetate (mitochondria)
PEP Phospho-enol pyruvate
PG3 Glyceraldehyde 3-phosphate
PHAC Phenyl acetaldehyde
PHE Phenylalanine
PHEx Phenylalanine (extracellular)
PHP Phenyl pyruvate
PYR Pyruvate
R5P Ribose 5-phosphate
Ru5P Ribulose 5-phosphate
S7P Sedoheptulose 7-phosphate
SHK Shikimate
SUC Succinate
X5P Xylose 5-phosphate
2PE 2-phenylethanol
2PEA 2-phenylethylacetate
BOD5 Biochemical oxygen demand
COD Chemical oxygen demand
EMsElementary modes
References
- 1.Consejo Regulador del Tequila (CRT) 2018. Información estadística.https://www.crt.org.mx/estadisticascrtweb/ (Accessed December 15, 2018. [Google Scholar]
- 2.López A., Davila G., León E., Villegas E., Gallardo J. Tequila vinasses: generation and full scale treatment processes. Rev. Environ. Sci. Biotechnol. 2010;9:109–116. [Google Scholar]
- 3.Ahmed O., Sulieman A.M.E., Elhardallou S.B. Physicochemical, chemical and microbiological characteristics of vinasse, A by-product from ethanol industry. Am. J. Biochem. 2013;3:80–83. [Google Scholar]
- 4.España-Gamboa E., Mijangos-Cortes J., Barahona-Perez L., Dominguez-Maldonado J., Hernández-Zarate G., Alzate-Gaviria L. Vinasses: characterization and treatments. Waste Manag. Res. 2011;29:1235–1250. doi: 10.1177/0734242X10387313. [DOI] [PubMed] [Google Scholar]
- 5.Robles-González V., Galíndez-Mayer J., Rinderknecht-Seijas N., Poggi-Varaldo H. Treatment of mezcal vinasses: a review. J. Biotechnol. 2012;157:524–546. doi: 10.1016/j.jbiotec.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Gamero A., Quintilla R., Groenewald M., Alkema W., Boekhout T., Hazelwood L. High-throughput screening of a large collection of non-conventional yeasts reveals their potential for aroma formation in food fermentation. Food Microbiol. 2016;60:147–159. doi: 10.1016/j.fm.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Pires E., Teixeira J., Brányik T., Vicente A. Yeast: the soul of beer’s aroma-a review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014;98:1937–1949. doi: 10.1007/s00253-013-5470-0. [DOI] [PubMed] [Google Scholar]
- 8.Carvajal-Zarrabal O., Nolasco-Hipólito C., Barradas-Dermitz D.M., Hayward-Jones P.M., Aguilar-Uscanga M.G., Bujang K. Treatment of vinasse from tequila production using polyglutamic acid. J. Environ. Manage. 2012;95(Supplement):S66–S70. doi: 10.1016/j.jenvman.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Dos Reis K.C., Arrizon J., Amaya-Delgado L., Gschaedler A., Schwan R.F., Silva C.F. Volatile compounds flavoring obtained from Brazilian and Mexican spirit wastes by yeasts. World J. Microbiol. Biotechnol. 2018;34:152. doi: 10.1007/s11274-018-2535-3. [DOI] [PubMed] [Google Scholar]
- 10.Seluy L.G., Isla M.A. A process to treat high-strength brewery wastewater via ethanol recovery and vinasse fermentation. Ind. Eng. Chem. Res. 2014;53:17043–17050. [Google Scholar]
- 11.Buitrón G., Carvajal C. Biohydrogen production from Tequila vinasses in an anaerobic sequencing batch reactor: effect of initial substrate concentration, temperature and hydraulic retention time. Bioresour. Technol. 2010;101:9071–9077. doi: 10.1016/j.biortech.2010.06.127. [DOI] [PubMed] [Google Scholar]
- 12.Salgado José M., Carballo Martínez E., Max B., Domínguez José M. Characterization of vinasses from five certified brands of origin (CBO) and use as economic nutrient for the xylitol production by Debaryomyces hansenii. Bioresour. Technol. 2010;101:2379–2388. doi: 10.1016/j.biortech.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Silva C.F., Arcuri S.L., Campos C.R., Vilela D.M., Alves J.G.L.F., Schwan R.F. Using the residue of spirit production and bio-ethanol for protein production by yeasts. Waste Manag. 2011;31:108–114. doi: 10.1016/j.wasman.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Christofoletti C.A., Escher J.P., Correia J.E., Marinho J.F.U., Fontanetti C.S. Sugarcane vinasse: environmental implications of its use. Waste Manag. 2013;33:2752–2761. doi: 10.1016/j.wasman.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Achmon Y., Ben-Barak Zelas Z., Fishman A. Cloning Rosa hybrid phenylacetaldehyde synthase for the production of 2-phenylethanol in a whole cell Escherichia coli system. Appl. Microbiol. Biotechnol. 2014;98:3603–3611. doi: 10.1007/s00253-013-5269-z. [DOI] [PubMed] [Google Scholar]
- 16.Chreptowicz K., Wielechowska M., Główczyk-Zubek J., Rybak E., Mierzejewska J. Production of natural 2-phenylethanol: from biotransformation to purified product. Food Bioprod. Process. 2016;100:275–281. [Google Scholar]
- 17.Kim T.-Y., Lee S.-W., Oh M.-K. Biosynthesis of 2-phenylethanol from glucose with genetically engineered Kluyveromyces marxianus. Enzyme Microb. Technol. 2014;61–62:44–47. doi: 10.1016/j.enzmictec.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Kim T.-Y., L Sang-Woo, Oh Min-Kyu. Biosynthesis of 2-phenylethanol from glucose with genetically engineered Kluyveromyces marxianus. Enzyme Microb. Technol. 2014;61-62:44–47. doi: 10.1016/j.enzmictec.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Tian X., Ye R., Wang J., Chen Y., Cai B., Guan S., Rong S., Li Q. Effects of aroma quality on the biotransformation of natural 2-phenylethanol produced using ascorbic acid. Electron. J. Biotechnol. 2015;18:286–290. [Google Scholar]
- 20.Etschmann M.M.W., Sell D., Schrader J. Production of 2-phenylethanol and 2-phenylethylacetate from L-phenylalanine by coupling whole-cell biocatalysis with organophilic pervaporation. Biotechnol. Bioeng. 2005;92:624–634. doi: 10.1002/bit.20655. [DOI] [PubMed] [Google Scholar]
- 21.Kondo T., Tezuka H., Ishii J., Matsuda F., Ogino C., Kondo A. Genetic engineering to enhance the Ehrlich pathway and alter carbon flux for increased isobutanol production from glucose by Saccharomyces cerevisiae. J. Biotechnol. 2012;159:32–37. doi: 10.1016/j.jbiotec.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Shen L., Nishimura Y., Matsuda F., Ishii J., Kondo A. Overexpressing enzymes of the Ehrlich pathway and deleting genes of the competing pathway in Saccharomyces cerevisiae for increasing 2-phenylethanol production from glucose. J. Biosci. Bioeng. 2016;122:34–39. doi: 10.1016/j.jbiosc.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 23.Wittmann C., Hans M., Bluemke W. Metabolic physiology of aroma-producing Kluyveromyces marxianus. Yeast. 2002;19:1351–1363. doi: 10.1002/yea.920. [DOI] [PubMed] [Google Scholar]
- 24.Yin S., Zhou H., Xiao X., Lang T., Liang J., Wang C. Improving 2-Phenylethanol production via ehrlich pathway using genetic engineered Saccharomyces cerevisiae strains. Curr. Microbiol. 2015;70:762–767. doi: 10.1007/s00284-015-0785-y. [DOI] [PubMed] [Google Scholar]
- 25.Hua D., Xu P. Recent advances in biotechnological production of 2-phenylethanol. Biotechnol. Adv. 2011;29:654–660. doi: 10.1016/j.biotechadv.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Fabre C.E., Blanc P.J., Goma G. Production of 2-Phenylethyl Alcohol by Kluyveromyces marxianus. Biotechnol. Prog. 1998;14:270–274. doi: 10.1021/bp9701022. [DOI] [PubMed] [Google Scholar]
- 27.Martin V., Boido E., Giorello F., Mas A., Dellacassa E., Carrau F. Effect of yeast assimilable nitrogen on the synthesis of phenolic aroma compounds by Hanseniaspora vineae strains. Yeast. 2016;33:323–328. doi: 10.1002/yea.3159. [DOI] [PubMed] [Google Scholar]
- 28.Chen P.-W., Theisen M.K., Liao J.C. Metabolic systems modeling for cell factories improvement. Curr. Opin. Biotechnol. 2017;46:114–119. doi: 10.1016/j.copbio.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Kaushal M., Chary K.V.N., Ahlawat S., Palabhanvi B., Goswami G., Das D. Understanding regulation in substrate dependent modulation of growth and production of alcohols in Clostridium sporogenes NCIM 2918 through metabolic network reconstruction and flux balance analysis. Bioresour. Technol. 2018;249:767–776. doi: 10.1016/j.biortech.2017.10.080. [DOI] [PubMed] [Google Scholar]
- 30.Rafieenia R., Chaganti S.R. Flux balance analysis of different carbon source fermentation with hydrogen producing Clostridium butyricum using Cell Net Analyzer. Bioresour. Technol. 2015;175:613–618. doi: 10.1016/j.biortech.2014.10.070. [DOI] [PubMed] [Google Scholar]
- 31.Robles-Rodriguez C.E., Bideaux C., Guillouet S.E., Gorret N., Cescut J., Uribelarrea J.-L., Molina-Jouve C., Roux G., Aceves-Lara C.A. Dynamic metabolic modeling of lipid accumulation and citric acid production by Yarrowia lipolytica. Comput. Chem. Eng. 2017;100:139–152. [Google Scholar]
- 32.Song H.-S., Ramkrishna D. Reduction of a set of elementary modes using yield analysis. Biotechnol. Bioeng. 2009;102:554–568. doi: 10.1002/bit.22062. [DOI] [PubMed] [Google Scholar]
- 33.Song H.-S., Ramkrishna D. When is the Quasi-Steady-State Approximation Admissible in Metabolic Modeling? When Admissible, What Models are Desirable? Ind. Eng. Chem. Res. 2009;48:7976–7985. [Google Scholar]
- 34.Comer A.D., Long M.R., Reed J.L., Pfleger B.F. Flux balance analysis indicates that methane is the lowest cost feedstock for microbial cell factories. Metab. Eng. Commun. 2017;5:26–33. doi: 10.1016/j.meteno.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cavallieri A.P., Baptista A.S., Leite C.A., Araujo M.L.Gd.C. A case study in flux balance analysis: lysine, a cephamycin C precursor, can also increase clavulanic acid production. Biochem. Eng. J. 2016;112:42–53. [Google Scholar]
- 36.Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31:426–428. [Google Scholar]
- 37.Arellano M., Gschaedler A., Alcazar M. Major volatile compounds analysis produced from mezcal fermentation using gas chromatography equipped headspace (GC–HS) In: Salih D.B., editor. Gas Chromatography in Plant Science, Wine Technology, Toxicology and Some Specific Applications. InTech; 2012. [Google Scholar]
- 38.APHA A.P.H.A. 22th ed. 2012. Standard Methods for the Examination of Water and Wastewater; pp. 1–134. [Google Scholar]
- 39.Voige W.H. Biochemical pathways: an atlas of biochemistry and molecular biology (ed. Michal, gerhard) J. Chem. Educ. 2012;77:163. [Google Scholar]
- 40.Hazelwood L.A., Daran J.-M., van Maris A.J., Pronk J.T., Dickinson J.R. The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008;74:2259–2266. doi: 10.1128/AEM.02625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Kamp A., Thiele S., Hädicke O., Klamt S. Use of CellNetAnalyzer in biotechnology and metabolic engineering. J. Biotechnol. 2017;261:221–228. doi: 10.1016/j.jbiotec.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Haibo Z., Mingle C., Xinglin J., Huibin Z., Cong W., Xin X., Mo X. De-novo synthesis of 2-phenylethanol by Enterobacter sp. CGMCC 5087. BMC Biotechnol. 2014;14:1–17. doi: 10.1186/1472-6750-14-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akita O., Ida T., Obata T., Hara S. Mutants of Saccharomyces cerevisiae producing a large quantity of β-Phenethyl alcohol and β-Phenethyl acetate. J. Ferment. Bioeng. 1990;69:125–128. [Google Scholar]
- 44.Jimenez-Marti E., del Olmo M.L. Addition of ammonia or amino acids to a nitrogen-depleted medium affects gene expression patterns in yeast cells during alcoholic fermentation. FEMS Yeast Res. 2008;8:245–256. doi: 10.1111/j.1567-1364.2007.00325.x. [DOI] [PubMed] [Google Scholar]
- 45.Adler P., Hugen T., Wiewiora M., Kunz B. Modeling of an integrated fermentation/membrane extraction process for the production of 2-phenylethanol and 2-phenylethylacetate. Enzyme Microb. Technol. 2011;48:285–292. doi: 10.1016/j.enzmictec.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Stark D., Zala D., Münch T., Sonnleitner B., Marison I.W., von Stockar U. Inhibition aspects of the bioconversion of l-phenylalanine to 2-phenylethanol by Saccharomyces cerevisiae. Enzyme Microb. Technol. 2003;32:212–223. [Google Scholar]
- 47.Uber G. Departamento de bioquímica y biología molecular. Universidad de Valencia, Valencia; España: 2006. Modificación genética de levaduras vínicias industriales paara mejorar la producción de un aroma secundario. [Google Scholar]
- 48.Camargo J.A., Alonso A. Contaminación por nitrógeno inorgánico en los ecosistemas acuáticos: problemas medioambientales, criterios de calidad del agua, e implicaciones del cambio climático. Ecosistemas. 2007;16(2):1–13. [Google Scholar]
- 49.Gschaedler A., Rodríguez-Garay B., Prado-Ramírez R., Flores Montaño J.L. 2nd ed. CIATEJ; 2015. Ciencia y tecnología del tequila: avances y perspectivas. [Google Scholar]
- 50.Sanclemente Reyes Oscar, Arboleda Mauricio, Trujillo Francis. Efecto del uso de melaza y microorganismos eficientes sobre la tasa de descomposición de la hoja de caña (Saccharum officinarum) Rev. Investig. Agrar. Y Ambient. 2011;2 [Google Scholar]
- 51.Pinal L., Cornejo E., Arellano M., Herrera E., Nuñez L., Arrizon J., Gschaedler A. Effect of Agave tequilana age, cultivation field location and yeast strain on tequila fermentation process. J. Ind. Microbiol. Biotechnol. 2009;36:655–661. doi: 10.1007/s10295-009-0534-y. [DOI] [PubMed] [Google Scholar]
- 52.Prado-Ramírez R., Gonzáles-Alvarez V., Pelayo-Ortiz C., Casillas N., Estarrón M., Gómez-Hernández H. The role of distillation on the quality of tequila. Int. J. Food Sci. Technol. 2005;40:701–708. [Google Scholar]
- 53.Díaz-Montaño D.M., Délia M.-L., Estarrón-Espinosa M., Strehaiano P. Fermentative capability and aroma compound production by yeast strains isolated from Agave tequilana Weber juice. Enzyme Microb. Technol. 2008;42:608–616. [Google Scholar]
- 54.Villanueva-Rodríguez S.J., Rodríguez-Garay B., Prado-Ramírez R., Gschaedler A. Academic Press; Oxford: 2016. Tequila: Raw Material, Classification, Process, and Quality Parameters. Encyclopedia of Food and Health; pp. 283–289. [Google Scholar]
- 55.Sandoval-Nuñez D., Arellano-Plaza M., Gschaedler A., Arrizon J., Amaya-Delgado L. A comparative study of lignocellulosic ethanol productivities by Kluyveromyces marxianus and Saccharomyces cerevisiae. Clean Technol. Environ. Policy. 2018;20:1491–1499. [Google Scholar]
- 56.García I.G., Venceslada J.L.B., Peña P.R.J., Gómez E.R. Biodegradation of phenol compounds in vinasse using Aspergillus terreus and Geotrichum candidum. Water Res. 1997;31:2005–2011. [Google Scholar]
- 57.Garrido F.V. Universidad Politécnica de Valencia; Valencia, España: 2011. Levaduras no- Saccharomyces para modular el aroma secundario de los vinos: Incremento del acetato de 2-feniletilo mediante cultivos iniciadores mixtos. Departamento de Tecnología de Alimentos. [Google Scholar]
- 58.Rollero S., Bloem A., Camarasa C., Sanchez I., Ortiz-Julien A., Sablayrolles J.-M., Dequin S., Mouret J.-R. Combined effects of nutrients and temperature on the production of fermentative aromas by Saccharomyces cerevisiae during wine fermentation. Appl. Microbiol. Biotechnol. 2015;99:2291–2304. doi: 10.1007/s00253-014-6210-9. [DOI] [PubMed] [Google Scholar]
- 59.Pires J.F., Ferreira G.M.R., Reis K.C., Schwan R.F., Silva C.F. Mixed yeasts inocula for simultaneous production of SCP and treatment of vinasse to reduce soil and fresh water pollution. J. Environ. Manage. 2016;182:455–463. doi: 10.1016/j.jenvman.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 60.Hye Ryun K., Jae-Ho K., Dong-Hoon B., Byung Hak A. Microbiological characteristics of wild yeast strain Pichia anomala Y197-13 for brewing makgeolli. Mycobiology. 2013;41(3):139–144. doi: 10.5941/MYCO.2013.41.3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]