Exogenous auxin triggers transverse patterning of cortical microtubule arrays through the TIR1/AFB receptor pathway.
Abstract
Auxin plays a central role in controlling plant cell growth and morphogenesis. Application of auxin to light-grown seedlings elicits both axial growth and transverse patterning of the cortical microtubule cytoskeleton in hypocotyl cells. Microtubules respond to exogenous auxin within 5 min, although repatterning of the array does not initiate until 30 min after application and is complete by 2 h. To examine the requirements for auxin-induced microtubule array patterning, we used an Arabidopsis (Arabidopsis thaliana) double auxin f-box (afb) receptor mutant, afb4-8 afb5-5, that responds to conventional auxin (indole-3-acetic acid) but has a strongly diminished response to the auxin analog, picloram. We show that 5 µm picloram induces immediate changes to microtubule density and later transverse microtubule patterning in wild-type plants, but does not cause microtubule array reorganization in the afb4-8 afb5-5 mutant. Additionally, a dominant mutant (axr2-1) for the auxin coreceptor AUXIN RESPONSIVE2 (AXR2) was strongly suppressed for auxin-induced microtubule array reorganization, providing additional evidence that auxin functions through a transcriptional pathway for transverse patterning. We observed that brassinosteroid application mimicked the auxin response, showing both early and late microtubule array effects, and induced transverse patterning in the axr2-1 mutant. Application of auxin to the brassinosteroid synthesis mutant, diminuto1, induced transverse array patterning but did not produce significant axial growth. Thus, exogenous auxin induces transverse microtubule patterning through the TRANSPORT INHIBITOR 1/AUXIN F-BOX (TIR1/AFB) transcriptional pathway and can act independently of brassinosteroids.
Plant morphology depends upon the growth properties of individual cells and their coordinated response to environmental stimuli such as light, gravity, and temperature. The axial growth of vascular plant tissues constitutes a principal aspect of morphogenesis, allowing shoots and roots to reach resource-rich environments. Axial growth requires the extension of shared side walls between adjoining cells with only limited expansion of the apical and basal walls orthogonal to the growth axis. The individual cells expand in response to turgor pressure, a nonvectoral force generating stress in the cell walls. The composition of the cell wall, and the spatial organization of the wall materials, in turn, provide a means to guide cell expansion to produce cell shape (Green, 1962). Wall polymers with a length scale of more than a few micrometers, such as cellulose, can produce cell walls with highly anisotropic material properties. Coalignment of cellulose in the wall, for example, can create a material that yields preferentially along the axis orthogonal to the cellulose alignment (Probine, 1965; Marga et al., 2005). The axial growth of plant roots and shoots takes great advantage of this mechanism, creating walls with distinct material properties leading to coordinated cell elongation (Baskin, 2001; Baskin, 2005; Cosgrove, 2005).
The Arabidopsis (Arabidopsis thaliana) hypocotyl is an important model for studying axial growth phenomena in flowering plants owing to the dramatic cell elongation in the absence of cell division and the sensitivity to environmental cues (Gendreau et al., 1997; Collett et al., 2000). Grown in the dark, an etiolated hypocotyl rapidly elongates, using the energy reserves in the seed to drive the cotyledons upward into the light (Fankhauser and Chory, 1997). Once light is detected, axial cell growth slows in favor of more radial cell growth (Liscum and Hangarter, 1993; Parks et al., 1998; Folta and Spalding, 2001) due to the action of light receptors and myriad downstream signaling targets discovered through forward genetic screens (Koornneef et al., 1980; Chory et al., 1989; Reed et al., 1993; Jiao et al., 2007). Suppressor screens, microarray analysis, and hormone treatments have provided evidence that hormone signals elicit cell growth downstream of light perception (Szekeres et al., 1996; Reed et al., 1998; Devlin et al., 2003; Lau and Deng, 2010). The major hormones involved in promoting early hypocotyl elongation are auxin, gibberellic acid, and brassinosteroid (Katsumi, 1985; Depuydt and Hardtke, 2011; Oh et al., 2014). Although a substantial number of papers have examined the gene expression changes associated with exogenous hormone treatments and receptor mutants, our present understanding of how cell growth (i.e. increase in size) is coordinated with the cytoskeleton to determine hypocotyl cell shape (i.e. cellular morphogenesis) is limited.
Auxin is a central regulator of cell growth, eliciting responses from seconds to hours and showing concentration-dependent effects on cell expansion (Rayle et al., 1970; Bates and Goldsmith, 1983; Kutschera and Schopfer, 1986; Rück et al., 1993; Teale et al., 2006). Auxin-induced growth in hypocotyl cells can be measured 15–20 min after treatment and is correlated with cell wall acidification or ‘acid growth’ (Romani et al., 1983; Theologis et al., 1985; Hager, 2003; Cosgrove, 2005; Falhof et al., 2016). The early auxin response was initially postulated to be controlled directly by AUXIN BINDING PROTEIN 1 (ABP1) in the absence of new transcription (Chen et al., 2014), but subsequent work has revealed that ABP1 is not required for auxin signaling (Enders et al., 2015; Gao et al., 2015). Recent work examining the almost immediate depolarization of the plasma membrane by auxin (Dindas et al., 2018; Paponov et al., 2019), indicates that auxin transport proteins, such as AUXIN TRANSPORTER PROTEIN 1 (AUX1), are required for depolarization and lead to transient fluctuations in a number of ion species. Other recent work shows that some rapidly transcribed SMALL AUXIN UPREGULATED RNA (SAUR) transcripts are translated and interact with plasma membrane proton ATPases to affect wall acidification, indicating a transcriptional pathway for acid growth (Spartz et al., 2014). Thus, the molecular mechanisms leading to the rapid auxin growth response are not completely defined, but could involve both nontranscriptional and transcriptional pathways.
The path from auxin perception to transcriptional response has been robustly described, and a family of six primary auxin receptors has been characterized (Dharmasiri et al., 2005; Parry et al., 2009). With increasing auxin concentration, the TRANSPORT INHIBITOR 1/AUXIN F-BOX (TIR1/AFB) receptors form complexes with a family of 29 AUXIN RESISTANT (Aux/IAA) nuclear coreceptors that interact with transcription factors such as the AUXIN RESPONSE FACTORS (ARFs; Gray et al., 2001; Weijers et al., 2005; Wang and Estelle, 2014). The auxin-dependent formation of the TIR1/AFB complex leads to the ubiquitinylation and degradation of the Aux/IAA coreceptor, releasing the associated transcription factors to regulate gene expression (Chandler, 2016). Hypocotyl growth, downstream from the TIR1/AFB Aux/IAA complex, is modulated primarily by three ARF transcription factors with overlapping function (Reed et al., 2018). Although auxin induces a substantial number of growth-related genes (Chapman and Estelle, 2009), there is presently no molecular pathway known that specifically regulates the cytoskeleton (Ulmasov et al., 1997; Salehin et al., 2015) or specifies cell elongation rather than diffuse growth (Kutschera and Niklas, 2016).
Microtubules are essential for specifying axial cell elongation in plants. Drugs that perturb the microtubule cytoskeleton, such as taxol or oryzalin, do not block cell growth per se, but lead to apparent swelling and distention in growing cells, indicating a block to the mechanisms controlling cellular morphogenesis (Baskin et al., 1994; Mathur, 2004). In flowering plants, microtubules attach laterally to the plasma membrane and form arrays in the absence of centrosomes (Newcomb, 1969; Hardham and Gunning, 1978; Ehrhardt and Shaw, 2006). The cortical microtubules are organized into patterns that influence cell shape by directing the deposition of new cell wall materials (Green, 1962; Cyr and Palevitz, 1995; Baskin, 2001). Cellulose synthases in the plasma membrane, which deposit cellulose microfibrils into the cell wall, track along microtubules, resulting in extended cellulose microfibrils that are oriented in the same direction as the cortical microtubules (Paredez et al., 2006; Crowell et al., 2010). Coalignment of microtubules transverse to the cell’s long axis leads to an accumulation of transverse cellulose fibers. The resultant anisotropic cell wall is hypothesized to be the dominant factor governing the observed anisotropic cell expansion in the hypocotyl and root (Green, 1962; Baskin, 2001; Lloyd and Chan, 2004; Mathur, 2004). How the cortical microtubules create a transverse coaligned pattern is, therefore, a key question for determining how developmental and environmental cues control morphogenesis on a cellular scale.
Exogenous auxin results in the formation of transverse microtubule patterns in light-grown hypocotyl cells (Katsumi, 1985). When combined with gibberellic acid (GA4), auxin (IAA) treatment was shown to override light and gravity responses in the light microscope, providing a means to investigate how wild-type Arabidopsis seedlings reorganize the cortical microtubule arrays into a transverse coaligned pattern (Vineyard et al., 2013). Combined IAA/GA4 treatment elicited both an early (∼5 min) and late (>30 min) microtubule response (Vineyard et al., 2013; Elliott and Shaw, 2018a, 2018b) in 6-d-old hypocotyl cells, consistent with prior observations of other auxin responses (Cleland, 1992; Abel et al., 1994; Oeller and Theologis, 1995; Schenck et al., 2010). The early response to IAA/GA4 included a decrease in the number of growing microtubule plus ends and suggested that a pretranscriptional effect was occurring before observed changes in microtubule array pattern. Beginning 30–60 min after IAA/GA4 addition, a transverse band of microtubules appears at the cell’s midzone, with the transverse coalignment spreading apically and basally to eventually repattern the entire cell face within 60–90 min (Vineyard et al., 2013). The loss of the longitudinally oriented microtubules after auxin treatment is specifically sensitive to cycloheximide (a translation inhibitor), suggesting that part of the hormone-induced microtubule patterning requires new gene expression (Elliott and Shaw, 2018a).
In this study, we examined the requirements for exogenous hormone induction of the transverse microtubule arrays in light-grown Arabidopsis hypocotyl cells. We hypothesized that the hormone treatments could act through nontranscriptional mechanisms leading to a cascade of changes in microtubule dynamics, ultimately forming a transverse pattern in time through self-organization (Ehrhardt and Shaw, 2006; Allard et al., 2010; Hawkins et al., 2010; Deinum et al., 2011; Mirabet et al., 2018). Alternatively, the hormone treatments could initiate new gene expression, providing new proteins that function on the microtubule array to more directly specify the array pattern type (Yuan et al., 1995; Sambade et al., 2012; Vineyard et al., 2013; Elliott and Shaw, 2018a).
RESULTS
Exogenous Auxin Induces Transverse Microtubule Arrays Using TIR1/AFB Receptors
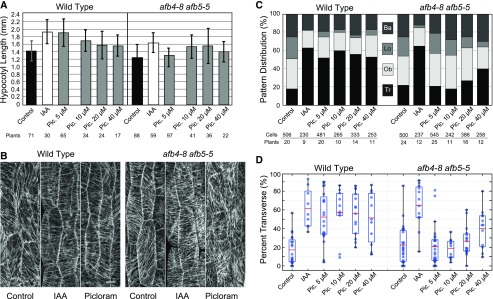
The cortical microtubule cytoskeleton in seedling hypocotyls reorganizes to a transverse coaligned pattern when exposed to 0.5 µm exogenous IAA. To investigate how exogenous auxin signals for transverse microtubule array coalignment, we took advantage of prior work showing that two of the Arabidopsis TIR1/AFB auxin receptors, AFB4 and AFB5, are specifically activated to initiate gene expression by the auxin analog, picloram (Greenham et al., 2011; Calderón Villalobos et al., 2012; Prigge et al., 2016). To determine effects on the microtubule arrays, we crossed the picloram-insensitive afb4-8/afb5-5 double mutant into our 35S-GFP-BETA-TUBULIN-1 (GFP-TUB1) microtubule marker lines. We previously established that 0.5 µm indole acetic acid (IAA) in 0.5× Murashige and Skoog (MS) liquid medium induces transverse microtubule array patterning in light-grown, 6-d-old seedlings by 2 h of application (Vineyard et al., 2013; Elliott and Shaw, 2018b). Importantly, 0.5 µm IAA in liquid medium induces, rather than retards, new hypocotyl growth over 72 h under lighted conditions, where enough growth accrues for accurate measurements (Vineyard et al., 2013). To identify a comparable picloram concentration for our experiments, we assayed picloram over a 5–40 µm range for hypocotyl growth response (Fig. 1A). When compared with solvent controls (1.43 ± 0.27 mm; n = 71), wild-type seedlings treated with 0.5 µm IAA showed a 35.2% length increase (1.94 ± 0.32 mm; n = 30; P < 0.001, 2-tailed Student’s t test of pooled replicates, see Supplemental Fig. 1 for ANOVA results), where 5 µm picloram produced a 33.4% increase in the wild type (1.91 ± 0.36 mm; n = 65; P < 0.001), indicating an equivalent effect on axial growth induction for the pooled data from replicate experiments (P = 0.734). Increasing picloram concentrations produced progressively less axial growth, suggesting that picloram is at, or above, the top range of its efficacy for hypocotyl growth induction at 5 µm (Fig. 1A).
Figure 1.
The synthetic auxin, picloram (Pic.), induces transverse microtubule patterning in wild-type Arabidopsis seedlings but not in the afb4-8/afb5-5 double auxin receptor mutant. A, Hypocotyl growth response to exogenous IAA or picloram for the wild type and afb4-8/afb5-5 double mutants under light-grown conditions. Mean length (in millimeters) and sd after 72-h treatment from d 6 to 9 for light-grown seedlings for the solvent control, 0.5 µm IAA, and 5, 10, 20, and 40 µm picloram on liquid medium. IAA and 5 µm picloram treatments show significant effects (P < 0.05, Student’s t test) on the wild type but only 0.5 µm IAA and not 5 µm picloram shows a significant effect on afb4-8/afb5-5. Number of plants per treatment are shown below graph. B, Representative images of epidermal hypocotyl cells of 6-d-old, light-grown wild-type and afb4-8/afb5-5 TUB1:GFP seedlings after 2 h treatment with control, 0.5 µm IAA, and 5 µm picloram. Scale bar = 10 µm. C, The distribution of microtubule array pattern in the epidermal cells of 6-d-old light-grown wild-type and afb4-8/afb5-5 hypocotyls after treatment with a solvent control, IAA, or picloram for 2 h. Ba, Basket, Lo, longitudinal; Ob, oblique; Tr, transverse. Number of cells/plants per treatment are shown below graph. D, The percentage of cells having transverse microtubule patterns broken out from (C) as box-and-whisker plots in wild-type and afb4-8/afb5-5 hypocotyls measured in (C). IAA and 5 µm picloram treatments show significant effects (P < 0.05, Student’s t test) on the wild type, but only IAA and not 5 µm picloram shows a significant effect on the afb4-8/afb5-5 mutant. Markers represent individual seedlings where the red bars equal the mean value and the box represents 25th to 75th percentiles.
The afb4-8/afb5-5 double mutant had slightly (12%) shorter hypocotyls (1.26 ± 0.24 mm; n = 88; P = 0.01) than wild-type plants under light-grown conditions. Treatment of the mutant with 0.5 µm IAA elicited a 31% increase in length (1.65 ± 0.26 mm; n = 59; P < 0.001), relative to the control, indicating that the double afb4-8/afb5-5 mutant responds to conventional auxin with a nearly identical growth response to wild type. Treatment of the double mutant with 5 µm picloram resulted in only a 4.8% length increase relative to the solvent control (1.32 ± 0.29 mm; n = 97; P = 0.131). Increasing the picloram concentration beyond 5 µm led to increases in hypocotyl length close to levels induced by IAA in the double mutant, even though picloram showed decreasing growth induction in the wild type. These data indicate that 5 µm picloram induces hypocotyl growth in the light-grown wild-type plant but has no significant effect on growth in the afb4-8/afb5-5 double mutant. Picloram growth assays at 5 µm were repeated on solid media plates for wild-type and afb4-8/afb5-5 double mutant seedlings expressing GFP-TUB1 (Supplemental Fig. 2) and showed comparable effects on growth induction to liquid assays.
To examine the requirement for AFB4/AFB5 in the auxin-induced transverse patterning of the microtubule arrays, we imaged both wild-type and afb4-8/afb5-5 TUB1-GFP hypocotyls after a 2-h incubation in solvent alone, 0.5 µm IAA, or 5 µm picloram (Fig. 1B). Microtubule array patterns were visually classified from epidermal cells in two adjacent cell files as predominantly transverse (0°–15°), oblique (16°–74°), longitudinal (75°–90°), or ‘basket’ (indeterminant) relative to the cell’s long axis (Vineyard et al., 2013; Elliott and Shaw, 2018b). Comparing the wild type and the afb4-8/afb5-5 double mutant for the solvent control revealed a roughly identical distribution of microtubule pattern types for control plants indicating that the absence of AFB4 and AFB5 function does not alter the ability of the seedlings to generate the array patterns (Fig. 1C). Breaking out the percentage of transverse patterns from the data sets for specific comparisons (Fig. 1D), the wild type had an average of 18% (n = 20 plants/506 cells) of the cells in a transverse coalignment compared with 22% (n = 24 plants/500 cells) for afb4-8/afb5-5 double mutants, an insignificant difference (P = 0.079; Z-test, see “Materials and Methods”). As positive controls, IAA treatment of the wild type significantly increased (P < 0.001) the percentage of transverse array patterns from 18% to 63% (n = 9 plants/230 cells) and significantly increased (P < 0.001) transverse patterns in the afb4-8/afb5-5 double mutants from 22% to 65% (n = 12 plants/237 cells), indicating that IAA is sufficient in both backgrounds to induce transverse array patterning to the same level (P = 0.59). Application of IAA at decreasing concentrations to wild-type and afb4-8/afb5-5 (Supplemental Fig. 3) plants indicated a lower threshold for maximum induction response between 0.05 and 0.25 µM for both genetic backgrounds.
To determine if picloram was sufficient to induce transverse microtubule array patterning in the wild type, we treated seedlings with 5 µm picloram and found 52% (n = 20 plants/481 cells; P < 0.001) of cells with transverse array patterns (Fig. 1, C and D), slightly less than with the 0.5 µm IAA treatment (P = 0.006). Increasing picloram concentrations resulted in a higher average percentage of transverse coaligned patterns for wild-type plants reaching a maximum of 60%, indicating that picloram shows a similar efficacy to IAA treatment. To determine if AFB4/AFB5 are necessary for the induction of transverse array patterning with picloram, we treated the afb4-8/afb5-5 double mutant with 5 µm picloram and found only 24% (n = 25 plants/545 cells) of arrays in a transverse pattern, an insignificant effect (P = 0.724) compared with 22% for solvent-treated controls (Fig. 1, C and D). Increasing the picloram concentration to 10 µm (19%; n = 11 plants/242 cells) resulted in no significant alteration in the percentage of cells exhibiting transverse microtubule patterns when compared with the double mutant control (P = 0.17). Higher picloram concentrations (20–40 µm) produced a higher percentage of transverse array patterns, but not to the same level as IAA. These data indicate that induction of the transverse microtubule array patterns by the synthetic auxin picloram, at 5 µm, requires the AFB4 or AFB5 auxin receptors.
Auxin Co-Receptor Mutants Have Defects in Auxin-Induced Microtubule Reorganization
The TIR1/AFB proteins act as auxin coreceptors through interaction with the 29-member Aux/IAA family (Dharmasiri et al., 2005). When auxin reaches a threshold concentration, the TIR1/AFB associates with an Aux/IAA in the nucleus resulting in the ubiquitin-mediated degradation of the Aux/IAA protein and subsequent modulation of transcription factors (Tan et al., 2007). Loss of function aux/iaa mutants do not show significant hypocotyl phenotypes, presumably due to functional redundancy (Nagpal et al., 2000; Muto et al., 2007). However, dominant negative alleles of three Aux/IAA genes, AXR2/IAA7 (axr2-1), AXR3/IAA17 (axr3-1), and AXR5/IAA1 (axr5-1), fail to correctly elongate their hypocotyls in the dark, suggesting a role in auxin-dependent axial growth (Nagpal et al., 2000). We speculated that if exogenous IAA induces transverse microtubule array patterning through a TIR1/AFB transcriptional pathway, one or more of these dominant Aux/IAA coreceptor mutants would be unable to reorganize the microtubule arrays correctly in response to IAA treatment. To test our hypothesis, we crossed our GFP-TUB1 line into the axr2-1, axr3-1, and axr5-1 mutants and examined the response of each mutant to exogenous auxin in homozygous mutant lines.
Because the mutant phenotypes were originally characterized in dark-grown seedlings, we first determined the response of each mutant to the addition of exogenous IAA at 0.5 µm under light-grown conditions using hypocotyl growth assays. Six-day-old seedlings were floated on a liquid bath containing a solvent control or 0.5 µm IAA for 72 h and measured for hypocotyl length (Fig. 2A). When compared with wild-type hypocotyls (1.38 ± 0.26 mm, n = 95), control axr2-1 hypocotyls were 9% longer (1.49 ± 0.26; n = 61; P = 0.008), axr3-1 hypocotyls were 31.9% longer (1.82 ± 0.32; n = 66; P < 0.001), and axr5-1 hypocotyls were 10.1% longer (1.52 ± 0.26 mm; n = 79; P < 0.001), on average (Fig. 2A). These data indicated that axr2-1 and axr5-1 mutants were only slightly different from the wild type, but axr3-1 was notably longer under light-grown conditions. IAA treatment produced a 13.8% length increase (1.60 ± 0.33 mm; n = 115; P < 0.001) in wild-type plants for these experiments (Fig. 2A). The same 0.5 µm exogenous IAA treatment produced no measurable new growth or potentially retarded growth over the 72 h incubation in the axr2-1 mutant (1.37 ± 0.28 mm; n = 58; P = 0.022). IAA treatment had no effect on hypocotyl growth in the axr3-1 mutant (1.83 ± 0.29 mm; n = 78; P = 0.816) when compared with controls but produced a 14.1% length increase in the axr5-1 mutant (1.76 ± 0.32 mm; n = 46; P = 0.003), comparable with the induction in the wild type.
Figure 2.
The dominant axr2-1 auxin coreceptor mutant blocks auxin-induced microtubule pattern reorganization. A, Hypocotyl growth assays for light-grown wild-type, axr2-1, axr3-1, and axr5-1 seedlings showing the mean (+/− std) length (mm) for light-grown seedlings treated from 6 to 9 d poststratification with a solvent control or 0.5 µm IAA on liquid. IAA showed significant effects (P < 0.05, Student’s t test) for wild-type and axr5-1 seedlings. Sample sizes are listed below the graph. B, The percentage of transverse (Tr; 0°–15°), oblique (Ob; 16°–75°), longitudinal (Lo; 76°–90°), and non–co-aligned microtubule array patterns (Ba) in 6-d-old hypocotyl cells treated with a solvent control or 0.5 µm IAA for 2 h. The sample sizes are shown below the graph. C, The percentage of cells within each hypocotyl (from [B]) displaying a transverse microtubule pattern. Blue markers represent each seedling. The red line shows the mean percentage, and the box designates the 25th and 75th percentiles. A significant induction (P < 0.05, Z-test) was observed for all genotypes. D, IAA concentration gradient growth assay (0.05–50 µm IAA) with wild-type and axr2-1 seedlings indicating an absence of response (*P > 0.05, Students t test) at all concentrations for the axr2-1 mutant. Mean hypocotyl length in millimeters is shown with error bars representing the sd. The sample sizes are listed below the graph. E, The percentage of each microtubule pattern in 6-d-old wild-type and axr2-1 GFP-TUB1 hypocotyls treated with a solvent control or 0.05–50 µm IAA for 2 h. The sample sizes are shown below the graph. F, The percentage of cells within each hypocotyl (from [E]) displaying a transverse microtubule pattern. Effect on the axr2-1 mutant is insignificant (P > 0.05, Students t test) below 50 µm IAA. Blue markers represent each seedling. The red line shows the mean percentage, and the box designates the 25th and 75th percentiles.
To determine if AXR2/IAA7, AXR3/IAA17, or AXR5/IAA1 is involved in auxin-induced microtubule patterning, we examined the distribution of microtubule array pattern types in epidermal hypocotyl cells from 6-d-old seedlings. Control plants for each mutant line treated with solvent contained the same four microtubule pattern types as the wild type with no evidence observed for novel array patterns (Fig. 2B). We noted that the control axr2-1 cells had a similar overall distribution of array patterns when compared with the wild type (Fig. 2B). Breaking the data out to compare the mean percentage of cells per hypocotyl having transverse patterns (Fig. 2C), we found that control wild-type seedlings (29%; n = 658 cells, 26 plants) had a slightly lower percentage than control axr2-1 mutants (36%; n = 660 cells, 27 plants; P = 0.006). Control seedlings for axr3-1 (44%; n = 261 cells, 10 plants; P < 0.001) and axr5-1 (43%; n = 521 cells, 17 plants; P < 0.001) mutants showed significantly higher percentages of transverse patterns than the wild type (Fig. 2C).
Incubation of 6-d-old seedlings with 0.5 µm IAA for 2 h significantly increased the number of wild-type cells exhibiting transverse patterns from 29% to 69% (n = 21 plants/523 cells; P < 0.001) where the axr2-1 mutant showed only a small and variable (8%; P = 0.004) increase from 36% to 44% (n = 24 plants/560 cells). In contrast, IAA treatment of the axr3-1 mutants increased the percentage of transverse patterns from 44% to 70% (n = 11 plants/255 cells; P < 0.001), a value more similar to the wild type. IAA treatment of the axr5-1 mutant resulted in a 22% change from 43% to 65% (n = 5 plants/176 cells; P < 0.001), again reaching a final percentage equal to the wild type (Fig. 2C).
Our results indicated that the dominant axr2-1 mutant was competent to create transverse microtubule arrays, but was not effectively induced by exogenous IAA to resume hypocotyl growth or increase the number of transverse array patterns to wild-type levels. To determine whether the dominant negative axr2-1 auxin coreceptor mutant was effectively blocked or was exhibiting lower sensitivity to exogenous IAA, we extended our analysis by applying IAA at 0.05–50 µm concentrations. In growth assays, wild-type hypocotyls increased in length with increasing IAA concentration, until 50 µm IAA, where we observed growth inhibition (Fig. 2D), similar to observations with high concentrations of picloram (Fig. 1A). We tested the axr2-1 mutant for growth induction with 0.5 µm picloram, using both liquid and solid media (Supplemental Fig. 2), and found no significant change from solvent controls. The IAA concentration series failed to induce hypocotyl elongation in the axr2-1 mutant at all concentrations after 72 h of treatment (Fig. 2D).
Examining the microtubule array patterns at 2-h after induction, treatment of the wild type with 0.05, 0.5, 5, or 50 µm IAA significantly increased the number of cells exhibiting transverse patterns in a concentration-dependent manner, from 27% (control; n = 6 plants/180 cells; P < 0.001) to 51% (0.05 µm IAA; n = 6 plants/140 cells; P < 0.001), 57% (0.5 µm IAA; n = 7 plants/209 cells; P < 0.001), 62% (5 µm IAA; n = 6 plants/177 cells; P < 0.001), and 80% (50 µm IAA; n = 5 plants/153 cells; P < 0.001; Fig. 2, E and F). In contrast, axr2-1 mutants showed insignificant changes in transverse patterning with increasing IAA concentration, from 40% (control; n = 9 plants/244 cells) to 32% (0.05 µm IAA; n = 7 plants/193 cells; P = 0.10), 35% (0.5 µm IAA; n = 3 plants/54 cells; P = 0.53), 36% (5 µm IAA; n = 7 plants/185 cells; P = 0.39), and 45% (50 µm IAA; n = 5 plants/119 cells; P = 0.31; Fig. 2, E and F). These data indicate that the dominant negative allele for the auxin nuclear AXR2/IAA7 coreceptor effectively blocks hypocotyl cells from responding correctly to exogenous auxin for axial growth induction and transverse microtubule patterning.
Early Microtubule Response to Exogenous Hormone Treatments
The combined addition of exogenous auxin with GA4 produced a rapid (∼5–10 min) and sustained decrease in the number of growing microtubule plus ends on the outer periclinal cell face (Vineyard et al., 2013). This prior observation suggested that an early, pretranscriptional response could be contributing to the reorganization of the microtubule array to a transverse coalignment. To test this hypothesis, we first examined the general requirement for the early response in wild-type plants using other plant hormones known to induce microtubule patterning changes in Arabidopsis hypocotyl cells (Katsumi, 1985). We imaged wild-type plants expressing the GFP-End Binding 1b (GFP-EB1) transgene before and after a 30-min treatment with solvent control or hormone. The average number of GFP-EB1 foci, representing growing microtubule plus ends from five sequential images taken at 0 or 30 min after induction, was divided into the area of the plant cell face. The percent difference in the density of growing microtubule plus ends was then determined for each cell (Fig. 3).
Figure 3.
Rapid effect of exogenous hormone treatments on the density of growing microtubule plus ends. The number of GFP-EB1 labeled microtubule (MT) plus ends per cell face area was counted (mean of 5 consecutive frames) before and after 30 min of treatment in liquid media on slide chambers, and the percent change was plotted per cell for the wild type (A) and the double afb4-8/afb5-5 auxin receptor mutant (B). Dots indicate a measurement from a single cell, and red bars are mean change for all cells. Control cells were treated with 10 µL 100% DMSO and 2 µL 50% ethanol in 2 mL bath. Hormone treatments included 0.5 µm IAA, 5 µm picloram, 10 µm GA4, 1 µm epibrassinolide (eBL), and combinations thereof. See text for statistical treatments.
The density of growing microtubule plus ends fluctuated over a substantial range for control plants creating an average difference close to 0 (Fig. 3A). Treatments with conventional auxin (0.5 µm IAA) or the auxin analog, picloram (5 µm), both produced a reduction in density (P < 0.01 in both cases, T-test and Ranksum test, see “Materials and Methods”) and to approximately the same extent (P = 0.006). Application of 10 µm GA4 alone did not produce a measurable change (P = 0.485) in the microtubule plus end density (Fig. 3A). The combined addition of IAA and GA4 reduced the density of growing plus ends (P = 0.018) but did not appear different than application of IAA alone (Fig. 3A). Brassinosteroid (eBL; Katsumi, 1985; Shibaoka, 1994) at 1 µm (Catterou et al., 2001) led to a measurable (P = 0.036) decrease in plus end density (Fig. 3A). Combined addition of eBL with IAA and GA4 did not change the apparent magnitude of the plus end density loss, but resulted in a higher consistency of response (Fig. 3A). These experiments indicate that auxin is not uniquely capable of eliciting this early microtubule response and that GA4 does not elicit the early response.
To determine if auxin could be working through TIR1/AFB receptors for this early response, we crossed the afb4-8/afb5-5 double mutant into our GFP-EB1 expressing lines. We determined that the double mutant shows a decrease in plus end density over time in solvent control experiments (Fig. 3B) even though the afb4-8/afb5-5 double mutant does not accumulate additional transverse array patterns under control conditions (Fig. 1C). Application of auxin, in the form of (0.5 µm) IAA or (5 µm) picloram, lead to approximately equivalent changes in plus end density (Fig. 3B). The decreases after IAA or picloram addition were not statistically separable from the effect of the mutant background itself (P = 0.084 and 0.076, respectively) given the biological variance in these experiments. Hence, picloram leads to a loss of plus end density in the wild type and appears to have an effect that is similar to IAA in the afb4-8/afb5-5 double mutant.
The axr2-1 Auxin Co-Receptor Mutant Does Not Block Brassinosteroid Signaling
The dominant axr2-1 auxin coreceptor mutant effectively blocked exogenous auxin from inducing transverse microtubule array patterning or new axial cell growth over a range of IAA concentrations (Fig. 2). Noting that eBL and GA4 both induce transverse array patterning in wild-type seedlings, we wondered if the eBL or GA4 signaling pathways were also blocked in the dominant axr2-1 mutant (Vierstra, 2009). To investigate this question, we asked if exogenous eBL or GA4 could stimulate growth and cortical microtubule rearrangements in the axr2-1 mutant (Reed et al., 2018). We additionally assayed the axr3-1 and axr5-1 mutants to see if the altered responses found for exogenous IAA were also observed with GA4 and eBL.
Growth induction assays were performed on 6-d-old light-grown seedlings treated with hormone for 72 h (Fig. 4A). GA4 at 10 µm did not have a strong effect on growth in the wild type (1.43 ± 0.25 mm; n = 81; P = 0.174) and had no measurable effect on axr2-1 (1.50 ± 0.36 mm; n = 56; P = 0.824) hypocotyls. However, GA4 did elicit measurable hypocotyl elongation in axr3-1 (1.95 ± 0.31 mm; n = 53; P = 0.028) and axr5-1 (1.69 ± 0.30 mm; n = 60; P < 0.001) seedlings, when compared with solvent controls (Figs. 2A and 4A). Exogenous eBL at 1 µm induced significant hypocotyl elongation in all genotypes tested (Fig. 4A). eBL induced a 45.1% increase in wild-type hypocotyl length (2.52 ± 0.46 mm; n = 107; P < 0.001) and a 24.2% increase in the axr2-1 mutant (1.90 ± 0.44 mm; n = 70; P < 0.001), suggesting a robust, but attenuated growth response in the mutant. We observed a 26.9% increase from the control in the axr3-1 mutant (2.49 ± 0.63 mm; n = 64; P < 0.001) where the most dramatic growth increase (65.2%) was observed in the axr5-1 mutant, suggesting hypersensitivity to eBL (2.99 ± 0.57 mm; n = 54; P < 0.001).
Figure 4.
Dominant Aux/IAA mutants remain responsive to brassinosteroids for growth induction and transverse microtubule patterning. A, Growth assays for wild-type, axr2-1, axr3-1, and axr5-1 seedlings showing the mean (+/− std) hypocotyl length for light-grown seedlings treated from 6 to 9 d postgermination with a solvent control (data from Fig. 2A), 10 µm GA4, or 1 µm eBL in liquid. GA4 showed significant effects on the axr3-1 and axr5-1 mutants where eBL showed significant effects on all genotypes (P < 0.05, Students t test). B, The percentage of transverse (Tr; 0–15°), oblique (Ob; 16–75°), longitudinal (Lo; 76–90°), and non–co-aligned microtubule array patterns (Ba) in 6-d-old hypocotyl cells treated with a solvent control, 10 µm GA4, or 1 µm eBL for 2 h. C, The percentage of cortical arrays showing a transverse pattern in each plant from (B), showing the distribution (blue dots), mean (red line), and boxed range (25th and 75th percentiles). GA4 showed significant effects on the wild type and the axr3-1 mutant where eBL showed significant effects on all genotypes (P < 0.05, Z-test).
Microtubule array patterning was assayed in 6-d-old light-grown hypocotyl cells (Fig. 4, B and C) using confocal microscopy of GFP-TUB1 expressing plants comparing solvent control with 10 µm GA4 or 1 µm eBL (Catterou et al., 2001) after 2-h treatment. GA4 alone induced a transition to transverse coalignment in the wild type from 29% in the control to 54% (n = 16 plants/360 cells; P < 0.001), in agreement with prior findings (Vineyard et al., 2013) and despite not producing an early loss of microtubule plus end density (Fig. 3). GA4 treatment only resulted in 30% (n = 16 plants/463 cells; P = 0.012) and 38% (n = 5 plants/133 cells; P = 0.11) of the hypocotyl cells showing a transverse pattern in the axr2-1 and axr5-1 mutants, respectively, indicating that the GA4 response is suppressed in these mutants. GA4 increased the percentage of cells with transverse microtubule patterning in the axr3-1 mutant from 44% in the control to 56% when treated (n = 4 plants/ 87 cells; P = 0.047), comparable with the wild type. Hence, exogenous GA4 at 10 µm elicited responses in the wild type and the axr3-1 mutant, but failed to produce responses in the axr2-1 and axr5-1 mutants.
The percentage of transverse microtubule arrays in wild-type seedlings treated with eBL increased from 29% (n = 26 plants/658cells) to 73% (n = 30 plants/794 cells; P < 0.001; Fig. 4C), nearly identical to IAA induction. eBL application to axr2-1 seedlings resulted in a clear increase in transverse microtubule patterns from 36% (control; n = 27 plants/660 cells) to 60% (n = 20 plants/435 cells; P < 0.001). There were significant increases in transverse microtubule patterning in the axr3-1 mutant (from 44% to 82%; n = 10 plants/ 261 cells; n = 9 plants/166 cells; P < 0.001) and, to a lesser degree, in the axr5-1 mutant (from 43% to 54%; n = 17 plants/ 521 cells; n = 7 plants/183 cells; P = 0.012), with eBL treatment. Thus, eBL induction of transverse microtubule array patterning is not blocked in the dominant axr2-1 auxin coreceptor mutant and shows similar induction properties to IAA in the axr5-1 mutant and possible hypersensitivity in the axr3-1 mutant.
Auxin Induces Transverse Microtubule Patterning Independent of Brassinosteroids
The induction of transverse patterning by eBL in the axr2-1 mutant suggested that brassinosteroids can act independently or potentially downstream of auxin to affect transverse microtubule patterning and hypocotyl growth. Based on these results, and studies indicating overlapping or interdependent transcriptional activity between IAA and eBL (Nemhauser et al., 2004; Vert et al., 2008; Oh et al., 2014), we wanted to determine if brassinosteroid signaling was required for these induced effects. To test this hypothesis, we crossed our GFP-TUB1 marker into the diminuto1 mutant, a loss-of-function steroid reductase mutant that is blocked for the production of active endogenous brassinosteroids (Takahashi et al., 1995; Klahre et al., 1998). To evaluate the growth response in light-grown seedlings, we performed hypocotyl growth assays on 6-d-old seedlings after 72 h in a liquid bath at a range (0.05–50 µm) of IAA concentrations (Fig. 5A). Wild-type controls showed increasing axial hypocotyl growth from 0.05 to 5 µm IAA with 50 µm showing no additional induction. Under our assay conditions, we found that hypocotyl elongation was either mildly retarded or showed no meaningful increases in the diminuto1 mutant with IAA until we reached 50 µm (0.88 ± 0.12 mm; n = 20; P < 0.001). These data confirm that eBL is critical for sustained hypocotyl elongation even when exogenous auxin is provided at a relatively high concentration.
Figure 5.
IAA induces transverse microtubule patterning in the absence of endogenous brassinosteroid production. A, Growth assays for the wild type and the brassinosteroid reductase mutant, diminuto1, showing the mean (+/− std) hypocotyl length for light-grown seedlings treated 72 h from 6 to 9 d poststratification with solvent control and 0.05–50 µm IAA on liquid. The sample sizes for each treatment are listed below the graph. IAA showed significant effects (P < 0.05, Students t test) in the wild type at all concentrations and at 50 µm for the diminuto1 mutant. B, The percentage of transverse (Tr; 0°–15°), oblique (Ob; 16°–75°), longitudinal (Lo; 76°–90°), and basket (Ba) microtubule array patterns in 6-d-old wild-type and diminuto1 GFP-TUB1 hypocotyl cells treated with a solvent control and 0.05–50 µm IAA for 2 h. The number of cells/plants counted are displayed below the graph. C, The percentage of cells in showing a transverse pattern in each hypocotyl from (B) with IAA having significant effects (P < 0.05, Z-test) at all concentrations. Blue markers represent each seedling. The red line shows the mean percentage, and the box designates the 25th and 75th percentiles.
To determine the effect on microtubule patterning, 6-d-old, light-grown diminuto1 GFP-TUB1 seedlings were treated with a solvent control or 0.05–50 µm IAA for 2 h and then imaged and visually scored for array pattern type (Fig. 5, B and C). Control diminuto1 mutants exhibited a distribution of pattern types highly similar to the wild type, where 30% (n = 19 plants/601 cells) of cells had transverse microtubule array patterns compared with 27% (n = 6 plants/180 cells) in the wild type (Fig. 5C; P = 0.469). Increasing concentrations of IAA produced a concentration-dependent response on transverse microtubule patterning in the diminuto1 mutant, similar to the response in wild-type seedlings (Fig. 5, B and C). For the wild type, application of 0, 0.05, 0.5, 5, and 50 µm IAA increased the percentage of transverse patterns from 30% (control; n = 19 plants/601 cells) to 41% (0.05 µm IAA; n = 10 plants/347 cells; P = 0.001), 57% (0.5 µm IAA; n = 17 plants/498 cells; P < 0.001), 65% (5 µm IAA; n = 10 plants/383 cells; P < 0.001), and 77% (50 µm IAA; n = 10 plants/312 cells; P < 0.001). These data show that exogenous auxin is perceived by cells in the diminuto1 mutant and induces the transition to transverse microtubule arrays even though the same signal does not produce sustained axial cell growth in the absence of brassinosteroid.
DISCUSSION
Auxin Functions Through TIR1/AFB Receptors to Induce Transverse Microtubule Patterning
The cortical microtubule arrays in flowering plants influence the distribution and orientation of cellulose polymers in the cell wall (Cyr, 1994; Paredez et al., 2006; Gutierrez et al., 2009; Szymanski and Cosgrove, 2009). The durable effects of oriented cellulose deposition on cell wall extension have been known for decades and provide an impetus for understanding how microtubule patterns are specified to influence cell shape (McFarlane et al., 2014). Epidermal hypocotyl cells extend axially and exhibit important changes in growth rate to produce hypocotyl bending in response to light and gravitational cues (Gendreau et al., 1997). Auxin, gibberellic acid, and brassinosteroids are key plant hormones for modulating axial hypocotyl extension (Collett et al., 2000; Depuydt and Hardtke, 2011; Oh et al., 2014). In this work, we have focused on determining how hypocotyl cells transduce the hormone signals leading to growth (i.e. increase in size) and to changes in the microtubule cytoskeleton to affect the correct cell shape (i.e. cellular morphogenesis).
Auxin, applied to 6-d-old, light-grown Arabidopsis seedlings, was previously shown to induce epidermal hypocotyl cells to form transversely coaligned microtubule arrays by 2 h with new axial cell growth accruing over the ensuing 2 to 3 d (Collett et al., 2000; Vineyard et al., 2013; Elliott and Shaw, 2018b). The transverse microtubule array patterning follows a reproducible temporal and spatial progression, showing a nearly immediate decrease in growing microtubule plus ends, followed by the appearance of transverse polymers at the cell’s midzone by 30–45 min, and completion of the transverse pattern by 90–120 min (Vineyard et al., 2013; Elliott and Shaw, 2018b). This stereotyped progression stands in contrast to the rapid reorganization from transverse to longitudinal patterns (15–45 min) observed when cells are brought from the dark into the light (Sambade et al., 2012; Lindeboom et al., 2013). We also found that light-grown cells treated with auxin and cycloheximide produced new transverse microtubules, but failed to suppress the continuous production of longitudinal microtubules on the outer periclinal cell face, resulting in ‘pinwheel’ array patterns (Elliott and Shaw, 2018). Taken together, these observations suggest that cortical microtubules can respond to stimuli through both transcriptional and nontranscriptional pathways. The results in this paper indicate that elevating the auxin concentration triggers the TIR1/AFB nuclear auxin receptors, working together with Aux/IAA coreceptors, to affect a reorganization of the microtubule array to a transverse coalignment without a requirement for early, pretranscriptional signaling to the cytoskeleton.
We propose from our results that elevating the auxin concentration leads to the previously defined interaction of TIR1/AFB and AUX/IAA coreceptors in the plant cell nucleus (Dharmasiri et al., 2005; Chapman and Estelle, 2009) that, in turn, signal for the reorganization of the microtubule array to reorganize into a transverse pattern. We provide genetic evidence that TIR1/AFB receptors (AFB4 and AFB5) are necessary and sufficient to cause transverse array patterning when activated by the auxin analog, picloram, at 0.5 µm concentration (Greenham et al., 2011; Calderón Villalobos et al., 2012; Wang and Estelle, 2014; Prigge et al., 2016). We found that conventional auxin (IAA) treatment of the afb4-8/afb5-5 mutant induced transverse microtubule arrays, indicating that the mutant was not impaired for transverse microtubule organization per se or specifically dependent on AFB4 or AFB5 for the cytoskeletal reorganization in response to auxin. We additionally found that the dominant axr2-1 auxin coreceptor mutant was functionally nonresponsive to both exogenous IAA (0.05–50 µm) or 0.5 µm picloram treatments for induction of transverse patterning where brassinosteroid induction showed, again, that this mutant was not impaired for microtubule array organization, per se. This result further indicates that auxin interaction with the TIR1/AFB and Aux/IAA coreceptors is clearly not the only mechanism by which the cell can initiate the reorganization of the microtubule arrays. We provide evidence that IAA, picloram, and eBL, but not GA4, elicit rapid changes to the density of growing microtubule plus ends, irrespective of genetic background. The effect does not correlate in all cases with later reorganization to the transverse pattern, suggesting that this early effect (Phase I in Vineyard et al., 2013) is not required for, or causal to, the array repatterning. Collectively, these data provide substantial evidence that elevating the auxin concentration induces transverse patterning through the TIR1/AFB Aux/IAA coreceptor system rather than through a direct transduction pathway in the cytoplasm. There still exists a formal possibility that exogenous IAA triggers a non–TIR1/AFB receptor in the afb4-8/afb5-5 mutant to induce transverse patterning, where that receptor pathway is specifically blocked in the dominant axr2-1 coreceptor mutant.
Evidence that Auxin Induces Transverse Microtubule Patterning Through a Transcriptional Pathway
TIR1/AFB receptors have only been shown to act within the nucleus and function as cofactors with Aux/IAA proteins to control gene expression through direct interactions with transcription factors (Spartz and Gray, 2008; Calderón Villalobos et al., 2012). We infer, therefore, that our data indicate a transcriptional pathway leading to transverse patterning in support of prior work showing cycloheximide sensitivity (Elliott and Shaw, 2018a). We provide additional evidence for this proposal using dominant mutants for the Aux/IAA coreceptors required for the auxin transcriptional response (Ulmasov et al., 1997; Nagpal et al., 2000; Weijers et al., 2005). Specifically, the axr2-1 mutant grew to a nearly wild-type light-grown hypocotyl length, showed a typical distribution of microtubule array pattern types (Fig. 2), and remained responsive to eBL for transverse patterning. This mutant, however, was severely defective or blocked for the induction of transverse microtubule patterns with IAA, even when IAA was applied at extreme levels (Fig. 2E). We interpret these observations to show that when exogenous IAA can no longer lead to the degradation of IAA7 (axr2-1), the transcription factors regulated by IAA7 are not released to control expression of proteins normally triggering the microtubule array reorganization. It is still possible that TIR1/AFB proteins are functioning in the cytoplasm as transducing agents, although the likelihood of this being a direct effect on the microtubules appears highly unlikely (Gray et al., 2001; Dharmasiri et al., 2005; Wang and Estelle, 2014).
Auxin has been shown to elicit changes in plant cells over a wide range of time scales, from seconds to hours (Cleland, 1992; Abel et al., 1994; Oeller and Theologis, 1995; Schenck et al., 2010). Much effort has been expended to understand the potential role of ABP1 in directly modulating intracellular events, including changes to the cell wall and cytoskeleton (Chen et al., 2014; Xu et al., 2014). Recent evidence indicates that rapid cellular responses to exogenous auxin, beginning with membrane depolarization, do not require ABP1 but more likely function through auxin transport proteins, such as AUX1 (Dindas et al., 2018; Paponov et al., 2019). Noting the magnitude of the reported auxin-induced membrane depolarization (Rück et al., 1993; Paponov et al., 2019), we speculate that the cortical microtubules could be responding within 5 min to a change in pH or to Ca2+, which have been observed after auxin treatment on the same time scale (Felle, 1988; Gehring et al., 1990; Monshausen et al., 2011). Cytoplasmic Ca2+ has been associated with directly modulating microtubule depolymerization (Cyr et al., 1987; Cyr, 1991) and initiates a substantial number of signal transduction pathways potentially linked to the cytoskeleton (Kao et al., 2000; Gardiner et al., 2001; Li and Xue, 2007; Mancuso et al., 2007; Li et al., 2011; Zhang et al., 2012; Bürstenbinder et al., 2017; Zhang et al., 2017; Wang et al., 2018). Therefore, it is possible that rapid changes to the microtubules could occur with elevated auxin concentrations that are unrelated to the transcription-dependent TIR1/AFB response leading to transverse patterning.
We found evidence that the early decrease in growing microtubule plus ends (Vineyard et al., 2013; Elliott and Shaw, 2018b) is not required for reorganization to a transverse pattern. GA4 produced an increase in transverse arrays, but did not alter the density of growing microtubule plus ends in cells (Figs. 3 and 4B). We found limited evidence that picloram signals for a loss of plus end density in the afb4-8/afb5-5 double mutant, comparable with the addition of IAA. Although equivocal owing to the variability of the measurements in the double mutant, the observation is consistent with the evidence that an early, pretranscriptional response is not causal to forming transverse microtubule patterns.
Auxin and Brassinosteroids Have Independent Routes to Array Reorganization
A substantial literature has accumulated showing the interdependency of auxin and eBL for directing hypocotyl elongation (Nemhauser et al., 2004; Hardtke et al., 2007; Vert et al., 2008; Oh et al., 2014; Tian et al., 2018). We observed that exogenous eBL induces the cortical microtubules to form transverse patterns to nearly the same extent as auxin addition (Figs. 1C, 2B, and 4B). In agreement with recent findings (Reed et al., 2018), application of eBL to the axr2-1 mutant in our experiments induced axial extension (Fig. 4A), although to a lesser degree than eBL addition to the wild type. Our work additionally showed that eBL treatment led to transverse microtubule arrays in the axr2-1 mutant even though IAA did not elicit this response (Figs. 2B and 4B). Therein, we propose that eBL either acts downstream of AXR2/IAA7 for initiating a transcriptional response or acts independently of the auxin pathway for transverse patterning. Auxin clearly does not require eBL to induce transverse arrays as our experiments with the diminuto1 mutant revealed (Fig. 5). The robust microtubule reorganization in the eBL synthesis mutant was somewhat unexpected since IAA in our experiments, and others (Choe et al., 1998), did not lead to substantial axial growth of hypocotyl cells in the diminuto1 seedlings (Fig. 5A). Therefore, auxin can function independently of eBL for induction of transverse patterning.
The diminuto1 experiments suggest that the induction of transverse microtubule patterning can, to some extent, be genetically separated from the induction of axial growth. The control diminuto1 seedlings showed a remarkably similar distribution of microtubule array pattern types to that of wild-type seedlings (Fig. 5B). Neither the physical size and geometry of the diminuto1 epidermal cells nor the absence of eBL during development altered the normal light-regulated microtubule patterning. Moreover, exogenous auxin prompted the same degree of transverse patterning (i.e. percentage of cells) as the wild type in the diminuto1 mutant, indicating that there is no requirement for coactivation of a pathway with eBL (Fig. 5B; Oh et al., 2014). IAA does elicit some cell enlargement in the diminuto1 mutant; therefore, our work does not rule out a possible connection of the microtubule patterning to cell expansion, per se, even if it does not lead to substantial axial hypocotyl elongation.
The axr3-1 and axr5-1 auxin coreceptor mutants, similar to axr2-1, responded to eBL with both an increase in cell elongation and an induction of transverse array patterning (Fig. 4). These mutants had longer hypocotyls in the light than wild-type and axr2-1 seedlings, where the axr5-1 mutant shows a remarkable elongation with eBL implying some level of hyper-sensitivity. We additionally observed that the induction of transverse patterns in the axr3-1 mutant by eBL was extremely robust and eliminated any obvious longitudinal arrays from the plants examined (Fig. 4B). We surmise, from these observations and, in conjunction with the diminuto1 mutant, that eBL is acting to scale the size of the seedling and is acting somewhat independently of the pathways affected by AXR2/IAA7, AXR3/IAA17, and AXR5/IAA1. These data stand in contrast with exogenous GA4 application where an induction of transverse patterning was observed in wild-type plants with almost no measurable increase in growth 3 d after 6-d-old plants were treated (Fig. 4). Unlike eBL, the GA4 effect on microtubule patterning was blocked in both the axr2-1 and axr5-1 mutants and potentially suppressed or mis-regulated in axr3-1 related to the overall increase in control seedlings. Hence, GA4 appears to require the correct activity of the Aux/IAA auxin coreceptors even when the dominant mutants are not blocking induction by auxin itself.
Pathways Leading to Transverse Microtubule Array Patterning
Our experiments provide new information about how plant cells transduce signals for changes to the organization of the microtubule cytoskeleton. The cortical microtubules are dynamic polymers, treadmilling from their site of nucleation to new locations around the cell. The outcome of microtubule-microtubule interactions at the cell cortex leads to a degree of self-organization that has been argued from simulation studies to specify the patterns formed by the array through time (Ehrhardt and Shaw, 2006; Allard et al., 2010; Hawkins et al., 2010; Deinum et al., 2011; Mirabet et al., 2018). Direct observations of cortical array pattern formation have, thus far, indicated that the majority of cortical microtubules forming nontransverse patterns on the outer periclinal cell face are generated on that outer periclinal cell face in a ‘split bipolar’ manner (Vineyard et al., 2013; Elliott and Shaw, 2018b). Thus, the array patterns appear to be generated more prominently from cell-face specific control of microtubule nucleation than a reliance on microtubule-microtubule interactions. Work in this manuscript indicates that the application of exogenous auxin, eBL, and to a lesser extent, GA4, stimulates a transition from the nontransverse split bipolar arrays to a transverse coalignment, even when axial cell growth is not substantially accelerated (e.g. the diminuto1 mutant with IAA and GA4 with the wild type). Hence, we speculate that there is a common downstream mechanism from these hormones leading to a change in microtubule nucleation on the outer periclinal array that controls the transition into a transverse coalignment.
Interestingly, we found no evidence in the literature for auxin, eBL, or GA4 mutants that substantially uncouple cell expansion from microtubule organization in a capacity to phenocopy the application of microtubule disrupting drugs (Baskin et al., 1994). Moreover, our hormone treatments of the dominant Aux/IAA mutants and the diminuto1 eBL mutant suggest control over the extent of axial cell growth (i.e. longer or shorter plants) but not a reprogramming of the cytoskeleton related to a new cell morphology (i.e. wider or misshaped plants). For the case of auxin signaling, a transcriptional pathway from IAA reception to proton efflux into the cell wall has been reported (Spartz et al., 2014). Elevated auxin levels lead to the very rapid production of proteins from SAUR transcripts (Theologis et al., 1985; Spartz et al., 2014) via the TIR1/AFB – Aux/IAA coreceptors acting on ARF transcription factors (Dharmasiri et al., 2005; Weijers et al., 2005; Parry et al., 2009). Specific SAUR proteins can activate plasma membrane proton pumps leading to acidification of the cell wall with consequent loosening of the wall materials and cell expansion (Spartz et al., 2014; Fendrych et al., 2016). Given our evidence that auxin functions through the TIR1/AFB receptors for transverse patterning of the cortical microtubules, we propose two possible models for how the downstream pathway coordinates cell expansion with the observed cytoskeletal organization. The transcriptional pathway could induce both the SAURs shown to activate proton pumps and a second set of genes to act on the microtubule arrays (i.e. a branched pathway). Alternatively, exogenous auxin could lead to SAUR expression followed by activation of plasma membrane proton pumps (Spartz et al., 2014; Fendrych et al., 2016) where the same change in pH that activates existing enzymes to loosen the cell wall material could signal, on either side of the plasma membrane, for the activation of proteins that act on the microtubules to change the array pattern.
MATERIALS AND METHODS
Plant Materials
Arabidopsis (Arabidopsis thaliana) seed was sterilized with a 19:1 (v/v) solution of 87.5% ethanol:30% H2O2 on #1 filter paper and dried in a sterile hood. Seed were sown on 1% (w/v) agar (Sigma-Aldrich) plates containing 0.5× MS medium (MS Basal Salt Mixture; Sigma-Aldrich). Plates were wrapped in metal foil and kept at 4°C for >24 h to synchronize germination. The foil was removed, and plates were oriented vertically under continuous light at 22°C for 6 d before experiments. Arabidopsis (Columbia-0) plants expressed a 35S:TUB1:GFP construct for imaging microtubule patterns and a 35S:GFP:EB1b transgene for visualizing growing microtubule plus-ends (Mathur et al., 2003). The afb4-8/afb5-5 double null mutant (gift from Mark Estelle) was crossed to plants expressing 35S:TUB1:GFP or 35S:GFP:EB1b and genotyped to confirm homozygous mutations. The axr2-1, axr3-1, and axr5-1 gain-of-function dominant negative mutants (gift of Mark Estelle and Jason Reed) were crossed with the 35S:TUB1:GFP plants. The diminuto1 mutant was ordered from the Arabidopsis Biological Resource Center (CS8100) and crossed with the 35S:TUB1:GFP plants.
Hormone Treatments
Seedlings were taken from agar germination plates after 6 d at 22°C and placed into liquid 0.5× MS medium for treatment. Auxin treatments were created using a 0.1 mm IAA (Sigma-Aldrich) stock dissolved in 100% dimethyl sulfoxide (DMSO; Sigma-Aldrich) or 0.1 mm picloram (Sigma-Aldrich) stock dissolved in 100% DMSO. A 10 mm GA4 (Sigma-Aldrich) stock dissolved in 1:1 (v/v) of ethanol and distilled water was made, and plants were treated with a 10 µm final concentration. A 200 mm eBL (Sigma-Aldrich) stock was made in 100% DMSO for dilution into 0.5× MS. For growth assays, plants were transferred from agar plates to liquid baths and floated on the liquid surface for 72 h in a lighted incubator at 22°C (Vineyard et al., 2013). For IAA assays, the medium was changed every 24 h to account for photolability. Liquid hormone baths were used for growth assays to better account for the effects of submerging seedlings in liquid (Vineyard et al., 2013) required for high numerical aperture imaging of the microtubules when determining pattern types. For length measurements, seedlings were arranged on 1% (w/v) agar plates and imaged using a stereo-microscope with a mounted camera. Growth assays for IAA and picloram were repeated on 0.5× MS agar plates by transferring 6-d-old seedlings directly to new plates with hormone and measuring hypocotyls after 72 h. For evaluation of microtubule pattern distributions, plants were placed in 2 mL of liquid hormone treatments and incubated in light at 22°C for 2 h. Then the seedlings were moved to a slide and cover glass with the same medium immediately before imaging (Vineyard et al., 2013). For time-lapse experiments to measure GFP-EB1 foci (plus-end density change), plants were transferred from agar plates to a glass slide with liquid 0.5× MS medium and mounted between slide and cover glass using silicon vacuum grease (Dow Corning) to form a chamber. The hypocotyl cells were imaged before treatment, and then the hormone treatments were flowed-in using a wick method and the same cells were imaged at 30 min after induction.
Confocal Microscopy
Microtubule patterns of epidermal hypocotyl cells expressing GFP-TUB1 were imaged using a Nikon A1 confocal laser scanning microscope with a Plan Fluor 40× 1.3 numerical aperture oil immersion objective lens. Growing microtubule plus-ends labeled with GFP-EB1b were imaged on a Leica SP8 laser scanning confocal microscope with a 60× 1.2 water immersion objective lens. GFP-based probes were excited using a 488-nm laser line, and excitation was collected using a spectrophotometric unit with the photomultiplier tube for the Nikon A1 and SMD hybrid detectors for the Leica SP8.
For microtubule array pattern distributions, we imaged hypocotyl cells expressing GFP-TUB1 to a minimum depth of 10 µm with the Nikon A1 laser scanning confocal microscope. Hypocotyls were imaged in tiled sections beginning with the root/hypocotyl junction and progressing to the apical meristem, and the sections were stitched together using the Nikon A1 software. Optical sections for reconstructed image projections were taken at 0.75 μm to 1.0 μm steps. Two-dimensional projections were created by converting proprietary Nikon files to TIF format and creating a maximum projection using ImageJ (NIH, Wayne Rasband).
For quantifying growing microtubule plus-end density changes, time-course experiments were performed on seedlings expressing EB1-GFP that were transferred from agar plates to microscope slides containing 0.5 ml of 0.5× MS medium. Cells were imaged to a depth of >5 µm using 0.75–1 µm intervals, and the image stack was projected to a single (maximum intensity) image to capture the entire outer periclinal cell surface. The process was repeated 5 times at 6–10 s intervals (average of 5 measurements) at time 0 and 30 min after induction to create the primary data set for determining the number of growing microtubule plus ends before and after treatment.
Image Processing and Data Analysis
For assaying the percentage of cells with transverse cortical microtubule arrays, the two-dimensional projections of stitched hypocotyl images were opened in a MATLAB (The Mathworks) scripted program for marking and tabulating pattern types (Vineyard et al., 2013). Microtubule patterns were visually scored in two adjacent cell files along the entire hypocotyl length to avoid bias in array organization for cell file. Tabulated pattern summaries were then used to create stacked bar graphs, depicting the percentage of each array pattern, and dot plots showing the percentage of transverse patterns for each hypocotyl with the arithmetic mean.
MATLAB (The Mathworks) scripts were developed as a series of graphical user interfaces for determination of cell face area and GFP-EB1 number. Hand selected cell boundary points were connected and interpolated to a one-pixel distance to create a cell perimeter. Perimeter positions, together with a micrometer-verified pixels per micrometer, were used for determining the projected cell face area. GFP-EB1 foci were identified within the cell boundary using a semiautomated difference of Gaussians approach. Users identified >6 EB1-GFP foci for input into an optimization routine that identified two sigma values for Gaussian functions that maximize local signal intensity over background after subtracting the Gaussian filtered images. Users set an optimized threshold value, and the EB1-GFP foci are identified and overlain on the original time-lapse images. All image sequences were subsequently reviewed and hand edited for errors such as double counting or missed foci. The mean number of identified foci from 5 consecutive time points was divided by the projected cell face area to estimate the final mean density of growing microtubule plus ends.
Growth measurements are reported as the arithmetic mean of pooled data from replicate experiments and the sd for the pooled population to provide a visual representation of the variance in the measured data. Student’s two-tailed t tests of the pooled data were used to test the hypothesis that any two populations of length measurements could have come from the same normally distributed population using ‘ttest2’ in MATLAB (The Mathworks). Secondary estimates for this probability are reported in supplemental data from multifactor ANOVA tests factoring out the effect of replicate experiments using StatPlus (AnalystSoft inc). Determining the probability that a treatment changes the percentage of transverse microtubule array patterns more than would be expected by random chance alone was estimated using a direct Z-test for each case. The number of transverse patterns out of the total population of cells was used as the test metric for each control and treatment and the final Z-test was used as the probability estimate. The Z-score was used in place of a χ2 test owing to the variability in the percentage of transverse patterns in each control case. The number of transverse microtubule patterns was divided by the total number of cells counted for each population to get the proportions (p1 and p2). The “normal” population mean (m) was equal to the total number of transverse microtubule patterns from both populations divided by all cells counted from control (n1) and treatment (n2). The Z-score was then calculated as Z = (p1 − p2)/ sqrt(m(1 − m) × (1/n1 + 1/n2)) for a normal distribution.
Estimating the probability that a given treatment changes the density of growing microtubule plus ends (EB1-GFP foci) more than would be expected by random chance requires either a nonparametric test or an assumption that the magnitude of change observed (percent difference between time 0 and 30 min) is normally distributed on a linear scale. Neither assumption is well met; therefore, the data are reported as dot plots with the mean difference for visual evaluation, and p values are reported from both a parametric two-tailed t test and a nonparametric ranksum test (Supplemental Fig. 1).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accessions numbers: At3g23050 (AXR2/IAA7), At1g04250 (AXR3/IAA17), At4g14560 (AXR5/IAA1), AT4G24390 (AFB4), AT5G49980 (AFB5), and AT3G19820 (diminuto1).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Data in tabular form comparing mean values, magnitude of effect, and p-values from different hypothesis tests for comparing control and treatment effects.
Supplemental Figure S2. Comparison of picloram treatment on seedling hypocotyl growth for wild type and mutant plants transferred to agar plates or to liquid medium.
Supplemental Figure S3. IAA concentration gradient effect on the axial growth and microtubule patterning in wild-type and afb4/afb5 mutant.
Acknowledgments
We thank Mark Estelle, Matthew E. Dwyer, and Roger Hangarter for advice on experiments; Jason Reed for seed lines; and Jim Powers for assistance at the Indiana University Light Microscopy Imaging Center.
Footnotes
This work was supported by National Science Foundation (NSF) MCB1615907 and MCB1157982 (to both authors) and Indiana University (IU) Carlos Miller Graduate Fellowship (to J.H.T.).
References
- Abel S, Oeller PW, Theologis A (1994) Early auxin-induced genes encode short-lived nuclear proteins. Proc Natl Acad Sci USA 91: 326–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard JF, Wasteneys GO, Cytrynbaum EN (2010) Mechanisms of self-organization of cortical microtubules in plants revealed by computational simulations. Mol Biol Cell 21: 278–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin TI. (2001) On the alignment of cellulose microfibrils by cortical microtubules: A review and a model. Protoplasma 215: 150–171 [DOI] [PubMed] [Google Scholar]
- Baskin TI. (2005) Anisotropic expansion of the plant cell wall. Annu Rev Cell Dev Biol 21: 203–222 [DOI] [PubMed] [Google Scholar]
- Baskin TI, Wilson JE, Cork A, Williamson RE (1994) Morphology and microtubule organization in Arabidopsis roots exposed to oryzalin or taxol. Plant Cell Physiol 35: 935–942 [PubMed] [Google Scholar]
- Bates GW, Goldsmith MHM (1983) Rapid response of the plasma-membrane potential in oat coleoptiles to auxin and other weak acids. Planta 159: 231–237 [DOI] [PubMed] [Google Scholar]
- Bürstenbinder K, Möller B, Plötner R, Stamm G, Hause G, Mitra D, Abel S (2017) The IQD family of calmodulin-binding proteins links calcium signaling to microtubules, membrane subdomains, and the nucleus. Plant Physiol 173: 1692–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón Villalobos LI, Lee S, De Oliveira C, Ivetac A, Brandt W, Armitage L, Sheard LB, Tan X, Parry G, Mao H, et al. (2012) A combinatorial TIR1/AFB-Aux/IAA co-receptor system for differential sensing of auxin. Nat Chem Biol 8: 477–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterou M, Dubois F, Schaller H, Aubanelle L, Vilcot B, Sangwan-Norreel BS, Sangwan RS (2001) Brassinosteroids, microtubules and cell elongation in Arabidopsis thaliana. II. Effects of brassinosteroids on microtubules and cell elongation in the bul1 mutant. Planta 212: 673–683 [DOI] [PubMed] [Google Scholar]
- Chandler JW. (2016) Auxin response factors. Plant Cell Environ 39: 1014–1028 [DOI] [PubMed] [Google Scholar]
- Chapman EJ, Estelle M (2009) Mechanism of auxin-regulated gene expression in plants. Annu Rev Genet 43: 265–285 [DOI] [PubMed] [Google Scholar]
- Chen X, Grandont L, Li H, Hauschild R, Paque S, Abuzeineh A, Rakusová H, Benkova E, Perrot-Rechenmann C, Friml J (2014) Inhibition of cell expansion by rapid ABP1-mediated auxin effect on microtubules. Nature 516: 90–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S, Dilkes BP, Fujioka S, Takatsuto S, Sakurai A, Feldmann KA (1998) The DWF4 gene of Arabidopsis encodes a cytochrome P450 that mediates multiple 22α-hydroxylation steps in brassinosteroid biosynthesis. Plant Cell 10: 231–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J, Peto C, Feinbaum R, Pratt L, Ausubel F (1989) Arabidopsis thaliana mutant that develops as a light-grown plant in the absence of light. Cell 58: 991–999 [DOI] [PubMed] [Google Scholar]
- Cleland RE. (1992) Auxin-induced growth of Avena coleoptiles involves two mechanisms with different pH optima. Plant Physiol 99: 1556–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett CE, Harberd NP, Leyser O (2000) Hormonal interactions in the control of Arabidopsis hypocotyl elongation. Plant Physiol 124: 553–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6: 850–861 [DOI] [PubMed] [Google Scholar]
- Crowell EF, Gonneau M, Vernhettes S, Höfte H (2010) Regulation of anisotropic cell expansion in higher plants. C R Biol 333: 320–324 [DOI] [PubMed] [Google Scholar]
- Cyr RJ. (1991) Calcium/calmodulin affects microtubule stability in lysed protoplasts. J Cell Sci 100: 311–317 [Google Scholar]
- Cyr RJ. (1994) Microtubules in plant morphogenesis: Role of the cortical array. Annu Rev Cell Biol 10: 153–180 [DOI] [PubMed] [Google Scholar]
- Cyr RJ, Bustos MM, Guiltinan MJ, Fosket DE (1987) Developmental modulation of tubulin protein and mRNA levels during somatic embryogenesis in cultured carrot cells. Planta 171: 365–376 [DOI] [PubMed] [Google Scholar]
- Cyr RJ, Palevitz BA (1995) Organization of cortical microtubules in plant cells. Curr Opin Cell Biol 7: 65–71 [DOI] [PubMed] [Google Scholar]
- Deinum EE, Tindemans SH, Mulder BM (2011) Taking directions: The role of microtubule-bound nucleation in the self-organization of the plant cortical array. Phys Biol 8: 56002. [DOI] [PubMed] [Google Scholar]
- Depuydt S, Hardtke CS (2011) Hormone signalling crosstalk in plant growth regulation. Curr Biol 21: R365–R373 [DOI] [PubMed] [Google Scholar]
- Devlin PF, Yanovsky MJ, Kay SA (2003) A genomic analysis of the shade avoidance response in Arabidopsis. Plant Physiol 133: 1617–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M (2005) The F-box protein TIR1 is an auxin receptor. Nature 435: 441–445 [DOI] [PubMed] [Google Scholar]
- Dindas J, Scherzer S, Roelfsema MRG, von Meyer K, Müller HM, Al-Rasheid KAS, Palme K, Dietrich P, Becker D, Bennett MJ, Hedrich R (2018) AUX1-mediated root hair auxin influx governs SCFTIR1/AFB-type Ca2+ signaling. Nat Commun 9: 1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt DW, Shaw SL (2006) Microtubule dynamics and organization in the plant cortical array. Annu Rev Plant Biol 57: 859–875 [DOI] [PubMed] [Google Scholar]
- Elliott A, Shaw SL (2018a) A cycloheximide-sensitive step in transverse microtubule array patterning. Plant Physiol 178: 684–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott A, Shaw SL (2018b) Microtubule array patterns have a common underlying architecture in hypocotyl cells. Plant Physiol 176: 307–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders TA, Oh S, Yang Z, Montgomery BL, Strader LC (2015) Genome sequencing of Arabidopsis abp1-5 reveals second-site mutations that may affect phenotypes. Plant Cell 27: 1820–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falhof J, Pedersen JT, Fuglsang AT, Palmgren M (2016) Plasma membrane H+-ATPase regulation in the center of plant physiology. Mol Plant 9: 323–337 [DOI] [PubMed] [Google Scholar]
- Fankhauser C, Chory J (1997) Light control of plant development. Annu Rev Cell Dev Biol 13: 203–229 [DOI] [PubMed] [Google Scholar]
- Felle H. (1988) Auxin causes oscillations of cytosolic free calcium and pH inZea mays coleoptiles. Planta 174: 495–499 [DOI] [PubMed] [Google Scholar]
- Fendrych M, Leung J, Friml J (2016) TIR1/AFB-Aux/IAA auxin perception mediates rapid cell wall acidification and growth of Arabidopsis hypocotyls. eLife 5: e19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folta KM, Spalding EP (2001) Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J 26: 471–478 [DOI] [PubMed] [Google Scholar]
- Gao Y, Zhang Y, Zhang D, Dai X, Estelle M, Zhao Y (2015) Auxin binding protein 1 (ABP1) is not required for either auxin signaling or Arabidopsis development. Proc Natl Acad Sci USA 112: 2275–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner JC, Harper JDI, Weerakoon ND, Collings DA, Ritchie S, Gilroy S, Cyr RJ, Marc J (2001) A 90-kD phospholipase D from tobacco binds to microtubules and the plasma membrane. Plant Cell 13: 2143–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring CA, Irving HR, Parish RW (1990) Effects of auxin and abscisic acid on cytosolic calcium and pH in plant cells. Proc Natl Acad Sci U S A 87: 9645–9649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendreau E, Traas J, Desnos T, Grandjean O, Caboche M, Höfte H (1997) Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol 114: 295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M (2001) Auxin regulates SCF(TIR1)-dependent degradation of AUX/IAA proteins. Nature 414: 271–276 [DOI] [PubMed] [Google Scholar]
- Green PB. (1962) Mechanism for plant cellular morphogenesis. Science 138: 1404–1405 [DOI] [PubMed] [Google Scholar]
- Greenham K, Santner A, Castillejo C, Mooney S, Sairanen I, Ljung K, Estelle M (2011) The AFB4 auxin receptor is a negative regulator of auxin signaling in seedlings (erratum: Curr Biol. 2015 Mar 16;25(6):819). Curr Biol 21: 520–525 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gutierrez R, Lindeboom JJ, Paredez AR, Emons AMC, Ehrhardt DW (2009) Arabidopsis cortical microtubules position cellulose synthase delivery to the plasma membrane and interact with cellulose synthase trafficking compartments. Nat Cell Biol 11: 797–806 [DOI] [PubMed] [Google Scholar]
- Hager A. (2003) Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: Historical and new aspects. J Plant Res 116: 483–505 [DOI] [PubMed] [Google Scholar]
- Hardham AR, Gunning BE (1978) Structure of cortical microtubule arrays in plant cells. J Cell Biol 77: 14–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke CS, Dorcey E, Osmont KS, Sibout R (2007) Phytohormone collaboration: Zooming in on auxin-brassinosteroid interactions. Trends Cell Biol 17: 485–492 [DOI] [PubMed] [Google Scholar]
- Hawkins RJ, Tindemans SH, Mulder BM (2010) Model for the orientational ordering of the plant microtubule cortical array. Phys Rev E Stat Nonlin Soft Matter Phys 82: 11911. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Lau OS, Deng XW (2007) Light-regulated transcriptional networks in higher plants. Nat Rev Genet 8: 217–230 [DOI] [PubMed] [Google Scholar]
- Kao Y-L, Deavours BE, Phelps KK, Walker RA, Reddy ASN (2000) Bundling of microtubules by motor and tail domains of a kinesin-like calmodulin-binding protein from Arabidopsis: Regulation by Ca2+/Calmodulin. Biochem Biophys Res Commun 267: 201–207 [DOI] [PubMed] [Google Scholar]
- Katsumi M. (1985) Interaction of a brassinosteroid with IAA and GA3 in the elongation of cucumber hypocotyl sections. Plant Cell Physiol 26: 615–625 [Google Scholar]
- Klahre U, Noguchi T, Fujioka S, Takatsuto S, Yokota T, Nomura T, Yoshida S, Chua N-H (1998) The Arabidopsis DIMINUTO/DWARF1 gene encodes a protein involved in steroid synthesis. Plant Cell 10: 1677–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Rolff E, Spruit C (1980) Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana (L.) Heynh. Z Pflanzenphysiol 100: 147–160 [Google Scholar]
- Kutschera U, Niklas KJ (2016) The evolution of the plant genome-to-morphology auxin circuit. Theory Biosci 135: 175–186 [DOI] [PubMed] [Google Scholar]
- Kutschera U, Schopfer P (1986) In-vivo measurement of cell-wall extensibility in maize coleoptiles: Effects of auxin and abscisic acid. Planta 169: 437–442 [DOI] [PubMed] [Google Scholar]
- Lau OS, Deng XW (2010) Plant hormone signaling lightens up: Integrators of light and hormones. Curr Opin Plant Biol 13: 571–577 [DOI] [PubMed] [Google Scholar]
- Li G, Xue HW (2007) Arabidopsis PLDzeta2 regulates vesicle trafficking and is required for auxin response. Plant Cell 19: 281–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang X, Qin T, Zhang Y, Liu X, Sun J, Zhou Y, Zhu L, Zhang Z, Yuan M, Mao T (2011) MDP25, a novel calcium regulatory protein, mediates hypocotyl cell elongation by destabilizing cortical microtubules in Arabidopsis. Plant Cell 23: 4411–4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeboom JJ, Nakamura M, Hibbel A, Shundyak K, Gutierrez R, Ketelaar T, Emons AMC, Mulder BM, Kirik V, Ehrhardt DW (2013) A mechanism for reorientation of cortical microtubule arrays driven by microtubule severing. Science 342: 1245533. [DOI] [PubMed] [Google Scholar]
- Liscum E, Hangarter RP (1993) Light-stimulated apical hook opening in wild-type Arabidopsis thaliana seedlings. Plant Physiol 101: 567–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd C, Chan J (2004) Microtubules and the shape of plants to come. Nat Rev Mol Cell Biol 5: 13–22 [DOI] [PubMed] [Google Scholar]
- Mancuso S, Marras AM, Mugnai S, Schlicht M, Zársky V, Li G, Song L, Xue H-W, Baluška F (2007) Phospholipase dzeta2 drives vesicular secretion of auxin for its polar cell-cell transport in the transition zone of the root apex. Plant Signal Behav 2: 240–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marga F, Grandbois M, Cosgrove DJ, Baskin TI (2005) Cell wall extension results in the coordinate separation of parallel microfibrils: Evidence from scanning electron microscopy and atomic force microscopy. Plant J 43: 181–190 [DOI] [PubMed] [Google Scholar]
- Mathur J. (2004) Cell shape development in plants. Trends Plant Sci 9: 583–590 [DOI] [PubMed] [Google Scholar]
- Mathur J, Mathur N, Kernebeck B, Srinivas BP, Hülskamp M (2003) A novel localization pattern for an EB1-like protein links microtubule dynamics to endomembrane organization. Curr Biol 13: 1991–1997 [DOI] [PubMed] [Google Scholar]
- McFarlane HE, Döring A, Persson S (2014) The cell biology of cellulose synthesis. Annu Rev Plant Biol 65: 69–94 [DOI] [PubMed] [Google Scholar]
- Mirabet V, Krupinski P, Hamant O, Meyerowitz EM, Jönsson H, Boudaoud A (2018) The self-organization of plant microtubules inside the cell volume yields their cortical localization, stable alignment, and sensitivity to external cues. PLOS Comput Biol 14: e1006011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen GB, Miller ND, Murphy AS, Gilroy S (2011) Dynamics of auxin-dependent Ca2+ and pH signaling in root growth revealed by integrating high-resolution imaging with automated computer vision-based analysis. Plant J 65: 309–318 [DOI] [PubMed] [Google Scholar]
- Muto H, Watahiki MK, Nakamoto D, Kinjo M, Yamamoto KT (2007) Specificity and similarity of functions of the Aux/IAA genes in auxin signaling of Arabidopsis revealed by promoter-exchange experiments among MSG2/IAA19, AXR2/IAA7, and SLR/IAA14. Plant Physiol 144: 187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal P, Walker LM, Young JC, Sonawala A, Timpte C, Estelle M, Reed JW (2000) AXR2 encodes a member of the Aux/IAA protein family. Plant Physiol 123: 563–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemhauser JL, Mockler TC, Chory J (2004) Interdependency of brassinosteroid and auxin signaling in Arabidopsis. PLoS Biol 2: E258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb EH. (1969) Plant microtubules. Annu Rev Plant Physiol 20: 253–288 [Google Scholar]
- Oeller PW, Theologis A (1995) Induction kinetics of the nuclear proteins encoded by the early indoleacetic acid-inducible genes, PS-IAA4/5 and PS-IAA6, in pea (Pisum sativum L.). Plant J 7: 37–48 [DOI] [PubMed] [Google Scholar]
- Oh E, Zhu J-Y, Bai M-Y, Arenhart RA, Sun Y, Wang Z-Y (2014) Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. eLife 3: e03031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paponov IA, Dindas J, Król E, Friz T, Budnyk V, Teale W, Paponov M, Hedrich R, Palme K (2019) Auxin-induced plasma membrane depolarization is regulated by auxin transport and not by AUXIN BINDING PROTEIN1. Front Plant Sci 9: 1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredez AR, Somerville CR, Ehrhardt DW (2006) Visualization of cellulose synthase demonstrates functional association with microtubules. Science 312: 1491–1495 [DOI] [PubMed] [Google Scholar]
- Parks BM, Cho MH, Spalding EP (1998) Two genetically separable phases of growth inhibition induced by blue light in Arabidopsis seedlings. Plant Physiol 118: 609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry G, Calderon-Villalobos LI, Prigge M, Peret B, Dharmasiri S, Itoh H, Lechner E, Gray WM, Bennett M, Estelle M (2009) Complex regulation of the TIR1/AFB family of auxin receptors. Proc Natl Acad Sci USA 106: 22540–22545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge MJ, Greenham K, Zhang Y, Santner A, Castillejo C, Mutka AM, O’Malley RC, Ecker JR, Kunkel BN, Estelle M (2016) The Arabidopsis auxin receptor F-box proteins AFB4 and AFB5 are required for response to the synthetic auxin picloram. G3: Genes, genomes. G3 (Bethesda) 6: 1383–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probine M. (1965) Chemical control of plant cell wall structure and of cell shape. Proc R Soc Lond B Biol Sci 161: 526–537 [Google Scholar]
- Rayle DL, Evans ML, Hertel R (1970) Action of auxin on cell elongation. Proc Natl Acad Sci USA 65: 184–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Elumalai RP, Chory J (1998) Suppressors of an Arabidopsis thaliana phyB mutation identify genes that control light signaling and hypocotyl elongation. Genetics 148: 1295–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J (1993) Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5: 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Wu M-F, Reeves PH, Hodgens C, Yadav V, Hayes S, Pierik R (2018) Three auxin response factors promote hypocotyl elongation. Plant Physiol 178: 864–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani G, Marriè M, Bonetti A, Cerana R, Lado P, Marre E (1983) Effects of a brassinosteroid on growth and electrogenic proton extrusion in maize root segments. Physiol Plant 59: 528–532 [Google Scholar]
- Rück A, Palme K, Venis MA, Napier RM, Felle HH (1993) Patch‐clamp analysis establishes a role for an auxin binding protein in the auxin stimulation of plasma membrane current in Zea mays protoplasts. Plant J 4: 41–46 [Google Scholar]
- Salehin M, Bagchi R, Estelle M (2015) SCFTIR1/AFB-based auxin perception: Mechanism and role in plant growth and development. Plant Cell 27: 9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambade A, Pratap A, Buschmann H, Morris RJ, Lloyd C (2012) The influence of light on microtubule dynamics and alignment in the Arabidopsis hypocotyl. Plant Cell 24: 192–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck D, Christian M, Jones A, Lüthen H (2010) Rapid auxin-induced cell expansion and gene expression: A four-decade-old question revisited. Plant Physiol 152: 1183–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibaoka H. (1994) Plant hormone-induced changes in the orientation of cortical microtubules: Alterations in the cross-linking between microtubules and the plasma membrane. Annu Rev Plant Biol 45: 527–544 [Google Scholar]
- Spartz AK, Gray WM (2008) Plant hormone receptors: New perceptions. Genes Dev 22: 2139–2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spartz AK, Ren H, Park MY, Grandt KN, Lee SH, Murphy AS, Sussman MR, Overvoorde PJ, Gray WM (2014) SAUR inhibition of PP2C-D phosphatases activates plasma membrane H+-ATPases to promote cell expansion in Arabidopsis. Plant Cell 26: 2129–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres M, Németh K, Koncz-Kálmán Z, Mathur J, Kauschmann A, Altmann T, Rédei GP, Nagy F, Schell J, Koncz C (1996) Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell 85: 171–182 [DOI] [PubMed] [Google Scholar]
- Szymanski DB, Cosgrove DJ (2009) Dynamic coordination of cytoskeletal and cell wall systems during plant cell morphogenesis. Curr Biol 19: R800–R811 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Gasch A, Nishizawa N, Chua N-H (1995) The DIMINUTO gene of Arabidopsis is involved in regulating cell elongation. Genes Dev 9: 97–107 [DOI] [PubMed] [Google Scholar]
- Tan X, Calderon-Villalobos LIA, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N (2007) Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446: 640–645 [DOI] [PubMed] [Google Scholar]
- Teale WD, Paponov IA, Palme K (2006) Auxin in action: Signalling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol 7: 847–859 [DOI] [PubMed] [Google Scholar]
- Theologis A, Huynh TV, Davis RW (1985) Rapid induction of specific mRNAs by auxin in pea epicotyl tissue. J Mol Biol 183: 53–68 [DOI] [PubMed] [Google Scholar]
- Tian H, Lv B, Ding T, Bai M, Ding Z (2018) Auxin-BR interaction regulates plant growth and development. Front Plant Sci 8: 2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vert G, Walcher CL, Chory J, Nemhauser JL (2008) Integration of auxin and brassinosteroid pathways by Auxin Response Factor 2. Proc Natl Acad Sci USA 105: 9829–9834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra RD. (2009) The ubiquitin-26S proteasome system at the nexus of plant biology. Nat Rev Mol Cell Biol 10: 385–397 [DOI] [PubMed] [Google Scholar]
- Vineyard L, Elliott A, Dhingra S, Lucas JR, Shaw SL (2013) Progressive transverse microtubule array organization in hormone-induced Arabidopsis hypocotyl cells. Plant Cell 25: 662–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Estelle M (2014) Diversity and specificity: Auxin perception and signaling through the TIR1/AFB pathway. Curr Opin Plant Biol 21: 51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijers D, Benkova E, Jäger KE, Schlereth A, Hamann T, Kientz M, Wilmoth JC, Reed JW, Jürgens G (2005) Developmental specificity of auxin response by pairs of ARF and Aux/IAA transcriptional regulators. EMBO J 24: 1874–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Dai N, Chen J, Nagawa S, Cao M, Li H, Zhou Z, Chen X, De Rycke R, Rakusová H, Wang W, Jones AM, et al. (2014) Cell surface ABP1-TMK auxin-sensing complex activates ROP GTPase signaling. Science 343: 1025–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Warn RM, Shaw PJ, Lloyd CW (1995) Dynamic microtubules under the radial and outer tangential walls of microinjected pea epidermal cells observed by computer reconstruction. Plant J 7: 17–23 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Lin F, Mao T, Nie J, Yan M, Yuan M, Zhang W (2012) Phosphatidic acid regulates microtubule organization by interacting with MAP65-1 in response to salt stress in Arabidopsis. Plant Cell 24: 4555–4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Qu Y, Wang Q, Song P, Wang P, Jia Q, Guo J (2017) Arabidopsis phospholipase D α1-derived phosphatidic acid regulates microtubule organization and cell development under microtubule-interacting drugs treatment. J Plant Res 130: 193–202 [DOI] [PubMed] [Google Scholar]