An apple malate transporter lacking a conserved C-terminal domain causes low fruit acidity.
Abstract
Malate accumulation in the vacuole largely determines apple (Malus domestica) fruit acidity, and low fruit acidity is strongly associated with truncation of Ma1, an ortholog of ALUMINUM-ACTIVATED MALATE TRANSPORTER9 (ALMT9) in Arabidopsis (Arabidopsis thaliana). A mutation at base 1,455 in the open reading frame of Ma1 leads to a premature stop codon that truncates the protein by 84 amino acids at its C-terminal end. Here, we report that both the full-length protein, Ma1, and its naturally occurring truncated protein, ma1, localize to the tonoplast; when expressed in Xenopus laevis oocytes and Nicotiana benthamiana cells, Ma1 mediates a malate-dependent inward-rectifying current, whereas the ma1-mediated transmembrane current is much weaker, indicating that ma1 has significantly lower malate transport activity than Ma1. RNA interference suppression of Ma1 expression in ‘McIntosh’ apple leaves, ‘Empire’ apple fruit, and ‘Orin’ apple calli results in a significant decrease in malate level. Genotyping and phenotyping of 186 apple accessions from a diverse genetic background of 17 Malus species combined with the functional analyses described above indicate that Ma1 plays a key role in determining fruit acidity and that the truncation of Ma1 to ma1 is genetically responsible for low fruit acidity in apple. Furthermore, we identified a C-terminal domain conserved in all tonoplast-localized ALMTs essential for Ma1 function; protein truncations into this conserved domain significantly lower Ma1 transport activity. We conclude that the truncation of Ma1 to ma1 reduces its malate transport function by removing a conserved C-terminal domain, leading to low fruit acidity in apple.
Acidity is a major contributor to apple (Malus domestica) fruit quality, including fruit overall taste and flavor (Sweetman et al., 2009; Etienne et al., 2013). Organic acids collectively are responsible for acidity, but malic acid accounts for more than 90% of the total acid and largely controls apple fruit acidity (Hulme and Wooltorton, 1957; Yamaki, 1984; Zhang et al., 2010). Most of the malic acid in apple fruit resides in the vacuole of the parenchyma cells (Yamaki, 1984), and its concentration shows a developmental pattern that peaks at 4 to 6 weeks after bloom followed by continuous decline to fruit harvest (Zhang et al., 2010). While malate synthesis and degradation can affect fruit malate level, apple fruit acidity appears to be primarily determined by intracellular transport of malate between the cytosol and the vacuole (Berüter, 2004; Sweetman et al., 2009; Etienne et al., 2013).
Malic acid accumulates in the vacuole via an acid-trap mechanism. In the cytosol, malic acid is present almost entirely in the dianion form, malate2−, due to a neutral or slightly alkaline pH. Pumping of H+ into the vacuole by both vacuolar H+-ATPase and H+-pyrophosphatase lowers the vacuolar pH, thereby instantly protonating any malate transported into the vacuole (Emmerlich et al., 2003; Kovermann et al., 2007; Schumacher and Krebs, 2010; Barbier-Brygoo et al., 2011; Meyer et al., 2011). This acid trapping effectively maintains the concentration gradient of malate across the tonoplast to drive its facilitated diffusion from the cytosol into the vacuole. Two types of malate transporters mediate this process. One is a tonoplast dicarboxylate transporter (Emmerlich et al., 2003; Hurth et al., 2005), and the other is an anion channel, which is a member of the so-called aluminum-activated malate transporter/channel (ALMT) family. Both Arabidopsis (Arabidopsis thaliana) ALMT9 and ALMT6 localize to the tonoplast and mediate malate transport into the vacuole (Kovermann et al., 2007; Meyer et al., 2011). In apple, the malic acid locus, Ma, which was named in 1978 (Visser and Verhaegh, 1978), was mapped to a region on linkage group 16 and accounts for 17% to 42% of the variation in apple fruit acidity (Maliepaard et al., 1998; Liebhard et al., 2003; Kenis et al., 2008; Xu et al., 2012). Two apple ALMT9 genes present in this genomic region are candidate genes underlying Ma and were named Ma1 and Ma2 (Bai et al., 2012; Khan et al., 2013). A single-nucleotide polymorphism at base 1,455 leads to the truncation of the Ma1 protein by 84 amino acids to ma1. Apples of genotype ma1ma1 have significantly lower acidity at fruit maturity than those of genotypes Ma1ma1 and Ma1Ma1, strongly suggesting that ma1 has lower malate transport activity than Ma1. However, the putative functional difference between Ma1 and ma1 has not been confirmed.
The ALMT family of proteins to which Ma1 belongs was named after TaALMT1, a plasma membrane (PM)-localized channel that facilitates the efflux of Al3+-chelating malate anions from wheat (Triticum aestivum) root apices, thereby mediating Al resistance (Delhaize et al., 1993a, 1993b; Sasaki et al., 2004). Arabidopsis AtALMT1, a homolog of TaALMT1, was soon found to play a similar role in Al3+ tolerance in Arabidopsis (Hoekenga et al., 2006). Recent work has shown that TaALMT1 activity is negatively regulated by γ-aminobutyric acid in addition to being actuated by Al3+ (Ramesh et al., 2015, 2018). This ALMT gene family is ubiquitously present in plants, and their physiological roles extend beyond the Al3+ detoxification process (Dreyer et al., 2012; Palmer et al., 2016; Sharma et al., 2016). There are 13 ALMTs in Arabidopsis (Delhaize et al., 2007), which can be phylogenetically grouped into three clades. Among these, clade II includes ALMT3, ALMT4, ALMT5, ALMT6, and ALMT9, all of which except ALMT3 localize to the tonoplast. Both AtALMT6 and AtALMT9 are involved in stomatal regulation. AtALMT6 is a calcium-activated malate channel mediating the transport of malate from the cytosol into the vacuole in guard cells. AtALMT9 was first found to mediate the malate current across the tonoplast (Kovermann et al., 2007), but more detailed characterization revealed that its chloride permeation is activated by the cytosolic malate level (De Angeli et al., 2013b). An AtALMT9 homolog in grape (Vitis vinifera), VvALMT9, is a vacuolar malate channel and is most likely involved in mediating malate and tartrate accumulation in the vacuole of grape berries (De Angeli et al., 2013a). In tomato (Solanum lycopersicum), SlALMT9 underlies a major quantitative trait locus (QTL) responsible for variations in malate accumulation among different genotypes, TOMATO FRUIT MALATE ON CHROMOSOME6, and a 3-bp insertion/deletion in its promoter region leads to a high-malate phenotype (Ye et al., 2017).
Based on a strong association between a mutation-led truncation in the C-terminal end of Ma1 and low fruit acidity in apple (Bai et al., 2012) and subsequent genotyping and phenotyping of segregating populations and Malus spp. germplasm (Khan et al., 2013; Ma et al., 2015; Jia et al., 2018; Verma et al., 2019), we hypothesized that the truncated version of Ma1, ma1, has significantly lower malate transport activity than the full-length protein Ma1. In this work, we compare the functionality of Ma1 and ma1 expressed both in Xenopus laevis oocytes and Nicotiana benthamiana cells. In combination with RNA interference (RNAi) suppression of Ma1 expression in apple and phenotyping of a large number of Malus spp. accessions from a diverse genetic background, we show that the premature stop codon-led truncation of Ma1 is genetically responsible for low acidity in apple, as it disrupts a highly conserved C-terminal domain essential for Ma1’s malate transport activity.
RESULTS
Ma1 Has a Higher Malate Transport Activity Than ma1 in X. laevis Oocytes
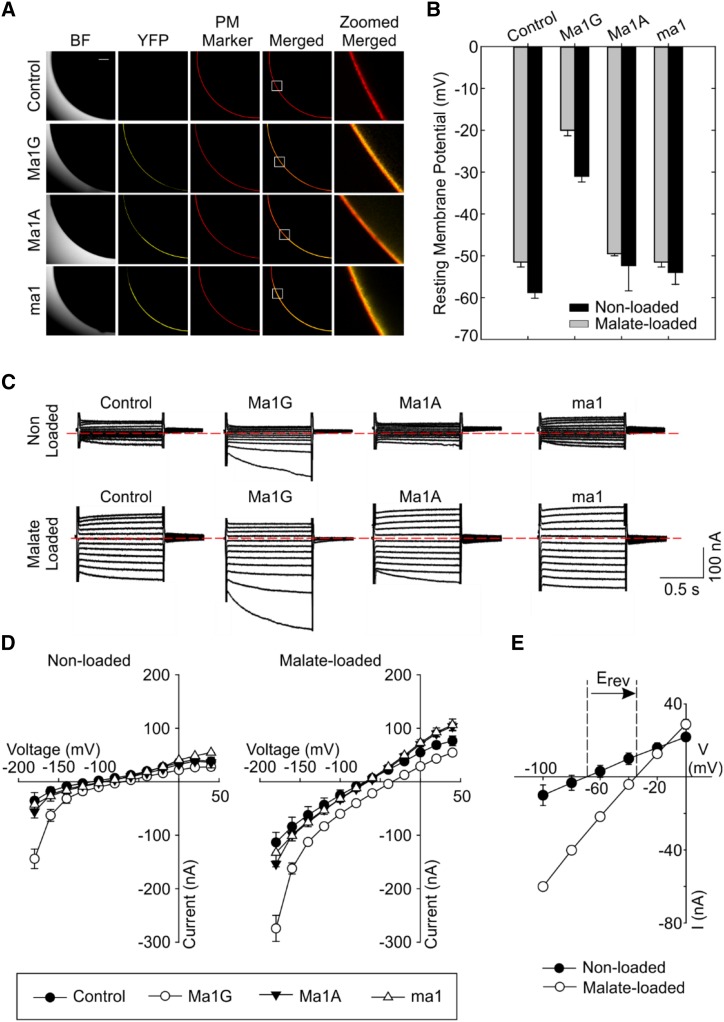
Our first goal was to investigate the functional difference between the putative ALMT transporters encoded by two naturally occurring ALMT alleles, Ma1 and ma1. The Ma1 gene (hereafter referred to as Ma1G) consists of a 1,708-bp open reading frame, which encodes a full-length ALMT transporter with 568 residues. The ma1 allele has a G-to-A substitution at position 1,455, which results in a W485Stop substitution, with the early stop codon truncating the last 84 C-terminal residues. In addition to the truncation, the ma1 allele has three additional residue substitutions: V272I, K429R, and V465A. Therefore, we generated an artificial allele, a truncated version of Ma1G, and named it Ma1A (i.e. the original Ma1G allele was truncated by introducing the W485Stop early stop via PCR). We functionally characterized the artificial Ma1A along with naturally occurring Ma1G and ma1 to dissect any potential effect of the other naturally occurring substitutions (i.e. residues 272, 429, and 465) on the functional changes resulting from the truncation. The initial functional characterization of Ma1G, Ma1A, and ma1 was carried out by heterologously expressing these genes in X. laevis cells and examining their electrophysiological properties. Confocal microscopy of oocytes injected with the complementary RNA (cRNA) of Ma1G, Ma1A, and ma1 fused with yellow fluorescent protein (YFP) revealed that these three proteins were properly expressed and localized to the PM of the oocyte, as indicated by their colocalization of the YFP signal with the deep red PM marker (Fig. 1A).
Figure 1.
Functional characterization of ALMT transporters encoded by Ma1G, ma1, and artificially truncated Ma1A genes in X. laevis oocytes. A, The expression and localization of Ma-YFP fusion proteins in oocytes. Deep Red was used as a PM marker. The white squares in the Merged signal indicate the 100-µm × 100-µm regions of interest shown in the last column. The scale bar for the bright field (BF), YFP, PM Marker, and Merged fluorescence signal columns is shown in the top left image; bar = 100 µm. B, RMPs recorded in control and Ma1G-, Ma1A-, and ma1-expressing cells. RMPs were recorded in cells nonloaded or loaded with malate by microinjecting cells with 50 nL of 100 mm sodium malate (increasing cytosolic malate2− activities by 4.5 mm) 2 to 3 h prior to the electrophysiological recordings. Data are means ± se. The numbers of cells nonloaded with malate are as follows: control (n = 9), Ma1G (n = 8), Ma1A (n = 10), and ma1 (n = 8); the numbers of cells loaded with malate are as follows: n = 8 for control, Ma1G, Ma1A, and ma1. C, Examples of currents elicited in response to holding potentials ranging from +40 to −180 mV (in 20-mV steps) recorded in control, Ma1G-, Ma1A-, and ma1-expressing cells, either nonloaded or loaded with malate. The zero-current level is indicated by the red dotted lines. D, Mean current-voltage (I/V) relationships constructed from steady-state current recordings with nonloaded (left) or malate-loaded (right) cells such as those shown in C. Data are means ± se. The numbers of cells nonloaded with malate are as follows: control (n = 9), Ma1G (n = 8), Ma1A (n = 10), and ma1 (n = 8); the numbers of cells loaded with malate are as follows: n = 8 for control, Ma1G, Ma1A, and ma1. E, Difference in the magnitude of Ma1G-mediated current and shift in the holding potential (Erev) at which the current reverses sign between nonloaded and malate-loaded X. laevis oocytes expressing Ma1G. The recording cell number is as follows: n = 8 for both nonloaded and malate-loaded cells.
Having validated the proper expression and cellular localization of these three proteins, we proceeded to perform the functional characterization of the untagged transporter. Cells expressing Ma1G had significantly less negative resting membrane potentials (RMPs) than those recorded in control cells (Fig. 1B). In contrast, cells injected with Ma1A or ma1 cRNA showed no significant differences in RMPs relative to the controls, regardless of the intracellular malate concentration. Given that all three proteins express and localize to the oocyte PM, the latter results suggest that Ma1G, but not Ma1A or ma1, encodes a functional membrane transporter that results in the reduction of the net internal negative charge (relative to that found in control cells) as a result of net anion efflux. Further characterization was performed using the conventional two-electrode voltage-clamp (TEVC) method. Under voltage clamp (Fig. 1, C–E), Ma1G-expressing cells mediated larger inward (i.e. negative) currents than those recorded in control cells. Increasing intracellular malate concentration (i.e. malate loading) resulted in an increase in the Ma1G-mediated inward current (Fig. 1, C and D), which was accompanied by a positive shift in Erev, the holding potential at which the current reverses sign (Fig. 1E). By convention, the Ma1G-mediated inward current is the product of either net positive charge influx or net negative charge efflux. Therefore, the increase in the magnitude of the Ma1G-mediated inward current upon increasing the intracellular malate activities was consistent with Ma1G inward current being the product of anion efflux. The latter was reinforced by the positive shift in Erev observed solely in Ma1G-expressing cells upon increasing the malate efflux (outwardly directed) driving force (i.e. increasing the intracellular malate activity). In contrast, the Erev and the magnitude of the inward currents recorded in cells expressing the Ma1A or ma1 proteins were not significantly different from those recorded in control cells, regardless of the intracellular malate status.
We also evaluated the substrate recognition of the Ma1G transporter to other organic anions. Large negative inward currents were also observed in Ma1G-expressing cells when they were preloaded with fumarate or citrate, rather than malate, prior to the electrophysiological recordings (Supplemental Fig. S1). These Ma1G-mediated inward currents in cells preloaded with fumarate resemble those recorded in malate-preloaded cells, while those recorded in cells preloaded with citrate were significantly smaller in magnitude, suggesting a lower permeability of citrate by Ma1G. These results are similar to the substrate selectivity described for ALMT members (Kovermann et al., 2007; Meyer et al., 2011), indicating that Ma1 is a typical ALMT member with conserved function in organic acid transport. Overall, the above results suggest that the full-length Ma1G allele encodes a functional malate transporter, while the two truncated transporters, Ma1A and ma1, have either a lower transport capacity or no functional activity at all (given the lack of differences relative to control cells), at least in X. laevis oocytes and under the ionic conditions tested. As Ma1G, Ma1A, and ma1 are all equally localized to the oocyte PM, the functional differences between Ma1G and ma1 result from the truncation of the full-length protein (and not from the naturally occurring V272I, K429R, and V465A residue substitutions), which ultimately translate into differences in cellular malate fluxes in planta.
Ma1 and ma1 Are Localized to the Tonoplast
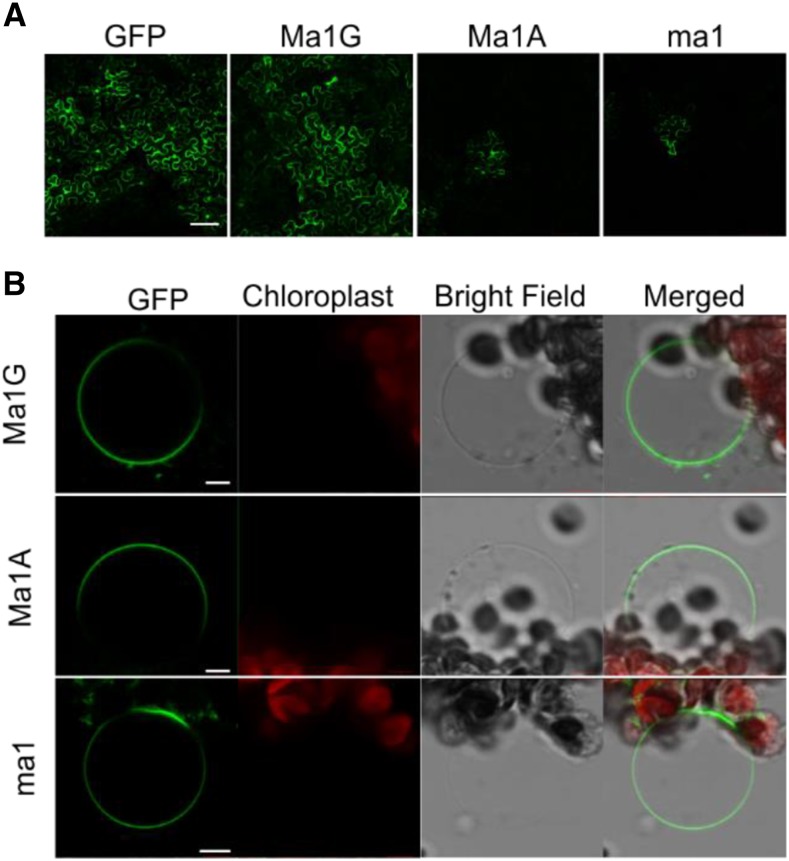
To determine the subcellular localization of Ma1G, Ma1A, and ma1 in planta, C-terminal GFP fusion constructs under the control of a 35S promoter were transiently expressed in N. benthamiana leaves via agroinfiltration. GFP fluorescence signals were detected for all three fusion proteins, but compared with the robust and widespread signal of Ma1G, the Ma1A and ma1 signals were consistently weaker and spatially restricted (Fig. 2A). To further investigate the proteins’ subcellular localization, leaf protoplasts were isolated from N. benthamiana leaves transiently transformed with the GFP fusion constructs and then lysed to release the intact vacuoles. The Ma1G-GFP, Ma1A-GFP, and ma1-GFP fluorescent signals all localized to the vacuole (Fig. 2B). The tonoplast localization of Ma1G, Ma1A, and ma1 proteins was confirmed in N-terminal GFP chimeras transiently expressed in N. benthamiana leaves (Supplemental Fig. S2), and the vacuolar localization of these chimeras resembled that obtained with the C-terminal GFP fusion proteins.
Figure 2.
Subcellular localization of Ma1G, Ma1A, and ma1 chimeras with the GFP protein fused to the C terminus. A, Example of the transient transformation efficiency of a 35S-driven cytosolic GFP (empty vector control), Ma1G-GFP, ma1-GFP, and Ma1A-GFP in A. tumefaciens-infiltrated N. benthamiana leaves. B, Tonoplast localization of the Ma1G, Ma1A, and ma1 GFP chimeras. Isolated vacuoles were obtained after lysis of N. benthamiana protoplasts transiently transformed with the latter constructs. Bars = 100 µm (A) and 10 µm (B).
Ma1 Has a Higher Malate Transport Activity Than ma1 in Plant Cells
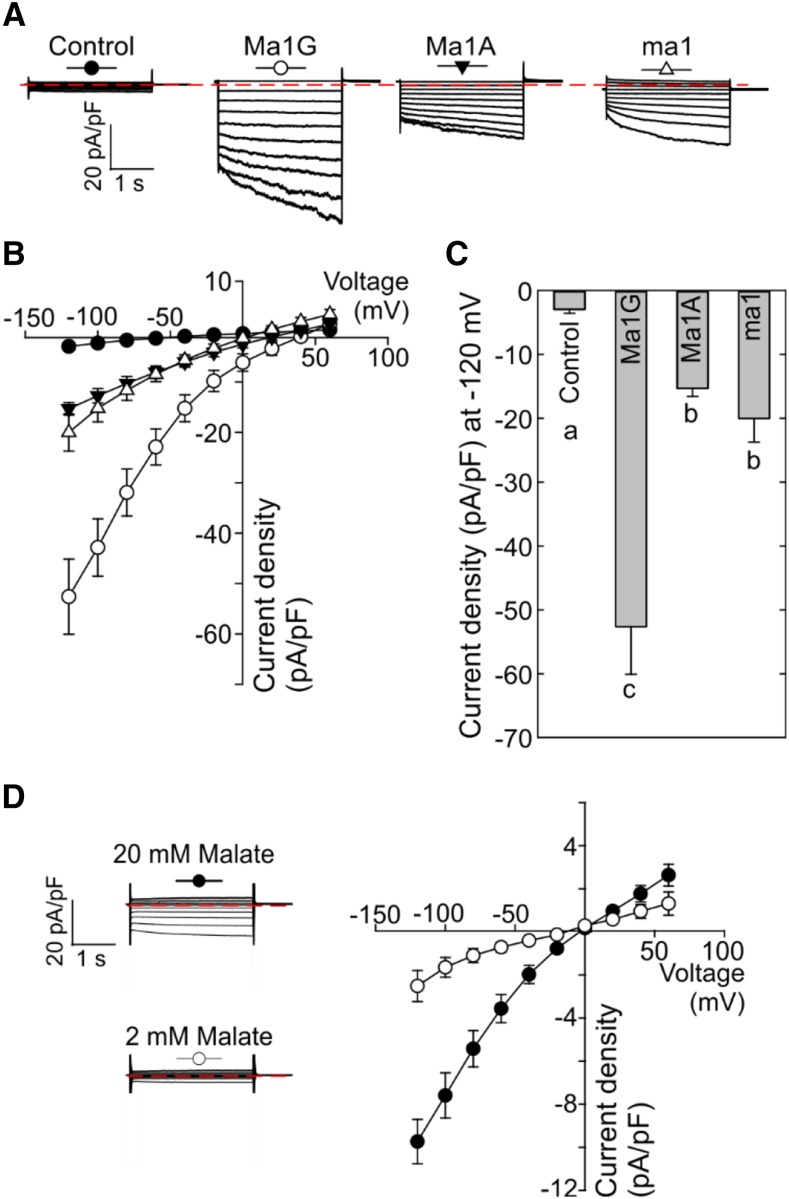
To further examine the functional differences among Ma1G, Ma1A, and ma1 in planta, we used the patch-clamp technique in vacuoles isolated from N. benthamiana leaves transiently expressing their GFP chimeras. Vacuoles from cells expressing Ma1G, Ma1A, or ma1 with similar fluorescence intensity were selected for patch-clamp experiments. Vacuoles expressing Ma1G had significantly larger inward current density in comparison with the lack of currents observed in control cells (Fig. 3, A–C). Smaller inward currents, yet significantly larger than those recorded in control cells, were detected in Ma1A- and ma1-expressing vacuoles. Reduction of the malate concentration on the cytosolic side (i.e. bath media) resulted in a pronounced reduction in the magnitude of the Ma1G-mediated inward currents (Fig. 3D), corroborating the malate permeability. Overall, these results were consistent with the functional differences inferred earlier from the data obtained in X. laevis oocytes (Fig. 1), confirming that Ma1G has a higher transport activity than the truncated Ma1A or ma1 protein. However, in contrast to the oocyte results, albeit being small, Ma1A- and ma1-mediated inward currents were significantly larger than those recorded in control cells. It appears that the patch-clamp system had a higher sensitivity than TEVC, allowing us to detect significant differences in current density between Ma1A/ma1 and the control cells. These differences suggest that although having a significantly lower current density than that of Ma1G, Ma1A and ma1 may still have some residual transport activity. Given that the current density comparisons were made among vacuoles with similar fluorescence, the differences are more likely due to intrinsic structural-functional differences rather than differences in protein abundance.
Figure 3.
Patch-clamp analysis of malate transport activity of Ma1G, Ma1A, and ma1 expressed in N. benthamiana. A, Representative recordings of whole-vacuole currents in control vacuoles (expressing empty vector) and vacuoles from cells expressing Ma1G, Ma1A, or ma1. Currents across the tonoplast were evoked in response to 3-s voltage pulses ranging from +60 to −120 mV in 20-mV steps. The zero-current level is indicated by the red dotted line. B, Mean current-voltage relationships from whole-vacuole currents constructed from steady-state current recordings as those shown in A for control vacuoles (n = 12) and vacuoles from cells expressing Ma1G (n = 10), Ma1A (n = 9), or ma1 (n = 10). The symbol for each curve corresponds to that depicted at the top of each set of traces in A. Data are means ± se. C, Bar graphs representing the current densities at −120 mV obtained by the patch clamping of control vacuoles and vacuoles from cells expressing Ma1G, Ma1A, or ma1. Different letters (a, b, and c) indicate significant differences between groups using Tukey’s honestly significant difference (HSD) test at P < 0.05 after ANOVA. Error bars denote se. D, The Ma1G-mediated current is dependent on the cytosolic malate concentration. Representative recordings of whole-vacuole currents from cells expressing Ma1G as the malate concentration in the bath solution (i.e. cytosolic) was decreased from 100 mm (top) to 20 or 2 mm. Currents were evoked using the same protocol described in A. Note that the time and current scales are identical to those shown in A.
RNAi Suppression of Ma1 Expression Leads to Lower Malate Levels in Apple Leaves and Fruit
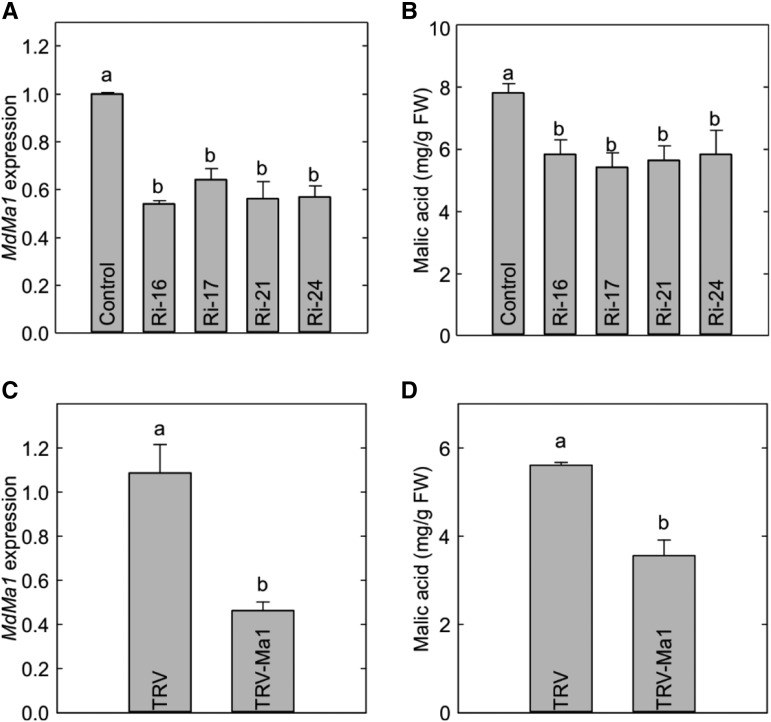
To elucidate the role of Ma1 in malate accumulation in apple cells, we transformed ‘McIntosh’ apple with a Ma1 RNAi construct. Four transgenic lines, Ri-16, Ri-17, Ri-21, and Ri-24, showing significantly lower Ma1 expression (as determined via reverse transcription quantitative PCR [RT-qPCR]), were selected among over 20 independently transformed lines (Fig. 4A). Consistent with reduced Ma1 expression, lower malate levels were detected in the leaves of all four RNAi lines compared with those measured in the wild-type control (Fig. 4B). Leaf sugar levels (sorbitol, Suc, Glc, and Fru) of these RNAi transgenic lines were also measured, but no significant difference was detected between the transgenic lines and the wild-type control (Supplemental Fig. S3, A–D). Transiently suppressing Ma1 expression in ‘Empire’ apple fruit (Ma1Ma1) using a tobacco rattle virus-based gene-silencing method decreased the expression of Ma1 and consequently lowered malic acid level in the fruit (Fig. 4, C and D), but sugar levels were not altered (Supplemental Fig. S3E). In addition, we also generated transgenic ‘Orin’ apple calli via agro-mediated transformation with the RNAi construct. RNAi suppression of Ma1 expression led to significantly lower malate levels in the transgenic calli compared with the control (Supplemental Fig. S4). These results are consistent with those obtained from the transgenic ‘McIntosh’ plants and ‘Empire’ fruit. Together, these findings indicate that Ma1 plays a key role in the accumulation of malate in apple cells, which is in agreement with its malate transport activity detected both in X. laevis oocyte cells and N. benthamiana vacuoles (Figs. 1 and 3).
Figure 4.
Reduced malate levels in the leaves of Ma1 RNAi lines of ‘McIntosh’ apple and fruit of ‘Empire’ apple with virus-induced silencing of Ma1. A, The relative expression of Ma1 in Ma1-RNAi transgenic lines in comparison with wild-type plants (control). B, The malic acid concentration in Ma1-RNAi transgenic lines and wild-type plants measured via gas chromatography-mass spectrometry analysis. C, The relative expression of Ma1 in mature ‘Empire’ apple fruit infiltrated with Agrobacterium tumefaciens GV3101 harboring an antisense Ma1 construct in a tobacco rattle virus (TRV)-based vector (TRV-Ma1) or TRV alone as a control. D, The malic acid concentration in mature ‘Empire’ apple fruit infiltrated with TRV-Ma1 or TRV alone as a control measured via gas chromatography-mass spectrometry analysis. Data are means ± se of three replicates with 20 leaves pooled from four in vitro shoots per replicate for ‘McIntosh’ and of five replicates with two fruits (three injection sites per fruit) per replicate for ‘Empire’. Different letters (a and b) indicate significant differences between genotypes using Tukey’s HSD test at P < 0.05 after ANOVA. FW, Fresh weight.
The Genotypes of Ma1 Determine Fruit Acidity in Diverse Malus Accessions
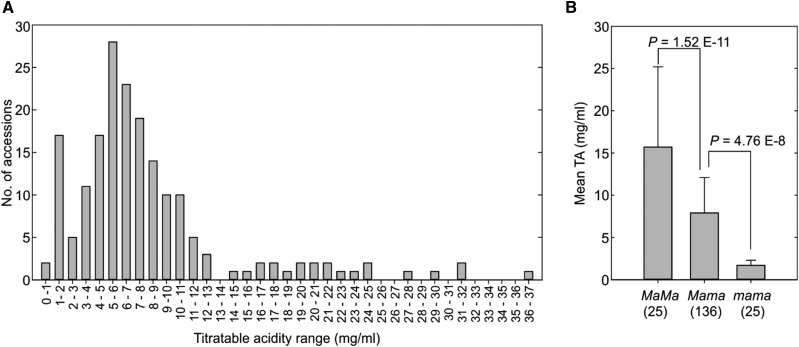
To examine if the premature stop codon mutation identified in Ma1 genetically determines fruit acidity in a more diverse genetic background, we phenotyped 186 apple germplasm accessions from 17 Malus species (Supplemental Table S1) for fruit titratable acidity (TA) over three consecutive growing seasons (2013–2015) and genotyped with marker CAPS1455 (Bai et al., 2012). Fruit acidity varied widely, with TA ranging from 0.7 to 36 mg mL−1 (Fig. 5A). The majority (164 or 88.2%) of the 186 accessions fell within a TA range from 0.7 to 13 mg mL−1. However, the remaining 22 (11.8%) accessions had TA > 13 mg mL−1, all of which are wild Malus accessions except for cv Russian (Supplemental Table S1).
Figure 5.
Fruit TA in 186 Malus accessions. A, Distribution of fruit TA assessed over three harvest seasons (2013–2015). B, One-way ANOVA of the Ma genetic effect on fruit TA in Malus accessions. The numbers in parentheses stand for the number of accessions in each genotype category. Data are means ± sd.
Based on marker CAPS1455, 25 (13.4%) of the 186 accessions were genotype Ma1Ma1, 136 (73.1%) were Ma1ma1, and another 25 (13.4%) were ma1ma1, estimating an evenly split frequency of 0.5 for alleles Ma1 and ma1 (Supplemental Table S1). By comparing the frequency distribution of the Ma1 genotypes and alleles among M. domestica, Malus hybrid, and others (Supplemental Table S1), we found that genotype Ma1ma1 was considerably enriched in M. domestica while Ma1Ma1 and ma1ma1 were reduced. This suggests that a positive selection for Ma1ma1 coupled with a negative selection against Ma1Ma1 and ma1ma1 might have occurred during apple domestication.
ANOVA of fruit acidity in genotypes Ma1Ma1, Ma1ma1, and ma1ma1 clearly demonstrated a major genetic effect of Ma1 on TA. The mean TA of ma1ma1 was 1.7 ± 0.6 mg mL−1 (n = 25), significantly lower than that of Ma1ma1 (7.9 ± 4.2 mg mL−1; n = 136, P = 4.76E−8), which in turn was significantly lower than that of Ma1Ma1 (15.7 ± 9.5 mg mL−1; n = 25, P = 1.52E−11), despite the mean estimates being likely compounded by year effect (Fig. 5B). Correspondingly, the juice pH value was significantly higher in ma1ma1 than that of Ma1ma1, which in turn was significantly higher than that of Ma1Ma1 (Supplemental Fig. S5). More importantly, the 25 ma1ma1 accessions that span over five Malus species, including 18 in M. domestica, three in Malus sieversii, two in M. hybrid, one in Malus asiatica, and one in Malus kirghisorum, had a narrow and low TA range (0.7–3.3 mg mL−1). Immunoblot analysis of Ma1/ma1 protein expression indicated similar protein levels in the fruit of different genotypes (ma1ma1, Ma1Ma1, Ma1ma1) at 5 weeks after bloom (peak malate level) and at maturity (Supplemental Fig. S6). The genotyping and phenotyping associations described above, taken together with the functional difference between Ma1G and ma1 recorded in oocytes, N. benthamiana cells, and transgenic apple tissues, clearly support the inference that the stop codon-led truncation in Ma1 is genetically responsible for low acidity in apple (Bai et al., 2012).
Ma1 Has a Highly Conserved C-Terminal Domain Essential for Its Malate Transport Activity
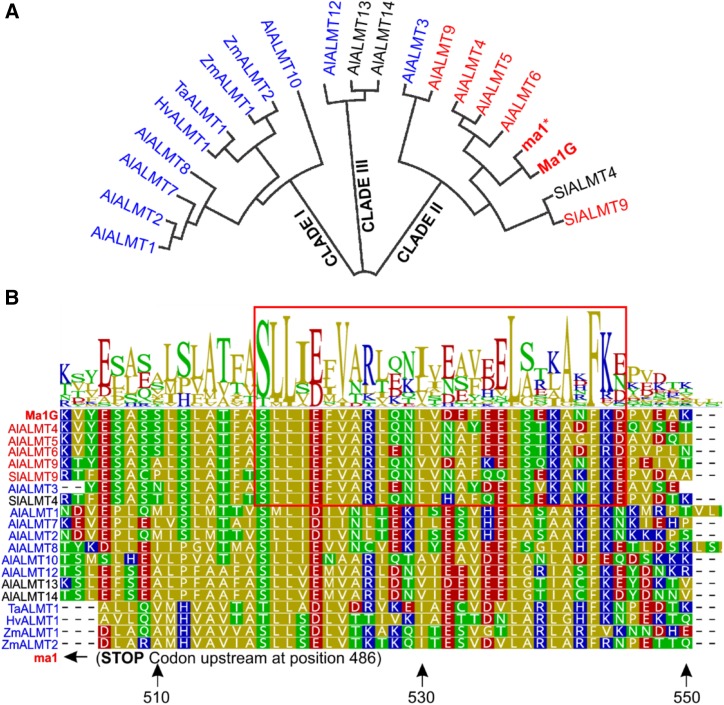
ALMTs are a family of membrane proteins unique to the plant kingdom. In Arabidopsis, this family consists of 13 members that are grouped mainly into three distinct clades based on amino acid sequence alignment (Kovermann et al., 2007). Phylogenetic analysis placed Ma1G into clade II (Fig. 6A), along with other tonoplast-localized proteins, including AtALMT4, AtALMT5, AtALMT6, and AtALMT9, as well as SlALMT9; the latter (SlALMT9) plays a key role in regulating the malate level in tomato fruits (Ye et al., 2017). Two other members of this clade, AtALMT3 and SlALMT4, localize to PM and endoplasmic reticulum, respectively (Sasaki et al., 2016; Maruyama et al., 2019). Based on amino acid sequence alignment, we identified a conserved domain in the C-terminal end downstream from the early stop codon leading to ma1 (Fig. 6B). This conserved domain spans approximately 45 amino acids, from 503 to 547, in Ma1G. Given the premature stop codon-led truncation, ma1 lacks this conserved C-terminal domain. Albeit being conserved among all three clades, this domain appears to have a higher degree of conservation among members of clade II.
Figure 6.
The conserved C-terminal domain of Ma1 and other tonoplast-localized ALMT members. A, Phylogenetic analysis of Ma1G, ma1, and the 13 ALMT Arabidopsis members, two tomato, two maize (Zea mays), one barley (Hordeum vulgare), and one wheat ALMT members based on amino acid sequence alignment by the neighbor-joining method using Geneious Tree Builder (https://www.geneious.com/). AtALMTs cluster into three groups: I, II, and III. Clade I contains ALMTs known to localize to the PM (colored in blue). Clade II contains ALMTs know to localize to the tonoplast (colored in red), including Ma1G and ma1. B, Sequence alignment and logo illustrating the degree of conservation in a C-terminal domain of the ALMTs described in A. The residue positions marked by the arrows at the bottom of the alignment are relative to the Ma1G sequence. The early stop codon in ma1 is located 17 residues upstream of the aligned sequences. The red box highlights the amino acid region 519 to 545 for the clade II ALMTs.
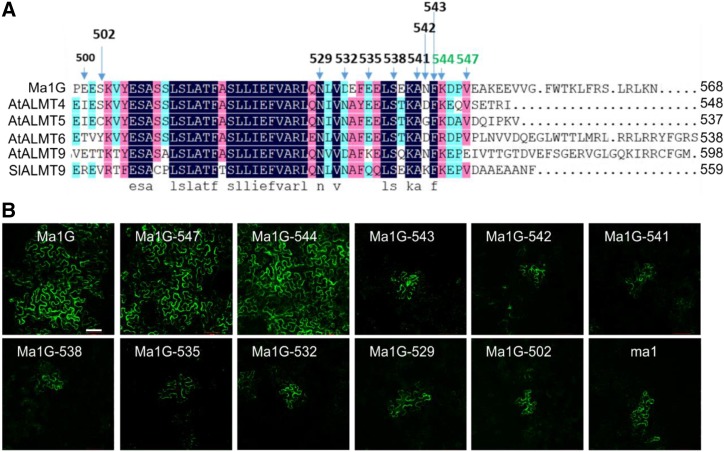
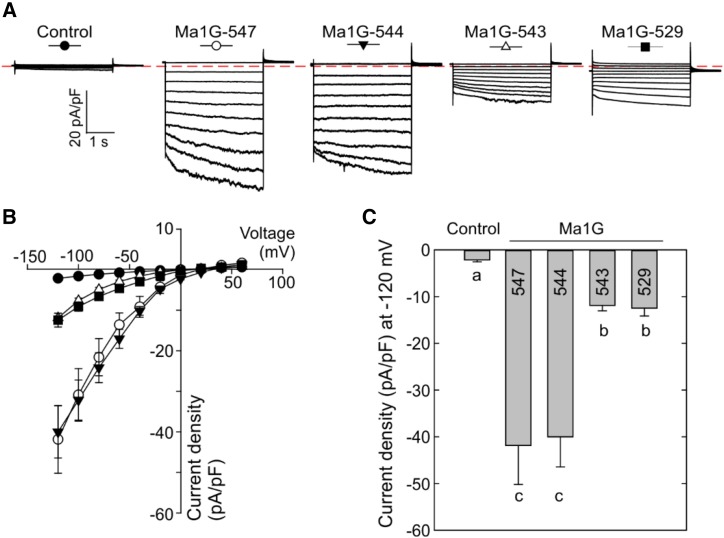
To examine the importance of the conserved C-terminal domain to the function of Ma1G, we generated a series of truncations at its C-terminal end. Initially, we examined three broad truncations that have the entire C-conserved terminal domain (Ma1G-502) or half of the domain (Ma1G-529) removed or the entire domain retained (Ma1G-547; Fig. 7A). Transient expression in N. benthamiana showed that Ma1G-547 had a widespread GFP signal similar to that of the full-length Ma1G, but both Ma1G-502 and Ma1G-529 had more restrictive GFP signals similar to those of Ma1A and ma1 (Fig. 7B). We then used the patch-clamp technique to assess the transport activity of these truncated proteins. Patch-clamp analysis demonstrated that Ma1G-547 had normal malate transport activity compared with Ma1G, but Ma1G-529, similar to ma1, had significantly lower transport activity (Fig. 8). These results indicate that the conserved C-terminal domain contains motifs required for the normal function of Ma1G. We then proceeded to generate a detailed series of C-domain truncations from Ma1G-529 to Ma1G-547 at an interval of three amino acids (Ma1G-532, Ma1G-535, Ma1G-538, Ma1G-541, and Ma1G-544) in an attempt to establish the minimum C domain required for normal Ma1G transport activity (Fig. 7). By using the distribution of GFP signal in transiently expressed N. benthamiana leaves as a proxy for GFP fusion protein function, we found widespread GFP signal for Ma1G-544-GFP, implying normal function for Ma1G-544, whereas the more restricted GFP signal for Ma1G-541-GFP suggests a significantly reduced function for Ma1G-541 (Fig. 7B). Further truncations from Ma1G-541 to Ma1G-544 at an interval of single amino acids showed that both Ma1G-542 and Ma1G-543 had more restricted GFP signals similar to that of ma1 (Fig. 7B). Subsequent patch-clamp analysis of Ma1G-544 and Ma1G-543 confirmed that Ma1G-544 has normal malate transport activity whereas Ma1G-543 has significantly reduced transport activity similar to that of Ma1G-529 (Fig. 8). These findings clearly indicate that the conserved C-terminal domain required for normal Ma1G transport activity ends at amino acid 544 and that removing one more residue from Ma1G-544 leads to strong reduction in Ma1G transport activity (Fig. 8).
Figure 7.
Expression of Ma1G C-terminal truncation mutants in N. benthamiana leaves. A, Sequence alignment of tonoplast-localized ALMT members, including Ma1 and ma1. The arrows above the alignment indicate the residue positions of the various experimental truncation sites tested. B, The expression of Ma1G-GFP, ma1-GFP, and various Ma1G C-terminal truncation mutants with a C-terminal fused GFP in the A. tumefaciens-infiltrated N. benthamiana leaves. Bar = 100 µm.
Figure 8.
Patch-clamp analysis of the malate transport activity of Ma1G C-terminal truncation mutants. A, Representative recordings of whole-vacuole currents in control vacuoles (expressing the empty vector) and vacuoles from cells expressing Ma1G-547, Ma1G-544, Ma1G-543, or Ma1G-529. Currents across the tonoplast were evoked in response to 3-s voltage pulses ranging from +60 to −120 mV in 20-mV steps. The zero-current level is indicated by the red dotted line. B, Mean current-voltage relationships from whole-vacuole currents constructed from steady-state current recordings as shown in A for control vacuoles (n = 10) and vacuoles from cells expressing Ma1G-547 (n = 8), Ma1G-544 (n = 10), Ma1G-543 (n = 8), or Ma1G-529 (n = 8). Data are means ± se. The symbol for each curve corresponds to that depicted at the top of each set of traces in A. C, Bar graphs representing the current densities at −120 mV obtained by the patch clamping of control vacuoles and vacuoles from cells expressing Ma1G-547, Ma1G-544, Ma1G-543, or Ma1G-529. Data are means ± se. Different letters indicate significant differences between groups using Tukey’s HSD test at P < 0.05 after ANOVA.
DISCUSSION
Malate is not only a key metabolite involved in glycolysis and the Krebs cycle, but its accumulation in the vacuole also largely determines the acidity of many fleshy fruits. Our earlier work showed that a mutation at base 1,455 in the Ma1 open reading frame leads to a premature stop codon that truncates the protein by 84 amino acids at its C-terminal end, and this truncation is strongly associated with low fruit acidity in apple (Bai et al., 2012). In this study, we have shown that the truncated protein, ma1, has significantly lower malate transport activity than the full-length protein, Ma1, and that this truncation is genetically responsible for low acidity in apple fruit across a large number of Malus accessions from a diverse genetic background. Furthermore, we have identified and characterized a conserved C-terminal domain that is essential for the malate transport activity of the Ma1 protein.
Truncation of Ma1 to ma1 Significantly Reduces Malate Transport Activity
Like several other members in clade II of the ALMT family, Ma1 is localized to the tonoplast (Fig. 2B; Supplemental Fig. S2B), consistent with its proposed role in mediating malate transport into the vacuole. Truncation caused by the premature stop codon did not alter the subcellular localization of the protein to the tonoplast (Fig. 2B; Supplemental Fig. S2B). These observations contrast with an earlier report suggesting that the truncation of Ma1 to ma1 results in a change in its subcellular localization from the tonoplast to the PM (Ma et al., 2015). However, the localization obtained in that earlier report was conducted via transient expression in onion (Allium cepa) epidermal cells without isolating protoplasts or releasing the vacuoles, and therefore it is considered less conclusive.
The GFP signal of Ma1A and ma1 fusion proteins in N. benthamiana was much more restricted in distribution than that of the full-length protein Ma1G (Fig. 2A). This pattern does not appear to be related to the fusion of GFP at the C-terminal end, as GFP fused at the N-terminal end of Ma1A and ma1 showed a similar restricted distribution (Supplemental Fig. S2A). The exact reason for this more restricted distribution is unclear, but most likely the lack of the C-terminal domain may have affected the targeting of the protein, or its stability, thereby increasing its turnover and/or degradation. However, truncation of Ma1 to ma1 in apple does not seem to affect the protein level, as different apple genotypes (Ma1Ma1, Ma1ma1, and ma1ma1) have similar protein expression at the peak malate level during fruit development (5 weeks after bloom) and at maturity (Supplemental Fig. S6).
Characterization of Ma1G, Ma1A, and ma1 in both X. laevis oocytes and N. benthamiana strongly supports that the truncation of Ma1 to ma1 leads to significant reduction in its malate transport activity. Electrophysiological analysis of Ma1G-, Ma1A-, and ma1-expressing X. laevis oocytes loaded with malate clearly shows that Ma1G is an inward-rectifying channel/transporter that mediates anion efflux from oocytes whereas Ma1A and ma1 are functionally impaired (Fig. 1). Consistent with the result obtained in oocytes, patch-clamp analysis showed that both Ma1A- and ma1-expressing N. benthamiana vacuoles have significantly lower transmembrane currents than those expressing Ma1G (Fig. 3). Although the GFP signal distribution and abundance in N. benthamiana leaves expressing Ma1G are different from those observed for Ma1A and ma1, the vacuoles used for the patch-clamp recordings had very similar GFP signal intensity. Therefore, it is unlikely that the variation in GFP fusion protein expression level is a contributing factor to the functional difference observed between Ma1G, Ma1A, and ma1. As the only difference between Ma1G and Ma1A is the truncation caused by the mutation of G-to-A at base 1,455 and no difference in function was detected between ma1 and Ma1A, the functional difference between Ma1G and ma1 is attributed to the truncation (i.e. Ma1 is a fully functional malate channel whereas ma1 functionality is hindered). This observation is consistent with an earlier finding that the excised fruit cortex tissue of a low-acid apple genotype (ma1ma1) took up less [14C]malate than that of a high-acid genotype (Ma1ma1; Berüter, 2004).
A Conserved C-Terminal Domain Is Essential for Ma1 Transport Activity
As for all other members of the ALMT family, secondary structure predictions suggest that the N-terminal half of the Ma1 protein assembles as a hydrophobic core consisting of six transmembrane domains followed by a mostly hydrophilic C-terminal half (Dreyer et al., 2012). This C-terminal half may contain one or two (depending on the algorithm) clusters of highly conserved hydrophobic residues. One such C-terminal hydrophobic cluster coincides with the conserved C-terminal domain identified in Ma1. A phylogenetic analysis of Ma1 along with Arabidopsis and other functionally characterized ALMTs, in combination with functional analysis via patch clamp, allowed us to perform a detailed functional-structural analysis of this conserved C-terminal domain (Figs. 6–8). We established that, although the terminal 24 amino acids do not appear to play a significant role in the functional integrity of Ma1, the highly conserved C-terminal domain is essential for its integrity, as increasing truncations lead to a reduction in malate transport activity to a level similar to that found in ma1. Interestingly, the importance of this domain has also been established for other ALMTs: truncations at residue 430 in the wheat TaALMT1 and 533 in the Arabidopsis AtALMT12 (with these residue positions being equivalent to residues 542 and 544 in the Ma1 protein according to the alignment shown in Fig. 6B) resulted in loss of the transport function when either of these two PM ALMTs was expressed in X. laevis oocytes (Ligaba et al., 2013; Mumm et al., 2013). Therefore, although this C-terminal domain appears to show a higher degree of conservation among ALMTs from clade II that contains the tonoplast-localized ALMTs, it is likely that the overall conservation of key residues (e.g. Lys-544, Phe-543, and Leu-542) across all clades is the driving force underlying the functional importance. The similarity in loss of function in truncations around this region most likely results from alterations in the overall structural integrity of ALMT family proteins rather than modifications of sites specific to a given clade. Our study also unveiled that the disruption of this putative membrane-anchoring domain (given its hydrophobic nature) in the Ma1 truncated proteins may alter the ALMT tertiary structure, as suggested by the reduced expression efficiency, at least in N. benthamiana cells. Therefore, we speculate that the disruption to this putative transmembrane domain may also compromise the proteins’ structure (i.e. folding), stability, trafficking, oligomerization, degradation, and/or turnover, resulting in a significant reduction in overall Ma1 transport activity (as suggested by the patch-clamp recordings from isolated vacuoles), rather than complete loss of function by the Ma1 protein subunit (as the X. laevis oocyte recordings would suggest). Clearly, understanding the exact role of the conserved C-terminal domain in the structure of the ALMT family warrants further work.
Premature Stop Codon-Led Mutation Is Genetically Responsible for Low Acidity in Apple
Genotyping and phenotyping of 186 apple accessions from a diverse genetic background of 17 Malus species clearly demonstrate that ma1ma1 genotypes have the lowest fruit acidity whereas Ma1Ma1 genotypes have the highest fruit acidity among all three genotypes, with Ma1ma1 genotypes being in the middle (Fig. 5). These data confirm the relationship between Ma1 genotypes and fruit acidity and the incomplete dominance of Ma1 over ma1 reported earlier (Bai et al., 2012; Xu et al., 2012) and from subsequent investigations by other groups on different segregating populations (Khan et al., 2013; Jia et al., 2018; Verma et al., 2019) as well as over a wide range of Malus accessions (Ma et al., 2015). In addition, RNAi suppression of Ma1 expression in ‘McIntosh’ apple leaves, ‘Empire’ apple fruit, and ‘Orin’ calli significantly decreased the malate level (Fig. 4; Supplemental Fig. S4). Taken together with the functional analyses of Ma1 and ma1 in both X. laevis oocytes and N. benthamiana, these findings provide strong evidence that Ma1 plays an important role in determining malate accumulation in apple and that the premature stop codon-led truncation of Ma1 to ma1 is genetically responsible for low fruit acidity in apple.
All 25 ma1ma1 genotype accessions had low fruit acidity with a narrow range (0.7–3.3 mg mL−1) in this study (Fig. 5A; Supplemental Table S1). Considering that these accessions span over five Malus species including both domesticated and wild apples and ma1 has very low malate transport activity (Figs. 1 and 3), the data strongly argue in favor of ma1 being the genetic cause for low acidity in apples with the ma1ma1 genotype. The only exception reported in the literature is ‘Belle de Boskoop’ apple, which has the ma1ma1 genotype but has a relatively high fruit acidity level (Ma et al., 2015). The high acidity appears to be related to a 10-fold higher expression of a gene encoding a tonoplast P3A H+‐ATPase that facilitates vacuolar acidification in the fruit (Ma et al., 2019). However, it should be noted that in the U.S. Department of Agriculture Malus repository in Geneva, New York, accession PI 300258 has the same name but is of the Ma1ma1 genotype based on marker CAPS1455. This suggests that, unless incorrect genotyping has occurred, the ‘Belle de Boskoop’ used in the study (Ma et al., 2019) is different from PI 300258.
Much larger variations in fruit acidity detected in the Ma1ma1 and Ma1Ma1 genotypes in this study could have resulted from several factors. First, within the same genotype of Ma1ma1 or Ma1Ma1, the expression level of Ma1 could be different (Bai et al., 2012). Also, single-nucleotide polymorphisms in addition to G/A substitutions at base 1,455 exist in the Ma1 allele, and these single-nucleotide polymorphisms could modify the function of Ma1, thereby altering its capacity for malate transport into the vacuole. Second, other genes have been reported to contribute to apple fruit acidity by modulating the expression or activity of Ma1 or tonoplast dicarboxylate transporter (tDT) and/or the primary vacuolar proton pumps. Two MYB transcription factors, MYB1 and MYB73, regulate the expression of tonoplast H+-ATPase, H+-PPase, tDT, and Ma1 in altering malate accumulation and vacuolar pH in apple (Hu et al., 2016, 2017). MYB44 expression also correlates with apple fruit acidity (Jia et al., 2018). In addition, a protein phosphatase2C (PP2C), PP2CH, inactivates three H+-ATPases and Ma1 via dephosphorylation, thereby reducing fruit malate accumulation, whereas a small auxin up-regulated RNA (SAUR) gene-encoded protein, SAUR37, interacts with PP2CH to suppress its activity, enhancing malate accumulation in apple (Jia et al., 2018). Third, a QTL originally identified in the cross ‘Fiesta’ × ‘Discovery’ on linkage group 8 was recently confirmed in pedigree-connected germplasm of 16 full-sib families representing nine important breeding parents. This QTL was named Ma3 and also plays a significant role in determining malate accumulation in apple (Liebhard et al., 2003; Verma et al., 2019). Ma3 and Ma1 follow an additive allele dosage model and jointly explain about 66% of the variation in apple fruit acidity (Verma et al., 2019). Finally, even for the same Malus accession, both variations in environmental factors and fruit maturity from year to year are expected to affect the malate level at harvest (Etienne et al., 2013; Verma et al., 2019). It is obvious that apple fruit acidity is under a complex genetic control involving many genes that interact with environmental factors. Nevertheless, as indicated by our data, Ma1 is a major player in this network.
CONCLUSION
Apple ALMT9, both its full-length protein Ma1 and its naturally truncated protein ma1, localizes to the tonoplast. Functional analyses of both proteins in oocytes, N. benthamiana, and apple, combined with genotyping and phenotyping of a large number of Malus accessions from a diverse genetic background, indicate that ma1 has significantly lower transport activity for malate than Ma1 across the tonoplast and that truncation of Ma1 to ma1 is genetically responsible for low fruit acidity in apple. A highly conserved C-terminal domain in the ALMTs is essential for the normal function of Ma1, and any truncation (naturally occurring or artificial) into this conserved domain leads to significant reduction in its malate transport activity.
MATERIALS AND METHODS
Plants and Growth Conditions
Full-length cDNAs of two naturally occurring Ma alleles, Ma1 and ma1, were cloned from ‘Usterapfel’ high-acid genotype (Ma1ma1) and ‘Brite Gold’ (ma1ma1) apple (Malus domestica), respectively, using PCR (primers are listed in Supplemental Table S2). ‘Usterapfel’ high-acid genotype trees were grown at Cornell Orchards on the Cornell campus in Ithaca, New York, and ‘Brite Gold’ apple trees were grown in the National Malus Germplasm Repository at Geneva, New York. The 186 Malus accessions from 17 species, including 123 M. domestica, 37 Malus hybrid, and 26 others for genotyping and phenotyping, were also grown in the National Malus Germplasm Repository (Supplemental Table S1). All these trees received standard horticulture and disease/pest control.
Nicotiana benthamiana plants for subcellular localization of Ma1 and ma1 and agroinfiltration were grown in Cornell Mix medium at one plant per pot (10 cm × 10 cm × 10 cm) in a controlled growth chamber at 24°C with 40% to 65% relative humidity under a 16-h photoperiod.
‘McIntosh’ apple in vitro shoot cultures and Ma1 RNAi lines were grown on Murashige and Skoog (MS) medium with 30 g L−1 Suc, 0.3 mg L−1 6-benzylaminopurine (6-BA), and 0.1 mg L−1 indole-3-butyric acid under cool-white fluorescent light (∼50 μmol m−2 s−1) in a 16-h photoperiod at 23°C.
Calli derived from ‘Orin’ apple used for genetic transformation and malate content assays were cultured on MS medium with 30 g L−1 Suc, 1.5 mg L−1 6-BA, and 0.5 mg L−1 indole-3-acetic acid at 25°C in the dark.
Transient Expression of Ma1G, Ma1A, and ma1 in Xenopus laevis Oocytes
The coding sequences of Ma1G, Ma1A, and ma1 were amplified and cloned into X. laevis oocyte expression vectors (with and without N-terminal YFP) by an advanced uracil excision-based cloning technique described previously by Nour-Eldin et al. (2006). cRNA was synthesized using the mMessage mMachine in vitro transcription kit following the manufacturer’s guidelines. For expression in oocytes, stage V and VI X. laevis oocytes were harvested and kept in ND96 solution (96 mm NaCl, 2 mm KCl, 1.8 mm CaCl2, 1 mm MgCl2, 2.5 mm sodium pyruvate, 5 mm HEPES, pH 7.5, 50 mg mL−1 gentamycin, and 0.4 g L−1 BSA). Oocytes were defolliculated by collagenase treatment in ND96 without CaCl2, gentamycin, and BSA and kept overnight at 18°C in complete ND96 solution. All animal procedures were performed in accordance with Cornell University IACUC Protocol number 2017-0139. Fifty nanoliters of cRNA of the desired construct was injected into each oocyte, and the injected oocytes were given 2 to 4 d for protein expression.
Transient Expression of Ma1 Genes in N. benthamiana
For transient overexpression of Ma1 genes with a C-terminal GFP or N-terminal GFP fusion protein, cDNAs were cloned into pGWB551 or pB7WGF2.0 vector via the Gateway recombination system (Invitrogen). The Agrobacterium tumefaciens-mediated infiltration of N. benthamiana leaves was conducted with slight modifications to that previously described (Yang et al., 2001). A. tumefaciens strain GV3101 carrying the different constructs was grown overnight in yeast extract peptone medium and transferred to one-half-strength MS liquid medium containing 50 µm acetosyringone for another 4 h until reaching an OD600 value of 0.4 to 0.6. The culture was then diluted 1:1 with 10 mm MES (pH 5.6), 10 mm MgCl2, and 150 µm acetosyringone and pressure infiltrated into the leaves of 4- to 5-week old N. benthamiana plants. After the infiltration, the plants were grown in a growth chamber at 24°C under 16 h of light/8 h of dark. The transformed leaves were used to extract protoplasts and vacuoles for confocal microscopy and patch-clamp experiments after 2 to 3 d.
Cellular Localization of Ma1G, Ma1A, and ma1
For YFP chimeras expressed in X. laevis oocytes, the YFP signal was detected on a confocal laser-scanning microscope (SP5; Leica) with PM stain (CellMask Plasma Membrane Stains, Deep Red C10064; Thermo Fisher Scientific) used as a marker for colocalization of the proteins in membrane. YFP and Deep Red were excited at 514 and 649 nm, and their emission signals were detected at 520 to 540 nm and 650 to 700 nm, respectively.
To detect the GFP signal of the fusion proteins in N. benthamiana leaves and determine the subcellular localization of Ma1G, Ma1A, and ma1, we isolated protoplasts and vacuoles of N. benthamiana leaves overexpressing these genes as described (Song et al., 2003) with minor modifications. N. benthamiana leaves were gently scratched on the abaxial side and floated in the enzymatic solution (0.3% [w/v] cellulose R-10, 0.03% [w/v] pectolyase Y-23, 1 mm CaCl2, 500 mm sorbitol, and 10 mm MES, pH 5.3) for 40 to 50 min at 25°C with shaking (60 rpm). The protoplasts were collected by centrifugation at 400g for 5 min, washed twice, and then resuspended in basic solution without any enzyme. Upon exposure to hypotonic medium (10 mm EGTA and 10 mm HEPES/Tris, pH 7.4, adjusted to 200 mOsmol kg−1 with sorbitol), protoplasts burst spontaneously to release vacuoles. The GFP signal and chlorophyll autofluorescence were examined using a confocal laser-scanning microscope (LSM 710; Carl Zeiss) at an excitation wavelength of 488 nm, and the emission signal was detected from 500 to 530 nm for GFP signal and 650 to 750 nm for chlorophyll autofluorescence signal.
Electrophysiological Recordings
Two-Electrode Voltage Analysis of X. laevis Oocyte Cells
Recordings were performed both in oocyte cells that were nonloaded or loaded with malate, with the latter being achieved by microinjecting cells with 50 nL of 100 mm sodium malate 2 to 3 h prior to the recordings. For substrate selectivity studies, sodium malate was replaced with equimolar amounts of fumarate or citrate. Whole-cell currents were recorded using a GeneClamp 500 amplifier (Axon Instruments) via the TEVC technique as previously described (Piñeros et al., 2008). Recordings were performed under constant perfusion in ND96 solution consisting of 96 mm NaCl, 1 mm KCl, 1.8 mm CaCl2, pH 7.5, with 5 mm HEPES/NaOH. Currents were elicited by voltage pulses stepped between +40 and −180 mV in 20-mV increments, with a 6-s rest at −40 mV between each test voltage. Steady-state current-voltage relationships were constructed by measuring the current amplitude at the end of the test pulse.
Patch Clamp of Isolated Vacuoles
Whole-vacuole malate currents were recorded using an Axopatch-200B amplifier (Axon Instruments) following a standard patch-clamp procedure as described elsewhere (Hamill et al., 1981; Kovermann et al., 2007). Patch pipettes were prepared from borosilicate glass capillaries with a flaming/brown micropipette puller (model P-87; Sutter Instrument) and polished using microforge CPM2 (Scientific Instruments). The bath chamber was fixed on an inverted fluorescence microscope (Axiovert 100; Zeiss). The standard bath solution contained 100 mm malic acid, 1 mm CaCl2, 1 mm EDTA, and 3 mm MgCl2 with pH adjusted to 7.5 using 1,3-bis[tris(hydroxymethyl)methylamino] propane. The pipette solution contained 10 mm malic acid, 1 mm CaCl2, and 3 mm MgCl2 with pH adjusted to 5.5 with 1,3-bis[tris(hydroxymethyl)methylamino] propane. The osmotic pressure of all solutions was adjusted to 500 mOsmol with d-sorbitol. The small bath chamber volume (less than 0.5 mL) allowed for rapid solution exchange, and vacuoles were released in the bath chamber upon exposure to hypotonic medium (10 mm EGTA and 10 mm HEPES/Tris at pH 7.4, adjusted to 200 mOsmol kg−1 with sorbitol). Three to 5 min later, the hypotonic medium was replaced with the bath recording solution and vacuoles with a diameter of 25 to 40 µm were selected for whole-cell patch-clamp experiment. Currents across the tonoplast were evoked in response to 3-s voltage pulses ranging from +60 to −120 mV in 20-mV steps, with a 2-s rest at 60 mV between each test voltage. Steady-state current-voltage relationships were constructed by measuring the current amplitude at the end of the test pulse Whole-vacuole series resistance and capacitance were partially compensated for by the amplifier. pCLAMP software (version 10.4; Axon Instruments) was used to acquire and analyze both TEVC and patch-clamp recorded currents. SigmaPlot 11.0 software (Systat Software) was used to draw current density voltage plots and analyze the data.
Phylogenetic Analysis
Phylogenetic analysis of protein sequences of Ma1G and ma1 was conducted along with the Arabidopsis (Arabidopsis thaliana) ALMT family members and TaALMT1, ZmALMT1, ZmALMT2, HvALMT1, and ALMT9 members from other species. The sequences were downloaded from The Arabidopsis Information Resource (https://www.arabidopsis.org/) and the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/). Then the phylogenetic tree was generated by the neighbor-joining method using Geneious Tree Builder (https://www.geneious.com/).
DNA and RNA Isolation
DNA was isolated from leaf tissues using a cetyl-trimethyl-ammonium bromide-based extraction protocol (Cullings, 1992; Allen et al., 2006). The genotypes of Ma in the 186 Malus accessions were determined using marker CAPS1455, which detects the premature stop codon mutation in ma1 (Bai et al., 2012; Supplemental Table S1).
Total RNA was extracted from apple leaves and calli using the modified cetyl-trimethyl-ammonium bromide method as described previously (Gasic et al., 2004). Two micrograms of total isolated RNA was reverse transcribed to cDNA using the iScript cDNA Synthesis Kit (Bio-Rad) after removing DNA with DNase I enzyme (Thermo Fisher Scientific). Then the cDNA was used for RT-qPCR analysis, which was performed with iQ SYBR Green Supermix in an iCycler iQ5 system (Bio-Rad) following the manufacturer’s instructions in triplicate using MdACTIN as an internal reference gene. The relative expression of each gene was calculated using the 2−ΔΔCT method (Livak and Schmittgen, 2001). All of the primers used in this study are listed in Supplemental Table S2.
Transformation of ‘McIntosh’ Apple Plants and ‘Orin’ Apple Calli with an RNAi Construct of Ma1
A 334-bp cDNA fragment specific to Ma1 was cloned into the pHannibal vector in both sense and antisense orientations using XhoI/KpnI and BamHI/XbaI restriction sites, respectively, and the entire cassette was excised and ligated into pCambia 2300 using SacI and PstI restriction sites. The resulting pCambia-Ma1RNAi construct was transformed into A. tumefaciens strain EHA105 with an additional virulence plasmid, pCH32 (Hood et al., 1993). Transformation of ‘McIntosh’ apple was conducted following the protocol described for ‘Royal Gala’ (Norelli et al., 1994; Hrazdina and Borejsza-Wysocki, 2003) as modified by Bolar et al. (1998). Briefly, ‘McIntosh’ shoots were subcultured on proliferation medium (Murashige and Skoog, 1962) at 4-week intervals. The youngest unfolded leaf was selected from in vitro shoots, and the entire leaf surface was wounded using a pair of nontraumatic forceps (Norelli et al., 1996) and cocultivated with the EHA105 (pCH32) harboring the pCambia-Ma1RNAi. Following cocultivation, the leaves were washed with distilled water containing one-half-strength MS salt mixture and cefotaxim sodium (500 mg L−1) to remove excess inoculum and placed on regeneration medium containing 25 mg L−1 kanamycin (Bolar et al., 1998). The regenerated shoots were propagated in vitro, and the expression of Ma1 was determined by RT-qPCR using RNA extracted from each line.
Transformation of ‘Orin’ apple calli with the pCambia-Ma1RNAi followed the protocol of An et al. (2012) with slight modifications. Both pCambia-Ma1RNAi and empty vector were transformed into A. tumefaciens strain GV3101 and screened with PCR. Three-week-old ‘Orin’ apple calli were incubated with the A. tumefaciens in liquid medium (MS + 1 mg L−1 2,4-dichlorophenoxyacetic acid [2,4-D] + 1 mg L−1 6-BA + 30 g L−1 Suc, pH 5.8–6) with shaking at 140 rpm at 25°C for 15 to 20 min. The calli were then filtered through a nylon mesh and cocultured on solid MS medium (MS + 1 mg L−1 2,4-D + 1 mg L−1 6-BA + 30 g L−1 Suc + 8 g L−1 agarose, pH 5.8–6) at 25°C. After 2 to 4 d, the transgenic calli were washed three times with sterile water and plated out on selection medium (MS + 1 mg L−1 2,4-D + 1 mg L−1 6-BA + 30 g L−1 Suc + 8 g L−1 agarose + 30 mg L−1 kanamycin + 250 mg L−1 cefotaxime, pH 5.8–6). The transformed calli were transferred onto a new selection medium after 20 to 30 d, and the established calli were confirmed via PCR. The selected transgenic calli were cultured for 3 weeks, and samples were taken for determining Ma1 transcript levels and malate levels. All primers used are listed in Supplemental Table S2.
Construction of an RNAi Viral Vector of Ma1 and Agroinfiltration of Apple Fruit
To generate a transient antisense expression vector for Ma1, a 200-bp fragment of the Ma1 coding sequence was cloned into the TRV vector in an antisense orientation under the control of the dual 35S promoter (Ratcliff et al., 2001; Hu et al., 2016). The construct TRV-Ma1 and helper vector TRV1 were transformed into A. tumefaciens strain GV3101. Positive A. tumefaciens clones were selected and cultured overnight in yeast extract peptone liquid medium. Subsequently, the agrobacteria were collected under 6,000 rpm centrifugation, resuspended in 10 mm MgCl2, 10 mm MES, and 150 mm acetosyringone, and then kept at room temperature for another 2 h. For TRV infections, separate A. tumefaciens cultures containing helper vector TRV1 and TRV-MdMa1 were mixed in a 1:3 ratio, and 200 μL of the mixture was infiltrated into each site of mature ‘Empire’ apple fruit using a 1-mL needleless syringe. After infiltration, fruits were kept at room temperature in the dark overnight and then moved into a 16°C incubator with lights. Each treatment (TRV-Ma1 and empty vector control) was replicated five times with two fruits per replicate in a completely randomized design. The injection regions of the fruit (three sites per fruit) were excised 2 weeks later, frozen in liquid nitrogen, and stored at −80°C for gene expression and malic acid analysis. Primers used for the TRV-Ma1 construct are listed in Supplemental Table S2.
Fruit Acidity and Malic Acid Measurements
Fruit TA was measured at harvest in three consecutive growing seasons from 2013 to 2015. To ensure similar maturity stage across samples, fruit were sliced into halves along the equator and then stained with an iodine and potassium iodine solution (I2 2.2 g and KI 8.8 g in 1 L) to select those reaching Cornell Starch Index 4.0 to 6.0 (Blanpied and Silsby, 1992). At least five to 10 such fruits were pooled and used for each accession. Fruit juice TA was quantified with an autotitrator (Metrohm 848 Titrino Plus and Metrohm 869 Compact Sample Changer).
To measure malate in Ma1G RNAi transgenic ‘McIntosh’ apple leaves, ‘Empire’ fruit, and ‘Orin’ apple calli, polar metabolites were extracted from 100 mg of frozen leaf or callus tissue in 75% (v/v) methanol with ribitol (0.12 mg per sample) added as an internal standard. Ten microliters of the aqueous phase was dried under vacuum without heat after fractionation of nonpolar metabolites into chloroform. The dried sample was derivatized with methoxyamine hydrochloride and N-methyl-N-trimethylsilyl-trifluoroacetamide sequentially for analysis on an Agilent 7890A GC/5975C MS device (Agilent Technology) as previously described (Wang et al., 2010).
Protein Extraction and Immunoblot Analysis
Proteins were extracted from fruit taken at 5 weeks after bloom and at maturity and quantified as described by Zheng et al. (2007). For immunoblot analysis, the proteins were incubated first with the primary antibody (Ma antibody, generated against peptide SESWGPAVRPKEYED in rabbit by Genescript) after 2,000-fold dilution with blocking buffer in Tris-buffered saline (SuperBlock, 37535; Thermo Fisher Scientific) and then with goat anti-rabbit secondary antibody (alkaline phosphatase conjugated, A3687; Sigma) after 5,000-fold dilution. The 1-Step NBT/BCIP substrate solution (34042; Thermo Fisher Scientific) was then added to the blot for 5 to 15 min until desired color developed. A Coomassie Brilliant Blue R-250 solution (161-0463; Bio-Rad)-stained SDS-PAGE gel was used to confirm equal sample loading.
Statistical Analysis
ANOVA followed by Tukey’s HSD test was conducted using software JMP Pro 12 (SAS) and Sigmaplot 11.0 (Systat Software).
Accession Numbers
Accession numbers are as follows. For ALMT members in Arabidopsis (https://www.arabidopsis.org/): AtALMT1 (AT1G08430), AtALMT2 (AT1G08440), AtALMT3 (AT1G18420), AtALMT4 (AT1G25480), AtALMT5 (AT1G68660), AtALMT6 (AT2G17470), AtALMT7 (AT2G27240), AtALMT8 (AT3G11680), AtALMT9 (AT3G18440), AtALMT10 (AT4G00910), AtALMT12 (AT4G17970), AtALMT13 (AT5G46600), and AtALMT14 (AT5G46610). For apple genes (https://www.rosaceae.org/): MdMa1 (MDP0000252114) and MdACTIN (MDP0000912745). For tomato genes (https://www.solgenomics.net/): SlALMT4 (solyc03g096820) and SlALMT9 (solyc06g072910). Others are ZmALMT1 (gene identifier 100037822), ZmALMT2 (gene identifier 3760032), TaALMT1 (gene identifier 543388), and HvALMT1 (gene identifier 100101500).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Anion selectivity of Ma1G.
Supplemental Figure S2. Subcellular localization of Ma1G, Ma1A, and ma1 chimeras with the GFP protein fused to the N terminus.
Supplemental Figure S3. Sugar levels in the leaves of Ma1 RNAi transgenic lines of ‘McIntosh’ apple and fruit of ‘Empire’ apple with virus-induced silencing of Ma1.
Supplemental Figure S4. Ma1 transcript level and malate concentration in ‘Orin’ apple calli in response to RNAi suppression of Ma1 expression.
Supplemental Figure S5. Fruit pH and its relationship with titratable acidity in 186 Malus accessions.
Supplemental Figure S6. The Ma1 protein level detected in the fruit of different apple genotypes (mama, MaMa, and Mama) at 5 weeks after bloom (35 d after bloom), peak malate level) and at maturity.
Supplemental Table S1. The genotype data from 186 Malus germplasm accessions.
Supplemental Table S2. List of primers used in this article.
Acknowledgments
We thank Dr. Markus Kellerhals and Dr. Josef Berüter at the Swiss Federal Research Station for Horticulture for the high-acid and low-acid ‘Usterapfel’ apples, Dr. Takaya Moriguchi of the National Institute of Fruit Tree Science in Japan for providing ‘Orin’ apple calli, and Dr. Robert Turgeon and Dr. Dagang Hu at Cornell University for critical reading of the article.
Footnotes
This work was supported in part by the USDA NIFA Agriculture and Food Research Initiative (grant 2014-67013-21660 to K.X., L.C., and M.A.P.).
Articles can be viewed without a subscription.
References
- Allen GC, Flores-Vergara MA, Krasynanski S, Kumar S, Thompson WF (2006) A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat Protoc 1: 2320–2325 [DOI] [PubMed] [Google Scholar]
- An XH, Tian Y, Chen KQ, Wang XF, Hao YJ (2012) The apple WD40 protein MdTTG1 interacts with bHLH but not MYB proteins to regulate anthocyanin accumulation. J Plant Physiol 169: 710–717 [DOI] [PubMed] [Google Scholar]
- Bai Y, Dougherty L, Li M, Fazio G, Cheng L, Xu K (2012) A natural mutation-led truncation in one of the two aluminum-activated malate transporter-like genes at the Ma locus is associated with low fruit acidity in apple. Mol Genet Genomics 287: 663–678 [DOI] [PubMed] [Google Scholar]
- Barbier-Brygoo H, De Angeli A, Filleur S, Frachisse JM, Gambale F, Thomine S, Wege S (2011) Anion channels/transporters in plants: From molecular bases to regulatory networks. Annu Rev Plant Biol 62: 25–51 [DOI] [PubMed] [Google Scholar]
- Berüter J. (2004) Carbohydrate metabolism in two apple genotypes that differ in malate accumulation. J Plant Physiol 161: 1011–1029 [DOI] [PubMed] [Google Scholar]
- Blanpied G, Silsby KJ (1992) Predicting Harvest Date Windows for Apples. Cornell Cooperative Extension, Ithaca, NY [Google Scholar]
- Bolar JP, Brown SK, Norelli JL, Aldwinckle HS (1998) Factors affecting the transformation of ‘Marshall McIntosh’ apple by Agrobacterium tumefaciens. Plant Cell Tiss Org Cult 55: 31–38 [Google Scholar]
- Cullings K. (1992) Design and testing of a plant‐specific PCR primer for ecological and evolutionary studies. Mol Ecol 1: 233–240 [Google Scholar]
- De Angeli A, Baetz U, Francisco R, Zhang J, Chaves MM, Regalado A (2013a) The vacuolar channel VvALMT9 mediates malate and tartrate accumulation in berries of Vitis vinifera. Planta 238: 283–291 [DOI] [PubMed] [Google Scholar]
- De Angeli A, Zhang J, Meyer S, Martinoia E (2013b) AtALMT9 is a malate-activated vacuolar chloride channel required for stomatal opening in Arabidopsis. Nat Commun 4: 1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Craig S, Beaton CD, Bennet RJ, Jagadish VC, Randall PJ (1993a) Aluminum tolerance in wheat (Triticum aestivum L.). I. Uptake and distribution of aluminum in root apices. Plant Physiol 103: 685–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Gruber BD, Ryan PR (2007) The roles of organic anion permeases in aluminium resistance and mineral nutrition. FEBS Lett 581: 2255–2262 [DOI] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Randall PJ (1993b) Aluminum tolerance in wheat (Triticum aestivum L.). II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol 103: 695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer I, Gomez-Porras JL, Riaño-Pachón DM, Hedrich R, Geiger D (2012) Molecular evolution of slow and quick anion channels (SLACs and QUACs/ALMTs). Front Plant Sci 3: 263–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerlich V, Linka N, Reinhold T, Hurth MA, Traub M, Martinoia E, Neuhaus HE (2003) The plant homolog to the human sodium/dicarboxylic cotransporter is the vacuolar malate carrier. Proc Natl Acad Sci USA 100: 11122–11126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne A, Génard M, Lobit P, Mbeguié-A-Mbéguié D, Bugaud C (2013) What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J Exp Bot 64: 1451–1469 [DOI] [PubMed] [Google Scholar]
- Gasic K, Hernandez A, Korban SS (2004) RNA extraction from different apple tissues rich in polyphenols and polysaccharides for cDNA library construction. Plant Mol Biol Rep 22: 437–438 [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391: 85–100 [DOI] [PubMed] [Google Scholar]
- Hoekenga OA, Maron LG, Piñeros MA, Cançado GM, Shaff J, Kobayashi Y, Ryan PR, Dong B, Delhaize E, Sasaki T, et al. (2006) AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc Natl Acad Sci USA 103: 9738–9743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood EE, Gelvin SB, Melchers LS, Hoekema A (1993) New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res 2: 208–218 [Google Scholar]
- Hrazdina G, Borejsza-Wysocki W (2003) Response of scab-susceptible (McIntosh) and scab-resistant (Liberty) apple tissues to treatment with yeast extract and Venturia inaequalis. Phytochemistry 64: 485–492 [DOI] [PubMed] [Google Scholar]
- Hu DG, Li YY, Zhang QY, Li M, Sun CH, Yu JQ, Hao YJ (2017) The R2R3-MYB transcription factor MdMYB73 is involved in malate accumulation and vacuolar acidification in apple. Plant J 91: 443–454 [DOI] [PubMed] [Google Scholar]
- Hu DG, Sun CH, Ma QJ, You CX, Cheng L, Hao YJ (2016) MdMYB1 regulates anthocyanin and malate accumulation by directly facilitating their transport into vacuoles in apples. Plant Physiol 170: 1315–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme A, Wooltorton L (1957) The organic acid metabolism of apple fruits: Changes in individual acids during growth on the tree. J Sci Food Agric 8: 117–122 [Google Scholar]
- Hurth MA, Suh SJ, Kretzschmar T, Geis T, Bregante M, Gambale F, Martinoia E, Neuhaus HE (2005) Impaired pH homeostasis in Arabidopsis lacking the vacuolar dicarboxylate transporter and analysis of carboxylic acid transport across the tonoplast. Plant Physiol 137: 901–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia D, Shen F, Wang Y, Wu T, Xu X, Zhang X, Han Z (2018) Apple fruit acidity is genetically diversified by natural variations in three hierarchical epistatic genes: MdSAUR37, MdPP2CH and MdALMTII. Plant J 95: 427–443 [DOI] [PubMed] [Google Scholar]
- Kenis K, Keulemans J, Davey MW (2008) Identification and stability of QTLs for fruit quality traits in apple. Tree Genet Genomes 4: 647–661 [Google Scholar]
- Khan SA, Beekwilder J, Schaart JG, Mumm R, Soriano JM, Jacobsen E, Schouten HJ (2013) Differences in acidity of apples are probably mainly caused by a malic acid transporter gene on LG16. Tree Genet Genomes 9: 475–487 [Google Scholar]
- Kovermann P, Meyer S, Hörtensteiner S, Picco C, Scholz-Starke J, Ravera S, Lee Y, Martinoia E (2007) The Arabidopsis vacuolar malate channel is a member of the ALMT family. Plant J 52: 1169–1180 [DOI] [PubMed] [Google Scholar]
- Liebhard R, Kellerhals M, Pfammatter W, Jertmini M, Gessler C (2003) Mapping quantitative physiological traits in apple (Malus × domestica Borkh.). Plant Mol Biol 52: 511–526 [DOI] [PubMed] [Google Scholar]
- Ligaba A, Dreyer I, Margaryan A, Schneider DJ, Kochian L, Piñeros M (2013) Functional, structural and phylogenetic analysis of domains underlying the Al sensitivity of the aluminum-activated malate/anion transporter, TaALMT1. Plant J 76: 766–780 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−Δ Δ CT method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Ma B, Liao L, Fang T, Peng Q, Ogutu C, Zhou H, Ma F, Han Y (2019) A Ma10 gene encoding P-type ATPase is involved in fruit organic acid accumulation in apple. Plant Biotechnol J 17: 674–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Liao L, Zheng H, Chen J, Wu B, Ogutu C, Li S, Korban SS, Han Y (2015) Genes encoding aluminum-activated malate transporter II and their association with fruit acidity in apple. Plant Genome 8: 1–14 [DOI] [PubMed] [Google Scholar]
- Maliepaard C, Alston FH, Van Arkel G, Brown LM, Chevreau E, Dunemann F, Evans KM, Gardiner S, Guilford P, Van Heusden AW (1998) Aligning male and female linkage maps of apple (Malus pumila Mill.) using multi-allelic markers. Theor Appl Genet 97: 60–73 [Google Scholar]
- Maruyama H, Sasaki T, Yamamoto Y, Wasaki J (2019) AtALMT3 is involved in malate efflux induced by phosphorus deficiency in Arabidopsis thaliana root hairs. Plant Cell Physiol 60: 107–115 [DOI] [PubMed] [Google Scholar]
- Meyer S, Scholz-Starke J, De Angeli A, Kovermann P, Burla B, Gambale F, Martinoia E (2011) Malate transport by the vacuolar AtALMT6 channel in guard cells is subject to multiple regulation. Plant J 67: 247–257 [DOI] [PubMed] [Google Scholar]
- Mumm P, Imes D, Martinoia E, Al-Rasheid KA, Geiger D, Marten I, Hedrich R (2013) C-terminus-mediated voltage gating of Arabidopsis guard cell anion channel QUAC1. Mol Plant 6: 1550–1563 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Norelli J, Aldwinckle H, Destéfano-Beltrán L, Jaynes J (1994) Transgenic ‘Mailing 26’apple expressing the attacin E gene has increased resistance to Erwinia amylovora. Euphytica 77: 123–128 [Google Scholar]
- Norelli J, Mills J, Aldwinckle H (1996) Leaf wounding increases efficiency of Agrobacterium-mediated transformation of apple. HortScience 31: 1026–1027 [Google Scholar]
- Nour-Eldin HH, Hansen BG, Nørholm MH, Jensen JK, Halkier BA (2006) Advancing uracil-excision based cloning towards an ideal technique for cloning PCR fragments. Nucleic Acids Res 34: e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AJ, Baker A, Muench SP (2016) The varied functions of aluminium-activated malate transporters: Much more than aluminium resistance. Biochem Soc Trans 44: 856–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeros MA, Cançado GM, Maron LG, Lyi SM, Menossi M, Kochian LV (2008) Not all ALMT1-type transporters mediate aluminum-activated organic acid responses: The case of ZmALMT1—an anion-selective transporter. Plant J 53: 352–367 [DOI] [PubMed] [Google Scholar]
- Ramesh SA, Kamran M, Sullivan W, Chirkova L, Okamoto M, Degryse F, McLaughlin M, Gilliham M, Tyerman SD (2018) Aluminum-activated malate transporters can facilitate GABA transport. Plant Cell 30: 1147–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh SA, Tyerman SD, Xu B, Bose J, Kaur S, Conn V, Domingos P, Ullah S, Wege S, Shabala S, et al. (2015) GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat Commun 6: 7879–7888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff F, Martin-Hernandez AM, Baulcombe DC (2001) Technical Advance. Tobacco rattle virus as a vector for analysis of gene function by silencing. Plant J 25: 237–245 [DOI] [PubMed] [Google Scholar]
- Sasaki T, Tsuchiya Y, Ariyoshi M, Nakano R, Ushijima K, Kubo Y, Mori IC, Higashiizumi E, Galis I, Yamamoto Y (2016) Two members of the aluminum-activated malate transporter family, SlALMT4 and SlALMT5, are expressed during fruit development, and the overexpression of SlALMT5 alters organic acid contents in seeds in tomato (Solanum lycopersicum). Plant Cell Physiol 57: 2367–2379 [DOI] [PubMed] [Google Scholar]
- Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H (2004) A wheat gene encoding an aluminum-activated malate transporter. Plant J 37: 645–653 [DOI] [PubMed] [Google Scholar]
- Schumacher K, Krebs M (2010) The V-ATPase: Small cargo, large effects. Curr Opin Plant Biol 13: 724–730 [DOI] [PubMed] [Google Scholar]
- Sharma T, Dreyer I, Kochian L, Piñeros MA (2016) The ALMT family of organic acid transporters in plants and their involvement in detoxification and nutrient security. Front Plant Sci 7: 1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WY, Sohn EJ, Martinoia E, Lee YJ, Yang YY, Jasinski M, Forestier C, Hwang I, Lee Y (2003) Engineering tolerance and accumulation of lead and cadmium in transgenic plants. Nat Biotechnol 21: 914–919 [DOI] [PubMed] [Google Scholar]
- Sweetman C, Deluc LG, Cramer GR, Ford CM, Soole KL (2009) Regulation of malate metabolism in grape berry and other developing fruits. Phytochemistry 70: 1329–1344 [DOI] [PubMed] [Google Scholar]
- Verma S, Evans K, Guan Y, Luby J, Rosyara U, Howard N, Bassil N, Bink M, van de Weg W, Peace C (2019) Two large-effect QTLs, Ma and Ma3, determine genetic potential for acidity in apple fruit: Breeding insights from a multi-family study. Tree Genet Genomes 15: 18–34 [Google Scholar]
- Visser T, Verhaegh J (1978) Inheritance and selection of some fruit characters of apple. I. Inheritance of low and high acidity. Euphytica 27: 753–760 [Google Scholar]
- Wang H, Ma F, Cheng L (2010) Metabolism of organic acids, nitrogen and amino acids in chlorotic leaves of ‘Honeycrisp’ apple (Malus domestica Borkh) with excessive accumulation of carbohydrates. Planta 232: 511–522 [DOI] [PubMed] [Google Scholar]
- Xu K, Wang A, Brown S (2012) Genetic characterization of the Ma locus with pH and titratable acidity in apple. Mol Breed 30: 899–912 [Google Scholar]
- Yamaki S. (1984) Isolation of vacuoles from immature apple fruit flesh and compartmentation of sugars, organic acids, phenolic compounds and amino acids. Plant Cell Physiol 25: 151–166 [Google Scholar]
- Yang KY, Liu Y, Zhang S (2001) Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc Natl Acad Sci USA 98: 741–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Wang X, Hu T, Zhang F, Wang B, Li C, Yang T, Li H, Lu Y, Giovannoni JJ, et al. (2017) An InDel in the promoter of Al-ACTIVATED MALATE TRANSPORTER9 selected during tomato domestication determines fruit malate contents and aluminum tolerance. Plant Cell 29: 2249–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li P, Cheng L (2010) Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ‘Honeycrisp’ apple flesh. Food Chem 123: 1013–1018 [Google Scholar]
- Zheng Q, Song J, Doncaster K, Rowland E, Byers DM (2007) Qualitative and quantitative evaluation of protein extraction protocols for apple and strawberry fruit suitable for two-dimensional electrophoresis and mass spectrometry analysis. J Agric Food Chem 55: 1663–1673 [DOI] [PubMed] [Google Scholar]