The Arabidopsis Small RNA Database is a user-friendly, web-based tool for exploring over 2,000 Arabidopsis sRNA-seq libraries.
Abstract
Small RNAs (sRNAs) play a wide range of important roles in plants, from maintaining genome stability and enhancing disease resistance to regulating developmental processes. Over the past decade, next-generation sequencing technologies have allowed us to explore the sRNA populations with unprecedented depth and accuracy. The community has accumulated a tremendous amount of sRNA sequencing (sRNA-seq) data from various genotypes, tissues, and treatments. However, it has become increasingly challenging to access these “big data” and extract useful information, particularly for researchers lacking sophisticated bioinformatics tools and expensive computational resources. Here, we constructed an online website, Arabidopsis Small RNA Database (ASRD, http://ipf.sustech.edu.cn/pub/asrd), that allows users to easily explore the information from publicly available Arabidopsis (Arabidopsis thaliana) sRNA libraries. Our database contains ∼2.3 billion sRNA reads, representing ∼250 million unique sequences from 2,024 sRNA-seq libraries. We downloaded the raw data for all libraries and reprocessed them with a unified pipeline so that the normalized abundance of any particular sRNA or the sum of abundances of sRNAs from a genic or transposable element region can be compared across all libraries. We also integrated an online Integrative Genomics Viewer browser into our Web site for convenient visualization. ASRD is a free, web-accessible, and user-friendly database that supports the direct query of over 2,000 Arabidopsis sRNA-seq libraries. We believe this resource will help plant researchers take advantage of the vast next-generation sequencing datasets available in the public domain.
Small RNAs (sRNAs) in plants, generally 20–24 nucleotides (nt) in size, are regulatory RNA molecules functioning in growth and developmental processes, as well as in protecting plants against viruses, transgenes, and transposable elements (TEs; Baulcombe, 2004; Carthew and Sontheimer, 2009; Chen, 2009; Ruiz-Ferrer and Voinnet, 2009; Bologna and Voinnet, 2014; Borges and Martienssen, 2015; Meyers and Axtell, 2019). There are two major classes of sRNAs: microRNAs (miRNAs) and small interfering RNAs (siRNAs; Axtell, 2013). For miRNA biogenesis, RNA polymerase II transcribes miRNA genes into long, single-stranded primary miRNAs, then these single-stranded primary miRNAs form imperfectly matched foldback structures and can be further processed by Dicer-like1 (DCL1) protein into mature miRNA/miRNA* duplexes (Carthew and Sontheimer, 2009; Voinnet, 2009; Axtell, 2013). In contrast, siRNAs are processed by DCLs other than DCL1, with their perfectly matched double-stranded precursors produced by RNA-dependent RNA polymerases (RDRs; Carthew and Sontheimer, 2009; Axtell, 2013). Transacting siRNAs (ta-siRNAs) are usually 21 nt in length and their biogenesis depends on RDR6 and DCL4 activity for their generation, triggered by either “two-hits” (Allen et al., 2005; Axtell et al., 2006) or a single 22-nt miRNA/siRNA (Fei et al., 2013). The production of 24-nt siRNAs is generally initiated by RNA polymerase IV (Pol IV) transcription, followed by RDR2 activity to form a complementary strand that is subsequently cleaved by DCL3. These 24-nt siRNAs can mediate transcriptional gene silencing by targeting DNA methylation and repressive histone modifications (Matzke and Mosher, 2014; Cuerda-Gil and Slotkin, 2016).
The development of next-generation sequencing (NGS) technologies has drastically improved sensitivity and accuracy in detecting sRNA molecules. A large number of sRNA sequencing (sRNA-seq) datasets have been stored in public databases, such as the Gene Expression Omnibus (GEO; Clough and Barrett, 2016) and the Sequence Read Archive (SRA; Leinonen et al., 2011), which include samples from various genotypes, tissues, and treatments. However, it is difficult to directly compare sRNA data across publications using the original results because different pipelines and methods were usually used to analyze these sRNA-seq libraries in each study. Thus, it is difficult for researchers, especially those with a wet-lab background, to take advantage of the vast collection of public NGS datasets.
RESULTS
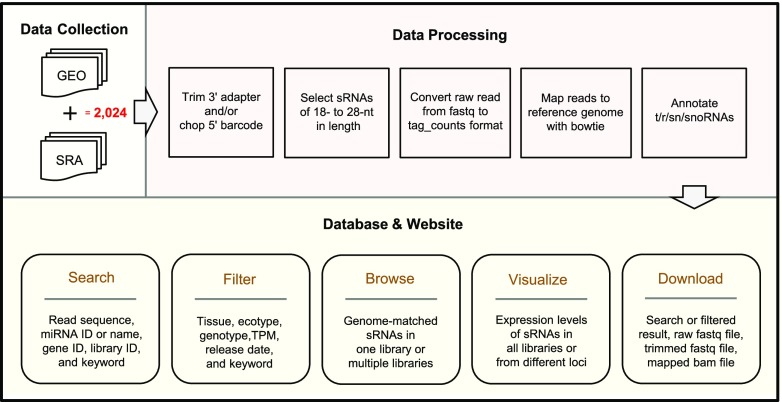
To address this challenge, here we present the Arabidopsis Small RNA Database (ASRD), an online database with integrated, multifaceted functions for exploring published Arabidopsis (Arabidopsis thaliana) sRNA-seq libraries (Fig. 1). ASRD currently hosts 2,024 sRNA-seq libraries collected from GEO and SRA databases. The raw sequencing data of these libraries were downloaded and processed with a unified pipeline so that we can compare the normalized abundances of sRNAs across all libraries. ASRD provides easy-to-access links to download the raw, trimmed, and mapped reads so that researchers can apply their favorite tools to these published libraries with ease. ASRD supports a “Google-like” search through querying of a single sRNA sequence, miRNA ID, miRNA name, miRNA sequence, gene ID, library ID, or library-related keyword (Fig. 2, A and B). ASRD also supports “Advanced options” for narrowing down the search area. All search results can be downloaded via a simple click and be further analyzed using Microsoft Excel. We also added a built-in online Integrative Genomics Viewer (IGV) interface (Robinson et al., 2011) to visualize and browse sRNA alignments (Fig. 2C). Functions of ASRD are described in the following sections (please see the online video tutorial for a step-by-step instruction on how to use the website):
Figure 1.
Overflow of the ASRD. A total of 2,024 publicly available Arabidopsis sRNA-seq libraries were collected from GEO and SRA databases, and processed with a unified pipeline for cross-library comparisons, and all the sRNA-related information can be accessed via keyword-based searching on the ASRD Web site (http://ipf.sustech.edu.cn/pub/asrd/).
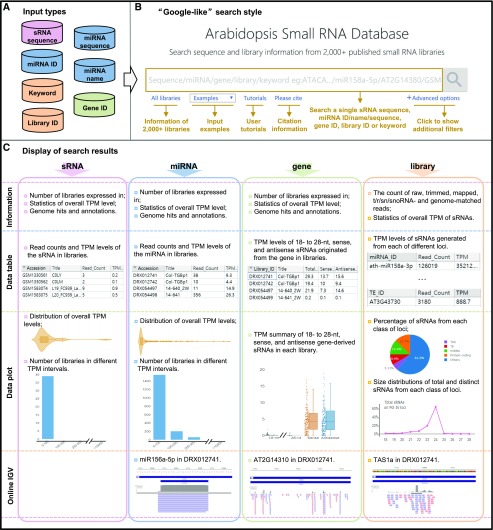
Figure 2.
Basic functions of ASRD. A, Input can be an sRNA sequence, an miRNA sequence, an miRNA ID, an miRNA name, a keyword, a library ID, or a gene ID. B, The introduction of ASRD main page functions. ASRD supports a “Google-like” search that allows the users to search each item in one single input box. “All libraries” shows the detailed information of libraries; “Examples” presents different types of queries; “Tutorials” links to instructions to users; “Advanced options” describes various additional filters. C, Display of search results, including the information, data table, data plot, and online IGV, based on different queries of “sRNA,” “miRNA,” “gene,” and “library.” All results can be downloaded to a local computer.
An “All libraries” Table
To give an overview of all 2,024 sRNA-seq libraries in ASRD (Supplemental Table S1), we provided a table with detailed information of each library, including description, genotype, ecotype, tissue, the counts of raw, transfer/ribosomal/small nuclear/small nucleolar RNAs (t/r/sn/snoRNA)-matched, genome-matched total and distinct reads, the transcripts per million (TPM) levels of sRNAs produced from miRNA, protein-coding (PC), and Pol IV loci. A table with more information (such as size distribution of total sRNAs and different classes of sRNAs in each library) can be downloaded via the link on the “All libraries” page.
Search by sRNA Sequence or miRNA ID/Name
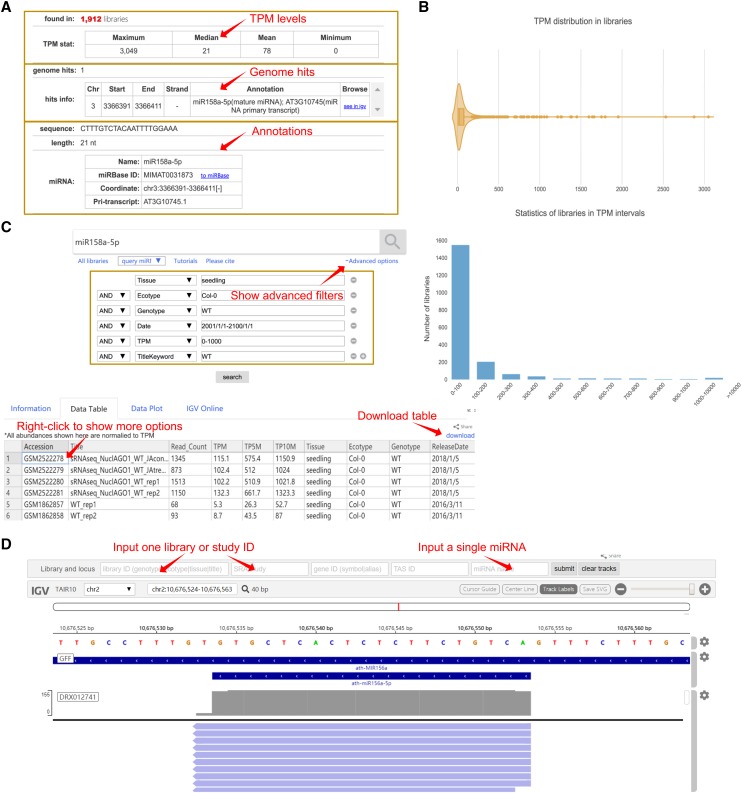
For querying the information of a single miRNA or sRNA, ASRD supports searches by a single sRNA sequence, miRNA ID, miRNA name, or miRNA sequence, and it will return the statistics of expression levels across all libraries. Taking the query of miR158a-5p as an example, Figure 3A shows the maximum, median, mean, and minimum TPM levels of miR158a-5p in all libraries. The violin plot shows the overall TPM distribution of miR158a-5p, and the bar diagram displays the number of miR158a-5p expressed libraries grouped by different TPM intervals (Fig. 3B). The result table with the raw count, TPM, TP5M, and TP10M of miR158a-5p in each library is also downloadable. Furthermore, users can narrow down the query results via the “Advanced options” by applying a combination of filters including tissue, ecotype, genotype, TPM level, keyword, and release date (Fig. 3C). The integrated online IGV interface can be used to browse the mapped miR158a-5p sequences on the genome in one or more libraries (Fig. 3D).
Figure 3.
Example of a query using miR158a-5p. A, Example data for miR158a-5p, such as the statistics of maximum, median, mean, and minimum TPM levels in all libraries, as well as genome hits and their annotations on miRBase. B, The violin plot shows the overall TPM distribution, and each point represents the TPM level of miR158a-5p in a library. The bar diagram displays the number of libraries in different TPM intervals. C, The result table displays the read count, TPM, TP5M, and TP10M levels of miR158a-5p in all libraries. The advanced options can be used to filter the results by tissue, ecotype, genotype, release date, TPM level, or keyword. Right-clicking on each column of this table shows more operations, such as adding, removing, or sorting a column, linking a library to the National Center for Biotechnology Information, or adding a library to online IGV. D, The online IGV browses the mapped miR158a-5p sequences in the DRX012741 library.
Search Library Using ID or Keyword
This function supports querying of a single library ID or library-related keyword. ASRD shows not only the library-related information gathered from the GEO or SRA database but also the statistics of sRNA expression levels across libraries. Read counts include raw, trimmed, mapped, t/r/sn/snoRNA-matched, genome-matched total and distinct reads. TPM levels are for sRNAs generated from miRNA, PC, Pol IV, TE, ta-siRNA loci (TAS), and others. The files of raw, trimmed, and mapped reads can be downloaded via a single click and fed into other analysis tools that the users prefer (Fig. 4A). For characterizing different classes of sRNAs, the pie diagram shows their corresponding percentage, and the line diagrams describe the size distributions of genome-matched total and distinct sRNAs (Fig. 4B). For a quick glance at each library, ASRD also provides the read counts and TPM levels of sRNAs from each of the miRNA, TAS, and “Top-100” PC, Pol IV, and TE loci ranked by TPM (Fig. 4C). The “Advanced options” can also be used to filter the search results by the TPM levels of sRNAs produced from different classes of loci.
Figure 4.
Examples of queries using library ID and gene ID. A to C, Search by library ID. A, The library information includes statistics of the raw, trimmed, mapped, t/r/sn/snoRNA-matched, genome-matched total and distinct reads, and the TPM levels of sRNAs generated from miRNA, TAS, Pol IV, TE, PC, and other classes of loci. B, The pie diagram exhibits the percentage of each class of sRNAs in the library, and the line diagrams show the size distributions of genome-matched total and distinct sRNAs from each class, with x axis indicating sRNA size (nt), and y axis showing the percentage of sRNAs. C, The tables display the read counts and TPM levels of sRNAs derived from each of miRNAs, TAS, Pol IV, TE, and the Top-100 abundant PCs. The advanced options additionally allow the users to filter the results by the TPM levels of sRNAs produced from different classes of loci. d and E, Search by gene ID. D, The diagram with scatters and boxes describes the TPM levels of sRNAs on the queried locus across all libraries. The gray, orange, and blue colors represent 18–28-nt sRNAs, sense, and antisense sRNAs, respectively. E, The table shows the TPM levels of 18–28-nt, sense, and antisense sRNAs generated from that locus in each library.
Search by Gene/TE ID
ASRD supports searches by any of the 38,621 gene/TE IDs annotated in the most recent Araport11 release (Cheng et al., 2017; Fig. 4A). To give an overview of sRNAs generated from a given locus, the “Data Plot” page shows the TPM levels of 18–28 nt sRNAs across all libraries (Fig. 4D), and the “Data table” page details the size distribution, sense, and antisense abundances in each library (Fig. 4E). This table can be downloaded for local use.
IGV Visualization
ASRD integrates an online IGV interface to browse genome-matched sRNAs in any number of libraries. Besides the links on the “Information” pages, the users can use several input boxes and a submit button to easily explore sRNAs at any genomic region in one or more libraries. We extended the IGV function to allow the users to submit a single library ID; genotype-, ecotype-, or tissue-related keyword; SRA study ID (to simultaneously select multiple libraries included in the same study); gene ID, gene symbol, or alias; TAS family members’ name and ID; and miRNA name.
DISCUSSION
Many excellent web-based resources have been developed for hosting sRNA data, such as the MPSS Web site (Nakano et al., 2006), the UCSC Genome Browser (Kent et al., 2002), the Anno-J Browser (Lister et al., 2008), and the EPIC-CoGe Browser (Nelson et al., 2018). Compared to these existing resources that are mostly designed for a single project or multiple projects, ASRD can easily and quickly extract the information from more than 2,000 Arabidopsis sRNA-seq libraries using a simple “Google-like” search and enables the direct comparison of sRNA abundances across different libraries by reprocessing all the raw data from scratch. In the future, we plan to update ASRD on a regular basis by adding more recently published sRNA-seq libraries, and we also intend to experiment with new functions that allow the users to upload their own sRNA-seq libraries and host them at ASRD.
MATERIALS AND METHODS
Data Collection, Processing, and Analysis
We collected Arabidopsis (Arabidopsis thaliana) sRNA-seq data published until July 2019 from GEO and SRA databases by searching with the following combinations of keywords: “([sRNA] OR [sRNAs] OR siRNA OR smallRNA OR smallRNAs OR miRNA OR sRNA OR sRNAs OR siRNAs OR miRNAs) and Arabidopsis.” We obtained a total of 2,024 nonredundant libraries from the NGS platform (Illumina) with raw sequencing data. Figure 1 describes the data collection, processing, and database construction pipeline. The raw datasets in SRA format were downloaded, processed, and analyzed by in-house scripts (all of our scripts are available upon request). In brief, we used the fastq-dump from the SRA Toolkit (v2.8.2; https://www.ncbi.nlm.nih.gov/books/NBK158900/) to convert raw data from sra to fastq format; if a 3′ adapter sequence was not provided, we would predict and trim it with the software tools DNApi (Tsuji and Weng, 2016) and Cutadapt v1.16 (Martin, 2011); if 5′ barcoding was used, we would also chopped the 5′ barcode sequence off; we then processed the remaining 18–28 nt reads in the fasta file to tag_count format. To annotate sRNA features, we mapped these reads to the Arabidopsis reference genome (The Arabidopsis Information Resource 10) using the program BowTie v1.2.1.1 (Langmead et al., 2009), allowing zero mismatches (−v 0) and multiple hits (−a). We used Araport11-annotated t/r/sn/snoRNAs to flag corresponding types of sRNAs in each bam file. After importing all sRNAs, our database contains 2,357,941,025 genome-matched sRNAs representing 254,678,199 distinct sRNAs.
Analysis of sRNA Abundance
The annotation of 426 mature Arabidopsis miRNAs, including both miRNA and miRNA* sequences (named as miRNA-5p and miRNA-3p), was obtained from the program miRbase (v22.1; Kozomara et al., 2019). Eight TAS loci (TAS1a, TAS1b, TAS1c, TAS2, TAS3, TAS3b, TAS3c, and TAS4) were used for calculating TAS abundance. The annotations of 38,621 genes were from Araport11. The list of 7,632 P4-siRNA loci was the same as described in Zhai et al. (2015). The 27,655 PC genes and 3,901 TEs annotated in Araport11 were used to calculate the abundance of PC gene-generating siRNAs and TE-generating siRNAs, respectively. The abundance of sRNAs in each library was calculated as TPM by normalizing to the total number of genome-matched reads excluding t/r/sn/snoRNA-derived ones. The TPM of sRNAs at a given locus was the sum of genome hits-normalized TPMs for all mapped reads at that locus.
Supplemental Data
The following supplemental materials are available.
Supplemental Table S1. The information of 2,024 publicly available libraries in ASRD.
Acknowledgments
We thank all the research groups that contributed sRNA-seq data to the public domain, and we apologize for not being able to cite all the related articles in the main text due to limited space. References for all libraries that we used are listed in Supplemental Table S1.
Footnotes
This work was supported by the National Key R&D Program of China Grant (2019YFA0903903), the National Natural Science Foundation of China (31871234 to J.Z.), the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2016ZT06S172), and the Shenzhen Sci-Tech Fund (KYTDPT20181011104005).
Articles can be viewed without a subscription.
References
- Allen E, Xie Z, Gustafson AM, Carrington JC (2005) microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121: 207–221 [DOI] [PubMed] [Google Scholar]
- Axtell MJ. (2013) Classification and comparison of small RNAs from plants. Annu Rev Plant Biol 64: 137–159 [DOI] [PubMed] [Google Scholar]
- Axtell MJ, Jan C, Rajagopalan R, Bartel DP (2006) A two-hit trigger for siRNA biogenesis in plants. Cell 127: 565–577 [DOI] [PubMed] [Google Scholar]
- Baulcombe D. (2004) RNA silencing in plants. Nature 431: 356–363 [DOI] [PubMed] [Google Scholar]
- Bologna NG, Voinnet O (2014) The diversity, biogenesis, and activities of endogenous silencing small RNAs in Arabidopsis. Annu Rev Plant Biol 65: 473–503 [DOI] [PubMed] [Google Scholar]
- Borges F, Martienssen RA (2015) The expanding world of small RNAs in plants. Nat Rev Mol Cell Biol 16: 727–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew RW, Sontheimer EJ (2009) Origins and mechanisms of miRNAs and siRNAs. Cell 136: 642–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. (2009) Small RNAs and their roles in plant development. Annu Rev Cell Dev Biol 25: 21–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Krishnakumar V, Chan AP, Thibaud-Nissen F, Schobel S, Town CD (2017) Araport11: A complete reannotation of the Arabidopsis thaliana reference genome. Plant J 89: 789–804 [DOI] [PubMed] [Google Scholar]
- Clough E, Barrett T (2016) The Gene Expression Omnibus Database. Methods Mol Biol 1418: 93–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuerda-Gil D, Slotkin RK (2016) Non-canonical RNA-directed DNA methylation. Nat Plants 2: 16163. [DOI] [PubMed] [Google Scholar]
- Fei Q, Xia R, Meyers BC (2013) Phased, secondary, small interfering RNAs in posttranscriptional regulatory networks. Plant Cell 25: 2400–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D (2002) The human genome browser at UCSC. Genome Res 12: 996–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozomara A, Birgaoanu M, Griffiths-Jones S (2019) miRBase: From microRNA sequences to function. Nucleic Acids Res 47(D1): D155–D162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen R, Sugawara H, Shumway M; International Nucleotide Sequence Database Collaboration (2011) The sequence read archive. Nucleic Acids Res 39: D19–D21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, O’Malley RC, Tonti-Filippini J, Gregory BD, Berry CC, Millar AH, Ecker JR (2008) Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell 133: 523–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17: 10–12 [Google Scholar]
- Matzke MA, Mosher RA (2014) RNA-directed DNA methylation: An epigenetic pathway of increasing complexity. Nat Rev Genet 15: 394–408 [DOI] [PubMed] [Google Scholar]
- Meyers BC, Axtell MJ (2019) MicroRNAs in plants: Key findings from the early years. Plant Cell 31: 1206–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M, Nobuta K, Vemaraju K, Tej SS, Skogen JW, Meyers BC (2006) Plant MPSS databases: Signature-based transcriptional resources for analyses of mRNA and small RNA. Nucleic Acids Res 34: D731–D735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ADL, Haug-Baltzell AK, Davey S, Gregory BD, Lyons E (2018) EPIC-CoGe: Managing and analyzing genomic data. Bioinformatics 34: 2651–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP (2011) Integrative genomics viewer. Nat Biotechnol 29: 24–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ferrer V, Voinnet O (2009) Roles of plant small RNAs in biotic stress responses. Annu Rev Plant Biol 60: 485–510 [DOI] [PubMed] [Google Scholar]
- Tsuji J, Weng Z (2016) DNApi: A De Novo Adapter Prediction Algorithm for small RNA sequencing data. PLoS One 11: e0164228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinnet O. (2009) Origin, biogenesis, and activity of plant microRNAs. Cell 136: 669–687 [DOI] [PubMed] [Google Scholar]
- Zhai J, Bischof S, Wang H, Feng S, Lee TF, Teng C, Chen X, Park SY, Liu L, Gallego-Bartolome J, et al. (2015) A one precursor one siRNA model for Pol IV-dependent siRNA biogenesis. Cell 163: 445–455 [DOI] [PMC free article] [PubMed] [Google Scholar]