Abstract
Fit testing procedure is required for filtering facepiece respirators (FFRs) to ascertain an acceptable fit between the skin and facepiece sealing surface. The present study seeks to compare the efficacy of Aloe vera (A. vera) and commercial BitrexTM as challenge agents of qualitative fit testing of particulate respirators. An herbal solution consisting of A. vera at seven different concentrations was developed. Threshold Screening Tests (TSTs) of A. vera solutions were compared to BitrexTM. To do so, solutions were administered randomly on a total of 62 participants. A placebo was also tested to ensure the taste response being valid. Statistical analysis was performed using R 3.2.5.0 software. There were no statistically significant differences between the A. vera (41.7, 58.3, 75, and 91.7 mg/ml) and BitrexTM threshold tests. Therefore, the minimum concentration of A. vera to develop the threshold solution was considered to be 41.7 mg/ml. When commercial products are expensive and unavailable, a cost-effective technique would be to replace A. vera solution with a commercial product as a challenge agent of qualitative fit testing of respirators.
Keywords: Qualitative Fit test, Challenge agents, Commercial and herbal solutions
Introduction
Efficient respiratory protection is dependent mainly on the filters or cartridges efficiency as well as appropriateness for users’ face and work environment1,2,3). According to the Occupational Safety and Health Administration (OSHA), it is mandatory to make sure there is an appropriate fit between a respirator’s facepiece and face seal. To meet the legal requirements, it is necessary to do fit testing for all subjects included in the Respiratory Protection Program (RPP) before the respirator selection4). The ability of the respirator to interface with the users’ face to protect the respiratory system against hazardous contaminants, named “Respirator fit”5).
There are mainly two assessment methods of respirator fit testing per OSHA standard (29 CFR 1910.134), to provide the expected level of protection for the wearers6): Qualitative fit test (QLFT) and Quantitative fit test (QNFT). QNFT uses an instrument to measure the fit factor by dividing the ambient particle concentration by the concentration of particles inside the facepiece of the respirator while the user performs a series of simulated work exercises4). QNFT has some advantages such as application to various classes of Respiratory Protective Equipment (RPE)7), documentation of numerical results, and no chance of deception8). However, due to high cost, time-consuming, skilled operator, unavailability5), annual recalibration, and reliance on a sampling adaptor or probed respirator, QLFT is widely being used as the preferred method4, 9, 10).
QLFT is a dichotomous test (pass/fail) that depends on the wearer’s olfactory or taste response to a challenge agent during the same series of exercises as the QNFT. Currently, there are three common challenge agents including isoamyl acetate; saccharin; and BitrexTM. Isoamyl acetate (IAA), a sweet (banana) smelling vapor is used in qualitative fit testing of respirators equipped with organic vapor cartridges; whereas, saccharin (sweet taste) and BitrexTM (bitter taste) are the challenge agents for fit testing of respirators equipped with particulate filters4). Moreover, BitrexTM, the trade name of the bitterest substance, denatonium benzoate (Smith Ltd., Montvale, NJ McFarland), generally used as an aversive agent in toxic household liquids to decrease the risk of accidental poisoning4).
The most substantial concern about the QLFT is its subjective nature. It is impossible to do fit testing for all subjects in events like pandemics11). It should be mentioned that the QLFT is simpler to use11, 12), and cheaper to set up and maintain12) than QNFT. But some of the challenge agents like BitrexTM lead to allergic (asthmatic) reactions13, 14), dermal and respiratory symptoms, anaphylactic reactions (angioedema and bronchospasm), and anxiety response or even an inability to detect the taste/smell of the agents13).
Remarkably, some qualifications should be considered for selecting a qualitative challenge agent as follows: cost-benefit, availability, safety, suitability for human exposure, and ability to use with any type of approved particular filter12). Commercial challenge agents would not be cost-beneficial or available, furthermore, they might be led to symptoms and complications in some subjects as mentioned above. Consequently, the efficacy of A. vera was compared to BitrexTM.
A. vera is a shining or bitter substance, bitter because of liquids such as Aloin, Aloe emodin, and related compounds in the leaves15,16,17). It is approved as Generally Recognized as Safe (GRAS) in the US by the Food and Drug Agency (FDA)16, 18) and World Health Organization (WHO)18, 19). It exerts beneficial effects on human health, including: anti-inflammatory17, 18, 20,21,22,23,24,25), antimicrobial18, 22,23,24, 26), antifungal17, 18), antioxidant and antitumor23, 27), anticancer17, 23, 26, 28), and immunomodulatory properties17, 23, 24). It seems that A. vera could be the right substitute for BitrexTM as a challenge agent. Also, as stated in ISO 16975-39), equivalent substances could be used as challenge agents if they behave similar and show identical results. Accordingly, the aim of this study was to compare the efficacy of A. vera against BitrexTM as a qualitative fit test agent.
Subjects and Methods
Study design
We conducted a single-blind, placebo-controlled, and interventional study at Shiraz University of Medical Sciences, Iran.
Participants
Sixty-two students (37 females and 25 males), mean age 23.45 ± 4.66 yr, using a proportional stratified sampling method based on their educational level, were recruited for the study. The participants were tested in the Industrial Safety laboratory of the School of Health.
First of all, the procedures of this study were approved by the Research Ethics Committee of Shiraz University of Medical Sciences (approval code IR.SUMS.REC.1396.191). Second, all participants were provided explanation about study procedures. Then, each was given written informed consent documentation. The criteria included an absence of the following: (1) allergy to any substance; (2) cold symptoms; (3) nasal congestion; (4) cardiovascular or respiratory diseases (asthma, shortness of breath, dyspnea);29) rhinoplasty surgery, or (6) other factors interfering with the sense of taste. If any participant developed a cold, the test session was postponed until his/her recovery.
Study procedure
All participants refrained from chewing gum, eating, and drinking (except for plain water) for 15 min prior to the test to ensure they could taste the threshold check solution (a diluted version of the fit test solution). Meanwhile, the placebo solution (distilled water) was tested among the solutions randomly to be sure the participants could distinguish it. To obtain reliable results, the test assessor reminded participants to drink only plain water. A 5-min break also allowed participants to clear their palates between test solutions. These adopted complementary tests were illustrated further:
1) To make certain the threshold check solutions hadn’t been contaminated, the microbial communications of the solutions were assessed. To do so, we utilized the Blood Agar for bacteria30) and Sabouraud Dextrose Agar31) (Merck Co., Germany) for fungus.
2) The taste of the solutions was evaluated continuously.
3) The optical molecular spectra of the solutions was observed regularly using Agilent Cary 60 spectrophotometer (Agilent Technologies, Santa Clara, CA, USA) at 25°C in the wavelength range of 200–800 nm to follow up the stability of the solutions regarding color, clarity and not construction of unstable colloids during the ten months which were presented in the Supplementary Figs. 1–8 (See online version).
4) The pH of the solutions was measured using a Metrohm pH meter model 827 (Metrohm, Herisau, Switzerland), according to manufacturer’s instructions (Table 1).
Table 1. Results of the Threshold Screening Tests (TSTs).
| Solutions | Concentration (mg/ml) |
pH | Threshold test | Number of sprays | Detection time (S) | |
|---|---|---|---|---|---|---|
| PassN (%) |
FailN (%) |
Mean ± SD | Mean ± SD | |||
| Placebo | -- | - | 44 (71) | 18 (29)* | 15.68 ± 8.04 | 22.08 ± 11.49 |
| BitrexTM | 0. 135 | 6.91 | 60 (96.8) | 2 (3.2) | 5.32 ± 4.18 | 7.96 ± 4.96 |
| A. vera | 10.7 | 7.50 | 52 (83.9) | 10 (16.1) | 7.54 ± 6.32 | 11.02 ± 7.58 |
| 18.8 | 7.11 | 43 (69.4) | 19 (30.6) | 7.65 ± 5.83 | 10.84 ± 6.8 | |
| 26.2 | 7.02 | 43 (69.4) | 19 (30.6) | 8.81 ± 7.22 | 13.58 ± 11.33 | |
| 41.7 | 6.70 | 53 (85.5) | 9 (14.5) | 7.04 ± 6.016 | 9.75 ± 6.97 | |
| 58.3 | 6.43 | 53 (85.5) | 9 (14.5) | 6.87 ± 4.87 | 9.43 ± 6.01 | |
| 75.0 | 6.25 | 57 (91.9) | 5 (8.07) | 6.23 ± 5.68 | 9.12 ± 7.43 | |
| 91.7 | 6.14 | 53 (85.5) | 9 (14.51) | 4.81 ± 3.07 | 7.27 ± 3.54 | |
*18 (29%) of the participants reported the placebo as a sweet, salty, or bitter taste.
Threshold check solution preparation
Nine different threshold check solutions, including A. vera (7 concentrations), a BitrexTM, and a placebo, were made. We found that limited amounts of A. vera could not significantly affect the taste of the solutions; therefore, they were considered for the range of applied concentrations of the A. vera threshold check solutions. The threshold check solutions were poured into identical bottles and labeled with unique codes by the administrator. Those solutions were randomly allocated to the participants based on the Latin Square Design (LSD)12). Accordingly, all possible permutation of solutions was 9 factorial (9!). Also, all participants were blinded to the ingredient and concentration of each solution.
BitrexTM threshold check solution
Moldex® Bitrex® Fit Test Kit Part number 0102 (Moldex Co., Culver, CA, USA) contained 0.0135% denatonium benzoate, 94.9865% water, and 5% sodium chloride32).
Aloe vera threshold check solution
We chose the A. vera as equivalent to BitrexTM. A. vera solutions were prepared by adding 1.28, 2.26, 3.15, 5, 7, 9, and 11 gr of A. vera powder to 120 ml of distilled water. The above mixtures were boiled at 100°C for 10 min. After the solutions had been cooled, they were passed through a stainless sieve (US Standard Sieve Mesh No. 200 (0.075 mm), Pars Sieve, Iran; ASTM E:11). Indeed, the final concentrations of the threshold check solutions 10.7, 18.8, 26.2, 41.7, 58.3, 75, and 91.7 mg/ml, respectively.
Threshold Screening Tests
Threshold Screening Tests (TSTs) were performed per OSHA respiratory protection standard, regulation 29 CFR 1910.1344). The TSTs were aimed to ensure the participants’ taste response being valid. Some accessories are required to do the TSTs, for instance, hood, nebulizer, and paper towel. The poly-coated test hood is approximately 12 inches (30.5 cm) in diameter by 14 inches (35.6 cm) and was placed over a participant’s head and positioned forward about 6 inches (15.25 cm) between the participant’s face and hood window. The 0.75 inch (1.9 cm) hole in front of the participant’s nose and mouth area accommodated the nebulizer nozzle and dispersed the aerosol with a 2.5 µm mass median aerodynamic diameter (MMAD) around the participant’s mouth9) (Fig. 1). Moreover, the nebulizers were periodically checked for aerosol generation while squeezing the bulbs. To do so, they were held against a dark background to ensure the visible aerosol appears. The nebulizers were disassembled based on the manufacturer’s manual and rinsed at regular intervals to prevent clogging. The hood was wiped frequently with a paper towel to clean any deposited solution.
Fig. 1.

Moldex fit test hood used in the current study.
Prior to starting the intervention, firstly, the participants were trained to position the hood over the head without donning a respirator, breathing only through their mouths slightly open with tongue extended and to report as soon as they could detect the taste (not smell) of the challenge agent, however, we didn’t notify the participants about the taste of the solutions (sweet, bitter, etc.). Secondly, the challenge agent was injected in the hood via inserting ten squeezes of the nebulizer bulb into the hole in front of the hood. This requires the bulb to fully collapse and expand during each squeeze cycle. Thirdly, the participants were asked if they could detect the taste of the solution. We applied up to a total of 30 sprays if required. Also, the taste threshold was recorded as ten, twenty, or thirty regardless of the numbers of actual performed squeezes (10, 20 or 30 sprays). In other words, if the participants could taste between 1–10, 11–20, or 21–30 sprays, their threshold levels were classified into one of the three classes of High (1), Medium (2), or Low (3), respectively. If the participants couldn’t detect the taste of the solution after 30 sprays, they were not sensitive to it and their threshold test was assigned as a failure; otherwise, a pass. The video of Moldex threshold screening procedure was played for the participants33).
Lastly, we recorded the study data such as threshold solution code, the concentration of test solutions (mg/ml), number of sprays, time required to elicit a taste response correctly (s), threshold level (1, 2 or 3), and test result (pass/fail).
Statistical analysis
The input variables were as follows: type and concentration of the solutions, age, and gender. The outcome variables included the results of TSTs (pass/fail). We took repeated measures on the participants; thus, the observations were correlated and the Mixed Effect Logistic Regression (MELR) model including random effects was proposed34). Noticeably, the model was adjusted for age and gender. We calculated the Kappa statistic (k), Brier score as the mean square error of taste detection34), and accuracy35) to compare the results of the TSTs. Meanwhile, a Receiver Operating Characteristic (ROC) curve analysis was obtained to evaluate the detectability of all threshold solutions against placebo (as a reference solution). The Area under the ROC Curve (AUC) was calculated to determine the overall performance of all threshold check solutions against the placebo35). Additionally, A. vera threshold solutions (bitter taste) were compared to BitrexTM (as a reference solution). A p-value of 0.05 was considered significant. All data analyses were performed using R 3.2.5.0 software.
Results
Table 1 shows the TSTs descriptive statistics results.
As shown, the majority of the participants could taste BitrexTM and A. vera (75 mg/ml) solutions (96.8% and 91.9%, respectively). As expected, all solutions (except for placebo) were tasted within close margin, in terms of the number of sprays and detection time(s).
The results of all threshold solutions tests against placebo are shown in Table 2. There were significant differences between the BitrexTM and A. vera solutions (41.7, 58.3, 75, and 91.7 mg/ml) and placebo. The odds ratio (OR) for taste detection of BitrexTM and A. vera (75 mg/ml) was calculated as 14.78 and 5.39, respectively. In addition, the accuracy score for the corresponding solutions was the highest as 84% (104/124) and 81% (101/124), respectively. The A. vera (75 mg/ml) held the lowest Brier score among all solutions.
Table 2. Comparison of threshold tests against placebo by MELR*.
| Solutions | Concentration (mg/ml) | Coefficient(β) | OR | 95% CI for OR | Accuracy | Brier Score | AUC | 95% CI for AUC | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | |||||||
| Commercial | 0.135 | 2.69** | 14.78 | 3.15 | 0.18 | 0.84 | 0.32 | 0.9 | 0.85 | 0.95 |
| BitrexTM | ||||||||||
| A. vera | 10.7 | 0.84 | 2.32 | 0.92 | 0.05 | 0.78 | 0.14 | 0.85 | 0.78 | 0.91 |
| 18.8 | −0.09 | 0.92 | 0.40 | 0.14 | 0.71 | 0.39 | 0.76 | 0.68 | 0.83 | |
| 26.2 | −0.09 | 0.92 | 0.40 | 0.39 | 0.72 | 0.31 | 0.78 | 0.71 | 0.85 | |
| 41.7 | 0.98*** | 2.65 | 1.03 | 0.31 | 0.78 | 0.14 | 0.85 | 0.79 | 0.92 | |
| 58.3 | 0.98*** | 2.65 | 1.03 | 0.14 | 0.78 | 0.13 | 0.85 | 0.79 | 0.92 | |
| 75.0 | 1.68*** | 5.39 | 1.78 | 0.13 | 0.81 | 0.05 | 0.89 | 0.84 | 0.95 | |
| 91.7 | 0.98*** | 2.65 | 1.03 | 0.048 | 0.78 | 0.11 | 0.86 | 0.8 | 0.92 | |
*Adjusted for age/gender, **p-value<0.0001, ***p-value<0.05. MELR: Mixed Effect Logistic Regression; CI: confidence interval; OR: odds ratio; AUC: Area under the ROC Curve.
The ROC curves for BitrexTM and A. vera threshold tests against placebo are given in Fig. 2. Accordingly, the ROC curve for A. vera threshold test was similar to that of BitrexTM (89.5% vs. 90.3%, respectively). The numbers represent the participants could detect the bitter taste of BitrexTM and A. vera (75 mg/ml) during TSTs. This suggests that the performance of A. vera threshold solution (75 mg/ml) was similar to that of BitrexTM.
Fig. 2.

Receiver operating characteristic (ROC) curves for threshold tests against placebo: BitrexTM(a), A. vera; 41.7 and 58.3 mg/ml (b), 75 mg/ml (c), 91.7 mg/ml (d).
The results of A. vera threshold tests against BitrexTM are summarized in Table 3. As seen, no significant differences were found between the A. vera (concentration range of 41.7 to 91.7 mg/ml) and BitrexTM threshold tests. Among all solutions, the OR for taste detection of A. vera (75 mg/ml) in comparison to BitrexTM was the highest (OR=0.37). The significant agreement between the BitrexTM and A. vera (75 mg/ml) threshold tests was calculated as the highest value (k=0.88).
Table 3. The results of A. vera threshold tests vs. commercial BitrexTM by MELR*.
| Solutions | Concentration (mg/ml) | Coefficient(β) | OR | 95% CI for OR | Kappa (k) | 95% CI for Kappa | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | |||||
| A. vera | 10.7 | −1.85** | 0.16 | 0.03 | 0.78 | 0.82 | 0.71 | 0.92 |
| 18.8 | −2.77*** | 0.06 | 0.01 | 0.29 | 0.69 | 0.57 | 0.82 | |
| 26.2 | −2.77*** | 0.06 | 0.01 | 0.29 | 0.69 | 0.57 | 0.82 | |
| 41.7 | −1.71 | 0.18 | 0.04 | 0.09 | 0.82 | 0.71 | 0.92 | |
| 58.3 | −1.71 | 0.18 | 0.04 | 0.09 | 0.85 | 0.75 | 0.94 | |
| 75.0 | −1.00 | 0.37 | 0.07 | 2.02 | 0.88 | 0.79 | 0.97 | |
| 91.7 | −1.71 | 0.18 | 0.04 | 0.09 | 0.85 | 0.75 | 0.94 | |
* Adjusted for age/gender, **p-value<0.05, ***p-value<0.0001. MELR: Mixed Effect Logistic Regression; CI: confidence interval; OR: odds ratio.
Figure 3 reveals the overall performance of BitrexTM and A. vera threshold tests against a placebo through comparing the correspondence AUC curves. Obviously, the AUC curves for those solutions overlapped. No significant differences were observed between the BitrexTM and A. vera threshold tests (p=0.66). Since the ROC curve for the A. vera (75 mg/ml) threshold solution moved to left and upside (BitrexTM), it is apparent that its efficacy is similar to that of BitrexTM and could be employed as a qualitative fit test agent. Interestingly, the Cost-Benefit Analysis (CBA) of the current study indicates that the cost and time required to access A. vera solution were remarkably less than BitrexTM one (Where cost is $84/100 gr versus $2/100 gr, and time between purchase and delivery is 1 business day versus 14 business days).
Fig. 3.
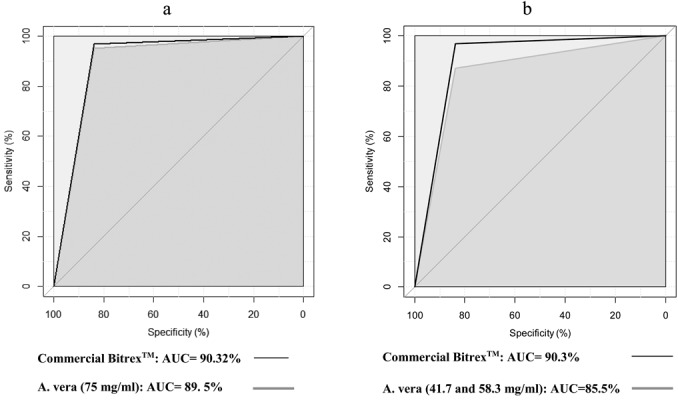
Comparison of area under the ROC curves (AUC) for threshold solutions: BitrexTM and A. vera; 75 mg/ml (a), 41.7, 58.3, and 75 mg/ml (b).
Discussion
The aim of this study is to investigate the efficacy of A. vera compared to BitrexTM as a qualitative challenge agent. The principal points found from this research will be discussed below in more detail.
First, the proposed MELR model demonstrated that there were no statistically significant differences between the BitrexTM and A. vera (41.7, 58.3, 75, and 91.7 mg/ml) threshold test results. This means that participants could detect the bitter taste using either BitrexTM or A. vera solution. The minimum concentration of A. vera to develop the threshold solution was considered be 41.7 mg/ml. Accordingly, A. vera solution could be made on-site with similar efficacy to that of BitrexTM.
Second, the degree of agreement between the results of BitrexTM and A. vera (75 mg/ml) threshold tests was considered very good (k=0.88) which highlights the high efficacy of A. vera in order to use as a qualitative fit test agent. This finding is consistent with the study of Brett G. Mitchell et al.11), which states that on-site made solutions could be replaced by commercial products for qualitative fit testing of respirators. The ability to produce A. vera solution on-site is a critical factor to making a key difference in the event of an influenza pandemic or similar respiratory diseases. If the testing solution was either not readily available or too costly, the facility could incur preventable loss of both profit and life.
Another important finding has to do with the fact that the majority of the participants tasted both the BitrexTM and A. vera (75 mg/ml) solutions. The OR for taste detection of these mentioned solutions was 14.78 and 5.39 times the OR for the placebo, respectively, meaning that the OR for taste detection of the BitrexTM was almost 2.70 times the OR for taste detection of A. vera (75 mg/ml) solution. This means that participants were 2.70 times more likely to detect the bitter taste of BitrexTM than that of A. vera (75 mg/ml) solution. The AUC for those solutions overlapped, which confirms that A. vera (75 mg/ml) solution has similar efficacy to that of BitrexTM.
Noticeably, BitrexTM was detected more often than A. vera (75 mg/ml) (96.8% vs. 91.9%). This is consistent with some published studies (Mullins, 1995; Roy T. McKay, 2000) that discussed easier detectability of BitrexTM compared with saccharin12, 36). The mean of the sprays’ number to detect the bitter taste of BitrexTM and A. vera (75 mg/ml) were almost the same (5.32, 6.23, respectively). We can also merge those two thoughts as “The CBA obtained from the laboratory test confirm that A. vera (75 mg/ml) could be utilized as a challenge agent of qualitative fit test, which is beneficial”.
The overall cost of A. vera was 42 times (97.6%) lower than that of BitrexTM. Additionally, the required time for preparation of A. vera fit test solution was lower than that of BitrexTM. In fact, on an average, 14 business days required for the commercial solution to be delivered. Whereas, the A. vera could be available about one business day.
Finally, a number of important limitations need to be considered. First, the TSTs of the current study were based on the subjective reaction to the challenge agents like the other qualitative fit test solutions. Second, any variation from the fit test protocol would invalidate the test results, including the hood size, changes in the concentration of the solutions, squeezing the nebulizer bulb, and the number of squeezes.
Conclusion
This study suggests that replacing of A. vera solution with a commercial product as a challenge agent in fit testing procedure would be a cost-effective technique; due to factors as performance, cost, and availability. It should be emphasized that the studied solutions are safe for microbial communications and some physicochemical properties of the solutions (taste, color or clearance) were monitored over the course of the study and we can safely assure that these properties are constant. To do so, it is feasible to utilize the herbal solutions (i.e., A. vera) as challenge agents for qualitative fit testing of particulate respirators.
Funding
The present article was extracted from the thesis written by Anahita Fakherpour, MSc., a student of Occupational Health and was financially supported by Shiraz University of Medical Sciences, Shiraz, Iran (Grant No. 1396-01-04-15787).
Conflicts of Interest
None declared.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge participants for their collaboration with our project.
References
- 1.Mueller W, Horwell CJ, Apsley A, Steinle S, McPherson S, Cherrie JW, Galea KS. (2018) The effectiveness of respiratory protection worn by communities to protect from volcanic ash inhalation. Part I: Filtration efficiency tests. Int J Hyg Environ Health 221, 967–76. [DOI] [PubMed] [Google Scholar]
- 2.Jahangiri M, Shahtaheri SJ, Adl J, Rashidi A, Kakooei H, Forushani AR, Nasiri G, Ghorbanali A, Ganjali MR. (2012) Preparation of activated carbon from walnut shell and its utilization for manufacturing organic-vapour respirator cartridge. Fresenius Environmental Bulletin 21, 1508–1514. [Google Scholar]
- 3.Jahangiri M, Adl J, Shahtaheri SJ, Rashidi A, Ghorbanali A, Kakooe H, Forushani AR, Ganjali MR. (2013) Preparation of a new adsorbent from activated carbon and carbon nanofiber (AC/CNF) for manufacturing organic-vacbpour respirator cartridge. Iran J Environ Health Sci Eng 10, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Title 29 CFR.1910.134. Respiratory protection program Standards- Fit Testing Procedures (Mandatory) Washington, Occupational Safety & Health Administration (OSHA), Government Publishing Office (2016) https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=STANDARDS&p_id=9780.
- 5.Rajhans GS, Pathak BP .(2002) Practical guide to respirator usage in industry, 2nd Ed., 1–190, Butterworth-Heinemann, Woburn. [Google Scholar]
- 6.Coffey CC, Lawrence RB, Campbell DL, Zhuang Z, Calvert CA, Jensen PA. (2004) Fitting characteristics of eighteen N95 filtering-facepiece respirators. J Occup Environ Hyg 1, 262–71. [DOI] [PubMed] [Google Scholar]
- 7.Health and Safety Executive (HSE 282/28) UK. www.hse.gov.uk/foi/internalops/ocs/200–299/282_28.pdf.
- 8.Introduction to Respirator Fit Testing. www.tsi.com/uploadedFiles/_Site_Root/Products/Literature/...Notes/iti_070.pdf.
- 9.International Organization for Standardization (ISO) ISO 16975-3, Respiratory protective devices—Selection, use and maintenance— Part 3: Fit Testing procedures. 2017. https://www.iso.org/standard/64513.html.
- 10.American National Standards Institute—Respirator fit testing methods (ANSI/AIHA Z88.10–2010) New York. https://www.aiha.org/publications-and-resources/BoKs/RPPA-FT/InteractiveVersion/Pages/Respirator-Fit-Testing.aspx.
- 11.Mitchell BG, Wells A, McGregor A, McKenzie D. (2012) Can homemade fit testing solutions be as effective as commercial products? Healthc Infect 17, 111–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mullins HE, Danisch SG, Johnston AR. (1995) Development of a new qualitative test for fit testing respirators. Am Ind Hyg Assoc J 56, 1068–73. [DOI] [PubMed] [Google Scholar]
- 13.Youakim S. (2007) Adverse reactions associated with respirator fit testing of healthcare workers in British Columbia, Canada: a review of compensation claim cases. Arch Environ Occup Health 62, 197–200. [DOI] [PubMed] [Google Scholar]
- 14.Coffey C, Zhuang Z, Campbell D. (1998) Evaluation of the Bitrix™ Qualitative Fit Test Method using N95 Filtering-Facepiece Respirators. J Int Soc Respir Prot 16, 48–55. [Google Scholar]
- 15.Ahlawat KS, Khatkar BS. (2011) Processing, food applications and safety of aloe vera products: a review. J Food Sci Technol 48, 525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh DM .(2008) Inspections, Compliance, Enforcement, and Criminal Investigations. Salud Natural Entrepreneurs, Inc. 4/8/16. https://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2016/ucm519400.htm.
- 17.Singh P, Rani B, Maheshwari R, Chauhan A. (2011) Diverse therapeutic applications of Aloe vera. J Adv Sci Res 2. [Google Scholar]
- 18.Ulbricht C, Armstrong J, Basch E, Basch S, Bent S, Dacey C, Dalton S, Foppa I, Giese N, Hammerness P, Kirkwood C, Sollars D, Tanguay-Colucci S, Weissner W. (2007) An evidence-based systematic review of Aloe vera by the natural standard research collaboration. J Herb Pharmacother 7, 279–323. [DOI] [PubMed] [Google Scholar]
- 19.Cepae BA .(1999) WHO monographs on selected medicinal plants, 2nd Ed., 1–295, World Health Organization, Geneva. [Google Scholar]
- 20.Ali S, Wahbi W. (2017) The efficacy of aloe vera in management of oral lichen planus: a systematic review and meta-analysis. Oral Dis 23, 913–8. [DOI] [PubMed] [Google Scholar]
- 21.Lakshmi P, Rajalakshmi P. (2011) Identification of phyto components and its biological activities of aloe vera through the gas chromatography-mass spectrometry. Int J Pharm 2, 247–9. [Google Scholar]
- 22.Lawrence R, Tripathi P, Jeyakumar E. (2009) Isolation, purification and evaluation of antibacterial agents from aloe vera. Braz J Microbiol 40, 906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joseph B, Raj SJ. (2010) Pharmacognostic and phytochemical properties of Aloe vera linn an overview. Int J Pharm Sci Rev Res 4, 106–10. [Google Scholar]
- 24.Somboonwong J, Duansak N. (2004) The therapeutic efficacy and properties of topical Aloe vera in thermal burns. J Med Assoc Thai 87 Suppl 4, S69–78. [PubMed] [Google Scholar]
- 25.Pothuraju R, Sharma RK, Onteru SK, Singh S, Hussain SA. (2016) Hypoglycemic and hypolipidemic effects of Aloe vera extract preparations: a review. Phytother Res 30, 200–7. [DOI] [PubMed] [Google Scholar]
- 26.Radha MH, Laxmipriya NP. (2014) Evaluation of biological properties and clinical effectiveness of Aloe vera: a systematic review. J Tradit Complement Med 5, 21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abo-Youssef AMH, Messiha BAS. (2013) Beneficial effects of Aloe vera in treatment of diabetes: comparative in vivo and in vitro studies. Bull Fac Pharm Cairo Univ 51, 7–11. [Google Scholar]
- 28.Feily A, Namazi MR. (2009) Aloe vera in dermatology: a brief review. G Ital Dermatol Venereol 144, 85–91. [PubMed] [Google Scholar]
- 29.https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=standards.
- 30.Krieg NR, Staley JT, Brown DR, Hedlund BP, Paster BJ, Ward NL, Ludwig W, Whitman WB .(2010) Bergey’s manual of systematic bacteriology, 2nd Ed., 1–976, Springer-Verlag, New York. [Google Scholar]
- 31.Chander J .(2017) Textbook of medical mycology, 4th Ed., 1–912, Jaypee Brothers Medical Ltd., New Delhi. [Google Scholar]
- 32.Moldex—Safety Data Sheets (SDS) of Bitrex sensitivity and fit test solutions MOLDEX https://www.moldex.com/resources/important-information/ material-safety-data-sheets-msds/.
- 33.Moldex Treshold Screening Tests (TSTs)https://www.youtube.coms/watch?v=xeeBRUC4UZs.
- 34.Agresti A .(2002) Categorical data analysis, 2nd Ed., 1–704, University of Florida, Gainesville. [Google Scholar]
- 35.Zhu W, Zeng N, Wang N. (2010) Sensitivity, specificity, accuracy, associated confidence interval and ROC analysis with practical SAS® implementations. North East SAS Users Group, Health Care and Life Sciences.
- 36.McKay RT, Davies E. (2000) Capability of respirator wearers to detect aerosolized qualitative fit test agents (sweetener and Bitrex) with known fixed leaks. Appl Occup Environ Hyg 15, 479–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


