Abstract
Purpose:
We evaluated the relationship between isocitrate dehydrogenase 1 (IDH1) mutation status and metabolic imaging in patients with nonenhancing supratentorial diffuse gliomas using 11C-methionine positron emission tomography (11C-MET PET).
Materials and Methods:
Between June 2012 and November 2017, we enrolled 86 (38 women and 48 men; mean age, 41.9 ± 13.1 years [range, 8-67 years]) patients with newly diagnosed supratentorial diffuse gliomas. All patients underwent preoperative 11C-MET PET. Tumor samples were obtained and immunohistochemically analyzed for IDH1 mutation status.
Results:
The mutant and wild-type IDH1 diffuse gliomas had significantly different mean maximum standardized uptake value values (2.73 [95% confidence interval, CI: 2.32-3.16] vs 3.85 [95% CI: 3.22-4.51], respectively; P = .004) and mean tumor-to-background ratio (1.90 [95% CI: 1.65-2.16] vs 2.59 [95% CI: 2.17-3.04], respectively; P = .007).
Conclusions:
11C-methionine PET can noninvasively evaluate the IDH1 mutation status of patients with nonenhancing supratentorial diffuse gliomas.
Keywords: glioma, 11C-MET PET, IDH1
Introduction
Cerebral gliomas are the most common primary malignant brain tumors, accounting for 29% of all primary and 81% of all malignant brain tumors.1 Previously, prognostic evaluations were based on the histological features of tumor tissues along with clinical variables, such as age and performance status. However, the clinical outcomes have greatly varied among patients regardless of having the same pathological classification. Although the histopathological classification of gliomas has a long history, it has high interobserver and intraobserver variability and does not adequately predict clinical outcomes.
Recently, several biomarkers have been discovered to play vital roles in tumor differentiation. Molecular characterizations have become even more relevant, with mutations of isocitrate dehydrogenase (IDH), tumor protein 53 (TP53), and α-thalassemia/mental retardation syndrome X-linked (ATRX); 1p/19q codeletion; and O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation being the important diagnostic and prognostic markers for tumors with similar morphology.2 Consequently, clinicians now increasingly rely on genetic classification to guide clinical decision-making.
Currently, World Health Organization (WHO) grades II to III are considered diffuse gliomas because of their similar genetic characteristics.3 Isocitrate dehydrogenase mutations are a characteristic of most diffuse gliomas and define a subtype associated with better prognosis.4 Isocitrate dehydrogenase mutations can be found in up to 80% of grades II to III gliomas and secondary glioblastomas but rarely in primary glioblastomas.3,4 Furthermore, compared to IDH-mutated tumors, grades II to III gliomas with wild-type IDH more frequently show molecular genetic alterations, which resemble those in glioblastomas.3,5 The genetic similarities between IDH wild-type glioblastomas and IDH wild-type diffuse gliomas lead to the same aggressive clinical behaviors.6 Compared to gliomas without IDH mutations, IDH-mutated gliomas progress slower over time and have better prognosis.4,7 Moreover, isocitrate dehydrogenase 1 (IDH1) mutation status can be used as a predictor of complete resection8 and median survival time.9 These associations have been justifiably accepted and have been shown to have a profound impact on the development of the new WHO Classification of central nervous system (CNS) tumors10 and on the updates on the recent CNS guidelines of the National Comprehensive Cancer Network. Generally, these biomarkers are detected by immunohistochemical analysis or genetic sequencing of postoperative pathological specimens. Therefore, identification of complementary imaging markers for the aforementioned critical biomarkers would be greatly valuable in providing more information for preoperative decision-making and maximally safe resection.
For decades, 11C-methionine positron emission tomography (11C-MET PET) has been applied for the diagnosis of gliomas;11 differentiation between tumor recurrence and radiation injury;12 and surgical13 and radiotherapy14 planning, owing to its strong contrast with background uptake in normal brain tissue. Moreover, the reported correlation of 11C-MET uptake with cell proliferation, expressions of Ki-67 and proliferating cell nuclear antigen, and microvessel density15 indicated its role as a marker of rapid tumor proliferation. Therefore, previous studies have applied 11C-MET PET to explore the association of 11C-MET uptake with preoperative tumor grading16 and the prognosis of gliomas.17
Although 11C-MET PET has shown a possible association between 11C-MET uptake and the molecular mechanisms of gliomas, the relationship between the key molecular biomarkers and 11C-MET uptake has been rarely studied. Molecular biomarkers, which are used for diagnosis and prognostication, must be identified by surgery. However, preoperative diagnosis and prognostication can alter the medical treatment strategy. Given the value of noninvasive preoperative evaluation of molecular biomarkers for clinical applications, we evaluated the relationship between 11C-MET PET uptake and IDH1 in patients with newly diagnosed diffuse supratentorial gliomas.
Material and Methods
Study Population
Between June 2012 and November 2017, we enrolled patients with newly diagnosed supratentorial diffuse glioma (WHO II and III) in this retrospective study. Patients who had previously undergone surgery, radiotherapy, or chemotherapy were excluded. Surgery was performed within a month of the latest diagnostic 11C-MET PET. Histological diagnosis was based on biopsy or tumor resection, as available. The study was approved by the institutional review board, and informed consent was obtained from all individual participants included in the study.
11C-Methionine Positron Emission Tomography and Computed Tomography
All patients underwent 11C-MET PET using a 3-dimensional (3D) PET scanner (Biograph 64; Siemens, Munich, Germany). Data acquisition began 15 minutes after an intravenous bolus injection of 370 MBq (10 mCi) 11C-MET. Special analysis and fasting were not required for 11C-MET PET because no collateral effects were reported. Before PET, computed tomography (CT) was performed for attenuation correction and image fusion. 11C-methionine PET images were obtained in a 15-minute static scan in 3D acquisition mode. The spatial resolution of the CT scanner in axial and tangential directions was 1.5 mm. Reconstructed imaging was achieved with filtered back projection and full width at a maximum of 3.5 mm using the manufacturer’s workstation and postreconstruction Gaussian 3D filter smoothing.
11C-Methionine Positron Emission Tomography/CT Data Processing
Semiquantitative analysis was performed using the maximum standardized uptake value (SUVmax) and mean tumor-to-background ratio (TBRmean). For SUVmax, 3D spherical volume of interest that covered the entire tumor was drawn, and the workstation automatically detected tumor boundaries. Further manual adjustments were then made to allow region of interest (ROI) to cover the whole tumor shown on the magnetic resonance imaging (MRI), and the SUVmax value inside the ROI was calculated. If the tumor was hypometabolic on 11C-MET PET, MRI only was used to delineate the tumor. For TBRmean, a circular ROI (7-mm diameter) was placed in the most intense area of the tumor in the center of the pixel with the maximum SUV, and in the unaffected corresponding contralateral region.18 If the tumor affected the mirroring region, the ROI was placed on the intact region of the contralateral hemisphere. Mean tumor-to-background ratio was defined as the mean uptake of radioisotope in the lesion ROI divided by the mean uptake in the reference ROI.
Histopathological and Immunohistochemical Examinations
Samples obtained by biopsy and tumor resection were fixed in 10% formalin and embedded in paraffin. The hematoxylin and eosin-stained sections of all samples were reviewed by 2 blinded neuropathologists and were categorized according to the WHO Classification of Tumors of the CNS. The IDH1R132H protein expression was evaluated to determine the IDH1 mutation status because antibodies against IDH1-mutated proteins are routinely used to detect IDH mutation.19 The histological definition was updated according to the WHO 2016 Classification of Tumors of the CNS, when all necessary data were available.
Statistical Analysis
Descriptive statistics were presented as mean with 95% confidence interval (CI) or mean with standard deviation. The relationships between 11C-MET PET parameters and IDH mutation status were assessed. Student t test was performed for 2-group comparisons, with adjustments for cases with unequal variances, as analyzed by Levene test. Value of P < .05 was considered statistically significant. SPSS software (version 21, IBM, Armonk, New York) was used for data analysis.
Results
Study Population
A total of 86 patients with newly diagnosed supratentorial diffuse gliomas were enrolled in this study and their descriptive data are summarized in Table 1. Isocitrate dehydrogenase mutations accounted for 55.8% (48 of 86) of all patients. Of the 61 patients diagnosed as WHO grade II glioma, 68.9% (42 of 61) had IDH1 mutation. Of the remaining 25 patients who were diagnosed as WHO grade III glioma, 24% (9 of 25) had IDH mutations. Of the enrolled patients, 22.1% (19 of 86) had a negative 11C-MET PET uptake. Eleven patients with photopenic defects could be identified among these 19 negative 11C-MET PET scans.
Table 1.
Patient Characteristics, Clinical Data, Pathologic Findings.a
| Characteristics | Value |
|---|---|
| Age, year | |
| Range | 8-67 |
| Mean | 41.9 ± 13.1 |
| Sex | |
| Male | 48 (55.8%) |
| Female | 38 (44.2%) |
| Surgical procedure | |
| Resection | 62 (72.1%) |
| Biopsy | 24 (27.9%) |
| WHO grade and histologic diagnosis | |
| WHO II | |
| Astrocytoma | 44 (51.2%) |
| Oligodendroglioma | 16 (18.6%) |
| Pleomorphic xanthoastrocytoma | 1 (1.2%) |
| WHO III | |
| Anaplastic astrocytoma | 25 (29.1%) |
| IDH1 mutation status | |
| IDH1 mutation | 48 (55.8%) |
| IDH1 wild-type | 38 (44.2%) |
Abbreviations: IDH1, isocitrate dehydrogenase 1; WHO, World Health Organization.
a Values are shown as the number of patients, unless otherwise indicated.
Relationship Between 11C-MET Uptake and Glioma Grade
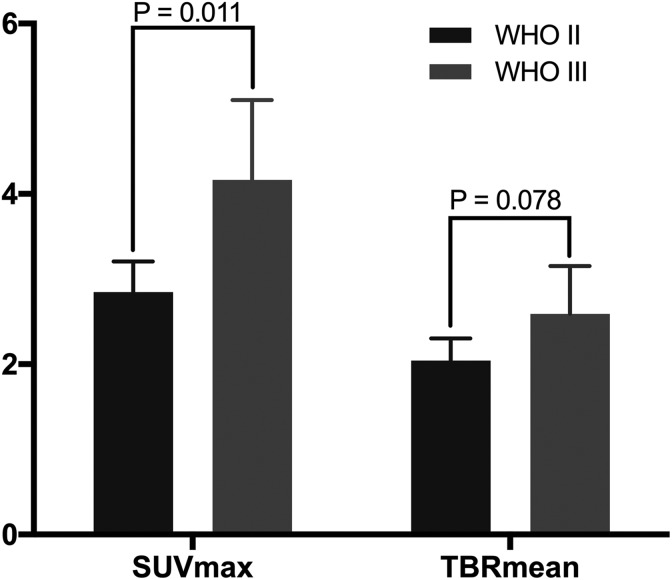
The 11C-MET uptake values were compared between the tumor grades. As shown in Figure 1, the SUVmax was significantly higher for grade III gliomas than for grade II gliomas (4.16 [95% CI: 3.29-5.05] vs 2.85 [95% CI: 2.49-3.22]; P = .011), whereas the TBRmean values were not significantly different between grade II and grade III gliomas (2.04 [95% CI: 1.80-2.32] vs 2.59 [95% CI: 2.08-3.13], respectively; P = .078).
Figure 1.
Relationship between 11C-MET uptake and glioma grade. The SUVmax of grade III gliomas is significantly higher than that of grade II gliomas (P = .011), whereas there was no significant difference in the TBR mean values of grades II and III gliomas (P = .078). 11C-MET indicates 11C-methionine; IDH1, isocitrate dehydrogenase 1; SUVmax, maximum standardized uptake value; TBRmean, mean tumor-to-background ratio.
Effect of the Oligodendroglial Component on the 11C-MET Uptake
In this study, gliomas with oligodendroglial component accounted for 18.6% (16 of 86) of all cases and were all grade II gliomas. Gliomas with oligodendroglial component and those without oligodendroglial component had no significant differences in SUVmax (2.89 [95% CI: 2.38-3.41] vs 3.31 [95% CI: 2.87-3.41], respectively; P = .232) and TBRmean (2.02 [95% CI: 1.71-2.30] vs 2.25 [95% CI: 1.97-2.57], respectively; P = .268).
Separate analyses of grade II gliomas showed that those with oligodendroglial component accounted for 22.5% (16 of 71). Gliomas with oligodendroglial component and those without oligodendroglial component had no significant differences in SUVmax (2.89 [95% CI: 2.41-3.38] vs 2.83 [95% CI: 2.39-3.31]; P = .896) and TBRmean values (2.02 [95% CI: 1.73-2.30] vs 2.05 [95% CI: 1.75-2.41]; P = .900).
Relationship Between 11C-MET Uptake and IDH1 Mutation Status
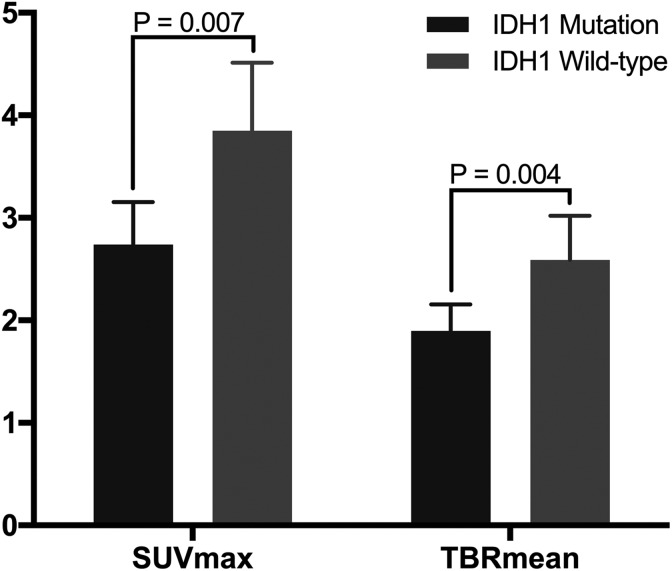
The IDH1 mutation status of the supratentorial diffuse gliomas and its relationships with the 11C-MET parameters were analyzed. As shown in Figure 2, compared to tumors with IDH1 mutation, wild-type IDH1 tumors had significantly higher SUVmax values (2.73 [95% CI: 2.32-3.16] vs 3.85 [95% CI: 3.22-4.51]; P = .004) and TBRmean values (1.90 [95% CI: 1.65-2.16] vs 2.59 [95% CI: 2.17-3.04]; P = .007). Representative cases are shown in Figure 3.
Figure 2.
Relationship between 11C-MET parameter values and IDH1 mutation status. Gliomas with mutant and wild-type IDH1 have significantly different SUVmax values (P = .007) and TBR mean values (P = .004). 11C-MET indicates 11C-methionine; IDH1, isocitrate dehydrogenase 1; SUVmax, maximum standardized uptake value; TBRmean, mean tumor-to-background ratio.
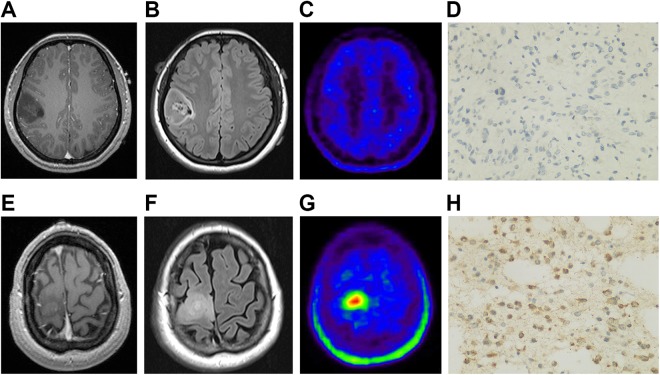
Figure 3.
Representative cases. A, T1-weighted MRI shows a low-intensity lesion in the right frontal lobe. B, Fluid-attenuated inversion-recovery MRI outlines the margin of the lesion. C, 11C-methionine PET shows weak accumulation in the lesion with SUVmax of 1.25 and TBRmean of 0.77. D, Surgery confirms the diagnosis of IDH1 mutated astrocytoma was confirmed. E, T1-weighted MRI shows a low-intensity lesion in the right frontal lobe. F, Fluid-attenuated inversion-recovery MRI outlines the margin of the lesion. G, 11C-MET PET shows strong accumulation in the lesion, with SUVmax of 8.45 and TBRmean of 3.25. H, Surgery confirms the diagnosis of IDH1 wild-type anaplastic astrocytoma was confirmed. 11C-MET PET indicates 11C-methionine positron emission tomography; IDH1, isocitrate dehydrogenase 1; MRI, magnetic resonance imaging; SUVmax, maximum standardized uptake; TBRmean, mean tumor-to-background ratio.
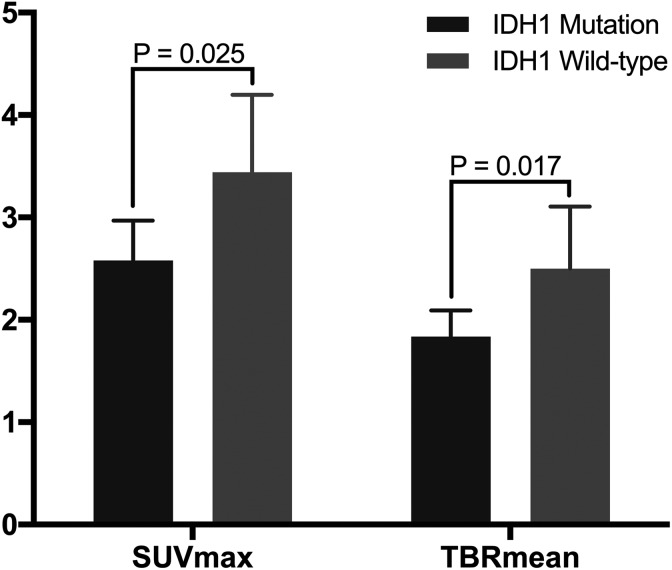
Separate analyses of grade II gliomas showed that, compared to tumors with IDH1 mutation, wild-type IDH1 tumors had significantly higher SUVmax values (2.58 [95% CI: 2.21-2.96] vs 3.44 [95% CI: 2.78-4.21]; P = .025) and TBRmean values (1.84 [95% CI: 1.60-2.11] vs 2.50 [95% CI: 1.98-3.08]; P = .017; Figure 4).
Figure 4.
Relationship between 11C-MET parameter values and IDH1 mutation status in WHO II gliomas. Gliomas with mutant and wild-type IDH1 have significantly different SUVmax values (P = .025) and TBRmean values (P = .017). 11C-MET indicates 11C-methionine; IDH1, isocitrate dehydrogenase 1; SUVmax, maximum standardized uptake value; TBRmean, mean tumor-to-background ratio; WHO, World Health Organization.
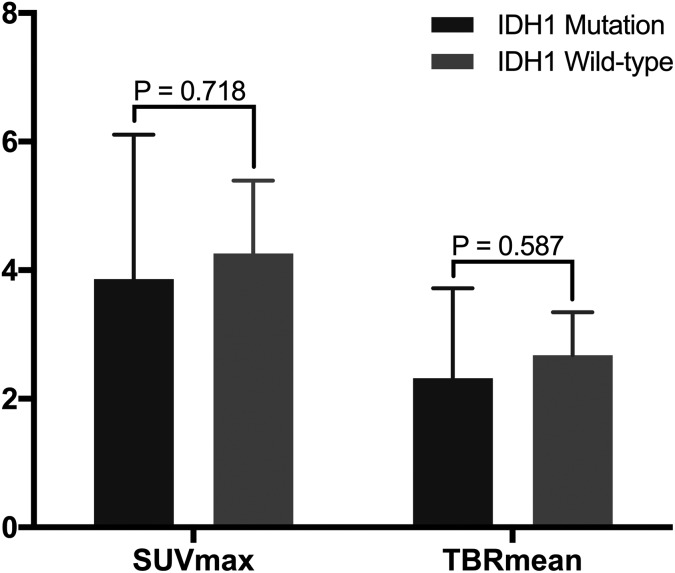
On the other hand, separate analyses of grade III supratentorial gliomas showed no such relationships between the 11C-MET parameters and IDH1 mutation status. Compared with tumors with IDH1 mutation, wild-type IDH1 tumors had similar SUVmax values (3.86 [95% CI: 2.13-5.65] vs 4.26 [95% CI: 3.26-5.27], respectively; P = .718) and TBRmean values (2.32 [95% CI: 1.28-3.42] vs 2.68 [95% CI: 2.09-3.27], respectively; P = .587; Figure 5).
Figure 5.
Relationship between 11C-MET parameter values and IDH1 mutation status in WHO III gliomas. Gliomas with mutant and wild-type IDH1 have no statistically significant difference in SUVmax values (P = .718) and TBRmean values (P = .587). 11C-MET indicates 11C-methionine; IDH1, isocitrate dehydrogenase 1; SUVmax, maximum standardized uptake value; TBRmean, mean tumor-to-background ratio; WHO, World Health Organization.
Discussion
The diagnosis and prognosis of diffuse gliomas had been primarily determined based on histopathological characteristics. However, the pathological classification of gliomas had been limited by sample heterogeneity. Because genomic analyses have dramatically increased our understanding of gliomas, we have been pursuing the trend of biomarker-driven glioma classification, which has been consistently proven to play a significant role in revealing the molecular characteristics and in the prognostication of gliomas. In particular, the mutation status of IDH, ATRX, TP53, and 1p/19q had been employed for the diagnosis and prognostication of diffuse gliomas, and MGMT promoter methylation status was reported to alter the chemotherapy plan.20
Prominent evidence has indicated that IDH mutations are early events in glioma formation before molecular distinctions drive specific differentiation and frequently occur in astrocytic and oligodendroglial lineages; examples of such mutations are 1p/19q codeletions in oligodendrogliomas and TP53 and ATRX mutations in astrocytomas.3 Isocitrate dehydrogenase mutations can be found in up to 80% of grades II to III gliomas and secondary glioblastomas but rarely in primary glioblastomas.3,4 Furthermore, compared with IDH-mutated grade II to III gliomas, those with wild-type IDH more frequently show molecular genetic alterations, such as epidermal growth factor receptor (EGFR), telomerase reverse transcriptase (TERT), Phosphatase And Tensin Homolog (PTEN), Cyclin Dependent Kinase Inhibitor 2A (CDKN2A), neurofibromatosis type 1 (NF1), and RB Transcriptional Corepressor 1 (RB1) alterations, which resemble the mutations in glioblastomas.3,5 Genetic similarities between IDH wild-type glioblastomas and IDH wild-type diffuse gliomas lead to the same aggressive clinical behaviors.6 Compared to gliomas without IDH mutations, IDH-mutated gliomas progress slower over time and have better prognosis.4,7 Meanwhile, gliomas that have relatively high 11C-MET uptake have been reported to have poor prognosis.21 This may explain the significantly higher 11C-MET uptake in gliomas with IDH1 wild-type than in those with mutants in our study.
Previously, 11C-MET PET had been used to predict the status of 1p/19q codeletion22-24 and MGMT promoter methylation,24,25 although the correlations remain controversial. Moreover, the correlation between IDH mutation status and 11C-MET uptake had been rarely studied. In this study, patients with newly diagnosed nonenhancing supratentorial diffuse gliomas were demonstrated to have higher SUVmax and TBRmean values when IDH1 mutation was present than when there was no IDH1 mutation. A prior study investigated the association between IDH1 mutation status in diffuse gliomas and 18F-fluorodeoxyglucose (18F-FDG) uptake, but the result showed that IDH1 mutation status did not correlate with 18F-FDG uptake.26 A possible explanation for this result was that the necrosis and blood–brain barrier (BBB) disruption in more aggressive gliomas resulted in a nonlinear increase in 18F-FDG uptake. A study using dynamic 18F-fluoroethyltyrosine (18F-FET) PET demonstrated that IDH mutations were more frequent in tumors with homogeneous increasing and focal decreasing time-activity curve but were rare in those exhibiting homogeneous decreasing time-activity curve.27 Verger et al28 have shown that 18F-FET PET had considerable diagnostic value for discriminating between astrocytomas and glioblastomas with mutant and wild-type IDH, suggesting that 18F-FET PET may be useful for noninvasive identification of IDH mutation status. However, another study that used static 18F-FET PET found no association between IDH mutation status and 18F-FET uptake in low-grade glioma patients.29 Using 3,4-dihydroxy-6-18F-fluoro-L-phynel-alanine (18F-FDOPA) PET, one study illustrated higher 18F-FDOPA uptake in gliomas with IDH mutation than in IDH wild-type gliomas.30 Previously, a study on 11C-MET PET indicated that IDH-mutated gliomas showed lower 11C-MET uptake, compared to that of IDH wild-type gliomas.24 This finding was contradictory to the findings of the previous study on 18F-FDOPA. The apparent discrepancy in the uptake patterns between 11C-MET and 18F-FDOPA may be associated with the metabolic profile of IDH-mutated gliomas. Isocitrate dehydrogenase mutation status is associated with elevated intracellular free amino acids, which can reduce efflux rate constant from the tumor tissue and affect the uptake of 18F-FDOPA via amino acid transporters that act as exchangers.31,32 In a previous study on 11C-MET, the samples included high-grade and recurrent gliomas with disrupted BBBs, the 11C-MET uptake in gliomas was influenced by passive diffusion in areas with BBB.33 In our study, all gliomas had intact BBBs and were shown as nonenhancing supratentorial diffuse gliomas on MRI. Therefore, methionine uptake was relatively less influenced by BBB disruption and only reflected the metabolic tumor. After ruling out the aforementioned factor, our findings consistently confirmed that 11C-MET uptake was significantly correlated with IDH1 mutation status in supratentorial diffuse glioma patients. However, the results were not significant in the subgroup of WHO III gliomas alone; this may be accounted for by the relatively few supratentorial WHO III gliomas in our study population.
In this study, the significant correlation of the 11C-MET PET parameters with IDH1 mutation status indicated the potential value of 11C-MET PET for noninvasive evaluation of IDH1 mutation status. Although IDH1 detection mainly relies on postoperative pathological examination, preoperative noninvasive detection methods can enable the development of more personalized treatment plans. Beiko et al8 found that IDH1 mutation was an independent predictor of complete resection and that IDH1-mutated gliomas were more frontally located. Kawaguchi et al34 suggested that IDH1-mutated grade III tumors without 1p/19q codeletion require a greater extent of resection. Maarten et al35 demonstrated that even very small postoperative volumes can have a strong negative effect on the overall survival of patients with IDH-mutated astrocytoma. A previous study on patients with IDH wild-type glioma reported that the median survival of patients with totally resected tumors was 2.86 years, whereas that for patients with partially resected tumors was 1.55 years.9 Moreover, compared to IDH wild-type tumors, IDH-mutated tumors were suggested to have higher response rate to chemotherapy and longer progression-free survival after radiation therapy or chemotherapy.36 Moreover, IDH mutations in low grade gliomas were shown to predict longer survival and response to temozolomide.33 Therefore, the IDH1 mutation status of gliomas can enable individualized treatment strategies and make preoperative noninvasive detection essential.
Because the treatment regimens and strategies vary among different tumor classes, noninvasive imaging methods that predict tumor grade can provide clinicians the directions for treatment before surgery. Relevant studies on 11C-MET PET have drawn different conclusions. Some studies have shown that 11C-MET PET had certain significance in tumor classification,16,37,38 whereas other studies have shown opposite results.39-41 In our study, the SUVmax of grade III gliomas was significantly higher than that of the grade II gliomas, whereas the results on TBRmean showed only a certain trend but did not reach a statistically significant difference. The differences among the studies may be due to the different sample compositions because different gliomas have some differences in 11C-MET uptake. Some literatures reported that 11C-MET uptake may be higher in oligodendrogliomas than in astrocytomas.17,42,43 However, in our study, the SUVmax and TBRmean did not significantly differ according to the presence of an oligodendroglial component. Perhaps, this was because gliomas with oligodendroglial component accounted for a small proportion of our samples, and all of them were WHO II gliomas.
Recently, Galldiks et al44 reported that within 100 patients with 8F-FET negative gliomas, a subgroup of patients who exhibit photopenic defects in 8F-FET uptake had an unfavorable outcome compared to patients with isometabolic 8F-FET uptake, and the multivariate survival analysis indicated that photopenic defects predict an unfavorable progression-free survival (PFS). Further follow-up imaging changes showed that photopenic IDH-mutant WHO II astrocytomas turned into 8F-FET positive faster than isometabolic IDH-mutant WHO II astrocytomas.45 The survival discrepancy could be explained with an inverted U-shaped curve, showing isometabolic gliomas with the most favorable PFS and both photopenic gliomas and 8F-FET positive gliomas on the adverse end of the PFS spectrum; however, the hypothesis remains to be confirmed by the overall survival analysis.46 Moreover, a study using 18F-FDOPA PET demonstrated patients with photopenic defects showed a tendency for a shorter PFS in comparison to patients with isometabolic 18F-FDOPA uptake.45 In our study, 11 patients were identified as photopenic defects among the 19 patients who had negative 11C-MET PET uptake. However, due to the small sample size and the lack of survival time data, further analysis was not feasible. Whether the relationship between 11C-MET uptake and survival time also has the same U-shaped curve as 8F-FET PET is worth further exploration.
Some limitations of our study should be noted. First, the study was retrospective with a relatively small sample size. Therefore, the prognostic value of 11C-MET PET must be validated in a larger prospective study. Second, the IDH1 mutations in our study were detected by immunohistochemistry. Although immunohistochemistry can detect most IDH1 mutations and is widely applied in clinical practice, genetic sequencing is needed to further improve the diagnostic accuracy.47,44
Conclusions
The 11C-MET uptake was significantly related with IDH1 mutation status in patients with supratentorial diffuse glioma. From a clinical perspective, 11C-MET PET can be used as an additional tool for noninvasive preoperative evaluation of the IDH1 mutation status of nonenhancing supratentorial diffuse gliomas.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.81672476) and Shanghai Sailing Program (No.16YF1415400).
Authors’ Note: Nijiati Kudulaiti and Huiwei Zhang contributed equally to this article and should be considered co-first authors. All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the National Natural Science Foundation of China (Nos.81672476), Shanghai Sailing Program (No.16YF1415400).
ORCID iD: Dongxiao Zhuang, MD, PhD  https://orcid.org/0000-0002-4964-6529
https://orcid.org/0000-0002-4964-6529
References
- 1. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 2014;16(suppl 4):iv1–63. doi:10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weller M, Pfister SM, Wick W, et al. Molecular neuro-oncology in clinical practice: a new horizon. Lancet Oncol. 2013;14:e370–e379. doi:10.1016/S1470-2045(13)70168-2. [DOI] [PubMed] [Google Scholar]
- 3. Cancer Genome Atlas Research Network, Brat DJ, Verhaak RG, Aldape KD, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372:2481–2498. doi:10.1056/NEJMoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi:10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N Engl J Med. 2015;372:2499–2508. doi:10.1056/NEJMoa1407279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Appin CL, Brat DJ. Biomarker-driven diagnosis of diffuse gliomas. Mol Aspects Med. 2015;45:87–96. doi:10.1016/j.mam.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 7. Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27:4150–4154. doi:10.1200/JCO.2009.21.9832. [DOI] [PubMed] [Google Scholar]
- 8. Beiko J, Suki D, Hess KR, et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 2014;16:81–91. doi:10.1093/neuonc/not159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aibaidula A, Chan AK, Shi Z, et al. Adult IDH wild-type lower-grade gliomas should be further stratified. Neuro Oncol. 2017;19:1327–1337. doi:10.1093/neuonc/nox078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi:10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 11. Kato T, Shinoda J, Nakayama N, et al. Metabolic assessment of gliomas using 11C-methionine, [18F] fluorodeoxyglucose, and 11C-choline positron-emission tomography. AJNR Am J Neuroradiol. 2008;29:1176–1182. doi:10.3174/ajnr.A1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shishido H, Kawai N, Miyake K, et al. Diagnostic value of 11C-methionine (MET) and 18F-fluorothymidine (FLT) positron emission tomography in recurrent high-grade gliomas; differentiation from treatment-induced tissue necrosis. Cancers. 2012;4:244–256. doi:10.3390/cancers4010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pirotte B, Goldman S, Dewitte O, et al. Integrated positron emission tomography and magnetic resonance imaging-guided resection of brain tumors: a report of 103 consecutive procedures. J Neurosurg. 2006;104:238–253. doi:10.3171/jns.2006.104.2.238. [DOI] [PubMed] [Google Scholar]
- 14. Matsuo M, Miwa K, Tanaka O, et al. Impact of [11C]methionine positron emission tomography for target definition of glioblastoma multiforme in radiation therapy planning. Int J Radiat Oncol Biol Phys. 2012;82:83–89. doi:10.1016/j.ijrobp.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 15. Glaudemans AW, Enting RH, Heesters MA, et al. Value of 11C-methionine PET in imaging brain tumours and metastases. Eur J Nucl Med Mol Imaging. 2013;40:615–635. doi:10.1007/s00259-012-2295-5. [DOI] [PubMed] [Google Scholar]
- 16. Hatakeyama T, Kawai N, Nishiyama Y, et al. 11C-methionine (MET) and 18F-fluorothymidine (FLT) PET in patients with newly diagnosed glioma. Eur J Nucl Med Mol Imaging. 2008;35:2009–2017. doi:10.1007/s00259-008-0847-5. [DOI] [PubMed] [Google Scholar]
- 17. De Witte O, Goldberg I, Wikler D, et al. Positron emission tomography with injection of methionine as a prognostic factor in glioma. J Neurosurg. 2001;95:746–750. doi:10.3171/jns.2001.95.5.0746. [DOI] [PubMed] [Google Scholar]
- 18. Kracht LW, Miletic H, Busch S, et al. Delineation of brain tumor extent with [11C]L-methionine positron emission tomography: local comparison with stereotactic histopathology. Clin Cancer Res. 2004;10:7163–7170. doi:10.1158/1078-0432.CCR-04-0262. [DOI] [PubMed] [Google Scholar]
- 19. Capper D, Reuss D, Schittenhelm J, et al. Mutation-specific IDH1 antibody differentiates oligodendrogliomas and oligoastrocytomas from other brain tumors with oligodendroglioma-like morphology. Acta Neuropathol. 2011;121:241–252. doi:10.1007/s00401-010-0770-2. [DOI] [PubMed] [Google Scholar]
- 20. Rodriguez FJ, Vizcaino MA, Lin MT Recent advances on the molecular pathology of glial neoplasms in children and adults. J Mol Diagn. 2016;18:620–634. doi:10.1016/j.jmoldx.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim S, Chung JK, Im SH, et al. 11C-methionine PET as a prognostic marker in patients with glioma: comparison with 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2005;32:52–59. doi:10.1007/s00259-004-1598-6. [DOI] [PubMed] [Google Scholar]
- 22. Saito T, Maruyama T, Muragaki Y, et al. 11C-methionine uptake correlates with combined 1p and 19q loss of heterozygosity in oligodendroglial tumors. AJNR Am J Neuroradiol. 2013;34:85–91. doi:10.3174/ajnr.A3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shinozaki N, Uchino Y, Yoshikawa K, et al. Discrimination between low-grade oligodendrogliomas and diffuse astrocytoma with the aid of 11C-methionine positron emission tomography. J Neurosurg. 2011;114:1640–1647. doi:10.3171/2010.11.JNS10553. [DOI] [PubMed] [Google Scholar]
- 24. Lopci E, Riva M, Olivari L, et al. Prognostic value of molecular and imaging biomarkers in patients with supratentorial glioma. Eur J Nucl Med Mol Imaging. 2017;44:1155–1164. doi:10.1007/s00259-017-3618-3. [DOI] [PubMed] [Google Scholar]
- 25. Okita Y, Nonaka M, Shofuda T. et al. (11)C-methinine uptake correlates with MGMT promoter methylation in nonenhancing gliomas. Clin Neurol Neurosurg. 2014;125:212–216. doi:10.1016/j.clineuro.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 26. Metellus P, Colin C, Taieb D, et al. IDH mutation status impact on in vivo hypoxia biomarkers expression: new insights from a clinical, nuclear imaging and immunohistochemical study in 33 glioma patients. J Neurooncol. 2011;105:591–600. doi:10.1007/s11060-011-0625-2. [DOI] [PubMed] [Google Scholar]
- 27. Thon N, Kunz M, Lemke L, et al. Dynamic 18F-FET PET in suspected WHO grade II gliomas defines distinct biological subgroups with different clinical courses. Int J Cancer. 2015;136:2132–2145. doi:10.1002/ijc.29259. [DOI] [PubMed] [Google Scholar]
- 28. Verger A, Stoffels G, Bauer EK, et al. Static and dynamic (18)F-FET PET for the characterization of gliomas defined by IDH and 1p/19q status. Eur J Nucl Med Mol Imaging. 2018;45:443–451. doi:10.1007/s00259-017-3846-6. [DOI] [PubMed] [Google Scholar]
- 29. Bette S, Gempt J, Delbridge C, et al. Prognostic value of O-(2-[18F]-Fluoroethyl)-L-tyrosine-positron emission tomography imaging for histopathologic characteristics and progression-free survival in patients with low-grade Glioma. World Neurosurg. 2016;89:230–239. doi:10.1016/j.wneu.2016.01.085. [DOI] [PubMed] [Google Scholar]
- 30. Verger A, Metellus P, Sala Q, et al. IDH mutation is paradoxically associated with higher (18)F-FDOPA PET uptake in diffuse grade II and grade III gliomas. Eur J Nucl Med Mol Imaging. 2017;44:1306–1311. doi:10.1007/s00259-017-3668-6. [DOI] [PubMed] [Google Scholar]
- 31. Verger A, Taieb D, Guedj E. Is the information provided by amino acid PET radiopharmaceuticals clinically equivalent in gliomas? Eur J Nucl Med Mol Imaging. 2017;44:1408–1410. doi:10.1007/s00259-017-3710-8. [DOI] [PubMed] [Google Scholar]
- 32. Kameyama M, Umeda-Kameyama Y. A kinetic solution for the paradoxical difference between F-Dopa and methionine. Eur J Nucl Med Mol Imaging. 2017;44:2328–2330. doi:10.1007/s00259-017-3796-z. [DOI] [PubMed] [Google Scholar]
- 33. Nojiri T, Nariai T, Aoyagi M, et al. Contributions of biological tumor parameters to the incorporation rate of L: -[methyl-(11)C] methionine into astrocytomas and oligodendrogliomas. J Neurooncol. 2009;93:233–241. doi:10.1007/s11060-008-9767-2. [DOI] [PubMed] [Google Scholar]
- 34. Kawaguchi T, Sonoda Y, Shibahara I, et al. Impact of gross total resection in patients with WHO grade III glioma harboring the IDH 1/2 mutation without the 1p/19q co-deletion. J Neurooncol. 2016;129:505–514. doi:10.1007/s11060-016-2201-2. [DOI] [PubMed] [Google Scholar]
- 35. Wijnenga MMJ, French PJ, Dubbink HJ, et al. The impact of surgery in molecularly defined low-grade glioma: an integrated clinical, radiological and molecular analysis. Neuro Oncol. 2017; 20 103–112. doi:10.1093/neuonc/nox176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cohen AL, Holmen SL, Colman H. IDH1 and IDH2 mutations in gliomas. Curr Neurol Neurosci. 2013;13:345 doi:10.1007/s11910-013-0345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sasaki M, Kuwabara Y, Yoshida T, et al. A comparative study of thallium-201 SPET, carbon-11 methionine PET and fluorine-18 fluorodeoxyglucose PET for the differentiation of astrocytic tumours. Eur J Nucl Med. 1998;25(9):1261–1269. [DOI] [PubMed] [Google Scholar]
- 38. Sadeghi N, Salmon I, Tang BN, et al. Correlation between dynamic susceptibility contrast perfusion MRI and methionine metabolism in brain gliomas: preliminary results. J Magn Reson Imaging. 2006;24:989–994. doi:10.1002/jmri.20757. [DOI] [PubMed] [Google Scholar]
- 39. Ceyssens S, Van Laere K, de Groot T, et al. [11C]methionine PET, histopathology, and survival in primary brain tumors and recurrence. AJNR Am J Neuroradiol. 2006;27(7):1432–1437. [PMC free article] [PubMed] [Google Scholar]
- 40. Moulin-Romsee G, D’Hondt E, de Groot T, et al. Non-invasive grading of brain tumours using dynamic amino acid PET imaging: does it work for 11C-methionine? Eur J Nucl Med Mol Imaging. 2007;34:2082–2087. doi:10.1007/s00259-007-0557-4. [DOI] [PubMed] [Google Scholar]
- 41. Galldiks N, Kracht LW, Berthold F, et al. [11C]-L-methionine positron emission tomography in the management of children and young adults with brain tumors. J Neurooncol. 2010;96:231–239. doi:10.1007/s11060-009-9953-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Herholz K, Holzer T, Bauer B, et al. 11C-methionine PET for differential diagnosis of low-grade gliomas. Neurology. 1998;50(5):1316–1322. [DOI] [PubMed] [Google Scholar]
- 43. Kato T, Shinoda J, Oka N, et al. Analysis of 11C-methionine uptake in low-grade gliomas and correlation with proliferative activity. AJNR Am J Neuroradiol. 2008;29:1867–1871. doi:10.3174/ajnr.A1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Galldiks N, Unterrainer M, Judov N, et al. Photopenic defects on O-(2-[18F]-fluoroethyl)-L-tyrosine PET: clinical relevance in glioma patients. Neuro Oncol. 2019;21:1331–1338. doi:10.1093/neuonc/noz083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Galldiks N, Verger A, Zaragori T, et al. Comment on “Hypometabolic gliomas on FET-PET—Is there an inverted U-curve for survival?”, Kamson 2019, Neuro-Oncology. Neuro Oncol. 2019. doi:10.1093/neuonc/noz173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kamson DO. Hypometabolic gliomas on FET-PET-is there an inverted U-curve for survival? Neuro Oncol. 2019;21:1221–1222. doi:10.1093/neuonc/noz122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Camelo-Piragua S, Kesari S. Further understanding of the pathology of glioma: implications for the clinic. Expert Rev Neurother. 2016;16:1055–1065. doi:10.1080/14737175.2016.1194755. [DOI] [PubMed] [Google Scholar]