Abstract
Background
Accumulating evidence has shown that the signal from spinal brain-derived neurotrophic factor/tyrosine receptor kinase B-K+-Cl− cotransporter-2 plays a critical role in the process of pain hypersensitivity. The activation of alpha-7 nicotinic acetylcholine receptors could have an analgesic effect on remifentanil-induced postoperative hyperalgesia. Nevertheless, whether intrathecal administration of PNU-120596, an alpha-7 nicotinic acetylcholine receptors selective type II positive allosteric modulator, before surgery could affect the duration of remifentanil-induced postoperative hyperalgesia remains unknown, and the effects of alpha-7 nicotinic acetylcholine receptors activation on the brain-derived neurotrophic factor/tyrosine receptor kinase B-K+-Cl− cotransporter-2 signal in the spinal dorsal horn of rats with remifentanil-induced postoperative hyperalgesia is still enigmatic.
Results
We demonstrated that the brain-derived neurotrophic factor/tyrosine receptor kinase B-K+-Cl− cotransporter-2 signal played a critical role in the development of remifentanil-induced postoperative hyperalgesia. Intrathecal administration of PNU-120596 (8 µg/kg, 15 min before surgery) was associated with earlier signs of recovery from remifentanil-induced postoperative hyperalgesia. Simultaneously, remifentanil-induced postoperative hyperalgesia-induced K+-Cl− cotransporter-2 downregulation was partly reversed and coincided with a decreased expression of brain-derived neurotrophic factor/tyrosine receptor kinase B in the spinal dorsal horn, approximately correlating with the time course of the nociceptive behavior. Moreover, intrathecal administration of the K+-Cl− cotransporter-2 inhibitor VU0240551 significantly reduced the analgesic effect of PNU-120596 on remifentanil-induced postoperative hyperalgesia.
Conclusions
The activation of alpha-7 nicotinic acetylcholine receptors induced a shorter duration of remifentanil-induced postoperative hyperalgesia by restoring the brain-derived neurotrophic factor/tyrosine receptor kinase B-K+-Cl− cotransporter-2 signal in the spinal dorsal horn of rats, which provides new insight into treatment in clinical postoperative pain management.
Keywords: Remifentanil, hyperalgesia, alpha-7 nicotinic acetylcholine receptors, K+-Cl− cotransporter-2, brain-derived neurotrophic factor/tyrosine receptor kinase B signal
Introduction
Although opioids are among the most effective analgesics in humans,1 there is growing evidence demonstrating that they also induce serious opioid-induced hyperalgesia after acute or chronic administration.2,3 As an ultrashort-acting μ-opioid receptor agonist, remifentanil is commonly used in daily clinical anesthesia. It provides adequate and controllable analgesia and has a rapid onset and short duration of action without accumulative effects after intravenous infusion.4 It has been reported that the intraoperative administration of remifentanil might induce hyperalgesia (RIH), worsen postoperative pain, and increase analgesic requirement after surgery. In addition, RIH could last for a considerable time, which would delay patients’ mobilization and rapid recovery of function.5–8 However, the underlying mechanisms and signal transduction pathways that mediate pain sensitization are not well known.
In recent years, it has been shown that disinhibition is the main cause of a series of pathological pain states, especially hyperalgesia.9–13 The dominant inhibitory neurotransmitter is GABA, and its main function is through activating GABA receptors. The cotransporter K+-Cl− cotransporter-2 (KCC2) plays a critical role in maintaining low Cl− concentration inside neurons of the central nervous system (CNS), and it is indispensable for postsynaptic inhibition caused by GABA. It has been reported that GABAergic disinhibition is caused by the activation of spinal brain-derived neurotrophic factor/tyrosine receptor kinase B-K+-Cl− cotransporter-2 (BDNF/trkB-KCC2) signal,9,11,14–16 which is the underlying mechanism of morphine-induced hyperalgesia and formalin-induced pain at the spinal level.11,17 Upregulation of spinal microglia BDNF promotes the decrease in KCC2 protein expression and transport activity on the surface of neurons, which results in hyperalgesia.11
Nicotinic acetylcholine receptors (nAChRs) are ligand-gated ion channels present in both the peripheral and CNSs. Multiple subtypes of nAChRs have been found to play a role in modulating pain transmission in the CNS,18 and (alpha-7 nAChRs) α7-nAChRs have been shown to be expressed in the microglia of the spinal dorsal horn.19–22 Agonist-mediated activation of α7-nAChRs has been shown to elicit anti-inflammatory and antinociceptive effects in rodents.23–27 Studies have demonstrated that α7-nAChRs selective type II positive allosteric modulator (PAMs; e.g. PNU-120596) produces anti-hyperalgesia in rats with carrageenan and complete Freund's adjuvant (CFA)-induced hindpaw inflammation.26 According to our previous study, 8 µg PNU-120596 was administered through the intrathecal route 24 h after remifentanil was subcutaneously infused during surgery, and the RIH in rats was alleviated through the downregulation of TNF-α, IL-6, and p-NR2B protein levels in the microglia of the spinal dorsal horn.28 However, it has not been reported whether intrathecal administration of PNU-120596 at 15 min before surgery could affect the duration of RIH, and the effects of PNU-120596 on KCC2 expression in the spinal dorsal horn of rats with RIH has not been elucidated. To address these questions, we first investigated the roles of BDNF/trkB-KCC2 signal in RIH in the spinal dorsal horn in rats. In addition, we explored the effects of presurgery intrathecal administration of PNU-120596 on the duration of RIH and the BDNF/trkB-KCC2 signal in the spinal dorsal horn.
Materials and methods
Animals
Adult male Sprague-Dawley rats weighing 220 to 250 g obtained from the Laboratory Animal Center of Drum Tower Hospital were used in all experiments. Animals were housed under 12-h light/dark conditions in a room with controlled temperature (22 ± 2℃) and relative humidity (55 ± 10%), and they had free access to food and water except during behavioral evaluation. All procedures were approved by the Experimental Animals Welfare and Ethics Committee of Affiliated Drum Tower Hospital of Medical Department of Nanjing University. All efforts were made to minimize the number of animals used and injuries to the animals.
Surgery
The rat model of postoperative pain was developed as previously described by Brennan et al.29,30 Briefly, the animals were anesthetized with sevoflurane delivered for 30 min through a nose mask (induction, 3.5% v/v; surgery, 3.0% v/v) in a sterile operating room. A 1-cm longitudinal incision was made through the skin and fascia, starting at 0.5 cm from the edge of the heel and extending toward the toes of the right hindpaw. The plantaris muscle was elevated using forceps and incised longitudinally, leaving the muscle origin and insertion intact. After hemostasis with gentle pressure, the skin was closed with two mattress sutures of 5-0 nylon. The wound site was covered with aureomycin ointment.
Drug administration
Remifentanil (batch number: 120801, Ren Fu Co, China) and sevoflurane (batch number: 08100931, Heng Rui Co., China) were supplied by the Department of Anesthesiology of Drum Tower Hospital (Nanjing, China). Remifentanil (80 µg/kg) was dissolved in saline (NaCl 0.9%) and infused subcutaneously over a period of 30 min (rate, 0.8 mL/h) using an apparatus pump. Control animals (sham-operated rats) underwent a sham procedure that consisted of the administration of sevoflurane plus the same volume of saline in identical conditions. BDNF-sequester TrkB/Fc (Sigma, USA) of 5 µg was dissolved in 10 µL saline. PNU-120596 (Sigma Chemical Co., St. Louis, MI), an α7-nAChRs agonist, was dissolved in 5% DMSO. The intrathecal injection of TrkB/Fc (5 µg) or PNU-120596 (8 µg) was performed 15 min before remifentanil was subcutaneously infused. A KCC2 inhibitor VU0240551 (Sigma, USA) was dissolved in 10 µL 0.01% DMSO.9,31 An intrathecal dose of 0.8 µg VU0240551 was administered 15 min before surgery in rats given PNU-120596. The intrathecal injections were made into the subarachnoid space on the midline between the L5 and L6 vertebrae in unanesthetized mice, according to the method of Hylden and Wilcox.32 A Hamilton microsyringe with a 30-gauge needle was used.
Behavioral testing
Assessment of mechanical allodynia
To assess mechanical allodynia, the paw withdrawal mechanical threshold (PWMT) was measured using a set of von Frey filaments. The animals were placed in plastic boxes (20 × 20 × 15 cm) with a wire mesh bottom (1 × 1 cm) and allowed to acclimatize for 30 min. Each von Frey filament was applied vertically to the plantar surface adjacent to the wound of right hindpaw for 6 to 8 s with sufficient force. Positive responses were defined as paw flinching or brisk withdrawal. There was a 5-min interval between withdrawal responses. The PWMT was determined by sequentially increasing and decreasing the stimulus strength as described previously.33 Each rat was tested five times per stimulus strength, and three or more positive responses of the lowest strength were considered as PWMT. The measurement of PWMT was conducted three times at each time point.
Assessment of thermal hyperalgesia
The paw withdrawal thermal latency (PWTL) was measured using a radiant thermal (BME410A, Institute of Biological Medicine, Academy of Medical Science, China). Rats were placed in clear plastic cages (20 × 15 × 15 cm) with a glass floor (3 mm thick) and allowed to acclimatize for 30 min before the test.34 A radiant heat source was focused on the plantar surface adjacent to the wound of the right hindpaw through the glass plate. Measurements of PWTL were recorded by a timer that was started by the activation of the heat source and stopped when withdrawal or licking of the hindpaw was detected with a photo-detector. The maximal cutoff time of 25 s was established to prevent tissue damage. There were five trials per rat with a 5-min interval between consecutive tests. The mean PWTL was obtained from the last three stimuli. At each time point, the detection of PWTL was performed three times.
Western blotting analysis
Rats were deeply anesthetized with 5% sevoflurane, and the dorsal horn of the spinal cord L4-L5 segments were removed rapidly and stored in liquid nitrogen. Tissue samples were homogenized in lysis buffer. The homogenate was centrifuged at 12,000 r/min for 10 min at 4℃, and supernatant was removed. The protein concentration was determined by the BCA Protein Assay Kit, following the manufacturer’s instructions. For KCC2, the proteins (70 µg) were prepared with sample buffer containing sulfhydryl-reducing agent, β-mercaptoethanol or not.35 Other proteins (70 µg) were separated on SDS-PAGE (6%–12%) and transferred onto a polyvinyl difluoride (PVDF) membrane. The filter membranes were blocked with 5% nonfat milk for 1 h at room temperature and incubated with the primary antibody KCC2 (1:1000; Sigma), anti-BDNF (1:1000; Sigma), and anti-trkB (1:1000, Abcam). The membrane was washed with tris-buffered saline+tween 20 (TBST) buffer and incubated for 1 h with the secondary antibody conjugated with horseradish peroxidase (1:5000, Jackson Immuno Research, USA) for 1 h at room temperature. Next, the immune complexes were detected using the ECL system (Millipore, Billerica, MA, USA). β-Actin was used as a loading control for total protein. The images of Western blot products were collected and analyzed by Quantity One V4.31 (Bio-Rad, USA).
Immunofluorescence assay
After deep anesthesia was induced, rats were fixed with 4% paraformaldehyde following perfusion with saline. The L4-L6 spinal segments were harvested, fixed overnight, and then cryopreserved in 30% sucrose. Using a cryostat (Leica, Buffalo Grove, IL, USA) 20 -µm-thick sections were cut at −20℃. Slices were blocked with 0.3% Triton X-100 in 10% goat serum and then incubated overnight at 4℃ with mouse anti-KCC2 (1:300; Sigma). After washing three times, sections were incubated in Alexa488-conjugated goat anti-mouse antibody (1:1200; Life Technologies) for 1 h. They were then washed with PBS, and the sections were mounted on glass slides, air-dried, and covered with coverslips by using Aquamount (Fisher Scientific, Ottawa, Canada). Images were taken by using the Leica TCS SP2 multiphoton confocal microscope (Leica Microsystems, Wetzlar, Germany). Images were randomly selected for further analysis.
Experimental design
In part 1, we explored the roles of BDNF/trkB-KCC2 signal in RIH in the spinal dorsal horn.
In section 1, the rats were randomly divided into four groups of equal numbers and received the following treatments: sevoflurane + subcutaneous infusion of saline (a treatment that does not alter nociceptive thresholds36) (control group), sevoflurane + subcutaneous infusion of 80 µg/kg remifentanil (remifentanil group), surgery performed under sevoflurane + subcutaneous infusion of saline (incision group), and surgery performed under sevoflurane + subcutaneous infusion of 80 µg/kg remifentanil (RIH group). The PWMT and PWTL were examined in all experimental groups at baseline (24 h before incision) and 4 h, in addition to postoperative days 1, 2, 4, 7, 10, 12, 14, and 21 (n = 8/group). According to the changes in the behavioral indicators of pain, the rats were randomly sacrificed to isolate the L4-L6 spinal segments for Western blotting (n = 4/group) and immunofluorescence assay (n = 4/group) on postoperative day 1.
In section 2, the rats were randomly selected into four groups of equal numbers and divided into the following groups: the control + saline group, the control + TrkB/Fc group, the RIH + saline group, and the RIH + TrkB/Fc group. The control + TrkB/Fc group and RIH + TrkB/Fc group were intrathecally injected with 5 µg TrkB/Fc (Sigma, USA) (dissolved in 10 µL saline) at 15 min before surgery, while the control + saline group and RIH + saline group were intrathecally injected with the same volume of saline. Animal pain behavior was assessed at baseline (24 h before incision) and 4 h, in addition to postoperative days 1, 2, 4, 7, 10, 12, 14, 21 (n = 8/group). On postoperative day 1, the rats were sacrificed to isolate L4-L6 spinal segments for Western blotting (n = 4/group) and immunofluorescence assay (n = 4/group).
In part 2, we investigated the effect of presurgery administration of PNU-120596 on the duration of RIH in rats and the interaction with BDNF, TrkB, and KCC2 expression in the spinal dorsal horn.
In section 1, the rats were randomly divided into four groups of equal numbers, namely the control + DMSO group, the control + PNU group, the RIH + DMSO group, and the RIH + PNU group. The control + PNU group and RIH + PNU group were intrathecally injected with 8 µg/kg PNU-120596 at 15 min before surgery, while the control + DMSO group and RIH + DMSO group were intrathecally injected with the same volume of DMSO. Pain behaviors were evaluated at baseline (24 h before incision) and 4 h, in addition to postoperative days 1, 2, 4, 7, 10, 12, 14, and 21 (n = 8/group). On postoperative day 1, the rats in the four groups were sacrificed to isolate L4-L6 spinal segments for Western blotting (n = 4/group) and immunofluorescence assay (n = 4/group). At baseline (24 h before incision) 4 h, in addition to postoperative days 1, 2, 4, 7, and 14, the rats in the RIH + DMSO group and RIH + PNU group were also sacrificed to isolate L4-L6 spinal segments for Western blotting (n = 4/group).
In section 2, the rats were randomly divided into four groups of equal numbers: the RIH + PNU group, the RIH + PNU + VU group, the RIH + DMSO group, and the RIH + VU group. The rats in the RIH + PNU + VU group were intrathecally injected with 0.8 µg VU0240551 (Sigma, USA) (dissolved in 10 µl 0.01% DMSO)9 at 15 min before surgery in PNU-120596-treated rats. Pain behaviors were evaluated at baseline (24 h before incision) and 4 h, in addition to postoperative days 1, 2, 4, 7, 10, 12, 14, and 21 days (n = 8/group). Each experiment was repeated at least four times.
Data analysis and statistics
SPSS statistics version 20 (IBM, Armonk, New York, NY, USA) was used for statistical analysis. Results are expressed as mean ± standard deviation. Two-way analysis of variance (ANOVA) followed by LSD post hoc comparison was used to compare the nociceptive thresholds to mechanical and thermal stimuli with group and time. Western blot results were compared by one-way ANOVA and two-way ANOVA (for multiple groups comparison) when necessary. Correlations between BDNF expression levels, KCC2 expression levels, and mechanical allodynia as well as thermal hyperalgesia were analyzed by Pearson’s coefficients. The figures were prepared by Graph Pad Prism Version-7.0 software. A value of P < 0.05 was considered significant.
Results
Changes in duration of remifentanil-induced postoperative hyperalgesia
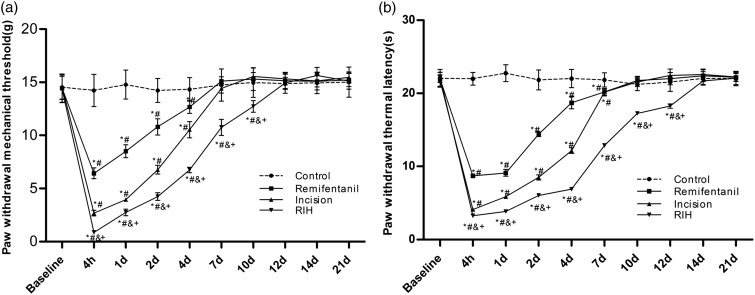
Before the operation, no significant differences were observed for PWMT and PWTL in all groups (P > 0.05). Compared with the baseline and control groups, the remifentanil administration (remifentanil group) and the plantar incision (incision group) induced a decrease in both PWMT (P < 0.05) and PWTL (P < 0.05) in the operated paw during the postoperative period. While intraoperative infusion of remifentanil (RIH group) significantly enhanced hyperalgesia induced by the plantar incision, this was manifested by a decrease in PWMT (P < 0.05) and PWTL (P < 0.05) compared with the remifentanil group and incision group. In all the three treatment groups, the maximal pronociceptive effects were observed between 4 h and two days. Surgery-induced or remifentanil-induced decrease in PWMT (P < 0.05) and PWTL (P < 0.05) returned to baseline or control levels on postoperative day 7 (PWMT) and 10 (PWTL), respectively. However, the durations of mechanical allodynia and thermal hyperalgesia in the RIH group were prolonged up to approximately 12 (PWMT) and 14 (PWTL) days, which were significantly longer than those in the remifentanil group and incision group (P < 0.05) (Figure 1(a) and (b)).
Figure 1.
The time course of the effects of RIH on nociceptive behaviors in rats. PWMT (a) and PWTL (b) were evaluated at 24 h before incision (baseline) and 4 h, 1, 2, 4, 7, 10, 12, 14, and 21 days after surgery was performed under remifentanil anesthesia in rats. Data are expressed as mean ± standard deviation (n = 8 in each group). *P < 0.05 compared with baseline; #P < 0.05 compared with the control group; &P < 0.05 compared with the remifentanil group; +P < 0.05 compared with the incision group.
Protein expression of BDNF and trkB in the spinal dorsal horn after RIH
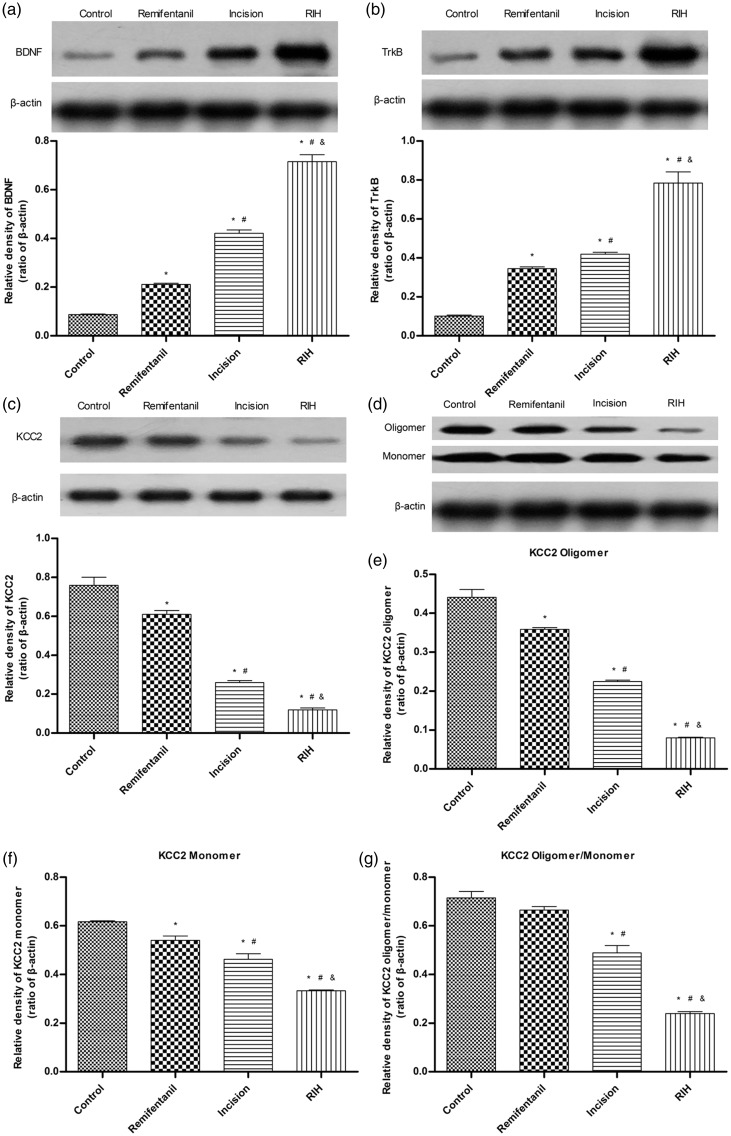
In Figure 2(a) and (b), the results of Western blotting showed that compared with the control group, the remifentanil group and incision group appeared to have upregulated BDNF and trkB protein levels on postoperative day 1 in the dorsal horn of L4-L6 of the spinal cord (P < 0.05), and the protein expression of BDNF and trkB in the RIH group were significantly greater (P < 0.05) than that in the remifentanil group or incision group.
Figure 2.
The expression of BDNF, TrkB, total KCC2, KCC2 oligomer, monomer, and oligomer/monomer ratios in the control group, remifentanil group, incision group, and RIH group one day after manipulation. (a) to (c) Representative Western blotting bands (top) and quantitative data (bottom) for analysis show the protein expression levels of BDNF, TrkB, and total KCC2; (d) Representative Western blotting bands of KCC2 oligomer and monomer in the spinal dorsal horn; (e) to (g) Quantification of the levels of KCC2 oligomer, monomer expression, and oligomer/monomer ratios. β-actin was used as an internal control. Data are expressed as mean ± standard deviation (n = 4 in each group). *P < 0.05 compared with the control group; #P < 0.05 compared with the remifentanil group; &P < 0.05 compared with the incision group.
Downregulation of KCC2 expression in the spinal dorsal horn after RIH
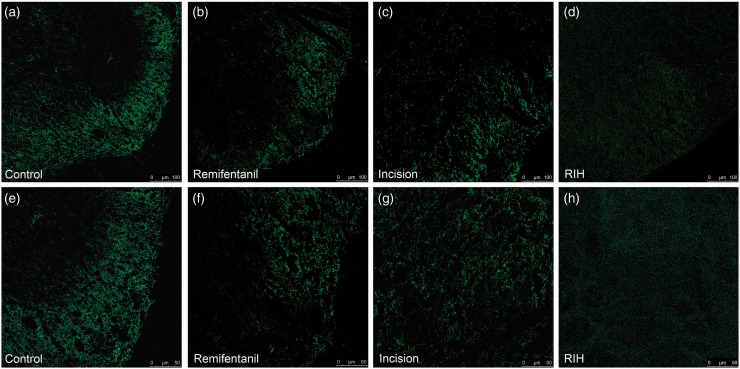
The expression levels of KCC2 in the dorsal horn of L4-L6 spinal segments of rats with RIH were markedly decreased on postoperative day 1 (P < 0.05) (Figure 2(c)). Furthermore, to explore whether RIH affected KCC2 oligomerization that was critical for the transport ability of KCC2, we assessed protein expression with a non-sulfhydryl-reducing sample buffer as previously described.35 The results showed that the KCC2 oligomer, monomer, and oligomer/monomer ratios were significantly downregulated after RIH in the spinal dorsal horn (P < 0.05) (Figure 2(d) to (g)). An immunofluorescence assay confirmed the decrease in KCC2 expression in the spinal dorsal horn after RIH (Figure 3).
Figure 3.
Immunofluorescence of KCC2 in the control group (a and e), remifentanil group (b and f), incision group (c and g), and RIH group (d and h) one day after manipulation. Expression of KCC2 in the spinal dorsal horn was detected by immunofluorescence and observed under a confocal microscope. Panels (a) to (h) show the representative immunofluorescence staining results of the four groups (n = 4 in each group). Note that KCC2 immunoreactivity in the spinal dorsal horn is less intense in the RIH group. Scale bars are 100 µm for panels (a) to (d) and 50 µm for panels (e) to (h).
BDNF-sequester TrkB/Fc significantly reversed the decrease in PWMT and PWTL and upregulated KCC2 expression following RIH
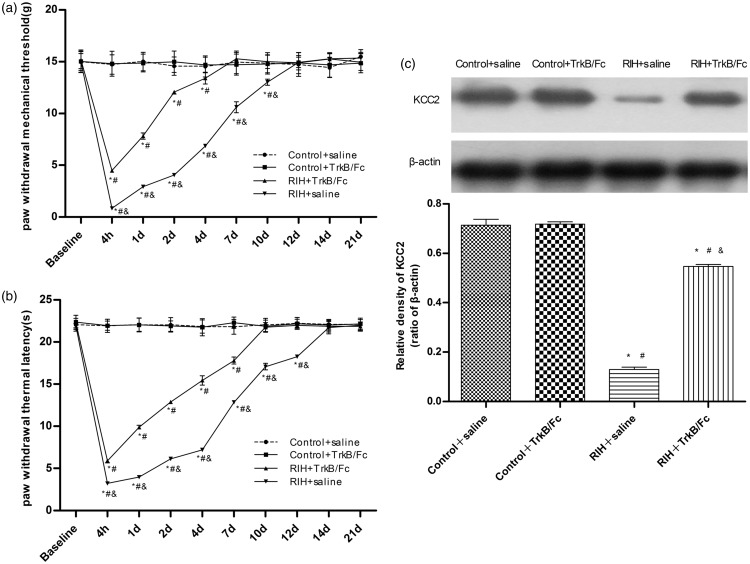
To further confirm the interaction between BDNF/trkB signal and KCC2 in the spinal dorsal horn of rats with RIH, we administered 5 µg of intrathecal TrkB/Fc15 at 15 min before surgery. No difference was detected between the control + TrkB/Fc group and control + saline group (P > 0.05). A comparison of PWMT and PWTL values between the RIH + saline group and control + saline (or TrkB/Fc) group revealed a significant difference after RIH (P < 0.05). Compared with the RIH + saline group, PWMT and PWTL in the RIH + TrkB/Fc group were significantly increased (P < 0.05); however, the values were still lower than groups who had received the sham procedure (P < 0.05). Moreover, intrathecal administration of TrkB/Fc markedly shortened the durations of mechanical allodynia and thermal hyperalgesia in rats with RIH to 7 days (PWMT) and 10 days (PWTL) (P < 0.05) (Figure 4(a) and (b)).
Figure 4.
The time course of the effects of intrathecal injection of TrkB/Fc on nociceptive behaviors in RIH rats, and the effects of intrathecal injection of TrkB/Fc on KCC2 expression in RIH rats one day after manipulation. PWMT (a) and PWTL (b) were evaluated at 24 h before incision (baseline) and 4 h, 1, 2, 4, 7, 10, 12, 14, and 21 days after surgery. Data are expressed as mean ± standard deviation (n = 8 in each group). *P < 0.05 compared with baseline; #P < 0.05 compared with the control + saline group; &P < 0.05 compared with the RIH + TrkB/FC group. (c) Representative Western blotting bands (top) and quantitative data (bottom) for analysis show the protein expression levels of KCC2 in the control + saline group, control + TrkB/Fc group, RIH + saline group, and RIH + TrkB/Fc group. β-actin was used as an internal control. Data are shown as mean ± standard deviation (n = 4 in each group). *P < 0.05 compared with the control + saline group; #P < 0.05 compared with control + TrkB/Fc group; &P < 0.05 compared with the RIH + saline group.
The Western blot analysis and immunofluorescence assay both showed that compared with the control + saline group and control + TrkB/Fc group, KCC2 expression was significantly downregulated in the RIH + saline group and RIH + TrkB/Fc group, while compared with the RIH + saline group, KCC2 expression in the RIH + TrkB/Fc group was noticeably upregulated (P < 0.05) (Figures 4(c) and 5).
Figure 5.
Immunofluorescence of KCC2 in the spinal dorsal horn after intrathecal injection of TrkB/Fc in RIH rats. Expression of KCC2 in the spinal dorsal horn was detected by immunofluorescence and observed under a confocal microscope. Panels (a) to (h) show the representative immunofluorescence staining results of the control + saline group (a and e), control + TrkB/Fc group (b and f), RIH + saline group (c and g), and RIH + TrkB/Fc group (d and h). Note that KCC2 immunoreactivity in the spinal dorsal horn is less intense in the RIH + saline group, and intrathecal injection of TrkB/Fc partly reversed the decrease in KCC2 expression. Scale bars are 100 µm for panels (a) to (d) and 50 µm for panels (e) to (h).
Intrathecal injections of PNU-120596 induced shorter duration of RIH in rats
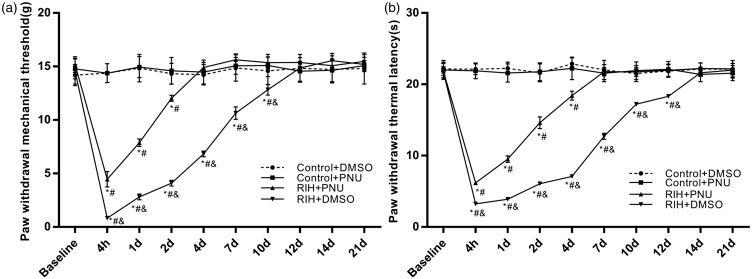
In our study, the intrathecal administration of PNU-120596 was carried out at 15 min before surgery, which is consistent with clinical preemptive analgesia. After intrathecal injections of 8 µg/kg of PNU-120596, no difference was detected between the control + DMSO group and control + PNU group (P > 0.05). A comparison of PMWT and PWTL values between the RIH + DMSO group and the sham-operated groups revealed significant differences after the surgery (P < 0.05). Compared with the RIH + DMSO group, PWMT and PWTL in the RIH + PNU group were significantly increased (P < 0.05); however, the values were still lower than in the sham-operated groups. After PNU-120596 administration, the mechanical allodynia and the thermal hyperalgesia in the RIH + PNU group had shorter durations (4 days and 7 days, respectively) than those in the RIH+DMSO group (12 days and 14 days, respectively) (Figure 6(a) and (b)).
Figure 6.
The time course of the effects of intrathecal injection of PNU-120596 on nociceptive behavior in RIH rats. PWMT (a) and PWTL (b) were evaluated at 24 h before incision (baseline) and 4 h, 1, 2, 4, 7, 10, 12, 14, and 21 days after surgery. Data are represented as mean ± standard deviation (n = 8 in each group). *P < 0.05 compared with baseline; #P < 0.05 compared with the control + DMSO group; &P < 0.05 compared with the RIH + PNU group.
The time course of the effects of PNU-120596 on the expression of BDNF and TrkB in the spinal dorsal horn of rats with RIH
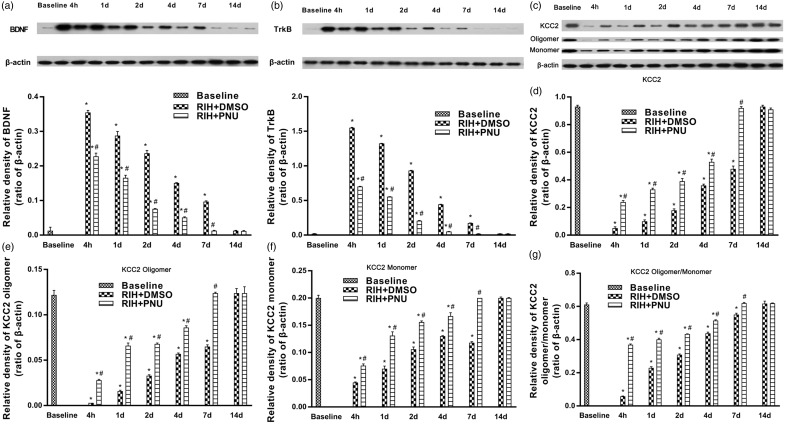
Western blot analysis revealed that intrathecal administration of PNU-120596 significantly inhibited RIH-induced upregulation of BDNF and trkB in the dorsal horn of the ipsilateral L4-L6 spinal segments of rats in the RIH + PNU group versus the RIH + DMSO group (P < 0.05), but the protein expression was still higher than in rats in the control + DMSO group and control + PNU group (P < 0.05). No difference was observed between the control + DMSO group and control + PNU group (P > 0.05) (Figure 7(f) and (g)). Figure 8(a) and (b) showed that at baseline, the protein expression of BDNF and trkB was detectable but was very low. The highest increase in BDNF and trkB expression was seen in the RIH + DMSO group on postoperative day 1. After preoperative intrathecal administration of PNU-120596, significant downregulation of BDNF or TrkB expression in the spinal dorsal horn was observed at 4 h and at 1, 2, 4, and 7 days post operation. The levels of protein expression showed earlier signs of recovery to baseline levels in the RIH + PNU group (7 days post operation) than in RIH + DMSO group (14 days post operation) (P < 0.05) (Figure 8(a) and (b)).
Figure 7.
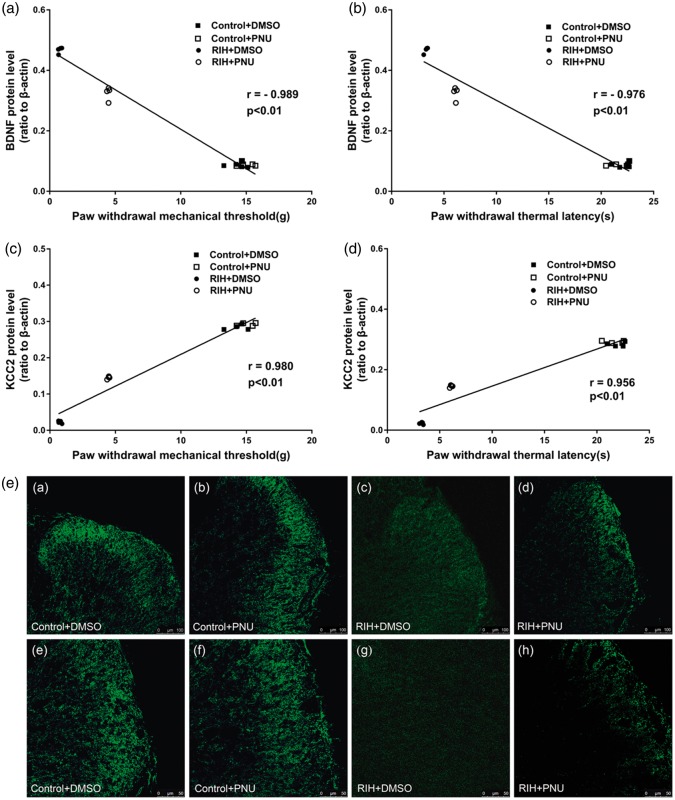
The effects of intrathecal injection of PNU-120596 on the expression of BDNF, TrkB, total KCC2, KCC2 oligomer, monomer expression, and oligomer/monomer ratios in the spinal dorsal horn of RIH rats one day after manipulation. (f) to (h) Representative Western blotting bands (top) and quantitative data (bottom) for analysis show the protein expression levels of BDNF, TrkB, and total KCC2; (i) Representative Western blotting bands of KCC2 oligomer and monomer in the spinal dorsal horn; (j) to (l) Quantification of the levels of KCC2 oligomer, monomer expression, and oligomer/monomer ratios. β-actin was used as an internal control. (g) Representative immunofluorescence of KCC2 in the control + DMSO group (a and e), control + PNU-120596 group (b and f), RIH + DMSO group (c and g), and RIH + PNU-120596 group (d and h) one day after manipulation. Expression of KCC2 in the spinal dorsal horn was detected by immunofluorescence and observed under a confocal microscope. Panels (a) to (h) show the representative immunofluorescence staining results of the four groups. Note that KCC2 immunoreactivity in the spinal dorsal horn is less intense in the RIH + DMSO group, and intrathecal injection of PNU-120596 partly reversed the decrease in KCC2 expression. Scale bars are 100 µm for panels (a) to (d) and 50 µm for panels (e) to (h). (a) to (d) Correlation analyses between BDNF expression levels, KCC2 expression levels and PWMT (g) as well as PWTL (s) on postoperative day 1 were conducted. Data are shown as mean ± standard deviation (n = 4 in each group). *P < 0.05 compared with the control + DMSO group; #P < 0.05 compared with the control + PNU-120596 group; &P < 0.05 compared with the RIH + DMSO group.
Figure 8.
Thetime course of the effects of PNU-120596 on the expression of BDNF, TrkB, total KCC2, KCC2 oligomer, monomer expression, and oligomer/monomer ratios in the spinal dorsal horn of RIH rats. Western blots for BDNF, TrkB, total KCC2, KCC2 oligomer, and monomer proteins in the RIH + DMSO group and RIH + PNU group were detected at baseline (24 h before surgery) and 4 h, 1, 2, 4, 7, and 14 days after surgery. (a) and (b) Representative Western blotting bands (top) and quantitative data (bottom) for analysis show the protein expression levels of BDNF and TrkB; (c) Representative Western blotting bands of total KCC2, KCC2 oligomer and monomer in the spinal dorsal horn; (d) to (g) Quantification of the levels of total KCC2, KCC2 oligomer, monomer expression, and oligomer/monomer ratios. β-actin was used as an internal control. Data are shown as mean ± standard deviation (n = 4 in each group). *P < 0.05 compared with baseline; #P < 0.05 compared with RIH + DMSO group of the same time point.
The time course of the effects of PNU-120596 on KCC2 expression in the spinal dorsal horn in rats with RIH
KCC2 expression in the dorsal horn of ipsilateral L4-L6 spinal segments in the RIH + PNU group was noticeably increased in comparison with the RIH + DMSO group (P < 0.05) (Figure 7(h)). Furthermore, compared with the RIH + DMSO group, the expression of total KCC2, KCC2 oligomer, monomer, and oligomer/monomer ratios were upregulated in the RIH + PNU group, but KCC2 oligomer and monomer expression was still lower than in sham-operated groups (P < 0.05) (Figure 7(i) to (l)). Time courses of protein expression of total KCC2, KCC2 oligomer and monomer, and oligomer/monomer ratios in the spinal dorsal horn showed that those of the RIH + PNU group returned to baseline levels on postoperative day 7, which is significantly earlier than in the RIH + DMSO group (postoperative day 14), approximately correlating with nociceptive behavior (P < 0.05) (Figure 8(c) to (g)). We also studied the effects of PNU-120596 on KCC2 expression in the spinal dorsal horn by immunofluorescence assay. A marked upregulation of KCC2 was found in the RIH + PNU group compared to the RIH + DMSO group but was still lower than in the sham-operated groups (P < 0.05) (Figure 7(e)).
Correlation analyses between BDNF expression levels, KCC2 expression levels and mechanical allodynia as well as thermal hyperalgesia were conducted. The results revealed that the BDNF expression levels were negatively correlated with the PWMT (P < 0.01, Pearson’s correlation coefficient (r) = −0.989, Figure 7(a)) and the PWTL (P < 0.01, r = −0.976, Figures 7(b) and 2(g)), but KCC2 expression levels were positively correlated with the PWMT (P < 0.01, r = 0.980, Figure 7(c)) and the PWTL (P < 0.01, r = 0.956, Figure 7(d)) on postoperative day 1 (n = 4 for each group).
KCC2 inhibitor VU0240551 reduced the analgesic effect of PNU-120596 on RIH
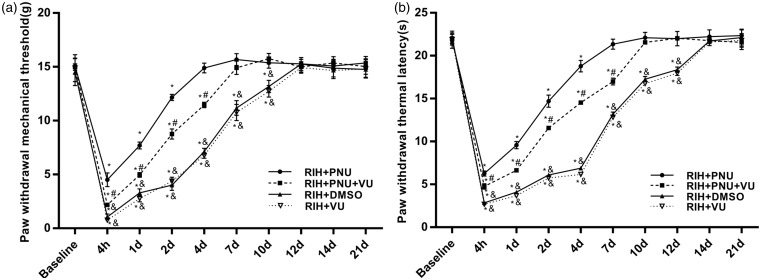
To further verify our hypotheses, we intrathecally administered 0.8 µg KCC2 inhibitor VU0240551 at 15 min presurgery in PNU-120596-treated rats. The results indicated that no significant differences were observed for PWMT and PWTL between the RIH + DMSO group and the RIH + VU group (P > 0.05) (Figure 9(a) and (b)), and the values in the two groups were noticeably decreased in comparison with the RIH + PNU + VU group (P < 0.05) (Figure 9(a) and (b)). Compared with the RIH + PNU group, PWMT and PWTL of the RIH + PNU + VU group were significantly lower (P < 0.05) (Figure 9(a) and (b)).
Figure 9.
The time course of the effects of intrathecal injection of VU0240551 on nociceptive behaviors in rats of the RIH + PNU group. PWMT (a) and PWTL (b) were evaluated at 24 h before incision (baseline) and 4 h, 1, 2, 4, 7, 10, 12, 14, and 21 days after surgery. Data are expressed as mean ± standard deviation (n = 8 in each group). *P < 0.05 compared with baseline; #P < 0.05 compared with the RIH + PNU group, &P < 0.05 compared with the RIH + PNU + VU group.
Discussion
It has been reported that intraoperative infusion of remifentanil is associated with postoperative hyperalgesia36,37 and increases postoperative analgesic requirements in both animal models38 and human clinical trials.39 Some patients who received intravenous infusion of high doses of remifentanil in combination with anesthesia felt an earlier and more intense surge of pain, which could last a long time after the operation. The long-lasting RIH not only increases patient suffering and morbidity but also delays rapid recovery and early discharge from hospital. Our previous study28 and other research38,40 have demonstrated that the activation of spinal α7-nAChR attenuates RIH, but PWMT and PWTL were measured only for two days after surgery, and the effect of activation of spinal α7-nAChRs on the duration of postoperative hyperalgesia was still unclear. In the present investigation, we assessed PWMT and PWTL for a longer time, until nociceptive thresholds in all groups returned to baseline values after manipulation. We observed that the intrathecal administration of α7-nAChRs type II PAMs (e.g. PNU-120596) significantly shorten the durations of both mechanical allodynia and thermal hyperalgesia in rats with RIH, which did affirm that activation of α7-nAchRs in the spinal dorsal horn plays an essential role in inhibiting the production and persistence of RIH.
One of the main findings of our study was the role of KCC2 in the spinal dorsal horn in the development of RIH. The data obtained to date suggest that in pain-signaling pathways, GABAergic inhibition loss plays a critical role in central sensitization.13,41 One main reason of the transition for this is the impaired intracellular chloride homeostasis due to the decreased activity and expression of KCC2, a postsynaptically restricted Cl−/K+ cotransporter that is crucial for postsynaptic inhibition mediated by GABAA receptors.42 Previous studies have shown that hyperalgesia-inducing treatment with morphine resulted in downregulation of the K+-Cl− cotransporter KCC2, impairing Cl− homeostasis in rat spinal lamina l neurons. Restoring the anion equilibrium potential reversed the morphine-induced hyperalgesia,11,40 and persistent pain resulted from the activation of a descending pain-facilitating pathway, which may be critical in the process of central sensitization.16,43 Pharmacological inhibition of KCC2 by its inhibitor, VU0240551, led to thermal hypersensitivity in normal rats,9 while rescuing the plasma membrane expression of KCC2 using CLP257, a KCC2-selective analog, alleviated hyperalgesia of neuropathic pain in rats,12 further indicating that KCC2 is necessary and sufficient for nociception modulation. However, the role of KCC2 in RIH remains to be elucidated. In our present experiment, we found a significant and long-lasting reduction in KCC2 expression in the spinal dorsal horn following RIH, and the time course of variations approximately coincided with the decline of nociceptive thresholds. More specifically, functional KCC2 exists mostly in the form of oligomers on the membrane surface of mature neurons, while altered KCC2 oligomerization and clusters would lead to loss of KCC2 transport activity,35,44 which is tightly associated with the modulation of nociception.11 In the RIH model, we also observed a significant decrease in KCC2 oligomerization, indicating that KCC2 downregulation was at least partly involved in the process of central sensitization in RIH, which may provide a novel option for the treatment of postoperative hyperalgesia.
Another important result was the modulation of BDNF/trkB signal on KCC2 in the spinal dorsal horn of rats with RIH. We observed a simultaneous time course of variations in KCC2 and BDNF/trkB signal in the spinal dorsal horn following RIH. It has been well-documented that BDNF is an important regulator for pain transmission in the spinal dorsal horn, which mainly functioned through its high-affinity trkB, namely the BDNF/trkB signal.45,46 The signal was originally found to downregulate KCC2 in a hippocampus culture,47 and later more direct supporting evidence verified that downregulation of KCC2 induced by the activation of BDNF/trkB signal was responsible for the disinhibition of GABAergic neurons under sensitized pain conditions, and blocking the signal between BDNF and trkB reversed hyperalgesia.11,15,16 In our experiment, blocking the BDNF/trkB signal by intrathecal administration of BDNF-sequester TrkB/Fc significantly attenuated the postoperative hyperalgesia and upregulated KCC2 expression in the spinal dorsal horn, to some extent indicating that the BDNF/trkB-KCC2 signal plays a key role in the development of RIH.
It has been well-documented that α7-nAChRs are expressed in microglia19–22 and are involved in promoting neuroprotection and the suppression of neuroinflammation by regulating microglia activation.48–51 According to our recent studies, spinal microglia were active in RIH rats,52 and α7-nAChRs activation induced by PNU-120596 has an anti-hyperalgesia effect in RIH.28 What’s more, the activation of α7-nAChRs attenuated posttraumatic stress disorder-related mechanical allodynia via the suppression of spinal microglia.48 While microglia activation may lead to secretion of specific messengers, including BDNF,11,53–55 which downregulates KCC2 protein expression impairing Cl− homeostasis, ultimately leading to hypersensitivity. To date, the mechanism of alleviation in morphine-induced hyperalgesia has been reported to involve the regulation of the BDNF/trkB-KCC2 signaling pathway in the microglia of the spinal dorsal horn.11 As an α7-nAchRs selective type II PAMs, PNU-12059656 could facilitate endogenous neurotransmission and enhance the efficacy and potency of an agonist without directly stimulating the agonist-binding sites.57,58 In our present experiment, it was observed that intrathecal administration of PNU-120596 significantly attenuated postoperative persistent pain; the BDNF/trkB-KCC2 signal was also partly reversed in the spinal dorsal horn. These findings suggest that after the activation of α7-nAchRs, the pain hypersensitivity induced by remifentanil maybe mediated by the same BDNF/trkB-KCC2 cascade in the microglia of the spinal dorsal horn, thus recapitulating the sequence of events described in morphine-induced hyperalgesia.11 Moreover, we observed a persistent upregulation of BDNF/trkB and a sustained downregulation of KCC2 in the spinal dorsal horn at all time points of evaluation, which lasted up to postoperative day 14. While the time courses of all the molecular changes were significantly shortened by the intrathecal injection of PNU-120596 and returned to baseline levels on postoperative day 7, which concurred with the complete recovery of nociceptive thresholds. We have reported previously that a subcutaneous infusion of remifentanil (40 µg/kg) during surgery enhanced postoperative hyperalgesia. In the present study, we used a higher dose of remifentanil (80 µg/kg) because it induces a greater pronociceptive effect and one which is maintained for a longer time.38 The maximal decrease in nociceptive thresholds were observed between 4 h and two days after manipulation, and the decrease lasted to postoperative day 14. Administration of a dose of 8 µg/kg of PNU-120596 at 15 min presurgery was carried out in accordance with the clinical preemptive analgesia and also previous reports.26,28 What’s more, KCC2 inhibitor VU0240551 administration significantly reduced the analgesic effect of PNU-120596 on RIH, which further confirmed the role of KCC2 in the analgesic effect of PNU-120596 on RIH.
There is a limitation in the current study. The present study did not further investigated the underlying mechanisms that how the activation of α7-nAChRs mediated the BDNF/trkB-KCC2 signal in the spinal dorsal horn. Further researches are needed to address the issue. Nevertheless, the primary objective of the current study was to evaluate the effectiveness of spinal activation of α7-nAChRs on the duration of RIH and the BDNF/trkB-KCC2 signal. Our finding would have implications for the development of novel therapeutic strategies for postoperative persistent pain.
Conclusion
In summary, in the present study, we explored the role of BDNF/trkB-KCC2 in the analgesic effect of α7-nAChRs selective type II PAMs (PNU-120596) on RIH. The data suggested that preoperative PNU-120596 intrathecal administration significantly shortened the duration of hyperalgesia in an animal model of RIH, and that BDNF/trkB-KCC2 signal in the spinal dorsal horn might contribute to the analgesic effects of PNU-120596.
Authors’ contributions
WG, WZ, and ZLM contributed to the study conception and design. WG, SSC, and YC performed the experiments and collected data. WG, WZ, and YSL analyzed the data and drafted the manuscript. XPG provided critical revisions of the article. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Youth Medical Experts Fund of Nanjing (Third Level) and the Key Subject of Anesthesiology in Jiangsu Province, China (XK 201140) and the National Nature Science Foundation of China (81171047, 81171048, 81070892, 81371207, 81300950, and 81300951).
References
- 1.White PF, Kehlet H. Improving postoperative pain management: what are the unresolved issues? Anesthesiology 2010; 112: 220–225. [DOI] [PubMed] [Google Scholar]
- 2.Mao J. Opioid-induced abnormal pain sensitivity: implications in clinical opioid therapy. Pain 2002; 100: 213–217. [DOI] [PubMed] [Google Scholar]
- 3.Simonnet G, Rivat C. Opioid-induced hyperalgesia: abnormal or normal pain? Neuroreport 2003; 14: 1–7. [DOI] [PubMed] [Google Scholar]
- 4.Burkle H, Dunbar S, Van Aken H. Remifentanil: a novel, short-acting, mu-opioid. Anesth Analg 1996; 83: 646–651. [DOI] [PubMed] [Google Scholar]
- 5.Troster A, Sittl R, Singler B, et al. Modulation of remifentanil-induced analgesia and postinfusion hyperalgesia by parecoxib in humans. Anesthesiology 2006; 105: 1016–1023. [DOI] [PubMed] [Google Scholar]
- 6.Singler B, Troster A, Manering N, et al. Modulation of remifentanil-induced postinfusion hyperalgesia by propofol. Anesth Analg 2007; 104: 1397–1403. Table of contents. [DOI] [PubMed] [Google Scholar]
- 7.Eisenach JC, Tong C, Curry RS. Failure of intrathecal ketorolac to reduce remifentanil-induced postinfusion hyperalgesia in humans. Pain 2015; 156: 81–87. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher D, Martinez V. Opioid-induced hyperalgesia in patients after surgery: a systematic review and a meta-analysis. Br J Anaesth 2014; 112: 991–1004. [DOI] [PubMed] [Google Scholar]
- 9.Austin TM, Delpire E. Inhibition of KCC2 in mouse spinal cord neurons leads to hypersensitivity to thermal stimulation. Anesth Analg 2011; 113: 1509–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janssen SP, Gerard S, Raijmakers ME, et al. Decreased intracellular GABA levels contribute to spinal cord stimulation-induced analgesia in rats suffering from painful peripheral neuropathy: the role of KCC2 and GABA(A) receptor-mediated inhibition. Neurochem Int 2012; 60: 21–30. [DOI] [PubMed] [Google Scholar]
- 11.Ferrini F, Trang T, Mattioli TA, et al. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl(-) homeostasis. Nat Neurosci 2013; 16: 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gagnon M, Bergeron MJ, Lavertu G, et al. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat Med 2013; 19: 1524–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavertu G, Cote SL, De Koninck Y. Enhancing K-Cl co-transport restores normal spinothalamic sensory coding in a neuropathic pain model. Brain J Neurol 2014; 137: 724–738. [DOI] [PubMed] [Google Scholar]
- 14.Dutheil S, Watabe I, Sadlaoud K, et al. BDNF signaling promotes vestibular compensation by increasing neurogenesis and remodeling the expression of potassium-chloride cotransporter KCC2 and GABAA receptor in the vestibular nuclei. J Neurosci Off J Soc Neurosci 2016; 36: 6199–6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miletic G, Miletic V. Loose ligation of the sciatic nerve is associated with TrkB receptor-dependent decreases in KCC2 protein levels in the ipsilateral spinal dorsal horn. Pain 2008; 137: 532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z, Wang X, Wang W, et al. Brain-derived neurotrophic factor-mediated downregulation of brainstem K+-Cl- cotransporter and cell-type-specific GABA impairment for activation of descending pain facilitation. Mol Pharmacol 2013; 84: 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuruga K, Hashimoto T, Kato R, et al. Plantar injection of formalin in rats reduces the expression of a potassium chroride cotransporter KCC2 in the spinal cord and a kinase inhibitor suppresses this reduction. Biomed Res 2016; 37: 243–249. [DOI] [PubMed] [Google Scholar]
- 18.Khan I, Osaka H, Stanislaus S, et al. Nicotinic acetylcholine receptor distribution in relation to spinal neurotransmission pathways. J Comp Neurol 2003; 467: 44–59. [DOI] [PubMed] [Google Scholar]
- 19.Shytle RD, Mori T, Townsend K, et al. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem 2004; 89: 337–343. [DOI] [PubMed] [Google Scholar]
- 20.De Simone R, Ajmone-Cat MA, Carnevale D, et al. Activation of alpha7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E2 in rat microglial cultures. J Neuroinflam 2005; 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takata K, Kitamura Y, Saeki M, et al. Galantamine-induced amyloid-{beta} clearance mediated via stimulation of microglial nicotinic acetylcholine receptors. J Biol Chem 2010; 285: 40180–40191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cordero-Erausquin M, Pons S, Faure P, et al. Nicotine differentially activates inhibitory and excitatory neurons in the dorsal spinal cord. Pain 2004; 109: 308–318. [DOI] [PubMed] [Google Scholar]
- 23.Abbas M, Rahman S. Effects of alpha-7 nicotinic acetylcholine receptor positive allosteric modulator on lipopolysaccharide-induced neuroinflammatory pain in mice. Eur J Pharmacol 2016; 783: 85–91. [DOI] [PubMed] [Google Scholar]
- 24.Loram LC, Harrison JA, Chao L, et al. Intrathecal injection of an alpha seven nicotinic acetylcholine receptor agonist attenuates gp120-induced mechanical allodynia and spinal pro-inflammatory cytokine profiles in rats. Brain Behav Immun 2010; 24: 959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bagdas D, Sonat FA, Hamurtekin E, et al. The antihyperalgesic effect of cytidine-5'-diphosphate-choline in neuropathic and inflammatory pain models. Behav Pharmacol 2011; 22: 589–598. [DOI] [PubMed] [Google Scholar]
- 26.Munro G, Hansen R, Erichsen H, et al. The alpha7 nicotinic ACh receptor agonist compound B and positive allosteric modulator PNU-120596 both alleviate inflammatory hyperalgesia and cytokine release in the rat. Br J Pharmacol 2012; 167: 421–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowley TJ, McKinstry A, Greenidge E, et al. Antinociceptive and anti-inflammatory effects of choline in a mouse model of postoperative pain. Br J Anaesth 2010; 105: 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W, Liu Y, Hou B, et al. Activation of spinal alpha-7 nicotinic acetylcholine receptor attenuates remifentanil-induced postoperative hyperalgesia. Int J Clin Exp Med 2015; 8: 1871–1879. [PMC free article] [PubMed] [Google Scholar]
- 29.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain 1996; 64: 493–501. [DOI] [PubMed] [Google Scholar]
- 30.Castel D, Willentz E, Doron O, et al. Characterization of a porcine model of post-operative pain. Eur J Pain 2014; 18: 496–505. [DOI] [PubMed] [Google Scholar]
- 31.Deisz RA, Wierschke S, Schneider UC, et al. Effects of VU0240551, a novel KCC2 antagonist, and DIDS on chloride homeostasis of neocortical neurons from rats and humans. Neuroscience 2014; 277: 831–841. [DOI] [PubMed] [Google Scholar]
- 32.Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol 1980; 67: 313–316. [DOI] [PubMed] [Google Scholar]
- 33.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Meth 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 34.Hargreaves K, Dubner R, Brown F, et al. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988; 32: 77–88. [DOI] [PubMed] [Google Scholar]
- 35.Blaesse P, Guillemin I, Schindler J, et al. Oligomerization of KCC2 correlates with development of inhibitory neurotransmission. J Neurosci Off J Soc Neurosci 2006; 26: 10407–10419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Celerier E, Gonzalez JR, Maldonado R, et al. Opioid-induced hyperalgesia in a murine model of postoperative pain: role of nitric oxide generated from the inducible nitric oxide synthase. Anesthesiology 2006; 104: 546–555. [DOI] [PubMed] [Google Scholar]
- 37.Chu LF, Cun T, Ngai LK, et al. Modulation of remifentanil-induced postinfusion hyperalgesia by the beta-blocker propranolol in humans. Pain 2012; 153: 974–981. [DOI] [PubMed] [Google Scholar]
- 38.Cabanero D, Campillo A, Celerier E, et al. Pronociceptive effects of remifentanil in a mouse model of postsurgical pain: effect of a second surgery. Anesthesiology 2009; 111: 1334–1345. [DOI] [PubMed] [Google Scholar]
- 39.Lenz H, Raeder J, Draegni T, et al. Effects of COX inhibition on experimental pain and hyperalgesia during and after remifentanil infusion in humans. Pain 2011; 152: 1289–1297. [DOI] [PubMed] [Google Scholar]
- 40.Campillo A, Cabanero D, Romero A, et al. Delayed postoperative latent pain sensitization revealed by the systemic administration of opioid antagonists in mice. Eur J Pharmacol 2011; 657: 89–96. [DOI] [PubMed] [Google Scholar]
- 41.Ford A, Castonguay A, Cottet M, et al. Engagement of the GABA to KCC2 signaling pathway contributes to the analgesic effects of A3AR agonists in neuropathic pain. J Neurosci Off J Soc Neurosci 2015; 35: 6057–6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coull JA, Boudreau D, Bachand K, et al. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 2003; 424: 938–942. [DOI] [PubMed] [Google Scholar]
- 43.Modol L, Cobianchi S, Navarro X. Prevention of NKCC1 phosphorylation avoids downregulation of KCC2 in central sensory pathways and reduces neuropathic pain after peripheral nerve injury. Pain 2014; 155: 1577–1590. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe M, Wake H, Moorhouse AJ, et al. Clustering of neuronal K+-Cl- cotransporters in lipid rafts by tyrosine phosphorylation. J Biol Chem 2009; 284: 27980–27988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melemedjian OK, Tillu DV, Asiedu MN, et al. BDNF regulates atypical PKC at spinal synapses to initiate and maintain a centralized chronic pain state. Mol Pain 2013; 9: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang HH, Zhang XQ, Xue QS, et al. The BDNF/TrkB signaling pathway is involved in heat hyperalgesia mediated by Cdk5 in rats. PloS One 2014; 9: e85536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rivera C, Li H, Thomas-Crusells J, et al. BDNF-induced TrkB activation down-regulates the K+-Cl- cotransporter KCC2 and impairs neuronal Cl- extrusion. J Cell Biol 2002; 159: 747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun R, Zhang W, Bo J, et al. Spinal activation of alpha7-nicotinic acetylcholine receptor attenuates posttraumatic stress disorder-related chronic pain via suppression of glial activation. Neuroscience 2017; 344: 243–254. [DOI] [PubMed] [Google Scholar]
- 49.Parada E, Egea J, Buendia I, et al. The microglial alpha7-acetylcholine nicotinic receptor is a key element in promoting neuroprotection by inducing heme oxygenase-1 via nuclear factor erythroid-2-related factor 2. Antioxid Redox Signal 2013; 19: 1135–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomsen MS, Mikkelsen JD. The alpha7 nicotinic acetylcholine receptor ligands methyllycaconitine, NS6740 and GTS-21 reduce lipopolysaccharide-induced TNF-alpha release from microglia. J Neuroimmunol 2012; 251: 65–72. [DOI] [PubMed] [Google Scholar]
- 51.Noda M, Kobayashi AI. Nicotine inhibits activation of microglial proton currents via interactions with alpha7 acetylcholine receptors. J Physiol Sci 2017; 67: 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun Y, Zhang W, Liu Y, et al. Intrathecal injection of JWH015 attenuates remifentanil-induced postoperative hyperalgesia by inhibiting activation of spinal glia in a rat model. Anesth Analg 2014; 118: 841–853. [DOI] [PubMed] [Google Scholar]
- 53.Ulmann L, Hatcher JP, Hughes JP, et al. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci off J Soc Neurosci 2008; 28: 11263–11268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beggs S, Trang T, Salter MW. P2X4R+ microglia drive neuropathic pain. Nat Neurosci 2012; 15: 1068–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trang T, Beggs S, Wan X, et al. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci Off J Soc Neurosci 2009; 29: 3518–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomsen MS, Mikkelsen JD. The alpha7 nicotinic acetylcholine receptor complex: one, two or multiple drug targets? Curr Drug Target 2012; 13: 707–720. [DOI] [PubMed] [Google Scholar]
- 57.Faghih R, Gfesser GA, Gopalakrishnan M. Advances in the discovery of novel positive allosteric modulators of the alpha7 nicotinic acetylcholine receptor. Recent Patent CNS Drug Discovery 2007; 2: 99–106. [DOI] [PubMed] [Google Scholar]
- 58.Bertrand D, Gopalakrishnan M. Allosteric modulation of nicotinic acetylcholine receptors. Biochem Pharmacol 2007; 74: 1155–1163. [DOI] [PubMed] [Google Scholar]