Infections by the ubiquitous Epstein-Barr virus (EBV) are associated with a wide spectrum of lymphomas and carcinomas. It has been well documented that activation levels of MAPKs are found in cancer cells to translate various external or intrinsic stimuli into cellular responses. Physiologically, the dual-specificity phosphates (DUSPs) exhibit great ability in regulating MAPK activities with respect to their capability of dephosphorylating MAPKs. In this study, we found that DUSPs were generally downregulated after EBV infection. EBV oncogenic latent membrane protein 1 (LMP1) suppressed DUSP6 and DUSP8 expression via MAPK pathway. In this way, LMP1-mediated MAPK activation was a continuous process. Furthermore, DUSP downregulation was found to contribute greatly to prevent apoptosis of EBV-infected cells. To sum up, this report sheds light on a novel molecular mechanism explaining how EBV maintains the unlimited proliferation status of the immortalized cells and provides a new link to understand EBV-induced B cell survival.
KEYWORDS: EBV
ABSTRACT
The strongest evidence of the oncogenicity of Epstein-Barr virus (EBV) in vitro is its ability to immortalize human primary B lymphocytes into lymphoblastoid cell lines (LCLs). Yet the underlying mechanisms explaining how the virus tempers the growth program of the host cells have not been fully elucidated. The mitogen-activated protein kinases (MAPKs) are implicated in many cellular processes and are constitutively activated in LCLs. We questioned the expression and regulation of the dual-specificity phosphatases (DUSPs), the main negative regulator of MAPKs, during EBV infection and immortalization. Thirteen DUSPs, including 10 typical and 3 atypical types of DUSPs, were tested. Most of them were downregulated after EBV infection. Here, a role of viral oncogene latent membrane protein 1 (LMP1) in limiting DUSP6 and DUSP8 expression was identified. Using MAPK inhibitors, we found that LMP1 activates extracellular signal-regulated kinase (ERK) or p38 to repress the expression of DUSP6 and DUSP8, with corresponding substrate specificity. Morphologically, overexpression of DUSP6 and DUSP8 attenuates the ability of EBV-immortalized LCL cells to clump together. Mechanistically, apoptosis induced by restoring DUSP6 and DUSP8 in LCLs indicated a novel mechanism for LMP1 to provide a survival signal during EBV immortalization. Collectively, this report provides the first description of the interplay between EBV genes and DUSPs and contributes considerably to the interpretation of MAPK regulation in EBV immortalization.
IMPORTANCE Infections by the ubiquitous Epstein-Barr virus (EBV) are associated with a wide spectrum of lymphomas and carcinomas. It has been well documented that activation levels of MAPKs are found in cancer cells to translate various external or intrinsic stimuli into cellular responses. Physiologically, the dual-specificity phosphates (DUSPs) exhibit great ability in regulating MAPK activities with respect to their capability of dephosphorylating MAPKs. In this study, we found that DUSPs were generally downregulated after EBV infection. EBV oncogenic latent membrane protein 1 (LMP1) suppressed DUSP6 and DUSP8 expression via MAPK pathway. In this way, LMP1-mediated MAPK activation was a continuous process. Furthermore, DUSP downregulation was found to contribute greatly to prevent apoptosis of EBV-infected cells. To sum up, this report sheds light on a novel molecular mechanism explaining how EBV maintains the unlimited proliferation status of the immortalized cells and provides a new link to understand EBV-induced B cell survival.
INTRODUCTION
Through evolution, Epstein-Barr virus (EBV) virus has adopted various mechanisms to persist in its host (1, 2). Primarily, EBV invades the host immune system by targeting B lymphocytes (3, 4). By preferentially infecting human memory B cells, which circulate in the peripheral blood in a dormant state, EBV enables its infection to become persistent and difficult to eradicate (1, 2, 4). EBV enters the latent phase immediately upon infection and, during latency, expresses only a small subset of viral genes, which may include EB nuclear antigens (EBNAs), latent membrane proteins (LMPs), and EBV-encoded RNAs (EBV-encoded small RNAs [EBERs] and BamHI-A rightward transcripts [BARTs]), to escape from immune surveillance (2). In vitro, EBV has the remarkable ability to immortalize B cells in lymphoblastoid cell lines (LCLs) (1, 2). As a result, these infected cells may divide and increase the amount of EBV that persists in the host. EBV-mediated B cell growth transformation is implicated in various malignancies. For instance, as the first defined human oncogenic virus, EBV infection has been reported to be associated with various lymphomas, such as Burkitt’s lymphoma (BL), Hodgkin lymphoma, NK/T-cell lymphoma, and diffuse-large-B cell lymphoma (DLBCL) (1, 2, 4). The EBV-immortalized LCL serves as an excellent model to study how the virus exploits host factors to promote pathogenesis.
In general, antigen-primed B cells are activated and expand in the germinal center (5). There, T helper cells activate CD40 on B cells and turn on the mitogen-activated protein kinase (MAPK) pathways (6, 7). However, to ensure that infected cells can proliferate and survive, EBV bypasses the default program of MAPK regulation. There are at least three major categories of MAPKs, namely, the extracellular signal-related kinases (ERKs), c-jun N-terminal kinases (JNKs), and p38 MAPKs (7). For specificity for the regulation of MAPK during EBV infection, MAPK pathways are constitutively activated by LMP1 and LMP2A and the viral lytic transactivator Zta (8–11). The latent membrane proteins LMP1 and LMP2A provide signals similar to those provided by ligand-free activated CD40 and the B cell receptor, respectively, driving the B cell proliferation that fuels B cell immortalization. In addition, Zta mimics a structural AP-1 protein and turns on not only viral lytic genes but also cellular signal transduction genes in a manner similar to that seen with ERK (12).
Thus, we sought to focus on the key negative regulators of MAPK and the dual-specificity phosphatases (DUSPs) and their roles in EBV infection. The 10 well-characterized, typical DUSPs (DUSP1, DUSP2, DUSP4, DUSP5, DUSP6, DUSP7, DUSP8, DUSP9, DUSP10, and DUSP16) interact with MAPKs through the kinase-interacting motif (KIM), within their Cdc25 homologous (CH2) domain at the N terminus, and dephosphorylate MAPK at both threonine/serine and tyrosine residues through their cysteine-containing catalytic C terminus, counteracting downstream signal cascades (13, 14). Fifteen other DUSPs, including DUSP3, DUSP14, DUSP19, and DUSP22, are atypical DUSPs that lack the kinase-recognizing N-terminal region and are deemed less extensively associated with MAPK deactivation (15). According to their subcellular location, typical DUSPs can be further grouped into three major categories as follows. DUSP1, DUSP2, DUSP4, and DUSP5 localize in the cell nucleus (type I). DUSP6, DUSP7, and DUSP9 are expressed in the cytoplasm (type II), whereas DUSP8, DUSP10, and DUSP16 can be found both inside and outside the cell nucleus (type III) (13, 14). The typical DUSPs of a given category have similar specificities for dephosphorylation of ERK, JNK, and p38. For instance, the typical type II DUSPs, i.e., DUSP6, DUSP7, and DUSP9, exhibit substrate specificity for ERK over JNK/p38, while the typical type III DUSPs, DUSP8, DUSP10, and DUSP16, are JNK/p38-specific phosphatases. Typical type I DUSPs show their corresponding substrate specificity regarding ERK, JNK, or p38 (13, 14). This study aimed to identify the role of DUSPs in EBV pathogenesis, seeking to determine how EBV orchestrates processing of MAPK signals to maintain the unlimited proliferative status in B cells.
RESULTS
Expression of DUSPs is downregulated during EBV immortalization.
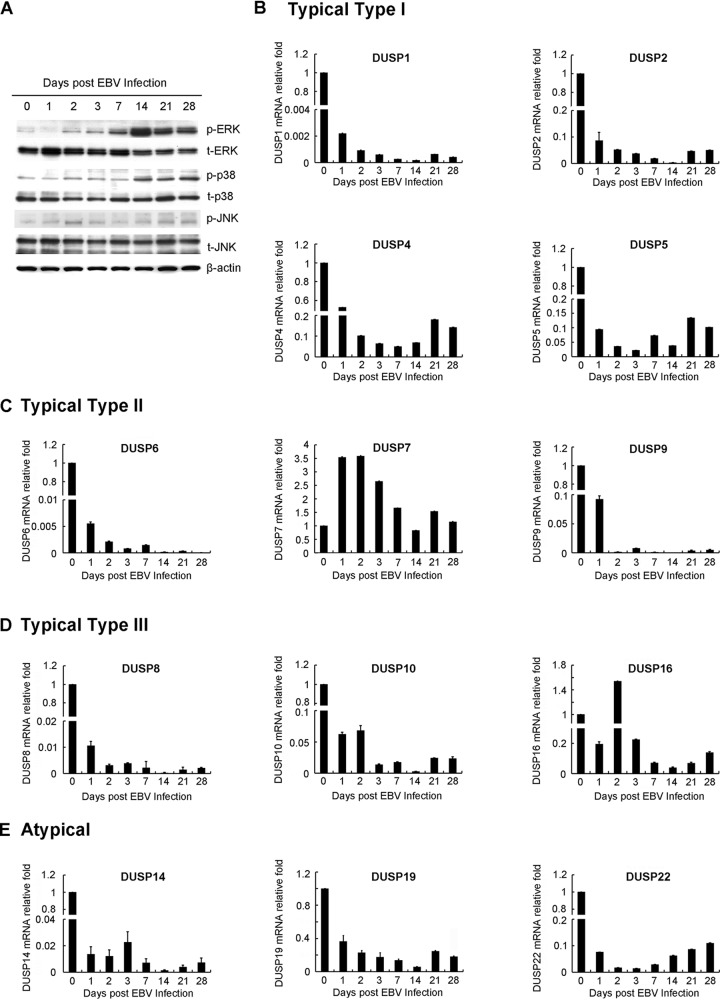
To evaluate how EBV infection and immortalization affect MAPK activation and DUSP expression in B lymphocytes, human B cells, purified using anti-CD19-coated beads, were infected with B95.8 strain EBV and harvested at the times indicated. As shown in Fig. 1A, phosphorylation of ERK, p38, and JNK gradually increased after EBV infection and immortalization, implying that the MAPKs were generally active during EBV immortalization (Fig. 1A). As shown in Fig. 1B to E, the expression of 13 DUSPs was determined by quantitative reverse transcription-PCR (RT-Q-PCR). Obviously, over 92% of the DUSPs tested (12/13), whether typical type I, II, or III DUSPs or atypical DUSPs, were downregulated after EBV immortalization at day 28, when LCLs were established (Fig. 1B to E) (16). The exception was DUSP7, whose expression showed no significant changes. Through the course of EBV infection, transcripts of DUSP1, DUSP2, DUSP6, DUSP8, DUSP9, DUSP10, and DUSP14 decreased more than 10-fold compared to uninfected B lymphocytes (Fig. 1B to E).
FIG 1.
Kinetic expression of MAPK activation and DUSPs during EBV immortalization. Human CD19+ B lymphocytes were purified from the buffy coat of a healthy blood donor. Cells were seeded in a 12-well plate at a density of 1 × 106 cells per well and infected with EBV. At 28 days postinfection, LCLs were established. RNA and proteins were harvested at the time points indicated. (A) Protein expression of MAPK activation, including phosphorylation of ERK (p-ERK), p38 (p-p38), and JNK (p-JNK), during EBV immortalization was detected by Western blotting. Total expression of MAPK (t-ERK, t-p38, and t-JNK) also is shown. β-Actin was the internal control. (B to E) Cells were also harvested for RT-Q-PCR for expression of DUSPs. The relative expression levels of DUSPs were compared to those of uninfected B lymphocytes after normalization with GAPDH (glyceraldehyde-3-phosphate dehydrogenase) expression. The DUSPs analyzed included (B) typical type I DUSPs (DUSP1, DUSP2, DUSP4, and DUSP5), (C) typical type II DUSPs (DUSP6, DUSP7, and DUSP9), (D) typical type III DUSPs (DUSP8, DUSP10, and DUSP16), and (E) atypical DUSPs (DUSP14, DUSP19, and DUSP22).
The fact that the depletion of DUSPs correlated with the activation of the MAPK pathway led us to explore whether MAPKs negatively modulate DUSPs. We took special interest in DUSPs with substrate specificity, such as DUSP6 (specific for ERK1/2) and DUSP8 (specific for JNK/p38), in order to elucidate a clearer pathway in their interplay with MAPK signaling.
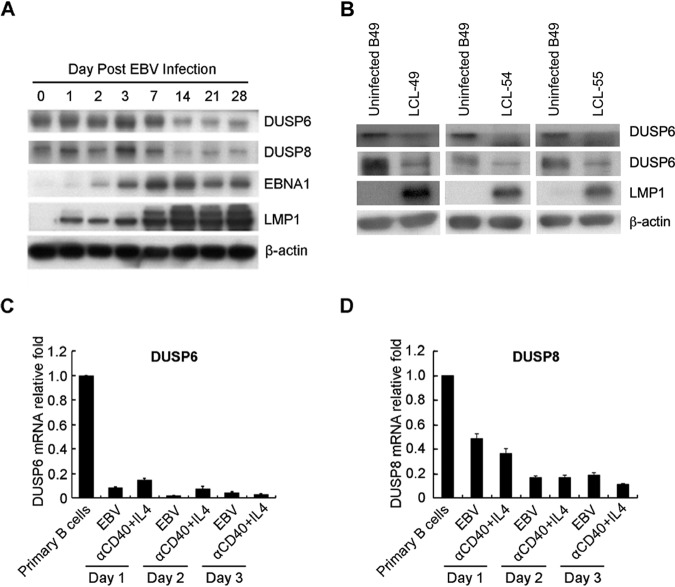
To eliminate potential individual bias of blood donors, expression of DUSP6 and DUSP8 was also analyzed in three different LCLs and in their uninfected B cell counterparts. We observed similar levels of suppression of DUSP6 and DUSP8 in all of these LCLs (Fig. 2A). In addition, the levels of kinetic expression of DUSP6 and DUSP8 proteins were analyzed in EBV-infected B cells purified from a healthy donor. As indicated in Fig. 2B, EBV infection and immortalization downregulated DUSP6 and DUSP8 at the protein level. B cells can be activated by stimuli other than EBV infection in response to pathogen infection (4). We therefore wondered about the expression of DUSP6 and DUSP8 that occurs when B cells receive T cell-dependent B cell activation signaling. Analyzing the RT-Q-PCR results, we found that anti-CD40/interleukin-4 (IL-4) could also downregulate both DUSP6 and DUSP8 (Fig. 2C and D). Our results were consistent with data reported previously from the laboratory of M. Rowe (17). Our results demonstrated that anti-CD40/IL-4 can reduce expression of DUSP6, which is consistent with the findings obtained by Rowe et al. using array and RT-Q-PCR analysis; furthermore, they found that only IL-4 was able to attenuate this downregulation. Taking the data together, we thought that the host gene alteration seen upon EBV infection was very similar to that exhibited by B cells treated with anti-CD40/IL-4 but might have been different from that seen with cells treated with IL-4 alone in some way.
FIG 2.
DUSP6 and DUSP8 depletion caused by EBV infection. (A) Cells were infected with B95.8 strain EBV for 28 days to establish LCLs. Cell lysates of infected and uninfected B cells were analyzed for DUSP6, DUSP8, and LMP1 expression by Western blotting. β-Actin was the internal control. (B) B lymphocytes were infected with strain B95.8 EBV and harvested on the day postinfection indicated. Immunoblots of DUSP6, DUSP8, EBNA1, and LMP1 are shown. β-Actin served as an internal control. (C and D) B cells were infected with EBV or treated with anti-CD40/IL-4 for 3 days. Total RNA was extracted each day and subjected to RT-Q-PCR to detect transcripts of (C) DUSP6 and (D) DUSP8. The relative expression levels were compared to those of untreated B lymphocytes after normalization with GAPDH expression.
EBV LMP1 suppresses the expression of DUSP6 and DUSP8.
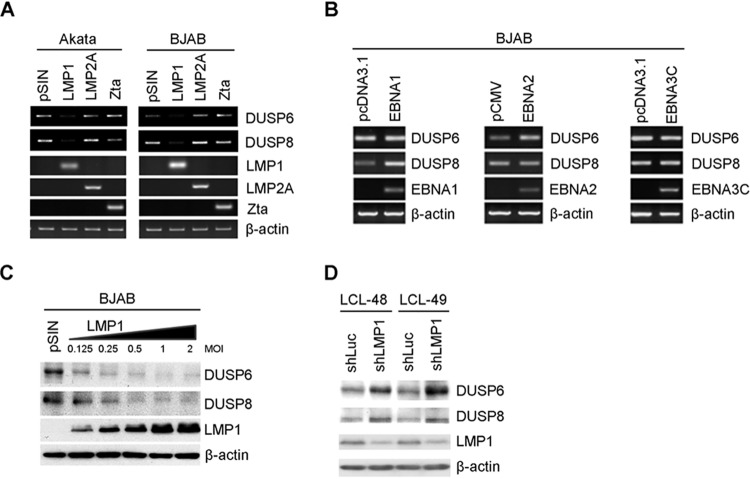
In order to characterize the interaction between EBV and the host cells in DUSP expression, we first identified which EBV gene product was responsible for the reduction of DUSP6 and DUSP8 expression. Viral products implicated in MAPK activation, including LMP1, LMP2A, and Zta, were primary candidates and were overexpressed in EBV-negative Burkitt’s lymphoma cells. We found that LMP1, whose activity mimics CD40-triggered signaling, was solely responsible for DUSP6 and DUSP8 suppression (Fig. 3A). Other viral genes tested, including LMP2A, Zta, EBNA1, EBNA2 and EBNA3C genes, did not produce the same effect (Fig. 3A and B). In addition, we found that the levels of downregulation of DUSP6 and DUSP8 were correlated with the increasing LMP1 expression amount, strengthening the hypothesis that LMP1 is associated with DUSP6 and DUSP8 downregulation (Fig. 3C). The data presented in Fig. 3D indicate that LMP1 is important for altered DUSP6 and DUSP8 expression because both phosphatases were upregulated in LCLs when LMP1 expression was repressed by the use of the short hairpin LMP1 (shLMP1) approach (10).
FIG 3.
DUSP6 and DUSP8 expression reduction caused by EBV LMP1. (A) EBV-negative Akata and BJAB lymphoma cells were infected with vector control lentivirus (pSIN) or lentiviruses that overexpressed LMP1, LMP2A, or Zta. DUSP6, DUSP8, LMP1, LMP2A, and Zta transcripts were analyzed by RT-PCR. β-Actin was detected as an internal control. (B) BJAB cells were electroporated with EBNA1, EBNA2, or EBNA3C. DUSP6, DUSP8, EBNA1, EBNA2, and EBNA3C transcripts were analyzed by RT-PCR. β-Actin was detected as an internal control. (C) BJAB cells were infected with LMP1 lentiviruses with increasing multiplicities of infection. Protein expression of DUSP6, DUSP8, and LMP1 was detected by Western blotting. β-Actin served as an internal control. (D) LCLs were infected with lentivirus containing shRNA targeting luciferase or LMP1. Data represent protein expression of DUSP6, DUSP8, and LMP1. β-Actin was detected as an internal control.
LMP1-mediated MAPK signaling drives the suppression of DUSP6 and DUSP8.
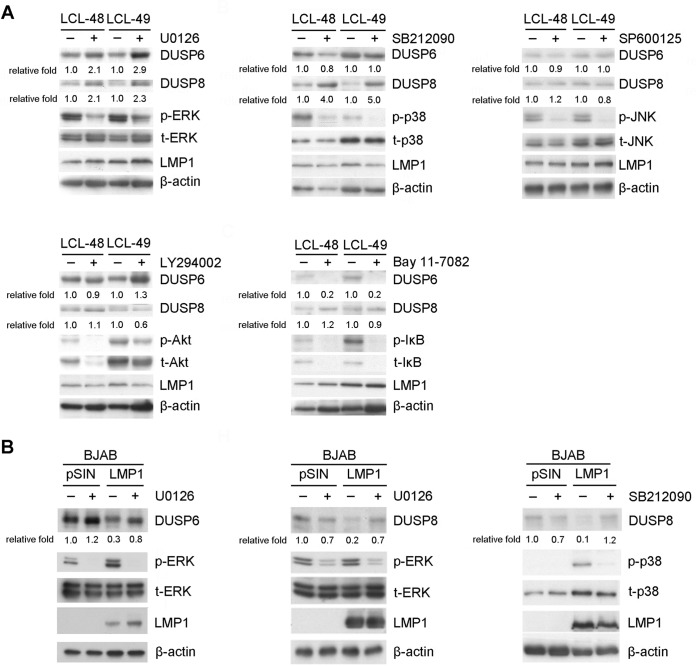
LMP1 mainly orchestrates cellular signal transduction through its C-terminal activating regions (CTARs), which associate with tumor necrosis factor receptor-associated factors, tumor necrosis factor receptor-associated death domain protein, and Janus-activated kinase 3 and then constitutively activate MAPK and NF-κB pathways (18, 19). To determine which pathway affects DUSP expression, LCLs were treated with several inhibitors of LMP1-activated signaling, namely, ERK, p38, JNK, Akt, and NF-κB, using the respective inhibitors U0126, SB212090, SP600125, LY294002, and BAY11-7082. As indicated in Fig. 4A, increases of DUSP6 and DUSP8 expression in LCLs were visible when ERK activation was inhibited but not during Akt, JNK, or NF-κB inhibition. Nonetheless, DUSP8 expression was obviously increased in the presence of p38 inhibitor (Fig. 4A). In other words, ERK activation was solely responsible for DUSP6 downregulation, while both ERK and p38 phosphorylation were crucial for DUSP8 reduction in LCLs.
FIG 4.
Signaling pathways involved in LMP1-mediated DUSP6 and DUSP8 suppression. (A) LCLs were treated with dimethyl sulfoxide (DMSO), 20 μM U0126 (ERK inhibitor), 20 μM SB212090 (p38 inhibitor), 25 μM SP600125 (JNK inhibitor), 20 μM LY294002 (Akt inhibitor), or 10 μM Bay11-7082 (NF-κB inhibitor) for 2 days. Detection of DUSP6, DUSP8, phosphorylated ERK (p-ERK), phosphorylated p38 (p-p38), phosphorylated JNK (p-JNK), phosphorylated Akt (p-Akt), phosphorylated IκB (p-IκB), total ERK (t-ERK), total p38 (t-p38), total JNK (t-JNK), total Akt (t-Akt), total IκB (t-IκB), and LMP1 was carried out by Western blotting. β-Actin served as an internal control. The fold relative expression data were calculated by comparing the levels of β-actin-normalized DUSP expression of the inhibitor-treated LCLs with those of the untreated LCLs. (B) BJAB cells were transduced with vector control (pSIN) or LMP1 via lentiviral infection and treated with DMSO, 20 μM U0126, or 20 μM SB212090 for 2 days. Detection of DUSP6, DUSP8, p-ERK, p-p38, t-ERK, t-p38, and LMP1 was carried out by Western blotting. The related level of expression of DUSP in vector control-transfected, untreated cells was set as a value of 1 to calculate the fold relative expression levels of DUSP6 and DUSP8.
LMP1 was overexpressed in EBV-negative B lymphoma cells, and the cells were treated with ERK and p38 inhibitors to interrogate the relationship between LMP1-mediated MAPK signaling and repression of DUSPs. As expected, in the presence of ERK inhibitor, LMP1 was unable to inhibit DUSP6 and DUSP8 protein expression (Fig. 4B). The p38 inhibitor also restored LMP1-mediated DUSP8 downregulation (Fig. 4B).
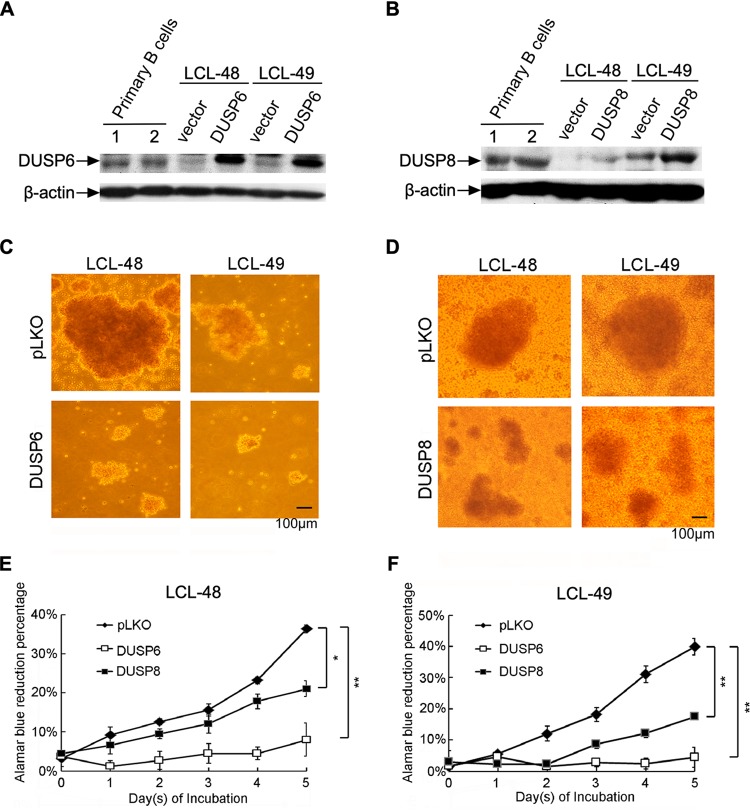
DUSP6 and DUSP8 induce phenotypic changes in LCLs.
DUSPs possess the prominent ability to inactivate MAPKs, which are involved in important cellular process, including cell proliferation, apoptosis, and survival (20). With the downregulation of DUSP expression pathways established, we wondered what the fate of LCLs would be under conditions of DUSP6 and DUSP8 expression. The levels of expression of DUSP6 and DUSP8 were detected in primary B cells, LCL, and LCL transduced with DUSP6- and DUSP8-containing lentivirus (Fig. 5A and B). The amounts of exogenous expression of DUSP6 and DUSP8 are shown in Fig. 5A and B. As shown in Fig. 5C and D, the LCL signature clumping mass shrank when DUSP6 and DUSP8 were overexpressed. Shrunken LCL clumps were detected in various scenarios, such as under conditions of decreased cell numbers and weakened cell-to-cell adhesion. Therefore, the cell proliferation rate was measured when DUSP6 and DUSP8 were overexpressed in LCLs. As indicated in Fig. 5E and F, expression of DUSP6 dramatically impaired LCL proliferation. DUSP8 also downregulated the proliferation of LCL cells, although to a lesser degree. Taken together, the results indicate that overexpression of DUSP6 and DUSP8 impaired the proliferation of LCLs, resulting in reduced LCL mass.
FIG 5.
Overexpression of DUSP6 and DUSP8 impaired the proliferation of LCLs. DUSP6 and DUSP8 were transduced into LCLs through lentiviral infection, and the cells were selected with G418 for 5 days. (A and B) Primary B cells or selected cells were analyzed by Western blotting to detect the indicated protein expression levels. (C and D) Selected cells were reseeded in 96-well plates at a density of 1 × 106 cells per ml. Photographs were taken under a bright-field microscope after 1 day of incubation. (E and F) Selected cells were reseeded in 96-well plates at a density of 1 × 105 cells per ml and incubated for 5 days. Proliferation rates of LCLs were determined by serial measurement of viable cell number every day via alamarBlue assay. (*, P<0.05; **, P<0.01 [Student's t test].)
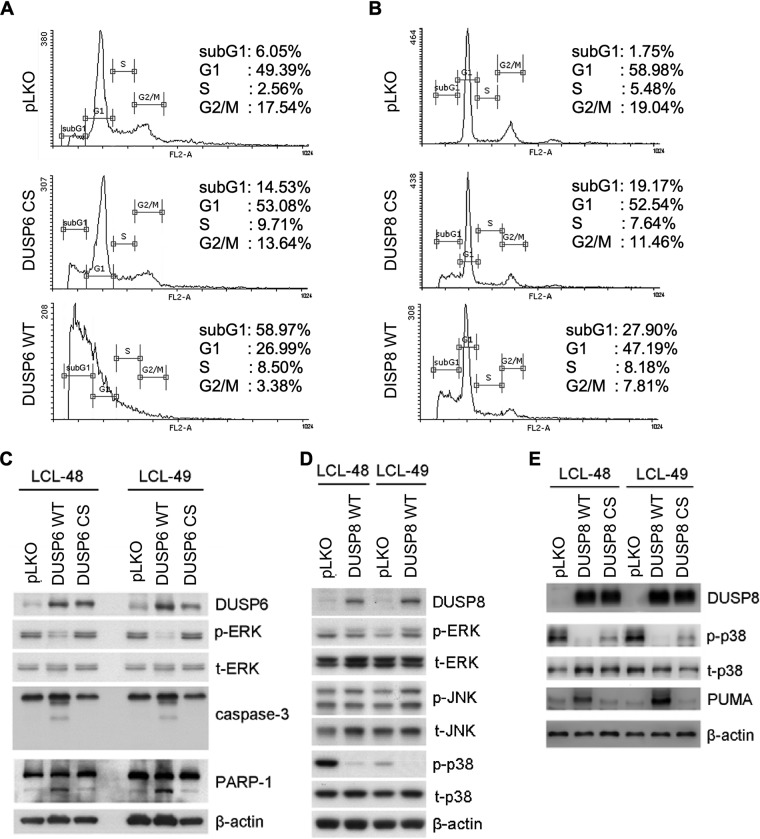
Restoration of DUSP6 and DUSP8 induced cell apoptosis in LCLs.
The mechanisms underlying overexpression of wild-type DUSP6 and DUSP8 (DUSP6 WT and DUSP8 WT) and mutants (DUSP6 C293S [CS] and DUSP8 CS) with a single point mutation in their C-terminal catalytic domain in LCL (21, 22) were explored. The apoptotic sub-G1 cells increased to a very small degree in DUSP6 CS but to a great degree in wild-type DUSP6 under conditions of overexpression, while DUSP8 expression in LCLs also slightly increased the numbers of apoptotic cells (Fig. 6A and B). Finally, the molecular mechanism underlying DUSP-mediated cell death was analyzed. Wild-type DUSP6 and DUSP8 (DUSP6 WT and DUSP8 WT) and mutants (DUSP6 CS and DUSP8 CS), with single point mutations in their C-terminal catalytic domain (23, 24), were transduced into LCLs. DUSP6 CS lacked the phosphatase-related ability to dephosphorylate ERK1/2 (Fig. 6C). According to the literature, DUSP8 has substrate affinity for JNK and p38 (13, 14). However, we found that DUSP8 dephosphorylated only p38, and not JNK, in LCLs (Fig. 6D), while its catalytic mutant partially restored the activation level of p38 (Fig. 6E). Moreover, expression of apoptotic proteins was detected in these transduced cells. As the data corresponding to the increased sub-G1 population may imply, indicators for apoptosis proteins, including cleaved caspase-3 and PARP-1, were seen only in WT DUSP6-transduced LCLs and not in DUSP6 CS-transduced cells (Fig. 6C). Of note, overexpression of DUSP8 induced expression of the proapoptotic PUMA, implying the occurrence of programmed cell death (Fig. 6E). These apoptotic effects of DUSP8 were also attenuated by the catalytic mutation (Fig. 6E). In summary, restoring DUSP6 and DUSP8 triggered LCL apoptosis.
FIG 6.
Apoptosis caused by overexpression of DUSP6 and DUSP8. (A and B) LCLs were overexpressed with wild-type DUSP6 (DUSP6 WT), wild-type DUSP8 (DUSP8 WT) or their catalytic mutants (DUSP6 CS and DUSP8 CS). Selected cells underwent PI staining, and cell cycle patterns were analyzed with flow cytometry. Cells were selected with G418, and their lysates were analyzed by Western blotting. (C) Protein expression of DUSP6, phosphorylated ERK (p-ERK), total ERK (t-ERK), caspase-3, cleaved caspase-3, PARP-1, and cleaved PARP-1 was detected by immunoblotting. β-Actin served as an internal control. (D) Protein expression of DUSP8, phosphorylated p38 (p-p38), total p38 (t-p38), phosphorylated JNK (p-JNK), and total JNK (t-JNK) was detected. β-Actin served as an internal control. (E) Protein expression of DUSP8, phosphorylated p38 (p-p38), total p38 (t-p38), and PUMA was detected by Western blotting. β-Actin served as the internal control. DUSP6 and DUSP8 were transduced into LCLs through lentiviral infection, and the cells were selected with G418 for 5 days. (A and B) Selected cells underwent PI staining, and cell cycle patterns were analyzed with flow cytometry. (C to E) LCLs were overexpressed with wild-type DUSP6 (DUSP6 WT) or wild-type DUSP8 (DUSP8 WT) or with their catalytic mutants (DUSP6 CS and DUSP8 CS). Cells were selected with G418, and their lysates were analyzed by Western blotting. (C) Protein expression of DUSP6, phosphorylated ERK (p-ERK), total ERK (t-ERK), caspase-3, cleaved caspase-3, PARP-1, and cleaved PARP-1 was detected by immunoblotting. β-Actin served as an internal control. (D) Protein expression of WT DUSP8, phosphorylated ERK (p-ERK), total ERK (t-ERK), phosphorylated JNK (p-JNK), total JNK (t-JNK), phosphorylated p38 (p-p38), and total p38 (t-p38) was detected. β-Actin served as an internal control. (E) Protein expression of wild-type DUSP8, DUSP8 CS, phosphorylated p38 (p-p38), total p38 (t-p38), and PUMA was detected by Western blotting. β-Actin served as the internal control.
DISCUSSION
DUSPs have been described as major negative regulators of MAPK signaling pathways (13, 15, 25, 26). Differential expression of DUSPs has been implicated in various types of leukemia and lymphoma. For example, DUSP4 deficiency was found in DLBCL patients (22) and DUSP16 was previously reported to be downregulated in BL and acute myeloid leukemia (27, 28). On the other hand, DUSP5 was found to be upregulated in mantle cell lymphoma (29) and DUSP7 was previously reported to be highly expressed in myeloid leukemia (30). Nonetheless, activation of a MAPK signaling pathway by EBV is essential for cellular transformation and the virus latent-lytic cycle switch (3). Notably, the relationship between EBV and DUSPs had not yet been reported. We were curious about the expression and biological function of DUSPs during EBV infection. Here, we took the advantage of the LCL model and observed the initiation steps of EBV-associated tumorigenesis closely. The LCLs made it possible for us to concentrate on the viral driving force of unlimited B cell proliferation, stripping away other host factors that may also contribute to B cell immortalization. Obviously, most DUSPs examined in this study were downregulated during EBV immortalization. Of interest, we found that the expression levels of DUSP6 and DUSP8, two DUSPs that differ in many aspects, were limited by LMP1 concordantly. Surprisingly, we found that LMP1 maintains the flow of MAPK activation by suppressing the expression of DUSP6 and DUSP8 and thus allows ERK and p38 to be constitutively active. Also of note, MAPK substrates of DUSPs were involved in the downregulation of their corresponding DUSPs. Specifically, the activity of ERK-inactivating DUSP6 was suppressed by LMP1-induced ERK activation while that of p38-inactivating DUSP8 was suppressed by LMP1-induced p38 activation.
In fact, DUSPs can be tightly controlled at various levels, namely, at the transcription, posttranslation, and epigenetic modification levels, in response to the MAPK signaling (21, 31, 32). In general, growth factors, serum, heat shock, and stress all can rapidly stimulate the expression of DUSPs but such responses are also quickly negatively regulated by MAPK (33). DUSP6 can be regulated by its substrate, ERK, via E26 transforming sequence 2 (ETS2) binding to its intron site, creating a positive-feedback loop (34). In addition, DUSP6 can be rapidly induced by platelet-derived growth factor (PDGF)-induced ERK, after which a DUSP-ERK negative-feedback loop occurs (35). Nonetheless, a recent study pointed out an ERK negative-feedback loop in which DUSP6 participated in promotion of pre-B cell transformation in acute lymphoblastic leukemia (36, 37). As for DUSP8, the other DUSP selected in this study, such a response can be induced by the presence of phorbol ester in leukemia cells and downregulated under conditions of oxidative stress in various cell types (12). To date, two types of DUSP6 gene regulation have been reported. In one example representing the first type, DUSP6 expression was found to be downregulated due to promoter hypermethylation in human pancreatic cancer (38). In the second, DUSP6 expression was found to be upregulated by ERK via an ETS factor bound to DUSP6 promoter (39, 40). In our case, inhibiting NF-κB decreased expression of DUSP6 instead of restoring DUSP expression. We observed that ERK was downregulated in the presence of NF-κB inhibitor and then downregulated ERK-mediated reductions of DUSP expression (data not shown). This hypothesis is consistent with the reports described above indicating that ERK can regulate DUSP expression. Negative feedback was also previously reported for DUSP8 and JNK/p38 under conditions in which cells faced external stresses, such as from arsenate, insulin, and growth factors (24). The great complexity of regulation of DUSPs could represent a way that abnormal cells adapt to cope with the intricate MAPK signal cascades. In the case of other viruses, the absence of DUSP1 promotes vaccinia virus replication (41). Kaposi’s sarcoma-associated herpesvirus (KSHV), another oncogenic herpesvirus, suppresses DUSP1 expression, which facilitates the induction of promigratory factors and cell invasiveness during endothelial cell infection (42).
In this study, restoring DUSP6 and DUSP8 expression downregulated proliferation of infected B cells and triggered apoptosis of LCLs. In summary, LMP1-mediated activation of ERK and p38 may reduce expression of DUSP6 and DUSP8, which control the survival of LCLs by regulating ERK and p38 phosphorylation. Notably, as one of the essential EBV oncogenic proteins, LMP1 is associated with the proliferation of EBV-infected cells through alteration of cellular genes such as receptor tyrosine kinases. For instance, LMP1 upregulates recepteur d’origine nantais (RON) tyrosine kinase via NF-κB and promotes cell proliferation (43, 44). It was reported recently that downregulation of LMP1-mediated erythropoietin-producing hepatocellular receptor A4 (EphA4) is involved in EBV lymphoproliferation and is correlated with poor prognosis among posttransplant lymphoproliferative disease and DLBCL patients (45).
In our series of studies, we revealed that cellular genes are largely manipulated by EBV during EBV-mediated B cell immortalization from many aspects, such as protein tyrosine kinase, cytokines, antiapoptotic products, etc. (9, 16, 44–47). Taken together, our results provide novel insights into MAPK signal transduction orchestrated by EBV, which could contribute greatly to the early stage of EBV-associated B cell transformation and thus could represent a novel therapeutic niche for EBV-associated B cell malignancies.
MATERIALS AND METHODS
B cell purification and EBV infection.
LCLs were established as previously described (16). Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood from anonymous donors (Taipei Blood Center of Taiwan Blood Service Foundation), and then CD19+ B cells were purified using Dynabeads (Invitrogen), according to the manufacturer’s instructions. Production of EBV virions (B95-8 strain) and infection of primary B cells by EBV have been described previously. Experiments involving human samples were approved by the Institutional Review Boards of National Taiwan University Hospital (Taipei, Taiwan).
Cell culture and treatments.
Akata and BJAB are EBV-negative Burkitt’s lymphoma-derived cell lines. LCLs were established from EBV-infected CD19-positive B cells. All B cell lines were cultured in complete RPMI medium (containing 10% fetal calf serum [FCS], 1 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin). MEK inhibitor (U0126), phosphatidylinositol 3-kinase (PI3K) inhibitor (LY294002), IκB-α phosphorylation inhibitor (BAY11-7082), JNK inhibitor (SP600125), and p38 inhibitor (SB202190) were purchased from Merck Millipore (Billerica, MA, USA). Anti-CD40 antibody (5C3; BD Bioscience) and IL-4 (R&D Systems) were used as previously described (47).
Construction of plasmids.
EBNA1 plasmid pCEP4 (Invitrogen) carries the simian virus 40 (SV40) promoter-driven hygromycin resistance (Hygr) gene and the EBNA1 gene. Plasmids pCMV-neo.BAM and pcDNA3.1-Hyg (+) (Invitrogen) carry the SV40 promoter-driven neomycin resistance (Neor) and Hygr genes, respectively. The plasmids expressing lentivirus-based full-length LMP1 (pSIN-LMP1) were described in our previous paper (8). Lentivirus-based LMP2A-expressing plasmid pSIN-LMP2A was described in our previous paper (46). Lentivirus-based Zta-expressing plasmid pSIN-Zta was constructed by insertion of a full-length Zta cDNA fragment into pSIN vector at the 5′ NdeI site and the 3′ MluI site. DUSP6-expressing plasmids (pLKO-DUSP6 and pLKO-DUSP6-C293S) and DUSP8-expressing plasmids (pLKO-DUSP8 and pLKO-DUSP8-C293S) were constructed from pCMV-Tag2B-DUSP6 and pCMV-Tag2B-DUSP6 ΔC293S, respectively, generously given by Jonathan D. Licht of Northwestern University Feinberg School of Medicine (Chicago, USA) (23), and pMT-SM-myc-M3/6 WT and pMT-SM-myc-M3/6 CS, generously given by George Panayoutou of the Alexander Fleming Biomedical Sciences Research Center (BSRC) (Vari, Greece) (24). Plasmids expressing the lentivirus-based constructs shLMP1 (5′-CCTAAGGTTAAGTCGCCCT-3′) and shLuciferase (shLuc; 5′-GCTCATTATTGCTCTCTAT-3′) were constructed by insertion of short hairpin RNA (shRNA) fragments into plasmid pLKO.1 through the 5′ AgeI and 3′ EcoRI sites.
Lentivirus infection.
The method of production and infection with lentiviruses was described previously (46). For lentivirus infection, 5 × 105 cells were infected with lentivirus at a multiplicity of infection (MOI) of 1.
Analysis by reverse transcription (RT)-PCR and quantitative PCR (Q-PCR).
Total RNA was isolated from cells by using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. Synthesis of cDNA was described in our previous paper (44). The cDNA was used as the template for PCR or quantitative PCR (qPCR) in the presence of primer pairs specific for each target, and the amplicon was detected using the following specific primers and probes: for DUSP6, PCR primers F-AGCAGCGACTGGAACGAGAA and R-TGTTGGACAGCGGACTACCAT; for DUSP8, PCR primers F-GTCAACATCTGCTGCTCCAA and R-GTAGAGGTGAGGCAGGATGC; for DUSP1, primers F-CGAGGCCATTGACTTCATAGA and R-AGGCAGATGGTGGCTGAC and probe 86; for DUSP2, primers F-GCCCACTGCCGTGTACTT and R-GCTGGTTTTGTCCCCTGTT and probe 66; for DUSP4, primers F-GACATCTGCCTGCTCAAAGG and R-CAAGGGCTCTGTGGCACT and probe 46; for DUSP5, primers F-ACAAATGGATCCCTGTGGAA and R-CCTTTTCCCTGACACAGTCAA and probe 5; for DUSP6, primers F-CGACTGGAACGAGAATACGG and R-TTGGAACTTACTGAAGCCACCT and probe 66; for DUSP7, primers F-CCCATCTCTGACCACTGGAG and R-CAGGACACCACACTTCTTGG and probe 14; for DUSP8, primers F-GACCATTGCGGAGCTCAT and R-TCATAGACCACCACGTCCTGT and probe 71; for DUSP9, primers F-TTCTTTCCGGAGGCCATT and R-ACAGTGACGGTGACAGAACG and probe 86; for DUSP9, primers F-AAGAGGCTTTTGAGTTCATTGAG and R-CAAGTAAGCGATGACGATGG and probe 64; and for DUSP16, primers F-TCTGAGGGAATTGGGAGGT and R-CCATTCCACAACAAAAGATGC and probe 71.
Western blotting and antibodies.
Proteins were analyzed by the use o fWestern blotting as previously described (46). The antibodies used in this study were as follows: LMP1 (S12 or CS1 to CS4) and DUSP6 (Ab54940; Abcam), DUSP8 (sc-271250; Santa Cruz Biotechnology), phospho-ERK Thr202/Tyr204 (E-4; Santa Cruz Biotechnology), ERK (K-23; Santa Cruz Biotechnology), phospho-Akt Ser473 (9271; Cell Signaling Technology), Akt (H136; Santa Cruz Biotechnology), phospho-IκB Ser32/36 (9246; Cell Signaling Technology), IκB (sc-203; Santa Cruz Biotechnology), phospho-p38 Thr180/Tyr182 (9211; Cell Signaling Technology), p38 (9212; Cell Signaling Technology), caspase 3 (9662; Cell Signaling Technology), PARP-1 (sc-8007; Santa Cruz Biotechnology), phospho-JNK Thr183/Tyr185 (9251; Cell Signaling Technology), total JNK (06-748; Upstate), PUMA (4976; Cell Signaling Technology), and β-actin (AC-15; Sigma-Aldrich).
alamarBlue assay.
Cells were seeded in 0.1 ml of culture medium (with 10% FCS) in 96-well flat-bottomed microtiter plates. At the time indicated, alamarBlue was added followed by incubation for an additional 4 h. Cell viability was measured by alamarBlue absorbance. Data shown are means ± standard errors of the means of results from three replicates per point.
Flow cytometry.
Cells (2 × 105) were harvested, washed with phosphate-buffered saline (PBS), resuspended in PBS, and fixed in 100% ethanol at –20°C. After being left to stand overnight, cell pellets were collected by centrifugation, resuspended in hypotonic buffer (0.5% Triton X-100, PBS, 0.2 μg/ml RNase A), and incubated at 37°C for 30 min. Then, propidium iodide (PI) solution was added and the mixture was allowed to stand on ice for 30 min. Fluorescence emitted from the PI-DNA complex was quantitated by FACScan cytometry (Becton Dickinson, San Jose, CA) after excitation of the fluorescent dye.
ACKNOWLEDGMENTS
We thank Tim J. Harrison of UCL Medical School (London, United Kingdom) for reviewing the manuscript critically. We also thank the Taipei Blood Center of Taiwan Blood Service Foundation for providing buffy coats from blood donors.
This work was supported by the Ministry of Science and Technology, Taiwan (grant 106-2320-B-002-024-MY3), and the National Health Research Institute, Taiwan (grant NHRI-EX105-10306BI).
K.-M.L. designed experiments, performed experiments, analyzed the data, and cowrote the manuscript; S.-J.L. designed experiments, performed experiments, analyzed the data, and cowrote the manuscript; J.-H.L. designed experiments, performed experiments, and analyzed the data; P.-Y.L. designed experiments, performed experiments, and analyzed the data; P.-L.T. performed experiments and analyzed the data; H.-E.W. performed experiments and analyzed the data; T.-H.Y. provided B cells; Y.-P.W. provided B cells; M.-R.C. provided reagents; C.-H.T. designed experiments and cowrote the manuscript.
We declare that we have no competing financial interests.
REFERENCES
- 1.Young LS, Rickinson AB. 2004. Epstein-Barr virus: 40 years on. Nat Rev Cancer 4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 2.Thorley-Lawson DA. 2015. EBV persistence–introducing the virus. Curr Top Microbiol Immunol 390:151–209. doi: 10.1007/978-3-319-22822-8_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng WH, Hong G, Delecluse HJ, Kenney SC. 2004. Lytic induction therapy for Epstein-Barr virus-positive B-cell lymphomas. J Virol 78:1893–1902. doi: 10.1128/jvi.78.4.1893-1902.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khanna R. 2015. EBV and memory B cells: an affair with consequences. Blood 126:2655–2656. doi: 10.1182/blood-2015-10-675439. [DOI] [PubMed] [Google Scholar]
- 5.Herzog S, Reth M, Jumaa H. 2009. Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signalling. Nat Rev Immunol 9:195–205. doi: 10.1038/nri2491. [DOI] [PubMed] [Google Scholar]
- 6.Lam N, Sugden B. 2003. CD40 and its viral mimic, LMP1: similar means to different ends. Cell Signal 15:9–16. doi: 10.1016/s0898-6568(02)00083-9. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, Liu HT. 2002. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- 8.Chang Y, Lee HH, Chen YT, Lu J, Wu SY, Chen CW, Takada K, Tsai CH. 2006. Induction of the early growth response 1 gene by Epstein-Barr virus lytic transactivator Zta. J Virol 80:7748–7755. doi: 10.1128/JVI.02608-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen SY, Lu J, Shih YC, Tsai CH. 2002. Epstein-Barr virus latent membrane protein 2A regulates c-Jun protein through extracellular signal-regulated kinase. J Virol 76:9556–9561. doi: 10.1128/jvi.76.18.9556-9561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eliopoulos AG, Young LS. 1998. Activation of the cJun N-terminal kinase (JNK) pathway by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP1). Oncogene 16:1731–1742. doi: 10.1038/sj.onc.1201694. [DOI] [PubMed] [Google Scholar]
- 11.Johansson P, Jansson A, Ruetschi U, Rymo L. 2010. The p38 signaling pathway upregulates expression of the Epstein-Barr virus LMP1 oncogene. J Virol 84:2787–2797. doi: 10.1128/JVI.01052-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen YR, Shrivastava A, Tan TH. 2001. Down-regulation of the c-Jun N-terminal kinase (JNK) phosphatase M3/6 and activation of JNK by hydrogen peroxide and pyrrolidine dithiocarbamate. Oncogene 20:367–374. doi: 10.1038/sj.onc.1204105. [DOI] [PubMed] [Google Scholar]
- 13.Jeffrey KL, Camps M, Rommel C, Mackay CR. 2007. Targeting dual-specificity phosphatases: manipulating MAP kinase signalling and immune responses. Nat Rev Drug Discov 6:391–403. doi: 10.1038/nrd2289. [DOI] [PubMed] [Google Scholar]
- 14.Kidger AM, Keyse SM. 2016. The regulation of oncogenic Ras/ERK signalling by dual-specificity mitogen activated protein kinase phosphatases (MKPs). Semin Cell Dev Biol 50:125–132. doi: 10.1016/j.semcdb.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patterson KI, Brummer T, O'Brien PM, Daly RJ. 2009. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J 418:475–489. doi: 10.1042/bj20082234. [DOI] [PubMed] [Google Scholar]
- 16.Tsai SC, Lin SJ, Chen PW, Luo WY, Yeh TH, Wang HW, Chen CJ, Tsai CH. 2009. EBV Zta protein induces the expression of interleukin-13, promoting the proliferation of EBV-infected B cells and lymphoblastoid cell lines. Blood 114:109–118. doi: 10.1182/blood-2008-12-193375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith N, Tierney R, Wei W, Vockerodt M, Murray PG, Woodman CB, Rowe M. 2013. Induction of interferon-stimulated genes on the IL-4 response axis by Epstein-Barr virus infected human b cells; relevance to cellular transformation. PLoS One 8:e64868. doi: 10.1371/journal.pone.0064868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eliopoulos AG, Young LS. 2001. LMP1 structure and signal transduction. Semin Cancer Biol 11:435–444. doi: 10.1006/scbi.2001.0410. [DOI] [PubMed] [Google Scholar]
- 19.Wang LW, Jiang S, Gewurz BE. 13 October 2017, posting date Epstein-Barr virus LMP1-mediated oncogenicity. J Virol doi: 10.1128/JVI.01718-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avruch J. 2007. MAP kinase pathways: the first twenty years. Biochim Biophys Acta 1773:1150–1160. doi: 10.1016/j.bbamcr.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peti W, Page R. 2013. Molecular basis of MAP kinase regulation. Protein Sci 22:1698–1710. doi: 10.1002/pro.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmid CA, Robinson MD, Scheifinger NA, Muller S, Cogliatti S, Tzankov A, Muller A. 2015. DUSP4 deficiency caused by promoter hypermethylation drives JNK signaling and tumor cell survival in diffuse large B cell lymphoma. J Exp Med 212:775–792. doi: 10.1084/jem.20141957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrison DJ, Kim MK, Berkofsky-Fessler W, Licht JD. 2008. WT1 induction of mitogen-activated protein kinase phosphatase 3 represents a novel mechanism of growth suppression. Mol Cancer Res 6:1225–1231. doi: 10.1158/1541-7786.MCR-08-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oehrl W, Cotsiki M, Panayotou G. 2013. Differential regulation of M3/6 (DUSP8) signaling complexes in response to arsenite-induced oxidative stress. Cell Signal 25:429–438. doi: 10.1016/j.cellsig.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Nunes-Xavier C, Romá-Mateo C, Ríos P, Tárrega C, Cejudo-Marín R, Tabernero L, Pulido R. 2011. Dual-specificity MAP kinase phosphatases as targets of cancer treatment. Anticancer Agents Med Chem 11:109–132. doi: 10.2174/187152011794941190. [DOI] [PubMed] [Google Scholar]
- 26.Lang R, Hammer M, Mages J. 2006. DUSP meet immunology: dual specificity MAPK phosphatases in control of the inflammatory response. J Immunol 177:7497–7504. doi: 10.4049/jimmunol.177.11.7497. [DOI] [PubMed] [Google Scholar]
- 27.Feurstein S, Rucker FG, Bullinger L, Hofmann W, Manukjan G, Gohring G, Lehmann U, Heuser M, Ganser A, Dohner K, Schlegelberger B, Steinemann D. 2014. Haploinsufficiency of ETV6 and CDKN1B in patients with acute myeloid leukemia and complex karyotype. BMC Genomics 15:784. doi: 10.1186/1471-2164-15-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S, Syed N, Taylor J, Smith P, Griffin B, Baens M, Bai M, Bourantas K, Stebbing J, Naresh K, Nelson M, Tuthill M, Bower M, Hatzimichael E, Crook T. 2010. DUSP16 is an epigenetically regulated determinant of JNK signalling in Burkitt’s lymphoma. Br J Cancer 103:265–274. doi: 10.1038/sj.bjc.6605711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ortega-Paino E, Fransson J, Ek S, Borrebaeck CA. 2008. Functionally associated targets in mantle cell lymphoma as defined by DNA microarrays and RNA interference. Blood 111:1617–1624. doi: 10.1182/blood-2007-02-068791. [DOI] [PubMed] [Google Scholar]
- 30.Levy-Nissenbaum O, Sagi-Assif O, Kapon D, Hantisteanu S, Burg T, Raanani P, Avigdor A, Ben-Bassat I, Witz IP. 2003. Dual-specificity phosphatase Pyst2-L is constitutively highly expressed in myeloid leukemia and other malignant cells. Oncogene 22:7649–7660. doi: 10.1038/sj.onc.1206971. [DOI] [PubMed] [Google Scholar]
- 31.Dong C, Davis RJ, Flavell RA. 2002. MAP kinases in the immune response. Annu Rev Immunol 20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Shepherd EG, Nelin LD. 2007. MAPK phosphatases–regulating the immune response. Nat Rev Immunol 7:202–212. doi: 10.1038/nri2035. [DOI] [PubMed] [Google Scholar]
- 33.Camps M, Nichols A, Arkinstall S. 2000. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J 14:6–16. doi: 10.1096/fasebj.14.1.6. [DOI] [PubMed] [Google Scholar]
- 34.Furukawa T, Sunamura M, Motoi F, Matsuno S, Horii A. 2003. Potential tumor suppressive pathway involving DUSP6/MKP-3 in pancreatic cancer. Am J Pathol 162:1807–1815. doi: 10.1016/S0002-9440(10)64315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jurek A, Amagasaki K, Gembarska A, Heldin CH, Lennartsson J. 2009. Negative and positive regulation of MAPK phosphatase 3 controls platelet-derived growth factor-induced Erk activation. J Biol Chem 284:4626–4634. doi: 10.1074/jbc.M808490200. [DOI] [PubMed] [Google Scholar]
- 36.Ahmad MK, Abdollah NA, Shafie NH, Yusof NM, Razak S. 2018. Dual-specificity phosphatase 6 (DUSP6): a review of its molecular characteristics and clinical relevance in cancer. Cancer Biol Med 15:14–28. doi: 10.20892/j.issn.2095-3941.2017.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shojaee S, Caeser R, Buchner M, Park E, Swaminathan S, Hurtz C, Geng H, Chan LN, Klemm L, Hofmann WK, Qiu YH, Zhang N, Coombes KR, Paietta E, Molkentin J, Koeffler HP, Willman CL, Hunger SP, Melnick A, Kornblau SM, Muschen M. 2015. Erk negative feedback control enables pre-B cell transformation and represents a therapeutic target in acute lymphoblastic leukemia. Cancer Cell 28:114–128. doi: 10.1016/j.ccell.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dawson CW, Laverick L, Morris MA, Tramoutanis G, Young LS. 2008. Epstein-Barr virus-encoded LMP1 regulates epithelial cell motility and invasion via the ERK-MAPK pathway. J Virol 82:3654–3664. doi: 10.1128/JVI.01888-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ekerot M, Stavridis MP, Delavaine L, Mitchell MP, Staples C, Owens DM, Keenan ID, Dickinson RJ, Storey KG, Keyse SM. 2008. Negative-feedback regulation of FGF signalling by DUSP6/MKP-3 is driven by ERK1/2 and mediated by Ets factor binding to a conserved site within the DUSP6/MKP-3 gene promoter. Biochem J 412:287–298. doi: 10.1042/BJ20071512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z, Kobayashi S, Borczuk AC, Leidner RS, Laframboise T, Levine AD, Halmos B. 2010. Dual specificity phosphatase 6 (DUSP6) is an ETS-regulated negative feedback mediator of oncogenic ERK signaling in lung cancer cells. Carcinogenesis 31:577–586. doi: 10.1093/carcin/bgq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caceres A, Perdiguero B, Gomez CE, Cepeda MV, Caelles C, Sorzano CO, Esteban M. 2013. Involvement of the cellular phosphatase DUSP1 in vaccinia virus infection. PLoS Pathog 9:e1003719. doi: 10.1371/journal.ppat.1003719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qin Z, Dai L, Defee M, Findlay VJ, Watson DK, Toole BP, Cameron J, Peruzzi F, Kirkwood K, Parsons C. 2013. Kaposi’s sarcoma-associated herpesvirus suppression of DUSP1 facilitates cellular pathogenesis following de novo infection. J Virol 87:621–635. doi: 10.1128/JVI.01441-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chou YC, Chen CL, Yeh TH, Lin SJ, Chen MR, Doong SL, Lu J, Tsai CH. 2012. Involvement of recepteur d’origine nantais receptor tyrosine kinase in Epstein-Barr virus-associated nasopharyngeal carcinoma and its metastasis. Am J Pathol 181:1773–1781. doi: 10.1016/j.ajpath.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 44.Chou YC, Lin SJ, Lu J, Yeh TH, Chen CL, Weng PL, Lin JH, Yao M, Tsai CH. 2011. Requirement for LMP1-induced RON receptor tyrosine kinase in Epstein-Barr virus-mediated B-cell proliferation. Blood 118:1340–1349. doi: 10.1182/blood-2011-02-335448. [DOI] [PubMed] [Google Scholar]
- 45.Huang YC, Lin SJ, Lin KM, Chou YC, Lin CW, Yu SC, Chen CL, Shen TL, Chen CK, Lu J, Chen MR, Tsai CH. 23 June 2016, posting date Regulation of EBV LMP1-triggered EphA4 downregulation in EBV-associated B lymphoma and its impact on patients’ survival. Blood doi: 10.1182/blood-2016-02-702530. [DOI] [PubMed] [Google Scholar]
- 46.Lin JH, Lin JY, Chou YC, Chen MR, Yeh TH, Lin CW, Lin SJ, Tsai CH. 2015. Epstein-Barr virus LMP2A suppresses MHC class II expression by regulating the B-cell transcription factors E47 and PU.1. Blood 125:2228–2238. doi: 10.1182/blood-2014-08-594689. [DOI] [PubMed] [Google Scholar]
- 47.Lin SJ, Wu SW, Chou YC, Lin JH, Huang YC, Chen MR, Ma N, Tsai CH. 2015. Novel expression and regulation of TIMP-1 in Epstein Barr virus-infected cells and its impact on cell survival. Virology 481:24–33. doi: 10.1016/j.virol.2015.02.015. [DOI] [PubMed] [Google Scholar]