Hepatitis delta virus is a defective RNA virus that requires hepatitis B virus envelope proteins (HBsAg) to fulfill its life cycle. Thus, HDV can only infect individuals at the same time as HBV (coinfection) or superinfect individuals who are already chronic carriers of HBV. The presence of HDV in the liver accelerates the progression of infection to fibrosis and to hepatic cancer. Since current treatments against HBV are ineffective against HDV, it is of paramount importance to study the interaction between HBV, HDV, and host factors. This will help unravel new targets whereby a therapy that is capable of simultaneously impeding both viruses could be developed. In this research paper, we evidence that the autophagy machinery promotes the replication of HBV and HDV at different steps of their life cycle. Notwithstanding their contribution to HBV release, autophagy proteins seem to assist HDV intracellular replication but not its secretion.
KEYWORDS: hepatitis delta virus (HDV), hepatitis B virus (HBV), autophagy, viral replication, chronic infection, ATG5
ABSTRACT
A substantial number of viruses have been demonstrated to subvert autophagy to promote their own replication. Recent publications have reported the proviral effect of autophagy induction on hepatitis B virus (HBV) replication. Hepatitis delta virus (HDV) is a defective virus and an occasional obligate satellite of HBV. However, no previous work has studied the relationship between autophagy and HDV. In this article, we analyze the impact of HBV and HDV replication on autophagy as well as the involvement of the autophagy machinery in the HDV life cycle when produced alone and in combination with HBV. We prove that HBxAg and HBsAg can induce early steps of autophagy but ultimately block flux. It is worth noting that the two isoforms of the HDV protein, the small HDAg (S-HDAg) and large HDAg (L-HDAg) isoforms, can also efficiently promote autophagosome accumulation and disturb autophagic flux. Using CRISPR-Cas9 technology to generate specific knockouts, we demonstrate that the autophagy machinery, specifically the proteins implicated in the elongation step (ATG7, ATG5, and LC3), is important for the release of HBV without affecting the level of intracellular HBV genomes. Surprisingly, the knockout of ATG5 and ATG7 decreased the intracellular HDV RNA level in both Huh7 and HepG2.2.15 cells without an additional effect on HDV secretion. Therefore, we conclude that HBV and HDV have evolved to utilize the autophagy machinery so as to assist at different steps of their life cycle.
IMPORTANCE Hepatitis delta virus is a defective RNA virus that requires hepatitis B virus envelope proteins (HBsAg) to fulfill its life cycle. Thus, HDV can only infect individuals at the same time as HBV (coinfection) or superinfect individuals who are already chronic carriers of HBV. The presence of HDV in the liver accelerates the progression of infection to fibrosis and to hepatic cancer. Since current treatments against HBV are ineffective against HDV, it is of paramount importance to study the interaction between HBV, HDV, and host factors. This will help unravel new targets whereby a therapy that is capable of simultaneously impeding both viruses could be developed. In this research paper, we evidence that the autophagy machinery promotes the replication of HBV and HDV at different steps of their life cycle. Notwithstanding their contribution to HBV release, autophagy proteins seem to assist HDV intracellular replication but not its secretion.
INTRODUCTION
Autophagy is a catabolic pathway that leads to the degradation of damaged organelles, protein aggregates, and intracellular microorganisms (1). More than 30 autophagy (ATG) proteins participate in the autophagy process that begins, upon induction, by the formation of a double-membrane phagophore that will, upon elongation and maturation, engulf cytoplasmic contents in the so-called autophagosome. The autophagy process is completed by the degradation and recycling of the engulfed materials by lysosomal enzymes following the fusion of autophagosomes with lysosomes (2).
The elongation and maturation of the phagophore require two ubiquitin-like conjugation systems. The first results in the formation of the autophagy elongation complex ATG5-12/16L1. In this process, ATG7, an E1 ubiquitin-like enzyme, captures ATG10, an E2 ubiquitin-like enzyme, and transfers it to ATG12 in order to trigger ATG5-ATG12 conjugation (3). To become functional, the ATG5-ATG12 conjugate requires a stoichiometric association with ATG16L1 (4). The second conjugation system allows the covalent addition of phosphatidylethanolamine (PE) to microtubule-associated protein 1 light chain 3 (LC3). The process is initiated by the cleavage of LC3 near its C terminus by the cysteine protease ATG4B. ATG7 then transfers ATG3 (E2 ubiquitin-like enzyme) to LC3 to form an active intermediate (ATG3-LC3). The recruitment of this intermediate at the phagophore permits the addition of PE by ATG3 with the help of the ATG5-12/16L1 complex, which acts as an E3 ubiquitin-like enzyme (5, 6). Therefore, the association of the elongation complex with the autophagosomal membrane dictates the site of LC3II formation (7, 8). The conjugation of the PE group to LC3 triggers its insertion in the inner and outer membranes of the autophagosome, where it is required for maturation. It should be noted that in this study, LC3 is referred to as LC3B, the most studied of the six LC3/GABARAP homologs.
Recently, several viruses, including hepatitis B virus (HBV) (9–14), have been demonstrated to subvert the antimicrobial catabolic activity of autophagy (xenophagy) by blocking autophagic flux and by using the autophagy proteins to promote their replication (15–17). Hence, the regulation of autophagy could represent a favorable antiviral target against a wide variety of viruses.
Hepatitis delta virus (HDV) is a defective virus that uses the envelope proteins of HBV to form its infectious particles (18). The genome of HDV is a circular, negative-strand RNA containing one open reading frame that codes for two isoforms of HDAg. The 24-kDa small HDAg (S-HDAg) isoform is expressed early in infection and believed to enhance the replication of the viral genome (19). Later in infection, the host double-stranded RNA adenosine deaminase (ADAR1) edits the amber stop codon (UAG) to a tryptophan codon (UGG). This leads to the C-terminal addition of 19 amino acids and, in turn, to the large isoform (27 kDa) of the protein (L-HDAg) (20). This extension contains a nuclear export signal (NES) and a prenylation motif (CXXX) (21, 22). These modifications have been demonstrated to initiate the formation and the secretion of infectious viral particles (23), notably by enabling the interaction between L-HDAg and S-HBsAg (23–25).
To date, no studies on the interplay between autophagy and the replication of HDV have been reported. Since HDV can propagate only in the presence of HBV and the autophagy machinery promotes HBV replication (11, 14, 26), we needed to assess to which extent autophagy was able to interfere with the HDV life cycle. In the present study, the induction of autophagy and its impact on HBV and HDV replication were analyzed and compared in cells expressing these viruses independently or simultaneously. Our results testify that HBV and HDV hijack the autophagy machinery to promote their replication at different steps of their life cycle.
RESULTS
HBsAg and HBxAg expressions induce the accumulation of autophagosomes in Huh7 cells.
Although a consensus has emerged on the ability of HBV to induce autophagy (reviewed in reference 27) and to benefit from its machinery (11), divergent findings indicate that either HBxAg or HBsAg is responsible for induction (9, 14). As such, we started our research by evaluating the induction of autophagy upon transfection with plasmids encoding the complete HBV genome of serotype ayw3 (genotype D) [pCIHBenv(+)] or an envelope-deficient expression vector [pCIHBenv(−)] (Fig. 1A). Interestingly, both plasmids increase the level of intracellular LC3II (72 h posttransfection), a marker of autophagosome formation. Aside from HBsAg, this result suggests that at least one viral protein induces LC3II accumulation. As expected, the autophagy inducer rapamycin also increases LC3II accumulation. We equally noticed that the level of p62, a protein degraded primarily through autophagy, was increased upon HBV expression, suggesting a disruption of autophagic flux. To identify more precisely which HBV proteins were actually responsible for modulating the autophagy process and because several reports identified HBsAg and HBxAg as autophagy inducers, we cloned these two genes into pCIneo in fusion with a C-terminal Flag tag. The transient expression of the tagged proteins in Huh7 cells was confirmed by Western blotting using an anti-Flag antibody (Fig. 1B). We also noticed that both S-HBsAg and HBxAg expression induced increased LC3II and p62, suggesting autophagosome accumulation with an alteration in autophagic flux. To confirm autophagosome accumulation, we visualized LC3 punctum formation using confocal microscopy upon cotransfection of Huh7 cells with plasmids encoding green fluorescent protein (GFP)-LC3 and S-HBsAg or HBxAg or the pCIneo plasmid as a control. Our results showed that both S-HBsAg and HBxAg from serotype ayw3 were able to trigger the accumulation of autophagosomes (Fig. 1C to E). It is worth noting that the ability of HBsAg and HBxAg to induce such accumulation seems to be additive when the viral proteins are expressed under the control of endogenous HBV promoters (Fig. 1F).
FIG 1.
HBsAg and HBxAg expressions induce the accumulation of autophagosomes in Huh7 cells. (A) Huh7 cells were transfected with pCIneo, pCIHBenv(+), or pCIHBenv(−) or treated for 24 h with rapamycin (Rap) (200 nM). Seventy-two hours after transfection, the levels of LC3II, p62, and β-actin were evaluated by Western blotting. (B) Huh7 cells were transfected with pCIneo, pCIHBx-Flag, or pCIHBs-Flag. After 72 h of transfection, the levels of LC3II, p62, and β-actin were evaluated by Western blotting. Moreover, the expressions of HBxAg and HBsAg were verified by Western blotting using an anti-Flag antibody. Cells transfected with pCIneo were treated or not with rapamycin. (C) Huh7 cells were cotransfected with pCIneo, pCIHBx-Flag, or pCIHBs-Flag and peGFP-LC3. As a positive control for autophagy induction, Huh7 cells cotransfected with pCIneo and peGFP-LC3 were treated with rapamycin (100 μM) for 3 h. At 72 h posttransfection, cells were fixed and stained with an anti-Flag antibody (red) or DAPI (blue). (D) The numbers of GFP-LC3 puncta per cell were calculated. (E and F) Huh7 cells were cotransfected with peGFP-LC3 and the plasmid pCIneo, pCIHBx-Flag, or pCIHBs-Flag, expressing the HBV proteins (S-HBsAg or HBxAg) through a cytomegalovirus (CMV) promoter (E), or the plasmid pCIneo, pT7HB2.7x(−) (L-M-S), or pT7HB2.7 (L-M-S-X), expressing the HBV envelope proteins and HBxAg from HBV endogenous promoters (F). The percentage of GFP-LC3-positive cells with punctate LC3 was calculated in transfected cells at 72 h posttransfection. Calculation was based on the count in 100 cells under each condition.
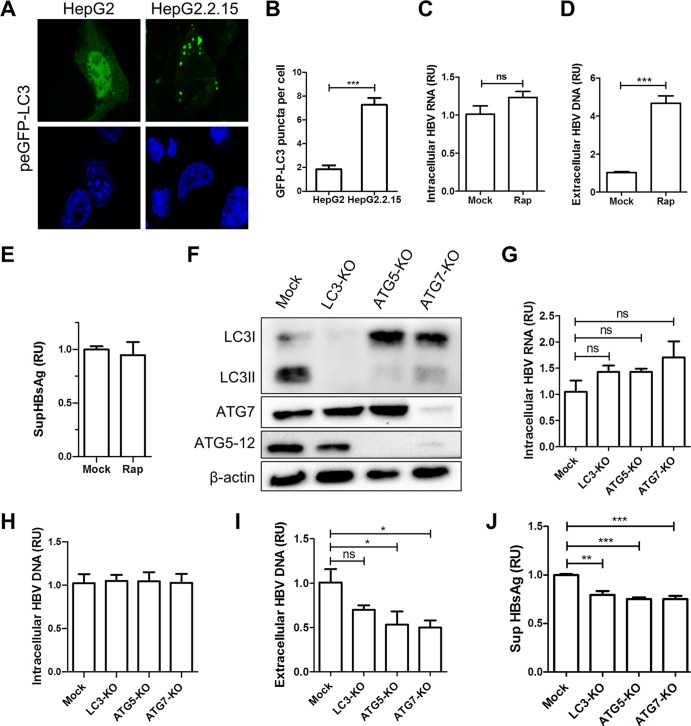
The autophagy machinery is required for HBV maturation/secretion.
To confirm the previously reported proviral effect of autophagy on the HBV life cycle, we used the well-established HepG2.2.15 cell line that harbors four tandem HBV genomic DNA copies of genotype D (ayw serotype) in its genome. HepG2.2.15 cells constitutively express HBV proteins and produce viral particles (28). As illustrated in Fig. 2A and B, the level of autophagosomes is elevated in HepG2.2.15 compared to HepG2 cells. This is likely due to the constitutive expression of HBV proteins. The pharmacological induction of autophagy with rapamycin (50 nM) for 48 h had no effect on the level of intracellular HBV RNA (Fig. 2C). Conversely, a >4-fold increase in HBV extracellular DNA was observed (Fig. 2D), without any significant influence on HBV RNA levels and subviral particle secretion (Fig. 2C and E). On balance, these results indicate that autophagy induction enhances the maturation/secretion of HBV infectious particles rather than the replication of its genome. To confirm these results, the replication of HBV was evaluated in HepG2.2.15 cells with a knockout for ATG7, ATG5, or LC3 (three autophagy proteins were involved in autophagosome formation and expansion). The knockout of these genes was performed via lentiviral delivery of CRISPR-Cas9 guide RNA (gRNA) constructs. Following transduction, knockout cell populations were enhanced by puromycin selection for 9 days, and the efficiency of the different knockouts was evaluated by Western blotting (Fig. 2F). Using this procedure, four HepG2.2.15 derivative cell populations (mock, ATG7 knockout [ATG7-KO], ATG5-KO, and LC3-KO) were obtained (Fig. 2F). As expected, ATG5-KO and ATG7-KO cells lost their ability to lipidate LC3I since both proteins were involved in the conjugation process of LC3. Likewise, the absence of ATG7 in ATG7-KO cells prevented ATG5 and ATG12 conjugation (Fig. 2F). We then measured the intracellular HBV RNA levels and the HBV extracellular virion concentrations in knockout HepG2.2.15 cells. Like the pharmacological activation of autophagy, the inhibition of autophagy did not have a significant effect on intracellular HBV RNA and DNA levels (Fig. 2G and H). However, the concentration of extracellular HBV virions decreased in LC3-KO, ATG5-KO, and ATG7-KO cells (Fig. 2I). In line with the data in Fig. 2D, these results provide evidence for the importance of the autophagy machinery for the accumulation of HBV virions in cell supernatants. They equally support previous reports that suggest that autophagy proteins are involved in nucleocapsid formation and/or envelopment (11, 14). Interestingly, a minor yet significant decrease in HBsAg secretion was observed in HepG2.2.15 KO cells. This indicates that the autophagy machinery could have participated in subviral particle secretion, although the induction of autophagy by rapamycin treatment did not enhance subviral particle secretion (compare Fig. 2E and J).
FIG 2.
Autophagy machinery is required for HBV maturation/secretion. (A and B) HepG2.2.15 and HepG2 cells were transfected with peGFP-LC3 for 72 h. The nuclei were stained with DAPI (blue). The numbers of GFP-LC3 puncta per cell were calculated (n = 74). (C to E) HepG2.2.15 cells were treated or not with rapamycin (50 nM). After 24 h of treatment, cells were washed and treated for another 24 h. Next, supernatants and cell lysates were harvested. The intracellular HBV RNA levels were measured by RT-qPCR. For monitoring the release of HBV virions, Dane particles from supernatants were immunoprecipitated with anti-preS1 antibody and protein A/G beads, and the viral genomes present in the virions were analyzed by qPCR. The release of HBsAg in the cell supernatants (SupHBsAg) was analyzed by an ELISA. The RT-qPCR, qPCR, and ELISA results are presented as relative units (RU) and compared to values for untreated HepG2.2.15 cells. Error bars show the standard errors of the means from four experiments, measured in triplicate. (F) HepG2.2.15 cells were transduced with lentivirus preparations targeting LC3, ATG5, and ATG7 for 9 days. The expression levels of LC3, ATG5, ATG7, and β-actin were verified by Western blotting. (G) Intracellular HBV RNAs were quantified by RT-qPCR. (H) Intracellular HBV DNAs were quantified by qPCR. (I) The extracellular HBV virions were immunoprecipitated with anti-preS1 antibody and protein A/G beads, and the viral DNA expression levels in the knockout populations were quantified by qPCR. (J) The secretion of HBsAg in the cell culture supernatant was measured by an ELISA. Data were measured in quadruplicate under each condition. RU, relative units; ns, not significant.
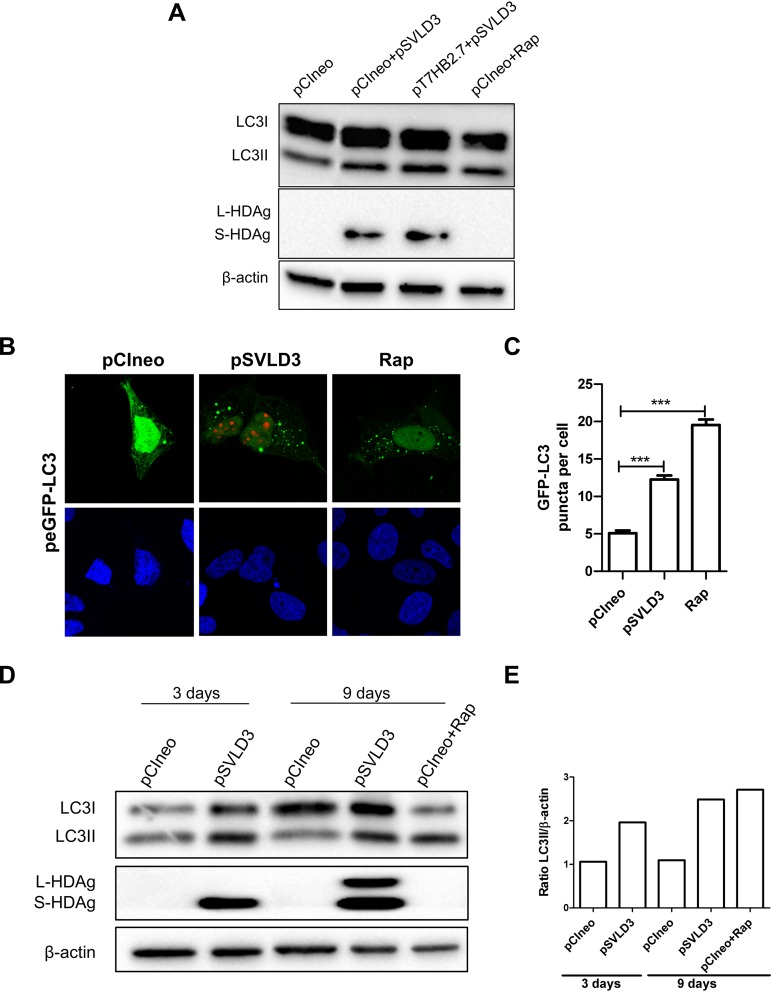
Hepatitis delta virus replication induces autophagosome accumulation in Huh7 cells.
Next, we assessed whether HDV replication was capable of triggering LC3 lipidation. This was explored by using Western blotting (Fig. 3A, D, and E) and confocal microscopy (Fig. 3B and C). To this end, Huh7 cells were transfected with pSVLD3. The latter is a plasmid that contains the complete HDV genome and is sufficient to initiate rolling-cycle replication of the viral genome (29), with or without the plasmid pT7HB2.7 encoding the three forms of HBV envelope proteins. The results indicate that HDV is able to enhance LC3II and autophagosome accumulation even in the absence of HBV envelope proteins. We then analyzed autophagy activity at two different time points (3 and 9 days posttransfection) to assess if LC3 lipidation was maintained during the entire replication cycle of HDV. As observed in Fig. 3D and E, the lipidation of LC3 was maintained during the course of HDV replication. It is worth noting that an enhanced lipidation of LC3I into LC3II can be observed at the time point of 9 days posttransfection, coinciding with a stronger production of HDAg. This finding suggests that although HBV can efficiently induce autophagosome accumulation, HDV has also evolved to trigger autophagosome formation.
FIG 3.
Hepatitis delta virus replication induces autophagosome accumulation in Huh7 cells. (A) Huh7 cells were transfected as indicated or treated with rapamycin (200 nM) for 24 h. At 72 h posttransfection, cells were lysed, and the levels of LC3, HDAg, and β-actin were evaluated by Western blotting. (B) Huh7 cells were cotransfected with pCIneo or pSVLD3 and peGFP-LC3. As a positive control for autophagy induction, Huh7 cells cotransfected with pCIneo and peGFP-LC3 were treated with rapamycin (100 μM) for 3 h. After 72 h, cells were fixed and then stained with anti-HDAg antibody from HDV+ human serum (red). The nucleus was stained with DAPI (blue). (C) The number of GFP-LC3 puncta per cell was calculated from confocal images (n = 100). (D) Huh7 cells were transfected with pCIneo or pSVLD3 or treated with rapamycin (200 nM) for 24 h. At 3 and 9 days posttransfection, cells were lysed, and the levels of LC3, HDAg, and β-actin were evaluated by Western blotting. (E) Ratios of LC3II/β-actin.
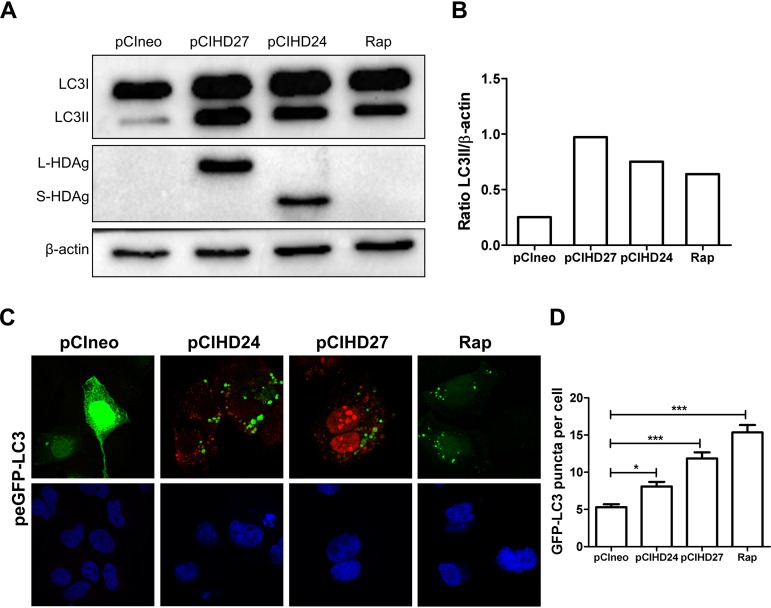
The two isoforms of HDAg can induce autophagosome accumulation in Huh7 cells.
HDV replication results in the expression of two isoforms of HDAg. S-HDAg (24 kDa) is expressed early upon infection. At a later stage, genome editing gives rise to L-HDAg (27 kDa) (20). To identify which of these two isoforms enhances LC3II formation, we transfected Huh7 cells with plasmids encoding either S-HDAg or L-HDAg (pCIHD24 and pCIHD27). Cells transfected with an empty plasmid or treated with rapamycin were used as negative and positive controls, respectively. Our results demonstrate that both S-HDAg and L-HDAg promote the accumulation of LC3II, suggesting that the effector domain is shared by the two isoforms of HDAg (Fig. 4A and B). To confirm the effect of HDAg isoforms on autophagosome formation, we analyzed GFP-LC3 punctum formation by confocal microscopy. Our findings show that S-HDAg and L-HDAg significantly increase the number of GFP-LC3 puncta per cell (Fig. 4C and D).
FIG 4.
The two isoforms of HDAg can induce autophagosome accumulation in Huh7 cells. (A) Huh7 cells were transfected with pCIneo or a plasmid coding for L-HDAg (pCIHD27) or S-HDAg (pCIHD24). As a control, Huh7 cells were treated with rapamycin (200 nM) for 24 h. At 72 h posttransfection, cells were lysed, and the levels of LC3, HDAg, and β-actin were evaluated by Western blotting. (B) Ratios of LC3II/β-actin. (C) Confocal images of Huh7 cells cotransfected with pCIneo, pCIHD27, pCIHD24, and peGFP-LC3. The S-HDAg and L-HDAg proteins were labeled using anti-HDAg antibody from HDV+ human serum (red). The nucleus was stained with DAPI (blue). (D) The number of GFP-LC3 puncta per cell was calculated (n = 100).
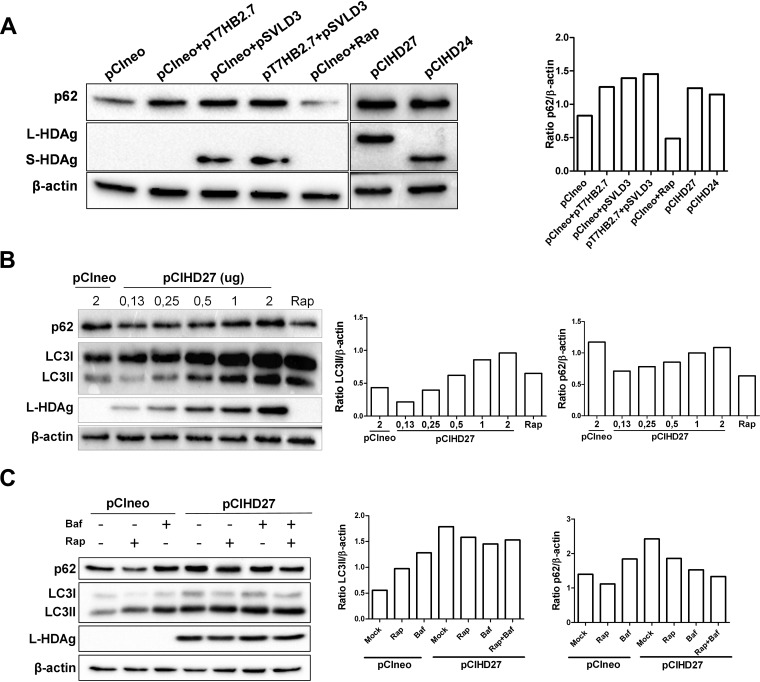
L-HDAg and S-HDAg expressions impede autophagic flux.
As shown in Fig. 1A, we observed that the expression of HBV proteins was sufficient to increase the level of p62, suggesting a reduction of autophagic flux, as previously reported (10, 13, 26). Indeed, the degradation of p62 is widely used as a marker to monitor autophagic flux since its LIR (LC3-interacting region) motif allows its degradation within autolysosomes (30, 31). The increase in intracellular autophagosomes upon HDV protein expression may result from reduced autophagic flux. To test this hypothesis, Huh7 cells were transfected with pSVLD3 and/or pT7HB2.7, and the level of p62 was analyzed by Western blotting (Fig. 5A). Even in the absence of HBV envelope protein expression, HDV replication increases the intracellular level of p62. This suggests that HDV proteins can reduce autophagic flux without the need for virion formation. Rapamycin treatment, known to induce complete autophagic flux, was used as a control. To confirm that the accumulation of p62 was due to HDV protein expression, cells were transfected with a plasmid encoding L-HDAg or S-HDAg, and their effect on autophagy was analyzed by Western blotting. Interestingly enough, the results show that the two HDV protein isoforms can efficiently block autophagic flux (Fig. 5A). To further explore whether the accumulation is a result of incomplete autophagic flux, a dose-response experiment was performed, in which the effect of increasing amounts of L-HDAg on the levels of LC3II and p62 was measured (Fig. 5B). For this experiment, pCIHD27 was selected over pCIHD24 due to the higher level of LC3II observed upon L-HDAg expression (Fig. 4B and D). As expected, the data show a clear L-HDAg dose-dependent accumulation of LC3II (Fig. 5B). It should be noted that the expression levels of L-HDAg and LC3II correlate with the amount of p62, strengthening our assumption that HDV proteins can negatively modulate a late step in autophagy.
FIG 5.
L-HDAg and S-HDAg expressions impede autophagic flux. (A) Huh7 cells were transfected as indicated or transfected with pCIneo and treated with rapamycin (200 nM) for 24 h. (Left) At 72 h posttransfection, the levels of p62, HDAg, and β-actin were evaluated by Western blotting. (Right) The ratios of p62/β-actin were quantified. (B) Huh7 cells were transfected with pCIneo and treated or not with rapamycin (200 nM for 24 h) or with increasing quantities of pCIHD27. (Left) After 72 h, the levels of expression of p62, LC3, L-HDAg, and β-actin were evaluated by Western blotting. (Right) The ratios of LC3II/β-actin and p62/β-actin were quantified. (C) Huh7 cells were transfected with pCIneo or pCIHD27 and treated or not with rapamycin and/or bafilomycin A1 (Baf), as indicated. (Left) After 72 h, cells were lysed, and the levels of p62, LC3, L-HDAg, and β-actin were evaluated by Western blotting. (Right) The ratios of LC3II/β-actin and p62/β-actin were also quantified.
To confirm the effect of L-HDAg expression on LC3II and p62 turnover, we treated Huh7 cells with rapamycin and/or bafilomycin A1. Treatment with bafilomycin A1 is known to abrogate autophagic flux completely through the inhibition of autolysosome acidification and autophagosome-lysosome fusion (32). It allows the direct comparison of protein turnover due to autophagic flux. Huh7 cells transfected with an empty plasmid (pCIneo) and treated with rapamycin or bafilomycin A1 were used as controls (Fig. 5C). As expected, separate treatments with rapamycin and bafilomycin A1 in pCIneo-transfected cells initiated a significant increase in the quantity of LC3II. However, only bafilomycin A1 treatment resulted in p62 accumulation. On the other hand, the expression of L-HDAg clearly induced the accumulation of LC3II and p62 regardless of drug treatments. Remarkably, the addition of bafilomycin A1 did not increase the level of p62. This means that L-HDAg was efficiently, or probably completely, blocking autophagic flux. These results confirm that HDV replication and its proteins promote the accumulation of autophagosomes and can effectively block the degradative action of autophagy.
The autophagy machinery promotes HDV replication.
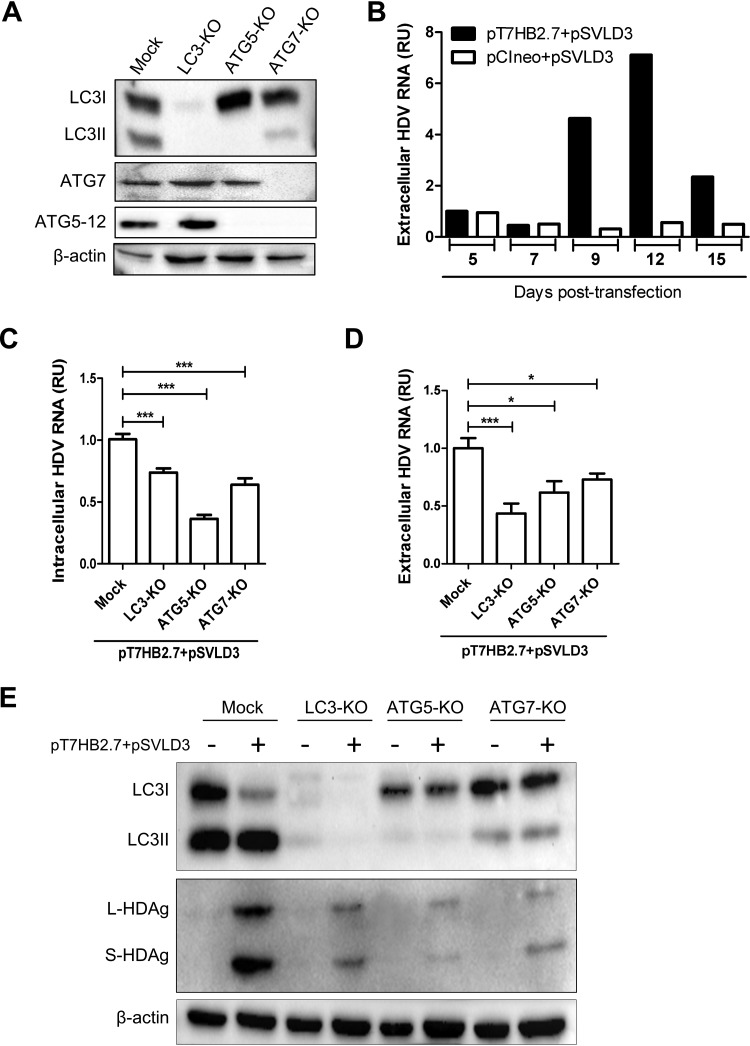
To understand whether the block of autophagy exerted by HDV was meant to allow viral replication, we sought to determine the effect of autophagy on the HDV life cycle. For this purpose, we produced Huh7 cells with a knockout of LC3, ATG5, or ATG7 using the same methodology as the one described above. The depletion of the targeted proteins was confirmed by Western blotting (Fig. 6A). Next, we analyzed the kinetics of HDV replication in wild-type Huh7 cells transfected with pT7HB2.7 and pSVLD3 and found that the peak of HDV particle production occurred at 9 to 12 days posttransfection (Fig. 6B). Therefore, day 9 posttransfection was chosen for the quantification of the intracellular replication and extracellular accumulation of the virus by reverse transcription-quantitative PCR (RT-qPCR) in the different cell populations (mock, LC3-KO, ATG5-KO, and ATG7-KO). Surprisingly, and unlike HBV, the knockout of autophagy genes resulted in a clear reduction of viral genome replication, as shown by the decrease in intracellular RNA (Fig. 6C). HDV replication was mainly affected by ATG5 depletion and mildly affected by ATG7 and LC3 depletion. This suggests that the autophagy elongation complex rather than autophagosome maturation acts as a proviral factor at the replication step of HDV. Such an observation was previously made for hepatitis C virus (HCV) (17, 33). By analyzing the impact of gene knockouts on the complete replication cycle of the virus, no additional inhibition was observed in Huh7 ATG5-KO cells. This means that the primary proviral role of ATG5 is at a stage prior to viral morphogenesis (Fig. 6D). Interestingly, in LC3-KO cells, we observed a clear additional reduction in extracellular HDV particles. This suggests that LC3 likely participates in a step subsequent to genome replication (ribonucleoprotein [RNP] formation, envelopment, or secretion). These results are in sharp contrast with the role of autophagy in the HBV life cycle, where autophagy modulation had no effect on HBV genome replication but was important at a later stage (Fig. 2). Next, we sought to monitor the effect of autophagic protein knockouts on L-HDAg and S-HDAg expression. The results depicted in Fig. 6E demonstrate that the depletion of the autophagy proteins severely reduces viral protein expression, which is in agreement with the observed effect on HDV replication. On the whole, these results demonstrate that ATG5 is positively regulating HDV replication, whereas LC3 acts as a proviral factor mainly at a later replication step.
FIG 6.
Autophagy machinery is required for HDV replication in Huh7 cells. (A) Huh7 cells were transduced with lentivirus to produce stable knockout populations for LC3, ATG5, and ATG7 genes. Western blotting was conducted to verify the knockout efficiencies. (B) Huh7 cells were cotransfected with pT7HB2.7 and pSVLD3 to produce HDV virions or with pSVLD3 and pCIneo. The supernatants were harvested at different time points to determinate the kinetics of secretion of HDV virions by RT-qPCR. (C to E) Huh7 cells knocked out for LC3, ATG5, or ATG7 were cotransfected with pT7HB2.7 and pSVLD3. (C and D) At 9 days posttransfection, intracellular (C) and extracellular (D) HDV RNAs were quantified by RT-qPCR. To control DNA plasmid contamination, the supernatants were treated with Benzonase. Error bars show the standard errors of the means from three independent experiments, measured in triplicate. (E) Cell lysates were used for Western blotting to evaluate the LC3, HDAg, and β-actin expression levels.
The autophagy machinery differentially affects the HBV and HDV replication cycle in cells expressing both viruses.
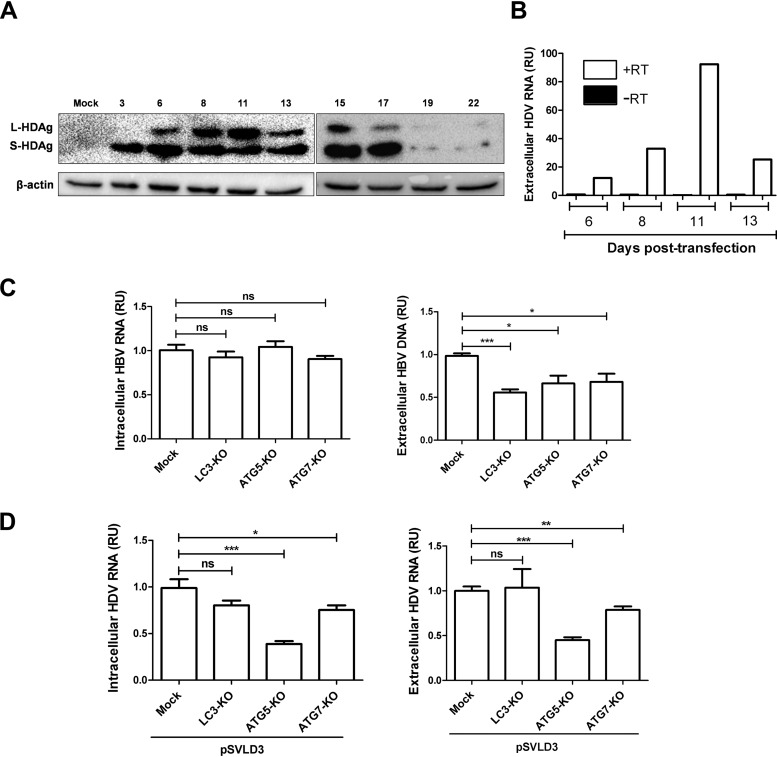
In patients, HDV can produce infectious particles only in HBV-infected hepatocytes. Nevertheless, in vitro characterization of host factors affecting HDV replication in cells harboring HBV replication has been rarely performed. To characterize simultaneously the impact of the autophagy machinery on the HBV and HDV life cycle, we first analyzed the kinetics of expression of S-HDAg and L-HDAg in HepG2.2.15 cells transfected with pSVLD3 starting from day 3 to day 22 posttransfection (Fig. 7A). The appearance of L-HDAg is known to correspond to HDV RNP formation and envelopment (34). As expected, the levels of L-HDAg expression observed (days 8 to 11) perfectly correlated with the extracellular HDV RNA level (Fig. 7B). The results also show that HDV replication is transient in HepG2.2.15 cells and mimics the replication cycle observed in Huh7 cells (Fig. 6B). Using a similar approach, we transfected HepG2.2.15 cells knocked out for LC3, ATG5, or ATG7 (Fig. 2) with pSVLD3 and analyzed viral replication and secretion at day 11 posttransfection. The results show that the replication of HDV did not modify the overall effect of autophagy proteins on the HBV replication cycle (Fig. 7C). Indeed, HBV genome replication remained unaffected by the knockout of autophagy proteins (Fig. 7C, left), whereas its maturation/secretion was still impaired (Fig. 7C, right). On the other hand, the knockout of ATG5 was detrimental for HDV genome replication (Fig. 7D, left), but no additional effect on its maturation was seen (Fig. 7D, right). Of note, the proviral effect of LC3 on HDV secretion detected in transfected Huh7 cells (Fig. 6D) was not observed in HepG2.2.15 cells. It is not clear whether this discrepancy is caused by cell tropism (Huh7 versus HepG2) or by the replication of both viruses in the same cells.
FIG 7.
Autophagy machinery differentially affects the HBV and HDV replication cycle in cells expressing both viruses. (A and B) The kinetics of HDV replication were evaluated in the presence of HBV replication. HepG2.2.15 cells were transfected with pSVLD3, and cells and supernatants were collected at different time points posttransfection. (A) Cell lysates from days 3 to 22 were subjected to Western blotting to evaluate the expression of HDAg and β-actin. (B) HDV RNAs from supernatants collected at 6 to 13 days posttransfection were quantified by RT-qPCR. To control for plasmid DNA contamination, the supernatants were treated with Benzonase, DNase treatment was carried out on columns during RNA extraction, and RT-qPCR was conducted with or without the reverse transcriptase enzyme. (C) HepG2.2.15 cells knocked out for LC3, ATG5, and ATG7 were transfected with pSVLD3. At 11 days posttransfection, cells and supernatants were harvested. (Left) To analyze intracellular HBV RNA, RT-qPCR was conducted. (Right) To measure the release of HBV virions, immunoprecipitated virions were subjected to qPCR. (D) Intracellular (left) and extracellular (right) HDV RNAs were quantified by RT-qPCR. Error bars show the standard errors of the means from three independent experiments, measured in triplicate.
DISCUSSION
There is accumulating evidence that HBV has evolved to subvert autophagy in order to promote its replication cycle in vitro and in vivo (reviewed in reference 27). However, to our knowledge, this is the first study that explores the relationship between autophagy and HDV, an occasional and obligate satellite of HBV. Importantly, the overall effect of HDV on autophagy induction and the modulatory effect of autophagy on HDV replication were confirmed in a model that produces both viruses (HBV and HDV). Such studies are required to identify host factors that impede the concomitant replication of HBV and HDV. Although HBV standard-of-care treatments are efficient in reducing HBV replication and therefore reducing HBV-related cirrhosis and hepatocellular carcinoma, they are ineffective in controlling HDV-related pathogenesis. Several previous reports suggested that HBxAg protein activates autophagy under starvation conditions via different pathways (9, 10, 12). Conversely, a different group suggested that HBsAg protein is responsible for the induction of autophagy in HBV infection (14). In this research, we provide evidence that both HBxAg and HBsAg contribute to autophagosome accumulation in Huh7 cells under normal nutritive conditions. Our results similarly demonstrate that S-HDAg and L-HDAg induce autophagosome accumulation. The strong LC3II accumulation obtained upon S-HDAg or L-HDAg expression indicates that HDV has evolved to trigger early steps in autophagy. It can be argued that autophagy is already promoted by HBV, thus decreasing the biological relevance of this observation. However, since the HDV replication cycle depends on autophagy machinery, it seems important that HDV proteins maintain efficient activation in case HBV proteins fail to provide sufficient levels of induction. We also show that HDV-induced autophagosome accumulation seems to be mainly due to incomplete autophagic flux, as evidenced by p62 accumulation, which was comparable to the one observed upon bafilomycin A1 treatment. Blocking of autophagic flux has been observed with several viruses (15, 35, 36) and is believed to favor viral replication by impeding xenophagy and enriching the level of autophagy factors involved at diverse levels of viral replication. In the case of HBV, our results agree with those of a recent study showing that autophagy machinery (mainly the elongation complex, ATG5-12/16L1) is important for a late step in the HBV life cycle (11). Although our experimental design could not precisely identify the stage that requires the autophagy machinery, it is likely a stage that follows HBV transcription and translation as well as reverse transcription, since the depletion of LC3, ATG5, and ATG7 did not affect the intracellular levels of HBV RNA and DNA but significantly reduced Dane particle secretion. It is important to note that HBsAg secretion was slightly reduced in HepG2.2.15 cells depleted for LC3, ATG5, and ATG7. This suggests that the autophagy machinery supports HBV subviral particle secretion. However, the addition of rapamycin did not increase subviral particle secretion, which suggests that the inherent level of autophagy present in HepG2.2.15 cells is sufficient to assist subviral particle secretion. We speculate that the autophagy machinery increases viral particle secretion by providing an additional source of membranes at the viral particle formation site or that HBV triggers incomplete autophagic flux that enhances cell secretory pathways.
The main objective of this study was to identify the role of autophagy in the HDV life cycle. For this purpose, Huh7 and HepG2.2.15 cells knocked out for LC3, ATG5, and ATG7 were established using CRISPR-Cas9 technology. ATG5 and LC3 were chosen in order to dissect the independent contributions of the two autophagy conjugation systems (ATG5-12 and LC3II) to HDV propagation. Indeed, these conjugation systems form the main autophagy machinery required for double-membrane vesicle elongation and maturation (37–39). We also included ATG7 in our study since its enzymatic activity (E1-like activating enzyme) is essential for the conjugation of both ATG12 and LC3I (40). The deletion of ATG5 significantly reduced the intracellular HDV RNA level, indicating that ATG5 acts as a proviral factor. The deletion of ATG7 and LC3 rendered a milder effect on the pool of intracellular HDV RNA, suggesting that ATG5 per se is specifically important. Therefore, the implication of its conjugation to ATG12 for viral replication would need further characterization. Interestingly, we observed a severe depletion of the intracellular level of HDAg in cells knocked out for LC3, ATG5, and ATG7. The level of depletion observed for the HDV proteins, particularly obvious in ATG5-KO cells, is higher than the level of depletion observed for the HDV RNA. This discrepancy is likely due to the fact that our RT-qPCR cannot decipher between the different forms of HDV RNA (genomic, antigenomic, and mRNA). On the whole, these results imply that ATG5, rather than the entire autophagy process, assists viral replication in a noncanonical manner at a replication step prior to RNP formation. Importantly, the proviral effect of ATG5 on HDV and that of ATG5-12 on HBV were maintained in HepG2.2.15 cells producing HBV and HDV. Since HDV replication occurs in the nucleus, the specific nuclear relocalization of autophagy proteins, such as ATG5, ATG12, and ATG16L1, to assist in HDV replication should be explored. It is worth mentioning that two different phenotypes for HDV secretion were observed in LC3-KO Huh7 and HepG2.2.15 cells. In Huh7 cells, LC3 was found to have additional effects on the release of HDV particles, whereas in HepG2.2.15 cells, LC3 had no effect on HDV secretion. Probably, this discrepancy is caused by an intrinsic difference in the cell lines or linked to the presence of HBV replication in HepG2.2.15 cells. As such, the putative proviral effect of LC3 or its homologs on HDV requires further investigation.
In summary, we have identified ATG5 as an important host factor for the replication of HDV and also provide the first evidence that targeting autophagy represents an approach to target the life cycles of HBV and HDV simultaneously.
MATERIALS AND METHODS
Cell culture, reagents, and antibodies.
The human hepatocellular carcinoma cell line Huh7 and the human embryonic kidney cell line HEK293T were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin. Concerning the production of HDV, Huh7 cells were cultured in Williams’ medium E (WME) supplemented with 10% fetal bovine serum, 100 mg/ml gentamicin, and GlutaMAX (Gibco). The human hepatoblastoma cell lines HepG2 and HepG2.2.15 were cultured in WME supplemented with 10% FBS, 100 mg/ml gentamicin, and GlutaMAX. Rapamycin, bafilomycin A1, and Benzonase were purchased from Sigma-Aldrich. The concentration of rapamycin used in each experiment was adjusted depending on the exposure time to minimize toxicity. Protein A/G-agarose plus was purchased from Santa Cruz. DNase was purchased from Qiagen. Rabbit polyclonal anti-LC3B (catalog number L7543) and anti-ATG5 (catalog number A0856) antibodies and mouse monoclonal anti-β-actin (catalog number A5441) and anti-Flag (catalog number F1804) (for immunofluorescence) antibodies were purchased from Sigma-Aldrich. The monoclonal antibody anti-SQSTM1 (catalog number H00008878) was purchased from Abnova. The rabbit polyclonal antibodies anti-ATG7 (catalog number D12B11) and anti-Flag (catalog number 2368) (for Western blotting) were purchased from Cell Signaling. Human serum against HDV was described previously (41). The anti-preS1 (catalog number Sc-57761) monoclonal antibody was purchased from Santa Cruz.
Plasmids and transfection.
The HBV recombinant plasmid pCIHBenv(−) was used for the production of HBV nucleocapsid, and the plasmid pCIHBenv(+) was used for the production of HBV virions, as previously described (42). The HBV expression vector pT7HB2.7 was used for the production of L-HBsAg, medium HBsAg (M-HBsAg), S-HBsAg, and HBxAg proteins under the control of the endogenous HBV promoters (42–44). The plasmid pT7HB2.7x(−) is derived from the pT7HB2.7 plasmid, where the start codon of HBxAg was mutated from AUG to GUG using the QuikChange lightning site-directed mutagenesis kit (Agilent). For the construction of pCIHBx-Flag and pCIHBs-Flag, the sequences coding for S-HBsAg and HBxAg were amplified by PCR from the pCIHBenv(+) plasmid and then inserted between the NotI and NheI restriction sites in the pCIneo plasmid (Promega). In parallel, a Flag tag was added to the C terminus of each protein. The primers used for S-HBsAg and HBxAg cloning are 5′-GGGAAGCGGCCGCTTAAATGTATACCCA-3′ and 5′-TATAGGCTAGCACCATGGAGAACATCACATCAG-3′, and 5′-AGGGAAGCGGCCGCTTAGGCAGAGGTGAA-3′ and 5′-TATAGGCTAGCACCATGGCTGCTAGGCTG-3′, respectively. The addition of the Flag tag to S-HBsAg and HBxAg was performed using the following primers: 5′-GGGAAGCGGCCGCTTACTTGTCGTCATCGTCTTTGTAGTCAATGTATACCCAAAG-3′ and 5′-AGGGAAGCGGCCGCTTACTTGTCGTCATCGTCTTTGTAGTCGGCAGAGGTGAAA-3′. The plasmid pSVLD3, containing three head-to-tail copies of the full-length HDV cDNA, was used for the replication of HDV RNA and the production of HDV RNP (24, 45, 46). The plasmids pCIHD24 and pCIHD27 were used for the expression of S-HDAg and L-HDAg, respectively (24). The peGFP-LC3 plasmid was kindly provided by Tamotsu Yoshimori. Transfection with plasmid DNA was carried out using Lipofectamine 2000 (Life Technologies). The quantities of DNA plasmids are indicated in the figure legends; otherwise, a quantity of 1.6 μg DNA plasmid was used for cells in 12-well plates. The efficiency of transfection was evaluated using the peGFP plasmid and visualized by immunofluorescence microscopy.
CRISPR-Cas9 knockouts.
Lentiviral constructs were used as vectors for the expression of Cas9 and small guide RNA (sgRNA). The lentiCRISPRv2, psPAX2, pMD2.G, and pRSV-REV plasmids were purchased from Addgene. Target sequences for the specific knockout of LC3B, ATG5, and ATG7 were identified using the E-CRISP online tool (http://www.e-crisp.org/), along with basic settings (Table 1), and cloned into the lentiCRISPRv2 plasmid. Lentiviral vectors were produced by the transfection of HEK293T cells with lentiCRISPRv2 derivatives, along with packaging plasmids psPAX2, pMD2.G, and pRSV-REV. The supernatants from these transfected cells were collected at day 2 posttransfection, clarified, and filtered (0.45 μm). Target cells (Huh7 or HepG2.2.15) were inoculated with the lentiviral constructs for 16 h in the presence of 8 μg/ml Polybrene (Sigma) and further cultured for 9 days in the presence of 1 μg/ml puromycin (Sigma). As controls, mock cells were transduced with lentiviral vectors containing no sgRNA sequence but still driving the expression of Cas9. Cells were then harvested, lysed, and analyzed by Western blotting.
TABLE 1.
Guide RNA primer sequences used in this study
| Gene | Guide RNA primer sequence |
|---|---|
| LC3B | Forward, CACCGGTGATAATAGAACGATACAA |
| Reverse, AAACTTGTATCGTTCTATTATCACC | |
| ATG5 | Forward, CACCGGATCACAAGCAACTCTGGAT |
| Reverse, AAACATCCAGAGTTGCTTGTGATCC | |
| ATG7 | Forward, CACCGGTATGATGAGAACATGGTGC |
| Reverse, AAACGCACCATGTTCTCATCATACC | |
Quantitative PCR.
(i) HDV. The supernatant was clarified by centrifugation at 1,200 rpm for 10 min prior to Benzonase treatment (30 min at 37°C). HDV RNA was extracted using a QIAamp viral RNA minikit (Qiagen) and reverse transcribed using iScript select cDNA (Bio-Rad). Quantification of HDV RNA was then conducted by TaqMan PCR. Intracellular HDV RNA was extracted using an Aurum total RNA minikit (Bio-Rad). The sequences for forward and reverse primers and the probe were 5′-TGGACGTGCGTCCTCCT-3′, 5′-TCTTCGGGTCGGCATGG-3′, and 5′-ATGCCCAGGTCGGAC-3′ (5′-6-carboxyfluorescein [FAM]/3′-6-carboxytetramethylrhodamine [TAMRA]), respectively, as previously described (41).
(ii) HBV. The supernatant was clarified by centrifugation at 1,200 rpm for 10 min prior to Benzonase treatment (30 min at 37°C). The treated supernatants containing Dane particles were subjected to immunoprecipitation using protein A/G-agarose plus beads and anti-preS1 antibody. Intracellular and extracellular HBV DNAs were extracted using a QIAamp viral DNA minikit (Qiagen). Intracellular HBV RNA was extracted using an Aurum total RNA minikit (Bio-Rad). The cDNA was obtained using iScript select cDNA (Bio-Rad). Quantification of HBV DNA and RNA was performed by TaqMan real-time PCR. The sequences for the forward and reverse primers and the probe were 5′-ACGTCCTTTGTTTACGTCCCG-3′, 5′-CCAACTCCTCCCAGTCTTTAAAC-3′, and 5′-FAM-TCAACGACCGACCTTGA-dabcyl-MBG-3′, respectively.
Indirect immunofluorescence and confocal microscopy.
For the detection of autophagosomes, Huh7 cells were cotransfected with peGFP-LC3 and plasmids as indicated in the figure legends. The fluorescence of GFP-LC3 was observed by confocal microscopy. Cells containing three or more GFP-LC3 dots were defined as autophagy-positive cells. The number of GFP-LC3 puncta per cell was calculated from 100 GFP-positive cells per sample unless otherwise indicated in the figure legends. For protein staining, Huh7 cells were cotransfected with the different plasmids as indicated in the figure legends. Forty-eight hours later, cells were trypsinized and grown on glass coverslips. After 24 h, cells were fixed using 4% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min and permeabilized with 0.5% Triton X-100 for 5 min. Next, coverslips were incubated with a blocking solution (PBS, 3% bovine serum albumin [BSA], 10% FBS) for 30 min at room temperature (RT), rinsed with PBS, and then incubated with the corresponding primary antibody (anti-HDAg antibody from HDV-positive [HDV+] human serum at a 1:1,000 dilution or mouse anti-Flag at 1:100) for 1 h at room temperature. After incubation, cells were rinsed with PBS and incubated with the corresponding secondary antibody (goat anti-human or anti-mouse Alexa Fluor 568 antibody) for 1 h at room temperature. After incubation, cells were rinsed with PBS and incubated with DAPI (4′,6-diamidino-2-phenylindole; Life Technologies) to stain cell nuclei. The coverslips were mounted on glass slides with Prolong Diamond antifade (Invitrogen) and analyzed using a Zeiss LSM 780 confocal microscope.
Western blotting.
Cells were lysed in lysis buffer (25 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% NP-40, 1 mM EDTA, 5% glycerol) supplemented with an EDTA-free complete protease inhibitor (Roche). A Pierce bicinchoninic acid (BCA) protein assay kit (Thermo Scientific) was used to normalize for total protein content in the different lysates. Equivalent amounts of protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad). The membranes were blocked for 30 min at room temperature with PBS–5% milk and then incubated overnight at 4°C with primary antibodies in PBS–0.1% BSA. Membranes were then washed with 0.4% Tween 20 in PBS and incubated for 1 h at RT with a horseradish peroxidase (HRP)-conjugated secondary antibody (goat anti-rabbit, goat anti-mouse, or goat anti-human IgG) in PBS–5% milk. Protein bands were revealed using the Clarity Western ECL reagent (Bio-Rad) using a ChemiDoc MP imaging system (Bio-Rad).
Enzyme-linked immunosorbent assay (ELISA).
HBsAg quantification in supernatants of cultured cells was carried out using the Murex HBsAg Version 3 kit (DiaSorin) as previously described (47).
Statistics.
To statically analyze the results, a two-tailed, unpaired t test was performed using GraphPad Prism 5. P values below 0.05 were considered statistically significant (*, P < 0.05; **, P < 0.01; ***, P < 0.001). The values are expressed as the means ± the standard errors of the means from at least three independent experiments.
ACKNOWLEDGMENTS
We thank Ahmed Fahmy for helpful comments on the manuscript and Tania Lucette Lognon Bomombé, and Jessy Trembley for technical support. We thank Ridha Bouich for proofreading the manuscript. The peGFP-LC3 construct was kindly supplied by Tamotsu Yoshimori. lentiCRISPRv2 puro was a gift from Brett Stringer (Addgene plasmid 98290). pRSV-Rev, psPAX2, and pMD2.G were gifts from Didier Trono (Addgene plasmids 12253, 12260, and 12259).
This work was supported by a grant from the NSERC of Canada to P.L. M.K. was supported by a scholarship from the Tunisian Ministry of Education.
REFERENCES
- 1.Klionsky DJ, Emr SD. 2000. Autophagy as a regulated pathway of cellular degradation. Science 290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noda NN, Inagaki F. 2015. Mechanisms of autophagy. Annu Rev Biophys 44:101–122. doi: 10.1146/annurev-biophys-060414-034248. [DOI] [PubMed] [Google Scholar]
- 3.Shpilka T, Mizushima N, Elazar Z. 2012. Ubiquitin-like proteins and autophagy at a glance. J Cell Sci 125:2343–2348. doi: 10.1242/jcs.093757. [DOI] [PubMed] [Google Scholar]
- 4.Mizushima N, Kuma A, Kobayashi Y, Yamamoto A, Matsubae M, Takao T, Natsume T, Ohsumi Y, Yoshimori T. 2003. Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the Apg12-Apg5 conjugate. J Cell Sci 116:1679–1688. doi: 10.1242/jcs.00381. [DOI] [PubMed] [Google Scholar]
- 5.Sakoh-Nakatogawa M, Matoba K, Asai E, Kirisako H, Ishii J, Noda NN, Inagaki F, Nakatogawa H, Ohsumi Y. 2013. Atg12-Atg5 conjugate enhances E2 activity of Atg3 by rearranging its catalytic site. Nat Struct Mol Biol 20:433–439. doi: 10.1038/nsmb.2527. [DOI] [PubMed] [Google Scholar]
- 6.Hanada T, Noda NN, Satomi Y, Ichimura Y, Fujioka Y, Takao T, Inagaki F, Ohsumi Y. 2007. The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J Biol Chem 282:37298–37302. doi: 10.1074/jbc.C700195200. [DOI] [PubMed] [Google Scholar]
- 7.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. 2000. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizushima N, Yoshimori T, Ohsumi Y. 2011. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol 27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 9.Wang P, Guo QS, Wang ZW, Qian HX. 2013. HBx induces HepG-2 cells autophagy through PI3K/Akt-mTOR pathway. Mol Cell Biochem 372:161–168. doi: 10.1007/s11010-012-1457-x. [DOI] [PubMed] [Google Scholar]
- 10.Zhong L, Shu W, Dai W, Gao B, Xiong S. 2017. Reactive oxygen species-mediated c-Jun NH2-terminal kinase activation contributes to hepatitis B virus X protein-induced autophagy via regulation of the Beclin-1/Bcl-2 interaction. J Virol 91:e00001-17. doi: 10.1128/JVI.00001-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doring T, Zeyen L, Bartusch C, Prange R. 2018. Hepatitis B virus subverts the autophagy elongation complex Atg5-12/16L1 and does not require Atg8/LC3 lipidation for viral maturation. J Virol 92:e01513-17. doi: 10.1128/JVI.01513-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang H, Da L, Mao Y, Li Y, Li D, Xu Z, Li F, Wang Y, Tiollais P, Li T, Zhao M. 2009. Hepatitis B virus X protein sensitizes cells to starvation-induced autophagy via up-regulation of beclin 1 expression. Hepatology 49:60–71. doi: 10.1002/hep.22581. [DOI] [PubMed] [Google Scholar]
- 13.Liu B, Fang M, Hu Y, Huang B, Li N, Chang C, Huang R, Xu X, Yang Z, Chen Z, Liu W. 2014. Hepatitis B virus X protein inhibits autophagic degradation by impairing lysosomal maturation. Autophagy 10:416–430. doi: 10.4161/auto.27286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Liu Y, Wang Z, Liu K, Wang Y, Liu J, Ding H, Yuan Z. 2011. Subversion of cellular autophagy machinery by hepatitis B virus for viral envelopment. J Virol 85:6319–6333. doi: 10.1128/JVI.02627-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi Y, Bowman JW, Jung JU. 2018. Autophagy during viral infection—a double-edged sword. Nat Rev Microbiol 16:341–354. doi: 10.1038/s41579-018-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson WT, Giddings TH Jr, Taylor MP, Mulinyawe S, Rabinovitch M, Kopito RR, Kirkegaard K. 2005. Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol 3:e156. doi: 10.1371/journal.pbio.0030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fahmy AM, Labonte P. 2017. The autophagy elongation complex (ATG5-12/16L1) positively regulates HCV replication and is required for wild-type membranous web formation. Sci Rep 7:40351. doi: 10.1038/srep40351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wedemeyer H, Hardtke S, Manns MP. 2013. Treatment of hepatitis delta. Clin Liver Dis (Hoboken) 2:237–239. doi: 10.1002/cld.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao M, Hsieh SY, Taylor J. 1990. Role of two forms of hepatitis delta virus antigen: evidence for a mechanism of self-limiting genome replication. J Virol 64:5066–5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casey JL. 2012. Control of ADAR1 editing of hepatitis delta virus RNAs. Curr Top Microbiol Immunol 353:123–143. doi: 10.1007/82_2011_146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glenn JS, Watson JA, Havel CM, White JM. 1992. Identification of a prenylation site in delta virus large antigen. Science 256:1331–1333. doi: 10.1126/science.1598578. [DOI] [PubMed] [Google Scholar]
- 22.Lee CH, Chang SC, Wu CH, Chang MF. 2001. A novel chromosome region maintenance 1-independent nuclear export signal of the large form of hepatitis delta antigen that is required for the viral assembly. J Biol Chem 276:8142–8148. doi: 10.1074/jbc.M004477200. [DOI] [PubMed] [Google Scholar]
- 23.Chang FL, Chen PJ, Tu SJ, Wang CJ, Chen DS. 1991. The large form of hepatitis delta antigen is crucial for assembly of hepatitis delta virus. Proc Natl Acad Sci U S A 88:8490–8494. doi: 10.1073/pnas.88.19.8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komla-Soukha I, Sureau C. 2006. A tryptophan-rich motif in the carboxyl terminus of the small envelope protein of hepatitis B virus is central to the assembly of hepatitis delta virus particles. J Virol 80:4648–4655. doi: 10.1128/JVI.80.10.4648-4655.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryu WS, Bayer M, Taylor J. 1992. Assembly of hepatitis delta virus particles. J Virol 66:2310–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sir D, Tian Y, Chen WL, Ann DK, Yen TS, Ou JH. 2010. The early autophagic pathway is activated by hepatitis B virus and required for viral DNA replication. Proc Natl Acad Sci U S A 107:4383–4388. doi: 10.1073/pnas.0911373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie M, Yang Z, Liu Y, Zheng M. 2018. The role of HBV-induced autophagy in HBV replication and HBV related-HCC. Life Sci 205:107–112. doi: 10.1016/j.lfs.2018.04.051. [DOI] [PubMed] [Google Scholar]
- 28.Sells MA, Chen ML, Acs G. 1987. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc Natl Acad Sci U S A 84:1005–1009. doi: 10.1073/pnas.84.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen PJ, Kalpana G, Goldberg J, Mason W, Werner B, Gerin J, Taylor J. 1986. Structure and replication of the genome of the hepatitis delta virus. Proc Natl Acad Sci U S A 83:8774–8778. doi: 10.1073/pnas.83.22.8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T. 2007. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 31.Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T. 2005. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mauvezin C, Neufeld TP. 2015. Bafilomycin A1 disrupts autophagic flux by inhibiting both V-ATPase-dependent acidification and Ca-P60A/SERCA-dependent autophagosome-lysosome fusion. Autophagy 11:1437–1438. doi: 10.1080/15548627.2015.1066957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fahmy AM, Khabir M, Blanchet M, Labonte P. 2018. LC3B is not recruited along with the autophagy elongation complex (ATG5-12/16L1) at HCV replication site and is dispensable for viral replication. PLoS One 13:e0205189. doi: 10.1371/journal.pone.0205189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee CZ, Chen PJ, Chen DS. 1995. Large hepatitis delta antigen in packaging and replication inhibition: role of the carboxyl-terminal 19 amino acids and amino-terminal sequences. J Virol 69:5332–5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Jiang K, Zhang Q, Meng S, Ding C. 2018. Autophagy in negative-strand RNA virus infection. Front Microbiol 9:206. doi: 10.3389/fmicb.2018.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Granato M, Santarelli R, Farina A, Gonnella R, Lotti LV, Faggioni A, Cirone M. 2014. Epstein-Barr virus blocks the autophagic flux and appropriates the autophagic machinery to enhance viral replication. J Virol 88:12715–12726. doi: 10.1128/JVI.02199-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romanov J, Walczak M, Ibiricu I, Schuchner S, Ogris E, Kraft C, Martens S. 2012. Mechanism and functions of membrane binding by the Atg5-Atg12/Atg16 complex during autophagosome formation. EMBO J 31:4304–4317. doi: 10.1038/emboj.2012.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walczak M, Martens S. 2013. Dissecting the role of the Atg12-Atg5-Atg16 complex during autophagosome formation. Autophagy 9:424–425. doi: 10.4161/auto.22931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanida I, Ueno T, Kominami E. 2004. LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol 36:2503–2518. doi: 10.1016/j.biocel.2004.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohsumi Y, Mizushima N. 2004. Two ubiquitin-like conjugation systems essential for autophagy. Semin Cell Dev Biol 15:231–236. doi: 10.1016/j.semcdb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Beilstein F, Blanchet M, Vaillant A, Sureau C. 2018. Nucleic acid polymers are active against hepatitis delta virus infection in vitro. J Virol 92:e01416-17. doi: 10.1128/JVI.01416-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blanchet M, Sureau C. 2006. Analysis of the cytosolic domains of the hepatitis B virus envelope proteins for their function in viral particle assembly and infectivity. J Virol 80:11935–11945. doi: 10.1128/JVI.00621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sureau C, Guerra B, Lee H. 1994. The middle hepatitis B virus envelope protein is not necessary for infectivity of hepatitis delta virus. J Virol 68:4063–4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ning X, Luckenbaugh L, Liu K, Bruss V, Sureau C, Hu J. 2018. Common and distinct capsid and surface protein requirements for secretion of complete and genome-free hepatitis B virions. J Virol 92:e00272-18. doi: 10.1128/JVI.00272-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuo MY, Chao M, Taylor J. 1989. Initiation of replication of the human hepatitis delta virus genome from cloned DNA: role of delta antigen. J Virol 63:1945–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sureau C, Taylor J, Chao M, Eichberg JW, Lanford RE. 1989. Cloned hepatitis delta virus cDNA is infectious in the chimpanzee. J Virol 63:4292–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blanchet M, Sinnathamby V, Vaillant A, Labonte P. 2019. Inhibition of HBsAg secretion by nucleic acid polymers in HepG2.2.15 cells. Antiviral Res 164:97–105. doi: 10.1016/j.antiviral.2019.02.009. [DOI] [PubMed] [Google Scholar]