During herpes simplex virus (HSV) latency, the viral genome is harbored in peripheral neurons in the absence of infectious virus but with the potential to restart infection. Advances in epigenetics have helped explain how viral gene expression is largely inhibited during latency. Paradoxically, at the same time, the view that latency is entirely silent has been eroding. This low-level noise has implications for our understanding of HSV latency and should not be ignored.
KEYWORDS: HSV, herpes simplex virus, latency
ABSTRACT
During herpes simplex virus (HSV) latency, the viral genome is harbored in peripheral neurons in the absence of infectious virus but with the potential to restart infection. Advances in epigenetics have helped explain how viral gene expression is largely inhibited during latency. Paradoxically, at the same time, the view that latency is entirely silent has been eroding. This low-level noise has implications for our understanding of HSV latency and should not be ignored.
INTRODUCTION
Herpes simplex viruses (HSV) 1 and 2 infect a very large fraction of the human population with rates of seropositivity for HSV-1 and -2 being in the order of 67% and 11%, respectively (1, 2). Their evolution is intertwined with ours, and while virus-host interactions are frequently described with war-like language, the endgame for a human versus HSV is best thought of as mutually assured survival. The survival of HSV is ensured by maintaining a lifelong infection of its host through latency in peripheral neurons. This strategy allows transgenerational persistence of the virus if the host can live without substantial harm and transmission as long as the symptoms of disease are not so severe that they preclude close contact with uninfected individuals.
HSV enters through breaks in the skin surface or mucosa via direct contact and quickly establishes acute infections in the skin at the same time as accessing nerves that innervate the infection site. The virus parasitizes axonal transport to move to the cell bodies of peripheral neurons, which are collected together in sensory and autonomic ganglia (3, 4). For these neurons, there are multiple fates, from productive infection at one end of the scale to the immediate establishment of latency at the other (5, 6). In addition, some neurons that initiate acute infection suppress this program and survive to join the pool of latently infected neurons (7–9). After infectious HSV is cleared, it is the neurons that have become latently infected that provide a reservoir of virus for future rounds of productive infection if reactivation occurs. The active state of productive or lytic infection is associated with a well-characterized cascade of lytic gene expression. By contrast, the latent state is viewed traditionally as being one of tight viral gene repression, and HSV has been considered a prototype for viral latency in general and indeed has inspired the initial approach to other viruses (10). The exceptions that prove this rule of viral repression is the expression of latency associated transcripts (LATs) and a set of microRNAs (miRNAs) that are transcribed from the same locus during latency (11). However, perhaps there are things to be applied to HSV now from the other herpesviruses. Epstein-Barr virus latency has long been associated with a set of gene expression programs. Likewise, in human cytomegalovirus, a more complex view of latency has emerged, complete with the expression of lytic genes (12, 13). The purpose of this Gem is to discuss the evidence for HSV latency as a more dynamic state in terms of viral activity, with the intent of provoking thought and discussion.
MODELS FOR PROBING HSV LATENCY
Before approaching this main topic, it is worth noting that our understanding of HSV latency maintenance and reactivation is derived from an array of experimental models. The pros and cons of many of these models have been described in detail in a comprehensive recent review (14), but they can be considered in three main groups. (i) Animal models investigated entirely in vivo. The mouse has become the most commonly used animal by far, but there are important contributions made by rabbit in particular and Guinea pig models. In all cases, introduction of the virus at a peripheral site leads to a natural history of infection that is faithful to human infection at least at the gross level. (ii) Explant models, which can be used exclusively to explore reactivation. These experimental systems rely on the observation that the removal, or explant, of ganglia that harbor latent HSV into cell culture leads to reproducible reactivation of virus. This system allows experimental intervention as well as observation and is perhaps best viewed as an extension of the animal model from which the ganglia are derived. (iii) Neuronal cell culture systems. These make use of an increasing array of neuron types derived from mice and rats of various ages (15–23) and increasingly human-derived neurons from embryonic as well as induced pluripotent stem cells (24–29). There also remains an important role for observation of clinical cases (30–32), even though these preclude manipulation, and care must be taken to ensure adequate sampling and not to conflate association with causation. The choice of the model depends upon the questions being posed, and each model tends to illuminate best or bring to the fore certain aspects of latency and reactivation. However, the availability of so many models, each offering a different vantage point on such a complex phenomenon should be a strength for the field. Ideally, the results across these models should be synthesized into a whole (33). However, this requires grappling with results that fail to fit easily into preferred paradigms and also asking which of our ideas sit well with our observations of human infection.
SILENCE DURING LATENCY MAKES SENSE
HSV was one of the first agents recognized as giving rise to repeated bouts of symptoms with apparently no sign of infection between these episodes. If one starts from a helicopter view of HSV pathogenesis, the idea that latency is a stable state that is largely silent makes intuitive sense. To begin, despite very high seropositivity levels across all human populations, relatively few individuals experience recurrent cold sores and even fewer would report that these are frequent. Further, this stable latency is reflected in the most frequently used animal model, the mouse (34). Aside from explant culture or transplantation, reactivation as demonstrated by infectious virus in mouse models is difficult to induce and if it occurs spontaneously, it is exceptionally rare. A teleological argument can also be advanced, in that the use of a nondividing cell type as the host for latency means that there is no requirement for HSV to maintain its genome through cell divisions, so there seems little biological pressure on the virus to be active. Beyond these broader views, advances in our understanding of the ways chromatin changes on the HSV genome drive all aspects of HSV infection provide an increasingly well-defined mechanism by which HSV lytic gene expression is quelled in latency (35, 36). All of the above leaves the impression that HSV infection is dominated by latency and that productive and latent phases are adequately viewed as binary, mutually exclusive states. This view suggests that what remain to be determined are the switches that flip between these states during establishment and reactivation. Indeed, recent reviews of HSV latency show that there is much biology to be learned through this approach (23, 33, 37). However, the views above are challenged by data showing that HSV latency in people is more dynamic in terms of frequency of reactivation. Further, what if the noise in viral gene expression that will inevitably occur despite repressive heterochromatin plays a more important role than anticipated? These two themes form the remainder of this Gem.
A NOISIER VIEW OF HUMAN INFECTION WITH HSV
It is now well appreciated that reactivation is far more frequent than is apparent from the observation of symptoms. It is also instructive to note how and why our view of this aspect of HSV natural history has changed. The first step was to move from observing symptoms to detecting infectious virus, which was necessitated by a clinical understanding that transmission was occurring in the absence of symptoms (38, 39). An early study that aimed to sample daily in a cohort of women with a history of genital herpes found that around a third of the episodes where virus was detected occurred in the absence of a lesion or prodrome (40). The duration of shedding was only a day in length for three quarters of cases. This led to a similar study that included individuals who were HSV-2 seropositive but did not report any symptoms at enrollment to remove a possible bias toward more severe infection in the original cohort. This study found that 70% of the subjects that had not previously reported symptoms shed HSV over the course of the study, which was an average of approximately 100 days. Further, virus was detected on an average of 3.8% and 6.4% of days sampled for individuals that were asymptomatic and symptomatic at the start of the study, respectively (41). Again, the length of episodes of subclinical shedding were typically short, with around 70% of instances being only a single day. The requirement for virus culture limits the sensitivity of detection, and by moving to quantitative real-time PCR, a study with a similar design found virus shedding was detected on 18% of the days across all subjects and 10% of days from those who considered themselves to be asymptomatic at enrollment (42). Finally, in a study where the intensity of sampling was increased to sample multiple areas by a clinic, 5 days a week for 4 weeks, while 40% of individuals were symptomatic, 82% shed virus from at least one site in the genital region on at least 1 day. Further, there were instances where viral genomes were detected in distinct areas with nonoverlapping innervation simultaneously, suggesting that there were reactivation events occurring concurrently in more than one ganglion (43). This implies that viral detection at the periphery by a single sample underestimates reactivation events. The above series of work was all based on genital herpes, and while HSV-1 was detected, HSV-2 reactivation was more frequent (44). However, a review that assayed the literature for studies of HSV shedding from the oral cavity came to a similar conclusion that the rate of frequency of shedding, which is evidence for reactivation, is highest in studies that sampled more often and used PCR detection (45). Indeed, across studies where sampling was done at least once a week for 3 weeks, the fraction of subjects shedding virus was 70.6% (45). This is supported by a recent study in which daily samples were taken for a median of 60 days that found shedding in 75% of a population of twins, where 85% reported a history of cold sores (46). Interestingly, shedding rates correlated well for twins that harbored the same virus but not where they had different strains, which at face value suggests that reactivation is linked to virus strain; however, there are other factors, such as time in life of initial infection, which was not controlled. Taken together, there is compelling evidence that in human infection, HSV-1 and HSV-2 reactivate very frequently and often in the absence of symptoms. As has been noted elsewhere, this also suggests that reactivation is often spontaneous, which we would define as occurring in the absence of obvious stimuli (14). This is an important insight, suggesting that subtle physiological cues or perhaps even cyclical events combined with fluctuating immune control are key determinants of the frequency of recurrent disease.
A NOISIER VIEW OF HSV LATENCY IN NEURONS
Just as closer observation led to an appreciation that reactivation in infected human patients is more frequent than originally thought, increasing attention has revealed more viral activity in neurons in models of HSV latency. The scene for this notion was set by an accumulation of reports over many years that viral lytic gene expression could be detected during latency in mice (47–55). This is despite latency being exceptionally stable in mouse models as noted above. As the sensitivity of methods to detect this activity have improved, these observations of lytic gene expression during latency have gone from being occasional and disputed to being routine in mouse models (9, 56–59). Most of the mouse studies to date aimed to find evidence of transcription only, and in the absence of work to map these RNAs, it remains unclear if they represent conventional messages controlled by lytic promoters or some other kind of transcripts. However, recently, two groups used sensitive reporter systems, for example, Cre expression under lytic promoters in Cre-reporter mice, to find that lytic proteins can be expressed during latency (9, 57). Further, a third group has suggested a role for a lytic protein (ICP0) during latency (58). Added to these findings, the observation that activated CD8+ T cells are found in close proximity to latently infected neurons in mice and humans suggests that viral antigens are being expressed and presented during latency (31, 60–62). These all suggest that at least some of the transcription being detected in these models results in lytic gene mRNAs that can be translated. That is not to say that these studies are without caveats. For example, the viruses used in Cre-marking studies used promoters placed in ectopic locations of the genome, and this might lead to over- or underactivity. Further, a recent study using postmortem human ganglia with viral genome-wide coverage did not find evidence for HSV transcription outside of the LAT region, though the sensitivity of detection would have been a limitation of this work (32).
A recent comprehensive review of latency came to the conclusion that the HSV activity seen in our model systems most likely represents abortive reactivation episodes (14). This view of dynamic latency suggests that reactivation attempts by the virus are relatively frequent but largely unsuccessful, being blocked at multiple levels. It also leaves the current model of productive versus latent infection as a binary switch largely intact. Others have left the door open to the possibility that viral lytic gene expression is a part of a latency itself; in other words, a latency program of gene expression (36). Sporadic but frequent abortive reactivation and a latency program of expression can be accommodated in a single model (Fig. 1), and recent data can be interpreted as supporting both ideas. When looking at the studies showing lytic gene expression during latency in mice, in the majority of cases, the viral activity is detected at the level of a whole ganglion. In such cases, it could be construed that the majority of lytic gene transcripts probably come from relatively few neurons that are undergoing reactivation at any one time. This is in sympathy with the idea of relatively frequent but abortive reactivation. However, one study used laser capture microscopy to look at gene expression in individual neurons, finding that transcripts of at least one of a set of nine lytic genes were expressed in around 60% of latently infected neurons (56). On the one hand, around half of these neurons were expressing lytic gene transcripts of multiple classes, which is consistent with the disordered pattern of gene expression that has come to characterize the earliest stages of reactivation (36, 63, 64). Further, a host-specific transcriptional response that included mRNA encoding several antiviral proteins was seen in the neurons expressing lytic genes, which might be construed as an effort to abort reactivation (56). On the other hand, it seems difficult to imagine that more than half of all latently infected neurons are engaged in an attempt to reactivate at any one time. Two studies that detected viral protein expression during latency likewise leave this question unanswered. The first used a Cre-reporter mouse system and found a very slow accumulation of neurons experiencing protein production from lytic promoters during latency, the rate being on average 1 neuron every 2 to 3 days (9). This would be consistent with very frequent but not constant reactivation. However, the second used luciferase driven from the viral genome and the amount of this protein expressed at the single time points examined suggest that derepression of the HSV genome is common (57). Finally, the finding that ICP0, a lytic gene product, is itself required for the maintenance of latency is consistent with the concept of a latency-specific program of viral protein expression (58).
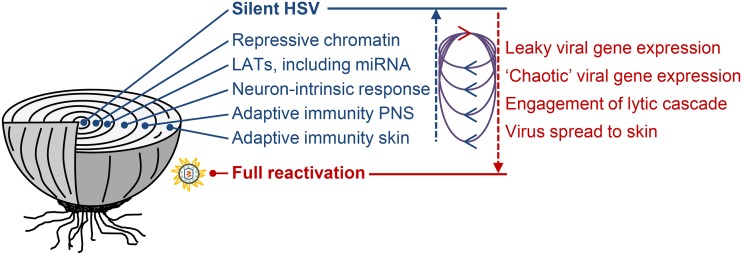
FIG 1.
HSV latency is maintained by multiple levels of regulation, shown here as layers of an onion. Full reactivation and shedding at the skin occur when the virus overcomes all of the layers. LATs and miRNAs are drawn as prolatency factors, but there may be RNA species or situations where expression of these predispose toward viral activity. The loops between the arrows pointing up toward silence and down toward reactivation represent cycling between viral activity and repression. These are drawn to suggest some mechanisms that drive the cycles, e.g., leaky lytic expression may drive a neuron-intrinsic and/or an adaptive immune response in the peripheral nervous system (PNS) that leads to a tightening of repression and re-enforcement of latency. A less conventional view is that the shortest of these cycles represents a program of gene expression that is a characteristic of latency itself, rather than being an aborted step toward reactivation.
This idea that gene expression might be regulated during latency per se rather than only during establishment and reactivation still remains somewhat beyond the mainstream. However, perhaps the nonuniform expression of LATs, the only highly abundant transcripts found in latently infected neurons, holds a further clue. It has been known for many years that LATs are not found in all neurons that harbor the HSV genome when examined at any one point in time (8, 65–67). However, this is typically seen as one of several types of heterogeneity across the pool of latently infected neurons. Many types of heterogeneity in HSV latency, for example, across neuronal subtype, copy number of latent genomes, and prior lytic gene expression, are inevitably stable over time (67–69). However, this does not imply that the roughly 30% of neurons expressing high levels of LATs seen at any one time can be assumed to represent a stable population. Indeed, one of the most intriguing but least commented on aspects of the first paper that used a Cre-marking mouse model was the continued accumulation of neurons marked by an HSV expressing Cre from the LAT promoter (8). Indeed, by day 30, the number of neurons marked by this virus approaches the total number thought to be latently infected in their model. This suggests that the establishment of latency is invariably associated with expression of LATs in every neuron, but only 30% of these cells are making LATs at any one time. If LATs are expressed in a cyclic fashion, that provides further evidence of a program of HSV transcription during latency that may conceivably then extend to miRNAs and low levels of lytic protein expression.
CONCLUSIONS AND FUTURE DIRECTIONS
Closer observation through more sensitive methods, increased intensity of sampling, and models that allow latently infected neurons to be examined without a large background of uninfected cells is revealing viral activity during latency that was previously hidden. We would argue that this greater level of activity has now been shown often enough that it must be a part of any broad explanation of HSV latency. The key question that remains is what this activity means and especially how it might help explain reactivation in humans, which we also now appreciate is more frequent than originally thought. While strong triggers for reactivation clearly exist, spontaneous reactivation seems to be the norm for HSV infections in people. So, our paradigms and models need to incorporate the possibility of subtle changes or perhaps even cyclic events that lead to reactivation to reflect this reality. Likewise, the possibility that there is a program of viral gene expression during latency, possibly regulated temporally in individual neurons, needs to be examined. For this, more sophisticated models are required so that we can observe gene expression and its consequences in latency in a way that is clearly separated from events that occur during the acute and establishment phases. Where recombinant viruses are used, for example in Cre-marking models, we need to aim for designs that reflect the native genome more faithfully. Further, our in vitro culture systems are becoming ever more refined and offer the possibility of serial observation over time. Finally, high-sensitivity studies of human ganglia are required as the ultimate reality check on our work in models. Notwithstanding all of the above, our view that HSV latency is a state that is characterized by high levels of viral repression remains intact. The exciting possibility is that the murmurs we are now appreciating will reveal targets or approaches that can be exploited to stop HSV roaring back to life in those people for whom frequent reactivation is a major impact on their health and wellbeing.
ACKNOWLEDGMENTS
We acknowledge the role of the Colorado Alphaherpesvirus Latency Society meetings in stimulating discussions that contributed to the ideas presented here.
D.C.T. is supported by a senior research fellowship (APP1104329) and project grant (APP1126599) from the Australian NHMRC; N.S. is supported by an International AGRTP Scholarship.
REFERENCES
- 1.Looker KJ, Magaret AS, May MT, Turner KM, Vickerman P, Gottlieb SL, Newman LM. 2015. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS One 10:e0140765. doi: 10.1371/journal.pone.0140765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. 2015. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One 10:e114989. doi: 10.1371/journal.pone.0114989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook ML, Stevens JG. 1973. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect Immun 7:272–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rødahl E, Stevens JG. 1992. Differential accumulation of herpes simplex virus type 1 latency-associated transcripts in sensory and autonomic ganglia. Virol 189:385–388. doi: 10.1016/0042-6822(92)90721-Z. [DOI] [PubMed] [Google Scholar]
- 5.Speck PG, Simmons A. 1991. Divergent molecular pathways of productive and latent infection with a virulent strain of herpes simplex virus type 1. J Virol 65:4001–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Speck PG, Simmons A. 1992. Synchronous appearance of antigen-positive and latently infected neurons in spinal ganglia of mice infected with a virulent strain of herpes simplex virus. J Gen Virol 73:1281–1285. doi: 10.1099/0022-1317-73-5-1281. [DOI] [PubMed] [Google Scholar]
- 7.Simmons A, Tscharke DC. 1992. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J Exp Med 175:1337–1344. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Proenca JT, Coleman HM, Connor V, Winton DJ, Efstathiou S. 2008. A historical analysis of herpes simplex virus promoter activation in vivo reveals distinct populations of latently infected neurones. J Gen Virol 89:2965–2974. doi: 10.1099/vir.0.2008/005066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell TA, Tscharke DC. 2016. Lytic promoters express protein during herpes simplex virus latency. PLoS Pathog 12:e1005729. doi: 10.1371/journal.ppat.1005729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawtell N, Thompson R. 2016. Herpes simplex virus and the lexicon of latency and reactivation: a call for defining terms and building an integrated collective framework [version 1; peer review: 2 approved]. F1000Res 5:2038. doi: 10.12688/f1000research.8886.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phelan D, Barrozo ER, Bloom DC. 2017. HSV1 latent transcription and non-coding RNA: a critical retrospective. J Neuroimmunol 308:65–101. doi: 10.1016/j.jneuroim.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Goodrum F. 2016. Human cytomegalovirus latency: approaching the Gordian knot. Annu Rev Virol 3:333–357. doi: 10.1146/annurev-virology-110615-042422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz M, Stern-Ginossar N. 2019. The transcriptome of latent human cytomegalovirus. J Virol 93:e00047-19. doi: 10.1128/JVI.00047-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bloom DC. 2016. Alphaherpesvirus latency: a dynamic state of transcription and reactivation. Adv Virus Res 94:53–80. doi: 10.1016/bs.aivir.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Wilcox CL, Johnson E. 1987. Nerve growth factor deprivation results in the reactivation of latent herpes simplex virus in vitro. J Virol 61:2311–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilcox CL, Johnson EM. 1988. Characterization of nerve growth factor-dependent herpes simplex virus latency in neurons in vitro. J Virol 62:393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danaher RJ, Jacob RJ, Miller CS. 1999. Establishment of a quiescent herpes simplex virus type 1 infection in neurally-differentiated PC12 cells. J Neurovirol 5:258–267. doi: 10.3109/13550289909015812. [DOI] [PubMed] [Google Scholar]
- 18.Arthur JL, Scarpini CG, Connor V, Lachmann RH, Tolkovsky AM, Efstathiou S. 2001. Herpes simplex virus type 1 promoter activity during latency establishment, maintenance, and reactivation in primary dorsal root neurons in vitro. J Virol 75:3885–3895. doi: 10.1128/JVI.75.8.3885-3895.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertke AS, Swanson SM, Chen J, Imai Y, Kinchington PR, Margolis TP. 2011. A5-positive primary sensory neurons are nonpermissive for productive infection with herpes simplex virus 1 in vitro. J Virol 85:6669–6677. doi: 10.1128/JVI.00204-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roehm PC, Camarena V, Nayak S, Gardner JB, Wilson A, Mohr I, Chao MV. 2011. Cultured vestibular ganglion neurons demonstrate latent HSV1 reactivation. Laryngoscope 121:2268–2275. doi: 10.1002/lary.22035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi M, Kim J-Y, Camarena V, Roehm PC, Chao MV, Wilson AC, Mohr I. 2012. A primary neuron culture system for the study of herpes simplex virus latency and reactivation. J Vis Exp 62:e3823. doi: 10.3791/3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhn MA, Nayak S, Camarena V, Gardner J, Wilson A, Mohr I, Chao MV, Roehm PC. 2012. A cell culture model of facial palsy resulting from reactivation of latent herpes simplex type I. Otol Neurotol 33:87–92. doi: 10.1097/MAO.0b013e31823dbb20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson AC, Mohr I. 2012. A cultured affair: HSV latency and reactivation in neurons. Trends Microbiol 20:604–611. doi: 10.1016/j.tim.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lafaille FG, Pessach IM, Zhang S-Y, Ciancanelli MJ, Herman M, Abhyankar A, Ying S-W, Keros S, Goldstein PA, Mostoslavsky G, Ordovas-Montanes J, Jouanguy E, Plancoulaine S, Tu E, Elkabetz Y, Al-Muhsen S, Tardieu M, Schlaeger TM, Daley GQ, Abel L, Casanova J-L, Studer L, Notarangelo LD. 2012. Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature 491:769–773. doi: 10.1038/nature11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Aiuto L, Prasad KM, Upton CH, Viggiano L, Milosevic J, Raimondi G, McClain L, Chowdari K, Tischfield J, Sheldon M, Moore JC, Yolken RH, Kinchington PR, Nimgaonkar VL. 2015. Persistent infection by HSV-1 is associated with changes in functional architecture of iPSC-derived neurons and brain activation patterns underlying working memory performance. Schizophr Bull 41:123–132. doi: 10.1093/schbul/sbu032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pourchet A, Modrek AS, Placantonakis DG, Mohr I, Wilson AC. 2017. Modeling HSV-1 latency in human embryonic stem cell-derived neurons. Pathogens 6:24. doi: 10.3390/pathogens6020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thellman NM, Botting C, Madaj Z, Triezenberg SJ. 2017. An immortalized human dorsal root ganglion cell line provides a novel context to study herpes simplex virus 1 latency and reactivation. J Virol 91:e00080-17. doi: 10.1128/JVI.00080-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thellman NM, Triezenberg SJ. 2017. Herpes simplex virus establishment, maintenance, and reactivation: in vitro modeling of latency. Pathogens 6:28. doi: 10.3390/pathogens6030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards TG, Bloom DC. 2019. Lund human mesencephalic (LUHMES) neuronal cell line supports herpes simplex virus 1 latency in vitro. J Virol 93:e02210-18. doi: 10.1128/JVI.02210-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Held K, Junker A, Dornmair K, Meinl E, Sinicina I, Brandt T, Theil D, Derfuss T. 2011. Expression of herpes simplex virus 1-encoded microRNAs in human trigeminal ganglia and their relation to local T-cell infiltrates. J Virol 85:9680–9685. doi: 10.1128/JVI.00874-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Velzen M, Jing L, Osterhaus AD, Sette A, Koelle DM, Verjans GM. 2013. Local CD4 and CD8 T-cell reactivity to HSV-1 antigens documents broad viral protein expression and immune competence in latently infected human trigeminal ganglia. PLoS Pathog 9:e1003547. doi: 10.1371/journal.ppat.1003547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LaPaglia DM, Sapio MR, Burbelo PD, Thierry-Mieg J, Thierry-Mieg D, Raithel SJ, Ramsden CE, Iadarola MJ, Mannes AJ. 2018. RNA-Seq investigations of human post-mortem trigeminal ganglia. Cephalalgia 38:912–932. doi: 10.1177/0333102417720216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzich JB, Cliffe AR. 2018. Strength in diversity: understanding the pathways to herpes simplex virus reactivation. Virology 522:81–91. doi: 10.1016/j.virol.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gebhardt BM, Halford WP. 2005. Evidence that spontaneous reactivation of herpes virus does not occur in mice. Virol J 2:67. doi: 10.1186/1743-422X-2-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kristie TM. 2015. Dynamic modulation of HSV chromatin drives initiation of infection and provides targets for epigenetic therapies. Virol 479–480:555–561. doi: 10.1016/j.virol.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cliffe AR, Wilson AC. 2017. Restarting lytic gene transcription at the onset of herpes simplex virus reactivation. J Virol 91:e01419-16. doi: 10.1128/JVI.01419-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roizman B, Whitley RJ. 2013. An inquiry into the molecular basis of HSV latency and reactivation. Annu Rev Microbiol 67:355–374. doi: 10.1146/annurev-micro-092412-155654. [DOI] [PubMed] [Google Scholar]
- 38.Mertz GJ, Schmidt O, Jourden JL, Guinan ME, Remington ML, Fahnlander A, Winter C, Holmes KK, Corey L. 1985. Frequency of acquisition of first-episode genital infection with herpes simplex virus from symptomatic and asymptomatic source contacts. Sex Transm Dis 12:33–39. doi: 10.1097/00007435-198501000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Rooney JF, Felser JM, Ostrove JM, Straus SE. 1986. Acquisition of genital herpes from an asymptomatic sexual partner. N Engl J Med 314:1561–1564. doi: 10.1056/NEJM198606123142407. [DOI] [PubMed] [Google Scholar]
- 40.Wald A, Zeh J, Selke S, Ashley RL, Corey L. 1995. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med 333:770–775. doi: 10.1056/NEJM199509213331205. [DOI] [PubMed] [Google Scholar]
- 41.Wald A, Zeh J, Selke S, Warren T, Ryncarz AJ, Ashley R, Krieger JN, Corey L. 2000. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med 342:844–850. doi: 10.1056/NEJM200003233421203. [DOI] [PubMed] [Google Scholar]
- 42.Tronstein E, Johnston C, Huang M-L, Selke S, Magaret A, Warren T, Corey L, Wald A. 2011. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA 305:1441–1449. doi: 10.1001/jama.2011.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnston C, Zhu J, Jing L, Laing KJ, McClurkan CM, Klock A, Diem K, Jin L, Stanaway J, Tronstein E, Kwok WW, Huang M-l, Selke S, Fong Y, Magaret A, Koelle DM, Wald A, Corey L. 2014. Virologic and immunologic evidence of multifocal genital herpes simplex virus 2 infection. J Virol 88:4921–4931. doi: 10.1128/JVI.03285-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnston C, Corey L. 2016. Current concepts for genital herpes simplex virus infection: diagnostics and pathogenesis of genital tract shedding. Clin Microbiol Rev 29:149–161. doi: 10.1128/CMR.00043-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller CS, Danaher RJ. 2008. Asymptomatic shedding of herpes simplex virus (HSV) in the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 105:43–50. doi: 10.1016/j.tripleo.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 46.Ramchandani MS, Jing L, Russell RM, Tran T, Laing KJ, Magaret AS, Selke S, Cheng A, Huang M-L, Xie H, Strachan E, Greninger AL, Roychoudhury P, Jerome KR, Wald A, Koelle DM. 2019. Viral genetics modulate orolabial herpes simplex virus type 1 shedding in humans. J Infect Dis 219:1058–1066. doi: 10.1093/infdis/jiy631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Green MT, Courtney RJ, Dunkel EC. 1981. Detection of an immediate early herpes simplex virus type 1 polypeptide in trigeminal ganglia from latently infected animals. Infect Immun 34:987–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kramer MF, Coen DM. 1995. Quantification of transcripts from the ICP4 and thymidine kinase genes in mouse ganglia latently infected with herpes simplex virus. J Virol 69:1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tal-Singer R, Lasner TM, Podrzucki W, Skokotas A, Leary JJ, Berger SL, Fraser NW. 1997. Gene expression during reactivation of herpes simplex virus type 1 from latency in the peripheral nervous system is different from that during lytic infection of tissue cultures. J Virol 71:5268–5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kramer MF, Chen SH, Knipe DM, Coen DM. 1998. Accumulation of viral transcripts and DNA during establishment of latency by herpes simplex virus. J Virol 72:1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen SH, Lee LY, Garber DA, Schaffer PA, Knipe DM, Coen DM. 2002. Neither LAT nor open reading frame P mutations increase expression of spliced or intron-containing ICP0 transcripts in mouse ganglia latently infected with herpes simplex virus. J Virol 76:4764–4772. doi: 10.1128/jvi.76.10.4764-4772.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feldman LT, Ellison AR, Voytek CC, Yang L, Krause P, Margolis TP. 2002. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc Natl Acad Sci U S A 99:978–983. doi: 10.1073/pnas.022301899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pesola JM, Zhu J, Knipe DM, Coen DM. 2005. Herpes simplex virus 1 immediate-early and early gene expression during reactivation from latency under conditions that prevent infectious virus production. J Virol 79:14516–14525. doi: 10.1128/JVI.79.23.14516-14525.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maillet S, Naas T, Crepin S, Roque-Afonso AM, Lafay F, Efstathiou S, Labetoulle M. 2006. Herpes simplex virus type 1 latently infected neurons differentially express latency-associated and ICP0 transcripts. J Virol 80:9310–9321. doi: 10.1128/JVI.02615-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giordani NV, Neumann DM, Kwiatkowski DL, Bhattacharjee PS, McAnany PK, Hill JM, Bloom DC. 2008. During herpes simplex virus type 1 infection of rabbits, the ability to express the latency-associated transcript increases latent-phase transcription of lytic genes. J Virol 82:6056–6060. doi: 10.1128/JVI.02661-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma JZ, Russell TA, Spelman T, Carbone FR, Tscharke DC. 2014. Lytic gene expression is frequent in HSV-1 latent infection and correlates with the engagement of a cell-intrinsic transcriptional response. PLoS Pathog 10:e1004237. doi: 10.1371/journal.ppat.1004237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nicoll MP, Hann W, Shivkumar M, Harman LER, Connor V, Coleman HM, Proença JT, Efstathiou S. 2016. The HSV-1 latency-associated transcript functions to repress latent phase lytic gene expression and suppress virus reactivation from latently infected neurons. PLoS Pathog 12:e1005539. doi: 10.1371/journal.ppat.1005539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raja P, Lee JS, Pan D, Pesola JM, Coen DM, Knipe DM. 2016. A herpesviral lytic protein regulates the structure of latent viral chromatin. mBio 7:e00633-16. doi: 10.1128/mBio.00633-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee JS, Raja P, Pan D, Pesola JM, Coen DM, Knipe DM. 2018. CCCTC-binding factor acts as a heterochromatin barrier on herpes simplex viral latent chromatin and contributes to poised latent infection. mBio 9:e02372-17. doi: 10.1128/mBio.02372-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Halford W, Gebhardt B, Carr D. 1996. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J Immunol 157:3542–3549. [PubMed] [Google Scholar]
- 61.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. 2003. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity 18:593–603. doi: 10.1016/S1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Lint AL, Kleinert L, Clarke SRM, Stock A, Heath WR, Carbone FR. 2005. Latent infection with herpes simplex virus is associated with ongoing CD8+ T-cell stimulation by parenchymal cells within sensory ganglia. J Virol 79:14843–14851. doi: 10.1128/JVI.79.23.14843-14851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Du T, Zhou G, Roizman B. 2011. HSV-1 gene expression from reactivated ganglia is disordered and concurrent with suppression of latency-associated transcript and miRNAs. Proc Natl Acad Sci U S A 108:18820–18824. doi: 10.1073/pnas.1117203108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim JY, Mandarino A, Chao MV, Mohr I, Wilson AC. 2012. Transient reversal of episome silencing precedes VP16-dependent transcription during reactivation of latent HSV-1 in neurons. PLoS Pathog 8:e1002540. doi: 10.1371/journal.ppat.1002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mehta A, Maggioncalda J, Bagasra O, Thikkavarapu S, Saikumari P, Valyi-Nagy T, Fraser NW, Block TM. 1995. In situ DNA PCR and RNA hybridization detection of herpes simplex virus sequences in trigeminal ganglia of latently infected mice. Virol 206:633–640. doi: 10.1016/S0042-6822(95)80080-8. [DOI] [PubMed] [Google Scholar]
- 66.Maggioncalda J, Mehta A, Su YH, Fraser NW, Block TM. 1996. Correlation between herpes simplex virus type 1 rate of reactivation from latent infection and the number of infected neurons in trigeminal ganglia. Virol 225:72–81. doi: 10.1006/viro.1996.0576. [DOI] [PubMed] [Google Scholar]
- 67.Sawtell NM. 1997. Comprehensive quantification of herpes simplex virus latency at the single-cell level. J Virol 71:5423–5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang L, Voytek CC, Margolis TP. 2000. Immunohistochemical analysis of primary sensory neurons latently infected with herpes simplex virus type 1. J Virol 74:209–217. doi: 10.1128/jvi.74.1.209-217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Margolis TP, Imai Y, Yang L, Vallas V, Krause PR. 2007. Herpes simplex virus type 2 (HSV-2) establishes latent infection in a different population of ganglionic neurons than HSV-1: role of latency-associated transcripts. J Virol 81:1872–1878. doi: 10.1128/JVI.02110-06. [DOI] [PMC free article] [PubMed] [Google Scholar]