The influenza A virus (IAV) envelope protein hemagglutinin binds α2,6- or α2,3-linked sialic acid as a host cell receptor. Bat IAV subtypes H17N10 and H18N11 form an exception to this rule and do not bind sialic acid but enter cells via major histocompatibility complex (MHC) class II.
KEYWORDS: influenza virus, receptor, viral entry
ABSTRACT
The influenza A virus (IAV) envelope protein hemagglutinin binds α2,6- or α2,3-linked sialic acid as a host cell receptor. Bat IAV subtypes H17N10 and H18N11 form an exception to this rule and do not bind sialic acid but enter cells via major histocompatibility complex (MHC) class II. Here, we review current knowledge on IAV receptors with a focus on sialoglycan variants, protein coreceptors, and alternative receptors that impact IAV attachment and internalization beyond the well-described sialic acid binding.
INTRODUCTION
Influenza A virus (IAV) particles possess envelopes that are densely covered with the viral glycoproteins hemagglutinin (HA) and neuraminidase (NA). HA is the viral receptor-binding protein that mediates attachment to and entry into host cells. Binding of IAV to a host cell is initiated by the interaction of HA with N-acetylneuraminic acid (known as sialic acid) linked to galactose on plasma membrane-resident glycoproteins or glycolipids (1, 2). The HA proteins of human and other mammalian IAVs preferentially bind to sialic acid with an α2,6 linkage to the penultimate sugar, whereas avian strains recognize α2,3-linked sialic acid (3–6). This difference in receptor binding specificity has far-reaching consequences for viral tropism, pathogenicity, and transmissibility and has been reviewed extensively (7–9). In addition, NA plays an important role in the IAV entry process. Its receptor-destroying activity is required for the release of virus particles from decoy receptors, which are present in respiratory mucus and on the cell surface (10–12). Furthermore, NA reduces the number of available sialic acid molecules near attachment sites and, thereby, mediates receptor gradient-dependent rolling of the virus particle on the cell surface (13, 14). Therefore, HA and NA densities on the virion surface, as well as their respective activities, are well balanced. This HA:NA balance is believed to contribute to IAV infectivity, pathogenicity, and host adaptation and is reviewed elsewhere (15, 16). The attachment of IAV to host cells is followed by internalization, which can occur through multiple redundant routes, namely, clathrin-dependent or clathrin-independent endocytosis as well as through macropinocytosis (17–20).
In this review, we discuss sialoglycan variants and potential coreceptors, which contribute to IAV attachment and internalization beyond the well-described α2,3- and α2,6-linked sialic acid receptor specificities of HA. In addition, we summarize experimental evidence suggesting that IAV binding and uptake can be mediated by host proteins and glycans independent of the canonical sialic acid-HA interaction.
SIALOGLYCAN CHARACTERISTICS THAT AFFECT IAV ENTRY AND ATTACHMENT
Sialic acid describes an acidic nine-carbon amino sugar that, in place of the amino group at position 5, contains either an N-acetyl or N-glycolyl group, yielding N-acetylneuraminic (Neu5Ac) or N-glycolylneuraminic (Neu5Gc) acid, respectively. In the context of IAV receptor specificity, sialic acid usually refers to Neu5Ac, as this is the commonly recognized variant of sialic acid. However, a number of HA proteins can also bind Neu5Gc, which is not present in humans or ferrets but is commonly found in ducks, pigs, and horses (21–25). In particular, the HAs of IAV isolates from ducks and pigs have been shown to display Neu5Gc binding, and for both animals, this feature seems relevant in vivo (21, 26). For example, while duck IAVs were found to bind efficiently to Neu5Ac and Neu5Gc and both sialic acid variants are present abundantly in the duck intestine, only recognition of Neu5Gc was associated with efficient replication in vivo. This might suggest that in ducks the functional IAV entry receptor(s) carry glycosylations with terminal Neu5Gc rather than Neu5Ac (21).
With advances in glycomics and structural methodologies over the past years, it has become evident that the linkage between sialic acid and the penultimate sugar moiety is not the sole determinant of HA binding. Structural studies have revealed that long glycans with multiple repeats of lactosamine units that carry α2,6-linked terminal sialic acid display an “umbrella-shaped” topology that is preferentially recognized by human-adapted IAVs. In contrast, glycans with α2,3-linked sialic acid or short α2,6 glycans, such as single lactosamine branches, adopt a “cone-like” topology and can be recognized by certain avian-adapted IAV strains (27–33). This suggests that it is not the sialic acid linkage per se but rather the glycan topology that determines the species specificity of IAV. Moreover, modifications such as sialylation, sulfation, or fucosylation of the glycans at position 2 (galactose), 3 (glucosamine or galactosamine), or even the core of the glycan were found to affect binding of different HA proteins substantially (5, 34–37). Consequently, one would predict that not every glycan with terminal α2,3- or α2,6-linked sialic acid is recognized by every strain of IAV with α2,3 or α2,6 receptor specificity, respectively. This was indeed observed when glycans isolated from pig or human lungs were analyzed for their capacity to bind different strains of IAV (34, 37). In summary, even though sialic acid and its linkage to galactose are essential for HA binding, the composition and topology of the glycosylation structure contribute to the receptor specificity of IAV.
SIALOGLYCAN-DEPENDENT CORECEPTORS
IAV can bind to sialoglycans in vitro in the absence of cellular proteins or lipids (38). Thus, it is assumed that in vivo IAV binds the sialic acid portion of N- or O-linked glycans on glycoproteins or on glycolipids. Not all binding events can lead to virus internalization, but this rather depends on the nature of the glycoprotein or glycolipid that is engaged by IAV. A study based on CHO lec1 cells that are deficient in complex N-linked glycans initially suggested that entry of IAV is strictly dependent on N-linked glycans (39). Furthermore, N-linked glycans terminating in sialic acid were found to be the preferred ligand for IAV when diverse carbohydrates isolated from swine lungs were tested in a glycan array (34). However, a later study showed that the strict dependence of IAV entry on N-linked glycans could not be confirmed with cell lines deficient in complex N-linked glycans other than CHO lec1 cells (40). Entry via endocytosis was only blocked in CHO lec1 cells but not the other N-linked glycan-deficient cell lines, namely, CHO 15B and HEK293S. Thus, N-linked glycans seem to be preferentially bound by IAV, but they are not essential for the uptake of the virus into the host cell, at least not for the endocytic route of internalization.
Moreover, several specific cellular glycoproteins have been implicated in IAV attachment and subsequent internalization; cell surface-expressed nucleolin was demonstrated to interact with IAV using a virus overlay protein binding assay (VOPBA), and interaction with HA was confirmed by pulldown experiments (41). Nucleolin was further shown to play a role during IAV internalization rather than attachment. In line with these findings, small interfering RNA (siRNA)-mediated knockdown of nucleolin reduced endocytosis of epidermal growth factor (EGF), transferrin, and IAV, indicating that nucleolin contributes to virus uptake after the virus attaches to host cells (41). However, the study did not further interrogate whether the binding of HA to nucleolin is sialic acid dependent. How, when, and where HA binding to nucleolin occurs and how the interaction triggers IAV internalization are currently not known.
For natural killer (NK) cells, the natural cytotoxicity receptors NK cell p44-related protein (NKP44) and NK cell p46-related protein (NKP46) have been shown to interact with HA in a sialic acid-dependent manner (42–44). Binding of the natural cytotoxicity receptors by HA was reported to result in NK cell activation (44), but a direct role of these receptors in virus attachment and internalization has not been described. Nevertheless, NK cells can be infected by IAV (45, 46), and hence, NKP44 and NKP46 could contribute to virus entry in this cell type.
Further evidence for the requirement of specific glycoproteins for IAV entry stems from a recent study by Fujioka and colleagues, which identified the voltage-dependent calcium channel Cav1.2 as a cellular entry factor that interacts with HA on the cell surface in a sialic acid-dependent manner (47). Interaction of IAV with Cav1.2 induced Ca2+ oscillations, which have been previously shown to contribute to virus internalization and infection (48). Moreover, it was suggested that IAV-Cav1.2 engagement might also occur in the absence of sialic acid, as mutations of potentially glycosylated asparagine residues on Cav1.2 only partially abrogated IAV binding. However, mechanistic insights into sialic acid-independent attachment of IAV to Cav1.2 and its consequences for virus entry are currently lacking, and it remains unclear how IAV binding to Cav1.2 induces Ca2+ influx. Nevertheless, the concept that virus binding triggers signaling cascades, which then orchestrate internalization of virus particles, is well accepted (48–55). An entry factor solely responsible for initiating these signaling cascades has not been described yet, and likely, several cellular factors contribute to internalization in a redundant fashion. Besides the calcium channel Cav1.2 described above, earlier studies have demonstrated a role for receptor tyrosine kinases (RTKs) in virus uptake into target cells. For example, treatment with RTK inhibitors reduces internalization of IAV virions (52). Epidermal growth factor receptor (EGFR) is a well-studied RTK family member, which is believed to play a central role during IAV internalization after virion attachment. The expression levels of EGFR positively correlate with IAV entry efficiency, and it was shown that EGFR and IAV colocalize on lipid raft structures at the cell surface followed by internalization of both IAV and EGFR. Disruption of lipid raft structures impaired IAV uptake and reduced subsequent viral growth (52). IAV attachment-induced EGFR and phosphatidylinositol 3-kinase (PI3K)/Akt activation was inhibited by sialidase treatment, and this was shown to hamper endocytosis of IAV but not EGF-dependent endocytosis of EGFR. These findings suggest that IAV and EGFR interact in a sialic acid-dependent manner (52). A recent study identified M85, a compound that targets EGFR and PIK3C2β, a class II PI3K, to potently reduce IAV internalization. Upon treatment with the inhibitor, IAV was retained at the cell surface or in close proximity thereof (56). These data further link RTK and PI3K pathways to the early stages of IAV uptake into target cells.
Another enzyme downstream of EGFR involved in IAV entry is phospholipase C-γ1 (PLC-γ1). Interestingly, EGFR-dependent phosphorylation of PLC-γ1 occurs only in response to infection with IAV of the H1N1 subtype but not with H3N2 viruses (57). Indeed, activation of PLC-γ1 positively influenced virus internalization (48, 57). These data allow for speculation that the precise internalization routes might differ between IAV subtypes.
In summary, a variety of cellular glycoproteins play important but seemingly redundant roles in virus entry. Some of these entry factors, such as nucleolin, NKP44/46, Cav1.2, and EGFR, could be considered coreceptors, as they might interact directly with HA and contribute to virion attachment and clustering at the cell surface (Fig. 1). Other factors could then be recruited to IAV-containing lipid raft structures and elicit signaling events, which trigger virion uptake and sorting into early endosomal compartments, a prerequisite for fusion and uncoating in late, acidified endosomes.
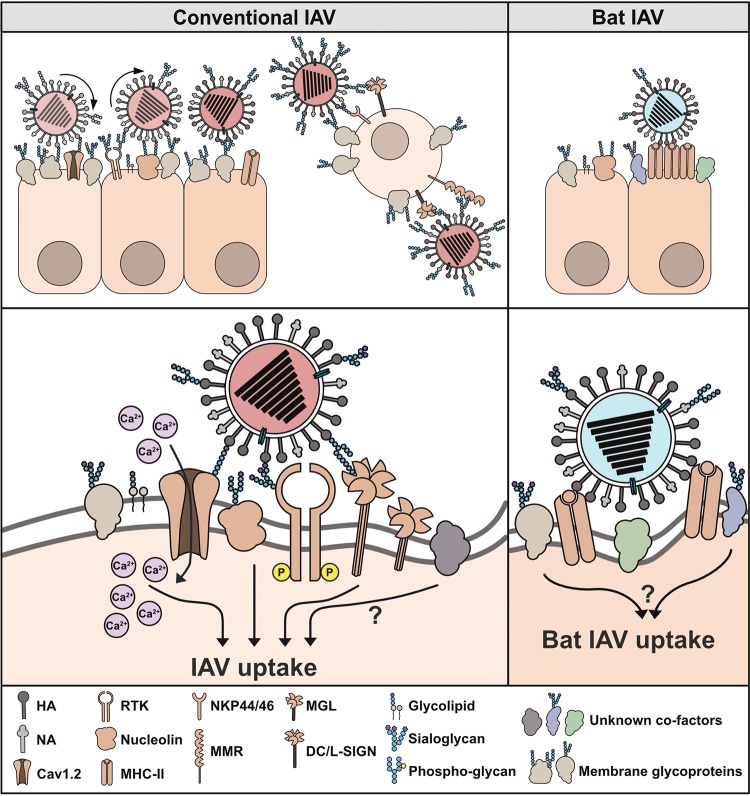
FIG 1.
Models for IAV attachment and uptake. Attachment of conventional IAV to sialoglycans and phospho-glycans on cellular glycoproteins and glycolipids is mediated by the viral HA. Well-balanced HA and NA activity allows the virus to “roll” along the cell surface until appropriate entry receptors are engaged. Sialic acid-independent IAV attachment may occur via C-type lectins (DC/L-SIGN, MMR, and MGL) and natural cytotoxicity receptors (NKP44/46) that are mainly expressed on immune cells. For virus internalization, IAV needs to bind to internalization receptors that initiate signaling events and facilitate IAV uptake. Internalization of conventional IAV may occur via sialic acid-dependent interactions with RTKs, nucleolin, and Cav1.2 and/or sialic acid-independent interactions with DC/L-SIGN, MGL, and MMR. In addition, yet unidentified entry receptors may exist. In contrast, bat IAV attachment and internalization are independent of sialoglycans and other glycans but strictly depend on major histocompatibility complex class II (MHC-II) expression. Clustering of MHC-II proteins by multivalent interactions with the bat IAV HAs might allow attachment and trigger uptake of virions. Other cofactors that might act in concert with MHC-II and contribute to bat IAV attachment and internalization remain to be identified.
SIALIC ACID-INDEPENDENT RECEPTORS
Besides the well-described HA-sialic acid binding, it was also suggested that sialic acid-independent interactions of the viral HA with the host cell can occur. In a recent study, the N-glycome from human lungs was isolated and used to assess binding of a panel of IAV strains from different hosts in a glycan array (37). Unexpectedly, the authors detected binding of IAV to nonsialylated phospho-glycans on this glycan array. Sialidase treatment of the array strongly reduced IAV binding to sialylated glycans and enhanced virus binding to phospho-glycans, whereas phosphatase treatment specifically blocked binding to phospho-glycans. Only a combination of both treatments abrogated IAV binding to the glycan array completely. As preincubation of IAV with sialic acid did not reduce IAV binding to phospho-glycans, the authors suggest that IAV possesses a binding site for phospho-glycans that is different from the canonical receptor binding site (RBS) in HA (37). While these exciting novel findings suggest that IAV can recognize phospho-glycans as alternative attachment receptors to sialoglycans in the human lung, it is so far unclear if such interactions would allow for internalization of the virus and, thus, represent a different type of entry receptor.
In addition to phospho-glycans, distinct cell surface proteins were described to bind IAV and facilitate virus infection in the absence of sialic acid. Interestingly, these proteins proposed as sialic acid-independent IAV receptors belong to the family of calcium-dependent (C-type) lectins and are mainly found on immune cells (58–63). Probably the most extensively studied cellular protein that has been implicated in sialic acid-independent IAV binding and infection is the dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN). DC-SIGN is known to bind complex mannose- and fucose-containing glycoconjugates and functions as a cellular adhesion molecule as well as pattern recognition receptor (PRR) to recognize fungal, bacterial, and viral pathogens (reviewed in reference 64). DC-SIGN has been described to promote the infection of various viruses, including phleboviruses (65), dengue virus (66), HIV (67), and hepatitis C virus (HCV) (68). Initially, Wang and colleagues observed that binding and infection of an avian H5N1 IAV positively correlated with expression levels of DC-SIGN on DCs and B cell lines (59). The authors proposed that N-linked glycans present on HA facilitate the interaction with DC-SIGN as an attachment factor and, thereby, enhance virus infection. Indeed, other IAV subtypes, including H1 and H3 viruses, were shown to bind and infect DC-SIGN-expressing cells in the absence of sialic acid (61, 62, 69). In addition to DC-SIGN, liver/lymph node-specific intercellular adhesion molecule 3-grabbing nonintegrin (L-SIGN), a protein that shares high sequence homology to DC-SIGN but is expressed on endothelial cells of liver and lymph nodes, has been demonstrated to bind IAV (61). IAV binding to DC-SIGN and L-SIGN is not necessarily subtype specific but rather depends on the extent of glycans present on HA and NA. Indeed, the amount of HA glycosylation correlates with virus infectivity on cells overexpressing DC-SIGN (62). In line with these results, a recombinant H1N1 virus with RBS mutations that abolish sialic acid binding was only rescued and propagated in cells that overexpressed DC-SIGN (62). Moreover, endocytosis-deficient L- and DC-SIGN were shown to retain IAV binding but strongly reduced susceptibility to IAV infection, suggesting that L-and DC-SIGN can act as authentic endocytic receptors for IAV (70).
Recently, Palomino-Segura et al. showed direct binding of recombinant HA proteins from different IAV strains to a recombinant SIGN-R1 protein, the murine homologue of DC-SIGN, and as expected, N-linked glycosylations on HA were shown to be essential for this interaction (69). As recombinant NA was also shown to bind SIGN-R1, it was suggested that both IAV glycoproteins contribute to IAV binding to C-type lectin receptors (69). The same study also provides evidence that SIGN-R1 on inflammatory DCs is required to capture virus particles in vivo, which leads to DC activation and secretion of inflammatory cytokines that recruit natural killer cells to control IAV infection in the murine tracheal mucosa.
Furthermore, several studies have described other C-type lectins, such as the macrophage mannose receptor (MMR) (58, 60), the macrophage galactose-type lectin 1 (MGL1) (60, 63), and langerin (71), to be exploited by IAV as entry receptors. MMR and MGL are both endocytic receptors expressed on macrophages and DCs (72, 73), which were shown to interact directly with IAV in a predominantly sialic acid-independent manner (60). Differences in glycosylation patterns of the surface glycoproteins from different IAV strains were shown to determine sensitivity to MMR and MGL binding in the absence of sialic acid receptors (58, 60, 63). Studies with sialic acid-deficient cell lines and internalization-defective MGL or langerin mutants revealed that both C-type lectins, besides mediating virus attachment, can also facilitate IAV internalization in a dynamin-dependent manner (63, 71).
Unlike HAs from conventional IAVs, bat-derived IAV HA subtypes (H17 and H18) are unique in their inability to bind sialic acid or other glycans (74–77). Consequently, bat IAVs significantly differ from conventional IAVs in terms of entry characteristics and receptor usage. Sialidase treatment of susceptible cell lines increased bat IAV entry and infection, suggesting that either removal of negatively charged sialic acid residues reduces electrostatic repulsion between the viral and cellular membrane (78) or enhances accessibility to the putative receptor (79). Recently, work from our group has identified the major histocompatibility complex class II (MHC-II) human leukocyte antigen DR isotype (HLA-DR) as an essential entry determinant for bat IAVs (80). Of note, HLA-DR homologs from multiple species, as well as other classical MHC-II proteins, namely, HLA-DQ and -DP, can confer susceptibility to bat IAV, and MHC-II expression was crucial for bat IAV infection in the nasal epithelium of mice (80). While bat IAV replication in mice was restricted to the upper respiratory tract, in fruit bats, H18N11 replication takes place in the follicle-associated epithelium of the gut lymphoid tissue and was shed at high titers in fecal samples, allowing transmission to naive contact bats (81). Moreover, the promiscuity of bat IAV HA regarding the use of MHC-II from several species suggests a risk for zoonotic transmission of bat IAV to other species. Indeed, bat IAV replication was experimentally shown in fruit bats, mice, and ferrets (80, 81). However, efficient virus replication and transmission of bat IAV were only observed in bats but not in other species, suggesting that bat IAV is poorly adapted to nonbat species (81). Unfortunately, large-scale serological studies in bats and other species are lacking, which complicates the assessment of the zoonotic potential of these IAV subtypes.
Even though proximity ligation assays (PLAs) and polykaryon formation assays indicate binding of bat IAV HA and MHC-II (80), the interaction sites on either MHC-II or bat IAV HA remain unknown. Direct binding of bat IAV HA to MHC-II has not been demonstrated, and detailed studies characterizing the MHC-II-dependent entry process of bat IAV have yet to be reported. Evidently, MHC-II is crucial for infection, but it is currently unclear whether interaction between H18 or H17 and MHC-II alone is the sole determinant of susceptibility to bat IAV infection or whether other, so far unknown cofactors are required to mediate MHC-II-dependent entry of bat IAV.
SUMMARY
For conventional IAV, attachment takes place mainly through interactions of viral HA with sialic acid, which can be found in two different variations, namely, Neu5Ac or Neu5Gc, depending on the host species. The binding preferences of HA to one or the other sialic acid variant, to the α2,6 or α2,3 linkage type, and to the composition and topology of the recognized glycan largely determine viral binding to host cells. Consequently, the presence or absence of sialoglycan variants has an impact on the IAV host range and tissue tropism. A small number of cellular entry factors have been described to contribute to IAV attachment through sialic acid-dependent interactions with HA (nucleolin, Cav1.2, and NKP44/46) and to internalization of attached particles (Cav1.2 and RTKs) (Table 1). IAV uptake is then largely dependent on second messengers and signaling pathways, which trigger endocytosis and macropinocytosis of attached virions. In addition, sialic acid-independent interactions with the host cell can occur through binding to phospho-glycans by a so far unknown mechanism, which is thought to be independent of the canonical RBS on the HA. Studies with IAV subtypes originating from bats revealed an MHC-II-dependent entry mechanism of bat IAV, which is entirely independent of sialic acid and further exemplifies the structural plasticity of the IAV HA. On immune cells, C-type lectins (DC-SIGN, MMR, MGL, and langerin) can contribute to sialic acid-independent virus attachment and internalization, which might be largely influenced by the virion glycosylation pattern.
TABLE 1.
Reported influenza A virus receptors
| Receptor | Reported role in IAV entry | Reference(s) |
|---|---|---|
| N-acetylneuraminic acid | Attachment receptor | 1–6 |
| N-glycolylneuraminic acid | Attachment receptor | 21–26 |
| Nucleolin | Internalization receptor | 35 |
| Cav1.2 | Attachment and internalization receptor | 41 |
| NKP44/46 | Putative attachment and internalization receptor | 36–38 |
| RTK | Internalization receptor | 46 |
| Phospho-glycans | Attachment receptor | 31 |
| DC/L-SIGN | Attachment and internalization receptor | 53, 55, 56, 63 |
| MMR | Attachment and internalization receptor | 52, 54 |
| MGL | Attachment and internalization receptor | 54, 57 |
| Langerin | Attachment and internalization | 65 |
| MHC-II | Entry determinant for bat IAV | 74 |
We, hence, propose a model of conventional IAV entry (Fig. 1) in which the virus utilizes a variety of different mechanisms to gain access to its target cells. Likely, for establishing contact with the host cell and for “rolling” along the cellular surface, no specific protein coreceptors are required. Instead, interactions of HA with sialoglycans and phospho-glycans present on various glycoproteins and glycolipids likely suffice for the early stages of virus entry. Of note, the affinity of HA for monovalent sialic acid-containing glycans is very low, with a dissociation constant (Kd) in the millimolar range (82, 83). Multivalent interactions between multiple HAs and sialic acid moieties increase binding strength and, thus, IAV attachment. Likely, the initial glycan-dependent attachment is followed by a secondary receptor engagement. In this second entry stage, multivalent attachment of IAV leads to clustering of specific cellular proteins with potentially redundant functions in lipid raft structures, which subsequently trigger and mediate uptake of virus particles. The identity of internalization receptors and the mechanism of uptake triggering remain to be discovered. This will likely require the application of novel technologies, including combinatorial CRISPR/Cas9 screening, and chemoproteomic approaches that cross-link virus-receptor interactions, such as HATRIC-based ligand receptor capture (HATRIC-LRC) (84, 85). Furthermore, much is left to learn about bat IAV entry. While we know that MHC-II plays a major role, no direct binding of H17 or H18 to MHC-II has been demonstrated, and it is unclear which other cellular proteins are involved in virus attachment and internalization.
ACKNOWLEDGMENT
We thank Ben Hale for critical reading of the manuscript.
REFERENCES
- 1.Gottschalk A. 1959. Chemistry of virus receptors, p 51–61. In Burnet FM, Stanley WM (ed), The viruses: biochemical, biological and biophysical properties. Academic Press, New York, NY. [Google Scholar]
- 2.Palese P, Shaw ML. 2007. Orthomyxoviridae: the viruses and their replication In Knipe DM, Howley PM (ed), Fields virology, 5th ed, vol 2 Lippincott Williams and Wilkins, Philadelphia, PA. [Google Scholar]
- 3.Rogers GN, Paulson JC. 1983. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology 127:361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- 4.Rogers GN, D'Souza BL. 1989. Receptor binding properties of human and animal H1 influenza virus isolates. Virology 173:317–322. doi: 10.1016/0042-6822(89)90249-3. [DOI] [PubMed] [Google Scholar]
- 5.Stevens J, Blixt O, Glaser L, Taubenberger JK, Palese P, Paulson JC, Wilson IA. 2006. Glycan microarray analysis of the hemagglutinins from modern and pandemic influenza viruses reveals different receptor specificities. J Mol Biol 355:1143–1155. doi: 10.1016/j.jmb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Yamada S, Suzuki Y, Suzuki T, Le MQ, Nidom CA, Sakai-Tagawa Y, Muramoto Y, Ito M, Kiso M, Horimoto T, Shinya K, Sawada T, Kiso M, Usui T, Murata T, Lin Y, Hay A, Haire LF, Stevens DJ, Russell RJ, Gamblin SJ, Skehel JJ, Kawaoka Y. 2006. Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Nature 444:378–382. doi: 10.1038/nature05264. [DOI] [PubMed] [Google Scholar]
- 7.de Graaf M, Fouchier RA. 2014. Role of receptor binding specificity in influenza A virus transmission and pathogenesis. EMBO J 33:823–841. doi: 10.1002/embj.201387442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imai M, Kawaoka Y. 2012. The role of receptor binding specificity in interspecies transmission of influenza viruses. Curr Opin Virol 2:160–167. doi: 10.1016/j.coviro.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long JS, Mistry B, Haslam SM, Barclay WS. 2019. Host and viral determinants of influenza A virus species specificity. Nat Rev Microbiol 17:67–81. doi: 10.1038/s41579-018-0115-z. [DOI] [PubMed] [Google Scholar]
- 10.Burnet FM. 1948. Mucins and mucoids in relation to influenza virus action. III. Inhibition of virus haemagglutination by glandular mucins. Aust J Exp Biol Med Sci 26:371–379. doi: 10.1038/icb.1948.38. [DOI] [PubMed] [Google Scholar]
- 11.Button B, Cai LH, Ehre C, Kesimer M, Hill DB, Sheehan JK, Boucher RC, Rubinstein M. 2012. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 337:937–941. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matrosovich MN, Matrosovich TY, Gray T, Roberts NA, Klenk HD. 2004. Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J Virol 78:12665–12667. doi: 10.1128/JVI.78.22.12665-12667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo H, Rabouw H, Slomp A, Dai M, van der Vegt F, van Lent JWM, McBride R, Paulson JC, de Groot RJ, van Kuppeveld FJM, de Vries E, de Haan CAM. 2018. Kinetic analysis of the influenza A virus HA/NA balance reveals contribution of NA to virus-receptor binding and NA-dependent rolling on receptor-containing surfaces. PLoS Pathog 14:e1007233. doi: 10.1371/journal.ppat.1007233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakai T, Nishimura SI, Naito T, Saito M. 2017. Influenza A virus hemagglutinin and neuraminidase act as novel motile machinery. Sci Rep 7:45043. doi: 10.1038/srep45043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrd-Leotis L, Cummings RD, Steinhauer DA. 2017. The interplay between the host receptor and influenza virus hemagglutinin and neuraminidase. Int J Mol Sci 18:E1541. doi: 10.3390/ijms18071541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaymard A, Le Briand N, Frobert E, Lina B, Escuret V. 2016. Functional balance between neuraminidase and haemagglutinin in influenza viruses. Clin Microbiol Infect 22:975–983. doi: 10.1016/j.cmi.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 17.de Vries E, Tscherne DM, Wienholts MJ, Cobos-Jiménez V, Scholte F, García-Sastre A, Rottier PJM, de Haan CAM. 2011. Dissection of the influenza A virus endocytic routes reveals macropinocytosis as an alternative entry pathway. PLoS Pathog 7:e1001329. doi: 10.1371/journal.ppat.1001329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sieczkarski SB, Whittaker GR. 2002. Influenza virus can enter and infect cells in the absence of clathrin-mediated endocytosis. J Virol 76:10455–10464. doi: 10.1128/jvi.76.20.10455-10464.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C, Zhuang X. 2008. Epsin 1 is a cargo-specific adaptor for the clathrin-mediated endocytosis of the influenza virus. Proc Natl Acad Sci U S A 105:11790–11795. doi: 10.1073/pnas.0803711105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rust MJ, Lakadamyali M, Zhang F, Zhuang X. 2004. Assembly of endocytic machinery around individual influenza viruses during viral entry. Nat Struct Mol Biol 11:567–573. doi: 10.1038/nsmb769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito T, Suzuki Y, Suzuki T, Takada A, Horimoto T, Wells K, Kida H, Otsuki K, Kiso M, Ishida H, Kawaoka Y. 2000. Recognition of N-glycolylneuraminic acid linked to galactose by the alpha2,3 linkage is associated with intestinal replication of influenza A virus in ducks. J Virol 74:9300–9305. doi: 10.1128/jvi.74.19.9300-9305.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pettersson S-O, Sivertsson R, Sjogren S, Svennerholm L. 1958. The sialic acids of hog pancreas. Biochim Biophys Acta 28:444–445. doi: 10.1016/0006-3002(58)90498-0. [DOI] [PubMed] [Google Scholar]
- 23.Naiki M. 1971. Chemical and immunochemical properties of two classes of globoside from equine organs. Jpn J Exp Med 41:67–81. [PubMed] [Google Scholar]
- 24.Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A. 1998. The molecular basis for the absence of N-glycolylneuraminic acid in humans. J Biol Chem 273:15866–15871. doi: 10.1074/jbc.273.25.15866. [DOI] [PubMed] [Google Scholar]
- 25.Broszeit F, Tzarum N, Zhu X, Nemanichvili N, Eggink D, Leenders T, Li Z, Liu L, Wolfert MA, Papanikolaou A, Martinez-Romero C, Gagarinov IA, Yu W, Garcia-Sastre A, Wennekes T, Okamatsu M, Verheije MH, Wilson IA, Boons GJ, de Vries RP. 2019. N-glycolylneuraminic acid as a receptor for influenza A viruses. Cell Rep 27:3284–3294.e6. doi: 10.1016/j.celrep.2019.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki T, Horiike G, Yamazaki Y, Kawabe K, Masuda H, Miyamoto D, Matsuda M, Nishimura SI, Yamagata T, Ito T, Kida H, Kawaoka Y, Suzuki Y. 1997. Swine influenza virus strains recognize sialylsugar chains containing the molecular species of sialic acid predominantly present in the swine tracheal epithelium. FEBS Lett 404:192–196. doi: 10.1016/s0014-5793(97)00127-0. [DOI] [PubMed] [Google Scholar]
- 27.Chandrasekaran A, Srinivasan A, Raman R, Viswanathan K, Raguram S, Tumpey TM, Sasisekharan V, Sasisekharan R. 2008. Glycan topology determines human adaptation of avian H5N1 virus hemagglutinin. Nat Biotechnol 26:107–113. doi: 10.1038/nbt1375. [DOI] [PubMed] [Google Scholar]
- 28.Stevens J, Blixt O, Tumpey TM, Taubenberger JK, Paulson JC, Wilson IA. 2006. Structure and receptor specificity of the hemagglutinin from an H5N1 influenza virus. Science 312:404–410. doi: 10.1126/science.1124513. [DOI] [PubMed] [Google Scholar]
- 29.Russell RJ, Kerry PS, Stevens DJ, Steinhauer DA, Martin SR, Gamblin SJ, Skehel JJ. 2008. Structure of influenza hemagglutinin in complex with an inhibitor of membrane fusion. Proc Natl Acad Sci U S A 105:17736–17741. doi: 10.1073/pnas.0807142105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gamblin SJ, Haire LF, Russell RJ, Stevens DJ, Xiao B, Ha Y, Vasisht N, Steinhauer DA, Daniels RS, Elliot A, Wiley DC, Skehel JJ. 2004. The structure and receptor binding properties of the 1918 influenza hemagglutinin. Science 303:1838–1842. doi: 10.1126/science.1093155. [DOI] [PubMed] [Google Scholar]
- 31.Eisen MB, Sabesan S, Skehel JJ, Wiley DC. 1997. Binding of the influenza A virus to cell-surface receptors: structures of five hemagglutinin-sialyloligosaccharide complexes determined by X-ray crystallography. Virology 232:19–31. doi: 10.1006/viro.1997.8526. [DOI] [PubMed] [Google Scholar]
- 32.Ha Y, Stevens DJ, Skehel JJ, Wiley DC. 2001. X-ray structures of H5 avian and H9 swine influenza virus hemagglutinins bound to avian and human receptor analogs. Proc Natl Acad Sci U S A 98:11181–11186. doi: 10.1073/pnas.201401198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ha Y, Stevens DJ, Skehel JJ, Wiley DC. 2003. X-ray structure of the hemagglutinin of a potential H3 avian progenitor of the 1968 Hong Kong pandemic influenza virus. Virology 309:209–218. doi: 10.1016/s0042-6822(03)00068-0. [DOI] [PubMed] [Google Scholar]
- 34.Byrd-Leotis L, Liu R, Bradley KC, Lasanajak Y, Cummings SF, Song X, Heimburg-Molinaro J, Galloway SE, Culhane MR, Smith DF, Steinhauer DA, Cummings RD. 2014. Shotgun glycomics of pig lung identifies natural endogenous receptors for influenza viruses. Proc Natl Acad Sci U S A 111:E2241–E2250. doi: 10.1073/pnas.1323162111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gambaryan AS, Tuzikov AB, Pazynina GV, Desheva JA, Bovin NV, Matrosovich MN, Klimov AI. 2008. 6-Sulfo sialyl Lewis X is the common receptor determinant recognized by H5, H6, H7 and H9 influenza viruses of terrestrial poultry. Virol J 5:85. doi: 10.1186/1743-422X-5-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevens J, Chen L-M, Carney PJ, Garten R, Foust A, Le J, Pokorny BA, Manojkumar R, Silverman J, Devis R, Rhea K, Xu X, Bucher DJ, Paulson JC, Paulson J, Cox NJ, Klimov A, Donis RO. 2010. Receptor specificity of influenza A H3N2 viruses isolated in mammalian cells and embryonated chicken eggs. J Virol 84:8287–8299. doi: 10.1128/JVI.00058-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byrd-Leotis L, Jia N, Dutta S, Trost JF, Gao C, Cummings SF, Braulke T, Muller-Loennies S, Heimburg-Molinaro J, Steinhauer DA, Cummings RD. 2019. Influenza binds phosphorylated glycans from human lung. Sci Adv 5:eaav2554. doi: 10.1126/sciadv.aav2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weis W, Brown JH, Cusack S, Paulson JC, Skehel JJ, Wiley DC. 1988. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature 333:426–431. doi: 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]
- 39.Chu VC, Whittaker GR. 2004. Influenza virus entry and infection require host cell N-linked glycoprotein. Proc Natl Acad Sci U S A 101:18153–18158. doi: 10.1073/pnas.0405172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Vries E, de Vries RP, Wienholts MJ, Floris CE, Jacobs MS, van den Heuvel A, Rottier PJ, de Haan CAM. 2012. Influenza A virus entry into cells lacking sialylated N-glycans. Proc Natl Acad Sci U S A 109:7457–7462. doi: 10.1073/pnas.1200987109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan C-M, Chu H, Zhang AJ, Leung L-H, Sze K-H, Kao RY-T, Chik KK-H, To KK-W, Chan JF-W, Chen H, Jin D-Y, Liu L, Yuen K-Y. 2016. Hemagglutinin of influenza A virus binds specifically to cell surface nucleolin and plays a role in virus internalization. Virology 494:78–88. doi: 10.1016/j.virol.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Ho JW, Hershkovitz O, Peiris M, Zilka A, Bar-Ilan A, Nal B, Chu K, Kudelko M, Kam YW, Achdout H, Mandelboim M, Altmeyer R, Mandelboim O, Bruzzone R, Porgador A. 2008. H5-type influenza virus hemagglutinin is functionally recognized by the natural killer-activating receptor NKp44. J Virol 82:2028–2032. doi: 10.1128/JVI.02065-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Achdout H, Arnon TI, Markel G, Gonen-Gross T, Katz G, Lieberman N, Gazit R, Joseph A, Kedar E, Mandelboim O. 2003. Enhanced recognition of human NK receptors after influenza virus infection. J Immunol 171:915–923. doi: 10.4049/jimmunol.171.2.915. [DOI] [PubMed] [Google Scholar]
- 44.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, Davis DM, Strominger JL, Yewdell JW, Porgador A. 2001. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature 409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 45.Mao H, Tu W, Liu Y, Qin G, Zheng J, Chan P-L, Lam K-T, Peiris JS, Lau Y-L. 2010. Inhibition of human natural killer cell activity by influenza virions and hemagglutinin. J Virol 84:4148–4157. doi: 10.1128/JVI.02340-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mao H, Tu W, Qin G, Law HK, Sia SF, Chan P-L, Liu Y, Lam K-T, Zheng J, Peiris M, Lau Y-L. 2009. Influenza virus directly infects human natural killer cells and induces cell apoptosis. J Virol 83:9215–9222. doi: 10.1128/JVI.00805-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujioka Y, Nishide S, Ose T, Suzuki T, Kato I, Fukuhara H, Fujioka M, Horiuchi K, Satoh AO, Nepal P, Kashiwagi S, Wang J, Horiguchi M, Sato Y, Paudel S, Nanbo A, Miyazaki T, Hasegawa H, Maenaka K, Ohba Y. 2018. A sialylated voltage-dependent Ca(2+) channel binds hemagglutinin and mediates influenza A virus entry into mammalian cells. Cell Host Microbe 23:809–818.e5. doi: 10.1016/j.chom.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 48.Fujioka Y, Tsuda M, Nanbo A, Hattori T, Sasaki J, Sasaki T, Miyazaki T, Ohba Y. 2013. A Ca(2+)-dependent signalling circuit regulates influenza A virus internalization and infection. Nat Commun 4:2763. doi: 10.1038/ncomms3763. [DOI] [PubMed] [Google Scholar]
- 49.Fujioka Y, Tsuda M, Hattori T, Sasaki J, Sasaki T, Miyazaki T, Ohba Y. 2011. The Ras-PI3K signaling pathway is involved in clathrin-independent endocytosis and the internalization of influenza viruses. PLoS One 6:e16324. doi: 10.1371/journal.pone.0016324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yanguez E, Hunziker A, Dobay MP, Yildiz S, Schading S, Elshina E, Karakus U, Gehrig P, Grossmann J, Dijkman R, Schmolke M, Stertz S. 2018. Phosphoproteomic-based kinase profiling early in influenza virus infection identifies GRK2 as antiviral drug target. Nat Commun 9:3679. doi: 10.1038/s41467-018-06119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tripathi S, Pohl MO, Zhou Y, Rodriguez-Frandsen A, Wang G, Stein DA, Moulton HM, DeJesus P, Che J, Mulder LCF, Yángüez E, Andenmatten D, Pache L, Manicassamy B, Albrecht RA, Gonzalez MG, Nguyen Q, Brass A, Elledge S, White M, Shapira S, Hacohen N, Karlas A, Meyer TF, Shales M, Gatorano A, Johnson JR, Jang G, Johnson T, Verschueren E, Sanders D, Krogan N, Shaw M, König R, Stertz S, García-Sastre A, Chanda SK. 2015. Meta- and orthogonal integration of influenza “omics” data defines a role for UBR4 in virus budding. Cell Host Microbe 18:723–735. doi: 10.1016/j.chom.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eierhoff T, Hrincius ER, Rescher U, Ludwig S, Ehrhardt C. 2010. The epidermal growth factor receptor (EGFR) promotes uptake of influenza A viruses (IAV) into host cells. PLoS Pathog 6:e1001099. doi: 10.1371/journal.ppat.1001099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ehrhardt C, Marjuki H, Wolff T, Nurnberg B, Planz O, Pleschka S, Ludwig S. 2006. Bivalent role of the phosphatidylinositol-3-kinase (PI3K) during influenza virus infection and host cell defence. Cell Microbiol 8:1336–1348. doi: 10.1111/j.1462-5822.2006.00713.x. [DOI] [PubMed] [Google Scholar]
- 54.Elbahesh H, Cline T, Baranovich T, Govorkova EA, Schultz-Cherry S, Russell CJ. 2014. Novel roles of focal adhesion kinase in cytoplasmic entry and replication of influenza A viruses. J Virol 88:6714–6728. doi: 10.1128/JVI.00530-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Constantinescu SN, Cernescu CD, Popescu LM. 1991. Effects of protein kinase C inhibitors on viral entry and infectivity. FEBS Lett 292:31–33. doi: 10.1016/0014-5793(91)80826-o. [DOI] [PubMed] [Google Scholar]
- 56.O’Hanlon R, Leyva-Grado VH, Sourisseau M, Evans MJ, Shaw ML. 2019. An influenza virus entry inhibitor targets class II PI3 kinase and synergizes with oseltamivir. ACS Infect Dis 5:1779–1793. doi: 10.1021/acsinfecdis.9b00230. [DOI] [PubMed] [Google Scholar]
- 57.Zhu L, Ly H, Liang Y. 2014. PLC-gamma1 signaling plays a subtype-specific role in postbinding cell entry of influenza A virus. J Virol 88:417–424. doi: 10.1128/JVI.02591-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reading PC, Miller JL, Anders EM. 2000. Involvement of the mannose receptor in infection of macrophages by influenza virus. J Virol 74:5190–5197. doi: 10.1128/jvi.74.11.5190-5197.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang S-F, Huang JC, Lee Y-M, Liu S-J, Chan Y-J, Chau Y-P, Chong P, Chen Y-MA. 2008. DC-SIGN mediates avian H5N1 influenza virus infection in cis and in trans. Biochem Biophys Res Commun 373:561–566. doi: 10.1016/j.bbrc.2008.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Upham JP, Pickett D, Irimura T, Anders EM, Reading PC. 2010. Macrophage receptors for influenza A virus: role of the macrophage galactose-type lectin and mannose receptor in viral entry. J Virol 84:3730–3737. doi: 10.1128/JVI.02148-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Londrigan SL, Turville SG, Tate MD, Deng YM, Brooks AG, Reading PC. 2011. N-linked glycosylation facilitates sialic acid-independent attachment and entry of influenza A viruses into cells expressing DC-SIGN or L-SIGN. J Virol 85:2990–3000. doi: 10.1128/JVI.01705-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hillaire MLB, Nieuwkoop NJ, Boon ACM, de Mutsert G, Vogelzang-van Trierum SE, Fouchier RAM, Osterhaus ADME, Rimmelzwaan GF. 2013. Binding of DC-SIGN to the hemagglutinin of influenza A viruses supports virus replication in DC-SIGN expressing cells. PLoS One 8:e56164. doi: 10.1371/journal.pone.0056164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ng WC, Liong S, Tate MD, Irimura T, Denda-Nagai K, Brooks AG, Londrigan SL, Reading PC. 2014. The macrophage galactose-type lectin can function as an attachment and entry receptor for influenza virus. J Virol 88:1659–1672. doi: 10.1128/JVI.02014-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Kooyk Y, Geijtenbeek TBH. 2003. DC-SIGN: escape mechanism for pathogens. Nat Rev Immunol 3:697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- 65.Lozach P-Y, Kühbacher A, Meier R, Mancini R, Bitto D, Bouloy M, Helenius A. 2011. DC-SIGN as a receptor for phleboviruses. Cell Host Microbe 10:75–88. doi: 10.1016/j.chom.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 66.Lozach P-Y, Burleigh L, Staropoli I, Navarro-Sanchez E, Harriague J, Virelizier J-L, Rey FA, Despres P, Arenzana-Seisdedos F, Amara A. 2005. Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN)-mediated enhancement of dengue virus infection is independent of DC-SIGN internalization signals. J Biol Chem 280:23698–23708. doi: 10.1074/jbc.M504337200. [DOI] [PubMed] [Google Scholar]
- 67.Curtis BM, Scharnowske S, Watson AJ. 1992. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc Natl Acad Sci U S A 89:8356–8360. doi: 10.1073/pnas.89.17.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pöhlmann S, Zhang J, Baribaud F, Chen Z, Leslie GJ, Lin G, Granelli-Piperno A, Doms RW, Rice CM, McKeating JA. 2003. Hepatitis C virus glycoproteins interact with DC-SIGN and DC-SIGNR. J Virol 77:4070–4080. doi: 10.1128/jvi.77.7.4070-4080.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Palomino-Segura M, Perez L, Farsakoglu Y, Virgilio T, Latino I, D’Antuono R, Chatziandreou N, Pizzagalli DU, Wang G, Garcia-Sastre A, Sallusto F, Carroll MC, Neyrolles O, Gonzalez SF. 2019. Protection against influenza infection requires early recognition by inflammatory dendritic cells through C-type lectin receptor SIGN-R1. Nat Microbiol 4:1930. doi: 10.1038/s41564-019-0506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gillespie L, Roosendahl P, Ng WC, Brooks AG, Reading PC, Londrigan SL. 2016. Endocytic function is critical for influenza A virus infection via DC-SIGN and L-SIGN. Sci Rep 6:19428. doi: 10.1038/srep19428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ng WC, Londrigan SL, Nasr N, Cunningham AL, Turville S, Brooks AG, Reading PC. 2016. The C-type lectin langerin functions as a receptor for attachment and infectious entry of influenza A virus. J Virol 90:206–221. doi: 10.1128/JVI.01447-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lennartz MR, Cole FS, Shepherd VL, Wileman TE, Stahl PD. 1987. Isolation and characterization of a mannose-specific endocytosis receptor from human placenta. J Biol Chem 262:9942–9944. [PubMed] [Google Scholar]
- 73.Ozaki K, Ii M, Itoh N, Kawasaki T. 1992. Expression of a functional asialoglycoprotein receptor through transfection of a cloned cDNA that encodes a macrophage lectin. J Biol Chem 267:9229–9235. [PubMed] [Google Scholar]
- 74.Tong S, Li Y, Rivailler P, Conrardy C, Castillo DA, Chen LM, Recuenco S, Ellison JA, Davis CT, York IA, Turmelle AS, Moran D, Rogers S, Shi M, Tao Y, Weil MR, Tang K, Rowe LA, Sammons S, Xu X, Frace M, Lindblade KA, Cox NJ, Anderson LJ, Rupprecht CE, Donis RO. 2012. A distinct lineage of influenza A virus from bats. Proc Natl Acad Sci U S A 109:4269–4274. doi: 10.1073/pnas.1116200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, Yang H, Chen X, Recuenco S, Gomez J, Chen L-M, Johnson A, Tao Y, Dreyfus C, Yu W, McBride R, Carney PJ, Gilbert AT, Chang J, Guo Z, Davis CT, Paulson JC, Stevens J, Rupprecht CE, Holmes EC, Wilson IA, Donis RO. 2013. New world bats harbor diverse influenza A viruses. PLoS Pathog 9:e1003657. doi: 10.1371/journal.ppat.1003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu X, Yu W, McBride R, Li Y, Chen L-M, Donis RO, Tong S, Paulson JC, Wilson IA. 2013. Hemagglutinin homologue from H17N10 bat influenza virus exhibits divergent receptor-binding and pH-dependent fusion activities. Proc Natl Acad Sci U S A 110:1458–1463. doi: 10.1073/pnas.1218509110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun X, Shi Y, Lu X, He J, Gao F, Yan J, Qi J, Gao GF. 2013. Bat-derived influenza hemagglutinin H17 does not bind canonical avian or human receptors and most likely uses a unique entry mechanism. Cell Rep 3:769–778. doi: 10.1016/j.celrep.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 78.Moreira EA, Locher S, Kolesnikova L, Bolte H, Aydillo T, Garcia-Sastre A, Schwemmle M, Zimmer G. 2016. Synthetically derived bat influenza A-like viruses reveal a cell type- but not species-specific tropism. Proc Natl Acad Sci U S A 113:12797–12802. doi: 10.1073/pnas.1608821113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoffmann M, Krüger N, Zmora P, Wrensch F, Herrler G, Pohlmann S. 2016. The hemagglutinin of bat-associated influenza viruses is activated by TMPRSS2 for pH-dependent entry into bat but not human cells. PLoS One 11:e0152134. doi: 10.1371/journal.pone.0152134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karakus U, Thamamongood T, Ciminski K, Ran W, Gunther SC, Pohl MO, Eletto D, Jeney C, Hoffmann D, Reiche S, Schinkothe J, Ulrich R, Wiener J, Hayes MGB, Chang MW, Hunziker A, Yanguez E, Aydillo T, Krammer F, Oderbolz J, Meier M, Oxenius A, Halenius A, Zimmer G, Benner C, Hale BG, Garcia-Sastre A, Beer M, Schwemmle M, Stertz S. 2019. MHC class II proteins mediate cross-species entry of bat influenza viruses. Nature 567:109–112. doi: 10.1038/s41586-019-0955-3. [DOI] [PubMed] [Google Scholar]
- 81.Ciminski K, Ran W, Gorka M, Lee J, Malmlov A, Schinkothe J, Eckley M, Murrieta RA, Aboellail TA, Campbell CL, Ebel GD, Ma J, Pohlmann A, Franzke K, Ulrich R, Hoffmann D, Garcia-Sastre A, Ma W, Schountz T, Beer M, Schwemmle M. 2019. Bat influenza viruses transmit among bats but are poorly adapted to non-bat species. Nat Microbiol 4:2298. doi: 10.1038/s41564-019-0556-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sauter NK, Bednarski MD, Wurzburg BA, Hanson JE, Whitesides GM, Skehel JJ, Wiley DC. 1989. Hemagglutinins from two influenza virus variants bind to sialic acid derivatives with millimolar dissociation constants: a 500-MHz proton nuclear magnetic resonance study. Biochemistry 28:8388–8396. doi: 10.1021/bi00447a018. [DOI] [PubMed] [Google Scholar]
- 83.Sauter NK, Hanson JE, Glick GD, Brown JH, Crowther RL, Park SJ, Skehel JJ, Wiley DC. 1992. Binding of influenza virus hemagglutinin to analogs of its cell-surface receptor, sialic acid: analysis by proton nuclear magnetic resonance spectroscopy and x-ray crystallography. Biochemistry 31:9609–9621. doi: 10.1021/bi00155a013. [DOI] [PubMed] [Google Scholar]
- 84.Najm FJ, Strand C, Donovan KF, Hegde M, Sanson KR, Vaimberg EW, Sullender ME, Hartenian E, Kalani Z, Fusi N, Listgarten J, Younger ST, Bernstein BE, Root DE, Doench JG. 2017. Orthologous CRISPR-Cas9 enzymes for combinatorial genetic screens. Nat Biotechnol 36:179–189. doi: 10.1038/nbt.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sobotzki N, Schafroth MA, Rudnicka A, Koetemann A, Marty F, Goetze S, Yamauchi Y, Carreira EM, Wollscheid B. 2018. HATRIC-based identification of receptors for orphan ligands. Nat Commun 9:1519. doi: 10.1038/s41467-018-03936-z. [DOI] [PMC free article] [PubMed] [Google Scholar]