Short abstract
Objective
To investigate the effect of the ATP-binding cassette transporter superfamily B member 1 gene (ABCB1) 3435C > T single nucleotide polymorphism (SNP) on docetaxel transportation in ovarian cancer cells.
Methods
ES-2 and SKOV3 cells were transfected with an ABCB1 3435C > T recombinant plasmid, and mRNA expression was detected by real-time PCR. The MTT assay was used to detect the toxicity of docetaxel. High-performance liquid chromatography determined the drug concentration in different cell models to evaluate intracellular accumulation, and a transmembrane resistance experiment was used to assess permeability and evaluate the effect of P-gp activity on drug transportation. A tumor-bearing mouse model was established to evaluate the effect of ABCB1 3435C > T on docetaxel resistance.
Results
P-gp was overexpressed in cells transfected with the ABCB1 3435C > T plasmid, leading to a significant increase in drug resistance to docetaxel. ABCB1 3435C/wild-type transfection significantly promoted the transport of docetaxel mediated by P-gp compared with ABCB1 3435T/mutant transfection.
Conclusion
P-gp encoded by the ABCB1 variant allele appears to be more efficient at transporting docetaxel compared with the wild-type allele. The ABCB1 3435C > T SNP dramatically affected the efflux ability of P-gp against docetaxel, and may influence P-gp expression and activity.
Keywords: Docetaxel, ABCB1, P-glycoprotein, polymorphism, ovarian cancer, drug transportation
Introduction
Docetaxel is a major anti-tumor drug used for the treatment of malignant tumors such as breast cancer, ovarian cancer, and non-small cell lung cancer. The remarkable efficacy of the drug makes it widely applicable, and its metabolism and clearance have received much attention.1,2 However, there are significant differences in the clinical pharmacokinetics and pharmacodynamics among individuals, and efficacy and adverse reactions also vary.
P-glycoprotein (P-gp) is a member of the ABC transporter family. Its main function is to pump substrates such as docetaxel out of the cell, and it is also involved in substrate absorption and distribution. P-gp is an important transporter in vivo that is widely distributed in the liver, brain, kidney, gastrointestinal tract, and other tissues. P-gp overexpression is the main cause of multidrug resistance in various human tissues.3 It is encoded by the ATP-binding cassette transporter superfamily B member 1 gene (ABCB1), which is over 100 kb, is localized on chromosome 7q21.1, and contains 28 exons. ABCB1 is highly expressed in various tumor tissues.4–6 Currently, more than 60 single nucleotide polymorphism (SNPs) in ABCB1 have been reported, with the most common being 1236 C > T, 2677G > T/A, and 3435C > T.
Different ABCB1SNPs can significantly influence the efficacy and adverse effects of paclitaxel.7,8 Vaclavikova et al.9 found that ABCB1 expression was down-regulated in 79.5% of cancer tissues, and was associated with the polymorphic mutations C3435T and C1236T. Lal et al.10 found that the clearance rate of paclitaxel in CC-GG-CC wild-type patients with C1236T, G2677T/A, and C3435T mutations was significantly increased compared with CT-GT-CT and TT-TT-TT mutants, while the plasma peak drug concentration was significantly decreased. Conversely, Fransson et al.11 reported that SNPs have only a small influence on the plasma concentration of paclitaxel, but that even a slight change of transfer or metabolism would affect the concentration of the main metabolic products of paclitaxel. The effect of ABCB1 on drug toxicity is therefore of greater importance than the pharmacokinetics of the drug itself. A study in Caucasian female patients with ovarian cancer12 found that the clearance rates of paclitaxel had no significant correlation with ABCB1 C1236T or its variants. However, only limited reports are available on the effect of SNPs on drug sensitivity in ovarian cancer cells.
In this study, ABCB1 (3435C > T) recombinant plasmids were constructed and transfected into docetaxel-resistant ES-2 and SKOV3 cells. The effect of ABCB1 (3435C > T) polymorphisms on the expression level of P-gp and the correlation of SNPs with drug susceptibility were investigated. Our findings provide a theoretical guidance for ovarian cancer clinical practice.
Materials and methods
Cell culture
ES-2 and SKOV3 cells, purchased from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China), were cultured in Dulbecco’s modified Eagle medium containing 1 g/L glucose and glutamine (GIBCO® Cell Culture, Carlsbad, CA, USA), 10% fetal bovine serum (GIBCO), and 1% neomycin (Gibco), at 37°C in an atmosphere of 5% CO2. Cells were seeded in a six-well plate at a density of 2000 cells/well and were assessed after 24 to 120 hours.
Construction of recombinant plasmids and cell transfection
The QuikChange II XL Site-directed Mutagenesis Kit (Agilent Technologies Inc., Santa Clara, CA, USA) was used to perform site-directed mutagenesis in the eukaryotic expression vector pcDNA 3.1 (GeneChem, Shanghai, China) to construct two recombinant plasmids: wild-type plasmid ABCB1 3435 C/wt and the mutant plasmid ABCB1 3435 T/mut. The point mutation primer was designed by Sangon Biotech (Shanghai, China). Sequencing and validation of recombinant plasmids were performed by Sanger sequencing (GATC Biotech AG, Konstanz, Germany).
ES-2 and SKOV3 cells (6–8 × 106 cells) were seeded in 25-cm culture dishes. The two recombinant plasmids (10 μg each) were transfected into both cell lines using Lipofectamine 2000 (Thermo Fisher Scientific Inc., Rockford, IL, USA). At 48 hours after transformation, 8 μg/mL puromycin was added to screen for stable transformants.
Western blot
Radioimmunoprecipitation assay (RIPA) lysis buffer with protease inhibitors was added to cells for protein extraction. The protein concentration was measured using a BCA kit. Samples were denatured and electrophoresed using 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis. Subsequently, proteins were transferred to polyvinylidene fluoride membranes and probed with rabbit anti-P-gp (1:1000; Sigma-Aldrich, St. Louis, MO, USA) and mouse anti-GAPDH (1:2000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) primary antibodies overnight at 4°C. Membranes were then incubated with anti-mouse (1:5000, Santa Cruz Biotechnology) and anti-rabbit secondary antibodies (1:5000, Santa Cruz Biotechnology), respectively, for 1 hour. Finally, immunoreactive bands were detected with Enhanced Chemiluminescence Plus reagent (Bio-Rad, Hercules, CA, USA). Band densities were analyzed with Quantity One software (Bio-Rad) and used to determine protein expression relative to that of GAPDH.
Real-time (RT-)PCR
To determine changes in P-gp mRNA expression, cDNA synthesis was performed using an RNA PCR kit (Takara Bio Inc., Shiga, Japan). Quantitative real-time PCR was carried out using a SYBR premix Ex TaqII kit (Takara Bio Inc.) according to the manufacturer’s instructions. Beta-actin was used as an internal control. RT-PCR was performed using a 7500HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA), and the threshold cycle (CT) number was determined using ABI 7500 Real-time PCR System SDS software v2.0.1. All reactions were performed in triplicate. Relative quantification of P-gp expression was calculated using the 2−ΔΔCt method. Primer information is given in Table 1.
Table 1.
Information about primers used in the study.
| Gene name and orientation | Primer sequence (5′–3′) | Primer length (bp) | Amplicon length (bp) |
|---|---|---|---|
| MDR1 | |||
| Sense | GGCACCAAAGACAACAGCTG | 20 | 286 |
| Anti-sense | ATGTCCTTTTCCAGCACCTC | 20 | |
| β-actin | |||
| Sense | TGTTTGAGACCTTCAACACCC | 21 | 529 |
| Anti-sense | AGCACTGTGTTGGCGTACAG | 20 |
MTT assay
The MTT assay was used to detect cell proliferation. Briefly, cells were seeded at a density of 1 × 104 cells/well of a 96-well plate and cultured for 48 hours. Each well was supplemented with 7.0 μmol/L docetaxel (Qilu Pharmaceutical Co. Ltd., Jinan, China), and 15 replicates were included for each group. Every 24 hours, cells from three replicates of each group were assessed by the MTT assay. The absorbance was read at 490/570 nm using a microplate reader. After obtaining optical density values for 5 days, the growth curve of cells under docetaxel selection was drawn.
Drug accumulation test
Cells were seeded in six-well plates at 5.0 × 105 cells/well and cultured for 4 hours. When cells were in the logarithmic phase, serum-free Dulbecco’s modified Eagle’s medium and 6.0 µmol/L docetaxel were added. Then, cyclosporin A (0.5 and 1.0 µM; Novartis Pharma AG, Basel, Switzerland) was added to inhibit drug transportation. After incubation at 37°C for 4 hours, cells were collected, washed with phosphate-buffered saline, centrifuged at 800 × g for 5 minutes, and the supernatant was discarded. The cell pellet was stored at −80°C until required.
Quantitative analysis of cell drug accumulation was performed using high-performance liquid chromatography (HPLC) as previously described.13 Briefly, 150 μL RIPA lysis buffer was added to cells and incubated in an ice bath for 30 minutes. Docetaxel was then extracted with glacial acetic acid and ethyl acetate. The supernatant was collected, and the solvents were evaporated using a nitrogen-drying apparatus (Ember, Shanghai, China). The obtained residue was dissolved in ethanol, then injected into the Agilent 1100 HPLC system (Waters Corp., Milford, MA, USA) with Agilent 4.6 mm × 250 mm columns (Waters Corp.). The amount of drug extracted from the cells was calculated using a standard curve (1.0–50 µM) and normalized to the total protein content of the cells.
Transmembrane transport analysis
The bidirectional transfer test was performed using cells seeded at a density of 1 × 104/well in a Transwell™ plate (3 μm). The transepithelial electrical resistance was measured using a Millicell electrical resistance system (Millipore, Billerica, MA, USA). To evaluate anti-tumor drug transport mediated by P-gp (A→B), HBSS-GH solution containing 7.0 μM docetaxel was added to the upper chamber (A) of the Transwell apparatus, and HBSS-GH was added to the lower chamber (B). Transport from B to A was used to measure the drug concentration from B to A. HBSS-FH solution containing 1.0 μmol/L cyclosporine A was added to upper and lower chambers to inhibit the activity of P-gp. According to previous studies,6,14–18 the apparent permeability value (Papp) was calculated by Papp = (ΔQ/Δt)/(C0×A), where ΔQ/Δt is the rate of drug transported to the receiving chamber; C0 is the initial concentration of the drug; and A is the permeability surface area. Both Papp of B→A and that of A→B were calculated. Each test was performed in triplicate.
In vivo assay
Male athymic nude mice (BALB/c-nu/nu) aged 4 to 6 weeks and weighing 18 to 22 g were purchased from Shanghai Slike Experimental Animals Co. (Shanghai, China). The mice were cared for according to Animal Facility Guidelines of the China Pharmaceutical University. Mice were randomized into three groups (n=10 per group): M0, control group; M1, implanted with SKOV3 cells transfected with ABCB1 3435 C/wt plasmids; and M2, implanted with SKOV3 cells transfected with ABCB1 3435T/mut plasmids. Cells were implanted into the subcutaneous tissue of the right groin of the mice. When the tumor volumes exceeded 100 mm3, docetaxel (8 mg/kg) was administered every 4 days for a total of five times. The animal body weight was recorded daily. On the last day (Day 21), mice were sacrificed after treatment, and their plasma and tissues were collected and stored at −80°C until analysis. The study protocol was approved by the Ethics Committee of Shandong Provincial Qianfoshan Hospital, Shandong University.
Statistical analysis
All experiments were performed in triplicate. SPSS 17.0 software (SPSS Inc., Chicago, IL, USA) was used to analyze the data. Measurement data were expressed as means ± standard deviations (SDs). Comparisons between groups were analyzed by the Student’s t-test. A P value less than 0.05 was considered statistically significant.
Results
Effect of the ABCB1 (3435C > T) polymorphism on P-gp protein expression
To investigate P-gp expression in cells, western blotting was performed. P-gp expression in ES-2 cells was increased 3.8-fold compared with the control group while that in SKOV3 cells was increased 7-fold (Figure 1).
Figure 1.
Analysis of P-gp protein expression. SKOV3/ES-2 cells were stably transfected with ABCB1 3435 C/wt or ABCB1 3435T/mut plasmids, and P-gp protein levels were detected by western blotting. Representative and quantitative western blot results are shown. M0: control; M1: cells transfected with ABCB1 3435 C/wt plasmids; M2: cells transfected with ABCB1 3435T/mut plasmids. *P<0.05, compared with M0.
Effect of the ABCB1 (3435C > T) polymorphism on P-gp mRNA expression
RT-PCR was performed to detect P-gp mRNA expression in cells. The expression of P-gp mRNA in both ES-2 and SKOV3 cells stably transfected with ABCB1 3435 C/wt and ABCB1 3435T/mut was significantly higher than in control cells (P < 0.01, Figure 2). P-gp mRNA was overexpressed in transfected cell lines, which was consistent with western blot findings.
Figure 2.
Analysis of P-gp mRNA expression. SKOV3/ES-2 cells were stably transfected with ABCB1 3435 C/wt or ABCB1 3435T/mut plasmids. P-gp mRNA levels were detected by RT-PCR. M0: control; M1: cells transfected with ABCB1 3435 C/wt plasmids; M2: cells transfected with ABCB1 3435T/mut plasmids. *P<0.05, compared with M0.
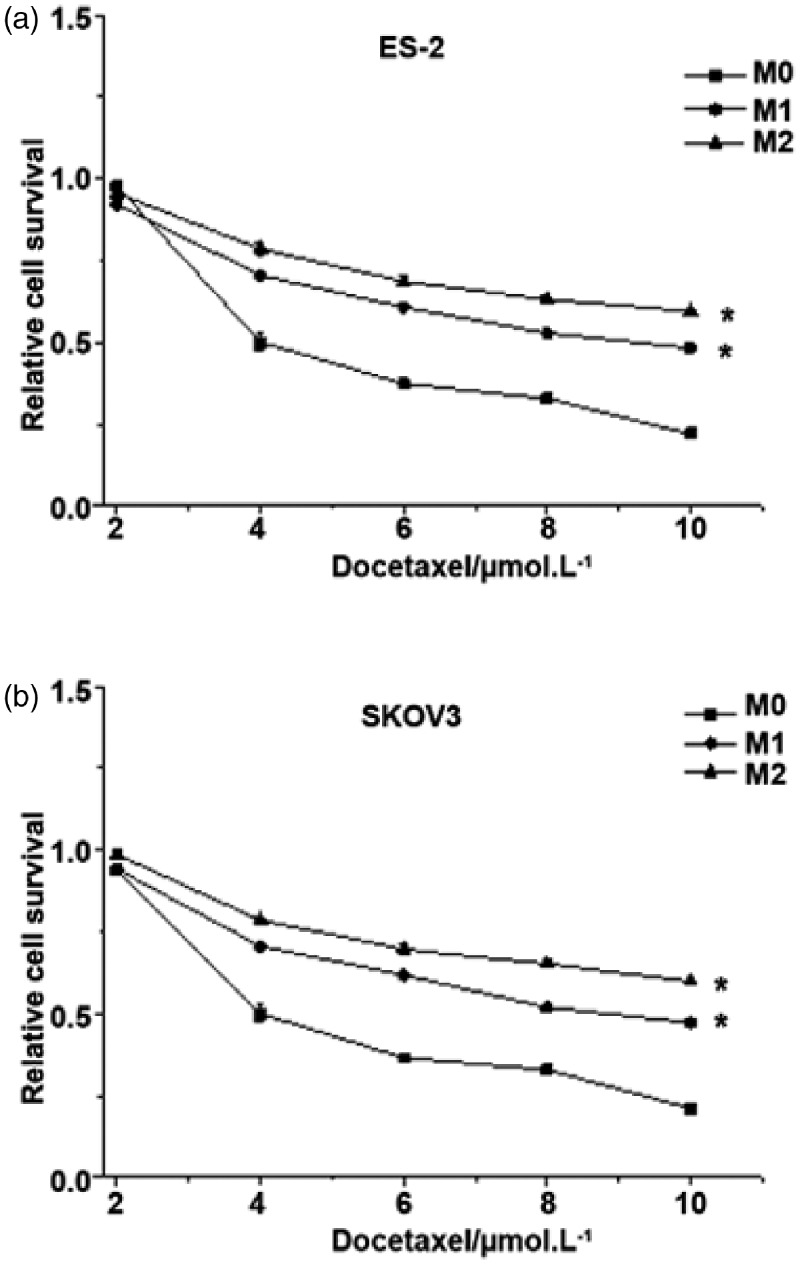
Effect of the ABCB1 (3435C > T) polymorphism on docetaxel cytotoxicity
The MTT assay was performed to determine the effect of docetaxel on cell proliferation. In both ES-2 and SKOV3 cell lines, the survival of cells stably transfected with ABCB1 3435T/mut was increased 1.11-fold compared with cells stably transfected with ABCB1 3435 C/wt (Figure 3). Additionally, compared with the control group, cells stably transfected with ABCB1 3435 C/wt or ABCB1 3435T/mut showed significantly higher cell survival (P < 0.05). This suggests that the drug resistance of cells to docetaxel is increased after ABCB1 (3435C > T) transfection and that ABCB1 SNPs are closely associated with cell sensitivity to drugs.
Figure 3.
The cytotoxicity of docetaxel on ES-2 and SKOV3 cells. SKOV3/ES-2 cells were transfected with ABCB1 3435 C/wt or ABCB1 3435T/mut plasmids. Cell survival was assessed using the MTT assay. M0: control; M1: cells transfected with ABCB1 3435 C/wt plasmids; M2: cells transfected with ABCB1 3435T/mut plasmids. *P<0.05, compared with M0.
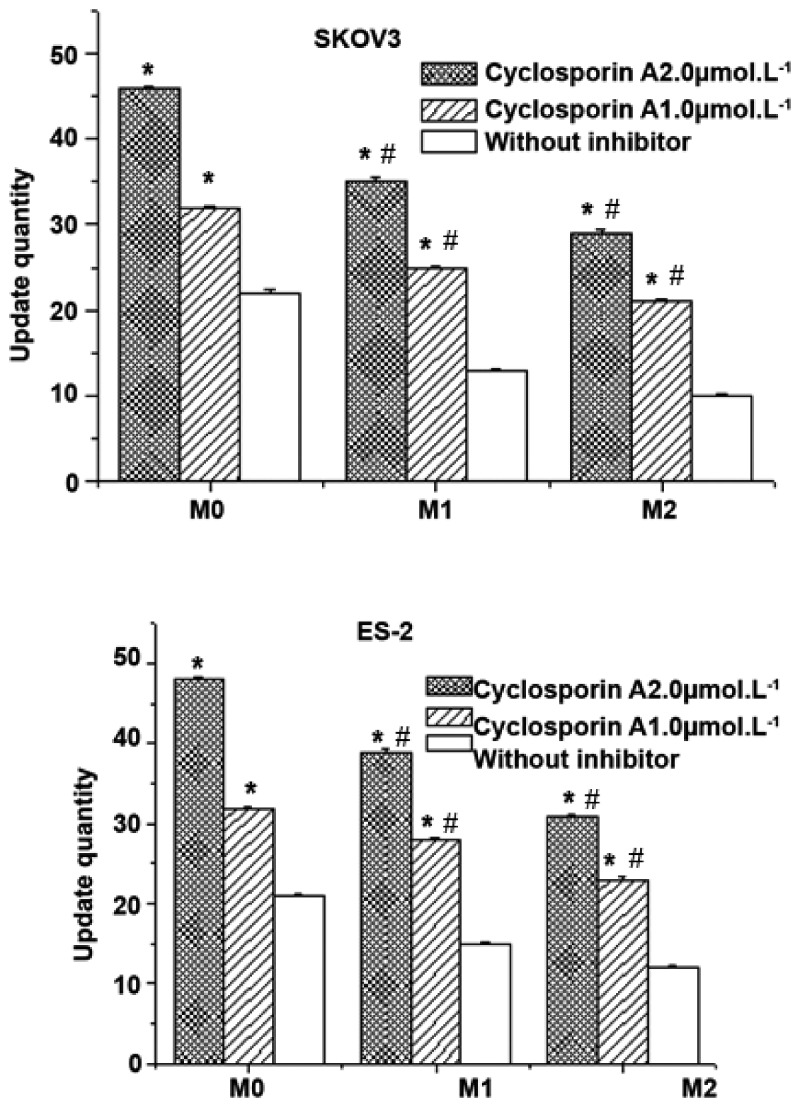
Effect of the ABCB1 (3435C > T) polymorphism on drug accumulation
HPLC was used to determine the drug concentration in cells following the application of cyclosporin A. After incubation with various concentrations of cyclosporin A for 30 minutes, docetaxel was added for 4 hours. In the existence of the inhibitor, the intake of docetaxel significantly increased in both control and transfected cells, and drug accumulation was positively correlated with the inhibitor concentration (P < 0.05, Figure 4). The intake of docetaxel in control cells was much higher than in stably transfected cells regardless of whether cyclosporin A was added. Moreover, transfection of ABCB1 3435T/mut reduced cellular drug accumulation compared with ABCB1 3435C/wt. These results indicate that ABCB1 3435T increases the drug transport rate mediated by P-gp, and that cyclosporin A reduces this rate in a dose-dependent manner.
Figure 4.
Analysis of docetaxel accumulation in ES-2 and SKOV3 cells. SKOV3/ES-2 cells were transfected with ABCB1 3435 C/wt or ABCB1 3435T/mut plasmids. Drug accumulation was assessed in the presence or absence of cyclosporin A. M0: control; M1: cells transfected with ABCB1 3435 C/wt plasmids; M2: cells transfected with ABCB1 3435T/mut plasmids. *P<0.05, compared with the group without inhibitor; #P<0.05, compared with M0.
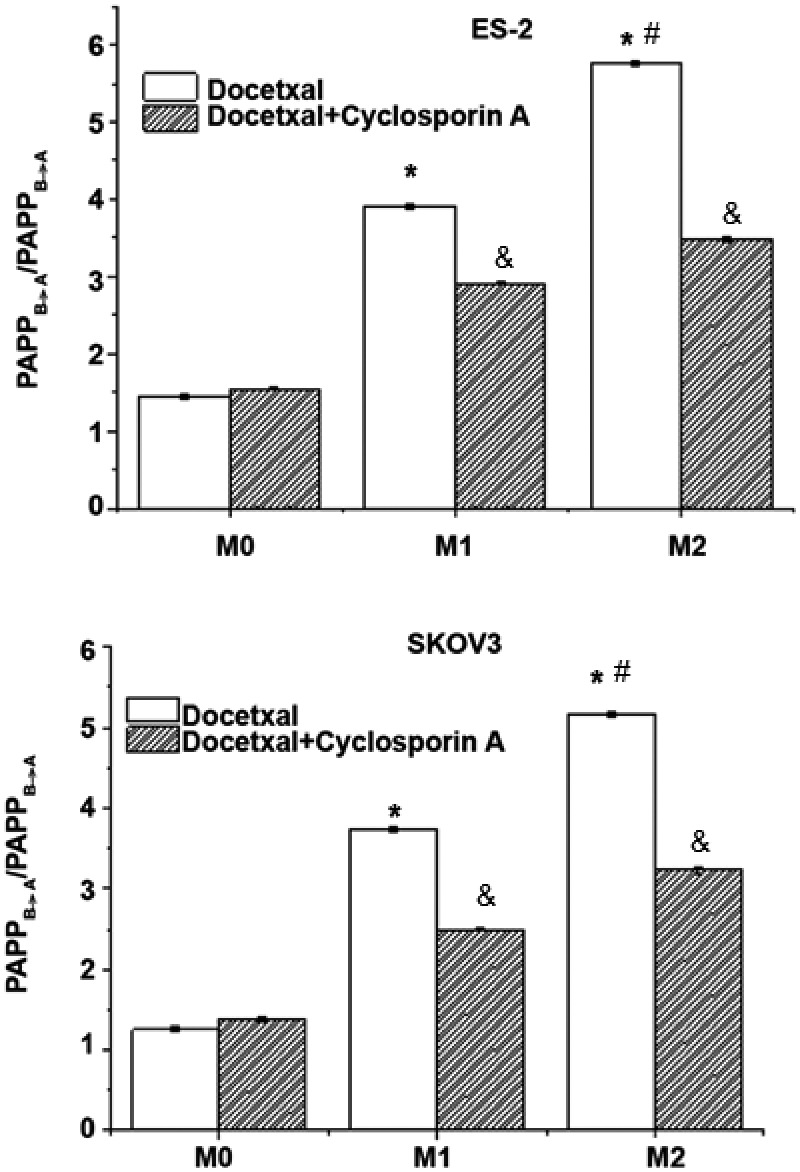
Effect of the ABCB1 (3435C > T) polymorphism on drug transmembrane transport
To investigate the transmembrane transport of docetaxel, a bidirectional transfer test was performed. Cells stably transfected with ABCB1 3435C/wt or ABCB1 3435T/mut showed strong resistance to docetaxel. The permeability ratio of docetaxel in control cells was > 1, indicating the drug transport direction. The transmembrane transport of docetaxel in cells stably transfected with ABCB1 3435C/wt or ABCB1 3435T/mut was significantly increased compared with control cells (P < 0.05), suggesting that the overexpression of P-gp increased drug transport. Additionally, the relative permeability ratio of docetaxel in cells stably transfected with ABCB1 3435T/mut was 1.47-fold higher than in cells stably transfected with ABCB1 3435C/wt (P < 0.05, Figure 5). Under the effect of cyclosporine A, transmembrane permeability in cells stably transfected with ABCB1 3435C/wt or ABCB1 3435T/mut decreased significantly (P < 0.05, Figure 5). These results indicate that ABCB1 (3435C > T) SNPs could promote the transport of P-gp-mediated docetaxel.
Figure 5.
Analysis of docetaxel transmembrane transport in ES-2 and SKOV3 cells. SKOV3/ES-2 cells were transfected with ABCB1 3435 C/wt or ABCB1 3435T/mut plasmids and docetaxel transmembrane transport was detected in the presence or absence of cyclosporin A. M0: control; M1: cells transfected with ABCB1 3435 C/wt plasmids; M2: cells transfected with ABCB1 3435T/mut plasmids. *P<0.05, compared with M0; #P<0.05, compared with M1; &P<0.05, compared with docetaxel.
Effect of the ABCB1 (3435C > T) polymorphism on drug resistance against docetaxel in transplanted tumors
To investigate the effect of ABCB1 (3435C > T) polymorphisms on tumor drug sensitivity, a tumor-bearing model was established to determine drug resistance against docetaxel by measuring tumor weight. ABCB1 (3435C > T) was found to significantly reduce the efficacy of docetaxel. On day 20, the average tumor weight of the control group was 0.4308 g compared with 0.9197 g in the ABCB1 3435 C/wt group and 1.9234 g in the ABCB1 3435T/mut group (P < 0.05, Figure 6). This indicated that ABCB1 (3435C > T) enhances docetaxel resistance in the ovarian cancer cells of tumor-bearing mice.
Figure 6.
The antitumor effect of docetaxel on mice transplanted with SKOV3 cells stably transfected with ABCB1 3435 C/wt or ABCB1 3435T/mut plasmids. The effect of docetaxel on transplanted tumors was evaluated by tumor weight. Gross morphology of tumors is shown in the upper panel and tumor weight is shown in the lower panel. M0: control; M1: cells stably transfected with ABCB1 3435 C/wt plasmids; M2: cells stably transfected with ABCB1 3435T/mut plasmids. *P<0.05, compared with M0.
Discussion
In 1976, Miano et al.19 first identified the ABCB1 transporter P-gp in drug-resistant Chinese hamster ovarian cells. Since then, many studies have shown that almost all human tumor cells express ABCB1 at varying levels. ABCB1 was found to be primarily expressed in colon cancer, rectal cancer, pancreatic cancer, adrenocortical carcinoma, and liver cancer cells, and to exhibit highly inducible expression in acute leukemia, lymphoma, and ovarian cancer.20–22 High ABCB1 expression is usually associated with poor prognosis in cancer patients.23,24 It affects the absorption, distribution, metabolism, and excretion of drugs25–27 such as the anticancer medicines colchicine, quinidine, tacrolimus, etoposide, doxorubicin, paclitaxel, and vinblastine.26 The over-expression of ABCB1 in cancer cells increases drug efflux and reduces drug concentrations, thus reducing the cytotoxicity of the drug against tumor cells and resulting in drug resistance.
ABCB1 C3435T is located in exon 26 and is a synonymous mutation.28,29 It does not cause remarkable changes in protein expression, but attenuates the efflux function of P-gp by altering the protein conformation.30 However, research showed that P-gp expression in the renal cortex and duodenum of individuals harboring ABCB1 3435TT was much lower than in those with wild-type ABCB1, indicating that P-gp protein expression is affected by the ABCB1 C3435T mutation in some tissues.31,32 Additionally, many studies reported a strong linkage disequilibrium between ABCB1 C3435T, G2677T/A, and C1236T, indicating that a specific haplotype synergistically regulates the expression and function of P-gp.33,34 ABCB1 (3435C > T) may also be associated with the treatment response and adverse effects of methotrexate,35 and the metabolism and disposal of substrates by P-gp.29,36
In the present study, two stable cell lines (ES-2 and SKOV3) transfected with ABCB1 3435 C/wt or ABCB1 3435T/mut plasmids were derived to study the effects of the ABCB1 (3435C > T) SNP on the transportation of docetaxel as mediated by P-gp. ABCB1 (3435C > T) plasmid transfection significantly increased the mRNA and protein expression of P-gp. The influence of ABCB1 SNPs on P-gp expression may involve several mechanisms, although these remain controversial.29,31,34,35,37 Hoffmeyer et al.31 first reported that ABCB1 SNPs affected the protein expression of P-gp, showing that ABCB1 (3435C > T) was closely associated with P-gp expression in the human duodenum. It has also been postulated that the effects of c3435T on the P-gp phenotype are mediated not only through changes in amino acid sequences,36,38,39 but also via epigenetic differences. Further studies are therefore warranted to clarify these mechanisms.
We conducted a docetaxel cytotoxicity test in our stable cell lines, and showed that the drug resistance of transfected cells was higher than that of control cells, while cells transfected with ABCB1 3435T/mut demonstrated greater drug resistance than those transfected with ABCB1 3435C/wt. Moreover, drug accumulation was higher in control cells than transfected cells, regardless of cyclosporine A addition, and was higher in the ABCB1 3435C/wt group than the ABCB1 3435T/mut group. This indicated that the ABCB1 3435 T/mut allele has a strong efflux effect on docetaxel.
We also found that changes in P-gp activity affected transmembrane transport. P-gp-mediated transport of docetaxel was promoted to a greater extent in cells carrying 3435T/mut than in ABCB1 3435 C/wt cells, although the transport rate of control cells was always lower than that of transfected cells in the presence or absence of P-gp inhibitor. This agrees with previous findings that ABCB1 mutations affect P-gp activity,29,37 thus altering the clearance of docetaxel. These results together suggest that ABCB1 over-expression is effective in transporting docetaxel out of cells.
Our in vivo experimental results were consistent with the in vitro findings in that drug resistance of mice implanted with transfected cells was stronger than in the control group. Mice implanted with ABCB1 3435T-containing cells showed a stronger drug resistance to docetaxel, probably because of reduced accumulation leading to decreased drug toxicity.
Conclusion
P-gp encoded by the ABCB1 variant allele appears to be more efficient at transporting docetaxel compared with the wild-type allele. Thus, the ABCB1 3435C > T SNP drastically influences P-gp expression and activity and its role in the efflux of docetaxel. Because the extent of drug resistance will affect the therapeutic effect, patients carrying the ABCB1 3435C > T SNP should be administered a combination of multiple drugs to avoid drug resistance.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethics approval and consent to participate
Ethical approval was not required for this study.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Evans WE, McLeod HL. Pharmacogenomics–drug disposition, drug targets, and side effects. N Engl J Med 2003; 348: 538–549. [DOI] [PubMed] [Google Scholar]
- 2.van Waterschoot RA, Lagas JS, Wagenaar E, et al. Absence of both cytochrome P450 3A and P-glycoprotein dramatically increases docetaxel oral bioavailability and risk of intestinal toxicity. Cancer Res 2009; 69: 8996–9002. [DOI] [PubMed] [Google Scholar]
- 3.Sun KX, Jiao JW, Chen S, et al. MicroRNA-186 induces sensitivity of ovarian cancer cells to paclitaxel and cisplatin by targeting ABCB1. J Ovarian Res 2015; 8: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian S, Zhang M, Chen X, et al. MicroRNA-595 sensitizes ovarian cancer cells to cisplatin by targeting ABCB1. Oncotarget 2016; 7: 87091–87099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun S, Cai J, Yang Q, et al. Prognostic value and implication for chemotherapy treatment of ABCB1 in epithelial ovarian cancer: a meta-analysis. PLoS One 2016; 11: e0166058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaidyanathan A, Sawers L, Gannon AL, et al. ABCB1 (MDR1) induction defines a common resistance mechanism in paclitaxel- and olaparib-resistant ovarian cancer cells. Br J Cancer 2016; 115: 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasile E, Tibaldi C, Leon GL, et al. Cytochrome P450 1B1 (CYP1B1) polymorphisms are associated with clinical outcome of docetaxel in non-small cell lung cancer (NSCLC) patients. J Cancer Res Clin Oncol 2015; 141: 1189–1194. [DOI] [PubMed] [Google Scholar]
- 8.Kim KP, Ahn JH, Kim SB, et al. Prospective evaluation of the drug-metabolizing enzyme polymorphisms and toxicity profile of docetaxel in Korean patients with operable lymph node-positive breast cancer receiving adjuvant chemotherapy. Cancer Chemother Pharmacol 2012; 69: 1221–1227. [DOI] [PubMed] [Google Scholar]
- 9.Vaclavikova R, Nordgard SH, Alnaes GI, et al. Single nucleotide polymorphisms in the multidrug resistance gene 1 (ABCB1): effects on its expression and clinicopathological characteristics in breast cancer patients. Pharmacogenet Genomics 2008; 18: 263–273. [DOI] [PubMed] [Google Scholar]
- 10.Lal S, Wong ZW, Sandanaraj E, et al. Influence of ABCB1 and ABCG2 polymorphisms on doxorubicin disposition in Asian breast cancer patients. Cancer Sci 2008; 99: 816–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fransson MN, Brugard J, Aronsson P, et al. Semi-physiologically based pharmacokinetic modeling of paclitaxel metabolism and in silico-based study of the dynamic sensitivities in pathway kinetics. Eur J Pharm Sci 2012; 47: 759–767. [DOI] [PubMed] [Google Scholar]
- 12.Bergmann TK, Brasch-Andersen C, Green H, et al. Impact of CYP2C8*3 on paclitaxel clearance: a population pharmacokinetic and pharmacogenomic study in 93 patients with ovarian cancer. Pharmacogenomics J 2011; 11: 113–120. [DOI] [PubMed] [Google Scholar]
- 13.Rezende VM, Rivellis A, Novaes MM, et al. Quantification of imatinib in human serum: validation of a high-performance liquid chromatography-mass spectrometry method for therapeutic drug monitoring and pharmacokinetic assays. Drug Des Devel Ther 2013; 7: 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang B, Li S, Meng X, et al. Inhibition of mdr1 by G-quadruplex oligonucleotides and reversal of paclitaxel resistance in human ovarian cancer cells. Tumour Biol 2015; 36: 6433–6443. [DOI] [PubMed] [Google Scholar]
- 15.Yang X, Iyer AK, Singh A, et al. MDR1 siRNA loaded hyaluronic acid-based CD44 targeted nanoparticle systems circumvent paclitaxel resistance in ovarian cancer. Sci Rep 2015; 5: 8509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Iyer AK, Singh A, et al. Cluster of differentiation 44 targeted hyaluronic acid based nanoparticles for MDR1 siRNA delivery to overcome drug resistance in ovarian cancer. Pharm Res 2015; 32: 2097–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnatty SE, Beesley J, Gao B, et al. ABCB1 (MDR1) polymorphisms and ovarian cancer progression and survival: a comprehensive analysis from the Ovarian Cancer Association Consortium and The Cancer Genome Atlas. Gynecol Oncol 2013; 131: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu H, Hong FZ, Li S, et al. Short hairpin RNA-mediated MDR1 gene silencing increases apoptosis of human ovarian cancer cell line A2780/Taxol. Chin J Cancer Res 2012; 24: 138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta 1976; 455: 152–162. [DOI] [PubMed] [Google Scholar]
- 20.Spitzwieser M, Pirker C, Koblmuller B, et al. Promoter methylation patterns of ABCB1, ABCC1 and ABCG2 in human cancer cell lines, multidrug-resistant cell models and tumor, tumor-adjacent and tumor-distant tissues from breast cancer patients. Oncotarget 2016; 7: 73347–73369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Genovese I, Ilari A, Assaraf YG, et al. Not only P-glycoprotein: Amplification of the ABCB1-containing chromosome region 7q21 confers multidrug resistance upon cancer cells by coordinated overexpression of an assortment of resistance-related proteins. Drug Resist Updat 2017; 32: 23–46. [DOI] [PubMed] [Google Scholar]
- 22.Vaclavikova R, Klajic J, Brynychova V, et al. Development of high‑resolution melting analysis for ABCB1 promoter methylation: Clinical consequences in breast and ovarian carcinoma. Oncol Rep 2019; 42: 763–774. [DOI] [PubMed] [Google Scholar]
- 23.Raspadori D, Damiani D, Michieli M, et al. CD56 and PGP expression in acute myeloid leukemia: impact on clinical outcome. Haematologica 2002; 87: 1135–1140. [PubMed] [Google Scholar]
- 24.Ren F, Chen R, Wang Y, et al. Paclitaxel-loaded poly(n-butylcyanoacrylate) nanoparticle delivery system to overcome multidrug resistance in ovarian cancer. Pharm Res 2011; 28: 897–906. [DOI] [PubMed] [Google Scholar]
- 25.Duan L, Yan Y, Sun Y, et al. Contribution of TRPV1 and multidrug resistance proteins in the permeation of capsaicin across different intestinal regions. Int J Pharm 2013; 445: 134–140. [DOI] [PubMed] [Google Scholar]
- 26.Wijdeven RH, Pang B, Assaraf YG, et al. Old drugs, novel ways out: Drug resistance toward cytotoxic chemotherapeutics. Drug Resist Updat 2016; 28: 65–81. [DOI] [PubMed] [Google Scholar]
- 27.Sun XY, Duan ZJ, Liu Z, et al. Inhibition of P-glycoprotein, multidrug resistance-associated protein 2 and cytochrome P450 3A4 improves the oral absorption of octreotide in rats with portal hypertension. Exp Ther Med 2016; 12: 3716–3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hemauer SJ, Nanovskaya TN, Abdel-Rahman SZ, et al. Modulation of human placental P-glycoprotein expression and activity by MDR1 gene polymorphisms. Biochem Pharmacol 2010; 79: 921–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimchi-Sarfaty C, Oh JM, Kim IW, et al. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science 2007; 315: 525–528. [DOI] [PubMed] [Google Scholar]
- 30.Komar AA. Silent SNPs: impact on gene function and phenotype. Pharmacogenomics 2007; 8: 1075–1080. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmeyer S, Burk O, von Richter O, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A 2000; 97: 3473–3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siegsmund M, Brinkmann U, Schaffeler E, et al. Association of the P-glycoprotein transporter MDR1(C3435T) polymorphism with the susceptibility to renal epithelial tumors. J Am Soc Nephrol 2002; 13: 1847–1854. [DOI] [PubMed] [Google Scholar]
- 33.Fung KL, Gottesman MM. A synonymous polymorphism in a common MDR1 (ABCB1) haplotype shapes protein function. Biochim Biophys Acta 2009; 1794: 860–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurata Y, Ieiri I, Kimura M, et al. Role of human MDR1 gene polymorphism in bioavailability and interaction of digoxin, a substrate of P-glycoprotein. Clin Pharmacol Ther 2002; 72: 209–219. [DOI] [PubMed] [Google Scholar]
- 35.Kathawala RJ, Wang YJ, Shukla S, et al. ATP-binding cassette subfamily B member 1 (ABCB1) and subfamily C member 10 (ABCC10) are not primary resistance factors for cabazitaxel. Chin J Cancer 2015; 34: 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang D, Johnson AD, Papp AC, et al. Multidrug resistance polypeptide 1 (MDR1, ABCB1) variant 3435C > T affects mRNA stability. Pharmacogenet Genomics 2005; 15: 693–704. [PubMed] [Google Scholar]
- 37.Amin ML. P-glycoprotein inhibition for optimal drug delivery. Drug Target Insights 2013; 7: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kimchi Sarfaty C, Oh JM, Kim IW, et al. Polymorphism in the ABCBI gene changes substrate specificity. Science 2007; 315: 525–525. [DOI] [PubMed] [Google Scholar]
- 39.Scotto KW. Transcriptional regulation of ABC drug transporters. Oncogene 2003; 22: 496–511. [DOI] [PubMed] [Google Scholar]