Abstract
Schaaf-Yang syndrome (SHFYNG) is caused by truncating mutations in the paternal allele of the MAGEL2 gene located in the Prader-Willi syndrome region. We report 5 newborns affected with SHFYNG in one family. Trio exome analysis revealed a heterozygous c.1996dupC frameshift mutation in MAGEL2 inherited from the unaffected father. The phenotypes showed strong resemblance, especially for severe respiratory disturbance requiring mechanical ventilation at birth. After discharge from the hospital, 4 of the patients died of respiratory insufficiency within 1 or 2 weeks after birth, and 1 child died after 110 days of aggravated apnea. Apnea or respiratory failure was the main cause of early death in this family. Respiratory distress is a common manifestation of SHFYNG, especially in patients with c.1996dupC mutations. Hypotonia is a main cause of respiratory disturbance, and we propose another possible cause affecting the respiratory center of the brain.
Keywords: MAGEL2, Respiratory failure, Whole-exome sequencing
Established Facts
Schaaf-Yang syndrome (SHFYNG) is caused by heterogeneous truncating mutations in the paternal allele of the MAGEL2 gene located at 15q11q13, the Prader-Willi syndrome region.
Respiratory distress is a common manifestation of SHFYNG, and patients with c.1996dupC variants show a higher prevalence of respiratory dysfunction compared to those without c.1996dupC variants.
Novel Insights
We report 5 family members affected with SHFYNG who displayed severe respiratory distress at birth and passed away within 4 months of life (7, 15, 110, 7, and 8 days) due to apnea or respiratory failure.
The underlying mechanisms for respiratory disease may include peripheral (muscular) and central (CNS respiratory center) causes, which require further study.
MAGEL2 is an imprinted paternally expressed gene located in the 15q11q13 Prader-Willi syndrome (PWS) critical region. Heterozygous truncating mutations in the paternal allele of MAGEL2 lead to Schaaf-Yang syndrome (SHFYNG; MIM 615547), which is characterized by intellectual disability/development delay, feeding difficulty, hypotonia, contractures, and behavioral abnormalities - an entity with some resemblance to PWS. A de novo nonsense mutation in MAGEL2 was found in 2 patients with a severe arthrogryposis phenotype [Mejlachowicz et al., 2015] and in a patient initially diagnosed with Opitz-C syndrome [Urreizti et al., 2017]. The SHFYNG phenotype can be highly variable. In a severe case, a patient affected with SHFYNG died of respiratory distress at postnatal day 2, and 3 fetuses died in utero diagnosed with arthrogryposis multiplex congenital [Mejlachowicz et al., 2015]. Thus, further investigation of the natural course of SHFYNG and its associated mortality is necessary.
We report 5 newborns affected with SHFYNG in one family with severe respiratory disturbance from at requiring respiratory support; after discharge from the hospital, 4 of them died of respiratory insufficiency after 1 or 2 weeks. One child died at 110 days after birth of aggravated apnea.
Clinical Report and Methods
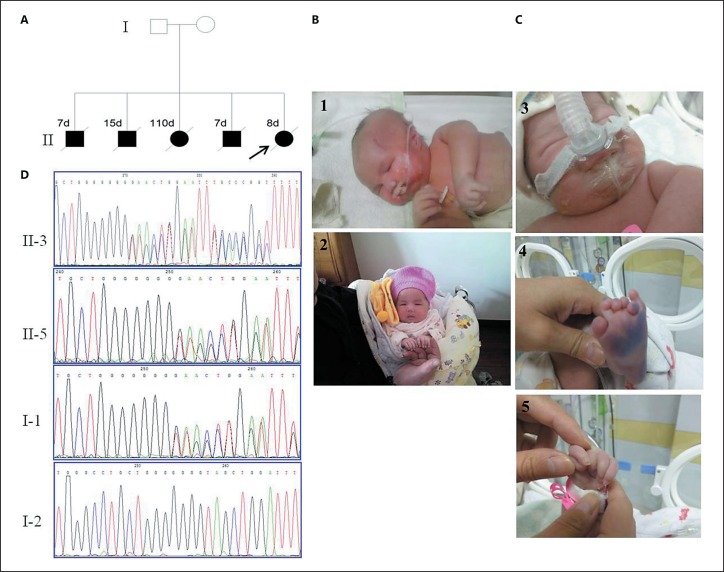
The proband was the fifth child of a nonconsanguineous healthy couple. The family history was striking (Fig. 1A). Four siblings died after birth of respiratory insufficiency (clinical presentation is listed in Table 1). The pregnancy was uneventful, and delivery occurred at 39 weeks' gestation by C-section due to breech presentation. Birth weight was 3,350 g. Neonatal anoxia occurred at birth, and after emergency treatment, she presented with irregular breathing, cyanosis, a poor response, and weak crying. She was admitted to the neonatal intensive care unit for respiratory and feeding support. The physical examination noted frontal bossing, low-set ears, microretrognathia, a high-arched palate, short neck, a wide nipple distance, decreased elbow extension of the upper arms, hypotonia, small hands, camptodactyly of the third and fourth fingers, and bilateral polydactyly. She died at postnatal day 8 after discharge from the hospital of respiratory distress (Fig. 1C).
Fig. 1.
A Pedigree of the family. B Facial features of patient II-3 at 2 days (B1) and 57 days (B2) old. C Clinical features of patient II-5 at 2 days (C3-C5) showing camptodactyly of the third and fourth fingers and polydactyly. D Heterozygous MAGEL2 mutations: Sanger sequencing of patients II-3 and II-5 and her parents, NM_019066.4 c.1996dupC (p.Gln666Profs*47).
Table 1.
The clinical presentation of the affected siblings in this family
| II-1 | II-2 | II-3 | II-4 | II-5 | |
|---|---|---|---|---|---|
| Sex | M | M | F | M | F |
| Gestation age | 36 week, 6 days | 36 weeks | 40 weeks, 4 days | 31 weeks | 39 weeks, 1 day |
| Birth weight, g | 2,600 | 3,290 | 3,450 | 1,790 | 3,350 |
| Respiratory disturbance | Neonatal anoxia, admitted to NICU, after rescue, respiratory and feeding support required | Tachypnea, cyanosis and foaming at the mouth soon after birth, admitted to NICU after rescue, respiratory and feeding support required | Weak crying, poor response, groan and foaming at the mouth soon after birth, admitted to NICU, feeding assistance required during hospital, after discharge, incidental apnea to frequent and aggravated apnea | Tachypnea, cyanosis occurred 50 min after birth, admitted to NICU after rescue, respiratory assistance required | Neonatal anoxia, after rescue, admitted to NICU, respiratory assistance required. |
| Neonatal pneumonia | + | + | + | − | + |
| Feeding difficulty | + | + | + | + | + |
| Hypotonia | + | + | − | + | + |
| Camptodactyly | Bilateral: 2nd–4th finger joint | Left: 3rd–4th finger joint | Left: 3rd–4th finger joint, decreased elbow extension | Left: 3rd–4th finger joint | Left: 3rd–4th finger joint, decreased elbow and knee extension |
| Polydactyly | − | − | − | − | Bilateral polydactyly |
| Hypogonadism | + | + | / | + | / |
| Facial dysmorphism | Robin sequence, low-set ears | Toothed gum, high-arched palate, microretrognathia, frontal bossing, small hands and feet | Micrognathia, high-arched palate, low-set ears | Frontal bossing, low-set ears, high-arched palate, short neck, small hands | |
| Age at death, days | 7 | 15 | 110 | 7 | 8 |
NICU, neonatal intensive care unit.
Whole-exome capture was performed on 3 μg of genomic DNA per individual using the xGen Exome Research Panel (Integrated DNA Technologies, Skokie, IL, USA). The resulting libraries were sequenced on a HiSeq 4000 system (Illumina, San Diego, CA, USA) according to the manufacturer's recommendations for paired-end 150-bp reads. The minimal amount of data was 8 Gb per sample. A standard bioinformatics pipeline applies GATK to call SNVs and small indels. This pipeline filtered out the high-frequency variants based on several population databases (the 1000 Genomes Project, ExAC, GnomAD, EVS, and an in-house database containing 500 exomes). Filtered variants were analyzed following different inheritance patterns to generate 3 candidate gene lists. The imprinted gene list was downloaded from Geneimprint (http://geneimprint.com/site/genes-by-species). Variants in the imprinted gene list were studied specifically based on the imprinted pattern. Selected variants in the family were confirmed by Sanger sequencing. Amplification of the corresponding genomic regions was conducted by a general PCR method. The primer sequences are available upon request.
Candidate variants were confirmed using Sanger sequencing. PCR primer sequences and protocols are available upon request. Amplified fragments were sequenced using a 96-capillary 3730xl system (Applied Biosystems, Foster City, CA, USA).
Results and Discussion
We report 5 siblings affected with SHFYNG with severe respiratory disturbance at birth. Subsequent trio exome analysis revealed a heterozygous frameshift variant in MAGEL2 - NM_019066.4:c.1996dupC (p.Gln666Profs*47) - previously described in other patients with SHFYNG inherited from the unaffected father. Sanger sequencing of PCR products with primers flanking the mutation confirmed the mutation in the 2 affected patients (II-3 and II-5) and showed that it was inherited from the unaffected father (Fig. 1D). c.1996dupC is a recurrent variant that has been found in 35 of 78 reported patients with SHFYNG [Schaaf et al., 2013; Buiting et al., 2014; Soden et al., 2014; Mejlachowicz et al., 2015; Fountain et al., 2017; Urreizti et al., 2017; Bayata et al., 2018; Jobling et al., 2018; Matuszewska et al., 2018; McCarthy et al., 2018a, b; Takuji et al., 2018; Tong et al., 2018].
All family members showed a high phenotypic similarity, and 4 children died within 2 weeks due to respiratory failure; 1 child died day 110 due to apnea. Apnea or respiratory failure was the main cause of early death in this family. To date, 3 individuals with SHFYNG have passed away due to the abovementioned symptoms, including a male patient with the c.1996dupC variant, who died at 9 months of age of an unknown cause of death, although apnea was suspected [Fountain et al., 2017]; another patient who carried a de novo variant in c.2118delT and died 2 days after birth of respiratory distress [Mejlachowicz et al., 2015], and another patient with the c.1996insC variant who died after 2 months due to apnea [Tong et al., 2018]. In a genotype-phenotype association study [McCarthy et al., 2018b], respiratory distress was found to be a common manifestation of SHFYNG; 58% of the patients required intubation during their lifetime, 55% required the use of mechanical ventilation, and 18% required a tracheostomy. Furthermore, patients with c.1996dupC mutations show a higher prevalence of respiratory dysfunction; 75% of the patients in the c.1996dupC cohort required intubation compared to 41% of the individuals with truncating variants other than c.1996dupC, and 71% of patients with a c.1996dupC variant required mechanical ventilation compared to 37% without c.1996dupC variants. These data demonstrate a high prevalence of respiratory distress in SHFYNG, especially in patients with c.1996dupC variants.
A major cause of respiratory disease is hypotonia, which is a common symptom in SHFYNG and PWS. In PWS, 88% of the patients present with hypotonia [Gunay-Aygun et al., 2001], and neonatal hypotonia is a nearly universal finding amongst patients with SHFYNG (97%). The higher prevalence of hypotonia in SHFYNG and PWS is most likely responsible for some complications in these 2 disorders, such as feeding difficulties, respiratory dysfunction, and skeletal abnormalities. In a Magel2-null mouse model, it was found that the loss of MAGEL2 contributed to hypotonia and musculoskeletal abnormalities in PWS and SHFYNG by reducing skeletal muscle strength and endurance in adult mice [Kamaludin et al., 2016]. Magel2 is highly expressed in the murine adult nervous system, strongly expressed in the tongue, and moderately expressed in the abdominal wall muscle, the diaphragm, and the vertebral axis muscle system. Therefore, the loss of Magel2 causes hypotonia in respiratory muscle, which results in decreased respiratory effort and hypoventilation [Kamaludin et al., 2016; McCarthy et al., 2018a].
In a previous report on PWS patients, 12% of the infants with PWS required intubation, and 33% required mechanical ventilation [Bar et al., 2017]. Children and adults with PWS have breathing defects with an irregular rhythm, frequent sleep apnea, and blunted respiratory responses to hypoxia and hypercapnia [Nixon and Brouillette, 2002]. Among several mouse models of PWS, only those with a Necdin deletion or Necdin (Ndn) knock out could reproduce the respiratory phenotype of PWS (central apnea and a blunted response to respiratory challenges). Serotonin contributes to CNS development and affects the maturation and function of the brainstem respiratory network. It has been suggested that Necdin may trigger breathing difficulties in PWS by affecting the serotonin system. Necdin deficiency in neonatal mice alters the serotonergic modulation of the respiratory rhythm generator [Zanella et al., 2008]. We further checked the variant list and found no pathogenic mutations in Ndn. The Magel2 gene is structurally similar to Ndn, and its mRNA is enriched in the mouse brain, especially within the hypothalamus and brainstem. Both Necdin Ndn and Magel2 are type II MAGE members and are adjacent to each other in the PWS region. These data raise the possibility that respiratory disease in patients with MAGEL2 truncating variants originates from dysregulated serotonin metabolism in the CNS respiratory system, which needs further study. However, severe respiratory deficiency in infants lacking MAGEL2 is not recapitulated in the Magel2-null mouse model [Kamaludin et al., 2016], which does not support the abovementioned speculation. Of course, the clinical features in the Magel2-null mouse model are less representative of the low prevalence of their respective phenotypes in human patients with truncating MAGEL2 mutations. It is hypothesized that the deletion of the entire MAGEL2 gene could have a milder effect than a truncating mutation, and the deletion of the complete paternal copy of the gene and promoter could lead to leaky expression of the maternal copy of the MAGEL2 gene, as suggested based on studies of mice lacking the paternal Magel2 copy [Matarazzo et al., 2017]. In addition, a patient with a MAGEL2 deletion was reported to have a milder phenotype than in those with PWS or SHFYNG with point mutations in MAGEL2 and without respiratory disturbance [Buiting et al., 2014]. It is possible that a mouse model with a truncating variant, which is not available so far, could provide more information.
In conclusion, we report 5 siblings with SHFYNG presenting with severe respiratory distress at birth and death within 4 months of life (7, 15, 110, 7, and 8 days) due to apnea or respiratory failure. The underlying mechanisms for respiratory disease may have central (CNS respiratory center) and peripheral (muscular) causes and require further study.
Statement of Ethics
This study was conducted according to institutional policies; written informed consent was obtained from the family for all clinical studies and for publication.
Disclosure Statement
The authors have no conflicts of interest to disclose.
Acknowledgment
The authors thank the patient's family for participating in the present study.
References
- Bar C, Diene G, Molinas C, Bieth E, Casper C, Tauber M. Early diagnosis and care is achieved but should be improved in infants with Prader-Willi syndrome. Orphanet J Rare Dis. 2017;12:118. doi: 10.1186/s13023-017-0673-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayat A, Bayat M, Lozoya R, Schaaf CP. Chronic intestinal pseudo-obstruction syndrome and gastrointestinal malrotation in an infant with Schaaf-Yang syndrome - expanding the phenotypic spectrum. Eur J Med Genet. 2018;61:627–630. doi: 10.1016/j.ejmg.2018.04.007. [DOI] [PubMed] [Google Scholar]
- Buiting K, Di Donato N, Beygo J, Bens S, von der Hagen M, et al. Clinical phenotypes of MAGEL2 mutations and deletions. Orphanet J Rare Dis. 2014;9:40. doi: 10.1186/1750-1172-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain MD, Aten E, Cho MT, Juusola J, Walkiewicz MA, et al. The phenotypic spectrum of Schaaf-Yang syndrome - 18 new affected individuals from 14 families. Genet Med. 2017;19:45–52. doi: 10.1038/gim.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunay-Aygun M, Schwartz S, Heeger S, O'Riordan MA, Cassidy SB. The changing purpose of Prader-Willi syndrome clinical diagnostic criteria and proposed revised criteria. Pediatrics. 2001;108:E92. doi: 10.1542/peds.108.5.e92. [DOI] [PubMed] [Google Scholar]
- Jobling R, Stavropoulos DJ, Marshall CR, Cytrynbaum C, Axford MM, et al. Chitayat-Hall and Schaaf-Yang syndromes: a common aetiology: expanding the phenotype of MAGEL2-related disorders. J Med Genet. 2018;55:316–321. doi: 10.1136/jmedgenet-2017-105222. [DOI] [PubMed] [Google Scholar]
- Kamaludin AA, Smolarchuk C, Bischof JM, Eggert R, Greer JJ, et al. Muscle dysfunction caused by loss of Magel2 in a mouse model of Prader-Willi and Schaaf-Yang syndromes. Hum Mol Genet. 2016;25:3798–3809. doi: 10.1093/hmg/ddw225. [DOI] [PubMed] [Google Scholar]
- Matarazzo V, Caccialupi L, Schaller F, Shvarev Y, Kourdougli N, et al. Necdin shapes serotonergic development and SERT activity modulating breathing in a mouse model for Prader-Willi syndrome. Elife. 2017;6:e32640. doi: 10.7554/eLife.32640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuszewska KE, Badura-Stronka M, Śmigiel R, Cabała M, Biernacka A, et al. Phenotype of two Polish patients with Schaaf-Yang syndrome confirmed by identifying mutation in MAGEL2 gene. Clin Dysmorphol. 2018;27:49–52. doi: 10.1097/MCD.0000000000000212. [DOI] [PubMed] [Google Scholar]
- McCarthy JM, McCann-Crosby BM, Rech ME, Yin J, Chen CA, et al. Hormonal, metabolic and skeletal phenotype of Schaaf-Yang syndrome: a comparison to Prader-Willi syndrome. J Med Genet. 2018a;55:307–315. doi: 10.1136/jmedgenet-2017-105024. [DOI] [PubMed] [Google Scholar]
- McCarthy J, Lupo PJ, Kovar E, Rech M, Bostwick B, Scott D, et al. Schaaf-Yang syndrome overview: report of 78 individuals. Am J Med Genet A. 2018b;176:2564–2574. doi: 10.1002/ajmg.a.40650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejlachowicz D, Nolent F, Maluenda J, Ranjatoelina-Randrianaivo H, Giuliano F, et al. Truncating mutations of MAGEL2, a gene within the Prader-Willi locus, are responsible for severe arthrogryposis. Am J Hum Genet. 2015;97:616–620. doi: 10.1016/j.ajhg.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon GM, Brouillette RT. Sleep and breathing in Prader-Willi syndrome. Pediatr Pulmonol. 2002;34:209–217. doi: 10.1002/ppul.10152. [DOI] [PubMed] [Google Scholar]
- Schaaf CP, Gonzalez-Garay ML, Xia F, Potocki L, Gripp KW, et al. Truncating mutations of MAGEL2 cause Prader-Willi phenotypes and autism. Nat Genet. 2013;45:1405–1408. doi: 10.1038/ng.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soden SE, Saunders CJ, Willig LK, Farrow EG, Smith LD, et al. Effectiveness of exome and genome sequencing guided by acuity of illness for diagnosis of neurodevelopmental disorders. Sci Transl Med. 2014;6:265ra168. doi: 10.1126/scitranslmed.3010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuji E, Nobuhiko O, Yoshinori I, Tomoki M, Mitsuru O, et al. Three patients with Schaaf-Yang syndrome exhibiting arthrogryposis and endocrinological abnormalities. Am J Med Genet A. 2018;176:707–711. doi: 10.1002/ajmg.a.38606. [DOI] [PubMed] [Google Scholar]
- Tong W, Wang Y, Lu Y, Ye T, Song C, et al. Whole-exome sequencing helps the diagnosis and treatment in children with neurodevelopmental delay accompanied unexplained dyspnea. Sci Rep. 2018;8:5214. doi: 10.1038/s41598-018-23503-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urreizti R, Cueto-Gonzalez AM, Franco-Valls H, Mort-Farre S, Roca-Ayats N, et al. A de novo nonsense mutation in MAGEL2 in a patient initially diagnosed as Opitz-C: similarities between Schaaf-Yang and Opitz-C syndromes. Sci Rep. 2017;7:44138. doi: 10.1038/srep44138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanella S, Watrin F, Mebarek S, Marly F, Roussel M, et al. Necdin plays a role in the serotonergic modulation of the mouse respiratory network: implication for Prader-Willi syndrome. J Neurosci. 2008;28:1745–1755. doi: 10.1523/JNEUROSCI.4334-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]