Abstract
Split-hand/foot malformation (SHFM) is a genetic limb anomaly disturbing the central rays of the autopod. SHFM is a genetically heterogeneous disorder with variable expressivity inherited as syndromic and nonsyndromic forms. We provide an update of the clinical and molecular aspects of nonsyndromic SHFM. This rare condition is highly complex due to the clinical variability and irregular genetic inheritance observed in the affected individuals. Nonsyndromic SHFM types have been reviewed in terms of major molecular genetic alterations reported to date. This updated overview will assist researchers, scientists, and clinicians in making an appropriate molecular diagnosis, providing an accurate recurrence risk assessment, and developing a management plan.
Keywords: Ectrodactyly, Limb malformation, Recent classification, SHFM, Skeletal disorder
Ectrodactyly or split-hand/foot malformations (SHFM) is a rare congenital malformation of the limbs, involving mostly the central rays of the autopods, median clefts in hands and feet, syndactyly, and metacarpal, metatarsal and phalangeal aplasia or hypoplasia. The hands and/or feet appear split into 2 halves with aplasia (failure of development) of the phalanx, metacarpal, and/or metatarsal bones of one or more fingers and/or toes as well as hypoplasia (underdevelopment) of the phalanges, metacarpals, and metatarsals (the bones leading up to the toes) [Duijf et al., 2003]. SHFM is inherited as an autosomal dominant, recessive, or X-linked entity, with a prevalence of 1 per 90,000 live births, and its clinical severity varies from patient to patient as well as between the limbs of the same patient (Fig. 1A, B) [Duijf et al., 2003; Elliott and Evans, 2006].
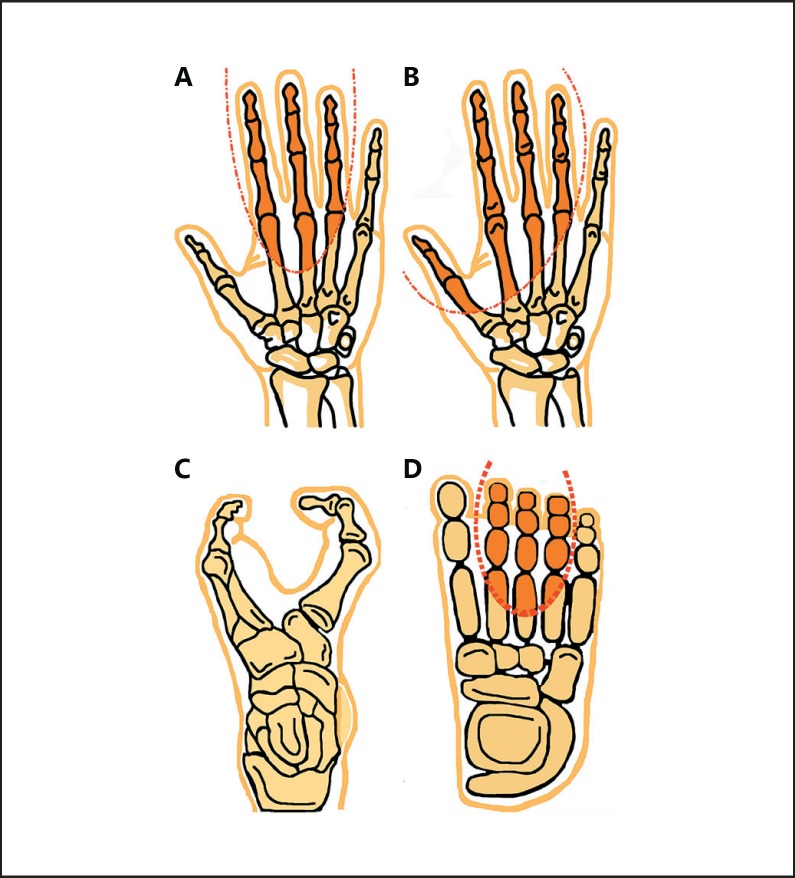
Fig. 1.
Schematic representation of different split-hand/foot malformation (SHFM) types based on the median cleavage. A SHFM showing aplasia of the central rays in hands and B aplasia of both preaxial and central rays, characterized as monodactyly. C Typical SHFM diagram showing a median cleft in the foot. D Diagram showing the area in the foot which may be affected in SHFM.
SHFM Classification
Twelve different types of SHFM have been mapped to different human chromosomes, including SHFM1 located in 7q21 (OMIM 183600) [Scherer et al., 1994], SHFM2 in Xq32 (OMIM 313350) [Faiyaz ul Haque et al., 1993], SHFM3 located in 10q24 (OMIM 246560) [Nunes et al., 1995; Gurrieri et al., 1996], SHFM4 in chromosome 3q27 (OMIM 605289) [Ianakiev et al., 2000], SHFM5 in 2q31 (OMIM 606708) [Boles et al., 1995], SHFM6 in 12q13.11q13 (OMIM 183600) [Ugur and Tolun, 2008], SHFM7 in 2q31.1 (MIM 616890) [Spielmann et al., 2016], SHFM8 in 19p13.11 [Umair et al., 2018] and a locus in chromosome 8q21.11q22.3 [Gurnett et al., 2006]. For these 12 loci, 5 genes including DLX5/DLX6 (MIM 600028, MIM 600030) for SHFM1 [Ullah et al., 2017], TP63 (MIM 603273) for SHFM4 [Ianakiev et al., 2000], WNT10B (MIM 601906) for autosomal recessive SHFM6 [Ugur and Tolun, 2008], ZAK (MIM 609479) for autosomal recessive SHFM7 [Spielmann et al., 2016], and EPS15L1 (MIM 616826) for autosomal recessive SHFM8 [Umair et al., 2018] have been identified (Table 1).
Table 1.
SHFM current classification
| SHFM type | Locus | OMIM | Causative gene/molecular mechanism | Chromosomal localization | Inheritance |
|---|---|---|---|---|---|
| Type 1 | SHFM1 | 183600 | Mutations in DLX5 and DLX6 | 7q21.2q21.3 | AD |
| Isolated SHFM | SHFM1 | 220600 | Homozygous mutation in DLX5 | 7q21.3 | AR |
| SHFM2 | 313350 | Unknown | Xq26 | XL | |
| SHFM3 | 246560 | Microduplications involving BTRC, POLL, and FBXW4 | 10q24 | AD | |
| SHFM4 | 605289 | TP63 mutations | 3q28 | AD | |
| SHFM5 | 606708 | Suspected dysregulation of HOXD cluster | 2q31 | AD | |
| SHFM6 | 225300 | WNT10B mutations | 12q13.12 | AR | |
| SHFM7 | 616890 | ZAK mutations | 2q31.1 | AR | |
| SHFM8 | 616826 | EPS15L1 microdeletions/mutations | 19p13.11 | AR | |
| Unknown | 8q21.11q22.3 | AR | |||
| Type 2 | SHFLD1 | 119100 | Unknown | 1q42.2q43 | AD |
| SHFM with long | SHFLD2 | 610685 | Unknown | 6q14.1 | AD |
| bone deficiency | SHFLD3 | 612576 | Microduplications involving BHLHA9 | 17p13.3 | AD |
AD, autosomal dominant; AR, autosomal recessive; SHFLD, split-hand/foot malformation with long bone deficiency; SHFM, split-hand/foot malformation; XL, X-linked.
Another form of SHFM known as SHFLD (split-hand/foot malformations with long bone deficiency) is dominantly inherited and genetically different from isolated forms of SHFM. To date, 3 types of SHFLD have been mapped to different human chromosomes including SHFLD1 in 1q42.2q43 (OMIM 119100) [Naveed et al., 2006], SHFLD2 in 6q14.1 (OMIM 610685) [Naveed et al., 2007], and SHFLD3 located in 17p13.1p13.3 (OMIM 612576) [Lezirovitz et al., 2008] (Table 1). SHFM has been described in association with other congenital malformations and is associated with more than 50 different syndromes (OMIM).
SHFM1
SHFM1 (MIM 183600) is characterized by deep median clefts, absence of central digital rays, and syndactyly. SHFM1 is dominantly transmitted and mapped for the first time to chromosome 7q21.3q22 [Scherer et al., 1994], harboring translocations, deletions, and inversions in this chromosomal region. The hallmark clinical features associated with SHFM1 include ectrodactyly, split hand/foot, aplasia or/and hypoplasia of single digital ray, triphalangeal thumbs, and lower limbs with road hallux and clinodactyly. The disorder is associated with variable expressivity and incomplete penetrance. It is usually caused by duplication, deletion, or rearrangement involving the DLX5, DSS1, and DLX6 genes as well as possible regulatory elements in the 7q21.3q22 chromosome region [Scherer et al., 1994].
A Yemeni family with 2 individuals affected by SHFM and hearing loss has been reported. The affected individuals had additional features such as a severe short stature, delayed walking, mild synophrys, cylindrical nails, tapered fingers, lower limb hypoplasia, clinodactyly, and asymmetrical short and deformed lower limbs. Molecular analysis using autozygome and whole-exome sequencing (WES) [Shamseldin et al., 2012] identified a homozygous missense mutation (c.533A>C; p.Gln178Pro) in the DLX5 gene that segregated with the disease phenotype. The disorder was termed as SHFM1 with sensorineural hearing loss (MIM 220600).
Ullah et al. [2017], using direct Sanger sequencing, identified a heterozygous missense variant (c.632T>A; p.Val211Glu) in the distal-less homeobox six (DLX6) gene, located in chromosome 7q21, causing SHFM1 in a Pakistani family. The affected individual exhibited features such as aplasia of carpals, metacarpals and phalanges with a classical central ray defect. Additional features included clubbed nails, short radius/ulna, anonychia, dental crowding, and synophrys [Ullah et al., 2017].
SHFM2
SHFM2 (MIM 313350) is clinically characterized by a median cleft in both feet, with bidactyly or monodactyly of both hands, mostly involving all 4 limbs. The mode of inheritance of SHFM2 is X-linked. Ahmad et al. [1987] clinically characterized a Pakistani family with 36 affected individuals in 7 generations (33 males and 3 females). The affected individuals had features such as metacarpal and phalangeal hypoplasia, syndactyly, and bone malformations of the hands. The homozygous females and hemizygous males had typical severe SHFM, with heterozygous females displaying a mild clinical presentation. Later, in the same family using linkage analysis, Faiyaz-Ul-Haque et al. [2005] narrowed down and identified a previously reported 22-Mb genetic interval in chromosome Xq24q26 to a 5.1-Mb region. The candidate gene is still unknown.
SHFM3
SHFM3 (MIM 246560) is transmitted as an autosomal dominant trait and accounts for classical SHFM phenotypes. Typical clinical features of affected individuals reported include dysplastic ears with hearing loss, cleft palate, face with maxillary hypoplasia and micrognathia, renal anomalies, ectrodactyly, clinodactyly, triphalangeal thumbs, preaxial polydactyly as well as ridged and dystrophic nails. In addition, intellectual disability has been observed in some patients [de Molleratet al., 2003].
About 20% of the SHFM3 cases are caused by duplications mapped to chromosome 10q24 (a 325 to 570-kb genomic region) and defined by submicroscopic duplications and complex rearrangements [de Molleratet al., 2003]. Rearrangements in several genes contribute to SHFM3 phenotypes such as FGF8, LBX1, BTRC, and DACTYLIN. The SHFM3 locus at 10q24 shows conservation of the syntenic region with the Dac region in mice (chromosome 19). The Dac mice exhibit the ectrodactyly phenotype with only certain genetic backgrounds [Chai, 1981; Kano et al., 2007].
SHFM4
SHFM4 (MIM 605289) displays complex phenotypes of hands and feet such as aplasia of the phalangeal, metacarpal and metatarsal bones, with or without syndactyly and webbing [Ianakiev et al., 2000]. Any pathogenic mutations in the TP63 gene (MIM 603273) cause SHFM4. The TP63 gene encodes a p63 protein, involved in the differentiation and regulation of the apical ectoderm ridge (AER) and ectodermal development [van Bokhoven et al., 2001; Berdón-Zapata et al., 2004]. The clinical presentation of affected individuals includes monodactyly, missing phalanges, metacarpals/metatarsals, thumb duplication, and syndactyly. To date, more than 120 mutations have been reported in the TP63 gene associated with several disorders such as limb-mammary syndrome, SHFM, cleft lip, ADULT syndrome, EEC syndrome, kidney disorders, AEC syndrome, and Rapp-Hodgkin syndrome (Table 2).
Table 2.
Clinical phenotypes associated with SHFM types
| No. | SHFM type | Gene/locus | Inheritance | Phenotype |
|---|---|---|---|---|
| 1a | SHFM1 | DLX5, DLX6 | AD | Ectrodactyly, split hand, aplasia of single digital ray, hypoplasia, triphalangeal thumbs, lower limbs with broad hallux and clinodactyly |
| 1b | SHFM1 with sensorineural hearing loss | DLX5 | AR | Short stature, mild scoliosis, sensorineural hearing loss, split hand/foot, cylindrical nails |
| 2 | SHFM2 | Xq26 | XL | Split hand/foot, monodactylous median cleft anomaly, partial syndactyly, metacarpal and phalangeal hypoplasia |
| 3 | SHFM3 | 10q24 | AD | Maxillary hypoplasia and micrognathia, dysplastic ears with hearing loss, cleft palate, renal anomalies, ectrodactyly, clinodactyly, ridged and dystrophic nails, and intellectual disability observed in some patients |
| 4 | SHFM4 | TP63 | AD | Split hand/foot, missing phalanges, monodactyly, triphalangeal thumb, syndactyly, and missing metacarpals and metatarsals |
| 5 | SHFM5 | 2q31 | AD | Monodactyly, penoscrotal hypoplasia, growth retardation, hypertelorism, cleft palate, microcephaly, microphthalmia, split hand malformation |
| 6 | SHFM6 | WNT10B | AR | Ectrodactyly (split hand/foot), with additional variable phenotype such as complex syndactyly and polydactyly also reported |
| 7 | SHFM7 with mesoaxial polydactyly | ZAK | AR | Split foot malformation, normal hands, hearing impairment, cutaneous syndactyly, and duplication of finger nail bed of fourth digit |
| 8 | SHFM8 | EPS15L1 | AR | Mild–severe split foot, missing metacarpals and metatarsals, complex preaxial syndactyly, underdeveloped digits and missing nail |
| 9 | SHFLD1 | 1q42.2q43 | AD | Cleft hand, absent tibia, absent middle finger, tetramonodactyly, transverse hemimelia, hypoplastic big toes, bifurcation of the femurs, cup-shaped ears and ulnar aplasia/hypoplasia |
| 10 | SHFLD2 | 6q14.1 | AD | Mild–severe skeletal defects involving upper and lower limbs, split hand/foot, syndactyly of fingers/toes, hypoplastic big toes, absence of middle phalanges, hypoplastic tibiae, beaked nose, and no cleft lip/palate or ectodermal dysplasia observed |
| 11 | SHFLD3 | 17p13.3p13.1 (BHLHA9 duplication) | AD | Ectrodactyly, oligodactyly, brachydactyly, syndactyly, camptodactyly, pes varus, club foot, tibial aplasia/hypoplasia, femoral bifurcation observed in some patients |
AD, autosomal dominant; AR, autosomal recessive; SHFLD, split-hand/foot malformation with long bone deficiency; SHFM, split-hand/foot malformation; XL, X-linked.
SHFM5
SHFM5 (MIM 606708) has been mapped to chromosome 2q31 [Boles et al., 1995], with the DLX1 and DLX2 genes closely related to this genomic region, but no mutations have been reported yet. It is inherited as an autosomal dominant disorder. Defective development of AER and ectrodactyly in mouse hind limbs were reported in the Dlx5 and Dlx6 double-knockout mouse [Restelli et al., 2014]. Goodman et al. [2002] suggested that as DLX1 and DLX2 genes are expressed in AER, they may be new candidate genes for SHFM5. Affected individuals with chromosomal rearrangements at the SHFM5 locus presented with features such as monodactyly or zeugopod, penoscrotal hypoplasia, and several other anomalies such as short stature, microphthalmia, hypospadias, microcephaly, and cleft palate (Table 2).
SHFM6
WNT10B (MIM 601906) has been reported as a causative gene for SHFM6 (MIM 225300). It has an autosomal recessive inheritance pattern and is reported in several consanguineous families [Ugur and Tolun, 2008; Khan et al., 2012; Aziz et al., 2014; Ullah et al., 2018]. The WNT10B gene is a WNT gene family member consisting of 18 other genes. WNT10B encodes a 389 amino acid protein. WNT proteins act as ligands in a variety of signaling pathways and play a major role in limb development and morphogenesis [Yang, 2003]. These proteins bind the low-density lipoprotein receptor and cell surface frizzled related proteins, which activate a conserved “canonical” signaling pathway [Peifer and Polakis, 2000] (Fig. 2). Proteins such as WNT6, WNT10a, and WNT10b involved in Wnt signaling are important for the maintenance and development of many tissues and organs including bones [Cadigan and Nusse, 1997]. During the development of the limb bud, the Wnt signaling pathway influences various mechanisms such as limb morphogenesis and patterning [Cawthorn et al., 2012].
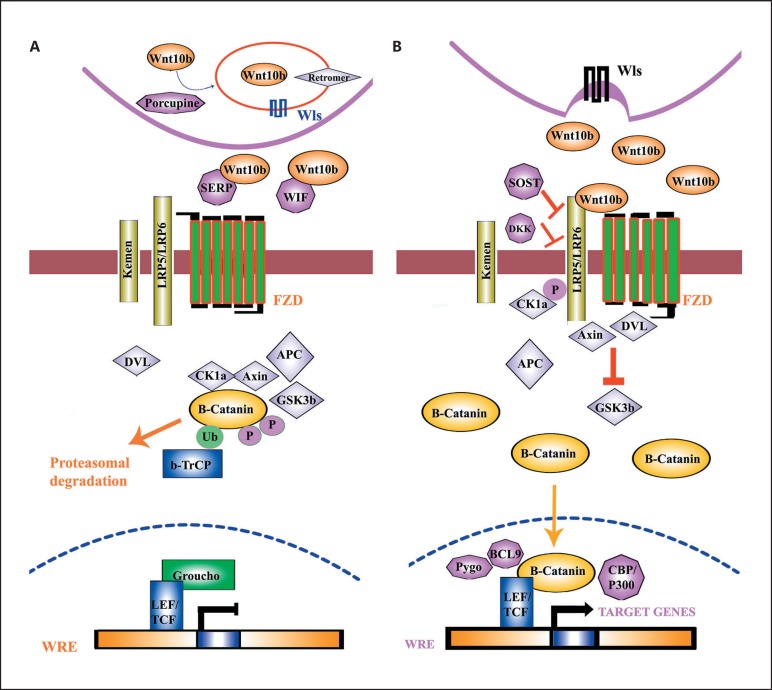
Fig. 2.
Canonical Wnt/β-catenin pathway. A Absent Wnt signal. B Present Wnt signal.
The disease-causing mutation in the WNT10B gene was first described by Ugur and Tolun [2008], identifying a homozygous missense mutation (Arg332Trp) in a Turkish consanguineous family. Later, a Swiss patient with a homozygous 4-bp duplication in WNT10B exhibited typical SHFM features [Blattner et al., 2010]. A novel homozygous mutation (Arg332Trp) in the WNT10B gene was detected by Khan et al. [2012] in a consanguineous Pakistani family, and Aziz et al. [2014] reported 2 Pakistani families with a homozygous 4-bp deletion (c.1165_1168delAAGT) and a homozygous 7-bp duplication (c.300_306dupAGGGCGG). Recently, Ullah et al. [2018] reported a recurrent duplication and a nonsense mutation in 4 Pakistani families segregating autosomal recessive SHFM6. To date, only 20 homozygous mutations have been reported in the WNT10B gene, with 8 being associated with SHFM (HGMD, 2018; Table 3). Affected individuals with a mutation in WNT10B present with, e.g., oligodontia, dental anomalies, and SHFM. Affected individuals with SHFM phenotypes include additional features such as polydactyly, complex cutaneous syndactyly, hypoplasia, aplasia of radial ray of the hands, and fixed flexion contractures (Table 2).
Table 3.
Mutations known to date in different genes responsible for nonsyndromic SHFM
| Gene mutation | Edna position | Protein position | Effect | Phenotype | ||||
|---|---|---|---|---|---|---|---|---|
| DLX5 | ||||||||
| Nonsense | c.115G>T | p.Glu39* | Stop codon | SHFM | ||||
| Missense | c.505G>A | p. Glu169Lys | Substitution | Hypogonadotropic hypogonadism | ||||
| Missense | c.533A>C | p.Gln178Pro | Substitution | SHFM | ||||
| Missense | c.558G>T | p. Gln186His | Substitution | SHFM | ||||
| Missense | c.576C>G | p.Ile192Met | Substitution | Pierre Robin sequence | ||||
| Missense | c.593A>C | p. Asn198Thr | Substitution | Hypogonadotropic hypogonadism | ||||
| Frame shift | c.482_485dupACCT | − | AS and PTC | SHFM | ||||
| Gross deletions | ~1 Mb incl. entire gene and DLX6 | − | AS and PTC | SHFM | ||||
| Gross deletions | ~8.478 Mb incl. entire gene, DLX6 and >50 others | − | AS and PTC | SHFM | ||||
| Gross deletions | 0.9 − 1.8 Mb incl. entire gene, DLX6 and DSS1 | − | AS and PTC | SHFM | ||||
| Gross insertion | 719 kb incl. entire gene and DLX6 | − | AS and PTC | SHFM | ||||
| WNT10B | ||||||||
| Missense | c.265G>A | p.Asp89Asn | Substitution | Dental anomalies | ||||
| Missense | c.475G>C | p.Ala159Pro | Substitution | Dental anomalies | ||||
| Missense | c.569C>G | p.Pro190Arg | Substitution | Oligodontia | ||||
| Missense | c.632G>A | p.Arg211Gln | Substitution | Oligodontia | ||||
| Missense | c.661C>T | p.Arg221Trp | Substitution | SHFM | ||||
| Missense | c.767G>A | p.Cys256Tyr | Substitution | Obesity | ||||
| Nonsense | c.786G>A | p.Trp262T* | Substitution | Oligodontia | ||||
| Missense | c.849C>A | p.Ile283Ile | Substitution | Oligodontia | ||||
| Missense | c.851T>G | p.Phe284Cys | Substitution | Oligodontia | ||||
| Missense | c.986C>G | p. Thr329Arg | Substitution | SHFM | ||||
| Missense | c.986C>A | p. Thr329Lys | Substitution | SHFM | ||||
| Missense | c.994C>T | p. Arg332Trp | Substitution | SHFM | ||||
| Missense | c.1052G>A | p. Arg351His | Substitution | Dental anomalies | ||||
| Missense | c.1087C>T | p. Arg363Cys | Substitution | Dental anomalies | ||||
| Splice site | c.338 − 1G>C | − | Substitution | SHFM | ||||
| Frame shift | c.695_697delACA | p. Asn232del | Small deletions | SHFM | ||||
| Frame shift | c.293_299dupAGGGCGG | − | Small deletions | SHFM | ||||
| Frame shift | c.458_461dupAGCA | − | Small deletions | SHFM | ||||
| TP63 | ||||||||
| Missense | c.191A>G | p. Gln25Arg | Substitution | Heterotaxy | ||||
| Missense | c.289C>T | p. Arg58Cys | Substitution | SHFM | ||||
| Missense | c.343G>T | p. Gly76Trp | Substitution | Limb-mammary syndrome | ||||
| Missense | c.386C>T | p. Ser90Leu | Substitution | Cleft lip | ||||
| Missense | c.386C>G | p. Ser90Trp | Substitution | Limb-mammary syndrome | ||||
| Missense | c.448G>A | p. Ala111Thr | Substitution | Cleft palate | ||||
| Missense | c.497C>T | p. c.497C>T | Substitution | ADULT syndrome | ||||
| Missense | c.518G>A | p. Gly134Asp | Substitution | Limb-mammary syndrome | ||||
| Missense | c.518G>T | p. Gly134Val | Substitution | ADULT syndrome | ||||
| Missense | c.598A>G | p. Lys161Glu | Substitution | SHFM | ||||
| Missense | c.602T>C | p. Leu162Pro | Substitution | EEC syndrome | ||||
| Missense | c.605A>G | p. Tyr163Cys | Substitution | EEC syndrome | ||||
| Missense | c.691T>G | p. Tyr192Asp | Substitution | EEC syndrome | ||||
| Missense | c.692A>G | p. Tyr192Cys | Substitution | EEC syndrome | ||||
| Missense | c.697A>G | p.Lys194Glu | Substitution | SHFM | ||||
| Missense | c.721G>A | p. Val202Met | Substitution | EEC syndrome | ||||
| Missense | c.728G>A | p. Arg204Gln | Substitution | EEC syndrome | ||||
| Missense | c.728G>T | p. Arg204Leu | Substitution | EEC syndrome | ||||
| Missense | c.727C>T | p. Arg204Trp | Substitution | EEC syndrome | ||||
| Missense | c.740A>G | p. His208Arg | Substitution | EEC syndrome | ||||
| Missense | c.739C>G | p. His208Asp | Substitution | EEC syndrome | ||||
| Missense | c.739C>T | p. His208Tyr | Substitution | EEC syndrome | ||||
| Missense | c.797G>A | p. Arg227Gln | Substitution | EEC syndrome | ||||
| Missense | c.797G>C | p. Arg227Pro | Substitution | EEC syndrome | ||||
| Missense | c.799G>A | p. Val228Ile | Substitution | Congenital anomalies of the kidney and urinary track | ||||
| Missense | c.923G>A | p. Cys269Tyr | Substitution | EEC syndrome | ||||
| Missense | c.929G>C | p. Ser271Thr | Substitution | EEC syndrome | ||||
| Missense | c.932G>A | p. Ser272Asn | Substitution | EEC syndrome | ||||
| Missense | c.932G>C | p. Ser272Thr | Substitution | EEC syndrome | ||||
| Missense | c.935G>A | p. Cys273Tyr | Substitution | EEC syndrome | ||||
| Missense | c.946A>T | p. Met277Leu | Substitution | Skeletal abnormality | ||||
| Missense | c.952C>T | p. Arg279Cys | Substitution | EEC syndrome | ||||
| Missense | c.953G>A | p. Arg279His | Substitution | EEC syndrome | ||||
| Missense | c.952C>A | p. Arg279Ser | Substitution | EEC syndrome | ||||
| Missense | c.955C>T | p. Arg280Cys | Substitution | SHFM | ||||
| Missense | c.956G>A | p.Arg280His | Substitution | SHFM | ||||
| Missense | c.956G>T | p. Arg280Leu | Substitution | SHFM | ||||
| Missense | c.955C>A | p.Arg280Ser | Substitution | EEC syndrome | ||||
| Missense | c.1010G>A | p. Arg298Gln | Substitution | ADULT syndrome | ||||
| Missense | c.1009C>G | p. Arg298Gly | Substitution | ADULT syndrome | ||||
| Missense | c.1028G>A | p.Arg304Gln | Substitution | EEC syndrome | ||||
| Missense | c.1028G>C | p. Arg304Pro | Substitution | EEC syndrome | ||||
| Missense | c.1027C>T | p. Arg304Trp | Substitution | EEC syndrome | ||||
| Missense | c.1033T>C | p. Cys306Arg | Substitution | EEC syndrome | ||||
| Missense | c.1034G>A | p. Cys306Tyr | Substitution | EEC syndrome | ||||
| Missense | c.1037C>A | p. Ala307Asp | Substitution | EEC syndrome | ||||
| Missense | c.1037C>G | p. Ala307Gly | Substitution | EEC syndrome | ||||
| Missense | c.1039T>A | p. Cys308Ser | Substitution | EEC syndrome | ||||
| Missense | c.1040G>A | p. Cys308Tyr | Substitution | AEC syndrome | ||||
| Missense | c.1042C>T | p. Pro309Ser | Substitution | EEC syndrome | ||||
| Missense | c.1046G>A | p. Gly310Glu | Substitution | SHFM | ||||
| Missense | c.1048A>G | p. Arg311Gly | Substitution | EEC syndrome | ||||
| Missense | c.1051G>A | p. Asp312Asn | Substitution | EEC syndrome | ||||
| Missense | c.1053C>A | p. Asp312Glu | Substitution | EEC syndrome | ||||
| Missense | c.1052A>G | p. Asp312Gly | Substitution | EEC syndrome | ||||
| Missense | c.1051G>C | p. Asp312His | Substitution | EEC syndrome | ||||
| Missense | c.1054A>G | p. Arg313Gly | Substitution | Cleft lip | ||||
| Missense | c.1061C>A | p. Ala315Glu | Substitution | EEC syndrome | ||||
| Missense | c.1063G>C | p. Asp316His | Substitution | AEC syndrome | ||||
| Missense | c.1646T>C | p. Ile510Thr | Substitution | Rapp-Hodgkin syndrome | ||||
| Missense | c.1655T>C | p. Phe513Ser | Substitution | AEC syndrome | ||||
| Missense | c.1654T>G | p. Phe513Val | Substitution | Rapp-Hodgkin syndrome | ||||
| Missense | c.1659A>T | p. Leu514Phe | Substitution | AEC syndrome | ||||
| Missense | c.1658T>C | p. Leu514Ser | Substitution | AEC syndrome | ||||
| Missense | c.1657T>G | p. Leu514Val | Substitution | AEC syndrome | ||||
| Missense | c.1670G>T | p. Gly518Val | Substitution | AEC syndrome | ||||
| Missense | c.1672T>C | p. Cys519Arg | Substitution | AEC syndrome | ||||
| Missense | c.1681T>C | p. Cys522Arg | Substitution | AEC syndrome | ||||
| Missense | c.1681T>G | p. Cys522Gly | Substitution | AEC syndrome | ||||
| Missense | c.1683T>G | p. Cys522Trp | Substitution | AEC syndrome | ||||
| Missense | c.1685T>C | p. Leu523Pro | Substitution | AEC syndrome | ||||
| Missense | c.1695C>A | p. Phe526Leu | Substitution | AEC syndrome | ||||
| Missense | c.1706G>T | p. Gly530Val | Substitution | AEC syndrome | ||||
| Missense | c.1709T>C | p. Leu531Pro | Substitution | Ankyloblepharon filiforme adnatum associated with Hay-Wells syndrome | ||||
| Missense | c.1714A>C | p. Thr533Pro | Substitution | AEC syndrome | ||||
| Missense | c.1724A>T | p. Gln536Leu | Substitution | AEC syndrome | ||||
| Missense | c.1727T>C | p. Ile537Thr | Substitution | AEC syndrome | ||||
| Missense | c.1739C>T | p. Ser541Phe | Substitution | AEC syndrome | ||||
| Missense | c.1739C>A | p. Ser541Tyr | Substitution | Rapp-Hodgkin syndrome | ||||
| Missense | c.1747G>T | p. Asp544Tyr | Substitution | AEC syndrome | ||||
| Missense | c.1751T>C | p. Leu545Pro | Substitution | AEC syndrome | ||||
| Missense | c.1766T>A | p. Ile550Asn | Substitution | EEC syndrome | ||||
| Missense | c.1769C>A | p.Pro551His | Substitution | AEC syndrome | ||||
| Missense | c.1769C>T | p. Pro551Leu | Substitution | AEC syndrome | ||||
| Missense | c.1781G>C | p. Arg555Pro | Substitution | AEC syndrome | ||||
| Missense | c.1790T>C | p. Ile558Thr | Substitution | AEC syndrome | ||||
| Missense | c.1799G>A | p. Gly561Asp | Substitution | Rapp-Hodgkin syndrome | ||||
| Missense | c.1799G>T | p. Gly561Val | Substitution | AEC syndrome | ||||
| Missense | c.1805T>C | p. Leu563Pro | Substitution | EEC syndrome | ||||
| Missense | c.1807G>C | p. Asp564His | Substitution | Cleft lip | ||||
| Missense | c.1904G>T | p. Gly596Val | Substitution | Ectodermal dysplasia | ||||
| Missense | c.1910G>T | p. Arg598Leu | Substitution | AEC syndrome | ||||
| Missense | c.1919A>T | p. Asp601Val | Substitution | AEC syndrome | ||||
| Nonsense | c.1974G>A | p. Trp619* | PTC | SHFM | ||||
| Nonsense | c.2011A>T | p. Lys632* | PTC | Limb-mammary syndrome | ||||
| Nonsense | c.2017C>T | Gln634* | PTC | SHFM | ||||
| Nonsense | c.2032G>T | p. Glu639* | PTC | SHFM | ||||
| Splice site | c.63 − 1G>C | − | Intronic | Prostate carcinoma | ||||
| Splice site | c.580 − 2A>C | − | Intronic | SHFM | ||||
| Splice site | c.580 − 2A>G | − | Intronic | EEC syndrome | ||||
| Splice site | c.1350 − 2A>G | − | Intronic | EEC syndrome | ||||
| Splice site | c.1747G>T | − | Intronic | AEC syndrome | ||||
| Regulatory sequence | c.*374G>A | − | Stop loss | Bladder cancer | ||||
| Regulatory sequence | c.*2345C>T | − | Stop loss | Bladder cancer | ||||
| Regulatory sequence | c.62 + 6895C>T | − | Stop loss | Lung adenocarcinoma | ||||
| Regulatory sequence | c.*20609A>G | − | Stop loss | Bladder cancer | ||||
| Regulatory sequence | c.62 + 33817C>T | − | Stop loss | Lung adenocarcinoma | ||||
| Small deletion | c.970_972delATT | p.Ile324del | FS | EECUT plus syndrome | ||||
| Small deletion | c.1338_1341delACTT | p.Leu446Phefs*20 | FS and PTC | Orofacial clefting | ||||
| Small deletion | c.1693_1694delTT | p.Phe565Hisfs*12 | FS and PTC | EEC syndrome | ||||
| Small deletion | c.1815delG | p.Gln606Serfs*98 | FS and PTC | Rapp-Hodgkin syndrome | ||||
| Small deletion | c.1827delA | p.Glu609Aspfs*95 | FS and PTC | Rapp-Hodgkin syndrome | ||||
| Small deletion | c.1838delC | p.Pro613Leufs*91 | FS and PTC | Rapp-Hodgkin syndrome | ||||
| Small deletion | c.1859delC | p.Pro620Glnfs*84 | FS and PTC | AEC syndrome | ||||
| Small deletion | c.1860_1861delAA | p.Ser621Glnfs*11 | FS and PTC | EEC syndrome | ||||
| Small deletion | c.1900delC | p.Arg634Glyfs*70 | FS and PTC | Rapp-Hodgkin syndrome | ||||
| Small deletion | c.1904delG | p.Gly635Valfs*69 | FS and PTC | Rapp-Hodgkin syndrome | ||||
| Small deletion | c.1963delC | p.Arg655Glufs*49 | FS and PTC | AEC syndrome | ||||
| Small deletion | c.1976delA | p.Asn659Metfs*45 | FS and PTC | Rapp-Hodgkin syndrome | ||||
| Small insertions | c.819_820dupCC | − | FS and PTC | Cleft lip and palate | ||||
| Small insertions | c.1572dupA | − | FS and PTC | Rapp-Hodgkin syndrome | ||||
| Small insertions | c.1689_1690insA | − | FS and PTC | EEC syndrome | ||||
| Small insertions | c.1718_1720dupTCT | − | FS and PTC | AEC syndrome | ||||
| Small insertions | c.1833_1843dup11 | − | FS and PTC | AEC/Rapp-Hodgkin syndrome | ||||
| Small indel | c.953_954delGCinsAA | − | FS and PTC | EEC syndrome | ||||
| Gross deletion | >19,1059 bp incl. exons 1 − 4 | − | FS and PTC | EEC syndrome | ||||
| DLX6 | ||||||||
| Missense | c.632T>A | p.Val211Glu | Substitution | SHFM | ||||
| ZAK | ||||||||
| Missense | c.1103T>G | p.Phe368Cys | Substitution | SHFM | ||||
| Gross deletion | exons 12 − 16del | − | Small protein | SHFM | ||||
| EPS15L1 | ||||||||
| Small deletion | c.409delA | p.Ser137Alafs*19 | FS and PTC | SHFM | ||||
FS, frame shift; PTC, premature termination codon; SHFM, split-hand/foot malformation.
WNT signaling plays a key role in the vertebrate limb development [Yang, 2003]. WNT forms a family of 19 highly conserved cysteine-rich signaling molecules, which plays an important role in osteoblastogenesis and bone formation [Pandur et al., 2002; Logan and Nusse, 2004]. The WNT name is derived from the first 2 members of the family: int-1 (mouse) and wingless (Drosophila) [Wodarz and Nusse, 1998].
The key functional role of the WNT pathway has been exclusively studied in the developing limb bud, which controls limb developmental processes such as dorsoventral limb identity, limb patterning, and limb morphogenesis [Galceran et al., 1999]. WNT signaling during late limb morphogenesis regulates the morphology and position of the limb development such as skeletal elements, tendons, and muscles [Yang, 2003]. Bone formation, through regulating chondrogenic differentiation as well as osteoblast proliferation, has also been associated with WNT signaling [Rudnicki and Brown, 1997; Hartmann and Tabin, 2001].
WNTs are secreted glycoproteins involved in the determination of cell fate and growth as well as acting as ligands in different pathways. Among these, canonical Wnt/β-catenin is the best understood. WNT10B plays an important role in the β-catenin/canonical Wnt pathway. In the process of WNT10B glycoproteins production, several players are involved [Miller et al., 1999]. The WNT10B protein is inactivated in the intercellular portion of the secreted frizzled-related protein or the Wnt inhibitory factor. Thus, WNT10B signals are absent, and the β-catenin is captured by the degradation complex containing the axin/conductin, glycogen synthase kinase 3b (GSK3b), and adenomatous polyposis coli (APC) as well as casein kinase 1a (CK1a). β-catenin is ubiquitinated, phosphorylated, and then degraded with the assistance of proteasome (Fig. 2A). Furthermore, in the nucleus, the Groucho (a transcriptional inhibitor) binds to lymphoid enhancer factor/T-cell factor transcription factors at the Wnt-responsive element and prevents transcription of Wnt target genes (Fig. 2A) [Miller et al., 1999].
Similarly, when the WNT10B molecules are secreted by the Wntless cells, it initiates the canonical Wnt signaling pathway. WNT10B binds and activates the low-density lipoprotein-related receptor protein and the Frizzled receptors. The degradation complex and the GSK3b are inactivated by the interface of axin with phosphorylated DVL and LRP5/6. The accumulation of β-catenin takes place in the cytoplasm, and it is translocated into the nucleus, where it forms a heterometric complex and activates Wnt target genes transcription (Fig. 2B). The interaction of WNT10B with other proteins has been illustrated (Fig. 3), demonstrating a strong interaction with other key players involved in skeletal development.
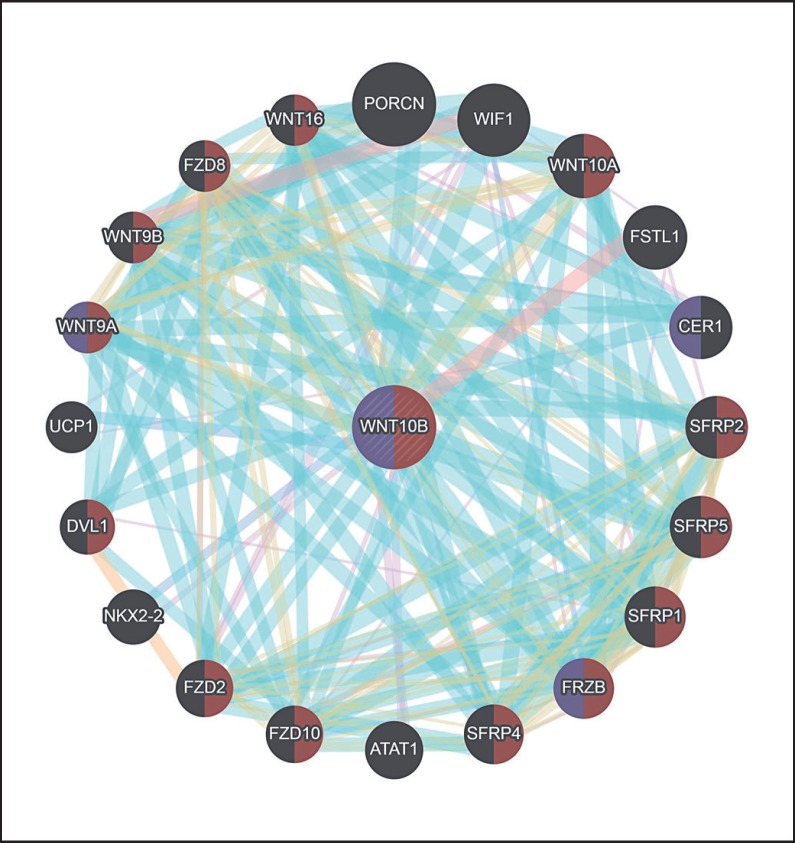
Fig. 3.
Schematic representation of WNT10B interaction with other key players involved in limb development in humans. Purple-shaded proteins indicate those involved in skeletal development, and brown-shaded proteins show the players involved in the Wnt signaling pathway (https://genemania.org/).
SHFM7/SFMMP
Split-foot malformation with mesoaxial polydactyly (SFMMP; 616890) was reported for the first time by Spielmann et al. [2016] in a Pakistani consanguineous family with 3 affected individuals and a Tunisian boy with unilateral/bilateral cutaneous syndactyly and SHFM. All affected individuals reported in the Pakistani family also had a bilateral sensorineural hearing impairment, but the Tunisian boy had normal hearing and normal psychomotor development. The affected individuals presented clinical features such as duplication of finger nail bed of the 4th digit, absent 3rd toe, and cutaneous syndactyly of the 1st and 2nd as well as the 4th and 5th toes. Interfamilial phenotypic variability was observed among the affected individuals of the same family.
Using SNP array, genotyping, and WES, Spielmann et al. [2016] identified homozygosity for a missense mutation in the ZAK gene in a Pakistani family that segregated with the disease phenotype, but it was not found in 180 Pakistani controls. In addition, a homozygous intragenic deletion in the ZAK gene was observed in the boy from Tunisia. Spielmann et al. [2016] showed that Zak is expressed in developing mice limbs and CRISPR/Cas-mediated knockout of the 2 Zak isoforms is embryonically lethal in mice. CRISPR/Cas-mediated knockout showed that ZAK is a key player in mammalian limb patterning and development [Spielmann et al., 2016].
SHFM8
Recently, Umair et al. [2018] presented the first direct evidence of involvement of the EPS15L1 gene (MIM 616826) causing mild to severe SHFM phenotypes in a consanguineous Pakistani family. The family had 2 affected individuals exhibiting SHFM features such as cleft hand deformity, agenesis at the metacarpal joint, dysplastic middle and distal phalanx of the lesser toe as well as preaxial and postaxial syndactyly. Whole-genome SNP array and WES identified a frameshift deletion (c.409delA) in exon 7 of the EPS15L1 gene that led to the formation of a premature stop codon (p.Ser137Alafs*19), which may have resulted in nonsense-mediated mRNA decay [Umair et al., 2018].
EPS15L1 is composed of 3 domains. Domain I consists of approximately 300 amino acids and an EF-hand-type calcium-binding domain. Domain II has heptad repeats of the coiled-coil domain, and Domain III exhibits a proline-rich region, consisting of a repeated aspartic acid-proline phenylalanine motif [Umair et al., 2018]. EPS15L1 chiefly functions as a substrate for tyrosine kinase activity of the epidermal growth factor receptor (EGFR) which is generally allied with limb morphogenesis [Seiler et al., 2015]. The EGFR signaling pathway is primarily involved in survival, growth, differentiation, and proliferation and is associated with limb development via AER. Both environmental factors and genetic defects may cause SHFM phenotypes by interfering with AER [Hsueh et al., 2015]. Any pathogenic mutation in the EPS15L1 gene may change the EPS15L1 protein dosage, reducing the substrate concentration. The reduced substrate may lead to a decreased tyrosine kinase activity of the EGFR.
Recently, it has been found that EPS15L1 displays a unique nonredundant role in the nervous system. In addition, in Eps15/Eps15l1 double-knockout mice, it has been shown to play a fundamental role during embryo development. All the developing embryos showed severe developmental delay, fused somites, a reduced midbrain-hindbrain boundary, and the absence of the limb bud [Milesi et al., 2019].
SHFLD
SHFLD known as split-hand/foot malformation with long-bone deficiency is dominantly inherited and genetically distinct from isolated forms of SHFM. SHFLD is mostly involved the deformity of the tibia and fibula and is associated with the duplication of the 17p13.3 locus. Currently, 3 types of SHFLD have been mapped to different human chromosomes including SHFLD1 located at chromosome 1q42.2q43 (OMIM 119 100) [Naveed et al., 2006], SHFLD2 at 6q14.1 (OMIM 610685) [Naveed et al., 2007], and SHFLD3 located in chromosomal region 17p13.1p13.3 (OMIM 612576) [Lezirovitz, et al., 2008].
Lezirovitz et al. [2008] mapped SHFLD for the first time in a large Brazilian family to an 841-kb interval at 17p13.1p13.3 (15). Later, Klopocki et al. [2012] revealed a defect in tandem duplication and narrowed down the region to only the single BHLHA9 gene. The Bhlha9 mouse and zebra fish expression pattern was restricted to the AER of the limb bud mesenchyme. Further, bhlha9 knockdown in zebrafish embryos revealed shortening of the pectoral fins and suggested an important role in limb development [Klopocki et al., 2012].
Diagnostic Aspects and Genetic Counseling
Patients presenting with SHFM features should be carefully diagnosed, clinically examined, and submitted to relevant cytogenetic and/or molecular testing. As described above, at least 12 SHFM types have been described in the literature [Umair et al., 2018]. Sporadic cases are mostly caused by de novo mutations exhibiting isolated SHFM features.
Considering the pathophysiology of variable expression, reduced penetrance, non-mendelian inheritance, and segregation falsification [Klopocki et al., 2012], genetic counseling, correct molecular diagnosis, and prenatal testing in SHFM cases are difficult and extremely challenging. Furthermore, variability of the phenotype between affected individuals of the same family makes it very difficult to diagnose the exact molecular etiology.
In the majority of isolated single cases with SHFM, conventional karyotyping can identify large chromosomal aberrations and thus reveal the disease phenotype. [Duijf et al., 2003; Elliott and Evans, 2006].
Direct Sanger sequencing of the TP63, DLX5, and DLX6 genes can solve most of the dominant cases [Ullah et al., 2017]. The TP63 mutations show highly variable expressivity and complete penetrance [Faiyaz-Ul-Haque et al., 2005]. Similarly, direct Sanger sequencing would be a suitable choice for families exhibiting rare autosomal recessive inheritance such as SHFM6 (WNT10B), SHFM7 (ZAK), and SHFM 8 (EPS15L1). Should Sanger sequencing fail to identify the disease-causing variant, whole-genome SNP array or WES could be used to identify the gene responsible for the disease.
Conclusion
The clinical and genetic heterogeneity of SHFM contributes to extremely challenging and difficult genetic counseling. Genetic alteration and appropriate molecular diagnosis responsible for SHFM is important for the entire family. Firstly, it would help the family to understand the genetic nature of the disease and develope proper risk management strategies for the disease. Secondly, molecular diagnosis would facilitate conscious family planning and support prenatal or preimplantation diagnosis. Finally, excluding all known disease-causing alterations, molecular diagnostic testing using next-generation sequencing provides an opportunity to solve unresolved cases (whole-exome or whole-genome sequencing), contributing to the identification of novel disease-causing candidate genes associated with SHFM.
References
- Ahmad M, Abbas H, Haque S, Flatz G. X-chromosomally inherited split-hand/split-foot anomaly in a Pakistani kindred. Hum Genet. 1987;75:169–173. doi: 10.1007/BF00591081. [DOI] [PubMed] [Google Scholar]
- Aziz A, Khan S, Zimri FK, Muhammad N, et al. Novel homozygous mutations in the WNT10B gene underlying autosomal recessive split hand/foot malformation in three consanguineous families. Gene. 2014;534:265–271. doi: 10.1016/j.gene.2013.10.047. [DOI] [PubMed] [Google Scholar]
- Berdón-Zapata V, Granillo-Alvarez M, Valdés-Flores M, García-Ortiz JE, Kofman-Alfaro S, Zenteno JC. p63 gene analysis in Mexican patients with syndromic and non-syndromic ectrodactyly. J Orthop Res. 2004;22:1–5. doi: 10.1016/S0736-0266(03)00166-9. [DOI] [PubMed] [Google Scholar]
- Blattner A, Huber AR, Röthlisberger B. Homozygous nonsense mutation in WNT10B and sporadic split-hand/foot malformation (SHFM) with autosomal recessive inheritance. Am J Med Genet A. 2010;152A:2053–2056. doi: 10.1002/ajmg.a.33504. [DOI] [PubMed] [Google Scholar]
- Boles RG, Pober BR, Gibson LH, Willis CR, McGrath J, et al. Deletion of chromosome 2q24-q31 causes characteristic digital anomalies: case report and review. Am J Med Genet. 1995;55:155–160. doi: 10.1002/ajmg.1320550204. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Cawthorn WP, Bree AJ, Yao Y, Du B, Hemati N, et al. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a β-catenin-dependent mechanism. Bone. 2012;50:477–489. doi: 10.1016/j.bone.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai CK. Dactylaplasia in mice a two-locus model for development anomalies. J Hered. 1981;72:234–237. doi: 10.1093/oxfordjournals.jhered.a109486. [DOI] [PubMed] [Google Scholar]
- de Mollerat XJ, Gurrieri F, Morgan CT, Sangiorgi E, Everman DB, et al. A genomic rearrangement resulting in a tandem duplication is associated with split handsplit foot malformation 3 (SHFM3) at 10q24. Hum Mol Genet. 2003;12:1959–1971. doi: 10.1093/hmg/ddg212. [DOI] [PubMed] [Google Scholar]
- Duijf P, van Bokhoven H, Brunner HG. Pathogenesis of split-hand/split-foot malformation. Hum Mol Genet. 2003;12:R51–R60. doi: 10.1093/hmg/ddg090. [DOI] [PubMed] [Google Scholar]
- Elliott AM, Evans JA. Genotype-phenotype correlations in mapped split hand foot malformation (SHFM) patients. Am J Med Genet A. 2006;140:1419–1427. doi: 10.1002/ajmg.a.31244. [DOI] [PubMed] [Google Scholar]
- Faiyaz ul Haque M, Uhlhaas S, Knapp M, Schüler H, Friedl W, et al. Mapping of the gene for X-chromosomal split-hand/split-foot anomaly to Xq26-q26.1. Hum Genet. 1993;91:17–19. doi: 10.1007/BF00230215. [DOI] [PubMed] [Google Scholar]
- Faiyaz-Ul-Haque M, Zaidi SH, King LM, Haque S, Patel M, et al. Fine mapping of the X-linked split-hand/split-foot malformation (SHFM2) locus to a 5.1-Mb region on Xq26.3 and analysis of candidate genes. Clin Genet. 2005;67:93–97. doi: 10.1111/j.1399-0004.2004.00369.x. [DOI] [PubMed] [Google Scholar]
- Galceran J, Fariñas I, Depew MJ, Clevers H, Grosschedl R. Wnt3a−/−− like phenotype and limb deficiency in Lef1−/− Tcf1−/− mice. Genes Dev. 1999;13:709–717. doi: 10.1101/gad.13.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman FR, Majewski F, Collins AL, Scambler PJ. A 117-kb microdeletion removing HOXD9-HOXD13 and EVX2 causes synpolydactyly. Am J Hum Genet. 2002;70:547–555. doi: 10.1086/338921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurnett CA, Dobbs MB, Nordsieck EJ, Keppel C, Goldfarb CA, et al. Evidence for an additional locus for split hand/foot malformation in chromosome region 8q21.11-q22.3. Am J Med Genet A. 2006;140:1744–1748. doi: 10.1002/ajmg.a.31375. [DOI] [PubMed] [Google Scholar]
- Gurrieri F, Prinos P, Tackels D, Kilpatrick MW, Allanson J, et al. A split hand-split foot (SHFM3) gene is located at 10q24-25. Am J Med Genet. 1996;62:427–436. doi: 10.1002/(SICI)1096-8628(19960424)62:4<427::AID-AJMG16>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Hartmann C, Tabin CJ. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell. 2001;104:341–351. doi: 10.1016/s0092-8674(01)00222-7. [DOI] [PubMed] [Google Scholar]
- Hsueh YL, Su YN, Lin HY, Lee CN, Shih JC. Array comparative genomic hybridization characterization of multiple interstitial deletions involving 7p22.1, 7q11.23, 7q21.3-q22.1, 19p13.3-p12, and 19q13.11-q13.43 in a fetus associated with split hand-foot malformation. Role of EPS15L1 in pathogenesis. Taiwan J Obstet Gynecol. 2015;54:455–458. doi: 10.1016/j.tjog.2014.12.009. [DOI] [PubMed] [Google Scholar]
- Ianakiev P, Kilpatrick MW, Toudjarska I, Basel D, Beighton P, Tsipouras P. Split-hand/split-foot malformation is caused by mutations in the p63 gene on 3q27. Am J Hum Genet. 2000;67:59–66. doi: 10.1086/302972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano H, Kurahashi H, Toda T. Genetically regulated epigenetic transcriptional activation of retrotransposon insertion confers mouse dactylaplasia phenotype. Proc Natl Acad Sci USA. 2007;104:19034–19039. doi: 10.1073/pnas.0705483104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Basit S, Zimri FK, Ali N, Ali G, et al. A novel homozygous missense mutation in WNT10B in familial split-hand/foot malformation. Clin Genet. 2012;82:48–55. doi: 10.1111/j.1399-0004.2011.01698.x. [DOI] [PubMed] [Google Scholar]
- Klopocki E, Lohan S, Doelken SC, Stricker S, Ockeloen CW, et al. Duplications of BHLHA9 are associated with ectrodactyly and tibia hemimelia inherited in non-Mendelian fashion. J Med Genet. 2012;49:119–125. doi: 10.1136/jmedgenet-2011-100409. [DOI] [PubMed] [Google Scholar]
- Lezirovitz K, Maestrelli , SR, Cotrim NH, Otto PA, Pearson PL, Mingroni-Netto RC. A novel locus for split-hand/foot malformation associated with tibial hemimelia (SHFLD syndrome) maps to chromosome region 17p13.1-17p13.3. Hum Genet. 2008;123:625–631. doi: 10.1007/s00439-008-0515-7. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Milesi C, Alberici P, Pozzi B, Oldani A, Beznoussenko GV, et al. Redundant and nonredundant organismal functions of EPS15 and EPS15L1. Life Sci Alliance. 2019;2:e201800273. doi: 10.26508/lsa.201800273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller , JR, Hocking AM, Brown JD, Moon RT. Mechanism and function of signal transduction by the Wnt/β-catenin and Wnt/Ca2+ pathways. Oncogene. 1999;18:7860–7872. doi: 10.1038/sj.onc.1203245. [DOI] [PubMed] [Google Scholar]
- Naveed M, Al-Ali MT, Murthy SK, Al-Hajali S, Al-Khaja N, et al. Ectrodactyly with aplasia of long bones (OMIM; 119100) in a large inbred Arab family with an apparent autosomal dominant inheritance and reduced penetrance: clinical and genetic analysis. Am J Med Genet A. 2006;140:1440–1446. doi: 10.1002/ajmg.a.31239. [DOI] [PubMed] [Google Scholar]
- Naveed M, Nath SK, Gaines M, Al-Ali MT, Al-Khaja N, et al. Genomewide linkage scan for split-hand/foot malformation with long-bone deficiency in a large Arab family identifies two novel susceptibility loci on chromosomes 1q42.2-q43 and 6q14.1. Am J Hum Genet. 2007;80:105–111. doi: 10.1086/510724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes ME, Schutt G, Kapur RP, Luthardt F, Kukolich M, et al. A second autosomal split hand/split foot locus maps to chromosome 10q24-q25. Hum Mol Genet. 1995;4:2165–2170. doi: 10.1093/hmg/4.11.2165. [DOI] [PubMed] [Google Scholar]
- Pandur P, Maurus D, Kühl M. Increasingly complex: new players enter the Wnt signaling network. BioEssays. 2002;24:881–884. doi: 10.1002/bies.10164. [DOI] [PubMed] [Google Scholar]
- Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis.- a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- Restelli M, Lopardo T, Lo Iacono N, Garaffo G, Conte D, et al. DLX5FGF8 and the Pin1 isomerase control ΔNp63α protein stability during limb development: a regulatory loop at the basis of the SHFM and EEC congenital malformations. Hum Mol Genet. 2014;23:3830–3842. doi: 10.1093/hmg/ddu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki JA, Brown AM. Inhibition of chondrogenesis by Wnt gene expression in vivo and in vitro. Dev Biol. 1997;185:104–118. doi: 10.1006/dbio.1997.8536. [DOI] [PubMed] [Google Scholar]
- Scherer SW, Poorkaj P, Allen T, Kim J, Geshuri D, et al. Fine mapping of the autosomal dominant split hand/split foot locus on chromosome 7 band q21.3-q22.1. Am J Hum Genet. 1994;55:12–20. [PMC free article] [PubMed] [Google Scholar]
- Seiler C, Gebhart N, Zhang Y, Shinton SA, Li YS, et al. Mutagenesis screen identifies agtpbp1 and eps15L1 as essential for T lymphocyte development in Zebrafish. PLoS One. 2015;10:e0131908. doi: 10.1371/journal.pone.0131908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamseldin HE, Faden MA, Alashram W, Alkuraya FS. Identification of a novel DLX5 mutation in a family with autosomal recessive split hand and foot malformation. J Med Genet. 2012;49:16–20. doi: 10.1136/jmedgenet-2011-100556. [DOI] [PubMed] [Google Scholar]
- Spielmann M, Kakar N, Tayebi N, Leettola C, Nürnberg G, et al. Exome sequencing and CRISPR/Cas genome editing identify mutations of ZAK as a cause of limb defects in humans and mice. Genome Res. 2016;26:183–191. doi: 10.1101/gr.199430.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugur SA, Tolun A. Homozygous WNT10b mutation and complex inheritance in split-hand/foot malformation. Hum Mol Genet. 2008;17:2644–2653. doi: 10.1093/hmg/ddn164. [DOI] [PubMed] [Google Scholar]
- Ullah A, Hammid A, Umair M, Ahmad W. A novel heterozygous intragenic sequence variant in DLX6 probably underlies first case of autosomal dominant split-hand/foot malformation type 1. Mol Syndromol. 2017;8:79–84. doi: 10.1159/000453350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah A, Gul A, Umair M, Ahmad F, et al. Homozygous sequence variants in the WNT10B gene underlie split hand/foot malformation. Genet Mol Biol. 2018;41:1–8. doi: 10.1590/1678-4685-GMB-2016-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umair M, Ullah A, Ahmad F, Shah K, Abbas S, Ahmad W. First direct evidence of involvement of a homozygous loss-of-function variant in the EPS15L1 gene underlying split-hand/split-foot malformation. Clin Genet. 2018;93:699–702. doi: 10.1111/cge.13152. [DOI] [PubMed] [Google Scholar]
- van Bokhoven H, Hamel BC, Bamshad M, Sangiorgi E, Gurrieri F, et al. p63 gene mutations in EEC syndrome, limb-mammary syndrome, and isolated split hand-split foot malformation suggest a genotype-phenotype correlation. Am J Hum Genet. 2001;69:481–492. doi: 10.1086/323123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signal transduction. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Yang Y. Wnts and wing: Wnt signaling in vertebrate limb development and musculoskeletal morphogenesis. Birth Defects Res C Embryo Today. 2003;69:305–317. doi: 10.1002/bdrc.10026. [DOI] [PubMed] [Google Scholar]