Abstract
Purpose
We detail a case of cobalt toxicity with visual and systemic complications, review the pathogenic process for the optic neuropathy and retinopathy, and discuss the controversy of metallic hip prosthesis.
Observations
A 59-year-old female with a history of multiple left hip arthroplasties presented to our clinic with bilateral visual loss. The year prior, she had failure of the hip implant necessitating revision surgery with placement of a chrome-cobalt head. A few months after surgery, she began experiencing blurred and “white, spotty” vision in both eyes in addition to hypothyroidism, cardiomyopathy and neuropathy. The possibility of the patient's symptoms being due to cobalt toxicity from her hip prosthesis was proposed and she was found to have a serum cobalt level >1000 μg/L (normal 0–0.9 ng/mL). Visual acuity was 20/600 in the right and 20/800 in the left eye. There was bilateral temporal optic disc pallor. Goldmann visual field testing demonstrated bilateral central scotomas, optical coherence tomography (OCT) showed severe ganglion cell layer-inner plexiform layer (GCLIPL) thinning and multifocal electroretinography (mfERG) demonstrated decreased amplitudes in both eyes. She underwent a total hip revision arthroplasty with extensive debridement of “black sludge” found within a pseudocapsule. Four days after surgery, cobalt serum levels had significantly decreased to 378 ng/mL. One month after surgery, she had significant improvement in visual acuity (20/150 right eye, 20/250 left eye), Goldmann visual field testing, and mfERG. OCT showed retinal nerve fiber thinning and persistent GCLIPL thinning in both eyes.
Conclusions and Importance
Excessive cobalt levels can result in systemic toxicity leading to visual changes, peripheral neuropathy, hearing loss, cognitive deficits, cardiomyopathy and hypothyroidism. In recent years it has become apparent that cobalt toxicity can be associated with metal-on-metal total hip arthroplasty, or the grinding effects of retained ceramic particles from a fractured ceramic head on a cobalt-chromium femoral head prosthesis.
Keywords: Cobalt toxicity, Optic neuropathy, Retinopathy, Metal on metal hip prosthesis, Ceramic hip prosthesis
1. Introduction
Cobalt, an essential trace element with an atomic number of 27, is a key component of vitamin B12 (i.e. cobalamin) that is involved in the production and regulation of red blood cells, platelet, DNA as well as fatty acid synthesis and energy production.1,2 Normal daily dietary intake ranges from 5 to 50 μg with normal plasma concentrations of <0.2 μg/L.3 However, excessive cobalt can produce systemic toxicity affecting multiple organ systems. Although not firmly established, it is believed that adverse effects related to cobalt toxicity typically only occur at levels of 7 μg/L or more.2,4
In the past, cobalt toxicity was predominantly seen in the form of pulmonary disease in industrial workers who inhaled large amounts of cobalt dust while drilling hard metal or polishing diamonds.5 More recently, systemic cobalt toxicity has been associated with metal-on-metal (MoM) total hip arthroplasty or the grating down of the cobalt-chromium ball (i.e. femoral head) due to retained ceramic particles from a failed ceramic femoral head prosthesis, causing the release of cobalt and chromium metal ions into the systemic circulation.5,6 Post-arthroplasty metallosis with systemic cobalt toxicity is a rare complication, but the morbidity and mortality related to this complication is extremely high.1,2 Systemic toxicity associated with excessive levels of cobalt include peripheral neuropathy, vision loss, sensorineural hearing loss, cognitive decline, cardiomyopathy, hypothyroidism, weakness, fatigue and polycythemia (Table 1).1,2,7, 8, 9, 10
Table 1.
Systemic manifestations of cobalt toxicity.
| Organ System | Clinical Manifestations |
|---|---|
| Cardiovascular System | Cardiomyopathy |
| Electrocardiographic changes | |
| Hypertension | |
| Tachycardia | |
| Peripheral and Central Nervous System | Sensorineural hearing loss |
| Tinnitus | |
| Vertigo | |
| Poor concentration | |
| Memory loss | |
| Disorientation | |
| Depression | |
| Irritability | |
| Fatigue | |
| Anxiety | |
| Tremor | |
| Incoordination | |
| Headaches | |
| Muscle weakness | |
| Peripheral neuropathy | |
| Gait disturbance | |
| Endocrine System | Hypothyroidism |
| Chronic Thyroiditis | |
| Hematological System |
Polycythemia |
| Respiratory System | Asthma |
| Hypersensitivity pneumonitis | |
| Interstitial pneumonia | |
| Dermatological System | Contact dermatitis |
| Acne | |
| Skin rashes |
Specifically, ocular cobalt toxicity, although uncommon, has seen an increase in the number of published cases due to rising use of cobalt-chromium hip implantations.6,8,9,11, 12, 13, 14, 15, 16, 17, 18 Four main types of materials are employed for total hip arthroplasty: MoM, metal-on-polyethylene (MoP), ceramic-on-ceramic (CoC), and ceramic-on-polyethylene (CoP).19 MoM hip implants were initially introduced in 1950 but lost popularity due to concern for metallosis. In the 1970s, MoP implants became widespread. However, due to polyethylene wear particles playing a role in osteolysis and loosening, alternative materials including CoC and CoP were developed. According to a review published in 2018, the most frequently materials used for total hip arthroplasty were cobalt-chromium alloys due to favorable strength and wear characteristics.19
We present a case of systemic cobalt toxicity in the setting of multiple total hip arthroplasty revision surgeries resulting in both an optic neuropathy and retinopathy in addition to hearing loss, thyroid dysfunction and cardiac conduction defect. We discuss the underlying pathomechanism of the visual loss from cobalt toxicity and review the debate of metallic hip prosthesis.
2. Case report
A 59-year-old female presented to our institution for evaluation of bilateral visual loss. Her medical history was notable for deep vein thrombosis and provoked pulmonary embolism after breast reduction surgery in October 2016.
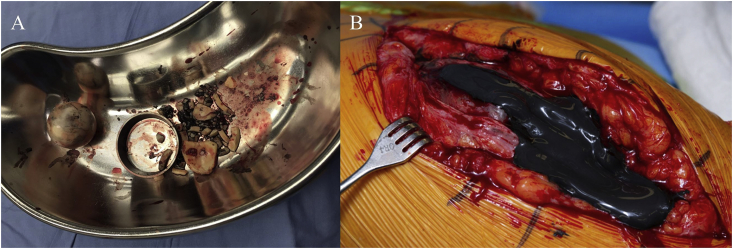
In 2010, she developed left hip pain and underwent hip arthroscopy at an outside institution. Due to the continued hip pain, in March 2011 she underwent a CoC total left hip arthroplasty. Starting in 2013, she noticed a “squeaking” sound in the left hip. By March 2017, while walking, she suddenly heard a “pop” in the left hip and felt the sensation of “walking on shattered glass.” She was diagnosed with a catastrophic failure of the hip implant due to ceramic femoral head fracture. Twelve days later, she underwent revision surgery during which she was noted to have extensive debris in the joint from the ceramic particles and a black metallic sludge, attributed to metallic debris produced from the friction between the titanium components of the implant and the fractured ceramic fragments (Fig. 1A). Extensive debridement was followed by placement of a cobalt-chrome femoral head prosthesis and a highly cross-linked polyethylene acetabular insert.
Fig. 1.
A. Ceramic fragments pigmented dark from the “black sludge” following March 2017 catastrophic failure of hip implant. B. Extensive black discoloration of subcutaneous tissue and black sludge material under the fascia was noted during the August 2018 hip revision surgery.
In the summer of 2017, she began to experience fatigue and hair loss. By December 2017, an audiology evaluation confirmed bilateral high-frequency hearing loss. One month later, she was diagnosed with hypothyroidism and sinus tachycardia. During this time, she also experienced severe headaches, fatigue, as well as numbness and paresthesias in the lower extremities. In May 2018, she was evaluated by a local optometrist for “white, spotty” vision in both eyes. Visual acuity was 20/20 in each eye with a normal fundus. One month later, she presented to a local emergency department for “more spots” in the vision, worsening hearing loss and tachycardia. Visual acuity was 20/250 in the right eye and 20/400 in the left eye. Paraneoplastic serology panel, lumbar puncture, magnetic resonance (MR) imaging and MR angiography of the brain with contrast were all negative or normal. Despite treatment with intravenous corticosteroids and plasma exchange for a possible autoimmune/inflammatory optic neuropathy, the vision in both eyes continued to worsen.
The possibility of cobalt toxicity from her hip prosthesis was suggested by the patient's daughter who coincidentally was watching a Netflix documentary entitled the “Bleeding Edge” and noted the similarities between her mother's symptoms and the patient, who had developed cobalt toxicity after a total hip arthroplasty.20 On August 2018, serum cobalt level was found to be > 1000 μg/L and chelation therapy with N-Acetylcysteine (NAC) was initiated. She was referred to our institution for urgent surgical consultation and consideration of left hip revision surgery. Repeat cobalt level was 953 μg/mL (normal 0–0.9 μg/mL), chromium 36.9 μg/mL (normal <0.3 μg/mL), and titanium 12 μg/mL (normal 0–1 μg/mL).
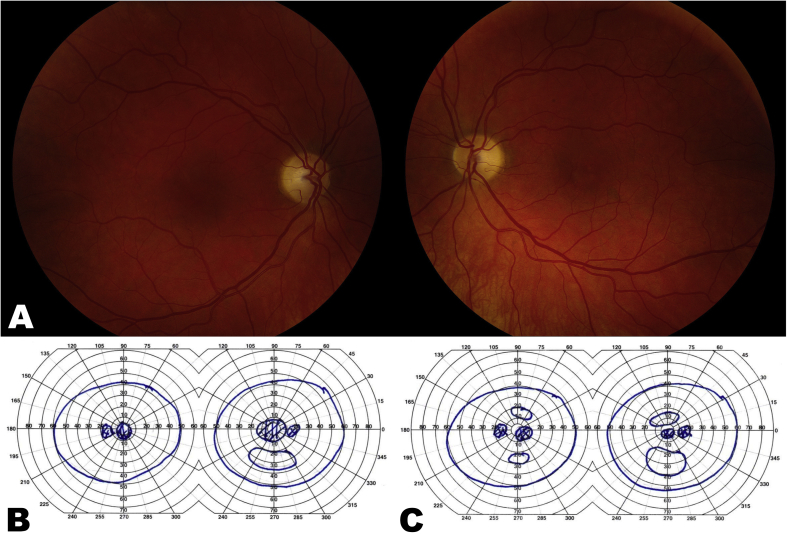
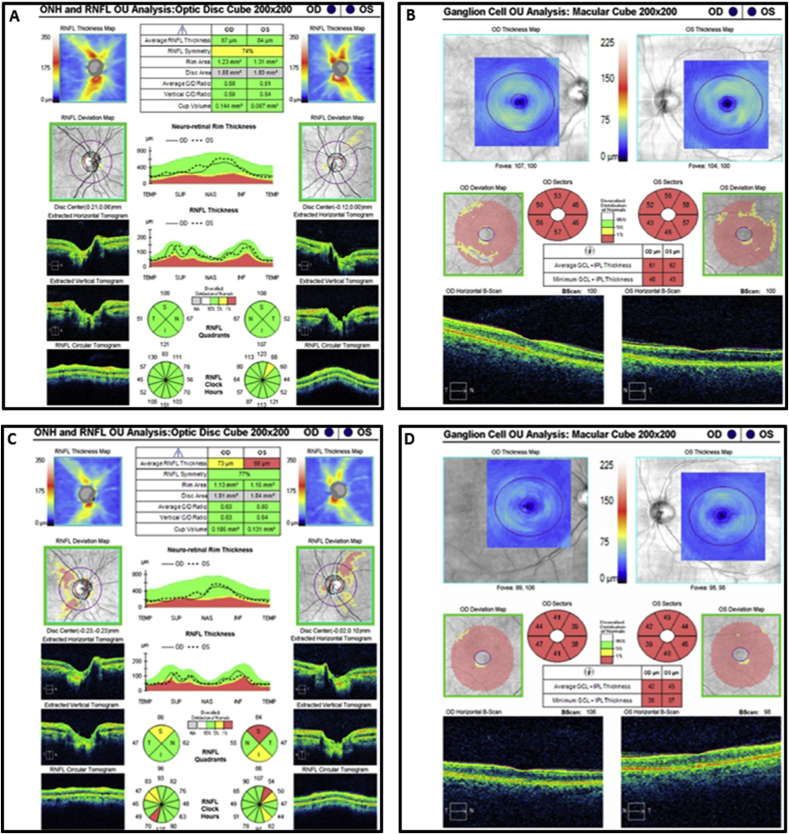
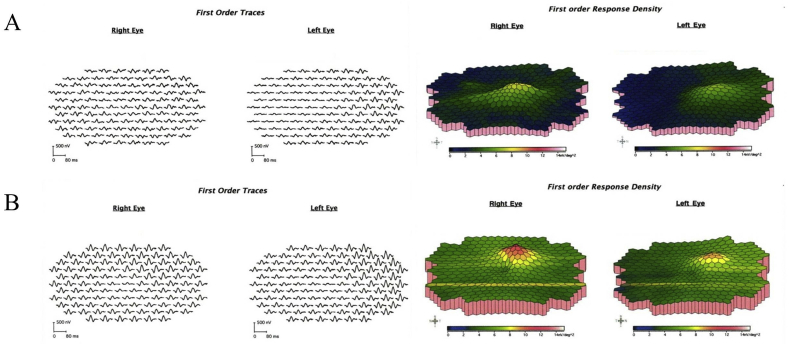
At the time of presentation to our neuro-ophthalmology clinic, she reported only being able to discern light and dark shapes. Visual acuity was 20/600 in the right eye and 20/800 in the left eye. She was unable to recognize any of the Ishihara pseudochromatic plates. Intraocular pressure were 13 mm Hg in both eyes. Pupils were sluggishly reactive to light bilaterally with no relative afferent pupillary defect. Slit-lamp examination revealed trace nuclear sclerotic cataracts and peripheral cortical changes in both eyes. Fundoscopy showed temporal optic disc pallor in both eyes with normal retina and vasculature (Fig. 2A). Goldmann visual field testing confirmed bilateral central scotomas (Fig. 2B). Optical coherence tomography (OCT) demonstrated normal peripapillary retinal nerve fiber layer (pRNFL) thickness in both eyes (Fig. 3A), but severe ganglion cell layer-inner plexiform layer (GCLIPL) thinning (Fig. 3B). Multifocal electroretinography (mfERG) showed decreased tracing amplitudes in both eyes (Fig. 4A).
Fig. 2.
A. Color fundus photographs showing bilateral temporal optic disc pallor prior to August 2018 hip revision surgery. B. Goldmann visual field testing demonstrates bilateral central scotomas prior to August 2018 hip revision surgery in August 2018. C. Goldmann visual field testing one month after the revision surgery demonstrates breaking up of the bilateral central scotomas. . (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
A. Optical coherence tomography of the retinal nerve fiber layer shows normal thickness in both eyes before the August 2018 revision surgery. B. Optical coherence tomography of the ganglion cell layer shows diffuse thinning in both eyes before the August 2018 revision surgery. C. Optical coherence tomography of the retinal nerve fiber layer shows moderate thinning in the right eye and severe thinning in the left eye one-month post revision surgery. D. Optical coherence tomography of the ganglion cell layer shows persistence of diffuse thinning in both eyes one-month post revision surgery.
Fig. 4.
A. Initial multifocal electroretinography (mfERG) four days after surgery demonstrates decreased tracing amplitude. B. Repeat mfERG one month after revision surgery shows improvement with an increase in the tracing amplitude.
A total left hip revision arthroplasty was performed that included insertion of a new acetabular component, exchange of the modular femoral head for one made of ceramic, extensive debridement, and excision of a pseudotumor and synovectomy. Intraoperatively, the surgeon noted extensive black staining of the subcutaneous tissues and when the fascia was incised there was flow of sludge-like black material (Fig. 1B). Four days after surgery, serum cobalt levels decreased to 378 μg/mL and chromium decreased to 17.5 μg/mL. One month after revision surgery, vision was 20/150 in the right eye and 20/250 in the left eye. Goldmann testing demonstrated a break-up of the central scotomas in both eyes (Fig. 2C). OCT showed moderate pRNFL thinning in the right eye and severe thinning in the left eye (Fig. 3C) with persistence of GCLIPL thinning in both eyes (Fig. 3D). Repeat mfERG demonstrated improvement with an increase in the tracing amplitudes in both eyes (Fig. 4B). Despite visual improvement, soon after the second revision surgery she was admitted to a local hospital for congestive heart failure due to non-ischemic cardiomyopathy. Nine months after surgery, visual acuity improved to 20/125 in both eyes. Automated visual field testing showed bilateral central scotomas. OCT demonstrated progressive pRNFL and GCLIPL layer thinning but no change in the mfERG.
3. Discussion
Our patient had findings of both an optic neuropathy and retinopathy secondary to cobalt toxicity. To date there have been only 11 cases published regarding visual loss associated with cobalt toxicity (Table 2).6,8,9,11, 12, 13, 14, 15, 16, 17, 18 Males outnumbered females (7 and 4, respectively) with age of the patients ranging from 39 to 71 years. Visual acuity data was not available in 6 patients. Documented improvement in vision was noted in 2 patients, stability in 2 patients and worsening in 1 patient. Cobalt levels ranged from 35 to 1078 μg/L.
Table 2.
Summary of the published cases presenting with ocular findings due to systemic cobalt toxicity.
| Age (years)/Gender | Pre-revision implant type | Post-revision implant type | C/Cr serum levels (μg/L) | Systemic findings | Ocular findings | Presenting visual acuity | Final visual acuity | |
|---|---|---|---|---|---|---|---|---|
| Steens et al.11 | 53/male | Ceramic on ceramic | Ceramic on metal | C: 398 Cr: 56 | Hearing loss, paresthesias | Optic atrophy and retinopathy both eyes | Could just recognize outlines and colors but could not read | Able to work on computer with strong glasses |
| Rizzetti et al.9 | 58/female | Ceramic on ceramic | Metal on polyethylene | C: 549 Cr: 54 |
Hearing loss, lower limb hyposthenia | MRI: Hyperintensity of optic nerves and tracts, VEP: abnormal both eyes | Not provided | Not provided |
| Tower et al.12 | 49/male | Metal on metal | Non-metal on metal | C: 35-122 Cr: 28-63 |
Cognitive disturbances, hearing loss, dyspnea, diastolic dysfunction, sleep apnea | Optic atrophy (eye not provided) | Not provided | Not provided |
| Apel et al.13 | 65/male | Ceramic on ceramic | Ceramic on metal | C: 446.4 Cr: 46 |
Malaise, cardiomyopathy, bulbar palsy, hypothyroidism | Poor visual acuity and color vision, ERG: abnormal both eyes | Right eye: 6/60 Left eye: 6/36 |
Right eye: 6/6 Left eye: 6/9 |
| Ng et al.14 | 39/female | Metal on metal | Not provided | C: 44.7 Cr: 30.9 |
Nausea, metallic taste | Bilateral ocular discomfort, photoreceptor-retinal pigment epithelium degeneration, paracentral scotoma, ICG: left eye chorioretinal, hypofluorescent lesions | Both eyes: 20/16 | Both eyes: 20/16 |
| Dahms et al.15 | 55/male | Ceramic on ceramic→ Metal on polyethylene |
Ceramic (unspecified) | C: 885 Cr: 49 |
Heart failure, hearing loss, hypothyroidism | Vision loss (eye not provided) | Not provided | Not provided |
| Harris et al.8 | 57/female | Ceramic on ceramic | Metal on polyethylene | C: 788.1 Cr: 140 |
Hip pain, fatigue, memory loss, lower extremity sensory loss, persistent tachycardia | Reduced visual acuity, subjective non-specific visual changes (eye not provided) | Not provided | Not provided |
| Grillo et al.6 | 66/male | Ceramic on ceramic | Metal on metal | C: 1078 Cr: not provided | Hearing loss, abdominal distention and ankle swelling, left hip pain, diarrhea, rash, incoordination, cognitive difficulties | Decreased visual acuity, poor color vision, and cecocentral scotoma left eye, ERG: normal | Right eye: 20/80 (History of amblyopia) Left eye: 20/30 |
Right eye: 20/80 (History of amblyopia) Left eye: 20/25 |
| Peters et al.17 | 71/male | Ceramic on ceramic | Metal on polyethylene | C: 596.5 Cr: 48.8 |
Abdominal pain, vomiting, diarrhea, hearing loss, visual impairment, vertigo, unintentional weight loss, hypothyroidism, cardiomyopathy | Nonspecific visual impairment (eye not provided) | Not provided | Not provided |
| Ho et al.16 | 63/male | Ceramic on ceramic | Not provided | C: 280-779 Cr: not provided |
Hearing loss, painful paresthesias, cardiomyopathy | Decreased visual acuity both eyes, MRI: hyperintensity of optic nerves | Right eye: 20/80 Left eye: 20/200 |
Both eyes: Count fingers |
| Weber et al.18 | 66/female | Ceramic (unspecified) | Metal on polyethylene | C: 411.5 Cr: not provided |
Depression, fatigue, unintentional weight loss, paresthesias, disequilibrium, hearing loss, cardiomyopathy | Decreased visual acuity with central scotomas both eyes, mild right relative afferent pupillary defect, ERG: severe cone dysfunction and low normal rod function | Both eyes: Count fingers | Both eyes: Count fingers |
The diagnosis of cobalt toxicity requires recognizing the potential of hip implant failure to cause visual loss in addition to systemic symptoms. In particular, a detailed surgical history should be obtained in all patients with unexplained visual loss. Ophthalmic findings of cobalt toxicity include decreased visual acuity, dyschromatopsia, central visual field defects, optic atrophy, decreased choroidal perfusion, macular dysfunction, visual evoked potential abnormalities, abnormal ERG, and optic nerve enhancement on MR imaging.1,6,16,21 Elevated cobalt blood levels will confirm the clinical suspicion. Based on the few cases reported in the literature, the prognosis of ocular cobalt toxicity is variable (Table 2). Mild degree of visual acuity improvement can occur in some patients, but other patients have irreversible visual loss. There is no proven treatment for cobalt toxicity aside from decreasing blood cobalt levels by either revising or replacing the failed hip implant as soon as the problem is recognized. As discussed below there is some evidence that NAC can prevent retinal ganglion cell (RGC) apoptosis.
Cobalt optic neuropathy and B12 deficiency optic neuropathy share similar ocular and systemic manifestations.22 This is due to the fact that cobalt is essential for the production of vitamin B12 (i.e cobalamin—a cobalt complex).23 It is unclear why cobalt excess manifests clinically similar to B12 deficiency. Both cobalt toxic optic neuropathy and B12 deficiency neuropathy are a consequence of disruption in mitochondrial oxidative phosphorylation.22 Some authors have suggested that cobalt optic neuropathy is secondary to RGC loss resulting from the oxidative stress on the mitochondria caused by the disturbance in the balance between the production and elimination of reactive oxygen species.5 Apostoli et al. demonstrated severe depletion of RGCs in New Zealand white rabbits receiving 1354–2708 μg/mL of cobalt chloride, while those who were exposed to high levels of both chromium and cobalt had less severe RGC loss, and those receiving only chromium did not have any significant RGC loss.5 These findings suggest that chromium may actually be protective in the setting of cobalt toxicity and may explain why in some patients there is only mild vision loss and/or vision recovery. Although our patient's titanium levels were also elevated initially, titanium toxicity is rare in humans as it is considered highly biocompatible compared to other metals.24 To our knowledge, there has been no published reports of elevated titanium levels and vision loss. Furthermore, cobalt exposed rabbits do not only have RGC loss but also have optic nerve axonal swelling and myelin thinning, similar to the pathophysiology of Leber hereditary optic neuropathy (LHON).5 Additionally, it has been noted that the pRNFL and the pre-laminar portion of the optic nerve may be particularly vulnerable to oxidative stress and apoptosis due to mitochondrial dysfunction.13 Some have suggested that the mitochondria also plays an important role in photoreceptor function in the setting of LHON.25 Thus, we propose that our patient's retinopathy is also related to the mitochondrial cytopathy caused by cobalt toxicity. Similar to our patient, Apel et al. showed ERG abnormalities in a patient with known cobalt-chromium toxicity due to a metal prosthesis with improvement after revision surgery.13 Their case and ours supports the notion that cobalt toxicity can also cause damage to the inner retinal layers.
The initial absence of pRNFL thinning in our patient was thought to be from the acute toxic effects of cobalt. During our patient's visual recovery, pRNFL and GCLIPL thickness decreased. The discordance between the GCLPL thinning and near normal pRNFL layer on presentation is suggestive of a mitochondrial cytopathy. This pattern has been observed in some patients with LHON, as well as ethambutol and linezolid toxicity.26,27 In the case of ethambutol optic neuropathy, it has been postulated that some axons, particularly in the papillomacular bundle, are able to recover function without undergoing apoptosis.28 In a case series of five patients, 4 had visual loss from early ethambutol optic neuropathy with normal pRNFL thickness.29 It has also been demonstrated that tobacco-alcohol induced toxic optic neuropathy can have initial normal pRNFL thickness, thought to be the result of pRNFL edema in the early phase of the pathological process.30
3.1. Cobalt toxicity and hip prosthesis
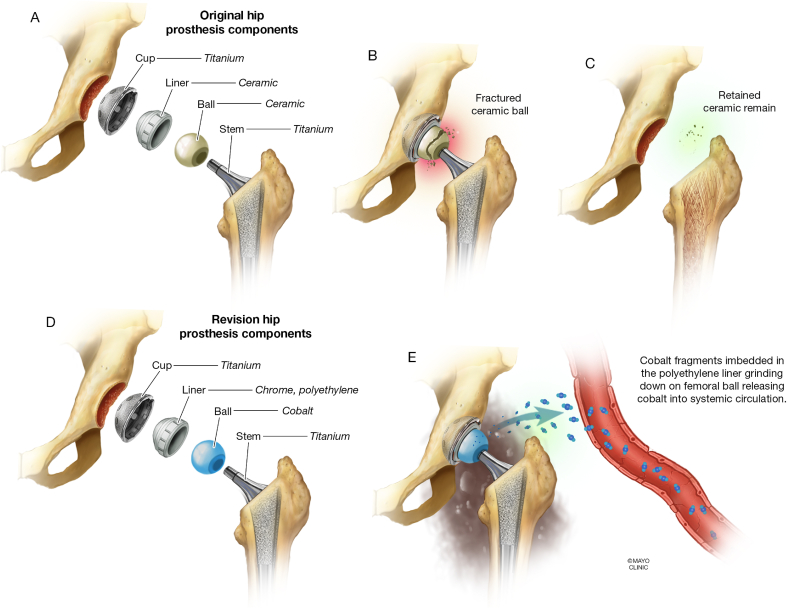
It is estimated that 60% of all total hip replacements require revision due to wear-related problems.7 The mechanical friction between the surfaces can lead to release of metal particles into the hip joint, and absorption into the systemic circulation (Fig. 5).7 The prosthesis surfaces can be made of different materials including metal (cobalt-chromium or stainless steel), ceramic or polyethylene.7,10 MoP and CoP tend to be less durable due to wear on the polyethylene component.7 In contrast, MoM (cobalt-chromium alloys) is known to be more durable.7 However, several studies have now shown that friction between residual microscopic fragments of ceramic and cobalt-chromium metal implants leads to excretion of cobalt and chromium into both the synovial fluid and bloodstream leading to potential systemic toxicity.2,4,8,17,18,31, 32, 33, 34 Bradberry et al. found that the greatest risk of systemic cobalt toxicity occurs in revision surgeries where a cobalt containing prosthesis replaces a failed ceramic prosthesis, rather than from the MoM wear in a first time prosthesis.7
Fig. 5.
A. Illustration of the original hip prosthesis with a ceramic ball (a.k.a. head) and a ceramic liner. B. Ceramic ball fracture in the setting of catastrophic failure of the hip implant. C. Retained ceramic fragments remain present at the site of the previous fracture despite debridement during revision surgery. D. After the first revision surgery, the components of the hip prosthesis included a cobalt ball and chrome, polyethylene liner. E. Ceramic fragments embedded into the polyethylene liner grinding down on the cobalt femoral ball releasing cobalt into the systemic circulation.
The main treatment of systemic cobalt toxicity is the revision or removal of the metallic hip prosthesis.31,35 Chelation with NAC can be temporarily used until surgery is performed.31,35 Yang et al. demonstrated the neuroprotective effects of NAC and that pretreating RGCs can counteract apoptosis by scavenging reactive oxygen species and targeting the hypoxia inducible factor-1 alpha pathway.35
3.2. Prevention of systemic cobalt toxicity
Because of the risk of developing systemic cobalt toxicity, some experts recommend obtaining whole blood and urine cobalt levels for those patients who previously had CoC prostheses, replaced with cobalt-chromium prosthesis.31 However, others have pointed out that elevated cobalt levels in otherwise asymptomatic patients may not be an adequate indication for revision.36
In addition, some have suggested ceramic components should replace failed ceramic prosthesis rather than using cobalt containing metal components.37,38 Trebse et al. have recommended “ceramic-on-ceramic bearings should be preferentially used at revision of fractured ceramic component.“37 They only recommend revision to a MoP if a “thorough synovectomy and pulsatile lavage” can be performed.37 Others completely oppose the use of polyethylene during revision surgery for a fractured ceramic component citing the potentially deadly effects of this combination due to the embedding of the retained ceramic particles into the polyethylene and subsequent grinding of the metal femoral head.38
The United States Food and Drug Administration (FDA) currently recommends patients with symptoms suggestive of hip implant failure or with systemic symptoms (skin rash, cardiomyopathy, renal dysfunction, thyroid abnormalities or neurologic such as hearing loss, visual disturbances, or cognitive decline) seek evaluation by an orthopedic surgeon for possible imaging of the hip implant and/or checking serum metal ion levels. Furthermore, the FDA recommends that asymptomatic patients with MoM hip prosthesis follow-up with their orthopedic surgeon every 1–2 years.39 However, the FDA has noted that there is no scientific evidence for checking levels of metal ions or hip imaging in asymptomatic patients.39 The United Kingdom, Canada and Australia have all disseminated public alerts regarding the risk of metallosis in the setting of MoM hip implants.40,41 However, at this time there are no specific guidelines regarding the use of polyethylene after ceramic failure.
4. Conclusions
Systemic cobalt toxicity in the setting of a hip prosthesis can cause significant morbidity and mortality. Based on this report and the few case reports in the literature it appears that ocular cobalt toxicity can involve either the optic nerve, retina or both. It is essential that orthopedic surgeons, ophthalmologists and patients be aware of the local and systemic symptoms of a malfunctioning hip in order to prevent the irreversible detrimental effects of cobalt toxicity.
Consent
The patient consented to publication of the case both in writing and orally.
Funding
None.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
None of the authors have any financial disclosures.
Acknowledgements
None.
References
- 1.Leyssens L., Vinck B., Van Der Straeten C., Wuyts F., Maes L. Cobalt toxicity in humans-A review of the potential sources and systemic health effects. Toxicology. 2017;387:43–56. doi: 10.1016/j.tox.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Paustenbach D.J., Tvermoes B.E., Unice K.M., Finley B.L., Kerger B.D. A review of the health hazards posed by cobalt. Crit Rev Toxicol. 2013;43(4):316–362. doi: 10.3109/10408444.2013.779633. [DOI] [PubMed] [Google Scholar]
- 3.Catalani S., Rizzetti M.C., Padovani A., Apostoli P. Neurotoxicity of cobalt. Hum Exp Toxicol. 2012;31(5):421–437. doi: 10.1177/0960327111414280. [DOI] [PubMed] [Google Scholar]
- 4.Kao C., Scalettar R., Bunning R.D. Two cases of metallosis from metal-on-polyethylene total hips: an emerging problem. Pharm Manag PM R. 2015;7(4):447–450. doi: 10.1016/j.pmrj.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Apostoli P., Catalani S., Zaghini A. High doses of cobalt induce optic and auditory neuropathy. Exp Toxicol Pathol. 2013;65:719–727. doi: 10.1016/j.etp.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Grillo L.M., Nguyen H.V., Tsang S.H., Hood D.C., Odel J.G. Cobalt-chromium metallosis with normal ERG: a case report and review. J Neuro Ophthalmol. 2016;36(4):383–388. doi: 10.1097/WNO.0000000000000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradberry S.M., Wilkinson J.M., Ferner R.E. Systemic toxicity related to metal hip prostheses. Clin Toxicol. 2014;52:837–847. doi: 10.3109/15563650.2014.944977. [DOI] [PubMed] [Google Scholar]
- 8.Harris A., Johnson J., Mansuripur P.K., Limbird R. Cobalt toxicity after revision to a metal-on-polyethylene total hip arthroplasty for fracture of ceramic acetabular component. Arthroplast Today. 2015;1(4):89–91. doi: 10.1016/j.artd.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rizzetti M.C., Liberini P., Zarattini G. Loss of sight and sound. Could it be the hip? Lancet. 2009;373(9668):1052. doi: 10.1016/S0140-6736(09)60490-6. [DOI] [PubMed] [Google Scholar]
- 10.Devlin J.J., Pomerleau A.C., Brent J., Morgan B.W., Deitchman S., Schwartz M. Clinical features, testing, and management of patients with suspected prosthetic hip-associated cobalt toxicity: a systematic review of cases. J Med Toxicol. 2013;9(4):405–415. doi: 10.1007/s13181-013-0320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steens W., von Foerster G., Katzer A. Severe cobalt poisoning with loss of sight after ceramic-metal pairing in a hip--a case report. Acta Orthop. 2006;77(5):830–832. doi: 10.1080/17453670610013079. [DOI] [PubMed] [Google Scholar]
- 12.Tower S.S. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(17):2847–2851. doi: 10.2106/JBJS.J.00125. [DOI] [PubMed] [Google Scholar]
- 13.Apel W., Stark D., Stark A., O'Hagan S., Ling J. Cobalt-chromium toxic retinopathy case study. Doc Ophthalmol. 2013;126(1):69–78. doi: 10.1007/s10633-012-9356-8. [DOI] [PubMed] [Google Scholar]
- 14.Ng S.K., Ebneter A., Gilhotra J.S. Hip-implant related chorio-retinal cobalt toxicity. Indian J Ophthalmol. 2013;61:35–37. doi: 10.4103/0301-4738.105053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahms K., Sharkova Y., Heitland P., Pankuweit S., Schaefer J.R. Cobalt intoxication diagnosed with the help of Dr House. Lancet. 2014;383(9916):574. doi: 10.1016/S0140-6736(14)60037-4. [DOI] [PubMed] [Google Scholar]
- 16.Ho V.M., Arac A., Shieh P.B. Hearing and vision loss in an older man. JAMA Neurol. 2018;75(11):1439–1440. doi: 10.1001/jamaneurol.2018.1868. [DOI] [PubMed] [Google Scholar]
- 17.Peters R.M., Willemse P., Rijk P.C., Hoogendoorn M., Zijlstra W.P. Fatal cobalt toxicity after a non-metal-on-metal total hip arthroplasty. Case Rep Orthop. 2017 doi: 10.1155/2017/9123684. 9123684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber K.P., Schweier C., Kana V., Guggi T., Byber K., Landau K. Wear and tear vision. J Neuro Ophthalmol. 2015;35(1):82–85. doi: 10.1097/WNO.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 19.Hu C.Y., Yoon T.R. Recent updates for biomaterials used in total hip arthroplasty. Biomater Res. 2018;22:33. doi: 10.1186/s40824-018-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.www.bleedingedgedoc.com accessed September 2019.
- 21.Lim C.A., Khan J., Chelva E., Khan R., Unsworth-Smith T. The effect of cobalt on the human eye. Doc Ophthalmol. 2015;130(1):43–48. doi: 10.1007/s10633-014-9469-3. [DOI] [PubMed] [Google Scholar]
- 22.Jefferis J.M., Hickman S.J. Treatment and outcomes in nutritional optic neuropathy. Curr Treat Options Neurol. 2019;21(1):5. doi: 10.1007/s11940-019-0542-9. [DOI] [PubMed] [Google Scholar]
- 23.Giedyk M., Goliszewska K., Gryko D. Vitamin B12 catalysed reactions. Chem Soc Rev. 2015;44(11):3391–3404. doi: 10.1039/c5cs00165j. [DOI] [PubMed] [Google Scholar]
- 24.Fage S.W., Muris J., Jakobsen S.S., Thyssen J.P. Titanium: a review on exposure, release, penetration, allergy, epidemiology, and clinical reactivity. Contact Dermatitis. 2016;74(6):323. doi: 10.1111/cod.12565. [DOI] [PubMed] [Google Scholar]
- 25.Kassem A., Karanjia R., McClelland C., Sadun A., Lee M.S. Unilateral cone-rod dysfunction and retinal thinning in a child carrying the 14484 mutation of Leber hereditary optic neuropathy. J AAPOS. 2019;23(2):104–106. doi: 10.1016/j.jaapos.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Lee J.Y., Han J., Seo J.G., Park K.A., Oh S.Y. Diagnostic value of ganglion cell-inner plexiform layer for early detection of ethambutol-induced optic neuropathy. Br J Ophthalmol. 2019;103(3):379–384. doi: 10.1136/bjophthalmol-2018-312063. [DOI] [PubMed] [Google Scholar]
- 27.Pilania R.K., Arora A., Agarwal A. Linezolid-induced mitochondrial toxicity presenting as retinal nerve fiber layer microcysts and optic and peripheral neuropathy in a patient with chronic granulomatous disease. Retin Cases Brief Rep. 2018 Jul 25 doi: 10.1097/ICB.0000000000000777. [DOI] [PubMed] [Google Scholar]
- 28.Masvidal D., Parrish R.K., Lam B.L. Structural-functional dissociation in presumed ethambutol optic neuropathy. J Neuroophthalmology. 2010;30(4):305–310. doi: 10.1097/WNO.0b013e3181e08ecb. [DOI] [PubMed] [Google Scholar]
- 29.Kim Y.K., Hwang J.M. Serial retinal nerve fiber layer changes in patients with toxic optic neuropathy associated with antituberculosis pharmacotherapy. J Ocul Pharmacol Therapeut. 2009;25(6):531–535. doi: 10.1089/jop.2009.0064. [DOI] [PubMed] [Google Scholar]
- 30.Moura F.C., Monteiro M.L. Evaluation of retinal nerve fiber layer thickness measurements using optical coherence tomography in patients with tobacco-alcohol-induced toxic optic neuropathy. Indian J Ophthalmol. 2010;58(2):143–146. doi: 10.4103/0301-4738.60087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox R.M., Willemse P., Rijk P.C., Hoogendoorn M., Zijlstra W.P. Fatal cobalt toxicity after total hip arthroplasty revision for fractured ceramic component. Clin Toxicol. 2016;54(9):874–877. doi: 10.1080/15563650.2016.1214274. [DOI] [PubMed] [Google Scholar]
- 32.Pelayo-de Tomas J.M., Novoa-Parra C., Gómez-Barbero P. Cobalt toxicity after revision total hip replacement due to fracture of a ceramic head. Rev Española Cirugía Ortopédica Traumatol. 2017;61(3):203–207. doi: 10.1016/j.recot.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Vasukutty N.L., Minhas T.H. Systemic effects of cobalt toxicity after revision hip replacement can manifest in intermediate to long term follow-up. Hip Int. 2016;26(4):e31–e34. doi: 10.5301/hipint.5000386. [DOI] [PubMed] [Google Scholar]
- 34.Zywiel M.G., Brandt J.M., Overgaard C.B., Cheung A.C., Turgeon T.R., Syed K.A. Fatal cardiomyopathy after revision total hip replacement for fracture of a ceramic liner. Bone Joint Lett J. 2013;95-B:31–37. doi: 10.1302/0301-620X.95B1.30060. [DOI] [PubMed] [Google Scholar]
- 35.Yang L., Tan P., Zhou W. N-acetylcysteine protects against hypoxia mimetic-induced autophagy by targeting the HIF-1α pathway in retinal ganglion cells. Cell Mol Neurobiol. 2012;32(8):1275–1285. doi: 10.1007/s10571-012-9852-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Lingen C.P., Ettema H.B., Timmer J.R., de Jong G., Verheyen C.C. Clinical manifestations in ten patients with asymptomatic metal-on-metal hip arthroplasty with very high cobalt levels. Hip Int. 2013;23(5):441–444. doi: 10.5301/hipint.5000054. [DOI] [PubMed] [Google Scholar]
- 37.Trebše R., Mihelič A., Levašič V., Cör A., Milošev I. Results of revision of total hip arthroplasty for alumina ceramic-on-ceramic bearing fracture. Hip Int. 2016;26(3):237–243. doi: 10.5301/hipint.5000340. [DOI] [PubMed] [Google Scholar]
- 38.Fernández-Valencia J.Á. Metal-on-polyethylene is not an option after the fracture of a ceramic component of a total hip arthroplasty. Hip Int. 2016;26(6):e56. doi: 10.5301/hipint.5000441. [DOI] [PubMed] [Google Scholar]
- 39.https://www.fda.gov/medicaldevices/productsandmedicalprocedures/implantsandprosthetics/metalonmetalhipimplants/ucm241604.htm accessed September 2019.
- 40.http://www.healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2012/14120a-eng.php accessed September 2019.
- 41.https://www.tga.gov.au/metal-metal-hip-replacement-implants accessed September 2019.