Abstract
Vasculogenic mimicry (VM) promotes tumor migration, metastasis, and invasion in various types of cancer, but the relationship between VM and these phenotypes remains undefined. In this study, we examined carcinoembryonic antigen cell adhesion molecule 1 (CEACAM1) as a novel target of VM. We found that ectopic expression of CEACAM1 in HT1080 human fibrosarcoma cells suppressed the formation of a VM-like network. Further, cell migration and proliferation were abated by the introduction of CEACAM1 into HT1080 cells. Conversely, knockout (KO) of the CEACAM1 gene in SK-MEL-28 melanoma cells, which normally express high levels of CEACAM1, inhibited formation of a VM-like network, which was covered on reintroduction of CEACAM1. These results suggest that CEACAM1 differentially regulates formation of the VM-like network between cancer cell types and implicate CEACAM1 as a novel therapeutic target in malignant cancer.
Keywords: Vasculogenic mimicry, CEACAM1, Cell migration, Cell proliferation, Melanoma, Fibrosarcoma
Abbreviations: VM, vasculogenic mimicry; CEACAM1, carcinoembryonic antigen cell adhesion molecule 1; DMEM, Dulbecco's modified Eagle's medium; PBS, phosphate-buffered saline; SDS, sodium dodecyl sulfate
Highlights
-
•
CEACAM1 is not expressed in HT1080 cells, and overexpression of CEACAM1 in HT1080 cells suppresses vasculogenic mimicry.
-
•
CEACAM1 is highly expressed in SK-MEL-28 cells, and deletion of CEACAM1 in SK-MEL-28 cells abolishes vasculogenic mimicry.
-
•
CEACAM1 regulates vasculogenic mimicry in a cell-dependent manner.
1. Introduction
Oxygen and nutrients are essential for tumor growth and are supplied primarily by angiogenesis, which recruits blood vessels to solid tumors through the stimulation of vascular endothelial cells [1]. However, recent studies have implicated vasculogenic mimicry (VM) as an alternative mechanism of neovascularization. VM is a phenomenon in which mosaic vessels from tumor cells [2]. Although VM does not require vascular endothelial cells, the vessels formed by tumor cells mimic the function of endothelial cells [3].
VM occurs in the extracellular matrix (ECM)-rich region, differentiating it from angiogenesis. Interactions between tumor cells and their surroundings are necessary for VM [4]. Further, VM provides growing tumors with sufficient blood perfusion, promotes cancer metastasis and progression, and is widely observed in various solid cancers, such as lung, breast, and melanoma [[5], [6], [7], [8]]. Thus, VM is a reasonable and attractive target for cancer therapy, like angiogenesis.
Carcinoembryonic antigen cell adhesion molecule 1 (CEACAM1), also known as biliary glycoprotein and CD66a, is a transmembrane glycoprotein that belongs to the CEACAM family. CEACAM1 is broadly expressed in epithelial, endothelial, lymphoid, and myeloid cells [9]. CEACAM1 forms homodimers and heterodimers with CEACAM5 or CEACAM6 for cell-cell adhesion and intracellular signaling [[10], [11], [12]]. CEACAM1 is differentially expressed, depending on the tumor type. For example, CEACAM1 is dysregulated in several tumors compared with normal tissues, such as colorectal, prostatic, and breast cancers, indicating that CEACAM1 functions as a tumor suppressor [[13], [14], [15]]. However, CEACAM1 is upregulated and promotes tumor progression in several tumors, such as melanoma and lung cancer [16,17], and the upmodulation of CEACAM1 increases microvascular density and correlates with the development of distant metastases in non-small-cell lung carcinoma [12], suggesting that CEACAM1 is oncogenic.
In this study, we examined the development of VM using common in vitro methods [[18], [19], [20]]. We established CEACAM1-overexpressing HT1080 cells and found that CEACAM1 suppresses VM-like network formation on Matrigel in HT1080 cells. Further, CEACAM1 negatively regulates cell proliferation, and migration. In contrast, knockout (KO) of CEACAM1 inhibits the development of VM-like networks and migration in SK-MEL-28 cells. Several roles for CEACAM1 have been reported in cancer malignancy; thus, our results indicate that CEACAM1 is a novel cell-dependent regulator of VM.
2. Materials and methods
2.1. Western blot
For the western blot analysis, we used a slightly modified version of a previous method [[21], [22], [23], [24], [25]]. Cells were lysed in lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% (w/v) SDS, 1% (v/v) Triton X-100, 1% (w/v) sodium deoxycholate, and 1 mM PMSF) at 4 °C with sonication. The lysates were centrifuged at 15,000×g for 10 min. Loading buffer (350 mM Tris-HCl, pH 6.8, 30% (w/v) glycerol, 0.012% (w/v) bromophenol blue, 6% (w/v) SDS, and 30% (v/v) 2-mercaptoethanol) was added to each lysate. After electrophoresis, proteins were transferred to polyvinylidene fluoride membranes and immunoblotted with anti-c-myc (9E10 hybridoma culture supernatant, Developmental Studies Hybridoma Bank, USA), anti-α-tubulin (#T5168, Merck KGaA, Germany), or anti-CEACAM1 (sc166453, Santa Cruz Biotechnology). Signals were detected by ECL using Western Lightning Plus-ECL (PerkinElmer, Inc., USA).
2.2. VM-like network formation assay
HT1080 and SK-MEL-28 cells, suspended in culture medium, were seeded at 1.6 × 104 cells/well or 2.0 × 104 cells/well in 96-well plates that precoated with 40 μL/well Matrigel (Corning Inc., USA) and cultured at 37 °C. These cells were photographed at 4 and 6 h after seeding. We quantified VM-like network formation as described [26].
2.3. Wound healing assay
HT1080 cells and SK-MEL-28 cells were seeded at 2 × 105 cells/well in 24-well plates, cultured for 24 h to approximately 90% confluence, and wounded using a yellow pipette tip (WATSON, Japan). After being washed twice with PBS, the cells were cultured in serum-free DMEM for 12 and 24 h. Photographs were taken of 4 independent areas, and the areas of migration were quantified using ImageJ 1.51 (National Institutes of Health, Bethesda, MD, USA) [27,28].
2.4. Cell culture, plasmid construction; establishment of CEACAM1-overexpressing HT1080, CEACAM1-KO SK-MEL-28, and CEACAM1-rescued SK-MEL-28 cells; RNA extraction and semiquantitative PCR; cell proliferation assay; and statistical analysis
Cell culture; plasmid construction; generation of CEACAM1-rescued SK-MEL-28, CEACAM1-overexpressing HT1080, and CEACAM1-KO SK-MEL-28 cells using the CRISPR/Cas9 system; RNA extraction and semiquantitative PCR; cell proliferation assay; and statistical analysis are described in Supplementary Materials and Methods.
3. Results
3.1. Expression of CEACAM1 in various cancer cell line cultures
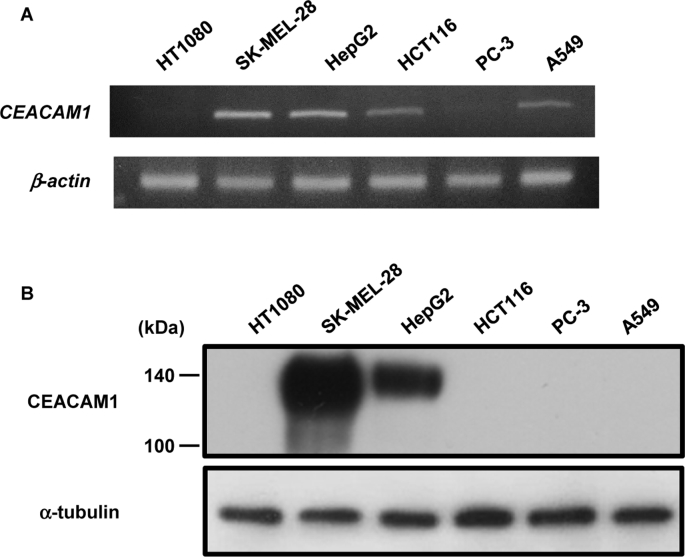
The expression of CEACAM1 varies, depending on the type of cancer [[13], [14], [15], [16], [17]]. We measured endogenous CEACAM1 levels by semi-quantitative RT-PCR and western blot in various cancer cell lines. CEACAM1 expression was high in SK-MEL-28 and HepG2 cells but absent in HT1080 and PC-3 cells (Fig. 1A and B). These results indicate that CEACAM1 expression depends on the cancer cell type.
Fig. 1.
Expression of endogenous CEACAM1 mRNA in various human cancer cell lines.
(A) Total mRNA was isolated from HT1080, SK-MEL-28, HepG2, HCT116, PC-3, and A549 cells, and semi-quantitative RT-PCR was performed. (B) HT1080, SK-MEL-28, HepG2, HCT116, PC-3, and A549 cells were cultured, and the cell lysates were immunoblotted with the indicated antibodies.
3.2. Suppression of VM-like network formation in HT1080 cells by CEACAM1 overexpression
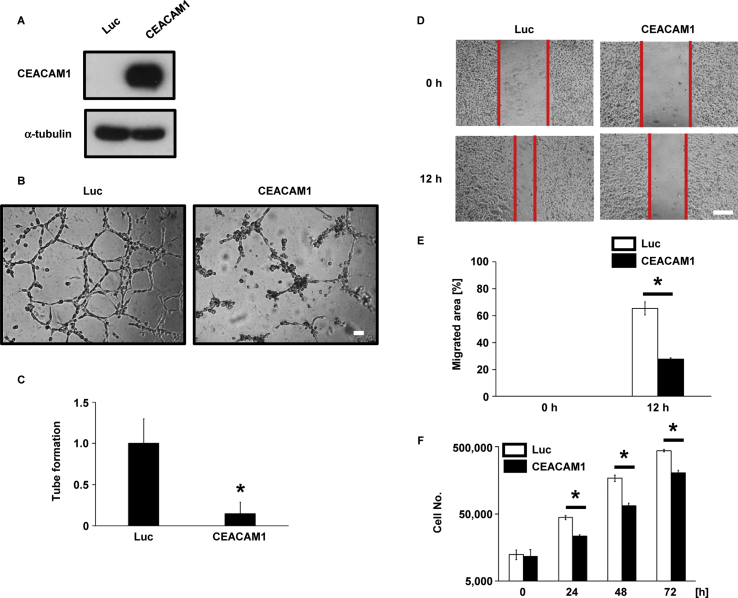
Because we reported that HT1080 cells have the potential to form a VM-like network on Matrigel [29], we used HT1080 cells to measure CEACAM1 function with regard to VM. To determine whether CEACAM1 affects formation of a VM-like network, we established a CEACAM1-overexpressing HT1080 cell line (Fig. 2A) and assessed its ability to form such a network on Matrigel. Network formation on Matrigel was significantly suppressed by the overexpression of CEACAM1 (Fig. 2B and C), suggesting that CEACAM1 inhibits VM-like network formation in HT1080 cells.
Fig. 2.
Effect of CEACAM1 overexpression on VM-like network formation in HT1080 cells.
(A) Generation of CEACAM1-overexpressing HT1080 cells. Control (Luc) and CEACAM1-overexpressing HT1080 cells were cultured, and the cell lysates were immunoblotted with the indicated antibodies. (B and C) Inhibition of VM-like network formation by overexpression of CEACAM1 in HT1080 cells. Cells were seeded on Matrigel-coated wells, photographs were taken at 6 h after seeding (B), and the number of tubes was counted in 6 independent randomly selected fields (C). Scale bar, 100 μm. (D and E) Suppression of cell migration by overexpression of CEACAM1 in HT1080 cells. Cells were scratched and photographed under an inverted microscope at 0 and 12 h after the scratch. Photographs of 4 independent areas were taken; (D) the quantitative analysis of the relative area of migration is shown (E). Scale bar, 500 μm. (F) Suppression of cell growth by CEACAM1 in HT1080 cells. Luc and CEACAM1-overexpressing HT1080 cells were seeded at 1.2 × 104 cells/well in 12-well plates and cultured for 3 h. Cells were recovered by trypsinization and counted using a hemocytometer every 24 h. Data shown are means ± SD. *P < 0.05.
Because CEACAM1 contributes to cancer migration [30], we examined whether CEACAM1 expression regulates cancer cell migration in HT1080 cells by wound-healing assay. The overexpression of CEACAM1 decreased the migration of HT1080 compared with control cells (Fig. 2D and E). Further, CEACAM1 expression significantly lowered the proliferation of HT1080 cells versus the control (Fig. 2F). These results demonstrate that CEACAM1 is a tumor suppressor in HT1080 cells.
3.3. Knockout of CEACAM1 inhibits VM-like network formation in melanoma cells
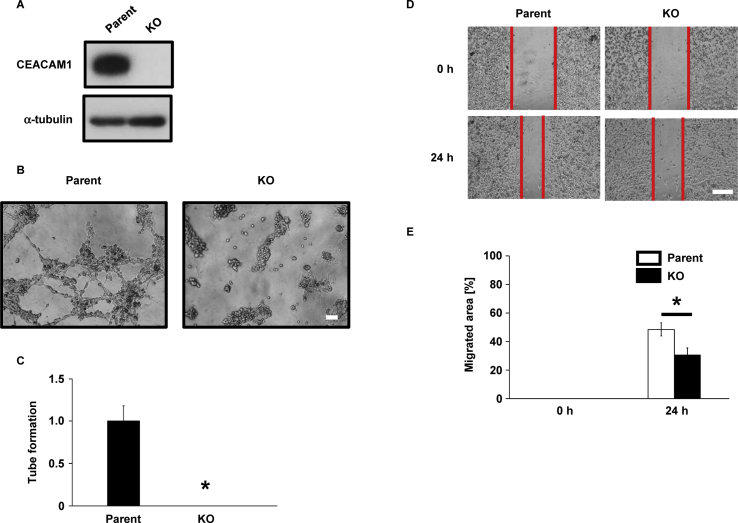
CEACAM1 is upregulated in human melanoma cells compared with human melanocytes and promotes cancer [16]. Consistent with this finding, CEACAM1 levels were higher in SK-MEL-28 cells than other cancer cell lines (Fig. 1A and B). To examine the function of CEACAM1 in VM, we established CEACAM1-KO SK-MEL-28 cells using the CRISPR/Cas9 system. Guide RNA sequences were designed at 2 close positions in exon 2 of CEACAM1 to avoid off-target risks. The resulting constructs were coinfected into Cas9-expressing SK-MEL-28 cells, and CEACAM1-KO clones were selected (Fig. 3A). Using the established cell line, we assessed the ability of CEACAM1-KO SK-MEL-28 cells to form VM-like networks. Network formation on Matrigel was completely inhibited by knockout of CEACAM1 (Fig. 3B and C), suggesting that CEACAM1 is necessary for VM-like network formation in SK-MEL-28 cells.
Fig. 3.
KO of CEACAM1 inhibits VM-like network formation in SK-MEL-28 cells.
(A) Generation of CEACAM1-KO SK-MEL-28 cells. Parental and CEACAM1-KO SK-MEL-28 cells were cultured, and the cell lysates were immunoblotted with the indicated antibodies. (B and C) VM-like network formation was completely abolished by KO of CEACAM1 in SK-MEL-28 cells. Cells were seeded on Matrigel-coated wells, photographs were taken at 4 h after seeding (B), and the number of tubes was counted in 6 independent, randomly selected fields (C). Scale bars, 100 μm. (D and E) Inhibition of cell migration in CEACAM1-KO SK-MEL-28 cells. Cells were scratched and photographed under an inverted microscope 0 and 24 h after the scratch. Photographs were taken of 4 independent areas (D); the quantitative analysis of the relative area of migration is shown (E). Scale bars, 500 μm. Data shown are means ± SD. *P < 0.05.
CEACAM1 promotes migration in melanoma cells [31]. Because the deletion of CEACAM1 inhibited VM-like network formation in SK-MEL-28 cells, we examined whether CEACAM1 regulates cancer cell migration and proliferation as in HT1080 cells. By wound-healing assay, migration was suppressed by knockout of CEACAM1 in SK-MEL-28 cells (Fig. 3D and E), but their proliferation did not change significantly (data not shown). These results indicate that CEACAM1 promotes migration in melanoma cells without altering cell growth.
3.4. CEACAM1 is essential for VM-like network formation in SK-MEL-28 melanoma
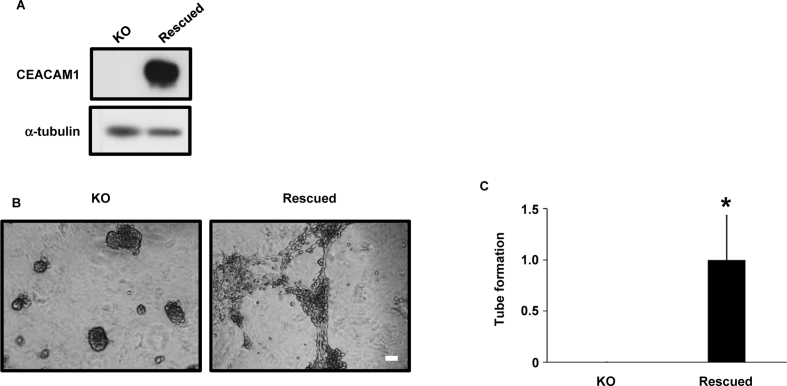
To confirm that CEACAM1 is necessary for VM formation, we performed CEACAM1 rescue experiments. We introduced Cas9-resistant CEACAM1 plasmids into CEACAM1-KO SK-MEL-28 cells transiently. Re-expression of CEACAM1 was confirmed by western blot (Fig. 4A). VM-like network formation was recovered on reintroduction of CEACAM1 (Fig. 4B and C), indicating that CEACAM1 is essential for VM-like network formation in SK-MEL-28 cells.
Fig. 4.
Reintroduction of CEACAM1 to CEACAM1-KO SK-MEL-28 cells recovers VM-like network formation.
(A) Reintroduction (rescued) of CEACAM1 to CEACAM1-KO SK-MEL-28 cells. CEACAM1-KO and CEACAM1-rescued SK-MEL-28 cells were cultured, and the cell lysates were immunoblotted with the indicated antibodies. (B and C) VM-like network formation was recovered in CEACAM1-rescued SK-MEL-28 cells. Cells were seeded on Matrigel-coated wells, photographs were taken at 24 h after seeding (B), and the number of tubes was counted in 6 independent, randomly selected fields (C). Scale bar, 100 μm. Data shown are means ± SD. *P < 0.05.
4. Discussion
There are many phenomena that are involved in the progression of cancer, such as the EMT, invasion, migration, angiogenesis, and VM, the latter of which has been proposed to contribute to malignancy in recent years. In this study, in examining a novel regulator of VM, we found that CEACAM1 is critical in its occurrence. We compared CEACAM1 levels in several cancer cell lines by semiquantitative RT-PCR and western blot (Fig. 1A and B). In previous studies, CEACAM1 expression was high in SK-MEL-28 and HepG2 cells [17,32], as in our results, whereas it was absent in HT1080 cells and PC-3 cells.
Thus, to examine the relationship between CEACAM1 and VM, we analyzed HT1080 cells, which do not express CEACAM1. Enforced expression of CEACAM1 in HT1080 cells suppressed VM-like network formation (Fig. 2A–C). Because CEACAM1 is a cell-cell adhesion molecule [10], it is presumed that it self-aggregates to suppress VM-like network formation. Moreover, VM formation is often observed in ECM-rich regions, and CEACAM1 is a cell surface receptor of ECM components, such as collagen, laminin, and fibronectin [33]. These results suggest that cell surface receptors, including CEACAM1, mediate VM.
We determined the effects of the overexpression of CEACAM1 on cancer cell functions, such as migration and proliferation. The migration and proliferation of CEACAM1-overexpressing HT1080 cells decreased (Fig. 2D–F). In several cancer cell lines, CEACAM1 expression correlated with low invasiveness and migration, such as multiple myeloma and breast cancer [30,34]. Consistent with the VM results in HT1080 cells, our study suggests that CEACAM1 overexpression inhibits cancer progression.
Conversely, in SK-MEL-28 cells, deletion of CEACAM1 suppressed VM-like network formation, which was recovered by reintroducing CEACAM1 (Fig. 4A–C). These results indicate that CEACAM1 is necessary to form the VM-like network in SK-MEL-28 cells. We also analyzed the effects of CEACAM1-KO on migration and proliferation. KO of CEACAM1 suppressed cell migration but not cell proliferation in SK-MEL-28 cells (Fig. 3D–F). Similarly, CEACAM1 does not affect cell proliferation in melanoma [35].
In this study, we have demonstrated that CEACAM1 is an important cell-dependent, negative and positive regulator of VM-like network formation. Notably, our data are consistent with previous reports-CEACAM1 is oncogenic in melanoma, and in other tumors that express low levels of CEACAM1, it acts as a tumor suppressor. Thus, the level of CEACAM1 might be a good diagnostic marker for tumors, and VM might contribute CEACAM1-mediated malignancy.
CRediT authorship contribution statement
Soichiro Hayashi: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing - original draft. Yoshiyuki Osada: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Validation, Writing - original draft. Kazuki Miura: Formal analysis, Project administration, Supervision, Validation, Writing - original draft. Siro Simizu: Conceptualization, Formal analysis, Funding acquisition, Project administration, Supervision, Validation, Writing - review & editing.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (C) under Grant Number JP18K06137 (to SS).
Footnotes
Transparency document related to this article can be found online at https://doi.org/10.1016/j.bbrep.2020.100734
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2020.100734.
Transparency document
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Warren B.A., Shubik P. The growth of the blood supply to melanoma transplants in the hamster cheek pouch. Lab. Invest. 1966;4:652–653. [PubMed] [Google Scholar]
- 2.Folberg R., Hendrix M.J., Maniotis A.J. Vasculogenic mimicry and tumor angiogenesis. Am. J. Pathol. 2000;156:361–381. doi: 10.1016/S0002-9440(10)64739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu W.B., Xu G.L., Jia W.D., Wang Prognostic significance and mechanisms of patterned matrix vasculogenic mimicry in hepatocellular carcinoma. Med. Oncol. 2011;28:S228–S238. doi: 10.1007/s12032-010-9706-x. [DOI] [PubMed] [Google Scholar]
- 4.Wang W., Lin P., Sun B. Epithelial-mesenchymal transition regulated by EphA2 contributes to vasculogenic mimicry formation of head and neck squamous cell carcinoma. BioMed Res. Int. 2014;2014:803914. doi: 10.1155/2014/803914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li W., Zong S., Shi Q. Hypoxia-induced vasculogenic mimicry formation in human colorectal cancer cells: involvement of HIF-1a, Claudin-4, and E-cadherin and Vimentin. Sci. Rep. 2016;6:37534. doi: 10.1038/srep37534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M., Zhao X., Zhu D. HIF-1α promoted vasculogenic mimicry formation in hepatocellular carcinoma through LOXL2 up-regulation in hypoxic tumor microenvironment. J. Exp. Clin. Canc. Res. 2017;36:1–14. doi: 10.1186/s13046-017-0533-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu S., Yu L., Cheng Z. Expression of maspin in non-small cell lung cancer and its relationship to vasculogenic mimicry. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2012;32:346–352. doi: 10.1007/s11596-012-0060-4. [DOI] [PubMed] [Google Scholar]
- 8.Zhou X., Gu R., Han X., Wu G., Liu J. Cyclin-dependent kinase 5 controls vasculogenic mimicry formation in non-small cell lung cancer via the FAK-AKT signaling pathway. Biochem. Biophys. Res. Commun. 2017;492:447–452. doi: 10.1016/j.bbrc.2017.08.076. [DOI] [PubMed] [Google Scholar]
- 9.Ling Y., Wang J., Wang L. Roles of CEACAM1 in cell communication and signaling of lung cancer and other diseases. Canc. Metastasis Rev. 2015;34:347–357. doi: 10.1007/s10555-015-9569-x. [DOI] [PubMed] [Google Scholar]
- 10.Obrink B. CEA adhesion molecules: multifunctional proteinswith signal-regulatory properties. Curr. Opin. Cell Biol. 1997;9:616–626. doi: 10.1016/S0955-0674(97)80114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oikawa S., Kuroki M., Matsuoka Y., Kosaki G., Nakazato H. Homotypic and heterotypic Ca++-independent cell adhesion activities of biliary glycoprotein, a member of carcinoembryonic antigen family, expressed on CHO cell surface. Biochem. Biophys. Res. Commun. 1992;186:881–887. doi: 10.1016/0006-291x(92)90828-9. [DOI] [PubMed] [Google Scholar]
- 12.Obrink B. On the role of CEACAM1 in cancer. Lung Canc. 2008;60:309–312. doi: 10.1016/j.lungcan.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Song J.H., Cao Z., Yoon J.H. Genetic alterations and expression pattern of CEACAM1 in colorectal adenomas and cancers. Pathol. Oncol. Res. 2011;17:67–74. doi: 10.1007/s12253-010-9282-6. [DOI] [PubMed] [Google Scholar]
- 14.Tilki D., Irmak S., Oliveira-Ferrer L. CEA-related cell adhesion molecule-1 is involved in angiogenic switch in prostate cancer. Oncogene. 2006;25:4965–4974. doi: 10.1038/sj.onc.1209514. [DOI] [PubMed] [Google Scholar]
- 15.Yang C., He P., Liu L. Down-regulation of CEACAM1 in breast cancer. Acta Biochim. Biophys. Sin. 2015;47:788–794. doi: 10.1093/abbs/gmv075. [DOI] [PubMed] [Google Scholar]
- 16.Ashkenazi S., Ortenberg R., Besser M., Schachter J., Markel G. SOX9 indirectly regulates CEACAM1 expression and immune resistance in melanoma cells. Oncotarget. 2016;7:30166–30177. doi: 10.18632/oncotarget.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dango S., Sienel W., Schreiber M. Elevated expression of carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) is associated with increased angiogenic potential in non-small cell lung cancer. Lung Canc. 2008;60:426–433. doi: 10.1016/j.lungcan.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Xia Y., Ca X.Y., Fan J.Q. Rho kinase inhibitor fasudil suppresses the vasculogenic mimicry of B16 mouse melanoma cells both in vitro and in vivo. Mol. Canc. Therapeut. 2015;14:1582–1590. doi: 10.1158/1535-7163.MCT-14-0523. [DOI] [PubMed] [Google Scholar]
- 19.Williamson S.C., Metcalf R.L., Trapani F. Vasculogenic mimicry in small cell lung cancer. Nat. Commun. 2016;7:13322. doi: 10.1038/ncomms13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hulin J.A., Tommasi S., Elliot D. MiR-193b regulates breast cancer cell migration and vasculogenic mimicry by targeting dimethylarginine dimethylaminohydrolase 1. Sci. Rep. 2017;7:13996. doi: 10.1038/s41598-017-14454-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simizu S., Suzuki T., Muroi M. Involvement of disulfide bond formation in the activation of heparanase. Canc. Res. 2007;67:7841–7849. doi: 10.1158/0008-5472.CAN-07-1053. [DOI] [PubMed] [Google Scholar]
- 22.Niwa Y., Suzuki T., Dohmae N., Simizu S. O-fucosylation of CCN1 is required for its secretion. FEBS Lett. 2015;589:3287–3293. doi: 10.1016/j.febslet.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Yasukagawa T., Niwa Y., Simizu S., Umezawa K. Suppression of cellular invasion by glybenclamide through inhibited secretion of platelet-derived growth factor in ovarian clear cell carcinoma ES-2 cells. FEBS Lett. 2012;586:1504–1509. doi: 10.1016/j.febslet.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Simizu S., Umezawa K., Takada M., Arber N., Imoto M. Induction of hydrogen peroxide production and Bax expression by caspase-3(-like) proteases in tyrosine kinase inhibitor-induced apoptosis in human small cell lung carcinoma cells. Exp. Cell Res. 1998;238:197–203. doi: 10.1006/excr.1997.3823. [DOI] [PubMed] [Google Scholar]
- 25.Matsuki W., Miyazaki S., Yoshida K. Synthesis and evaluation of biological activities of vibsanin A analogs. Bioorg. Med. Chem. Lett. 2017;27:4536–4539. doi: 10.1016/j.bmcl.2017.08.059. [DOI] [PubMed] [Google Scholar]
- 26.Tang J., Wang J., Fan L. cRGD inhibits vasculogenic mimicry formation by down-regulating uPA expression and reducing EMT in ovarian cancer. Oncotarget. 2016;7:24050–24062. doi: 10.18632/oncotarget.8079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komai K., Niwa Y., Sasazawa Y., Simizu S. Pirin regulates the epithelial to mesenchymal transition independently of Bcl3-Slug signaling. FEBS Lett. 2015;589:738–743. doi: 10.1016/j.febslet.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 28.Ishida K., Wierzba M.K., Teruya T., Simizu S., Osada H. Novel heparan sulfate mimetic compounds as antitumor agents. Chem. Biol. 2004;11:367–377. doi: 10.1016/j.chembiol.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Kawahara R., Niwa Y., Simizu S. Integrin β1 is an essential factor in vasculogenic mimicry of human cancer cells. Canc. Sci. 2018;109:2490–2496. doi: 10.1111/cas.13693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu J., Liu B., Ma S. Characterizing the tumor suppressor role of CEACAM1 in multiple myeloma cell. Physiol. Biochem. 2018;45:1631–1640. doi: 10.1159/000487730. [DOI] [PubMed] [Google Scholar]
- 31.Ullrich N., Heinemann A., Nilewski E. CEACAM1-3S drives melanoma cells into NK cell-mediated cytolysis and enhances patient survival. Canc. Res. 2015;75:1897–1907. doi: 10.1158/0008-5472.CAN-14-1752. [DOI] [PubMed] [Google Scholar]
- 32.Hokari M., Matsuda Y., Wakai T. Tumor suppressor carcinoembryonic antigen-related cell adhesion molecule 1 potentates the anchorage-independent growth of human hepatoma HepG2 cells. Life Sci. 2007;81:336–345. doi: 10.1016/j.lfs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Gerstel D., Wegwitz F., Jannasch K. CEACAM1 creates a pro-angiogenic tumor microenvironment that supports tumor vessel maturation. Oncogene. 2011;30:4275–4288. doi: 10.1038/onc.2011.146. [DOI] [PubMed] [Google Scholar]
- 34.Yang C., Cao M., Liu Y. Inhibition of cell invasion and migration by CEACAM1-4S in breast cancer. Oncol. Lett. 2017;14:4758–4766. doi: 10.3892/ol.2017.6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ebrahimnejad A., Streichert T., Nollau P. CEACAM1 enhances invasion and migration of melanocytic and melanoma cells. Am. J. Pathol. 2004;165:1781–1787. doi: 10.1016/S0002-9440(10)63433-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.