Abstract
Objective:
The immune makers including CD4+CD25+ T cells, natural killer cells, and T cells subgroup were retrospectively analyzed to find the relationship between apatinib and the immune system in the patients treated with apatinib.
Method:
Forty-two patients with advanced malignant tumors orally took apatinib as treatment and 16 patients with the same situation did not take apatinib as a control group. These patients were all included in the study, and they orally received apatinib 500 mg daily as monotherapy or combination. The treatment was continued until disease progression or intolerable toxicity. CD4+CD25+ T cells, natural killer cells, and T cells subgroup were detected before and 1 month after therapy for all the patients. The relationship between the changing number of immune cells and progression-free survival was analyzed in this study.
Result:
For the apatinib group, the rate of CD4+CD25+ T cells significantly increased (P = .048). The median progression-free survival was 3.25 months for the 42 patients. The median progression-free survival in the patients with the rate of CD4+CD25+ T cells increased and decreased was 5.8 months and 2.9 months, respectively (P = .012). Multivariate analysis found the increased rate of CD4+CD25+ T cells was an independent prognostic factor for a longer progression-free survival. The rate of natural killer cells and T cells subgroup did not change much after apatinib therapy, and they were not independent prognostic factors for progression-free survival.
Conclusion:
The rate of CD4+CD25+ T cells is very important in patients with apatinib treatment. The changing number of CD4+CD25+ T cells may be a good indicator for apatinib prognosis. Natural killer cells and T cells subgroup did not change much after apatinib, and they were not independent prognostic factors for progression-free survival.
Keywords: CD4+CD25+ T cells, NK cells, T cells subgroup, apatinib, malignant tumor
Background
Apatinib is a kind of small-molecule inhibitor of vascular endothelial growth factor receptor 2 (VEGFR-2), the evidences coming from clinical study had demonstrated its encouraging anticancer activity in many malignant tumors, such as gastric cancer,1 colorectal cancer,2 esophagus cancer,3 ovarian cancer,4 and sarcoma.5 In clinic, we found the effect of apatinib varied widely in the different patients. Some patients obtained satisfied effect, and the others did not get any benefit from the drug in the same pathological type tumour. According to the mechanism of the drug, the difference of the clinical effects may be caused by the different expression of VEGFR-2. However, some unknown indicators may influent this different clinical efficacy. Apatinib have an extensive antitumor activity, and the oncologists did not need to check any markers to use it, so it is widely used in a variety of tumors now. So, this status quo cause the drug abuse to some extent, and some patients did not get benefit from the drug. As a result, for the oncologist there is an urgent need to identify some markers, which can help screening patients and predicting the prognosis.
Traditionally, some chemotherapy drugs can cause temporarily impaired blood cell production in the marrow and depressed immune system functions. However, in recent years, many studies have found some chemotherapy drugs can enhance the immunogenicity of the patients with tumor.6 Up to now, the influence of apatinib in the immune system has not reported. So, we create the hypothesis that there is some relationship between apatinib and the immune system, and we may try to find some immunity indicators which can be used to select the right patient. And then we may find some immune prognostic indicators for the use of apatinib.
In this study, we retrospectively analyzed the relationship between apatinib and the immune system. We attempt to analyze the immune markers, which included CD4+CD25+ T cells, natural killer (NK) cells, and T cells subgroup, in patients who take apatinib as treatment. And we try to find some immunity indicators which may be used for selecting the right patient or to find some immune prognostic indicators for the use of apatinib.
Methods
Patients
Between February 2017 and March 2018, 69 patients who took apatinib in Anhui Provincial Cancer Hospital (The First Affiliated Hospital of University of Science and Technology of China) were included in the study. Progression-free survival (PFS) time could be followed up of the patients. All the patients were diagnosed with advanced malignant tumor, and previously took more than one regime of treatment at least. Consents were obtained from all patients before the therapy of apatinib. In our department, the blood test of immune cells is a conventional checking for all patients. We got the rate of serum CD4+CD25+ T cells, NK cells, and T cells subgroup data from our hospital information system (HIS) system. The patients were treated with apatinib alone or combination. CD4+CD25+ T cells, NK cells, and T cells subgroup were detected in 3 days before receiving the apatinib treatment, and 1 month after treatment for all the patients. Forty-two of 69 patients who finished the immune cells detection before therapy and 1 month after therapy were included in the study. Of the 42 patients, 12 patients were gastric cancer. The other 30 patients were consisted of 9 patients with esophageal squamous cell carcinoma, 13 patients with colorectal cancer, 3 patients with ovarian cancer, 3 patients with sarcoma, 1 patient with malignant melanoma, and 1 patient with invasive thymoma. The median age was 57 years, the range from 24 to 76 years. The ratio of males and females in the study was 1.5:1 (25 males and 17 females). Seventeen patients had received regimens of apatinib in combination with one chemotherapy drug, the other 25 patients took apatinib as monotherapy. Fourteen patients had taken one regime of chemotherapy treatment previously and the other 28 patients had taken multiline chemotherapies before apatinib. Thirty-four of 42 patients had more than 1 organ metastasis. The primary analysis data consisted of age, gender, the rate of serum CD4+CD25+ T cells, the rate of serum NK cells and the rate of serum T cells subgroup, diagnosis, the therapy line of apatinib, chemotherapy regimens, and the number of metastatic organ. We also choose 16 patients who did not take apatinib as a control group (nonapatinib group). All of the 16 patients were diagnosed with advanced malignant tumor and did not take any therapy before the first time immune cells checking.
Immune Cell Detection Method
The rate of CD4+CD25+ T cells, NK cells, and T cells subgroup were all measured by flow cytometry. The following antibodies were used in our study: anti-CD4 antibody (Abcam, Cambridge, UK), anti-CD25 antibody (Abcam, Cambridge, UK), anti-CD127 antibody (Abcam, Cambridge, UK), anti-CD3 antibody (Abcam, Cambridge, UK), anti-CD16 antibody (Abcam, Cambridge, UK), anti-CD56 antibody (Abcam, Cambridge, UK), anti-CD8 antibody (Abcam, Cambridge, UK), and anti-CD45 antibody (Abcam, Cambridge, UK). Flow cytometry analysis was performed on a FACScan (BD Biosciences, Mountain View, California) using CellQuest software.
Statistical Analysis
Statistical analysis was conducted with SPSS version 16.0 (SPSS Inc, Chicago, Illinois). The difference of immune cells was analyzed by t test. The χ2 test was used as appropriate for the comparison of variables. The PFS was calculated by the Kaplan-Meier method, and compared by log-rank test. Cox proportional hazards regression model was performed to evaluate the prognostic factors for PFS. All statistical tests were 2 sided, and P values <.05 was considered statistically significant in all tests.
Results
In this study, 42 patients had finished the blood test of CD4+CD25+ T cells, NK cells, and T cells subgroup before and 1 month after apatinib therapy. The other 16 patients had also finished the above examination before and 1 month after chemotherapy (nonapatinib group). The results of immune cells were recorded in percentage form. For the apatinib group, the median age was 57 years, PFS was 3.25 months. There are 32 patients still alive at the end of the follow-up period (April 27, 2018), so the overall survival was not analyzed in this article. The median value of CD4+CD25+ T cells, NK cells, and T cells subgroup before the treatment was 12.06%, 16.75%, and 74.45%, respectively. The relationship between immune cells and patients’ characteristics is shown in Table 1. The number of patients having gastric cancer with elevated rate of T cells subgroup was larger than that of the other patients with malignant tumor (χ2 = 4.200, P = .040). The patients with more than 1 metastatic sites had a higher rate of NK cells than those with the single metastatic patients (χ2 = 5.559, P = .018). Kaplan-Meier method was used to analyze the relationship between PFS and the following factors. The factors were CD4+CD25+ T cells, NK cells, and T cells before therapy, gender, age, diagnosis, the therapy line of apatinib, monotherapy or combination, the number of metastatic sites. The results showing the above factors did not display a substantial correlation with PFS (Tables 2 and 3). In the control group, the characteristics of 16 patients and the relationship between immune cells was in Table 4. The median PFS in the control group was 5.95 months, we did not find the relationship of CD4+CD25+ T cells, NK cells, and T cells subgroup in different ages, genders, diagnosis, and the number of tumor metastasis.
Table 1.
Characteristics of 42 Patients and Relationship Between Immune Cells.
| Clinicopathologic Data | n | CD4+CD25+ | χ2 | P | NK | χ2 | P | T | χ2 | P | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥12.06 | <12.06 | ≥16.75 | <16.75 | ≥74.45 | <74.45 | ||||||||
| Age (years) | |||||||||||||
| ≤60 | 23 | 12 | 11 | 0.096 | .757 | 10 | 13 | 0.865 | .352 | 12 | 11 | 0.096 | .757 |
| >60 | 19 | 9 | 10 | 11 | 8 | 9 | 10 | ||||||
| Gender | |||||||||||||
| Male | 25 | 10 | 15 | 2.471 | .116 | 14 | 11 | 0.889 | .346 | 13 | 12 | 0.099 | .753 |
| Female | 17 | 11 | 6 | 7 | 10 | 8 | 9 | ||||||
| Diagnosis | |||||||||||||
| GC | 12 | 8 | 4 | 1.867 | .172 | 4 | 8 | 1.867 | .172 | 9 | 3 | 4.200 | .040 |
| Other | 30 | 13 | 17 | 17 | 13 | 12 | 18 | ||||||
| Line | |||||||||||||
| 2 | 14 | 9 | 5 | 1.714 | .190 | 5 | 9 | 1.714 | .190 | 9 | 5 | 1.714 | .190 |
| ≥3 | 28 | 12 | 16 | 16 | 12 | 12 | 16 | ||||||
| Therapy | |||||||||||||
| Single drug | 25 | 14 | 11 | 0.889 | .346 | 12 | 13 | 0.099 | .753 | 13 | 12 | 0.099 | .753 |
| Combined chemotherapy | 17 | 7 | 10 | 9 | 8 | 8 | 9 | ||||||
| Metastasis | |||||||||||||
| 1 | 8 | 6 | 2 | 2.471 | .116 | 1 | 7 | 5.559 | .018 | 2 | 6 | 2.471 | .116 |
| ≥2 | 34 | 15 | 19 | 20 | 14 | 15 | 19 | ||||||
Abbreviations: GC, gastric cancer; NK, natural killer
Table 2.
Kaplan-Meier Analysis of the Relationship Between PFS and Clinical Characteristics (Apatinib Group).
| Clinical Characteristics | Case | mPFS | 95% CI | P |
|---|---|---|---|---|
| Gender | ||||
| Male | 25 | 5.2 | 4.122-6.278 | .272 |
| Female | 17 | 2.6 | 0.675-4.525 | |
| Age (years) | ||||
| ≤60 | 23 | 4.2 | 1.999-6.400 | .839 |
| >60 | 19 | 4.6 | 2.116-7.084 | |
| Diagnosis | ||||
| Gastric cancer | 12 | 4.6 | 0.934-8.266 | .360 |
| The other cancer | 30 | 4.5 | 3.020-5.980 | |
| The therapy line of apatinib | ||||
| Second line | 14 | 4.4 | 3.934-4.866 | .983 |
| More than second line | 28 | 5.2 | 2.947-7.453 | |
| The number of metastases organ | ||||
| One | 8 | 4.5 | 0.000-9.792 | .464 |
| More than one | 34 | 4.4 | 2.281-6.519 | |
Abbreviations: CI, confidence interval; mPFS, median progression-free survival.
Table 3.
Influence of Serum CD4+CD25+ T Cells, NK Cells, and T Cells Subgroup in PFS (Apatinib Group).
| Immune Cells | Case | mPFS (Months) | 95% CI | P |
|---|---|---|---|---|
| CD4+CD25+ cells | ||||
| ≥12.06 | 21 | 4.2 | 2.104-6.296 | .620 |
| <12.06 | 21 | 5.2 | 2.778-7.622 | |
| CD4+CD25+CD127low/− | ||||
| ≥5.255 | 21 | 4.2 | 1.726-6.674 | .444 |
| <5.255 | 21 | 4.5 | 4.112-4.888 | |
| NK cells | ||||
| ≥16.75 | 21 | 4.6 | 3.855-5.345 | .673 |
| <16.75 | 21 | 3.3 | 0.681-5.345 | |
| T cells | ||||
| ≥74.45 | 21 | 4.5 | 1.972-7.028 | .519 |
| <74.45 | 21 | 4.4 | 2.120-6.680 | |
| Th cells | ||||
| ≥41.95 | 21 | 4.2 | 1.962-6.438 | .224 |
| <41.95 | 21 | 5.4 | 2.845-7.955 | |
| Ts cells | ||||
| ≥29.1 | 21 | 5.2 | 3.917-6.483 | .132 |
| <29.1 | 21 | 4.2 | 1.794-6.606 | |
| Th/Ts | ||||
| ≥1.495 | 21 | 4.2 | 1.794-6.606 | .197 |
| <1.495 | 21 | 5.2 | 3.913-6.487 |
Abbreviations: CI, confidence interval; mPFS, median progression-free survival; NK, natural killer.
Table 4.
The Characteristics of 16 Patients and the Relationship Between Immune Cells(Nonapatinib Group).
| Clinicopathologic data | n | CD4+CD25+ | χ2 | P | NK | χ2 | P | T | χ2 | P | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥ 12.06 | <12.06 | ≥16.75 | <16.75 | ≥ 74.45 | < 74.45 | ||||||||
| Age (years) | |||||||||||||
| ≤60 | 10 | 5 | 5 | 0.423 | .633 | 5 | 5 | 0.000 | 1.000 | 3 | 7 | 0.019 | 1.000 |
| >60 | 6 | 2 | 4 | 3 | 3 | 2 | 4 | ||||||
| Gender | |||||||||||||
| Male | 8 | 4 | 4 | 0.254 | 1.000 | 7 | 1 | 9.000 | .001 | 1 | 7 | 2.618 | .282 |
| Female | 8 | 3 | 5 | 1 | 7 | 4 | 4 | ||||||
| Diagnosis | |||||||||||||
| GC | 5 | 1 | 4 | 1.667 | .308 | 3 | 2 | 0.291 | 1.000 | 1 | 4 | 0.428 | 1.000 |
| Other | 11 | 6 | 5 | 5 | 6 | 4 | 7 | ||||||
| Metastasis | |||||||||||||
| 1 | 5 | 4 | 1 | 3.883 | .106 | 2 | 3 | 0.291 | 1.000 | 3 | 2 | 2.798 | .245 |
| ≥2 | 11 | 3 | 8 | 6 | 5 | 2 | 9 | ||||||
Abbreviations: GC, gastric cancer; NK, natural killer.
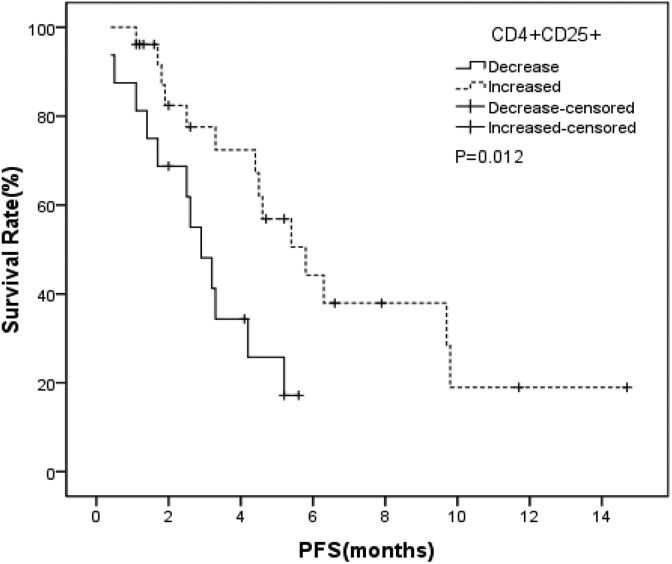
After 1 month therapy of apatinib, the rate of CD4+CD25+ T cells elevated significantly (T = −2.042, P = .048). Further analysis found the rate of CD4+CD25+CD127-/low cells, a subtype of CD4+CD25+ T cells, was increased significantly after 1 month therapy of apatinib (T = −2.996, P = .005). This phenomenon was not found in the rate of NK cells and T cells (Table 5). T cells are divided into Th cells and Ts cells. In this study, we did not find that the 2 subtypes have a distinct change after the therapy. Further analysis found that the patients with CD4+CD25+ T cells increased had a long PFS than those with CD4+CD25+ T cells decreased (5.8 months vs 2.9 months, P = .012; Table 6 and Figure 1). In multivariable analyses, the rate of CD4+CD25+ T cells increased after therapy was an independent influential factor of PFS, and it was the only prognostic factor for PFS in the patients who receiving apatinib as treatment in this study (Table 7). We also used multivariable analysis method to analyze the role of the rate of CD4+CD25+ T cells, NK cells, T cells before and after 1 month therapy in PFS, but we did not find any of the above factors was an independent prognostic factor. In the nonapatinib group, we also found the rate of CD4+CD25+ T cells elevated significantly (T = −2.223, P = .026), but we did not find this phenomenon had correlation with PFS. In multivariable analysis, none of the change of the CD4+CD25+ T cells, NK cells, and T cells was an independent prognostic factor for PFS (Table 8).
Table 5.
The Difference of Immune Cells Before and After Therapy (Apatinib Group).
| Immune Cells | CD4+CD25+ | CD4+CD25+CD127low/- | NK | T | Th | Ts | Th/Ts |
|---|---|---|---|---|---|---|---|
| Before | 13.50±5.53 | 5.37±2.19 | 18.25±8.51 | 72.21±11.62 | 40.32±10.93 | 30.68±10.29 | 1.50±0.71 |
| After | 15.36±6.12 | 6.34±2.19 | 17.36±9.75 | 73.21±11.34 | 40.74±11.08 | 30.97±10.53 | 1.56±0.86 |
| T | −2.042 | −2.996 | 0.760 | −0.906 | −0.428 | −0.416 | 0.874 |
| P | .048 | .005 | .452 | .370 | .671 | .680 | .387 |
Abbreviation: NK, natural killer.
Table 6.
The Influence of Serum CD4+CD25+ T Cells, NK Cells, and T Cells Subgroup Before and After Therapy in PFS (Apatinib Group).
| Immune Cells | Case | mPFS (months) | 95% CI | P |
|---|---|---|---|---|
| CD4+CD25+ cells | ||||
| Increased | 26 | 5.8 | 3.635-7.965 | .012 |
| Decrease | 16 | 2.9 | 1.804-3.999 | |
| NK cells | ||||
| Increased | 19 | 3.3 | 1.233-5.367 | .697 |
| Decrease | 23 | 4.6 | 3.438-5.762 | |
| T cells | ||||
| Increased | 25 | 4.4 | 2.588-6.212 | .880 |
| Decrease | 17 | 4.5 | 1.258-7.742 |
Abbreviations: CI, confidence interval; NK, natural killer; PFS, progression-free survival.
Figure 1.
The median progression-free survival of regulatory T cells increased and decreased group (apatinib group).
Table 7.
Multivariate Analysis of PFS in 42 Patients (Apatinib Group).
| Prognostic Factor | Disease-Free Survival (PFS) | ||
|---|---|---|---|
| Odds Ratio | 95% CI | P Value | |
| Age | 1.349 | 0.524-3.473 | .534 |
| Gender | 2.187 | 0.740-6.459 | .157 |
| CD4+CD25+ T cells change | 0.342 | 0.124-0.947 | .039 |
| NK cells change | 0.814 | 0.285-2.329 | .701 |
| T cells change | 0.963 | 0.374-2.480 | .937 |
| The therapy regime | 1.277 | 0.440-3.709 | .653 |
| Diagnosis | 2.597 | 0.869-7.758 | .087 |
| Metastatic organ number | 2.127 | 0.509-8.882 | .301 |
| The line of therapy | 1.008 | 0.347-2.930 | .998 |
Abbreviations: CI, confidence interval; NK, natural killer; PFS, progression-free survival.
Table 8.
Multivariate Analysis of PFS in 16 Patients (Nonapatinib Group).
| Prognostic Factor | Disease-Free Survival (PFS) | ||
|---|---|---|---|
| Odds Ratio | 95% CI | P Value | |
| Age | 3.656 | 0.790-16.913 | .097 |
| Gender | 7.101 | 0.185-272.001 | .292 |
| CD4+CD25+ T cells change | 1.832 | 0.288-11.652 | .521 |
| NK cells change | 2.322 | 0.037-149.726 | .690 |
| T cells change | 1.221 | 0.138-10.815 | .858 |
| Diagnosis | 0.592 | 0.076-4.583 | .615 |
| Metastatic organ number | 0.906 | 0.158-5.185 | .911 |
Abbreviations: CI, confidence interval; NK, natural killer.
Discussion
For many malignant tumors there were always no effective medicines available in the late stage. Apatinib is a small molecule which could inhibit VEGF-stimulated endothelial cell migration and proliferation and decrease tumor microvascular density.7,8 It was approved and launched in People’s Republic of China in 2014 as a subsequent-line treatment for patients with advanced gastric cancer. And the clinical trials of this drug in other tumors are currently undergoing in clinic. In our clinical work, we find apatinib is a new strategy for the treatment of a variety of solid tumors, such as non-small cell lung cancer, breast cancer, and hepatocellular carcinoma, esophageal cancer, sarcoma, and so on. During the apatinib medication, we have noticed an interesting phenomenon. Some patients have obtained good results, and these patients always have a very long PFS after the apatinib treatment. However, the others have the disease progression rapidly and die very soon. As apatinib was approved in advanced gastric cancer, it was also chosen for the other types of tumors because there was no effective medicines available, as well as intolerable chemotherapy. This status quo may be related to the poor prognosis. But in our work, we also find some patients with a good eastern cooperative oncology group (ECOG) cannot get a good result from apatinib. What’s more, up to now there is no effective prognostic indicator for the use of apatinib. This phenomenon makes us think apatinib may have an influence on immune system, and the immune system may be an important factor for the therapeutic effect.
CD4+CD25+ T cells is a subpopulation of suppressor T cells defined based on expression of CD4 and CD25. CD4+CD25+ T cell is an important subtype of T lymphocytes. It’s a group of regulatory T cells and is differ from Th1 and Th2 cells. CD4+CD25+ T cells, 5% to 10% of peripheral CD4+T cells, resemble anergic cells in vitro, playing an important role in peripheral immune tolerance, and playing a key role in the development of tumors.9-13 The immunosuppressive function of CD4+CD25+ T cells had gained widespread attention. Numerous studies have shown that CD4+CD25+ T cells play critical roles in maintaining homeostasis, mediating tolerance to allografts and surveillance of tumor. Previous studies showed elevated level of CD4+CD25+ T cells in tumor draining lymph nodes, including cervical, endometrial, gastric cancers, and melanoma.14-16
The depletion of CD4+CD25+ T cells can disrupt immunological unresponsiveness to autologous tumors, thus mediated effective immune responses to tumor cells.17,18 The CD4+CD25+ T cells can suppress all subsets of Vα24+NKT cells, which including Vα24+CD4-CD8- cells, Vα24+CD4+ and Vα24+CD8+, in both proliferation and cytokine production, such as interferon γ, interleukin-4 (IL-4), IL-13, and IL-10.19 So the elevated rate of CD4+CD25+ T cells suppressed the cytotoxic activity of Vα24+NKT cells and weakened the action of antitumor. Study had found CD4+CD25+ T cells was an important contributor to the development of tumors immune tolerance and they play a critical role in the induction of tolerance to tumor associated antigens and suppression of antitumor immunity.20 CD4+CD25+ T cells consist of natural Tregs (nTregs) and peripherally induced Tregs (iTregs).21 Natural Treg, with Foxp3 expression, are thymus derived, constitutively express CD25, and their mechanism of suppression is cell contact dependent.22 Induced Treg, adaptive Treg, is developing in the periphery, which Foxp3 is considered unstable. Induced Treg cells variably express CD25, and they work by cells contact and cytokine dependent mechanism.23,24 Researchers found CD4+CD25+ T cells limited beneficial responses by suppressing sterilizing immunity and compromising anti-tumor immunity.25 Many studies showed high rate of CD4+CD25+ Treg cells was closely related with poor prognosis.26,27 The levels of these cells in malignant tumor patients were higher than in non-neoplastic patients.28 Up to now, the inhibitory cytokines of IL-10, IL-35, and transforming growth factor β are known as the 3 key mediators of Tregs function.29,30
Given the above, increased levels of CD4+CD25+ T cells in the peripheral blood of patients with cancer were detected as compared to normal healthy control. And the previous research suggested the higher rate of CD4+CD25+ T cells should be connected with poor outcome. But in our study, we found the patients with CD4+CD25+ T cells increased after apatinib treatment had a better outcome. This means the elevated Treg cells were closely associated with longer PFS. But the rate of NK cells and T cells subgroup did not change much after apatinib, and they were not an independent prognostic factor for PFS. The result is contrary to conventional wisdom. This is an interesting phenomenon, and the mechanisms are worthy of further investigation.
Apatinib is a small molecule which could inhibit VEGF-stimulated endothelial cell migration. Vascular endothelial growth factor receptor 1 has been implicated in macrophage chemotaxis and tumor cell survival and invasion. Vascular endothelial growth factor receptor 2 is the primary angiogenic receptor, and apatinib is working on VEGFR2, hence inhibiting the formation of new blood vessels in the tumor tissues and the growth of tumor cells. Marek et al 31 found the CD4+ T cells produce significantly a high amount of VEGF in patients with type 1 diabetes. These VEGF maybe induce VEGFR overexpression, so that it can increase the efficiency of apatinib. A study found anti-VEGF(R) drug axitinib can increase the number of naïve CD8(+) T cells and central memory CD4+ and CD8+T.32 The phenomenon in our study may demonstrate that immunogenicity is activated by apatinib. A study found33 low dose chemotherapeutic drugs can be used clinically to reduce Treg-mediated immunosuppression. Apatinib may reduce inhibitory effect of Treg-mediated immunosuppression, and then the patients obtain a good therapeutic effect.
Conclusion
CD4+CD25+ T cells are very important for patients with apatinib treatment. The changing number of CD4+CD25+ T cells may be a good indicator for apatinib, even for anti-VEGF(R) drug prognosis.
Abbreviations
- IL
interleukin
- NK
natural killer
- mPFS
median progression-free survival
- PFS
progression-free survival
- VEGFR
vascular endothelial growth factor receptor
Footnotes
Authors’ Note: Our study was approved by the Ethics Committee of Anhui Provincial Hospital (The First Affiliated Hospital of University of Science and Technology of China West District; approval no. 2018-17). All patients provided written informed consents prior to enrollment in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Medical Scientific Research Foundation of Anhui Province, China (1808085MH234) (1408085MH179).
ORCID iD: Hui-Qin Luo  https://orcid.org/0000-0001-7550-3719
https://orcid.org/0000-0001-7550-3719
References
- 1. Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013;31(26):3219–3225. [DOI] [PubMed] [Google Scholar]
- 2. Liang L, Wang L, Zhu P, et al. Pilot study of apatinib as third-line treatment in patients with heavily treated metastatic colorectal cancer. Clin Colorec Can. 2018;17(3):30519–30594. [DOI] [PubMed] [Google Scholar]
- 3. Li J, Wang L. Efficacy and safety of apatinib treatment for advanced esophageal squamous cell carcinoma. Onco Targ Ther. 2017;10:3965–3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miao M, Deng G, Luo S, et al. A phase II study of apatinib in patients with recurrent epithelial ovarian cancer. Gynecol Oncol. 2018;148(2):286–290. [DOI] [PubMed] [Google Scholar]
- 5. Xie L, Guo W, Wang Y, Yan T, Ji T, Xu J. Apatinib for advanced sarcoma: results from multiple institutions’ off-label use in China. BMC Can. 2018;18(1):396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu WM, Fowler DW, Smith P, Dalgleish AG. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer. 2010;102(1):115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011; 102(7):1374–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li J, Zhao X, Chen L, et al. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer. 2010;10:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193(11):1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levings MK, Sangregorio R, Roncarolo MG. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193(11):1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dieckmann D, Plottner H, Berchtold S, et al. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193(11):1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167(3):1245–1253. [DOI] [PubMed] [Google Scholar]
- 13. Ng WF, Duggan PJ, Ponchel F, et al. Human CD4(+)CD25(+) cells: a naturally occurring population of regulatory T cells. Blood. 2001;98(9):2736–2744. [DOI] [PubMed] [Google Scholar]
- 14. Kawaida H, Kono K, Takahashi A, et al. Distribution of CD4+CD25high regulatory T-cells in tumor-draining lymph nodes in patients with gastric cancer. J Surg Res. 2005;124(1):151–157. [DOI] [PubMed] [Google Scholar]
- 15. Matsuura K, Yamaguchi Y, Ueno H, Osaki A, Arihiro K, Toge T. Maturation of dendritic cells and T-cell responses in sentinel lymph nodes from patients with breast carcinoma. Cancer. 2006;106(6):1227–1236. [DOI] [PubMed] [Google Scholar]
- 16. Viguier M, Lemaître F, Verola O, et al. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173(2):1444–1453. [DOI] [PubMed] [Google Scholar]
- 17. Onizuka S, Tawara I, Shimizu J, et al. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59(13):3128–3133. [PubMed] [Google Scholar]
- 18. Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163(10):5211–5218. [PubMed] [Google Scholar]
- 19. Azuma T, Takahashi T, Kunisato A, Kitamura T, Hirai H. Human CD4+CD25+ regulatory T cells suppress NKT cell functions. Cancer Res. 2003;63(15):4516–4520. [PubMed] [Google Scholar]
- 20. Chen X, Du Y, Lin X, et al. CD4+CD25+ regulatory T cells in tumor immunity. Int Immunopharmacol. 2016;34:244–249. [DOI] [PubMed] [Google Scholar]
- 21. Ohkura N, Hamaguchi M, Morikawa H, et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012;37(5):785–799. [DOI] [PubMed] [Google Scholar]
- 22. Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. [DOI] [PubMed] [Google Scholar]
- 23. Zelenay S, Lopes-Carvalho T, Caramalho I, et al. Foxp3+ CD25−CD4 T cells constitute a reservoir of committed regulatory cells that regain CD25 expression upon homeostatic expansion. Proc Natl Acad Sci U S A. 2005;102(11):4091–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cassis L, Aiello S, Noris M. Natural versus adaptive regulatory T cells. Contrib Nephrol. 2005;146:121–131. [DOI] [PubMed] [Google Scholar]
- 25. Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shen X, Li N, Li H, Zhang T, Wang F, Li Q. Increased prevalence of regulatory T cells in the tumor microenvironment and its correlation with TNM stage of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2010;136(11):1745–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fu J, Xu D, Liu Z, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132(7):2328–2339. [DOI] [PubMed] [Google Scholar]
- 28. Hontsu S, Yoneyama H, Ueha S, et al. Visualization of naturally occurring Foxp3+ regulatory T cells in normal and tumor-bearing mice. Int Immunopharmacol. 2004;4(14):1785–1793. [DOI] [PubMed] [Google Scholar]
- 29. von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6(4):338–344. [DOI] [PubMed] [Google Scholar]
- 30. Wing JB, Sakaguchi S. Multiple Treg suppressive modules and their adaptability. Front Immunol. 2012;3:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marek N, Myśliwiec M, Raczyńska K, Zorena K, Myśliwska J, Trzonkowski P. Increased spontaneous production of VEGF by CD4+ T cells in type 1 diabetes. Clin Immunol. 2010;137(2):261–270. [DOI] [PubMed] [Google Scholar]
- 32. Du Four S, Maenhout SK, Benteyn D, et al. Disease progression in recurrent glioblastoma patients treated with the VEGFR inhibitor axitinib is associated with increased regulatory T cell numbers and T cell exhaustion. Cancer Immunol Immunother. 2016;65(6):727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mills KH, McGuirk P. Antigen-specific regulatory T cells—their induction and role in infection. Semin Immunol. 2004;16(2):107–117. [DOI] [PubMed] [Google Scholar]