Abstract
The current study was aimed to study the effect of curcumin on the expression levels of brain glucose transporter 1 protein (GLUT1) and femoral muscle glucose transporter 4 protein (GLUT4), in addition to study its possible therapeutic role in ameliorating insulin resistance and the metabolic disturbance in the obese and type 2 diabetic male albino Wistar rat model. Diabetes was induced by a high-fat (HF) diet with low dose streptozotocin (STZ). Curcumin was administered intragastrically for 8 weeks (80 mg/kg BW/day). The HF-diet group developed obesity, hyperglycemia, hyperinsulinemia, reduced liver glycogen content with significant dyslipidemia. In the diabetic control group, hyperglycemia and insulin resistance high calculated homeostasis model assessment (HOMA-IR-index score) were pronounced, with reductions in liver and muscle glycogen contents, concomitant with dyslipidemia and significantly elevated malondialdehyde levels in liver and pancreas. GLUT1 and GLUT4 were down-regulated in the obese and the diabetic control groups, respectively. Curcumin, showed glucose-lowering effect and decreased insulin resistance, dyslipidemia and malondialdehyde levels in both tissues, it increased liver & muscle glycogen contents, compared to the diabetic control. Curcumin significantly up-regulated GLUT4 gene expression, compared to the diabetic control group. In conclusions, these results indicate a therapeutic role of curcumin in improving the diabetic status, obesity and enhancing the expression of GLUT4 gene.
Keywords: Curcumin, Insulin resistance, GLUT1, GLUT4
1. Introduction
Insulin resistance (IR) in obesity patients with Type 2 diabetes mellitus (T2DM) associated diseases such as hypertension and hyperlipidemia are well-known as major health problems worldwide (Yuzefovych et al., 2019). Aging and IR considered as risk factors for the development of T2DM (Dos Santos et al., 2012). T2DM characterized by hyperglycemia in perspective to IR found to affect at least 285 million worldwide and its frequency is expected to increase continuously (Khan et al., 2019). T2DM is vigorously linked to obesity and pathological lipid deposition in the non-adipose tissues, which is suggested in contributing the development of IR (Amrutkar et al., 2015). In the glucose metabolism, insulin is the main regulatory function in multiple tissues such as adipocyte, liver and muscle through redistributing glucose transporter (GLUT4), which regulates the insulin stimulated glucose transport in adipose tissues and skeletal muscles. Glucose transporter protein content is altered under pathological conditions such T2DM (Prabhakar and Doble, 2011, Summers et al., 1998). Once insulin binds to its receptor, it regulates different cellular responses such as glucose uptake, glycogen synthesis and lipogenesis mainly in the targeting tissue. IR in both muscle and adipocyte tissues results in the dysregulation in hormonal control, which leads to the prone of T2DM disease. The pathogenesis mechanisms of IR is poorly discussed (Mlinar et al., 2007). The facilitative Glucose transport across cellular membrane via the GLUT protein family. GLUT1 protein is accountable uptake in cells. GLUT4 protein forms the cytoplasm vesicles through the sarcolemmal plasma membrane. However, both GLUTs are major glucose transporters (Carbo and Rodríguez, 2019). Mueckler (Mueckler, 2001) in his study has already acknowledged the connection between the IR and GLUT4 in the skeletal muscle.
Some natural products as herbs have been used as a source for novel medicine due to their ability to treat enormous types of human diseases. The chemical therapeutic treatments found to have dangerous side-effects, which could not noticed with herbal medicines, therefore it has grabbed the more attention (Maheshwari et al., 2006, Metzler et al., 2013, Witkin and Li, 2013). Turmeric is known to be the one of the important herbs and its active component is known to be Curcumin, which is used as treatment for the different human diseases. Biological and pharmacological effects of Curcumin have been previously described both in vivo and in vitro (Pivari et al., 2019). The efficiency of Curcumin involves diabetes, cancer and cardiovascular diseases; however, abundant studies have been documented that Curcumin was more effective than Turmeric in treating T2DM (Lin et al., 2014, Phan et al., 2001, Piwocka et al., 2002). Numerous biological and pleotropic activities were involved with curcumin as anti-proliferative, anti-inflammatory anti-bacterial and anti-oxidant agents (Rivera-Mancía et al., 2018). Curcumin contents of polyphenol-enriched fraction showed increase in the anti-diabetic effect through increasing GLUT4 levels in C2C12 skeletal muscle myotube cells (Accurso, 2004, Aggarwal and Sung, 2009, Babu and Srinivasan, 1995, Babu and Srinivasan, 1997, Suryanarayana et al., 2005). Although, numerous studies have demonstrated the curcumin effect on insulin signaling gene expression (AMPK, ERK, IRS1, PI3K) and GLUT4 uptake from the cytoplasm to the cell-membrane (Mlinar et al., 2007, Accurso, 2004, Aggarwal and Sung, 2009, Babu and Srinivasan, 1995, Babu and Srinivasan, 1997, Suryanarayana et al., 2005, Arun and Nalini, 2002). Limited or no studies were documented with the combination of curcumin and its effect on GLUT4 gene expression analysis. Due to the fact that Insulin has the major regulatory role in glucose metabolism in different tissues, it looks crucial to study the effect of curcumin on the expression level of GLUT1 and GLUT4 genes.
2. Materials and methods
2.1. Study details
This study has been carried out with 35 adult-male albino Wistar rats (150–170 g, each). They were obtained from the breeding unit of Ophthalmology National Institute (ONI), Cairo, Egypt. In plastic cages, animals were housed under standard conditions (12 h light-dark cycles, at 22 °C). Normal diet (N-diet) or high-fat diet (HF-diet) were provided to rats for a couple of weeks prior to the experiment. The HF-diet consisted of 37.70% (wt/wt) corn starch, 10% sucrose, 20% protein (casein), high-fats (20% lard and 2% soy-bean oil), 5%cellulose, 1% vitamin mixture, 4% AIN-93 mineral premix, 0.3% L-methionine. The normal chow diet consisted of the same ingredients except using 52.70% corn starch and 7% soy-bean oil instead of lard (Piwocka et al., 2002). Animals were maintained according to the rules and regulations of the Ain Shams University Ethics Committee for Experimental Animals.
Curcumin (Sigma-Aldrich Chemie Gmbh, Steinheim, Germany) was liquefied in pure water and an oral dose of 80 mg was given per day (Murugan and Pari, 2007).
2.2. Study design
Animals were divided into 5 groups with 7 rats each.
Group-I is defined as Normal rats; throughout the experiment, rats were fed-up with N-diet.
Group-II is non-diabetic HF-diet control rats; only citrate-buffer were given to the rats.
Group-III: Non-diabetic Curcumin treated rats; Curcumin was given regularly for 8-weeks by gastric intubation after 48-hours of STZ injection (Sigma Chemical Co., St. Louis, USA).
Group-IV is known as Diabetic control rats; each rat injected with 35 mg/kg BW STZ dissolved in citrate buffer (pH 4.5).
Group-V is defined as diabetic-Curcumin treated rats; Rats were treated as the previous diabetic control group, then Curcumin was given on the regular basis for 8 weeks.
2.3. Sample collection
Every weak, body weight of all rats was measured throughout the experiment. After completion of 10 weeks, rats with holding sustenance for 16 h. Serum was separated and stored at −4 °C. Muscle and pancreas were separated and frozen in the liquid nitrogen for further biochemical analysis.
Gene expression analysis was performed with GLUT1 and GLUT4 genes using muscle and brain tissues.
2.4. Biochemical analysis
At the end of the study, serum glucose concentrations were determined by colorimetric methods, after the enzymatic reaction with peroxidase (Biodiagnostic, Cairo, Egypt), and insulin levels via Immulite insulin kit (on Immulite 1000, Siemens Medical Solutions, Diagnostic Products Corporation, Los Angeles, CA, USA). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as the product of the fasting serum glucose (mg/dl) and fasting insulin levels (µU/ml) divided by a constant (405). Glycogen concentrations of liver and muscular (from thigh) tissues were determined as described by Plummer (Aggarwal and Sung, 2009). Serum total protein was estimated using Biuret test (Diamond kit, Cairo, Egypt), triglycerides, total cholesterol, high density lipoprotein cholesterol (HDL-Ch) and low density lipoprotein cholesterol (LDL-Ch) were determined by a colorimetric method (Diamond kits, Cairo, Egypt) (Khan et al., 2019). Liver & pancreas malondialdehyde (MDA) contents were measured by preparation of 10% homogenates in 1.15% KCl, centrifuged at 1000g at 4 °C for 20 min, and the resultant supernatants were used for the assay as described by Yoshioka et al. (1979).
2.5. Gene-expression studies
RNA extraction was performed using 100 mg of rats’ tissue (muscle and brain) were collected in the RNAlater solution. Using the RNA isolation kit, RNA was extracted as per the kit provided by RNeasy mini kit. Nanodrop was used to measure the concentrations and purity of RNA (Khan et al., 2015, Khan et al., 2015). cDNA was obtained from a sample of 1 μg of tissue- derived RNA on a conventional thermal cycler. This cDNA will be applied for (RT-PCR). Both the GLUT1 and GLUT4 specific primers oligonucleotides will be used for the synthesis. PCR amplification will be performed in the triplicates in a total volume of 20 μl with SYBR green master-mix. The 18S rRNA house keeping gene was used for the normalization.
2.6. Statistical analysis
Analyses were performed with SPSS version 16 software for Windows (SPSS, Chicago, IL). Data were expressed as mean ± S.E. Differences between groups were tested for statistical significance using one-way analysis of variance (ANOVA), followed by Tukey’s test. A P < 0.05 was considered significant for all data analyses.
3. Results
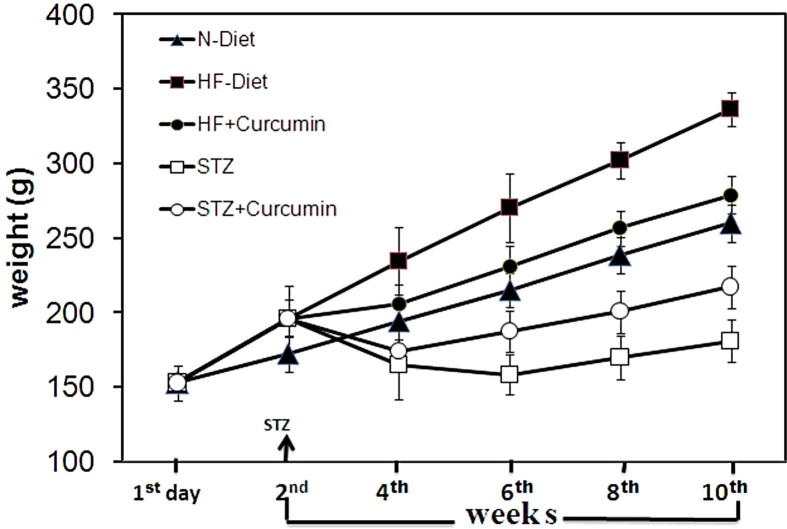
The initial body weight was found to be similar in all groups. Gradual and significant increase in obesity was detected in HF-Diet groups compared to N-Diet groups (p < 0.001). Significant weight loss was observed in diabetes-control rats compared to obesity rats (p < 0.001). In obesity rats, significant weight loss was detected using the Curcumin, however, it recovers strongly from the body weight loss in the diabetic rats starting from sixth week until the completion of the experiment (Table 1 and Fig. 1).
Table 1.
Final body weight, serum glucose and insulin concentrations, HOMA-IR, liver & muscle glycogen contents, and serum total protein, in experimental groups of rats.
| Groups | |||||
|---|---|---|---|---|---|
| Parameters | HF-Diet |
||||
| N-diet Control | HF-diet Control | Curcumin | Diabetic (STZ) | Diabetic(STZ) + Curumin | |
| Final body weight (g) | 259.57 ± 2.18 | 336.29 ●●● ±1.52 | 278.57+++±3.05 | 180.86+++ ±4.12 | 216.86***±2.60 |
| Glucose (mg/dl) | 72.70 ± 1.65 | 88.41●● ±2.50 | 73.66++ ±1.78 | 291.55+++ ±4.77 | 160.77***±2.34 |
| Insulin (µU/ml) | 2.49 ± 0.18 | 3.66●● ±0.17 | 2.45+++±0.18 | 1.67+++ ±0.14 | 1.90 ± 0.22 |
| HOMA-IR | 0.45 ± 0.04 | 0.80● ±0.04 | 0.44+±0.03 | 1.21 ++±0.11 | 0.76***±0.09 |
| Liver glycogen (mg/g fresh tissue) | 35.98 ± 1.60 | 29.52●●±0.99 | 30.70 ± 0.93 | 17.90+++ ±0.62 | 22.37*±0.30 |
| muscle glycogen (mg/g fresh tissue) | 6.67 ± 0.32 | 6.04 ± 0.29 | 5.94 ± 0.28 | 1.97+++±0.21 | 3.34***±0.21 |
| Total protein (g/dl) | 8.03 ± 0.17 | 7.48 ± 0.22 | 7.51 ± 0.21 | 3.91+++±0.09 | 5.53***±0.21 |
Data are presented as mean ± S.E. (n = 7).
Significantly different from N-diet control: ●p < 0.05; ●●p < 0.01; ●●●p < 0.001.
Significantly different from HF-diet control: +p < 0.05; ++p < 0.01; +++p < 0.001.
Significantly different from non-treated diabetic control:* p < 0.05;*** p < 0.001.
Fig. 1.
Measurement of the body weight on Day1. After couple of weeks and on fourth, sixth, eighth and tenth weeks of the experiment with STZ supervision day.
Hyperglycemic were observed with non-diabetic obese rats (p < 0.001); results of HOMA-IR confirmed them as IR when compared to the N-diet group (Table 1). The STZ diabetogenic rats clubbed with HF-diet shows IR with clear T2DM through severe hyperglycemia; detects in HOMA-IR (Table 1). Glucose levels were decreased after Curcumin treatment and the status of IR was decreased using HOMA-IR levels in diabetes and obese groups (p < 0.05–0.001).
In the obese rats, the liver glycogen content was decreased (Table 1). T2DM rats also revealed significant reduction in glycogen and serum total protein content compared to non-diabetic obese controls (p < 0.001). While diabetic rats Curcumin treatment increased glycogen content in liver compared to non-treated diabetic groups (p < 0.001).
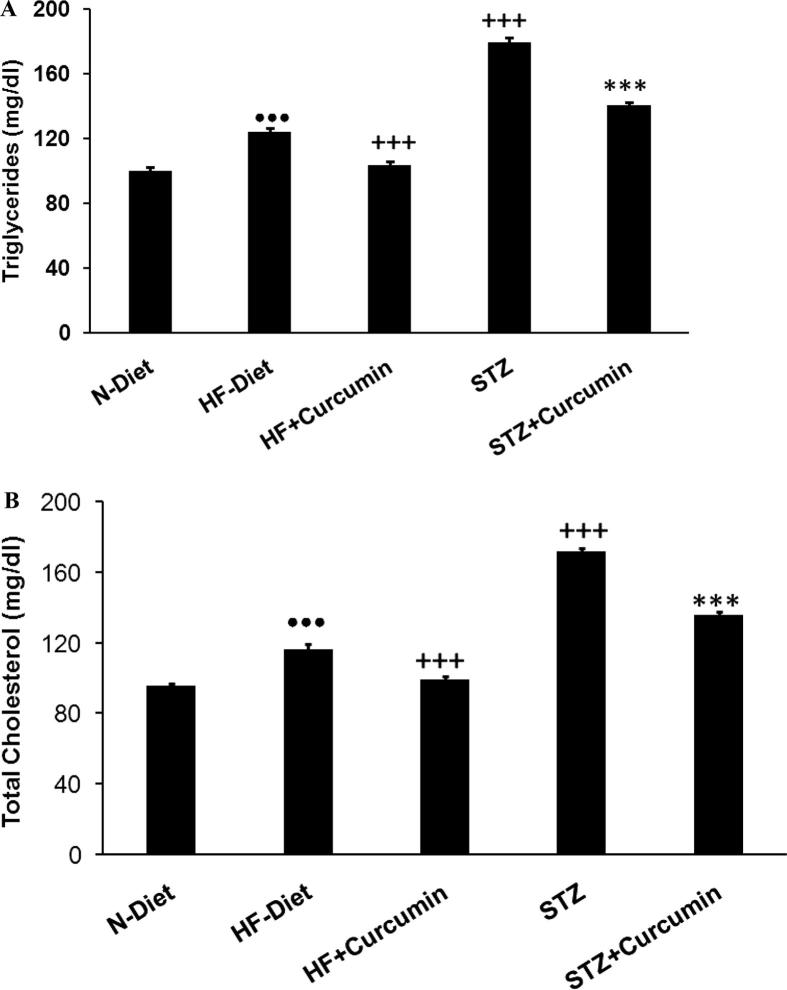
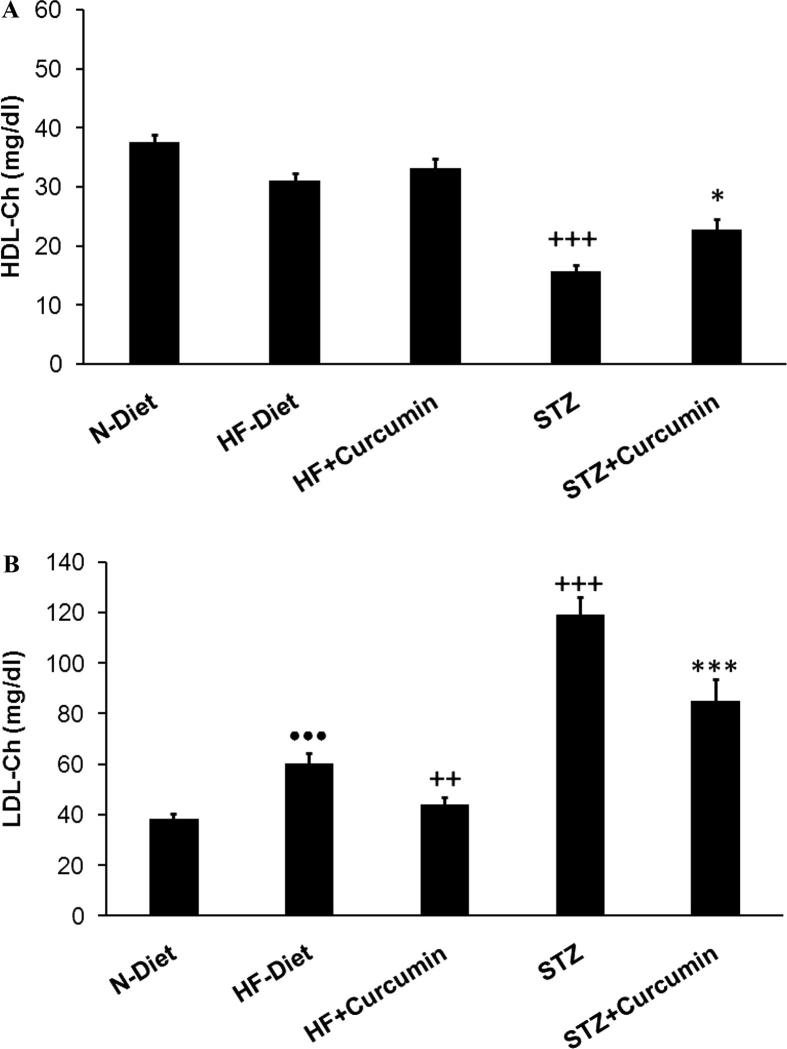
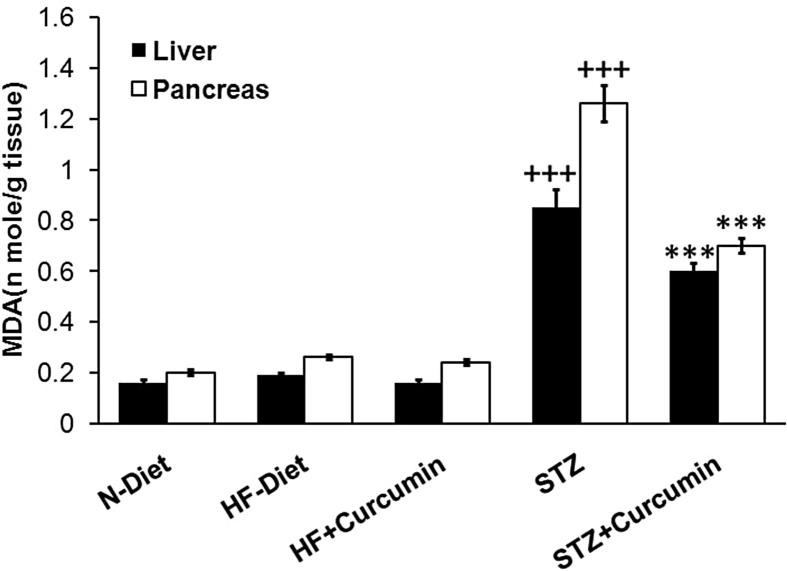
Hypertriglyceridemia, hypercholesterolemia, and high LDL-Ch levels were observed in obese rats (p < 0.001). Dyslipidemia was more pronounced in diabetic rats than obese control group with low levels of HDL-C (Figs. 2A, B and 3A and B). In obese and T2DM groups, the Curcumin treatment has lowered the triglycerides, total cholesterol, and LDL-Ch, while it elevates HDL-C levels compared to diabetic control group. MDA-levels were elevated in liver and pancreas of T2DM group (p < 0.001), these effects were reversed significantly via administration of Curcumin (Fig. 4).
Fig. 2.
(A) serum-TG and (B) serum-TC levels were measured in H-diet non-diabetic vs diabetic rats treated with curcumin and controls. N-diet normal group rats were refereed as controls.
Fig. 3.
(A) HDL-c serum and (B) LDL-c levels measured in H-diet non-diabetic; diabetic rats were treated with curcumin treatment. Controls were referred to as N-diet normal group.
Fig. 4.
MDA levels in both liver and pancreas measured in H-diet non-diabetic; diabetic rats were treated with curcumin by controls as N-diet group.
RT-PCR was used to perform comparative gene expression analysis. This experiment showed that the obese and diabetic rats exhibited down-regulation of both glucose transporter genes. Compared to normal control group, down-regulation for GLUT1 gene expression of the HF-diet control and the diabetic control was observed with a value of 0.20 and 0.09, respectively. Down-regulation of GLUT4 expression was also observed with values of 0.16 and 0.001 for HF-diet control and the diabetic control, respectively compared to normal control group (Table 2).
Table 2.
Average CT & 18S rRNA average CT measured, and ΔCT, ΔΔCT & fold difference calculated, for both Glut1&Glut4; in the N-diet, the HF-diet control group and the control diabetic group. The N-diet control group was considered as the reference control.
| Groups | Glut1 |
Glut4 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glut1 average CT | 18S rRNA Average CT | ΔCT | ΔΔCT | 2−ΔΔCt | Glut4 Average CT | 18S rRNA Average CT | ΔCT | ΔΔCT | 2−ΔΔCt | |
| N-diet | 31.22 | 28.16 | 3.06 | 0 | 1 | 36.28 | 35.43 | 0.85 | 0 | 1 |
| HF-diet | 30.33 | 24.97 | 5.36 | 2.30 | 0.20 | 33.64 | 30.13 | 3.51 | 2.66 | 0.16 |
| Diabetic control | 30.65 | 24.19 | 6.46 | 3.40 | 0.09 | 33.65 | 23.12 | 10.53 | 9.68 | 0.001 |
Where ΔCT = Glut1 or Glut4 CT- 18S rRNA CT
ΔΔCT = ΔCT treated -ΔCT nontreated(corresponding control).
2−ΔΔCt = Fold difference in Glut 1 or Glut 4 relative to the nontreated control.
However, Curcumin-treated and STZ-induced diabetic groups showed down-regulation of GLUT1 gene expression analysis in brain tissue, which reached 0.99 and 0.47 of HF-diet control group, respectively (Table 3). Moreover, down-regulation of the GLUT4 gene expression reaching 0.02 compared to control group was observed after Curcumin treatment. These results indicated that diabetogenesis down-regulated the gene expression, reaching 0.01 of the corresponding HF-diet control group (Table 3). The Curcumin-treated diabetic group, showed down-regulation of GLUT1 gene expression of 0.81 that of the diabetic control. However, Curcumin caused up-regulation of GLUT4 gene expression. The expression change was 8.07 folds that of the diabetic control (Table 4).
Table 3.
Average CT & 18S rRNA average CT measured, and ΔCT, ΔΔCT & fold difference calculated, for both Glut1&Glut4; in the HF-diet control group, nondiabetic control groups treated with curcumin, and the nontreated diabetic group. The HF-diet control group was considerd as the reference control.
| Groups | Glut1 |
Glut4 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glut1 average CT | 18S rRNA average CT | ΔCT | ΔΔCT | 2−ΔΔCt | Glut4 average CT | 18S rRNA average CT | ΔCT | ΔΔCT | 2−ΔΔCt | |
| Diabetic control | 30.65 | 24.19 | 6.46 | 0 | 1 | 33.65 | 23.12 | 10.53 | 0 | 1 |
| Curcumin | 30.06 | 23.30 | 6.76 | 0.3 | 0.81 | 31.97 | 24.51 | 7.46 | −3.07 | 8.40 |
Where ΔCT = Glut1 or Glut4 CT- 18S rRNA CT
ΔΔCT = ΔCT treated -ΔCT nontreated(corresponding control).
2−ΔΔCt = Fold difference in Glut 1 or Glut 4 relative to the nontreated control.
Table 4.
Average CT & 18S rRNA average CT measured, and ΔCT, ΔΔCT & fold difference calculated, for both Glut1&Glut4; in the diabetic control group, and diabetic groups treated with curcumin. The diabetic control group was considerd as the reference control.
| Groups | Glut1 |
Glut4 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glut1 average CT | 18S rRNA average CT | ΔCT | ΔΔCT | 2−ΔΔCt | Glut4 average CT | 18S rRNA average CT | ΔCT | ΔΔCT | 2−ΔΔCt | |
| HF-diet | 30.33 | 24.97 | 5.36 | 0 | 1 | 33.64 | 30.13 | 3.51 | 0 | 1 |
| Curcumin | 30.81 | 25.44 | 5.37 | 0.01 | 0.99 | 32.37 | 22.95 | 9.42 | 5.91 | 0.02 |
| Diabetic control | 30.65 | 30.65 | 24.19 | 1.10 | 0.47 | 33.65 | 23.12 | 10.53 | 7.02 | 0.01 |
Where ΔCT = Glut1 or Glut4 CT- 18S rRNA CT.
ΔΔCT = ΔCT treated -ΔCT nontreated(corresponding control).
2−ΔΔCt = Fold difference in Glut 1 or Glut 4 relative to the nontreated control.
4. Discussion
The current study, Curcumin treatment lowered the insulin level, significant hyperglycemia and acute IR; in H-diet and STZ diabetic rats compared in HF-diet control group + T2DM. However, this study was found to be in accordance with the Herlein et al. (2010).
In this study, Curcumin treatment strongly improves glucose level and body weight of the diabetic-rats (Kuhad and Chopra, 2007). The combination of Curcumin and tetrahydro-Curcumin dose of 80 mg/kg BW for 45 days has decreased the glucose levels in STZ/NA-induced rats in T2DM (Murugan and Pari, 2007, Murugan and Pari, 2007). Thus, these anti-hyperglycemic effects of Curcuminoids could be the indirect actions, which results in lowering the plasma levels in free fatty-acids in the liver, which lowers the hepatic glucose production (Pongchaidecha et al., 2009). It has been documented that, Curcumin to improves the insulin release through induction of β-cell in the electrical activity (Best et al., 2007).
Seo et al. (2008) confirmed the role of Curcumin treatment in the glycogen of both liver and muscle in diabetic groups could be linked to the glucokinase activity and glycogen content, which has been repressed through enzyme G6PD and phosphoenolpyruvate carboxykinase. Curcumin is known to increase the liver glycogen synthesis in fasting BALB/c through inhibition of glycogen synthase kinase-3-β (Bustanji et al., 2009). The current study recommends the advancement of Curcumin treatment in IR increases the serum and protein levels and confirms positive investigation (Murugan and Pari, 2007).
The hypolipidemic effect on both Curcumin and tetrahypocurcumin on STZ6/NA diabetic rats has been documented. Their treatments resulted in lowering the serum and liver cholesterol, TG, phospholipids, free fatty-acids, TC, VLDL and LDL-C levels. HDL-c levels were lowers in diabetic rats, which leads to the normalization after the treatment. Our study founds be similar to previous studies (Pongchaidecha et al., 2009, Seo et al., 2008). However, other study confirms Curcumin stimulates the hepatic cholesterol-7α-hydroxylase activity, which reflects the high levels of hepatic LDL-R and lipoprotein lipase; thus, leads to the hypocholesterolemic and hypotriglyceridemic effects in the animals with diabetes (Babu and Srinivasan, 1997).
This study also documents the reduction of lipid-peroxidation levels, which might be due to the presence of β-diketone and phenolic hydroxyl groups. Curcumin has antioxidant and anti-inflammatory properties more effective than vitamin-E (Aggarwal and Shishodia, 2006) via increasing the intra-cellular glutathione, normalizing antioxidant enzymes and scavenging of free-radicals by preventing the lipid peroxidation (Osawa and Kato, 2005). Moreover, low levels of oxidative stress by diabetic rats, which may be attributed to lower the influx of glucose into the polyol pathway. This leads to high levels of NADPH-NAPD ratio and an elevated activity in the antioxidant enzyme glutathione peroxidase (Aggarwal et al., 2005). This protective effect of Curcumin against oxidative-stress is in agreement with previous study using type1 diabitic rats (Amin et al., 2010).
Down-regulation of GLUT1 gene was observed in the brain tissue in HF-diet and the diabetic control groups compared to the N-diet control group, could be due to IR and obesity, which arises in HF-diet treatment and STZ-management. The current study results were similarly documented in the previous study with the IR.
Pardridge et al. (1990) concludes that GLUT1 gene expression was reduced by 77% in the STZ-induced diabetes rats in a weak when compared in the non-diabetic rats. The similar experiment performed for 8 weeks shows the 60% of GLUT1 expression levels when compared with non-diabetic rats (Badr et al., 2000). GLUT1 gene expression studies were majorly reported its down-regulation in the brains of diabetic rats (Hou et al., 2007, Iwata et al., 2010).
In the diabetic rats, chronic hyperglycemia was documented with down-regulation in both GLUT1 and GLUT3 mRNA and protein expression levels in the brain, which is known to have the negative effect in the glucose levels. The down-regulation is known to maintain the abnormal levels of hyperglycemia for long-term to evade cell-damage occurs with the excess glucose enters into the cells is known as self-protective mechanism in the body, which lowers the glucose transport (Iwata et al., 2010).
The GLUT4 gene expression analysis showed down-regulation in the femoral muscles in both the diabetic and obese non-diabetic control group compared to normal-diet group, these results are similar to previous studies (Higashida et al., 2009, Shoghi et al., 2008, Zhang et al., 2008).
GLUT4 mRNA was decreased in the triceps in the skeletal muscle in the HF-diet rats in L6 myotubes. These results indicate HF-diet may lower the GLUT4 gene transcription and/or degrade the GLUT4 mRNA (Higashida et al., 2009).
The expression of GLUT4 was evidently moderated in Zucker Diabetic fatty rats compared to control (Shoghi et al., 2008) and low levels of GLUT4 protein expression was found in muscle, liver and adipose tissue in T2DM rat model (Zhang et al., 2008).
In the present study, down-regulation of GLUT1 and GLUT4 genes have been recognized with Curcumin treatment, which is accredited to high fat-levels in obesity. The GLUT1 expression studies in non-treated diabetic group was down-regulated to 0.47 in HF-diet of control group and GLUT4 expression analysis in the non-diabetic group was down regulated to 0.01 in control obese group of HF-diet.
The current study reported that Curcumin treatment downregulated GLUT1 expression in diabetic rats, this is the first to documentation for this result. The GLUT4 protein levels obtained through western-blotting found to be increased after Curcumin treatment, suggesting the activation of MI-mAChR for increasing levels of glucose uptake by phospholipase C–phosphoinositide 3-kinase (PLC-PI3K) pathway in the skeletal muscle (Cheng et al., 2009). Cheng et al. (2009) documented another possible mechanism of Curcumin stimulation of GLUT4 expression depending on Kramer et al. study (Kramer et al., 2001), they indicated probable mechanism for anti-hyperglycemic effect of rosiglitazone, which was mediated by peroxisome proliferator-activated receptor gamma (PPAR-γ) activation. PPAR-γ has been reported to increase the expression and translocation of the glucose transporters GLUT1 and GLUT4 to liver and skeletal muscle cells and lower in the plasma glucose levels. Prakobwong et al. (2011) study documented the Curcumin enhances the expression of PPAR-γ in human biliary cancer cells. This could tempt one to suggest that, Curcumin has a potential role in activating down-stream insulin signaling pathway as its anti-hyperglycemic property.
5. Conclusion
Curcumin ameliorated IR, the metabolic derangements recorded in obesity and diabetes, and decreased lipid peroxidation in the diabetic status. Curcumin up-regulated Glut4 expression compared to the diabetic group. Future studies of more glucose transporter genes are advocated to clarify the mechanism(s) of the pathogenesis of type 2 diabetes.
Footnotes
Peer review under responsibility of King Saud University.
References
- Accurso F. Curcumin and cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 2004;39(3):235. doi: 10.1097/00005176-200409000-00001. [DOI] [PubMed] [Google Scholar]
- Aggarwal B.B., Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006;71(10):1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Aggarwal B.B., Kumar A., Aggarwal M.S., Shishodia S. Curcumin derived from turmeric (Curcuma longa): a spice for all seasons. Phytopharm. Cancer Chemoprevent. 2005;23:351–387. [Google Scholar]
- Aggarwal B.B., Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol. Sci. 2009;30(2):85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Amin K.A., Hameid H.A., II, Elsttar A.A. Effect of food azo dyes tartrazine and carmoisine on biochemical parameters related to renal, hepatic function and oxidative stress biomarkers in young male rats. Food Chem. Toxicol. 2010;48(10):2994–2999. doi: 10.1016/j.fct.2010.07.039. [DOI] [PubMed] [Google Scholar]
- Amrutkar M., Cansby E., Chursa U., Nuñez-Durán E., Chanclón B., Ståhlman M., Fridén V., Mannerås-Holm L., Wickman A., Smith U., Bäckhed F. Genetic disruption of protein kinase STK25 ameliorates metabolic defects in a diet-induced type 2 diabetes model. Diabetes. 2015;64(8):2791–2804. doi: 10.2337/db15-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arun N., Nalini N. Efficacy of turmeric on blood sugar and polyol pathway in diabetic albino rats. Plant Foods Hum. Nutr. 2002;57(1):41–52. doi: 10.1023/a:1013106527829. [DOI] [PubMed] [Google Scholar]
- Babu P.S., Srinivasan K. Influence of dietary curcumin and cholesterol on the progression of experimentally induced diabetes in albino rat. Mol. Cell. Biochem. 1995;152(1):13–21. doi: 10.1007/BF01076459. [DOI] [PubMed] [Google Scholar]
- Babu P.S., Srinivasan K. Hypolipidemic action of curcumin, the active principle of turmeric (Curcuma longa) in streptozotocin induced diabetic rats. Mol. Cell. Biochem. 1997;166(1–2):169–175. doi: 10.1023/a:1006819605211. [DOI] [PubMed] [Google Scholar]
- Badr G.A., Tang J., Ismail-Beigi F., Kern T.S. Diabetes downregulates GLUT1 expression in the retina and its microvessels but not in the cerebral cortex or its microvessels. Diabetes. 2000;49(6):1016–1021. doi: 10.2337/diabetes.49.6.1016. [DOI] [PubMed] [Google Scholar]
- Best L., Elliott A.C., Brown P.D. Curcumin induces electrical activity in rat pancreatic β-cells by activating the volume-regulated anion channel. Biochem. Pharmacol. 2007;73(11):1768–1775. doi: 10.1016/j.bcp.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Bustanji Y., Taha M.O., Almasri I.M., Al-Ghussein M.A., Mohammad M.K., Alkhatib H.S. Inhibition of glycogen synthase kinase by curcumin: investigation by simulated molecular docking and subsequent in vitro/in vivo evaluation. J. Enzyme Inhib. Med. Chem. 2009;24(3):771–778. doi: 10.1080/14756360802364377. [DOI] [PubMed] [Google Scholar]
- Carbo R., Rodríguez E. A glucose-insulin-potassium solution improves glucose intake in hypoxic cardiomyocytes by a differential expression of glucose transporters in a metabolic syndrome model. J. Biosci. 2019;44(1):19. [PubMed] [Google Scholar]
- Cheng T.C., Lin C.S., Hsu C.C., Chen L.J., Cheng K.C., Cheng J.T. Activation of muscarinic M-1 cholinoceptors by curcumin to increase glucose uptake into skeletal muscle isolated from Wistar rats. Neurosci. Lett. 2009;465(3):238–241. doi: 10.1016/j.neulet.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Dos Santos J.M., Benite-Ribeiro S.A., Queiroz G., Duarte J.A. The effect of age on glucose uptake and GLUT1 and GLUT4 expression in rat skeletal muscle. Cell Biochem. Funct. 2012;30(3):191–197. doi: 10.1002/cbf.1834. [DOI] [PubMed] [Google Scholar]
- Herlein J.A., Fink B.D., Sivitz W.I. Superoxide production by mitochondria of insulin-sensitive tissues: mechanistic differences and effect of early diabetes. Metabolism. 2010;59(2):247–257. doi: 10.1016/j.metabol.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashida K., Higuchi M., Terada S. Dissociation between PGC-1α and GLUT-4 expression in skeletal muscle of rats fed a high-fat diet. J. Nutr. Sci. Vitaminol. 2009;55(6):486–491. doi: 10.3177/jnsv.55.486. [DOI] [PubMed] [Google Scholar]
- Hou W.K., Xian Y.X., Zhang L., Hong L.A.I., Hou X.G., Xu Y.X., Ting Y.U., Xu F.Y., Jun S.O.N.G., Fu C.L., Zhang W.W. Influence of blood glucose on the expression of glucose transporter proteins 1 and 3 in the brain of diabetic rats. Chin. Med. J. 2007;120(19):1704–1709. [PubMed] [Google Scholar]
- Iwata N., Okazaki M., Kamiuchi S., Hibino Y. Protective effects of oral administrated ascorbic acid against oxidative stress and neuronal damage after cerebral ischemia/reperfusion in diabetic rats. J. Health Sci. 2010;56(1):20–30. [Google Scholar]
- Khan Imran Ali, Jahan Parveen, Hasan Qurratulain, Rao Pragna. Genetic confirmation of T2DM meta-analysis variants studied in gestational diabetes mellitus in an Indian population. Diabetes & Metabolic Syndrome: Clin. Res. Rev. 2019;13(1):688–694. doi: 10.1016/j.dsx.2018.11.035. [DOI] [PubMed] [Google Scholar]
- Khan I.A., Poornima S., Jahan P., Rao P., Hasan Q. Type 2 diabetes mellitus and the association of candidate genes in asian indian population from Hyderabad, India. J. Clin. Diagnostic Res.: JCDR. 2015;9(11) doi: 10.7860/JCDR/2015/14471.6855. Gc01-5. Epub 2015/12/18. PubMed PMID: 26673680; PubMed Central PMCID: PMCPMC4668434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan Imran Ali, Vattam Kiran Kumar, Jahan Parveen, Mukkavali Kamal Kiran, Hasan Qurratulain, Rao Pragna. Correlation between KCNQ1 and KCNJ11 gene polymorphisms and type 2 and post-transplant diabetes mellitus in the Asian Indian population. Genes Diseases. 2015;2(3):276–282. doi: 10.1016/j.gendis.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer D., Shapiro R., Adler A., Bush E., Rondinone C.M. Insulin-sensitizing effect of rosiglitazone (BRL-49653) by regulation of glucose transporters in muscle and fat of Zucker rats. Metabol.-Clin. Exp. 2001;50(11):1294–1300. doi: 10.1053/meta.2001.27202. [DOI] [PubMed] [Google Scholar]
- Kuhad A., Chopra K. Curcumin attenuates diabetic encephalopathy in rats: behavioral and biochemical evidences. Eur. J. Pharmacol. 2007;576(1–3):34–42. doi: 10.1016/j.ejphar.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Lin M.L., Lu Y.C., Chen H.Y., Lee C.C., Chung J.G., Chen S.S. Suppressing the formation of lipid raft-associated Rac1/PI3K/Akt signaling complexes by curcumin inhibits SDF-1α-induced invasion of human esophageal carcinoma cells. Mol. Carcinog. 2014;53(5):360–379. doi: 10.1002/mc.21984. [DOI] [PubMed] [Google Scholar]
- Maheshwari Radha K., Singh Anoop K., Gaddipati Jaya, Srimal Rikhab C. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78(18):2081–2087. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Metzler M., Pfeiffer E., Schulz S.I., Dempe J.S. Curcumin uptake and metabolism. BioFactors (Oxford, England) 2013;39(1):14–20. doi: 10.1002/biof.1042. Epub 2012/09/22. PubMed PMID: 22996406. [DOI] [PubMed] [Google Scholar]
- Mlinar B., Marc J., Janež A., Pfeifer M. Molecular mechanisms of insulin resistance and associated diseases. Clin. Chim. Acta. 2007;375(1–2):20–35. doi: 10.1016/j.cca.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Mueckler M. Insulin resistance and the disruption of Glut4 trafficking in skeletal muscle. J. Clin. Investig. 2001;107(10):1211–1213. doi: 10.1172/JCI13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugan P., Pari L. Influence of tetrahydrocurcumin on erythrocyte membrane bound enzymes and antioxidant status in experimental type 2 diabetic rats. J. Ethnopharmacol. 2007;113(3):479–486. doi: 10.1016/j.jep.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Murugan P., Pari L. Influence of tetrahydrocurcumin on hepatic and renal functional markers and protein levels in experimental type 2 diabetic rats. Basic Clin. Pharmacol. Toxicol. 2007;101(4):241–245. doi: 10.1111/j.1742-7843.2007.00109.x. [DOI] [PubMed] [Google Scholar]
- Osawa T., Kato Y. Protective role of antioxidative food factors in oxidative stress caused by hyperglycemia. Ann. N. Y. Acad. Sci. 2005;1043(1):440–451. doi: 10.1196/annals.1333.050. [DOI] [PubMed] [Google Scholar]
- Pardridge W.M., Triguero D., Farrell C.R. Downregulation of blood-brain barrier glucose transporter in experimental diabetes. Diabetes. 1990;39(9):1040–1044. doi: 10.2337/diab.39.9.1040. [DOI] [PubMed] [Google Scholar]
- Phan T.T., See P., Lee S.T., Chan S.Y. Protective effects of curcumin against oxidative damage on skin cells in vitro: its implication for wound healing. J. Trauma Acute Care Surg. 2001;51(5):927–931. doi: 10.1097/00005373-200111000-00017. [DOI] [PubMed] [Google Scholar]
- Pivari F., Mingione A., Brasacchio C., Soldati L. Curcumin and type 2 diabetes mellitus: prevention and treatment. Nutrients. 2019;11(8):1837. doi: 10.3390/nu11081837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwocka K., Bielak-Mijewska A.N.N.A., Sikora E. Curcumin induces caspase-3-independent apoptosis in human multidrug-resistant cells. Ann. N. Y. Acad. Sci. 2002;973(1):250–254. doi: 10.1111/j.1749-6632.2002.tb04643.x. [DOI] [PubMed] [Google Scholar]
- Pongchaidecha A., Lailerd N., Boonprasert W., Chattipakorn N. Effects of curcuminoid supplement on cardiac autonomic status in high-fat–induced obese rats. Nutrition. 2009;25(7–8):870–878. doi: 10.1016/j.nut.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Prabhakar Pranav Kumar, Doble Mukesh. Effect of natural products on commercial oral antidiabetic drugs in enhancing 2-deoxyglucose uptake by 3T3-L1 adipocytes. Therapeutic Adv. Endocrinol. 2011;2(3):103–114. doi: 10.1177/2042018811411356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakobwong S., Gupta S.C., Kim J.H., Sung B., Pinlaor P., Hiraku Y., Wongkham S., Sripa B., Pinlaor S., Aggarwal B.B. Curcumin suppresses proliferation and induces apoptosis in human biliary cancer cells through modulation of multiple cell signaling pathways. Carcinogenesis. 2011;32(9):1372–1380. doi: 10.1093/carcin/bgr032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Mancía S., Trujillo J., Chaverri J.P. Utility of curcumin for the treatment of diabetes mellitus: evidence from preclinical and clinical studies. J. Nutrit. Intermed. Metabol. 2018;14:29–41. [Google Scholar]
- Seo K.I., Choi M.S., Jung U.J., Kim H.J., Yeo J., Jeon S.M., Lee M.K. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol. Nutr. Food Res. 2008;52(9):995–1004. doi: 10.1002/mnfr.200700184. [DOI] [PubMed] [Google Scholar]
- Shoghi K.I., Gropler R.J., Sharp T., Herrero P., Fettig N., Su Y., Mitra M.S., Kovacs A., Finck B.N., Welch M.J. Time course of alterations in myocardial glucose utilization in the Zucker diabetic fatty rat with correlation to gene expression of glucose transporters: a small-animal PET investigation. J. Nucl. Med. 2008;49(8):1320–1327. doi: 10.2967/jnumed.108.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers S.A., Garza L.A., Zhou H., Birnbaum M.J. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol. Cell. Biol. 1998;18(9):5457–5464. doi: 10.1128/mcb.18.9.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryanarayana P., Saraswat M., Mrudula T., Krishna T.P., Krishnaswamy K., Reddy G.B. Curcumin and turmeric delay streptozotocin-induced diabetic cataract in rats. Invest. Ophthalmol. Vis. Sci. 2005;46(6):2092–2099. doi: 10.1167/iovs.04-1304. [DOI] [PubMed] [Google Scholar]
- Witkin J.M., Li X. Curcumin, an active constiuent of the ancient medicinal herb Curcuma longa L.: some uses and the establishment and biological basis of medical efficacy. CNS Neurol. Disord.-Drug Targets (Formerly Curr. Drug Targets-CNS Neurol. Disord.) 2013;12(4):487–497. doi: 10.2174/1871527311312040007. [DOI] [PubMed] [Google Scholar]
- Yoshioka T., Kawada K., Shimada T., Mori M. Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. Am. J. Obstet. Gynecol. 1979;135(3):372–376. doi: 10.1016/0002-9378(79)90708-7. [DOI] [PubMed] [Google Scholar]
- Yuzefovych L.V., Pastukh V.M., Ruchko M.V., Simmons J.D., Richards W.O., Rachek L.I. Plasma mitochondrial DNA is elevated in obese type 2 diabetes mellitus patients and correlates positively with insulin resistance. PLoS ONE. 2019;14(10):e0222278. doi: 10.1371/journal.pone.0222278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Xu Y.C., Guo F.J., Ye M., Li M.L. Anti-diabetic effects of cinnamaldehyde and berberine and their impacts on retinol-binding protein 4 expression in rats with type 2 diabetes mellitus. Chin. Med. J. 2008;121(21):2124–2128. [PubMed] [Google Scholar]