Summary
Gaze shifts, the directing of the eyes to an approaching predator, preferred food source, or potential mate, have universal biological significance for the survival of a species. Our knowledge of gaze behavior is based primarily on visually triggered responses, whereas head orientation triggered by auditory stimuli remains poorly characterized. Common marmoset (Callithrix jacchus) is a diurnal, small-bodied (∼350 g), New World monkey species, known for its rich behavioral repertoires during social interactions. We used a lightweight head tracking system to measure marmosets' reflexive head orientations toward a natural stimulus presented from behind. We found that marmoset could rotate its head at angular velocities above 1,000°/s and maintained target accuracy for a wide range of rotation amplitudes (up to 250°). This unusual, saccadic head orienting behavior offers opportunities for understanding the many biological factors that have shaped the evolution of sensorimotor controls of gaze orientation by the primate brain.
Subject Areas: Biological Sciences, Neuroscience, Behavioral Neuroscience, Sensory Neuroscience
Graphical Abstract

Highlights
-
•
Marmosets can make rapid, reflexive head turns in response to natural stimuli
-
•
The peak velocity of marmoset head turns can exceed that of primate eye saccades
-
•
When the environment is lit, head movements are faster than when it is dark
Biological Sciences; Neuroscience; Behavioral Neuroscience; Sensory Neuroscience
Introduction
In natural viewing conditions, gaze direction often involves head movements. The contribution of the head movement toward the target of the gaze increases as the targeted location approaches or goes beyond the species' oculomotor range (OMR) (Tomlinson and Bahra, 1986a, Freedman and Sparks, 1997, Freedman, 2008, Blakemore and Donaghy, 1980). Head and eye movements exhibit different kinetics and timing depending on the visibility of a target, despite being initiated by coordinated central control signals (Gandhi and Katnani, 2011). Humans and non-human primates, such as rhesus macaques, tend to direct their gaze with slow head movements coupled with rapid eye movements (Bizzi, 1988, Freedman, 2008). The eyes typically lead the head when a target occurs at an unpredictable but visible location. However, when the target location is predictable the head may move earlier than the eyes (Guitton, 1988, Bizzi et al., 1971).
Multiple mechanisms may contribute to the difference in eye and head speeds. One likely explanation lies in the difference in the mechanical load for motor effectors or muscles. The head's weight makes its inertia difficult to quickly overcome for fast head movements (Bartz, 1966), even though neck muscles are activated earlier or, at least, no later than eye muscles (Bizzi et al., 1971, Bizzi et al., 1972, Zangemeister and Stark, 1982b). In support of this argument, many small animals (e.g., birds) rotate their head quickly with their eyes either fixed in the head or having limited motility (Land, 1999, Land, 2015, Knudsen et al., 1979). Second, it is also possible that the relative contribution to reorientation of the gaze by the eyes and head was shaped by the primate brain's evolution. The selection pressure that gave rise to speedy eye movement may have been missing in small animals with lightweight, easily moveable heads, whereas increased eye motility may have evolved to compensate for primates' larger, heavier heads. More generally, phylogenetically older mammalian species tend to orient their gaze through body or head rotation, whereas more advanced species use rapid eye movement (Roucoux and Crommelinck, 1988). Third, the speed difference between head and eye could be affected by the ecological and behavioral significances of sensory signals that initiate gaze changes. Primates have frontal, foveal vision that provides high-resolution visual information to the brain. To redirect the gaze to a threat from behind, animals must rotate the head (and sometimes, the body as well) toward an estimated source direction using auditory or other non-visual information (McCluskey and Cullen, 2007). A rapid change in the gaze direction would need to be made deliberately because sight of any target in the current view is temporarily lost. Therefore, fast head turns might not be a cost-effective strategy for predators, whereas a very necessary one for prey. Given the phylogenetic relationship among primates and different predation pressures they face, it is unlikely that gaze orientation of different primate species follows similar predictable patterns during flight-or-fight response. Combining the factors that contribute to head speed (i.e., body mass, eye motility, and sensory drive), one might predict that small primates with limited eye motility may evolve rapid head movements to improve their chance of survival (McCrea and Gdowski, 2003, Mitchell et al., 2014). Existing data, however, do not offer direct confirmation of this hypothesis. One primary reason is that studies of gaze orientation predominantly use visual signals in relatively larger, phylogenetically more advanced primates such as humans and macaque monkeys (Bizzi, 1988, Freedman, 2008). Although comparative studies have revealed a correlation between the width of best vision and auditory spatial acuity (Heffner, 2004), the role of auditory-mediated, spatial location sensitivity, especially in the rear field, is not well understood for primate gaze orientation, and in particular, the types of reflexive movements to a sudden-onset natural stimulus coming from behind.
The common marmoset (Callithrix jacchus) is a New World monkey species with a small head (∼3.5 cm in width) and frontally positioned eyes. Marmosets naturally inhabit the rainforest canopy and face predation pressures from reptiles and raptors that could come from any direction (Stevenson and Rylands, 1988), so spatial awareness is vital to their survival. Early studies have described two sets of interesting but different head movement patterns as part of their explorative behavioral repertoire, “head-cocking” and “head-jerking.” “Head-cocking” involves rolling the head around the rostral-caudal axis without changing fixation (Stevenson and Poole, 1976, Kaplan and Rogers, 2006). This visual behavior is not unique to marmosets, and has been observed in other small primates as well (e.g., galagos) (Menzel, 1980, Rogers et al., 1993). “Head-jerking” involves rotating the head around the vertical axis to change the direction of gaze (Reekie et al., 2008). Marmosets have more restricted eye motility than other New World monkeys, Old World monkeys, and humans as reflected by their OMR (marmosets, ∼10°; squirrel monkeys, 20–25°; macaques, 40–50°; humans ∼55°) (Mitchell et al., 2014, Guitton and Volle, 1987, McCrea and Gdowski, 2003, Freedman, 2008). With these physical and behavioral features, marmosets are an adequate model for testing whether small primates have evolved to use rapid head movement. This study focuses on marmosets' head orienting responses (“head-jerking”). Experiments were designed to investigate the following two questions: (1) to what extent their head movement can offset their limitations in eye movement to reorient gaze and (2) what types of sensory factors support this offset if it occurs.
Results
We measured the kinetics of head-turning behaviors of untrained marmosets over large head rotations in response to signals coming from behind. A marmoset sat in a sound-attenuated testing room with its head free and the rest of its body, below the neck, constrained by a custom-designed primate chair (Figure 1A). To accommodate their small head size, lightweight inertial sensors (3-axis gyroscopes) were directly mounted to the marmosets' heads and the digitized angular velocity signals of yaw, roll, and pitch rotations were sent wirelessly (at an ∼175 Hz sampling rate per axis) to a computer outside the testing room for real-time data display and storage (see Supplemental Information - Transparent Methods). The angular amplitude was calculated offline by integrating an event-triggered velocity signal over a time window of ∼1.31 s, centered on the peak-velocity time.
Figure 1.
Experimental Setup
(A) The head-tracking system allows real-time signal encoding/decoding and wireless signal transmission between the sensor station inside the testing room and the base station outside.
(B) Top: The relative position of a forward-facing head and door inside the testing room. The initial head position before the door-open event was coded offline into one of four quadrants based on video recording collected simultaneously throughout an experiment. Bottom: The normalized amplitude and the spectrogram of the sounds made by opening and closing the door. Signals were collected using an audio recorder (96-kHz, 24-bit, Zoom H4n).
(C) Sample trace of the angular velocity and rotation amplitude of yaw rotation. Red dots indicate peak velocity and maximum amplitude.
To gauge the human capacity for head motility, large gaze shifts (>120°) can be achieved by asking a subject to orient from an off-center position toward a remembered target position as fast as possible (Guitton and Volle, 1987). In studies of non-human primates, such verbal instructions are of no use; the novelty of the stimulus is therefore essential for eliciting reflexive, large head turns. Compared with visual methods for eliciting the small saccades permitted by eye motility, audiovisual stimuli are often used to evoke large gaze shifts in a less controlled manner (e.g., banging on a door, Tomlinson and Bahra, 1986a). We initially used natural sounds, including animal vocalizations and noises, to trigger reflexive head turns. Marmosets adapted quickly to these stimuli and rarely showed reflexive responses to sound onset. We discovered that suddenly opening the testing door behind the animal (at a predictable location with unpredictable timing) could evoke reliable head turns in marmosets. To ensure sound insulation, the test room door has a double-walled, heavyweight structure. Opening the door produces a broadband noise burst with peak energy centered at 10 kHz (Figure 1B). Marmosets can hear ultrasonic sounds up to 36 kHz, and their hearing is most sensitive around 8 kHz (Osmanski and Wang, 2011). Compared with other sound stimuli we used initially, a door-opening stimulus was more successful because it is behaviorally relevant. The sound of door opening is a consequential signal, followed often by the appearance of a human experimenter who could either bring food or retrieve the animal.
Figure 1C shows the angular velocity and amplitude of the head movement of one marmoset (M1) during a trial response (see also Videos S1 and S2). The door-opening events occurred twice (labeled by black triangles) and a saccadic head turn was triggered each time with a short latency (200 and 133 ms, respectively). For both responses, the marmoset was initially facing the front-left corner of the room. The fast head turns in the horizontal plane (yaw) brought the animal to face the door in one sweeping motion with peak angular velocity exceeding 1,000°/s and rotation amplitude close to 180°. Rapid head movements at such a fast speed were only observed twice for the head-turn data collected using other sound stimuli (692 turns for marmoset M1 and 609 turns for marmoset M2) as shown in later results.
Video shows the first head turn in Figure 1C.
Video shows the second head turn in Figure 1C.
Results reported in this study were based on the data collected using the door-opening stimuli from two adult male marmosets (∼4 years old). They had similar body weight (∼460 g) and similar width of the head (3.75 cm for M1 and 3.64 cm for M2, radiograph measurement). Because the head orientation we tested requires turning from front to back, revolving around the vertical axis, yaw rotation is the dominant component of head movement, and the majority of roll and pitch rotations are small in amplitude (<50°). Thus the resulting analyses will focus on head rotations in yaw. Results of roll and pitch rotation will be described for purposes of comparison.
Speed, Amplitude, and Accuracy
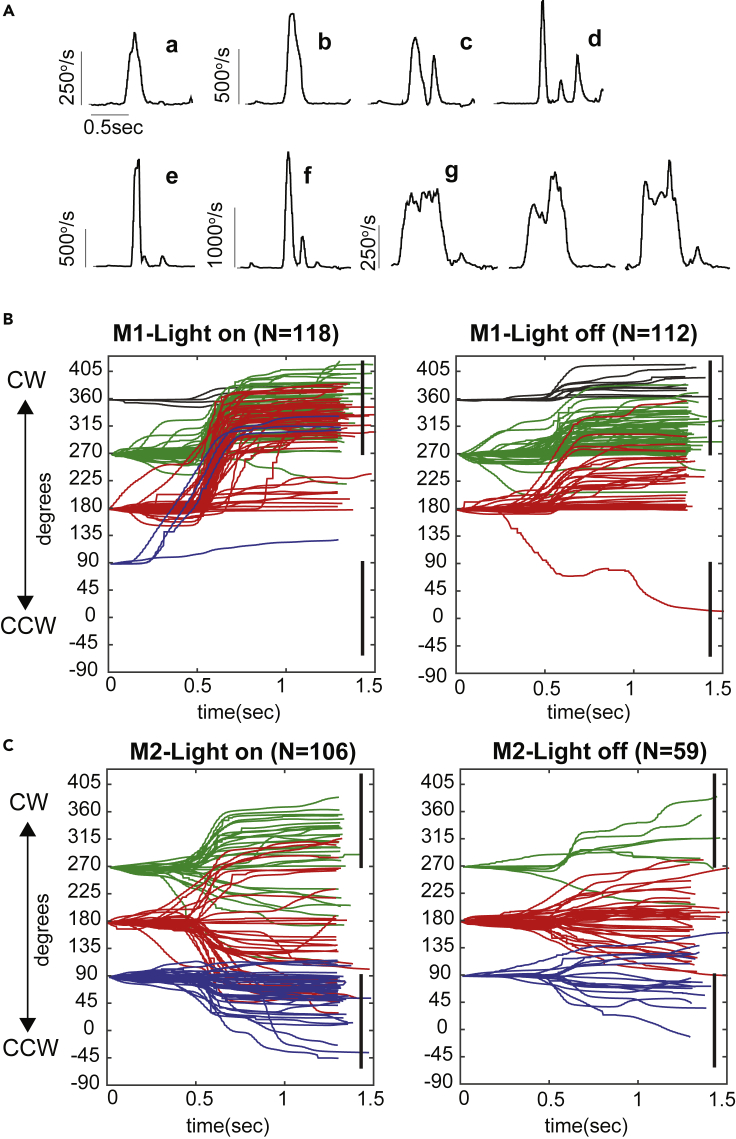
Saccade-like head turning with quick acceleration and deceleration were observed in both marmosets (M1 and M2). Figure 2A shows example velocity profiles for head turns of varying speed. The majority of turns (>85%) were completed with single or double saccades; three or more consecutive saccadic turns (e.g., panel d in Figure 2A) were rare. In general, fast turns were associated with large rotation amplitude. We found that head-turn amplitude is affected by the initial position and idiosyncratic motor patterns of the marmoset head. M2 could turn either clockwise (CW) or counter-clockwise (CCW) toward the door, whereas M1 almost exclusively made CW turns. Many of the superfast head turns we recorded (>1,500°/s) were associated with M1 rotating its head starting from the front-left quadrant (180°) of the room (e.g., Figure 2A, panels e and f). Interestingly, even when its initial head position was within the back-left quadrant (90°), M1 still made CW rotations despite the fact that turning CCW would result in a shorter angular distance to the door. To make such a large gaze shift (∼225°), M1 accelerated its head to reach peak velocity and maintained this velocity for the duration of head turn. We observed three such instances, shown in panel g of Figure 2A (also see Video S3). In macaque monkeys, a plateau-like velocity profile of gaze has been reported for large target range (see Figure 7 in Tomlinson and Bahra, 1986a). They were achieved through combined eye-head movement in the same direction, where the head accelerates and decelerates in between a double eye saccade. As such, three consecutive short-duration, “bell-shaped” velocity profiles (eye + head + eye) combined into one long-duration, “flat-topped” gaze velocity needed for large-gaze amplitude. It is remarkable that marmoset's head movement alone exhibits this velocity profile. However, because eye movement was not simultaneously measured, whether the eyes could further modify the gaze remains unclear, although it is worth noting that marmoset's ocular range is limited (∼10° when measured during natural viewing in head-restrained marmosets, Mitchell et al., 2014) and constitutes a small fraction of the magnitude of their head turn we observed in this study.
Figure 2.
Head Velocity and Amplitude
(A) Example saccade velocities in yaw. Panels a and b, single saccade; panel c, double saccade; panel d, triple saccade; panels e and f, superfast saccade; panel g, plateau velocity saccade.
(B and C) Head rotation amplitudes in yaw during light-on and light-off conditions for marmosets M1 and M2, respectively. All traces were aligned based on peak velocity (See Transparent Methods), and the time zero refers to the relative start time of a segment, not the stimulus time. The results color code the initial positions of the head before the door-open events. Head turns with a minimum movement of 20° and endpoint saccade within the angle ranges of vertical lines (door direction) are considered Hit responses.
Similar to humans, marmosets are diurnal and naturally seek food and engage in social play during daylight. For humans, eye saccades during sound source localization are less accurate in the dark than in lit environments when tested during the light cycle (Platt and Warren, 1972). To understand whether this also applies to marmoset head saccades, we measured their head-turn responses during trials when the house light inside the testing room was turned on or off. When the house light was on, marmosets could see their surroundings and opening the door did not provide any additional visual cues when animals were facing forward. When the house light was turned off, the testing room was almost completely dark. Opening the door let in the light from outside, thus providing additional visual cues for the door-opening event. Figures 2B and 2C show the head rotation amplitudes of M1 and M2, respectively. The amplitude plots clearly demonstrate the two marmosets' idiosyncratic head-turn strategies mentioned above (CW versus CCW). When the environment was visible (light-on), both marmosets achieved high rates of accuracy orienting toward the door direction (black vertical lines; see Transparent Methods). Without any visual stimulation before the door was open (light-off), both marmosets often failed to initiate large enough head turns to reach the door direction (see Videos S4 and S5). This rotate-to-the-door behavior was stereotypical and salient, followed always by a fixation at the door direction for at least half of a second (see extended “endpoints” over time in head trajectories in Figure 2B, light-on condition).
Table 1 summarizes the percentage hit rates of head-turn responses for different lighting conditions and different initial head positions. The majority of data were collected when the animal faced forward before the door was opened from behind. Although marmoset M1 made more accurate turns than M2 in orientating toward the direction of the door in both lit and dark conditions, the effects of light on their responses were consistent. The total hit rate for both marmosets dropped by about 30% from lit to dark conditions. This contrasting behavior is more apparent when the initial head position was at front-left (180°). At this initial position, the door was out of sight for marmosets and thus the orienting behaviors were triggered by auditory cues generated from opening the door from behind. Moreover, the peak velocities for the hit responses were also affected. When initially facing forward (front-left and front-back), M1 made significantly faster head turns in lit than dark conditions (p < 10−3, Wilcoxon rank-sum test). M2 also made significantly faster head turns when the light was on, but only for the front-left initial position (p < 0.05, Wilcoxon rank-sum test). When the light was off, M2 seldom made hit responses at each position, so the validity of statistical comparisons is weak.
Table 1.
Numbers of Door-Open Stimuli and the Hit Rates of Head-Turn Responses
| House Light | Starting Position % Hit |
90° Back-Left | 180° Front-Left | 270° Front-Right | 0°/360° Back-Right | Overall Hit Rate | |
|---|---|---|---|---|---|---|---|
| M1 | ON N = 118 | # Stimulus | 4 | 56 | 55 | 3 | |
| CW | 75% | 83.9% | 90.9% | 100% | 87.3% | ||
| CCW | NA | NA | NA | NA | |||
| OFF N = 112 | # Stimulus | 0 | 39 | 64 | 9 | ||
| CW | NA | 12.8% | 65.6% | 100% | 50.9% | ||
| CCW | NA | 2.6% | NA | NA | |||
| M2 | ON N = 106 | # Stimulus | 43 | 35 | 28 | 0 | |
| CW | 0% | 11.4% | 67.9% | NA | 55.7% | ||
| CCW | 23.6% | 31.4% | 0% | NA | |||
| OFF N = 59 | # Stimulus | 16 | 36 | 7 | 0 | ||
| CW | 0% | 5.6% | 57.1% | NA | 22.4% | ||
| CCW | 8.5% | 2.8% | 0% | NA |
The data were partitioned based on lighting conditions, initial head positions, and the direction of head turns.
CW, clockwise; CCW, counter-clockwise.
Bolded are related to Stimulus information (#Stimulus) and those not bolded are related to responses.
Main Sequence
Figure 3A shows the relationship between peak velocity and amplitude of marmoset head movements in yaw in response to door-opening stimuli. In primates, the duration, velocity, and amplitude of eye/head movement obey a stereotypical relationship, known as the main sequence (Freedman and Sparks, 1997, Bahill et al., 1975, Zangemeister and Stark, 1982a, Zangemeister and Stark, 1982b). A linear relationship beween velocity and amplitude suggests that motor control of gaze modulates velocity to determine the amplitude rather than the duration of saccade. For single saccades (filled symbols), marmoset head movements conform to this rule. The distribution of the raw data and the linear regression analysis also confirm that both marmosets made larger, more rapid saccades in lit conditions. Notably, the reduction in speed and amplitude from lit to dark conditions mostly occurred when the animals were initially facing forward (red and green symbols) before the door was opened. Similar to the head rotation in yaw, the main sequences for roll and pitch rotations followed a linear relationship and showed reduced speed and amplitude in the dark condition. The slopes of the regression fits for pitch (kpitch) and roll (kroll), which indicate the relative gain in velocity per amplitude change, are summarized here. For M1, kpitch = 5.46 and 3.28, kroll = 7.24 and 4.22; for M2, kpitch = 4 and 2.29, kroll = 5.24 and 5.65; respectively, for lit and dark conditions. Linear regressions reached the significance level (p < 10−3) for all axes of rotations in both marmosets.
Figure 3.
Main Sequence Analysis between Horizontal Rotation Amplitude and Peak Angular Velocity of Two Marmosets in Light-on and Light-off Conditions
(A) Response to the door-opening stimuli. The results are color-coded for four different initial head positions. Filled circles, single saccade; open circles, double or triple saccades. Linear regression fits were performed on single saccades only.
(B) Responses to auditory stimuli presented from loudspeakers.
Results in Figure 3A also indicate that single saccades occurred more often in the lit than in the dark conditions. On average, the percent of single head saccades dropped from 58.5% (light on) to 39.3% (light off) for M1 and from 36.8% (light on) to 27.1% (light off) for M2. Previous work in macaque monkeys indicated that animal motivation affects eye saccade strategies (Tomlinson and Bahra, 1986a). Fast and large gaze shifts through single saccades were seen when the animals were highly motivated. When less motivated, an animal made successive small saccades to reorient its gaze. Along this line of thought, both marmosets appeared to be more motivated using their rapid, single head saccade in target localization when they could see their surroundings before the stimulus occurred.
In a subset of experiments, we also measured head movements of M1 and M2 when auditory stimuli (broadband noise and marmoset vocalization) were broadcast on and off from one of the two loudspeakers positioned in front (±45°). As mentioned earlier, typical visual and auditory stimulation used in laboratory settings often failed to evoke stimulus-onset-locked, head-turn responses, as marmosets adapted quickly to these stimuli. Unlike the “door-open” event, animal behaviors do not show a clear stimulus-response correlation in response to typical auditory stimuli, which is critical for evaluating orienting behaviors. Nevertheless, head speed and amplitude appeared to obey a linear relationship. Figure 3B shows the main sequence in yaw of M1 and M2. Compared with the door-opening responses shown in Figure 3A, there is a reduced gain (slope of regression) for both light-on and light-off results, indicating slower head turns for similar rotation ranges in response to these auditory signals presented from loudspeakers.
Reaction Time
In addition to reduced speed and accuracy, head responses to door-opening stimuli also became more sluggish in the dark. When scoring reaction times based on visual inspections of video recordings, inspectors detected higher rates of no-turn responses to door-opening events in the dark condition (78.5%, 88 of 112 events for M1 and 83.1%, 49 of 59 events for M2) than those in the lit condition (18.6%, 22 of 118 events for M1 and 34.9%, 37 of 106 events for M2). Among those detected head-turn responses, reaction times of M1 relative to the door-opening events were faster when the environment was visible (median, 200 ms for lit versus 333 ms for dark; p < 0.01, Wilcoxon rank-sum test). For M2, the difference was not statistically significant (median, 200 ms for lit versus 233 ms for dark).
As observed in both humans and macaque monkeys, the head moves earlier, relative to gaze onset, as the target range gets larger and the target location is predictable (Zangemeister and Stark, 1982a, Freedman and Sparks, 1997). Given that there is a strong positive correlation between head amplitude and head speed in our data (Figure 3A), we hypothesized that faster velocity and larger amplitude should both lead to decreasing reaction times. Figure 4 shows the relationship between the maximal speed measured from the head tracker and the latency of head-turn responses measured from visual inspection. The negative relationship is only statistically significant for light-on results, whereas short-latency, rapid head turns were scarcely seen in the light-off results. On the other hand, no significant correlations were detected between amplitude and latency (light-on p = 0.07; light-off p = 0.81; Spearman's correlation). Re-examining the data in Figure 3A, we could see that some large-amplitude rotations were achieved through multiple head turns (open circles), whose maximal velocities are slower than those of single head saccade. Overall, these reaction time results suggest that the neural commands for initiating head turns (premotor signals) and those innervating neck muscles (motor signals) act more precisely in lit than dark environment when rapid, but not necessarily large, head turns are needed for directing gaze to a fixed location target.
Figure 4.
Relationship between Response Latency and Speed of Head-Turn Responses to Door-Opening Stimuli in Light-on and Light-off Conditions
Results were pooled from detected head-turn responses of M1 and M2 from visual inspections of the data.
Discussion
Comparative Analysis of Head Speed and Head Amplitude in Primates
Compared with other primates, marmoset head orientation stands out for its speed and large rotation amplitude. Tested at its limit, a human head may approach speeds of 600°/s (Guitton and Volle, 1987, Zangemeister et al., 1981) and a macaque head could achieve an average speed of 700°/s for a horizontal 120° rotation (Tomlinson and Bahra, 1986a). For similar radial distances, the two marmosets we tested could voluntarily make rapid head turns above 1,000°/s for M1 and above 500°/s for M2. Their speed of head turns increased further as the magnitude of their head turns extended beyond 120°, greater than what is possible for humans. To understand these differences, it is important to realize that primate species vary widely in their body mass, locomotion patterns (leaping or brachiating), habitats (arboreal versus terrain), and lifestyles (diurnal versus nocturnal). These environmental and physiological factors could interdependently shape the evolution of the primate gaze system. In particular, small body size is not a reliable predictor of speed. The semicircular canal system, which helps stabilize the gaze during locomotion, is suggested as an alternative predictor of agility. A recent study on 91 extant and recently extinct primate species shows that species that move rapidly (e.g., tarsiers and marmosets) have larger canal sizes than those that move slowly (e.g., lorises) once the body size is accounted for (Spoor et al., 2007).
As the majority of eye-head gaze studies use visual targets positioned within the visual field in humans and macaque monkeys, data for across-species comparisons are scarce. Figure 5 shows the main sequences of eye and head measured in a few primates including the data from the present study in marmosets. The selected four species have increasingly large body mass (average adult, marmosets ∼350 g, squirrel monkeys ∼800 g, macaques ∼6 kg, and humans ∼80 kg) as well as OMR as mentioned earlier. This comparative difference suggests that while most primate species have sharp vision, their eye motility appears to grow with head/body size during primate evolution, although marmosets are one of the few primate species that undergo “dwarfism” with evolutionary reductions in body mass (Montgomery and Mundy, 2013).
Figure 5.
Main Sequence of Eye and Head Movement in Four Primate Species
Despite at least 10-fold differences in body mass, the main sequence of eye movement is remarkably similar among marmosets, macaques, and humans (within 15°, dashed lines in Figure 5). In contrast, their head speed varies considerably for visible and small-amplitude targets (solid lines, comparison possible within 50°), in which small-bodied marmosets and squirrel monkeys make faster head turns than large-bodied macaques and humans. However, the differences in speed between marmoset M1 and M2, which had similar body weight and head size, showed as much variations as the between-species difference. These comparative data suggest that, whereas head size may set the biomechanical upper limit for head speed, other factors, such as animal motivation and stimulus saliency, can influence head speed within the “allowable” range. Human studies show that when clear verbal instructions were given to command head turns toward fixed targets as quickly as possible, head movement was still highly variable and influenced by the subject's intention (Stark et al., 1980, Zangemeister et al., 1981). Between the two marmosets we tested, motivation appeared to affect the occurrence, accuracy, and latency of head turns. Our results also showed the effects of stimulus saliency when comparing head turns observed between door-opening stimuli and other sounds (Figure 3).
Another between-species difference is marmosets' greater incidence of large-amplitude head rotations. While the lack of data in larger primates may be related to difficulties in data collection, large head turns are not an uncommon behavioral trait among arboreal primitive primates (i.e., prosimians). For example, tarsiers have forward-facing eyes with limited motility. Their gaze shifts are achieved almost entirely using the head supported by unique spinal morphology (Ankel-Simons, 2010). Marmosets can move their eyes, but their OMR is small (<10°), and so is the amplitude of eye saccade (median ∼3.6°) when tested in head-restrained conditions (Mitchell et al., 2014). Similar to tarsiers, marmosets are arboreal and face predators coming from above and below. Arguably, the eye anatomy of tarsiers and marmosets may be the result of selective pressures that demand that these two primate species adopt a head-based gaze strategy in rapidly orienting to threats in surrounding environments. The selective pressure might be less critical for larger primates. Conversely, it could also be that the motility of a small head is sufficient for the functional adaptation of marmosets, thus reducing the selective pressure for developing large eye movements. Future comparative data on primate species with different sizes but from similar family (e.g., lemurs) will be helpful in testing these hypotheses.
Eye Movement
Several features of marmoset head movement observed in this study resemble eye saccades. This includes the “bell-shaped” velocity profile, multiple saccades for large rotation amplitude, and the effects of animal motivation on saccade behavior. One hypothesis is that marmoset head movement serves as part of the reflexive saccade system extending beyond the field of vision for responding to threats in surrounding environments. This study, however, did not measure marmoset eye movement during head turning. Thus the angular range at which gaze shift switches from using an ocular-centric to a head-centric motor strategy as observed in other larger primates (∼30° in macaques, Phillips et al., 1995; and ∼45° in humans, Guitton and Volle, 1987) remains unknown for the marmosets we tested. Nevertheless, due to its limited ocular motility, marmoset eye movement would only contribute a small fraction to gaze shift observed in this study (maximal head amplitude 250° for M1 and 150° for M2). On the other hand, eye movement is important to gaze stabilization during head turns through the vestibulo-ocular reflex (VOR) or other neural controls, depending on gaze amplitude. In cats and macaques, VOR ceases to act for large gaze amplitude (>40°); rather eye and head appear to be controlled by interactive motor programs (Guitton, 1988, Tomlinson and Bahra, 1986b). Recent studies show that eye saccade behaviors in dealing with new information appear to differ between marmosets and macaques. When viewing novel images, marmosets make more frequent saccades with a shorter fixation time, resulting in a longer overall looking time than macaques (Nummela et al., 2018). Target identification in a natural scene is a dynamic process that requires “multiple looks” at a potential target to refine the analysis. This process can be complicated during self-motion with simultaneous involvements of eye-centered visual spatial information and head-centered auditory spatial information (Vliegen et al., 2004). For future work, it is of great interest to know the sequences of eye saccade made before, during, and after the ballistic head movements in marmosets to reveal how sensory update modifies gaze behaviors.
Auditory Orienting Response
A large portion of rapid, large head turns observed in this study were triggered by auditory information from door-opening events when marmosets facing away from the door direction. Arguably, marmosets could do this because of their small head size. A small head not only reduces the inertia of head movements but also affects sensory functions important to gaze control. Comparative data show that small heads result in a smaller inter-ocular distance and a smaller VOR during gaze control (McCrea and Gdowski, 2003). A small head also results in a smaller inter-aural distance, which in turn leads to a smaller dynamic range of the timing cue (inter-aural time difference, ITD) for localizing low-frequency sounds. As such, compared with humans, animals with smaller heads tend to rely less on ITDs and more on inter-aural-level differences (ILD) to localize sound sources (Heffner, 2004).
The magnitude of ILDs, however, is only sufficiently sizable for high-frequency sounds. Unsurprisingly, the hearing ranges of small-headed mammalian animals, including rodents and marmosets, are at least an octave higher than humans. Other than ILDs, high-frequency sounds also provide spectral cues for distinguishing between the front/back, up/down direction of sound source locations (Blauert, 1997). As an arboreal species, such three-dimensional spatial hearing seems crucial to the survival of marmosets. In the auditory cortex of marmosets, the sound-location selectivity of single neurons is not restricted to the frontal, horizontal domain of the visual field, but extends to up-down and front-back dimensions, surrounding the head (Remington and Wang, 2018, Zhou and Wang, 2012). However, it remains unknown whether the spatially extended location selectivity is present in the superior colliculus (SC) of marmosets as well. In this midbrain structure, eye/head movements are coordinated using combined auditory, visual, and motor maps (Meredith and Stein, 1983, Meredith and Stein, 1986, Meredith and Stein, 1996, Knudsen and Brainard, 1995). In barn owls, which use the head to orient gaze, electrical stimulation in the optic tectum (avian analog of SC) induced head saccades with systemically organized direction and amplitude (Du Lac and Knudsen, 1990). Whether marmoset SC contains a motor map that guides three-dimensional, saccadic head movement remains an interesting topic for future investigation.
Head Orientation as a Multisensory Response
Head orientation is also influenced by multisensory factors. To respond by orienting toward the door, a marmoset has to use integrated auditory and visual information along with memory to initiate its responses. Intriguingly, we found that rapid head turns in marmosets are linked to visibility of the sensory environment. When head turns were initiated from auditory cues coming from behind the head, there is a pronounced reduction in speed and accuracy for head saccades in the dark relative to the lighted environment. This reduced accuracy could be attributed to multiple reasons. One potential explanation is that, as a diurnal prey species, the marmoset is reluctant to move if light is not provided during the light cycle. Studies show that marmosets demonstrate a lack of “cognitive impulsivity” in decision making (Adriani et al., 2013). We found a lack of motion in some trials in the dark (e.g., Video S4). However, we also found that the marmosets scanned the environment in looking for the sound source in the dark (e.g., Video S5).
This raises another potential explanation for reduced accuracy and prolonged reaction time in dark. That is, sound-evoked head orientation requires a visual spatial reference. In humans, blindfolding increases sound localization errors in the horizontal plane for stimuli presented in front of, to the side of, and behind subjects' heads (Shelton and Searle, 1980). More directly relevant to this study, human eye saccades for sound source localization are less accurate in the dark than in lighted environments (Platt and Warren, 1972). Both previous studies suggest that sound source localization benefits from a guiding visual spatial reference. If marmoset head saccades also depend on the spatial references provided by the visual or “textured” environment, these reference-related, visual influences are more pronounced when the target is outside the field of vision. This suggests that gaze control in marmosets requires sensitivity over a broad and panoramic multisensory spatial domain.
In summary, the unusual rapid and large marmoset head movements observed in this study go beyond demonstrating the polygenetic gaze orientation behaviors in primates. Gaze control is a complex and dynamic process that involves an interplay of the sensory, motor, and cognitive nervous systems. The marmoset model offers a unique opportunity for future studies in understanding these mechanisms and more importantly, how the evolution of the primate brain has engaged specialized processing of biologically significant directional information to guide gaze orientation.
Limitations of the Study
-
•
The accuracy of the head movements reported in the current study is limited. Initial head direction was coarsely defined based on visual inspection and the target range of the door direction was rather wide. For future studies, we plan to improve the inertial sensor functions to simultaneously encode the initial head position and kinetics.
-
•
Eye movement was not measured in this study. This limits the understanding of the interaction and the extent of coupling and decoupling between eye and head movements for auditory orienting responses.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
Rachele McAndrew and Kyle Labban assisted with animal preparation during data collection. Kelvin Tran and Maggie Zhou examined the video recordings and calculated response times (latency) of head turns. Drs. Brad May and M. Torben Pastore provided insightful discussions on the interpretations of the results. Elizabeth Teret offered suggestions to the manuscript preparation. We appreciate their inputs to our work. This research was supported by a grant from National Science Foundation (NSF BCS-1539376 to Y.Z.).
Author Contributions
S.P., S.S., and Y.Z. designed the study. S.P. and Y.Z. performed the experiments. S.P. and Y.Z. analyzed the data. Y.Z. wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: February 21, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100837.
Data and Code Availability
https://data.mendeley.com/datasets/mh5t8fzbbf/draft?a=3759893f-38d7-49b7-a9d8-e8d25174b6eb.
Supplemental Information
References
- Adriani W., Romani C., Manciocco A., Vitale A., Laviola G. Individual differences in choice (in) flexibility but not impulsivity in the common marmoset: an automated, operant-behavior choice task. Behav. Brain Res. 2013;256:554–563. doi: 10.1016/j.bbr.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Ankel-Simons F. Elsevier; 2010. Primate Anatomy: An Introduction. [Google Scholar]
- Bahill A.T., Clark M.R., Stark L. The main sequence, a tool for studying human eye movements. Math. Biosci. 1975;24:191–204. [Google Scholar]
- Bartz A.E. Eye and head movements in peripheral vision: nature of compensatory eye movements. Science. 1966;152:1644–1645. doi: 10.1126/science.152.3729.1644. [DOI] [PubMed] [Google Scholar]
- Bizzi E. Sensory System I. Springer; 1988. Eye-head coordination. [Google Scholar]
- Bizzi E., Kalil R.E., Morasso P. Two modes of active eye-head coordination in monkeys. Brain Res. 1972;40:45–48. doi: 10.1016/0006-8993(72)90104-7. [DOI] [PubMed] [Google Scholar]
- Bizzi E., Kalil R.E., Tagliasco V. Eye-head coordination in monkeys: evidence for centrally patterned organization. Science. 1971;173:452–454. doi: 10.1126/science.173.3995.452. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Donaghy M. Co-ordination of head and eyes in the gaze changing behaviour of cats. J. Physiol. 1980;300:317–335. doi: 10.1113/jphysiol.1980.sp013164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauert J. MIT Press; 1997. Spatial Hearing: The Psychophysics of Human Sound Localization. [Google Scholar]
- Du Lac S., Knudsen E.I. Neural maps of head movement vector and speed in the optic tectum of the barn owl. J. Neurophysiol. 1990;63:131–146. doi: 10.1152/jn.1990.63.1.131. [DOI] [PubMed] [Google Scholar]
- Freedman E.G. Coordination of the eyes and head during visual orienting. Exp. Brain Res. 2008;190:369. doi: 10.1007/s00221-008-1504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman E.G., Sparks D.L. Eye-head coordination during head-unrestrained gaze shifts in rhesus monkeys. J. Neurophysiol. 1997;77:2328–2348. doi: 10.1152/jn.1997.77.5.2328. [DOI] [PubMed] [Google Scholar]
- Gandhi N.J., Katnani H.A. Motor functions of the superior colliculus. Annu. Rev. Neurosci. 2011;34:205–231. doi: 10.1146/annurev-neuro-061010-113728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitton D. Eye-head coordination in gaze control. In: Peterson B.W., Richmond F.J., editors. Control of head movement. Oxford University Press; 1988. [Google Scholar]
- Guitton D., Volle M. Gaze control in humans: eye-head coordination during orienting movements to targets within and beyond the oculomotor range. J. Neurophysiol. 1987;58:427–459. doi: 10.1152/jn.1987.58.3.427. [DOI] [PubMed] [Google Scholar]
- Heffner R.S. Primate hearing from a mammalian perspective. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004;281:1111–1122. doi: 10.1002/ar.a.20117. [DOI] [PubMed] [Google Scholar]
- Kaplan G., Rogers L.J. Head-cocking as a form of exploration in the common marmoset and its development. Dev. Psychobiol. 2006;48:551–560. doi: 10.1002/dev.20155. [DOI] [PubMed] [Google Scholar]
- Knudsen E., Brainard M. Creating a unified representation of visual and auditory space in the brain. Annu. Rev. Neurosci. 1995;18:19–43. doi: 10.1146/annurev.ne.18.030195.000315. [DOI] [PubMed] [Google Scholar]
- Knudsen E.I., Blasdel G.G., Konishi M. Sound localization by the barn owl (Tyto-Alba) measured with the search coil technique. J. Comp. Physiol. 1979;133:1–11. [Google Scholar]
- Land M.F. Motion and vision: why animals move their eyes. J. Comp. Physiol. A. 1999;185:341–352. doi: 10.1007/s003590050393. [DOI] [PubMed] [Google Scholar]
- Land M.F. Eye movements of vertebrates and their relation to eye form and function. J. Comp. Physiol. A. 2015;201:195–214. doi: 10.1007/s00359-014-0964-5. [DOI] [PubMed] [Google Scholar]
- McCluskey M.K., Cullen K.E. Eye, head, and body coordination during large gaze shifts in rhesus monkeys: movement kinematics and the influence of posture. J. Neurophysiol. 2007;97:2976–2991. doi: 10.1152/jn.00822.2006. [DOI] [PubMed] [Google Scholar]
- McCrea R.A., Gdowski G.T. Firing behaviour of squirrel monkey eye movement-related vestibular nucleus neurons during gaze saccades. J. Physiol. 2003;546:207–224. doi: 10.1113/jphysiol.2002.027797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel C.R. Head-cocking and visual perception in primates. Anim. Behav. 1980;28:151–IN10. doi: 10.1016/s0003-3472(80)80020-0. [DOI] [PubMed] [Google Scholar]
- Meredith M.A., Stein B.E. Interactions among converging sensory inputs in the superior colliculus. Science. 1983;221:389–391. doi: 10.1126/science.6867718. [DOI] [PubMed] [Google Scholar]
- Meredith M.A., Stein B.E. Visual, auditory, and somatosensory convergence on cells in superior colliculus results in multisensory integration. J. Neurophysiol. 1986;56:640–662. doi: 10.1152/jn.1986.56.3.640. [DOI] [PubMed] [Google Scholar]
- Meredith M.A., Stein B.E. Spatial determinants of multisensory integration in cat superior colliculus neurons. J. Neurophysiol. 1996;75:1843–1857. doi: 10.1152/jn.1996.75.5.1843. [DOI] [PubMed] [Google Scholar]
- Mitchell J.F., Reynolds J.H., Miller C.T. Active vision in marmosets: a model system for visual neuroscience. J. Neurosci. 2014;34:1183–1194. doi: 10.1523/JNEUROSCI.3899-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S.H., Mundy N.I. Parallel episodes of phyletic dwarfism in callitrichid and cheirogaleid primates. J. Evol. Biol. 2013;26:810–819. doi: 10.1111/jeb.12097. [DOI] [PubMed] [Google Scholar]
- Nummela S.U., Jutras M.J., Wixted J.T., Buffalo E.A., Miller C.T. Recognition memory in marmoset and macaque monkeys: a comparison of active vision. J. Cogn. Neurosci. 2018;31:1318–1328. doi: 10.1162/jocn_a_01361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmanski M.S., Wang X. Measurement of absolute auditory thresholds in the common marmoset (Callithrix jacchus) Hear. Res. 2011;277:127–133. doi: 10.1016/j.heares.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J., Ling L., Fuchs A., Siebold C., Plorde J. Rapid horizontal gaze movement in the monkey. J. Neurophysiol. 1995;73:1632–1652. doi: 10.1152/jn.1995.73.4.1632. [DOI] [PubMed] [Google Scholar]
- Platt B.B., Warren D.H. Auditory localization: the importance of eye movements and a textured visual environment. Percept. Psychophys. 1972;12:245–248. [Google Scholar]
- Reekie Y.L., Braesicke K., Man M.S., Roberts A.C. Uncoupling of behavioral and autonomic responses after lesions of the primate orbitofrontal cortex. Proc. Natl. Acad. Sci. U S A. 2008;105:9787–9792. doi: 10.1073/pnas.0800417105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington E.D., Wang X. Neural representations of the full spatial field in auditory cortex of awake marmoset (Callithrix jacchus) Cereb. Cortex. 2018;29:1199–1216. doi: 10.1093/cercor/bhy025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers L., Stafford D., Ward J. Head cocking in galagos. Anim. Behav. 1993;45:943–952. [Google Scholar]
- Roucoux A., Crommelinck M. Control of head movement during visual orientation. In: Peterson B.W., Richmond F.J., editors. Control of head movement. Oxford University Press; 1988. [Google Scholar]
- Shelton B.R., Searle C.L. The influence of vision on the absolute identification of sound-source position. Percept. Psychophys. 1980;28:589–596. doi: 10.3758/bf03198830. [DOI] [PubMed] [Google Scholar]
- Spoor F., Garland T., Jr., Krovitz G., Ryan T.M., Silcox M.T., Walker A. The primate semicircular canal system and locomotion. Proc. Natl. Acad. Sci. U S A. 2007;104:10808–10812. doi: 10.1073/pnas.0704250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark L., Zangemeister W., Edwards J., Grinberg J., Jones A., Lehman S., Lubock P., Narayan V., Nystrom M. Head rotation trajectories compared with eye saccades by main sequence relationships. Invest. Ophthalmol. Vis. Sci. 1980;19:986–988. [PubMed] [Google Scholar]
- Stevenson M.F., Poole T.B. An ethogram of the common marmoset (Calithrix jacchus jacchus): general behavioural repertoire. Anim. Behav. 1976;24:428–451. doi: 10.1016/s0003-3472(76)80053-x. [DOI] [PubMed] [Google Scholar]
- Stevenson M.F., Rylands A.B. The marmosets, genus Callithrix. In: Mittermeier R.A., Rylands A.B., Coimbra-Filho A.F., Da Fonseca G.A.B., editors. Vol. 2. World Wildlife Fund; 1988. pp. 131–222. (Ecology and behavior of neotropical primates). [Google Scholar]
- Tomlinson R., Bahra P. Combined eye-head gaze shifts in the primate. I. Metrics. J. Neurophysiol. 1986;56:1542–1557. doi: 10.1152/jn.1986.56.6.1542. [DOI] [PubMed] [Google Scholar]
- Tomlinson R., Bahra P. Combined eye-head gaze shifts in the primate. II. Interactions between saccades and the vestibuloocular reflex. J. Neurophysiol. 1986;56:1558–1570. doi: 10.1152/jn.1986.56.6.1558. [DOI] [PubMed] [Google Scholar]
- Vliegen J., Van Grootel T.J., Van Opstal A.J. Dynamic sound localization during rapid eye-head gaze shifts. J. Neurosci. 2004;24:9291–9302. doi: 10.1523/JNEUROSCI.2671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangemeister W., Stark L. Types of gaze movement: variable interactions of eye and head movements. Exp. Neurol. 1982;77:563–577. doi: 10.1016/0014-4886(82)90228-x. [DOI] [PubMed] [Google Scholar]
- Zangemeister W.H., Jones A., Stark L. Dynamics of head movement trajectories: main sequence relationship. Exp. Neurol. 1981;71:76–91. doi: 10.1016/0014-4886(81)90072-8. [DOI] [PubMed] [Google Scholar]
- Zangemeister W.H., Stark L. Gaze latency: variable interactions of head and eye latency. Exp. Neurol. 1982;75:389–406. doi: 10.1016/0014-4886(82)90169-8. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Wang X. Level dependence of spatial processing in the primate auditory cortex. J. Neurophysiol. 2012;108:810–826. doi: 10.1152/jn.00500.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video shows the first head turn in Figure 1C.
Video shows the second head turn in Figure 1C.
Data Availability Statement
https://data.mendeley.com/datasets/mh5t8fzbbf/draft?a=3759893f-38d7-49b7-a9d8-e8d25174b6eb.