Abstract
The study is to scrutinize andrographolides with Indoleamine 2,3-dioxygenase (IDO) inhibitory potential, its molecular mechanism against streptozotocin (STZ) diabetic retinopathy (DR) in Wistar rats. Oxidative stress markers such as Kynurenine metabolites, retinal histopathological changes have been studied. Further, IDO gene expression and docking studies have been performed. Andrographolide treated rats have been reducing the level of thiobarbituric acid reactive substances and protein carbonyls Kynurenine metabolites with an improvement in the level of GSH and expression of IDO as revealed by morphological changes in inner and outer nuclear layer of the retina. The current results of this study have been generated information about an activity of the andrographolide in the essential pocket of IDO. Our results explain, involving IDO and andrographolide would constitute an attempt to identify natural products with therapeutic value and further studies in this direction would be of immense significance in the administration of diabetes and its related problems.
Keywords: Diabetes, Andrographolide, Oxidative stress, Metabolites, GSH
1. Introduction
Diabetes Mellitus (DM) is a chronic and metabolic-disorder categorized through Insulin deficiency. Globally, diabetes affects more than 425 million subjects and hypoglycemia is a common complication leads to insulin treatment and persistence, high blood glucose levels can lead to diabetic neuropathy, nephropathy, retinopathy and additional complications (Cho et al., 2018, Khan et al., 2019). DM complications are common and disabling (Hinder et al., 2019). Diabetes Retinopathy (DR) becomes a serious ocular and one of the micro-vascular complications which affect the visual loss. (Gucciardo et al., 2019, Ha et al., 2019). Glucose levels which are measured with glycated hemoglobin levels (HbA1c); abnormal values will lead to the greater risk of developing retinopathy (Pusparajah et al., 2016). Diagnosis is confirmed with vascular abnormalities in the retina, has been categorized into (i) proliferative and (ii) non-proliferative diabetic retinopathies. Confirmation of earlier stage in DR is based on clinical symptoms as retinal hemorrhages, microaneurysms, venous caliber changes and intraretinal microvascular abnormalities is defined as “non-proliferative DR. Whereas, proliferative DR is an advanced stage” in DR, characterized through the hallmark feature of pathologic preretinal neovascularization; the DR patient may experience severe vision impairment (Duh et al., 2017, Wang and Lo, 2018). Based on the present scientific status, the pathogenesis of DR is still unclear, which can be complicated to multiple factors (Zhong et al., 2019).
The predominance of retinopathy expands gradually with aging and associated through hypertension, hyperglycemia, hyperlipidemia, anemia and pregnancy. The retina is metabolically dynamic tissue and insulin inadequacy and hyperglycemia is supposed to unfavorable influence the normal physiology. A number of biochemical, hematological and immune mechanisms were reported to involve in the vascular disruption connected with retinopathy. A numerous biochemical routes have been suggested to elucidate the pathogenesis of DR that may consist of increased polyol pathway, increased advanced glycation end products (AGE) formation, activation of protein kinase (PKC) in serum samples of DR (Chen et al., 2016, Farrar, 2016).
Tryptophan “is an essential amino acid used in protein synthesis mainly metabolized through the kynurenine/methoxyindole pathways; a source of NAD+ is an essential cofactor in energy metabolism” and important way to degrade the tryptophan which generates numerous metabolites is known as kynurenines (KPm) (Favennec et al., 2015, Christensen et al., 2018). The initial step in kynurenine pathway is connected with tryptophan 2,3-dioxygenease or indoleamine 2,3-dioxygenase (IDO) (Davis and Liu, 2015), which catalyze the conversion of” (Larkin et al., 2016). IDO is a monometric hemoprotein with a molecular weight of 45 kDa (Obayashi et al., 2016, Badawy, 2017). IDO is responsible for degrading tryptophan, which is significantly, affected to decrease in the serotonin synthesis and also raises the tryptophan production catabolizes through the neurotoxic properties as kynurenine, xanthurenic and quinolinic acid. This IDO activation can be casual factor linked up with both depression and diabetes (da Silva Dias et al., 2016). Kynurenine pathway metabolites have been also reported in human eye and the direction with Kynurenine is associated with DR and IDO as a rate limiting enzyme of the kynurenine pathway, suggesting blockage of IDO assumes impact in the management of DR. Earlier studies have mainly focused on investigating natural products of plant sources of restrained IDO; which was increase in the serum level of tryptophan and kynurenines were reported and still now there were no adequate pharmacological treatment for managing diabetic complications (Munipally et al., 2011).
Andrographolide “(3-(-{decahydro-6-hydroxy-5-(hydroxymethyl0-5,8 α-dimethyl-2-ethylene-1-napthalenyl} ethylidene] dihydro-4-hydroxy-2(3H)-furanone) (C20H30O5) is a natural diterpenoid lactone, the major compound isolated from medicinal herb Andrographis paniculata Nees, family member of Acanthacea. It appears as crystalline bicyclic diterpenoid colorless lactone with bitter taste (Yu et al., 2015). It is also documented as antioxidant, antiplatelet, antihyperglycemic, immunomodulatory and anticancer properties (Subramanian et al., 2008). Andrographis paniculata is the previous therapeutic agent for diabetes which was emphasized for its anti-oxidation, hepatoprotective and anti-inflammatory effects (Yu et al., 2003). Ethanol extraction of these plants can reduce the blood glucose levels in T1DM rats; unreported antidiabetic results were available in T2DM (Nugroho et al., 2012); exhibits hypoglycemic effects in STZ-diabetics rat (Nugroho et al., 2013). Andrographolide exhibits remarkably tremendous scope of biological activities and it was observed to be useful in blocking carbon tetrachloride induced liver injury in rats” and mice and it also has a specific role in anticipating oxygen radical production in STZ actuated rats (Chen et al., 2018). In the light of the aforesaid value of andrographolide, the present experiments have been intended to understand the efficacy and defensive mechanism of andrographolide’s supplementation to the experimental DR rats. So, the current study was carried out with andrographolides and IDO inhibitory potential and its molecular mechanism against STZ- DR in Wistar rats.
2. Materials and methods
2.1. Invivo experiments
2.1.1. Extraction of retina
The combination of ketamine and xylazine provides the safe anesthesia and these compounds has been implemented in our experiment for rats. Once the rats were sacrificed by carbon dioxide asphyxiation, the eye balls were separated through posterior approach and stored for the further analysis. The retinal tissue was isolated from eye and optimized in 0.1 M phosphate buffer (PB; pH-7.4) and various centrifugations were performed from 3000 to 10,000 rpm at 5–10 mins. With the help of PBS, paraformaldehyde and Triton-X, the complete protocol of extraction of retina was carried out from the previous publications (Ullmann et al., 2012, Ren et al., 2018). The extracted supernatant was further utilized for IDO activity.
2.1.2. 2IDO activity assay
In our study, the IDO activity assay was performed based on the methodology of Kanth et al. (2009) studies. To perform this assay, we have prepared 200 µl reaction mixture with 50 mM potassium PB (pH6.5), 20 mM sodium ascorbate, 10 µM methylene blue, 100 µg/mL catalase and 200 µM trypsin. The reaction mixtures were incubated at different temperatures. The supernatant of aliquots was used for HPLC apparatus with the combination of different solvents. Kynurenic acid was quantified through the known concentration of the standard compound kynurenine.
2.1.3. IDO inhibition assay
In this assay, 200 µl of standard examine medium were prepared with the final concentrations of (i) 0.2 M of ascorbic acid, (ii) 0.5 mM methylene blue (iii) 0.5 M potassium phosphate buffer (PPB), (iv) 5 mg/mL of catalase, (v) 100 µg/mL of milli-Q-water, (vi) 4 mL of tryptophan, (vii) 20 µl of retina sample, and (viii) 10 µl of DMSO solutions. The carried out experiment has produced the amount of Kynurenine, which was determined by the standard control of Kynurenine and the inhibition levels were in the form of percentages and andrographolide, were determined considering the 100% IDO activity without any inhibitor (Southan et al., 1996). One of the documented materials “1-Methyl-Tryptophan” was used as the reference material (Hou et al., 2007, Lewis et al., 2017). The andrographolide concentration result in 50% inhibition (IC50) was calculated using regression analysis with the log concentration of compound and (vs) inhibition percentage.
2.1.4. Kynurenine standard curve
200 µl of standard medium was primed with the combination of 20 µl of 0.5 M PPB with pH6.5; “20 µl of 0.2 M ascorbic acid; 4 µl of 0.5 mM methylene blue; 4 µl of 5 mg/mL catalase; 132 µl of MilliQ water; and 20 µl of different solutions of Kynurenine solutions with the final concentrations of 0–1–5–7.5–10–25–50–75–100 µM”. The 40 × 2 = 80 µl of medium was centrifuged around 11,000 rpm-15 min for HPLC (40 µl) and fluorescence assay (40 µl). The Kynurenine formation was monitored at 480 nm (Matin et al., 2006).
2.2. In-vitro experiments
2.2.1. Animal study cum breeding
The ethical approval for the animals was received in accordance with the Institute Animal ethical committee. We have attained 40 rats from the Animal House. The Wistar rats (Rattus norvegicus) were three months old with an average body weight of 220 ± 8 g. The experiment was carried out for 2 months and animals were divided into 4 groups and each group 10 rats were included. Group-I was considered as a control group; Group-II as Diabetic rats; Group-III as Diabetic rats treated with andrographolide at 1.5 mg/kg of body weight and Group-IV is treated as diabetic rats with andrographolide at 4.5 mg/kg of body weight. The protocol was implemented with polycarbonate cages, in which rats were preserved in a room with 12 h/ white and black cycles, moistures at 45–60%at the temperature of 20 ± 2 °C (Nistiar et al., 2012). AIN-93 diet was provided for each rat (Santos et al., 2015).
2.2.2. Biochemical assay
In the Wistar rats, the diabetes was persuaded in overnight fasting for 3 days through the solitary intraperitoneal injection of STZ (35 mg/kg). Using the anesthesia by retro-orbital puncture method for Wistar rats, blood was collected for measuring the plasma glucose levels using glucose oxidase and peroxidase kit method. This complete protocol for plasma glucose, carboxyl, and estimation of reduced glutathione and thiobarbituric acid reactive substances (TBARS) assays, were adopted from our previous studies (Kumar et al., 2015).
2.2.3. Histological assays
The stored eye ball would be used for the histological analysis which are enucleated cells and post-fixed in 10% formaldehyde for 4 h. Using various concentrations (30%, 50%, 75% and 100%) of alcohol gradients, tissues were hydrated and rinsed with distilled water for 15–20 min. Tissues were cleared with 2 xylene changes for 10 mins and kept in wax for 55 °C. In the melted wax, tissues were embedded at 60 °C with L-blocks. Approximately, 10 µm eyeball slices were sectioned using the leica, hematoxylin-eosin stained with captured with light microscope. The complete protocol has been documented with Huang et al. (2018) studies.
2.2.4. Gene expression assay
Using Trizol reagent, RNA was extracted from 100 mg of lenses, which was dissected from the eyeball. The purity of RNA concentrations was measured with NanoDrop spectrophotometer. The extracted RNA was transcribed into reverse cDNA, was further used for reverse transcriptase-polymerase chain reaction (RT-PCR) using GAPDH-600 bp and IDO-277 bp primers (20 pmoles) (Kanth et al., 2009) studies. The GAPDH gene was used as house-keeping gene for normalization. Amplification conditions are as follows; Initial denaturation as 95 °C-5 mins; denaturation 95 °C-30 s (GAPDH:55 °C-30 s and IDO:48.2 °C-100 s) extension and final extension took place at 72 °C-10 min. Using 2% agarose gel, PCR products were electrophoresed with ethidium bromide stained and bands were visualized with UV-trans illuminator (Syngene gel doc, USA). The 100% concordance rate was observed in the expression of candidate genes (GAPDH), which are induced in diabetic rats (Kanth et al., 2009, Huang et al., 2018).
2.2.5. Molecular docking assay
The molecular docking studies are performed with andrographolide in the active site of the IDO using accelrys discovery studio 2.5. Andrographolide was minimized into least energy confirmation and taken for docking study. The crystal structure of IDO (PDB: 2DOT) was downloaded from the Protein Data Bank (PBD; http://www.pdb.org) and protein structure minimized utilizing charm force field. Docking was done through CDOCKER module and after docking poses was viewed with DS viewer.
2.2.6. Statistical analysis
Using SPSS software (version 21.0), one-way ANOVA analysis was performed within the groups of data followed with post hoc-test (multiple comparisons). The p values less than 0.05 (p < 0.05) were considered as significant difference.
3. Results
3.1. Inhibition of IDO by andrographolide
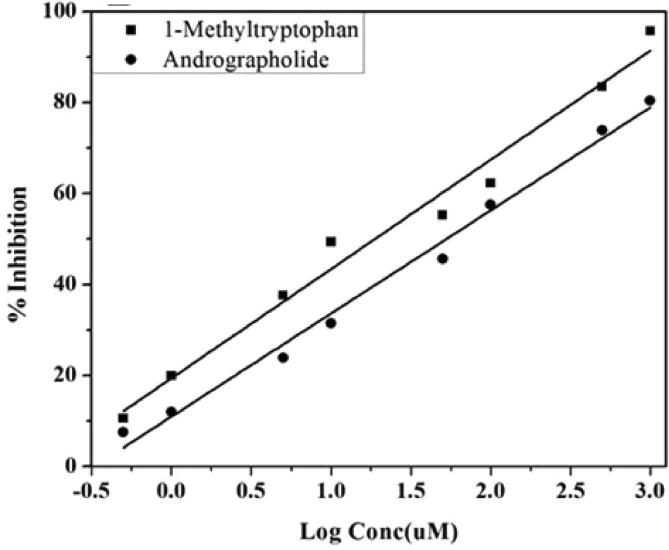
IDO activity is characterized “as the amount of enzyme producing nmol/hr kynurenine. Andrographolide was tried for its inhibitory action against rat retina IDO and IC50 values were compared with that of 1-methyltryptophan, a known inhibitor” of IDO which has an IC50 value of 18 ± 0.36 µg/mL. While, andrographolide inhibited rat retina IDO value were 56 ± 0.09 µg/mL (Fig. 1).
Fig. 1.
Inhibition of Indoleamine 2, 3-dioxygenase (IDO) by Andrographolide. Legend: Representative graph showing the different concentrations of Andrographolide resulting in 50% inhibition (IC50) was determined by non-linear regression analysis of log concentration of Andrographolide versus percentage inhibition. The data are the mean ± SD (n = 10).
3.2. Andrographolide prevents STZ induced diabetic retinopathy in rats
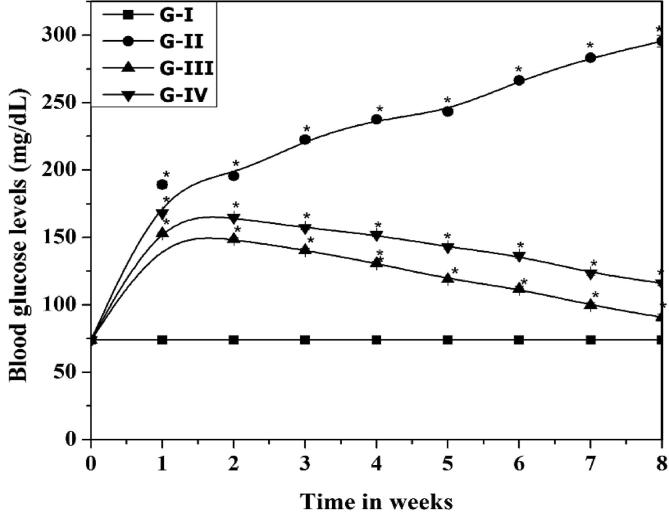
The improved food intake in diabetic rats in the “groups 2, 3 and 4 were compared to group 1 of control. In spite of improved food intake, the body weight of diabetic group-2 rats was decreased (155 ± 13 mg) compared to control group (210 ± 8 mg) (Group-I) and andrographolide fed rats” (Group-3, 197 ± 8 mg and Group-4, 178 ± 15 mg). Moreover, there is an increase in blood glucose concentration which has been abated by feeding with andrographolide to diabetic rats (Fig. 2). At the end of the two months, nourishing of andrographolide to diabetic rats, observed a decline in IDO activity in group-2, 3 and 4 in a dose-dependent pattern.
Fig. 2.
The effect of Andrographolide on blood glucose levels. Legend: Fasting blood glucose levels were estimated in different groups of rats (G I to G IV). Control (G-I), STZ-treated (G-II), STZ-treated + 1.5 mg/kg andrographolide (G-III) and STZ-treated + 4.5 mg/kg andrographolide (G-IV). The data are the mean ± SD (n = 10). *Statistically significant from G I (analyzed by ANOVA; p ≤ 0. 05).
3.3. Oxidative stress markers
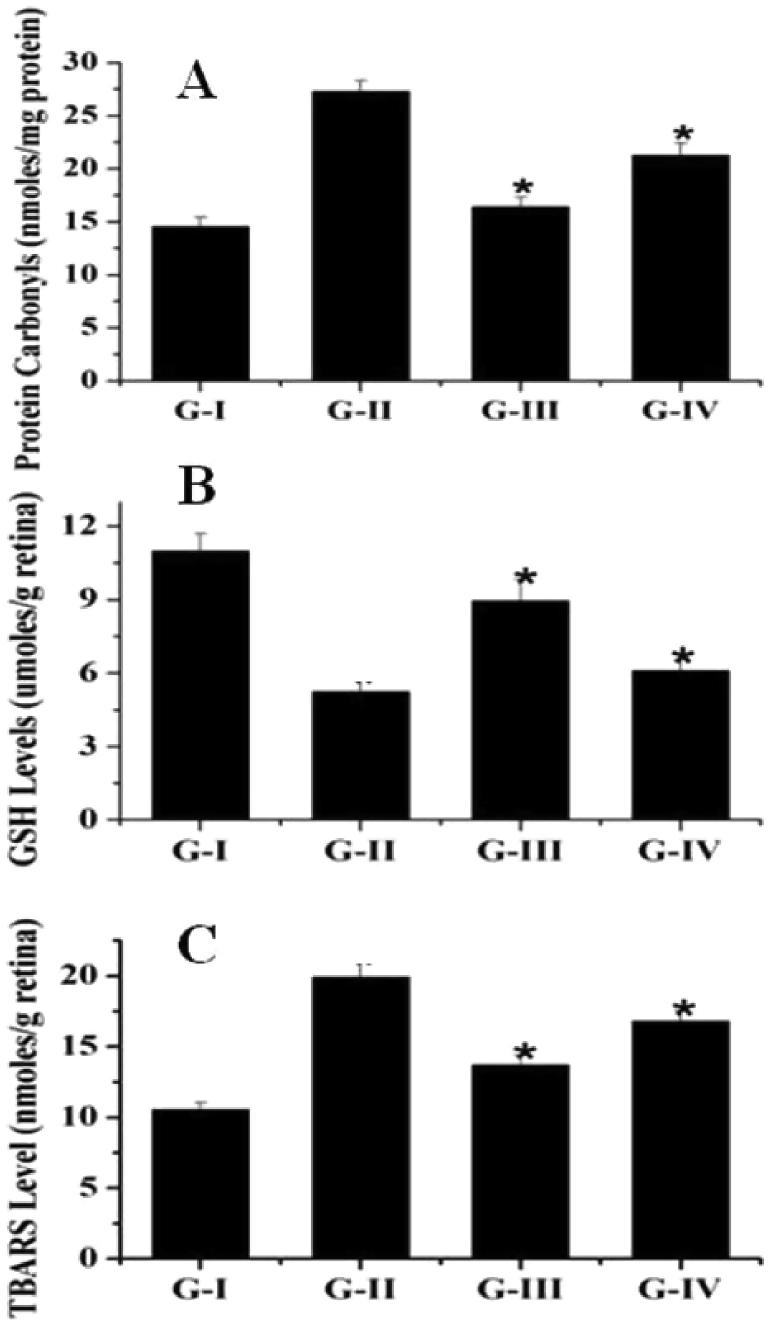
The levels of protein carbonyls, TBARS were raised up and reduced glutathione as decline. The groups 3 & 4 fed with andrographolide i.e. 1.5 and 4.5 mG/kg body weights were respectively found to have diminished TBARS in GSH levels (Fig. 3).
Fig. 3.
The protective effect of Andrographolide on oxidative markers in STZ induced diabetic rats. Legend: Effect of Andrographolide on Protein Carbonyls (A), GSH (B), and TBARS (C) level in different groups. Control (G-I), STZ-treated (G-II), STZ-treated + 1.5 mg/kg andrographolide (G-III) and STZ-treated + 4.5 mg/kg andrographolide (G-IV). Data are mean ± S. D (n = 3). * denotes that the data is significantly different from G-I at P < 0.05.
3.4. Estimation of kynurenine metabolites
Our study revealed the significant elevated levels of tryptophan metabolites (KYN, KYNA and 3-HKYN) “in diabetic group rats (Group –II) with respect to control rats (Group-I)”. These metabolites were found to decrease with feeding of andrographolide in groups III & IV with increased protection at lower dose i.e. 1.5 mg/kg body weight (Table 1).
Table 1.
Concentration of Tryptophan (Trp), kynurenine (KYN), Kynurenic acid (Kyn) and 3-hydroxykynurenine (3HKYN) in the retinas of different groups.
| Group | TRP (µM) | KYN (µM) | KYNA (nM) | 3HKYN (µM) |
|---|---|---|---|---|
| Group-I (Control) | 40.56 ± 2.0 | 2.06 ± 0.13 | 0.17 ± 0.024 | 4.18 ± 0 0.05 |
| Group-II (STZ-Treated) | 44.03 ± 2.9 | 4.00 ± 0.12 | 0.38 ± 0.01 | 7.28 ± 0.18 |
| Group-III (STZ + 1.5 mg/kg Andrographolide) | 42.65 ± 9.4* | 2.25 ± 0.43* | 0.20 ± 0.024* | 4.71 ± 0.27* |
| Group-IV (STZ + 4.5 mg/kg Andrographolide) | 43.84 ± 3.1 | 2.96 ± 0.15 | 0.31 ± 0.02 | 6.47 ± 0.29 |
3.5. Morphological changes in retina
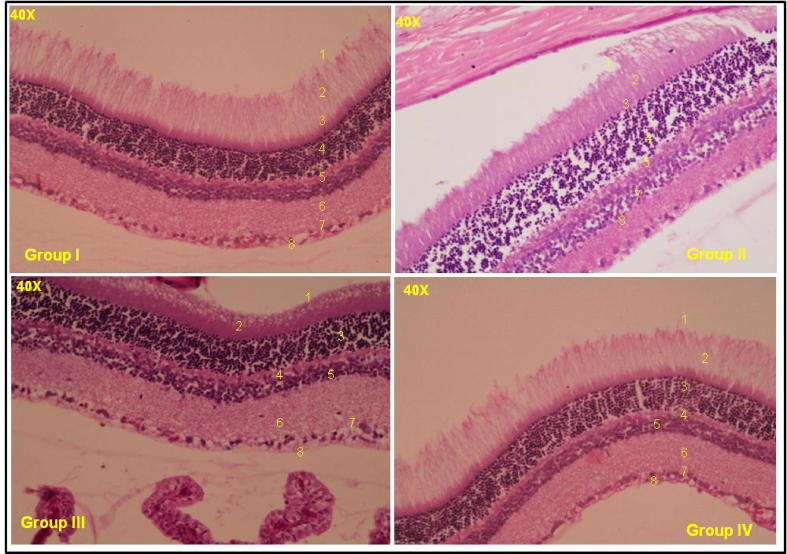
Morphological modifications have been noticed in inner nuclear layer (INL), outer nuclear layer (OPL) and pigment layer in diabetic group-2 compared with respective age matched control rats (Group-1, Fig. 4). The rat retinas (Group-I) showed normal intact of retinal sub layers whereas diabetic control (Group-II) retinas found to have disturbed architecture. Little retrieval was observed in 4.5 mg/kg body weight andrographolide fed rats (Group-4) but positive recovery was detected with 1.5 mg/kg body weight andrographolide fed rats (Group-III) suggesting an effective defensive potential against diabetic induced alterations.
Fig. 4.
Histological examination of retinas in Andrographolide treated diabetic rats. Legend: Effect of Andrographolide on Histology in different groups. Control (G-I), STZ-treated (G-II), STZ-treated + 1.5 mg/kg andrographolide (G-III) and STZ-treated + 4.5 mg/kg andrographolide (G-IV).
3.6. Gene expression analysis in retina
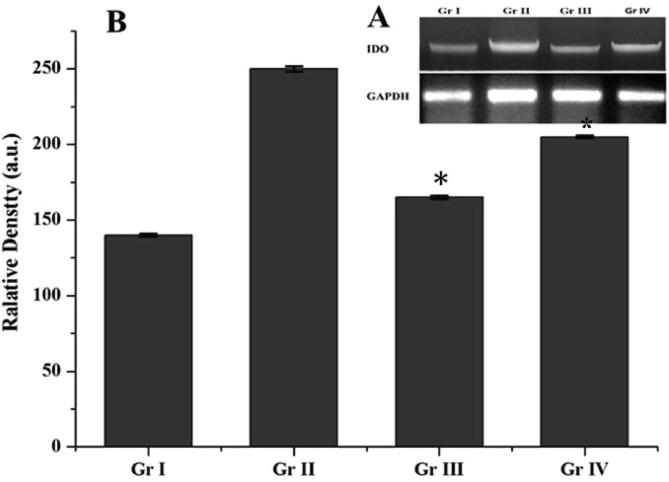
To support our observation with regard to the morphological and biochemical changes we have performed RT-PCR on retinal tissues of different groups with primers corresponding to IDO and one housekeeping gene GAPDH. Fig. 5 explains RT-PCR amplified products showing significant d diabetic control rats (Group-II). The current analysis reveals the lowers in expression of IDO mRNA in the diabetic group which has been administered between groups 3 and 4 i.e., 1.5 and 4.5 mg/kg body weight of andrographolides.
Fig. 5.
Expression levels of Indoleamine 2, 3-dioxygenase (IDO) gene in Andrographolide treated diabetic rats. Legend: Increase in IDO expression in diabetic Rat retina. (A) Representative Agarose gel analysis showing the IDO gene expression in different groups of retinas. (B) Data are presented as the relative density of IDO gene expression compared with that of GAPDH. The data are the mean ± SD (n = 10). *Statistically significant from G-I (analyzed by ANOVA; p ≤ 0. 05).
3.7. Docking of IDO
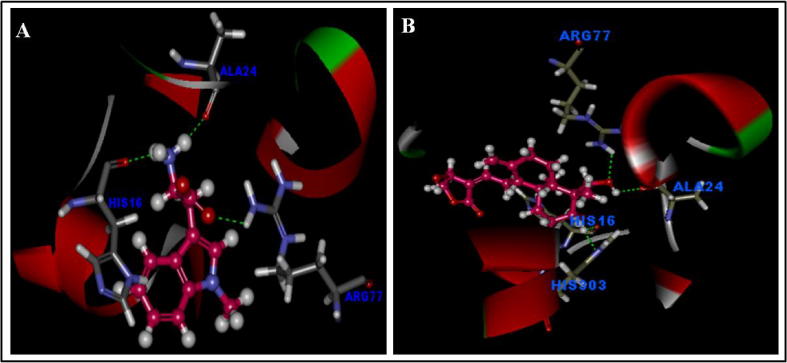
We anticipated the tertiary structure of IDO and stimulated docking to andrographolide. using accelrys Discovery /studio 2.5 (DS), we searched for IDO residues that would bind to andrographolide. The molecular docking of the IDO-ligand complexes was well-performed with andrographolide stably posed in the pocket of IDO by Discovery Studio 2.5. To validate the binding patterns and selective inhibition of IDO through andrographolide, docking studies were performed and it was confirmed as andrographolide conceivably connects with IDO at the active site residues Arg-77, Ala-24 and His-16 (Hydrogen bonds) with CDOCKER energy 45.24 kcal/mol at the same time 1-methyltryptophan known inhibitor of IDO also showing the similar hydrogen bonding interactions with active site residues Arg-77, Ala-24, His-16 and His-303 with CDOCKER 36.45 kcal/mol (Fig. 6).
Fig. 6.
Molecular docking of Indoleamine 2, 3-dioxygenase (IDO) and docking interactions with Andrographolide. Legend: Computational prediction of the structure of IDO and docking stimulation with 1-methyltryptophan (A) and Andrographolide (B) interactions bound to IDO active site amino acids.
4. Discussion
Diabetes is connected with the combination of multiple factors such as carbohydrates, fats and protein metabolism (Khan et al., 2019). Numerous treatments are accessible for different forms of diabetes. Presently, era of medicinal plants (alkaloids, carotenoids, flavonoids and glycosides) treatment is recommended which comprises of anti-diabetic effects (Mujeeb et al., 2014). Animal model analysis is the accurate which remains indispensable for discovering, optimizing and validating novel therapeutics for the safe use in humans (Fang et al., 2019). STZ-induced hyperglycaemia in animals is used as a good model for the initial screening of plants and plant derived compounds against diabetes (Saravanan et al., 2009). STZ is known as broad spectrum of antibiotic, which induces diabetics; thus elevates the glucose levels which indicates the inadequate release of Insulin (Aboulthana et al., 2018). The STZ induced diabetes is described as extreme weight loss in body affected by gluconeogenesis, catabolism of fats and proteins, increased muscle wasting, loss of tissue proteins and loss of degradation of structural proteins (Naidu et al., 2015, Naidu et al., 2016). STZ-induction also caused in substantial growth in the level and reduction in their weight. The oral administration Andrographolide was halting the sustained hyperglycemia and reverting back the body weight and blood glucose to that of control animals. This antihyperglycemic property of Andrographolide could be ascertained to its antioxidant components (Lin et al., 2009).
Experimental induced hyperglycemic condition contributes to the pathophysiology of diabetic cellular metabolism in dual way, one being an increased production of ROS and other being the reduction of antioxidant defense. The combination of both leads to cellular and tissue damage promoted in various diabetic complications (Saravanan and Ponmurugan, 2011). Free radicals might also be formed via the auto-oxidation of unsaturated fatty acids in plasma and membrane lipids and respond with other polyunsaturated fatty acids of membrane leading to lipid peroxidation that will in turn results in elevated production of free radicals” (Levy et al., 1999).
Previous reported that the free radical improved lipid peroxidation and associated impairment of tissue antioxidant marker enzymes in diabetic animals (Saravanan and Ponmurugan, 2011). Oral administration of strawberry extracts exactly did the same as we have found reduction in the levels of hydroperoxides and TBARS that were elevated in diabetic rats. Similarly, oral treatment of Andrographolide boosted the cellular antioxidant defense by restoring the derangement of antioxidant markers such as GSH during diabetes. These attributes of andrographolide could be due to its total antioxidant capacity. Andrographolide mediated stabilization of antioxidant marker enzymes were already reported by Lin et al. (2009) and our findings endorse the earlier report.
Andrographolide and its inhibitory potential against IDO have been investigated. Supplementation of andrographolide in dose dependant manner was found to effectively ameliorate retinopathy as revealed by the changes in histology and oxidative markers. We hypothesize that andrographolide’s protective influence on retina could be linked to the IDO and tryptophan metabolites have been received much consideration currently by the researchers because of it prooxidant property and “their contribution in diabetic complications such as cataract and retinopathy (Koch et al., 1982, Wegener et al., 2002). In tryptophan pathway kynurenine is the first stable intermediate (Takikawa et al., 2001) and this kynurenine undergoes deamination or irreversible transamination forming the Kynurenic acid (KYNA), a known prooxidant” (Thomas and Stocker, 1999, Takikawa et al., 2001) significant increase in the concentration of KYNA in rats of group-2 and group-4 compared to group-1and group-3. In DR, IDO caused an oxidative stress which caused an increase in kynurenine metabolites in the eye (Chiarugi et al., 1999).
Therefore, 3-HKYN might become an “internal source of H2O2 which is believed to play an important role in the oxidative damage often related with DR. In the present study the 3-HKYN levels have been found to be higher in Group-2rats when compared to control subjects. This remark suggests that tryptophan pathway metabolites play a key role and they may perhaps be involved in the DR arbitrated through the mechanism of oxidative stress. Oxidative stress indicators for example TBARS and protein carbonyl levels were found to increase significantly in STZ induced diabetic rats. Supplementation of andrographolide normalized the levels to some extent but definitely not nearer to those of controls. Further, Supplementation of andrographolide in a dose-dependent manner had restored GSH levels, suggesting its protective value. It has been suggested that diabetic control rats have recovered increased kynurenine metabolites in retina and therefore it may be speculated that the observed alterations could be due either to a rise in the concentration of the substrate or to the enhancement of the first stage of its enzymatic degradation. The former possibility seems less probable, since no difference in the “concentration of tryptophan in the retinas of different groups of rats was observed.
Among numerous pharmacological targets pointed to combat DR, inhibition of IDO activity has received a better consideration in recent times. The elevated expression of mRNA encoding “IDO (P < 0.05) in diabetic control rats (group-2, when compared with control animals (group-1) clearly proposes a role of IDO enzyme in DR” and perhaps a possible recovery by a lower dose i.e. 1.5 mg kg/ b. wt of andrographolide rather than a high dose 4.5 mg kg/ b.wt. The useful effect of strict glycemic control in counteractive action of diabetic complications has been well established. However, the compounds/molecules that can significantly delay or prevent the onset the development of diabetic complications have not been completely investigated. Our computational docking examines have uncovered essential information about the role of the inhibitor in the binding pocket of IDO.
Legend-enzyme interaction investigation found that the interaction binding residues of andrographolide (Ala-77, Ala-24, and His-16) were the primary contributors to the receptor-ligand communication and our outcomes likewise propose that andrographolide binds favorably to the active site of IDO as appeared in Fig. 6.
5. Conclusion
The present investigation suggested the administration of andrographolide inhibit IDO and consequential changes in Kynurenine metabolites and can be considered as the agent that controls the diabetic complications.
Acknowledgments
Acknowledgements
“The authors (SM and KAAG) express their sincere appreciation to the Deanship of Scientific Research at the King Saud University for its funding of this research through the Research Group Project No. RG-1435-012”.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aboulthana W.M., El-Feky A.M., Ibrahim N.E.-S., Sahu R.K., El-Sayed A.E.-K.B. Evaluation of the pancreatoprotective effect of nannochloropsis oculata extract against streptozotocin-induced diabetes in rats. J. Appl. Pharm. Sci. 2018;8(06):046–058. [Google Scholar]
- Badawy A.A. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int. J. Tryptophan Res. 2017;10 doi: 10.1177/1178646917691938. 1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Ovbiagele B., Feng W. Diabetes and stroke: epidemiology, pathophysiology, pharmaceuticals and outcomes. Am. J Med. Sci. 2016;351(4):380–386. doi: 10.1016/j.amjms.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Zhang J., Yi R., Mu J., Zhao X., Yang Z. Hepatoprotective effects of lactobacillus on carbon tetrachloride-induced acute liver injury in mice. Int. J. Mol. Sci. 2018;19(8):2212. doi: 10.3390/ijms19082212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarugi A., Rapizzi E., Moroni F., Moroni F. The kynurenine metabolic pathway in the eye: studies on 3-hydroxykynurenine, a putative cataractogenic compound. FEBS Lett. 1999;453(1–2):197–200. doi: 10.1016/s0014-5793(99)00724-3. [DOI] [PubMed] [Google Scholar]
- Cho N., Shaw J., Karuranga S., Huang Y., da Rocha Fernandes J., Ohlrogge A., Malanda B. Idf diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diab. Res. Clin. Practice. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- Christensen M.H., Fadnes D.J., Røst T.H., Pedersen E.R., Andersen J.R., Våge V., Ulvik A., Midttun Ø., Ueland P.M., Nygård O.K. Inflammatory markers, the tryptophan-kynurenine pathway, and vitamin b status after bariatric surgery. PloS One. 2018;13(2):e0192169. doi: 10.1371/journal.pone.0192169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Dias I.C., Carabelli B., Ishii D.K., de Morais H., de Carvalho M.C., De Souza L.E.R., Zanata S.M., Brandão M.L., Cunha T.M., Ferraz A.C. Indoleamine-2, 3-dioxygenase/kynurenine pathway as a potential pharmacological target to treat depression associated with diabetes. Mol. Neurobiol. 2016;53(10):6997–7009. doi: 10.1007/s12035-015-9617-0. [DOI] [PubMed] [Google Scholar]
- Davis I., Liu A. Taylor & Francis; 2015. What is the Tryptophan Kynurenine Pathway and Why is it Important to Neurotherapeutics? [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duh E.J., Sun J.K., Stitt A.W. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight. 2017;2(14) doi: 10.1172/jci.insight.93751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J.-Y., Lin C.-H., Huang T.-H., Chuang S.-Y. In vivo rodent models of type 2 diabetes and their usefulness for evaluating flavonoid bioactivity. Nutrients. 2019;11(3):530. doi: 10.3390/nu11030530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar D. Hyperglycemia in pregnancy: prevalence, impact, and management challenges. Int. J. Women's Health. 2016;8:519. doi: 10.2147/IJWH.S102117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favennec M., Hennart B., Caiazzo R., Leloire A., Yengo L., Verbanck M., Arredouani A., Marre M., Pigeyre M., Bessede A. The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity. 2015;23(10):2066–2074. doi: 10.1002/oby.21199. [DOI] [PubMed] [Google Scholar]
- Gucciardo E., Loukovaara S., Korhonen A., Lehti K. An ex vivo tissue culture model for fibrovascular complications in proliferative diabetic retinopathy. JoVE (Journal of Visualized Experiments) 2019;143:e59090. doi: 10.3791/59090. [DOI] [PubMed] [Google Scholar]
- Ha M., Choi S.Y., Kim M., Na J.K., Park Y.-H. Diabetic nephropathy in type 2 diabetic retinopathy requiring panretinal photocoagulation. Korean J. Ophthalmol. 2019;33(1):46–53. doi: 10.3341/kjo.2018.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinder L.M., Sas K.M., O’Brien P.D., Backus C., Kayampilly P., Hayes J.M., Lin C.-M., Zhang H., Shanmugam S., Rumora A.E. Mitochondrial uncoupling has no effect on microvascular complications in type 2 diabetes. Sci. Reports. 2019;9(1):881. doi: 10.1038/s41598-018-37376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou D.-Y., Muller A.J., Sharma M.D., DuHadaway J., Banerjee T., Johnson M., Mellor A.L., Prendergast G.C., Munn D.H. Inhibition of indoleamine 2, 3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007;67(2):792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- Huang B., Liang J.-J., Zhuang X., Chen S.-W., Ng T.K., Chen H. Intravitreal injection of hydrogen peroxide induces acute retinal degeneration, apoptosis, and oxidative stress in mice. Oxidative Med. Cell. Longevity. 2018 doi: 10.1155/2018/5489476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanth V.R., Lavanya K., Srinivas J. Elevated expression of indoleamine 2, 3-dioxygenase (ido) and accumulation of kynurenic acid in the pathogenesis of stz-induced diabetic cataract in wistar rats. Curr. Eye Res. 2009;34(4):274–281. doi: 10.1080/02713680902725954. [DOI] [PubMed] [Google Scholar]
- Khan I.A., Jahan P., Hasan Q., Rao P. Genetic confirmation of t2dm meta-analysis variants studied in gestational diabetes mellitus in an indian population. Diab. Metabolic Syndrome. 2019;13(1):688–694. doi: 10.1016/j.dsx.2018.11.035. [DOI] [PubMed] [Google Scholar]
- Koch H.-R., Ohrloff C., Bours J., Riemann G., Dragomirescu V., Hockwin O. Separation of lens proteins in rats with tryptophan deficiency cataracts. Exp. Eye Res. 1982;34(4):479–486. doi: 10.1016/0014-4835(82)90019-7. [DOI] [PubMed] [Google Scholar]
- Kumar M.P., Sankeshi V., Naik R.R., Thirupathi P., Das B., Raju T. The inhibitory effect of isoflavones isolated from caesalpinia pulcherrima on aldose reductase in stz induced diabetic rats. Chemico-biological Interact. 2015;237:18–24. doi: 10.1016/j.cbi.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Larkin P.B., Sathyasaikumar K.V., Notarangelo F.M., Funakoshi H., Nakamura T., Schwarcz R., Muchowski P.J. Tryptophan 2, 3-dioxygenase and indoleamine 2, 3-dioxygenase 1 make separate, tissue-specific contributions to basal and inflammation-induced kynurenine pathway metabolism in mice. Biochim. Biophys. Acta (BBA)-General Subjects. 2016;1860(11):2345–2354. doi: 10.1016/j.bbagen.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy Y., Zaltzberg H., Ben-Amotz A., Kanter Y., Aviram M. Β-carotene affects antioxidant status in non-insulin-dependent diabetes mellitus. Pathophysiology. 1999;6(3):157–161. [Google Scholar]
- Lewis H.C., Chinnadurai R., Bosinger S.E., Galipeau J. The ido inhibitor 1-methyl tryptophan activates the aryl hydrocarbon receptor response in mesenchymal stromal cells. Oncotarget. 2017;8(54):91914. doi: 10.18632/oncotarget.20166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F., Wu S., Lee S., Ng L. Antioxidant, antioedema and analgesic activities of andrographis paniculata extracts and their active constituent andrographolide. Phytotherapy Res.: Int. J. Devoted to Pharmacol. Toxicol. Eval. Nat. Prod. Derivat. 2009;23(7):958–964. doi: 10.1002/ptr.2701. [DOI] [PubMed] [Google Scholar]
- Matin A., Streete I.M., Jamie I.M., Truscott R.J., Jamie J.F. A fluorescence-based assay for indoleamine 2, 3-dioxygenase. Analytical biochemistry. 2006;349(1):96–102. doi: 10.1016/j.ab.2005.10.039. [DOI] [PubMed] [Google Scholar]
- Mujeeb F., Bajpai P., Pathak N. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of aegle marmelos. BioMed Res. Int. 2014 doi: 10.1155/2014/497606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munipally P.K., Agraharm S.G., Valavala V.K., Gundae S., Turlapati N.R. Evaluation of indoleamine 2, 3-dioxygenase expression and kynurenine pathway metabolites levels in serum samples of diabetic retinopathy patients. Arch. Physiol. Biochem. 2011;117(5):254–258. doi: 10.3109/13813455.2011.623705. [DOI] [PubMed] [Google Scholar]
- Naidu P.B., Ponmurugan P., Begum M.S., Mohan K., Meriga B., RavindarNaik R., Saravanan G. Diosgenin reorganises hyperglycaemia and distorted tissue lipid profile in high-fat diet–streptozotocin-induced diabetic rats. J. Sci. Food Agric. 2015;95(15):3177–3182. doi: 10.1002/jsfa.7057. [DOI] [PubMed] [Google Scholar]
- Naidu P.B., Uddandrao V.S., Naik R.R., Suresh P., Meriga B., Begum M.S., Pandiyan R., Saravanan G. Ameliorative potential of gingerol: promising modulation of inflammatory factors and lipid marker enzymes expressions in hfd induced obesity in rats. Mol. Cell. Endocrinol. 2016;419:139–147. doi: 10.1016/j.mce.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Nistiar F., Racz O., Lukacinova A., Hubkova B., Novakova J., Lovasova E., Sedlakova E. Age dependency on some physiological and biochemical parameters of male wistar rats in controlled environment. J. Environ. Sci. Health, Part A. 2012;47(9):1224–1233. doi: 10.1080/10934529.2012.672071. [DOI] [PubMed] [Google Scholar]
- Nugroho A.E., Andrie M., Warditiani N.K., Siswanto E., Pramono S., Lukitaningsih E. Antidiabetic and antihiperlipidemic effect of andrographis paniculata (burm. F.) nees and andrographolide in high-fructose-fat-fed rats. Indian J. Pharmacol. 2012;44(3):377. doi: 10.4103/0253-7613.96343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A.E. Nugroho, N.Y. Lindawati, K. Herlyanti, L. Widyastuti, S. Pramono, 2013. Anti-diabetic effect of a combination of andrographolide-enriched extract of andrographis paniculata (burm f.) nees and asiaticoside-enriched extract of centella asiatica l. In high fructose-fat fed rats. [PubMed]
- Obayashi Y., Ozaki Y., Goto S., Obayashi S., Suzumori N., Ohyama F., Tone S., Sugiura-Ogasawara M. Role of indoleamine 2, 3-dioxygenase and tryptophan 2, 3-dioxygenase in patients with recurrent miscarriage. Am. J. Reprod. Immunol. 2016;75(1):69–77. doi: 10.1111/aji.12434. [DOI] [PubMed] [Google Scholar]
- Pusparajah P., Lee L.-H., Abdul Kadir K. Molecular markers of diabetic retinopathy: potential screening tool of the future? Front. Physiol. 2016;7:200. doi: 10.3389/fphys.2016.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J.L., Yu Q.X., Liang W.C., Leung P.Y., Ng T.K., Chu W.K., Pang C.P., Chan S.O. Green tea extract attenuates lps-induced retinal inflammation in rats. Sci. Reports. 2018;8(1):429. doi: 10.1038/s41598-017-18888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos J.F., Amaral M.S., Oliveira S.L., Barbosa J.P., Cabral-Jr C.R., Melo I.S., Bueno N.B., Freitas J.D., Sant’ana A.G., Ataíde T.R. Dietary intake of ain-93 standard diet induces fatty liver with altered hepatic fatty acid profile in wistar rats. Nutricion Hospitalaria. 2015;31(5):2140–2146. doi: 10.3305/nh.2015.31.5.8597. [DOI] [PubMed] [Google Scholar]
- Saravanan G., Ponmurugan P. Ameliorative potential of s-allyl cysteine on oxidative stress in stz induced diabetic rats. Chemico-Biol. Interact. 2011;189(1–2):100–106. doi: 10.1016/j.cbi.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Saravanan G., Ponmurugan P., Kumar G.P.S., Rajarajan T. Antidiabetic properties of s-allyl cysteine, a garlic component on streptozotocin-induced diabetes in rats. J. Appl. Biomed. 2009;7(7):151–159. [Google Scholar]
- Southan M., Truscott R., Jamie J., Pelosi L., Walker M., Maeda H., Iwamoto Y., Tone S. Structural requirements of the competitive binding site of recombinant human indoleamine 2, 3-dioxygenase. Med. Chem. Res. 1996;6(5):343–352. [Google Scholar]
- Subramanian R., Asmawi M., Sadikun A. Effect of andrographolide and ethanol extract of andrographis paniculata on liver glycolytic, gluconeogenic, and lipogenic enzymes in a type 2 diabetic rat model. Pharm. Biol. 2008;46(10–11):772–780. [Google Scholar]
- Takikawa O., Littlejohn T.K., Truscott R.J. Indoleamine 2, 3-dioxygenase in the human lens, the first enzyme in the synthesis of uv filters. Exp. Eye Res. 2001;72(3):271–277. doi: 10.1006/exer.2000.0951. [DOI] [PubMed] [Google Scholar]
- Thomas S.R., Stocker R. Redox reactions related to indoleamine 2, 3-dioxygenase and tryptophan metabolism along the kynurenine pathway. Redox Report. 1999;4(5):199–220. doi: 10.1179/135100099101534927. [DOI] [PubMed] [Google Scholar]
- Ullmann J.F., Moore B.A., Temple S.E., Fernández-Juricic E., Collin S.P. The retinal wholemount technique: a window to understanding the brain and behaviour. Brain, Behavior Evol. 2012;79(1):26–44. doi: 10.1159/000332802. [DOI] [PubMed] [Google Scholar]
- Wang W., Lo A. Diabetic retinopathy: Pathophysiology and treatments. Int. J. Mol. Sci. 2018;19(6):1816. doi: 10.3390/ijms19061816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener A., Golubnitschaja O., Breipohl W., Schild H., Vrensen G.M. Effects of dietary de? Ciency of selective amino acids on the function of the cornea and lens in rats. Amino Acids. 2002;23(1–3):337–342. doi: 10.1007/s00726-001-0147-x. [DOI] [PubMed] [Google Scholar]
- Yu B.-C., Chen W.-C., Cheng J.-T. Antihyperglycemic effect of andrographolide in streptozotocin-induced diabetic rats. Planta Med. 2003;69(12):1075–1079. doi: 10.1055/s-2003-45185. [DOI] [PubMed] [Google Scholar]
- Yu Z., Lu B., Sheng Y., Zhou L., Ji L., Wang Z. Andrographolide ameliorates diabetic retinopathy by inhibiting retinal angiogenesis and inflammation. Biochim. Biophys. Acta (BBA)-General Subjects. 2015;1850(4):824–831. doi: 10.1016/j.bbagen.2015.01.014. [DOI] [PubMed] [Google Scholar]
- Zhong Y., Yue S., Wu J., Guan P., Zhang G., Liu L., Chen L. Association of the serum total cholesterol to triglyceride ratio with diabetic retinopathy in chinese patients with type 2 diabetes: a community-based study. Diabetes Therap. 2019:1–8. doi: 10.1007/s13300-019-0579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]