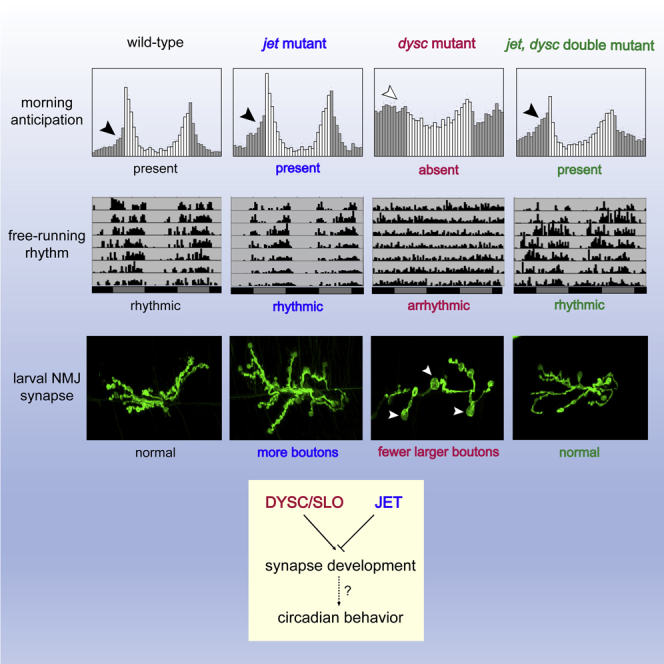
Summary
Circadian output genes act downstream of the clock to promote rhythmic changes in behavior and physiology, yet their molecular and cellular functions are not well understood. Here we characterize an interaction between regulators of circadian entrainment, output, and synaptic development in Drosophila that influences clock-driven anticipatory increases in morning and evening activity. We previously showed the JETLAG (JET) E3 ubiquitin ligase resets the clock upon light exposure, whereas the PDZ protein DYSCHRONIC (DYSC) regulates circadian locomotor output and synaptic development. Surprisingly, we find that JET and DYSC antagonistically regulate synaptic development at the larval neuromuscular junction, and reduced JET activity rescues arrhythmicity of dysc mutants. Consistent with our prior finding that DYSC regulates SLOWPOKE (SLO) potassium channel expression, jet mutations also rescue circadian and synaptic phenotypes in slo mutants. Collectively, our data suggest that JET, DYSC, and SLO promote circadian output in part by regulating synaptic morphology.
Subject Areas: Chronobiology, Developmental Biology, Developmental Neuroscience
Graphical Abstract
Highlights
-
•
Loss of DYSC differentially impacts morning and evening oscillators
-
•
Reduced JET activity rescues the dysc and slo arrhythmic phenotype
-
•
Reduced JET activity causes synaptic defects at the larval NMJ
-
•
JET opposes DYSC and SLO function at the NMJ synapse
Chronobiology; Developmental Biology; Developmental Neuroscience
Introduction
Many aspects of adult behavior and physiology are subject to circadian regulation, including rhythmic alterations in locomotor activity that persist in the absence of changing environmental cues such as light or temperature. Forward genetic screens in the fruit fly, Drosophila melanogaster, have been of fundamental importance in elucidating the genetic underpinnings and molecular principles by which circadian clocks function and are entrained by the external environment (Allada and Chung, 2010, Axelrod et al., 2015, Franco et al., 2018, Tataroglu and Emery, 2015, Tategu et al., 2008). At the core of the Drosophila clock is a transcription-translation feedback loop, in which CLOCK (CLK) and CYCLE (CYC) activate transcription of their own inhibitors, PERIOD (PER) and TIMELESS (TIM). The molecular clock functions in approximately 150 neurons in the adult Drosophila brain (Nitabach and Taghert, 2008, Veleri et al., 2007). In standard 12 h light–12 h dark (LD) and constant 25°C conditions, Drosophila exhibit increased locomotor activity before light onset (morning anticipation) and offset (evening anticipation). These behavioral changes are driven by the circadian clock, and distinct groups of clock neurons in the lateral regions of the Drosophila brain, termed morning and evening oscillators, control morning and evening anticipation, respectively (Grima et al., 2004, Stoleru et al., 2004). However, whether the outputs of these distinct groups of clock neurons are regulated via the same molecular and circuit mechanisms is unclear.
Light is an important entraining factor for the Drosophila clock and synchronizes circadian oscillations via degradation of TIM, thus resetting the negative feedback component of the clock. This process is mediated by a molecular machinery comprised of the blue-light photo-receptor CRYPTOCHROME (CRY) and the F box protein JETLAG (JET), a component of an E3 ubiquitin ligase complex (Emery et al., 1998, Koh et al., 2006, Peschel et al., 2009, Stanewsky et al., 1998). Blue light activates CRY and induces the association of JET with both CRY and TIM, which facilitates proteasome-mediated degradation of TIM. To date, the sole known function for JET is in the light-induced TIM degradation pathway.
Several genes have been identified that, when mutated, result in arrhythmic locomotor patterns despite normal cycling of clock proteins (Dockendorff et al., 2002, Suh and Jackson, 2007, Williams et al., 2001), suggesting that these genes impact rhythmicity by regulating either the output of clock neurons or the function or development of downstream circuit components. We previously characterized an output gene termed dyschronic (dysc) (Jepson et al., 2012), a Drosophila homolog of whirlin/DFNB31. Human DFNB31 encodes a PDZ-domain containing protein linked to Usher syndrome, a form of deaf-blindness (Mburu et al., 2003). dysc mutants exhibit arrhythmic locomotor behavior in constant dark (DD) conditions despite the persistence of wild-type oscillations in the molecular clock, and restoring dysc expression in clock neurons is not sufficient to rescue arrhythmicity (Jepson et al., 2012). This suggests DYSC functions in circuit components downstream of clock neurons for rhythmic behavior, although it may also function in clock neurons. DYSC binds to and regulates the expression of the calcium-activated voltage-gated potassium channel SLOWPOKE (SLO), the Drosophila ortholog of the mammalian Slo1 BK potassium channel (Jepson et al., 2012). As with dysc mutants, loss-of-function mutations in slo result in arrhythmicity (Fernandez et al., 2007). Furthermore, using the Drosophila larval neuromuscular junction (NMJ) as a model system, we demonstrated a role for DYSC in synaptic development (Jepson et al., 2014). We found that loss of DYSC results in alterations in synaptic morphology including increased bouton size and decreased bouton number (Jepson et al., 2014). Consistent with the regulation of SLO expression by DYSC, slo mutants exhibit NMJ phenotypes that overlap with those observed in dysc mutants. Together, these results demonstrate roles for DYSC and SLO in the regulation of circadian output and synaptic development.
Here we show that the morning and evening oscillators exhibit distinct functional requirements for DYSC under varying light conditions and demonstrate an unexpected interaction between JET, DYSC, and SLO in regulating both circadian rhythms and synaptic development. Mutations in jet, but not cry, significantly rescue the arrhythmic phenotype of both dysc and slo mutants in DD, suggesting a role for JET in the circadian output pathway. Intriguingly, defects in dysc and slo mutant synapses are also suppressed by jet mutations, and reducing JET levels enhances synaptic growth at the NMJ. We find that DYSC, SLO, and JET act as regulators of synaptic morphology, suggesting a common cellular locus for their activity. Collectively, these data suggest that JET, DYSC, and SLO influence circadian output in part by regulating synaptic morphology.
Results
Loss of DYSC Differentially Impacts Morning and Evening Oscillators
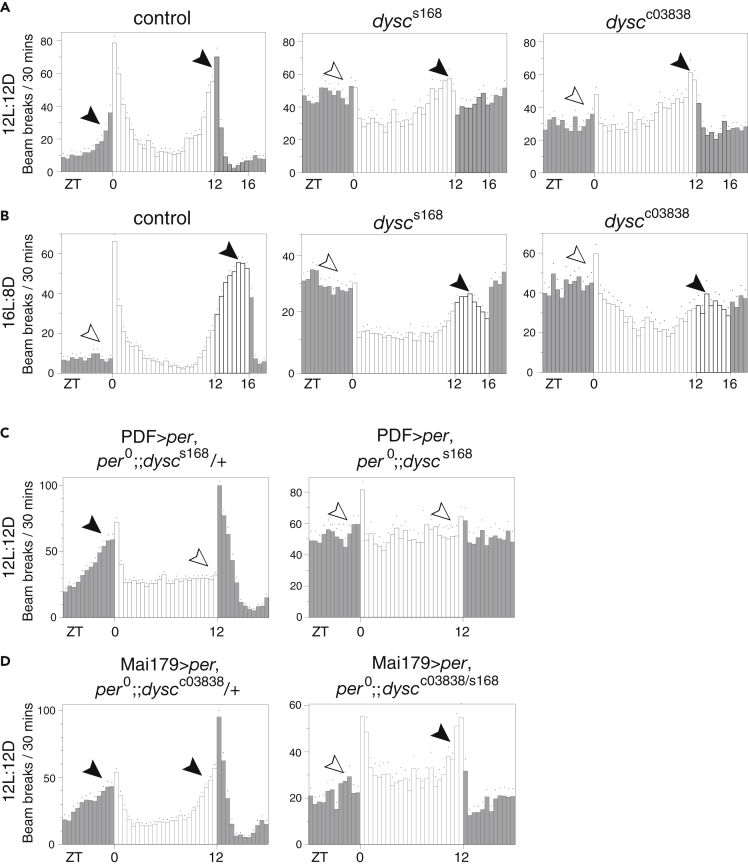
Distinct clusters of clock neurons control rhythmic behavior under different environmental conditions (Grima et al., 2004, Stoleru et al., 2004, Stoleru et al., 2007, Zhang et al., 2010). We previously reported that dysc mutants are arrhythmic under DD conditions (Jepson et al., 2012). This indicates that DYSC is required for proper output of small lateral ventral neurons (s-LNvs) expressing the neuropeptide PDF, which controls rhythmicity in DD (Renn et al., 1999). PDF-positive s-LNvs are referred to as the morning oscillator due to their role in anticipatory increase in locomotor activity before light onset (Grima et al., 2004). To examine the role of DYSC in additional clock neuron clusters, we assayed dysc mutants under different light conditions. As expected, dysc mutants did not exhibit morning anticipation in standard 12 h:12 h LD conditions (Figures 1A and S1A), confirming defective output of the morning oscillator. However, dysc mutants showed robust evening anticipation (Figures 1A and S1A), suggesting DYSC is not essential for the output of the CRY+, PDF-negative fifth s-LNv, and dorsal lateral neurons (LNds), referred to as the evening oscillator (Grima et al., 2004). To confirm that DYSC is not required for the output of the evening oscillator, we examined locomotor behavior under prolonged photoperiod conditions (16 h: 8 h LD). The evening peak of activity occurs at or near the light offset under 12 h:12 h LD, but a few hours before the light offset under 16 h:8 h LD (Majercak et al., 1999, Schlichting et al., 2016, Yoshii et al., 2009). We observed clear evening peaks of activity in both wild-type controls and dysc mutants a few hours before the light offset under 16 h:8 h LD (Figure 1B). Relative to 12 h:12 h LD, evening peaks were delayed by 2–3 hr in 16 h:8 h LD in both control and dysc mutants. These results suggest an essential role of DYSC for the behavioral output of the morning but not the evening oscillator in alternating light-dark conditions.
Figure 1.
Loss of DYSC Differentially Impacts Morning and Evening Oscillators
(A) Activity profiles of control and dyscs168 or dyscc03838 homozygous adult males in 12 h:12 h LD conditions. Bars represent average beam breaks per 30 min. Dots represent SEM. Gray bars represent dark periods, and white bars represent light periods. In this and subsequent figures, filled and open arrowheads represent the presence and absence of anticipation, respectively. N = 38–54.
(B) Activity profiles of males of indicated genotypes under extended photoperiod (16 h:8 h LD) conditions. N = 28–53.
(C and D) Activity profiles of per0 flies in which per is restored in the morning oscillator using PDF-Gal4 (C) or in both morning and evening oscillators using Mai179-Gal4 (D). The heterozygous dysc background (left) is used as a control for the dysc homozygous or transheterozygous (right) background. N = 24–66. Quantification of morning and evening anticipation is presented in Figure S1.
To further examine the differential role of DYSC in the output of morning and evening oscillators, we analyzed the effects of restoring per expression in distinct groups of clock neurons of per0 mutants harboring wild-type or mutant alleles of dysc. Previous studies have shown that expressing per only in the morning oscillator is sufficient to restore morning anticipation in LD, whereas expressing per only in the evening oscillator is sufficient to restore evening anticipation in LD (Grima et al., 2004, Stoleru et al., 2004). We therefore compared the effects of restoring per expression in different groups of clock cells in per0, dyscs168 double mutant flies, vs per0, dysc s168/+ flies. We previously showed that dysc heterozygotes behave indistinguishably from wild-type control flies (Jepson et al., 2012), and as expected, restoring per in LNvs using PDF-Gal4 was sufficient to rescue morning anticipation in per0, dysc s168/+ flies (Figures 1C and S1B). In contrast, it was not sufficient to rescue morning anticipation in per0, dyscs168 double mutants, providing additional evidence that dysc is necessary for the output of the morning oscillator. Next, we used the Mai179-Gal4 driver to restore per expression in both the morning and evening oscillators (Grima et al., 2004). Although this was sufficient to rescue morning and evening anticipation in a dysc heterozygous background, only evening anticipation was rescued in a dysc s168/c03838 transheterozygous background (Figures 1C and S1C). These results confirm that dysc is required for the output of the morning oscillator but not the evening oscillator in LD.
Mutations in jet, but Not cry, Rescue the dysc Arrhythmic Phenotype
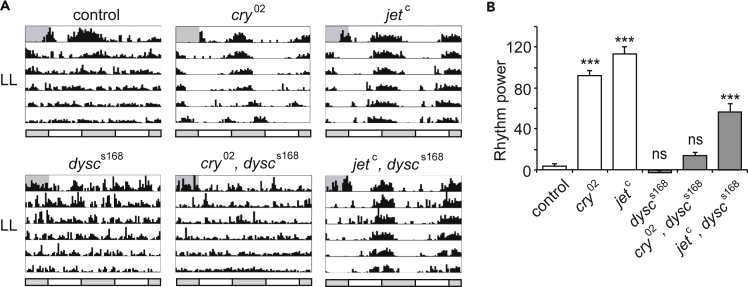
Although dysc mutants exhibit clear evening anticipation, the pattern of anticipation is different from that of control flies. Although control flies rapidly increase their activity before the light offset over a few hours, dysc mutants gradually increase their activity over several hours (Figure 1A). To further examine the evening oscillator output in dysc mutants, we assayed locomotor rhythmicity in LL in a cry mutant background, which allows the evening oscillator to drive rhythmic behavior (Koh et al., 2006, Picot et al., 2007, Stanewsky et al., 1998). Double mutants for dysc and cry were arrhythmic in LL conditions (Figures 2A and 2B), suggesting that the evening oscillator is dependent on DYSC under constant light conditions. Together, our data show that although the evening oscillator in dysc mutants is capable of driving a gradual evening anticipation in LD conditions, it is unable to support rhythmic behavior in LL. The loss of DYSC may have a relatively minor impact on behavior in LD conditions where the clock is reset on a daily basis but a stronger impact on rhythmic behavior over several days in constant conditions.
Figure 2.
Reduced JET Rescues Arrhythmicity of dysc Mutants in LL
(A) Representative actograms of males of indicated genotypes in LL.
(B) Mean rhythm strength (power) of males of indicated genotypes in LL. Although dysc single mutants are arrhythmic in LL, jet, dysc double mutants exhibit moderate rhythm strength. N = 28–66. Bars represent mean ± SEM. ***p < 0.0005, one-way Brown-Forsythe and Welch ANOVA for unequal variances followed by Dunnett T3 test relative to controls.
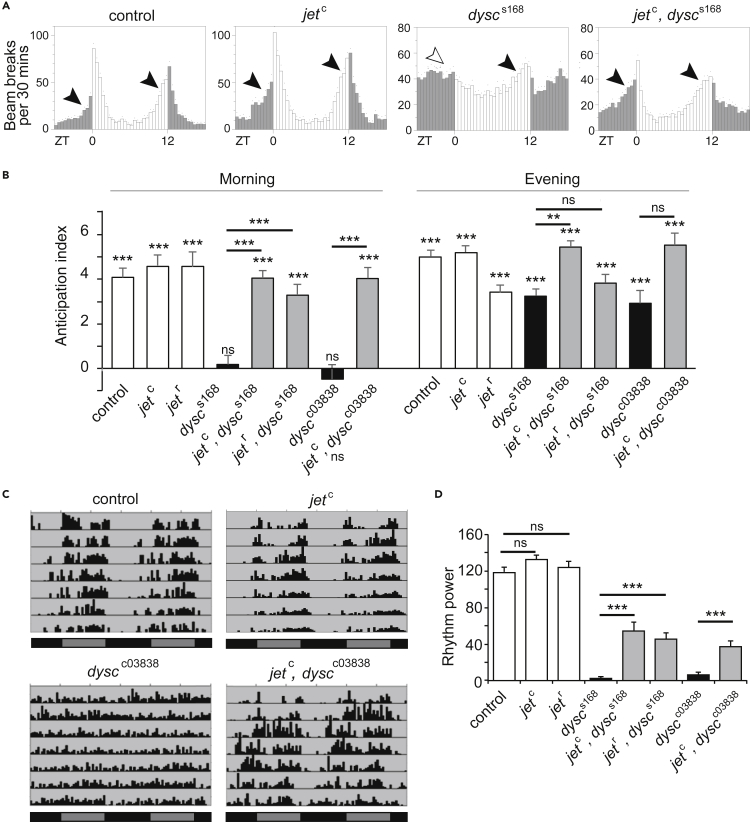
Because CRY and JET act in the same pathway to regulate light-dependent TIM degradation and both cry and jet mutants are rhythmic in LL (Koh et al., 2006, Picot et al., 2007, Stanewsky et al., 1998), we expected jet, dysc double mutants to phenocopy cry, dysc double mutants in LL. Unexpectedly, however, we found that a hypomorphic mutation in jet (jetc) robustly restored rhythmicity to dysc mutants in LL (Figures 2C and 2D). To our further surprise, introduction of jetc fully rescued morning anticipation in dysc mutants under LD conditions (Figures 3A and 3B). We observed morning anticipation in jet, dysc double mutants using two independent alleles of jet (jetc and jetr) and dysc (dyscs168 and dyscc03838) (Jepson et al., 2012, Koh et al., 2006). These results suggest that reduced JET activity restores output of both the morning and evening oscillator in dysc mutants. To further analyze morning oscillator output in jet, dysc double mutants, we next examined rhythmicity in DD. As in LL, we found a substantial rescue of locomotor rhythmicity in jet, dysc double mutants (Figures 3C and 3D). Together, these data demonstrate that reductions in JET activity at least partially rescue all known circadian phenotypes of the dysc mutant and reveal a function for JET in regulating circadian output that is independent of its canonical role in light entrainment.
Figure 3.
Reduced JET Function Restores Morning Anticipation in LD and Rhythmicity in DD of dysc Mutants
(A) Activity profiles of control males, as well as jetc single, dyscs168 single, and jetc, dyscs168 double mutants under 12 h:12 h LD conditions. Morning anticipation, absent in dysc single mutants, is present in jet, dysc double mutants.
(B) Morning and evening anticipation index for indicated genotypes. Anticipation index is defined as the slope of the best-fitting regression line for the activity counts over a period of 6 h prior to a light-dark transition. N = 23–59. Bars represent anticipation index ±standard error. Statistics above the lines indicate whether the two genotypes are different from each other, whereas those above the bars indicate whether anticipation index of each genotype is significantly different from 0.
(C) Representative actograms of control males as well as jetc single, dyscc03838 single, and jetc, dyscc03838 double mutants under constant dark (DD). Black bars indicate subjective night, and dark gray bars indicate subjective day.
(D) Mean power of rhythmicity of control adult males of indicated genotypes. N = 32–72. Bars represent mean ± SEM. **p < 0.005***p < 0.0005, ns: not significant, two-way ANOVA (genotype and morning vs evening as the two main factors) followed by Tukey's post-hoc tests for pairwise comparisons; t test with Bonferroni correction for comparing each anticipation index to 0 (B); one-way Brown-Forsythe and Welch ANOVA for unequal variances followed by Dunnett T3 test for the indicated pairwise comparisons (D).
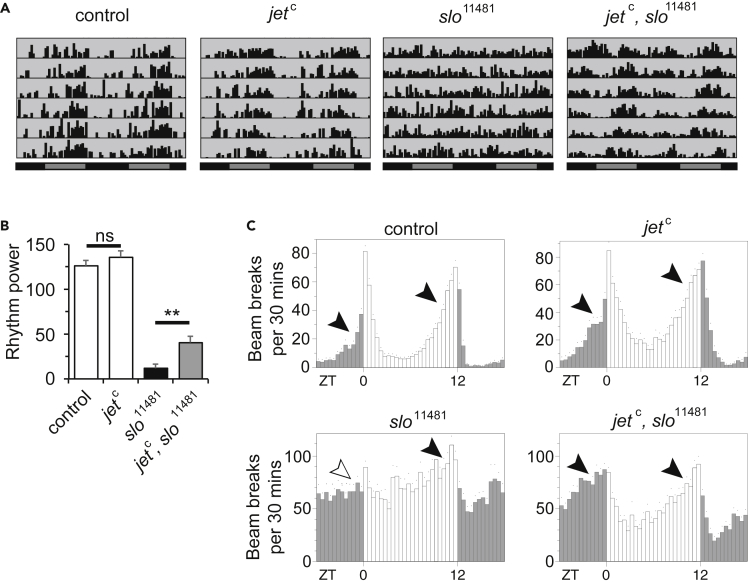
Reduced JET Activity Rescues Circadian Phenotypes of slo Mutants
We previously showed that in dysc mutants, the protein levels of the SLO BK potassium channel are markedly reduced, although not completely eliminated. The slo transcript levels are not affected (Jepson et al., 2012), suggesting DYSC post-transcriptionally regulates the expression of the SLO channel. Mutations in slo also lead to arrhythmic locomotor behavior (Fernandez et al., 2007), suggesting that DYSC influences circadian output through its regulation of SLO. Because JET acts in the protein degradation pathway, a simple hypothesis is that reductions in JET activity protect SLO channels from proteasomal degradation following loss of DYSC, thus maintaining rhythmic behavior. This hypothesis yields a clear prediction that mutations in jet should be unable to rescue the arrhythmic phenotype of slo null mutants. We thus examined circadian function in jet, slo double mutants using flies harboring a P element insertion in the second exon of the slo locus that acts as a null allele (slo11481) (Jepson et al., 2014). As with dysc mutants, slo11481 homozygotes were arrhythmic in DD (Figures 4A and 4B) and showed significant evening anticipation but no morning anticipation in LD (Figures 4C and S2). Intriguingly, in both DD and LD conditions, morning oscillator output was restored in jetc, slo11481 double mutants (Figures 4 and S2). Another simple hypothesis that DYSC itself is a substrate of JET is also unlikely because jet mutations rescue the circadian phenotypes of dyscc03838 null mutants. In addition, dyscs168 mutants express only a short isoform of dysc that does not play a role in circadian rhythms and lack the isoforms that support rhythmic behavior (Jepson et al., 2012). Together, our data indicate that SLO and DYSC are not substrates of JET and demonstrate a role for JET that intersects with both DYSC and SLO to modulate circadian output.
Figure 4.
Reduced JET Function Restores Morning Anticipation in LD and Rhythmicity in DD of slo Mutants
(A) Representative actograms of control, jetc, slo11481 and jet, slo11481 males in DD.
(B) Mean power of rhythmicity of control, jetc, slo11481 and jetc, slo11481 males in DD. N = 26–48. Bars represent mean ± SEM. **p < 0.005, ns: not significant, one-way Brown-Forsythe and Welch ANOVA for unequal variances followed by Dunnett T3 test for all pairwise comparisons, only one of which is shown for clarity.
(C) Activity profiles of control males, as well as slo11481 single and jetc, slo11481 double mutants under 12 h:12 h LD conditions. N = 35–43. Quantification of morning and evening anticipation is presented in Figure S2.
Reduced JET Activity Rescues Synaptic Defects in dysc and slo Mutants
In the adult Drosophila brain, DYSC localizes to both neuronal tracts as well as the synaptic neuropil (Jepson et al., 2012), and at the larval neuromuscular junction (NMJ), DYSC exhibits a punctate presynaptic expression pattern in synaptic boutons and localizes closely to active zones, the sites of neurotransmitter release (Jepson et al., 2014, Wagh et al., 2006). We previously demonstrated that DYSC plays an important role in modulating a range of synaptic parameters, with loss of DYSC resulting in a reduction in the number of synaptic boutons coupled with an increase in bouton size (Jepson et al., 2014). Thus, we were interested to determine whether reduced JET function could modify the synaptic effects of loss of DYSC.
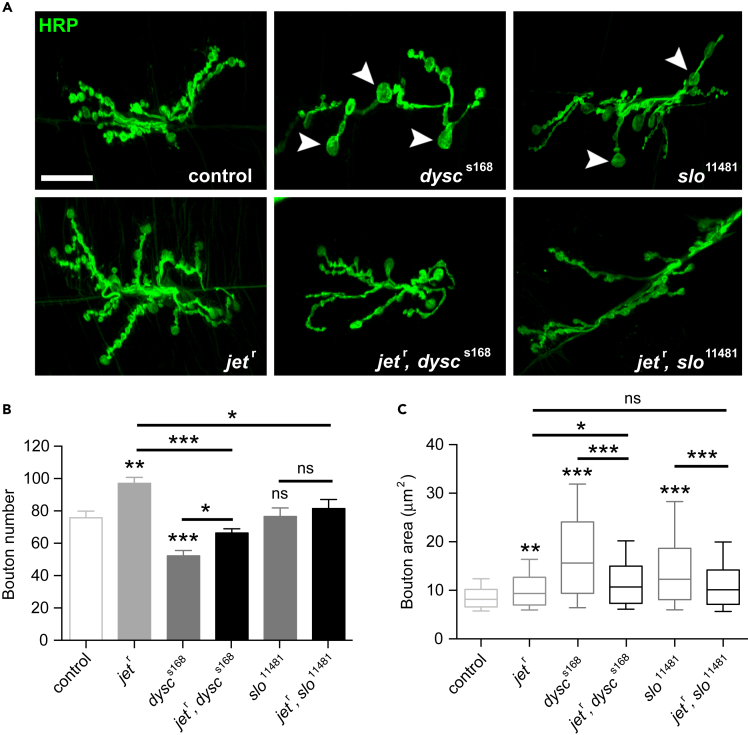
To do so, we examined synaptic morphology at the third instar NMJ in wild-type larvae compared with jet and dysc single mutant larvae and jet, dysc double mutants. Consistent with our previous data, loss of DYSC resulted in a significant reduction in synaptic bouton number and enlarged boutons (Figure 5). Interestingly, reduced JET activity resulted in a marked increase in bouton number, revealing a previously uncharacterized role for JET in synaptic development (Figures 5A and 5B). As expected from the opposite effects on bouton number, reduced JET compensated for the loss of DYSC, and jet, dysc double mutants had an essentially wild-type number of boutons (Figures 5A and 5B). Intriguingly, despite the fact that reduced JET activity resulted in a slight but significant increase in bouton size, it nonetheless partially rescued the much greater increase in bouton size of dysc mutants (Figures 5A and 5C), demonstrating an interaction between the two genes for the regulation of bouton size. As expected from the similarities between dysc and slo bouton size phenotypes, reduction of JET activity also rescued the large bouton size phenotype of slo mutants (Figures 5A and 5C). As previously shown, unlike dysc mutants, slo mutants show a wild-type number of boutons. Interestingly, loss of SLO can rescue the increased bouton number phenotype of jet mutants (Figures 5A and 5B), further demonstrating opposing roles of slo and jet for synapse development. Thus, JET acts in opposition to DYSC and SLO in a common pathway regulating synaptic morphology.
Figure 5.
Mutations in jet Rescue Synaptic Defects in dysc and slo Homozygous Larvae
(A) Representative confocal z-stacks showing HRP-labeled synapses from muscle 6/7, segment 3, of the NMJ of the third instar larvae. Note the enlarged boutons in dyscs168 and slo11481 mutant synapses (arrowheads), which are no longer present in jetr, dyscs168 and jetr, slo11481 double mutant synapses. Scale bar, 20 μm.
(B) Average number of synaptic boutons in control, jetr, dyscs168, slo11481 single and double mutant synapses. Bars represent mean ± SEM. N = 16–30.
(C) Box plot illustrating distribution of synaptic bouton areas in the indicated genotypes. Whiskers/lines represent 10th, 25th, median, 75th, and 90th percentiles of the population distribution. N = 188–391. *p < 0.05, **p < 0.005, ***p < 0.0005, ns: not significant, one-way Brown-Forsythe and Welch ANOVA for unequal variances followed by Sidak post-hoc test (B), and Kruskal-Wallis test followed Dunn post-hoc test (C). For both (B) and (C), 7 indicated pairwise post-hoc tests were performed to compare controls against single mutants and double mutants against single mutants.
Our discovery that DYSC has a role in larval synaptic development raises the possibility that arrhythmic behavior of adult dysc mutants may stem from developmental defects in the formation of circadian circuits. To determine whether DYSC functions during development or adulthood for rhythmic behavior, we used GeneSwitch, an RU486-inducible Gal4-derived protein that allows temporal control of transgenic expression. Adult-specific expression of a dysc transgene using the pan-neuronal elav-GeneSwitch (elav-GS) driver restored rhythmicity to dysc mutants but did so in a partial manner (Figure S3; compare with Figure 3D). Together, our results suggest that DYSC acts acutely at the adult stage to drive rhythmic behavior but may also have a role in the development of neural circuits downstream of the clock cell network.
Discussion
The present investigation began with the interesting observation that although dysc mutants are arrhythmic in DD and lack morning anticipation in LD, they show robust evening anticipation in LD. This led us to hypothesize that DYSC is required for the proper output of the morning oscillator but not of the evening oscillator. Consistent with this hypothesis, restoring per expression in the evening oscillator is sufficient to produce evening anticipation in per, dysc double mutants, but restoring per expression in the morning oscillator is not sufficient for morning anticipation. These results demonstrate that the two oscillators have distinct molecular requirements for robust output. The differential requirements could be due to recruitment of distinct downstream circuit elements or a differential impact on the connectivity between the oscillators and common downstream circuit elements. A recent work has demonstrated that the morning and evening oscillators recruit a common set of dopaminergic relay neurons and premotor centers in the ellipsoid body of the central complex to produce morning and evening peaks of activity (Liang et al., 2019). Although several circuit components downstream of the morning oscillator have been identified (Cavanaugh et al., 2014, Luo and Sehgal, 2012), it is not clear whether they are also downstream of the evening oscillator and how they connect to the dopamingeric relay neurons. It will therefore be interesting to determine the circuit mechanisms underlying the differential impact of the loss of DYSC on the morning and evening oscillators.
Interestingly, although DYSC is dispensable for robust evening anticipation in LD, it is required for rhythmic behavior in constant light conditions, suggesting that the requirement for DYSC changes depending on the lighting condition. Although dysc mutants exhibit robust evening anticipation, the pattern of anticipation is different from that of control flies (i.e., gradual vs. rapid increase in activity before lights off). This finding suggests that even under LD conditions, DYSC plays a role in behavioral output of the evening oscillator, albeit a non-essential one. In constant light conditions, the loss of DYSC, in combination with the absence of daily resetting of the clock, may result in more pronounced circadian defects.
Examination of dysc mutants in cry or jet mutant background revealed previously unknown roles of JET in circadian output and synaptic development, distinct from its known role for light entrainment of the clock. The fact that reduced JET, but not reduced CRY, can support rhythmic behavior of dysc mutants in LL and that reduced JET also rescues dysc arrhythmicity in DD suggests that JET plays a role in maintaining rhythmic behavior in constant conditions. Furthermore, jet mutants exhibit an increase in synaptic bouton number at the NMJ, providing direct evidence of a role for JET in synaptic development.
DYSC and SLO bind to each other and regulate each other's expression, and dysc and slo mutants have similar circadian and synaptic phenotypes (Jepson et al., 2012, Jepson et al., 2014). Thus, it is not surprising that jet mutations can also rescue slo mutant phenotypes. Not only can jet mutations rescue dysc and slo circadian and enlarged bouton phenotypes, dysc and slo mutations can also rescue the increased bouton number phenotype of jet mutants. This highlights the antagonistic nature of the interaction between JET and the DYSC-SLO complex.
Our finding that jet mutations can rescue null mutations of dysc and slo indicate that DYSC and SLO are not substrates of JET. Thus, an important future goal would be to identify the substrates of JET relevant for circadian output and synaptic development. This in turn may help us to understand how JET, DYSC, and SLO interact and whether the same substrates mediate their roles in circadian output and synaptic development.
The fact that all three molecules function in both circadian rhythms and synaptic development suggest that the two processes are closely linked. Clock neurons as well as downstream circuit components in dysc and slo mutants may have synaptic defects analogous to those observed at the NMJ, which may mediate their circadian phenotypes. Similarly, compensatory synaptic alterations in clock and downstream neurons caused by jet mutations may underlie their ability to restore rhythmicity in dysc and slo mutants. It would be fruitful to investigate whether the antagonistic functions of JET vs DYSC and SLO during larval synaptic development have counterparts in the adult brain, especially in clock and circadian output neurons. Previous research found that the dorsal projections of the adult sLNv neurons in slo mutants exhibit altered arborization patterns (Fernandez et al., 2007), which provides partial support for the hypothesis that synaptic defects in adult neurons underlie circadian phenotypes. Because slo and dysc share several, but not all, synaptic phenotypes at the larval NMJ (Jepson et al., 2014), analysis of multiple aspects of synaptic morphology, e.g, the arborization pattern and synaptic puncta size, in adult clock neurons as well as downstream neurons may be required to discover adult synaptic phenotypes relevant to circadian behavior.
In summary, our research reveals previously unknown roles of JET in opposition to DYSC and SLO for circadian behavior and synaptic development. The functions of the closest mammalian homolog of JET, F box and LRR protein 15 (FBXL15), in circadian rhythms and synaptic development are unknown. It will therefore be intriguing to examine whether FBXL15 and the mammalian homologs of DYSC and SLO, Whirlin and Slo1, respectively, similarly function in an antagonistic manner to regulate circadian rhythms and synaptic development.
Limitations of the Study
The present research has examined the synaptic phenotypes of the dysc, slo, and jet mutants at the larval NMJ. Whether the roles these genes play in synaptic morphology are relevant for adult circadian behavior remains unclear. We performed an analysis of the dorsal projections of the adult sLNvs in dysc mutants, but the results were inconclusive. Detailed analysis of synapses of clock neurons and downstream circuits would be required to determine whether there is a direct link between synaptic and circadian functions of the DYSC/SLO complex and JET.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Drs. Charlotte Helfrich-Förster, Haig Kesheshian, Francois Rouyer, and Amita Seghal, Harvard Medical School Stock Center, and the Bloomington Stock Center for fly stocks, and M. Boudinot and Dr. Francois Rouyer for the FaasX software. This work was supported by grants from the National Institutes of Health (R01GM088221 and R01NS086887 to K.K.).
Author Contributions
Conceptualization and Methodology: A.L., J.E.C.J., and K.K. Investigation: A.L., J.E.C.J., and O.A. Writing—Original Draft: A.L., J.E.C.J., and K.K. Writing—Review & Editing: O.A. Funding Acquisition and Supervision: K.K.
Declaration of Interests
The authors declare no competing interests.
Published: February 21, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100845.
Data and Code Availability
Data will be made available upon request.
Supplemental Information
References
- Allada R., Chung B.Y. Circadian organization of behavior and physiology in Drosophila. Annu. Rev. Physiol. 2010;72:605–624. doi: 10.1146/annurev-physiol-021909-135815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod S., Saez L., Young M.W. Studying circadian rhythm and sleep using genetic screens in Drosophila. Methods Enzymol. 2015;551:3–27. doi: 10.1016/bs.mie.2014.10.026. [DOI] [PubMed] [Google Scholar]
- Cavanaugh D.J., Geratowski J.D., Wooltorton J.R., Spaethling J.M., Hector C.E., Zheng X., Johnson E.C., Eberwine J.H., Sehgal A. Identification of a circadian output circuit for rest:activity rhythms in Drosophila. Cell. 2014;157:689–701. doi: 10.1016/j.cell.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockendorff T.C., Su H.S., McBride S.M., Yang Z., Choi C.H., Siwicki K.K., Sehgal A., Jongens T.A. Drosophila lacking dfmr1 activity show defects in circadian output and fail to maintain courtship interest. Neuron. 2002;34:973–984. doi: 10.1016/s0896-6273(02)00724-9. [DOI] [PubMed] [Google Scholar]
- Emery P., So W.V., Kaneko M., Hall J.C., Rosbash M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell. 1998;95:669–679. doi: 10.1016/s0092-8674(00)81637-2. [DOI] [PubMed] [Google Scholar]
- Fernandez M.P., Chu J., Villella A., Atkinson N., Kay S.A., Ceriani M.F. Impaired clock output by altered connectivity in the circadian network. Proc. Natl. Acad. Sci. U S A. 2007;104:5650–5655. doi: 10.1073/pnas.0608260104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco D.L., Frenkel L., Ceriani M.F. The underlying genetics of Drosophila circadian behaviors. Physiology (Bethesda) 2018;33:50–62. doi: 10.1152/physiol.00020.2017. [DOI] [PubMed] [Google Scholar]
- Grima B., Chelot E., Xia R., Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- Jepson J.E., Shahidullah M., Lamaze A., Peterson D., Pan H., Koh K. dyschronic, a Drosophila homolog of a deaf-blindness gene, regulates circadian output and Slowpoke channels. PLoS Genet. 2012;8:e1002671. doi: 10.1371/journal.pgen.1002671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson J.E., Shahidullah M., Liu D., le Marchand S.J., Liu S., Wu M.N., Levitan I.B., Dalva M.B., Koh K. Regulation of synaptic development and function by the Drosophila PDZ protein Dyschronic. Development. 2014;141:4548–4557. doi: 10.1242/dev.109538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh K., Zheng X., Sehgal A. JETLAG resets the Drosophila circadian clock by promoting light-induced degradation of TIMELESS. Science. 2006;312:1809–1812. doi: 10.1126/science.1124951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Ho M.C.W., Zhang Y., Li Y., Wu M.N., Holy T.E., Taghert P.H. Morning and evening circadian pacemakers independently drive premotor centers via a specific dopamine relay. Neuron. 2019;102:843–857.e4. doi: 10.1016/j.neuron.2019.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Sehgal A. Regulation of circadian behavioral output via a MicroRNA-JAK/STAT circuit. Cell. 2012;148:765–779. doi: 10.1016/j.cell.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majercak J., Sidote D., Hardin P.E., Edery I. How a circadian clock adapts to seasonal decreases in temperature and day length. Neuron. 1999;24:219–230. doi: 10.1016/s0896-6273(00)80834-x. [see comments] [DOI] [PubMed] [Google Scholar]
- Mburu P., Mustapha M., Varela A., Weil D., El-Amraoui A., Holme R.H., Rump A., Hardisty R.E., Blanchard S., Coimbra R.S. Defects in whirlin, a PDZ domain molecule involved in stereocilia elongation, cause deafness in the whirler mouse and families with DFNB31. Nat. Genet. 2003;34:421–428. doi: 10.1038/ng1208. [DOI] [PubMed] [Google Scholar]
- Nitabach M.N., Taghert P.H. Organization of the Drosophila circadian control circuit. Curr. Biol. 2008;18:R84–R93. doi: 10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- Peschel N., Chen K.F., Szabo G., Stanewsky R. Light-dependent interactions between the Drosophila circadian clock factors cryptochrome, jetlag, and timeless. Curr. Biol. 2009;19:241–247. doi: 10.1016/j.cub.2008.12.042. [DOI] [PubMed] [Google Scholar]
- Picot M., Cusumano P., Klarsfeld A., Ueda R., Rouyer F. Light activates output from evening neurons and inhibits output from morning neurons in the Drosophila circadian clock. PLoS Biol. 2007;5:e315. doi: 10.1371/journal.pbio.0050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn S.C., Park J.H., Rosbash M., Hall J.C., Taghert P.H. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- Schlichting M., Menegazzi P., Lelito K.R., Yao Z., Buhl E., Dalla Benetta E., Bahle A., Denike J., Hodge J.J., Helfrich-Forster C., Shafer O.T. A neural network underlying circadian entrainment and photoperiodic adjustment of sleep and activity in Drosophila. J. Neurosci. 2016;36:9084–9096. doi: 10.1523/JNEUROSCI.0992-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanewsky R., Kaneko M., Emery P., Beretta B., Wager-Smith K., Kay S.A., Rosbash M., Hall J.C. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell. 1998;95:681–692. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- Stoleru D., Peng Y., Agosto J., Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- Stoleru D., Nawathean P., Fernandez Mde L., Menet J.S., Ceriani M.F., Rosbash M. The Drosophila circadian network is a seasonal timer. Cell. 2007;129:207–219. doi: 10.1016/j.cell.2007.02.038. [DOI] [PubMed] [Google Scholar]
- Suh J., Jackson F.R. Drosophila ebony activity is required in glia for the circadian regulation of locomotor activity. Neuron. 2007;55:435–447. doi: 10.1016/j.neuron.2007.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataroglu O., Emery P. The molecular ticks of the Drosophila circadian clock. Curr. Opin. Insect Sci. 2015;7:51–57. doi: 10.1016/j.cois.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tategu M., Nakagawa H., Hayashi R., Yoshida K. Transcriptional co-factor CDCA4 participates in the regulation of JUN oncogene expression. Biochimie. 2008;90:1515–1522. doi: 10.1016/j.biochi.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Veleri S., Rieger D., Helfrich-Forster C., Stanewsky R. Hofbauer-Buchner eyelet affects circadian photosensitivity and coordinates TIM and PER expression in Drosophila clock neurons. J. Biol. Rhythms. 2007;22:29–42. doi: 10.1177/0748730406295754. [DOI] [PubMed] [Google Scholar]
- Wagh D.A., Rasse T.M., Asan E., Hofbauer A., Schwenkert I., Durrbeck H., Buchner S., Dabauvalle M.C., Schmidt M., Qin G. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Williams J.A., Su H.S., Bernards A., Field J., Sehgal A. A circadian output in Drosophila mediated by neurofibromatosis-1 and Ras/MAPK. Science. 2001;293:2251–2256. doi: 10.1126/science.1063097. [DOI] [PubMed] [Google Scholar]
- Yoshii T., Wulbeck C., Sehadova H., Veleri S., Bichler D., Stanewsky R., Helfrich-Forster C. The neuropeptide pigment-dispersing factor adjusts period and phase of Drosophila's clock. J. Neurosci. 2009;29:2597–2610. doi: 10.1523/JNEUROSCI.5439-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liu Y., Bilodeau-Wentworth D., Hardin P.E., Emery P. Light and temperature control the contribution of specific DN1 neurons to Drosophila circadian behavior. Curr. Biol. 2010;20:600–605. doi: 10.1016/j.cub.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon request.