Abstract
Due to the endless emergence of drug resistant pathogens, there is a constant need for new therapeutic agents for clinical use. The identification of active components in natural products and determining the efficacy of these active components has become the current focus of pharmacological research. The present study aimed to evaluate the anthelmintic and antimicrobial activities of Indigofera oblongifolia leaf extract (ILE) against the earthworm Allolobophora caliginosa, the gram-positive bacteria (Bacillus cereus, Streptococcus pneumoniae, and Staphylococcus aureus), the gram-negative bacteria (Klebsiella pneumoniae, Pseudomonas aeruginosa and Escherichia coli) and the yeast Candida albicans. Methanolic extract of I. oblongifolia leaf was obtained and the total phenolics and flavonoids in ILE were determined. The anthelmintic study was carried out to determine the time to paralysis and time to death of worms using three doses (100, 200, and 300 mg/mL) of ILE. Also, Kirby-Bauer disk diffusion susceptibility method was used to determine the antimicrobial activity of ILE. The results showed that ILE induces paralysis and death of A. caliginosa at all concentration tested faster than the reference drug, Albendazole. Additionally, ILE exhibited prominent antimicrobial activity against all gram-positive bacteria tested but almost no significant activity against the gram-negative bacteria, except K. pneumoniae. ILE showed close similarity to the spectrum of chloramphenicol and cefoxitin activities. Furthermore, C. albicans was highly susceptible to the leaf extracts. Our results showed that ILE is an effective anthelmintic and antimicrobial agent.
Keywords: Anthelmintic, Antimicrobial, Indigofera oblongifolia, Bacteria
1. Introduction
Helminths are considered to be one of the most common infectious agents in humans in developing countries. Helminthiasis, an infestation of helminths in the body, is a neglected tropical disease that mostly affects people in poor countries. In addition, helminths are widely prevalent in livestock, subsequently causing losses in the agricultural sector (McSorley and Maizels, 2012). Several studies have already reported that helminths cause long-term chronic infections in both humans and animals by regulating immune-mediated inflammation (Elliott et al., 2007, Elliott and Weinstock, 2012). However, the complete eradication of helminth infection remains a distant goal, due to the lack of effective vaccines, limited pharmacological efficacy of current drugs, emerging drug resistance, and rapid reinfection in environments where transmission cannot be interrupted.
The overuses of antibiotics induce a rapid emergence of multidrug-resistant pathogens. Morbidity, mortality, the duration of hospitalization, and healthcare costs could be increase due to the antimicrobial resistance (Cosgrove et al., 2005). Both gram-positive bacteria including Staphylococcus aureus (MRSA) and multidrug-resistant (MDR) Mycobacterium tuberculosis, and gram-negative bacteria including extended-spectrum beta-lactamase (ESBLs)-producing bacteria, have become major global healthcare problems in the twenty-first century (van Duin and Paterson, 2016).
For fungi infections, a few classes of antifungal drugs are available, so the emergence of resistance to single drug classes and now multidrug resistance greatly hampers patient management. Among them, Candida and Aspergillus species show increasing resistance against several drugs. Therefore drug resistance, in particular, Candida, is one of the greatest challenges to clinical success (Whaley et al., 2017).
Since ancient times, natural products have been used for the treatment of several infectious diseases. These products including medicinal plant extract are promising sources for the development of novel therapies against diseases (Mehlhorn, 2014). The leaf extract of Indigofera oblongifolia is one such medicinal plant of the family Fabaceae, which is used for medicinal purposes due to its analgesic and anti-inflammatory properties (Sharif et al., 2005). It is also reported to have antioxidant activity and is commonly used as an antimalarial agent present in leaf active components (polyphenols, flavonoids, and organic acids) (Abdel Moneim, 2016, Dkhil et al., 2019). The present study aimed to evaluate the anthelmintic and the antimicrobial activities of ILE against the earth worm Allolobophora caliginosa, the yeast pathogen Candida albicans and gram-positive and gram-negative bacteria.
2. Materials and methods
2.1. Preparation of the plant extract
I. oblongifolia leaves were obtained from Jazan Province in the southwest region of the Kingdom of Saudi Arabia. The identity of this species (voucher specimen number 9028) was confirmed by Dr. Pandalayil (Department of Botany and Microbiology, College of Science, King Saud University). Leaves were air dried at 40 °C and ground into a powder. The dried leaves were extracted using 70% methanol, by incubating the powder at 4 °C for 24 h with intermittent stirring. ILE was filtered and then evaporated to completely dry the sample using a vacuum evaporator (Heidolph, Germany). The dry residue was then dissolved in distilled water and used in this experiment.
To prepare ILE stock, leaves were weighed, and the percentage yield of the extraction was determined (relative to the starting dry weight of the leaves). Stock solution for the antimicrobial activity test was prepared using 100% dimethylsulfoxide (DMSO) at 25 mg/mL. The resultant solution was subsequently diluted to 12.5 mg/mL, 6.25 mg/mL, 3.125 mg/mL, and 1.56 mg/mL using 100% DMSO.
2.2. Phytochemical analysis
The amount of total phenolics and flavonoids in ILE were determined according to procedures reported by Kim et al., 2003, Dewanto et al., 2002, respectively.
2.3. Anthelmintic test
The anthelmintic study was carried out using three doses (100, 200, and 300 mg/mL) of ILE against the earthworm Allolobophora caliginosa as described by Ajaiyeoba et al. (2001). Briefly, eight worms of nearly the same size were treated with identical doses of ILE and subsequently the time of worm paralysis and death was determined. Time for paralysis of worms was considered as the time period to observe the absence of any sort of worm movement after worm treatment with ILE, except when the worms were shaken vigorously. Time for death of worms was recorded after ascertaining that worms neither moved when shaken vigorously nor when dipped in warm water (50 °C) followed with fading away of their body colors (Ajaiyeoba et al., 2001). Albendazole suspension (10 mg/mL) was used as the reference drug in both cases (paralysis and death of worms) (Murugamani et al., 2012). Distilled water was used as the negative control. All extracts and drug solutions were freshly prepared before the start of the experiment.
2.4. Microorganisms tested
The antimicrobial activity of ILE was screened against three gram-positive bacteria [Bacillus cereus (ATCC 13061), Streptococcus pneumoniae (ATCC 27336), and Staphylococcus aureus (ATCC 25923)], three gram-negative bacteria [Klebsiella pneumoniae (ATCC 27736), Pseudomonas aeruginosa (ATCC 27853), and Escherichia coli (ATCC 25922)], and the yeast strain Candida albicans (NCPF-3179). Test microorganisms were purchased from MediaMark Europe (Grenoble, CEDE, France) and maintained on appropriate agar plates in accordance with the manufacturer’s instructions. Subsequently, colonies of each strain were resuspended in 10% glycerol, frozen, and stored at −80 °C.
2.5. Antimicrobial assay
Antimicrobial activity of ILE was determined using the Kirby-Bauer disk diffusion susceptibility method (Bauer et al., 1966, Bonev et al., 2008). Bacteria strains were cultured overnight on Müller–Hinton agar at 37 °C, while yeasts were cultured on Sabouraud dextrose agar at 25 °C. Suspension of each microorganism was prepared in physiological saline solution (0.9%) using the McFarland optical density of 0.5, and 200 µL of each microorganism was evenly spread on the appropriate medium using sterile cotton swabs. Disks of Whatman filter paper grade 3 were prepared using a regular paper puncher (size of disk: 6 µM) and were sterilized in an autoclave. Six dried filter paper disks were evenly distributed on the agar plates. Stock or diluents (20 µL) of leaf extract was dropped in the center of each filter paper disk (in 2 steps, each 10 µL). An equal volume of 100% DMSO was used as a negative control. The plates were left uncovered for 20 min inside a sterilized safety cabinet in order to dry the solutions. The plates were then covered and incubated at 37 °C for 24 h and 27 °C for 48 h for bacteria and yeast, respectively. After incubation, the plates were observed for the formation of a clear inhibition zone around the disk, which would be indicative of the presence of antimicrobial activity. The diameter of the zone of inhibition was measured and recorded. All experiments were done in duplicate and repeated thrice.
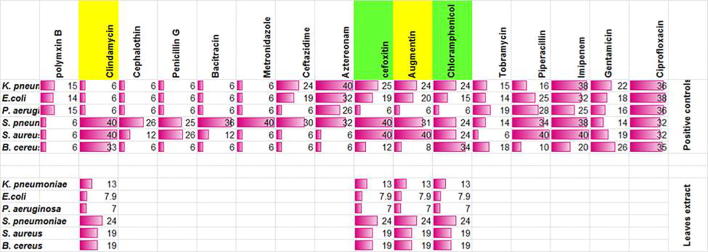
In order to compare the bacterial spectrum and susceptibility profile of the leaf extract of I. oblongifolia with commercially available antibiotics, sixteen antibiotics were selected, and the susceptibilities of the selected gram-positive and gram-negative bacteria to the antibiotics were determined. The spectrum of activity of the leaf extract was then compared with the spectrum of activity of each of these antibiotics. Among these antibiotics, the spectrum of the leaf extract showed some similarity to the spectrum of clindamycin and augmentin.
3. Results
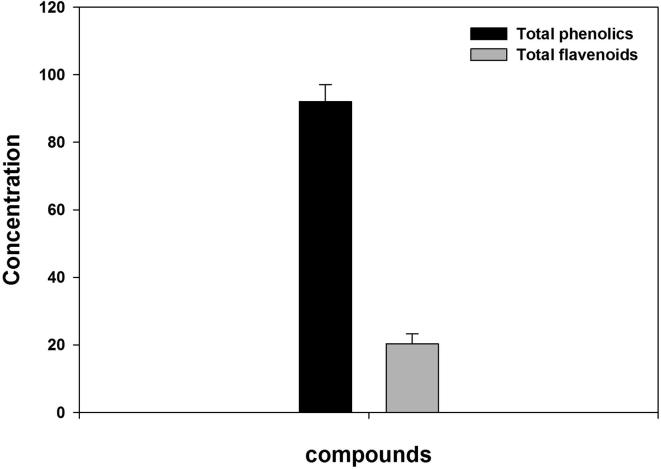
Pytochemical analysis revealed that phenolic and flavonoid compounds are present in the leaf extract of I. oblongifolia (Fig. 1). Treatment of the worms with 100 mg/ml of ILE induced worm paralysis and death significantly in comparison to the negative control, in less than 25 min and 60 min of exposure, respectively (Table 1). Furthermore, increased concentration of ILE resulted in reduction of time of paralysis and death (see time of paralysis and death for the concentrations 200 mg/ml and-300 mg/ml in table 1) Thus, this effect was dose dependent as increased of ILE concentration is inversely proportional to time of paralysis and death. Interestingly, the effect of higher concentrations of ILE (200 mg/ml and-300 mg/ml) on worm paralysis and death were stronger than that caused by the positive control (see time of paralysis and death Albendazole in table 1).
Fig. 1.
Total phenolic and flavonoid compounds present in ILE. Phenolic compounds were measured as mg of gallic acid equivalents per gram of the sample. Flavonoid compounds were measured as mg of quercetin equivalents per gram of the sample.
Table 1.
Anthelminthic action of I. oblongifolia leaf extract.
| Treatment | Time to paralysis (min) | Time to death (min) |
|---|---|---|
| Vehicle control | – | – |
| ILE (100 mg/ml) | 23 ± 3 | 56 ± 4 |
| ILE (200 mg/ml) | 8.5 ± 1 | 15 ± 2 |
| ILE (300 mg/ml) | 6.3 ± 1 | 8.6 ± 1 |
| Albendazole (10 mg/ml) | 20 ± 2 | 25 ± 1 |
Values are means ± SD. N = 8 in each group.
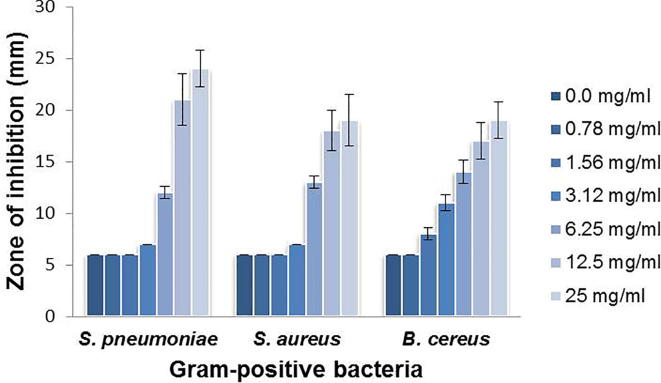
The antimicrobial activity of ILE was also assessed against gram-positive bacteria at different concentrations of the extract using the Kirby-Bauer disk diffusion susceptibility method. Estimation of the diameter of the zones of inhibition produced by serial dilutions of the leaf extract (ranging from 0.0 to 25 mg/mL) against the gram-positive bacteria tested is shown in Fig. 2. The leaf extracts of I. oblongifolia exhibited potent antimicrobial activity against all the gram-positive bacteria tested (B. cereus, S. pneumoniae, and S. aureus). The concentration of the extract was proportional to the degree of inhibition, in particular, at concentrations of 3.12, 5.25, 12.5, and 25 mg/mL (Fig. 2). These results clearly indicate that ILE has an anti-gram-positive bacterial effect.
Fig. 2.
Antimicrobial activity of ILE against selected gram-positive bacterial pathogens: S. pneumoniae, S. aureus, and B. cereus. Appropriate dilutions of the leaf extract were used, and the diameter of the zone of inhibition was measured. Values are expressed as mean ± SD of three independent experiments.
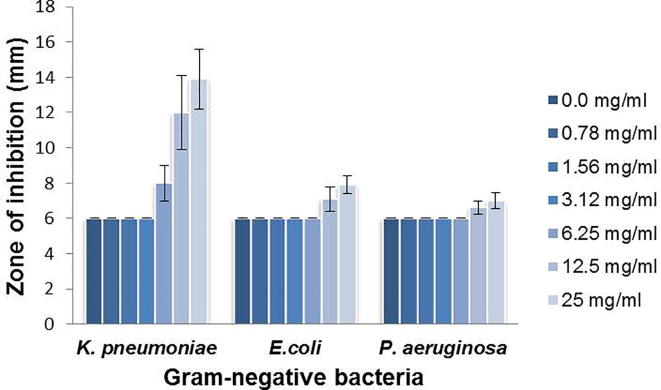
Additionally, the antimicrobial activity of the leaf crude extracts from I. oblongifolia were tested against gram-negative bacteria – K. pneumoniae, P. aeruginosa and E. coli. Interestingly, K. pneumoniae was highly sensitive to ILE (Fig. 3). The diameter of the zone of inhibition was also proportional to the concentration of the extract (see the diameter of zone of inhibition at 5.25, 12.5 and 25 mg/mL in Fig. 3). In contrast, the leaf extract showed no significant anti-bacterial activity against P. aeruginosa and E. coli. Thus, the leaf extract of I. oblongifolia is only effective against K. pneumoniae among the tested gram-negative bacteria.
Fig. 3.
Antimicrobial activity of the methanolic extract of ILE against selected gram-negative bacterial pathogens: K. pneumoniae, E. coli, and P. aeruginosa. Dilutions similar to those used in Fig. 2 were applied, and the diameter of zone of inhibition was measured.
Interestingly, the leaf extract showed greater similarity to the spectrum of chloramphenicol and cefoxitin activities (boxed in green, Fig. 4). Thus, ILE likely has a broad-spectrum of activity, which is comparable to known commercial antibiotics.
Fig. 4.
Antimicrobial activity of common antibiotics against gram-positive and gram-negative bacteria. ILE showed close activity to chloramphenicol and cefoxitin (boxed in green). Bars are correlated to the diameter of zones of inhibition (as indicated in numbers). Values of diameters are expressed as mean ± SD of three independent experiments. All experiments were done in duplicate and repeated thrice.
Furthermore, we tested the antifungal activity of ILE against the yeast pathogen C. albicans. Our results for C. albicans showed high susceptibility to the leaf extract (Fig. 5). This inhibitory activity was even stronger than those observed against the bacterial organisms tested above. Similarly, the increase in extract concentration led to a further boost in the inhibitory activity against C. albicans at concentration tested (Fig. 5). These results clearly indicate that ILE acts as an antifungal compound, in particular, has considerable activity against C. albicans. Altogether, the results showed ILE has antimicrobial activity against gram positive bacteria, the gram-negative K. pneumoniae, and yeast pathogen C. albicans in addition to its activity as anthelmintic.
Fig. 5.
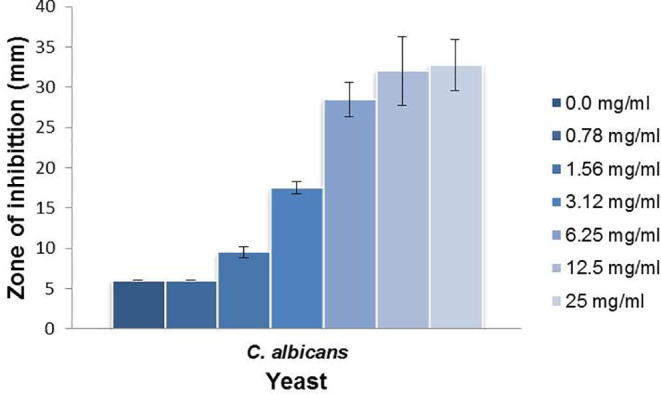
Antimicrobial activity of the methanolic extract of ILE against the yeast pathogen C. albicans. Dilutions similar to those used in Fig. 2 were applied, and the diameter of zone of inhibition was measured. Values are expressed as mean ± SD of three independent experiments.
4. Discussion
The traditional treatment of nematode infections using conventional anthelmintic drugs has yielded approximately 294 million dollars in veterinary market revenue in 2004 (Molento et al., 2004). The consequences of this are much greater than the rising costs of livestock management. There is no clear evidence that synthetic anthelmintics do not leave residues in meat, which can, in turn, pose potential public health hazards (Rodrigues et al., 2007). The search for novel anthelmintic plant extracts such as ILE can contribute towards the development of phytotherapeutic products that are cost effective, safe, and more accessible and offer a lower risk of resistance than the conventional therapeutic arsenal being currently employed.
In this study, ILE exhibited anthelmintic activity against A. caliginosa and antimicrobial activity against gram-positive bacteria, C. albicans, and one gram negative-bacterial strain (K. pneumoniae). However, there is no information reported thus far about anthelmintic activity and the antimicrobial activity of the leaf extract of I. oblongifolia, whereas different studies have reported the antimicrobial activities of the leaf extract of other Indigofera species. Our results are similar to other findings regarding the antibacterial activity of leaf extract of I. tinctoria, which exhibits inhibitory activity against gram-positive bacteria (B. cereus, S. pneumoniae, and S. aureus) but not against gram-negative bacteria (P. aeruginosa, and E. coli) (Renukadevi et al., 2011). K. pneumoniae and yeast were not tested in the study (Renukadevi et al., 2011). Esimone et al. (1999) reported the antimicrobial activity of the aqueous extract of I. dendroides leaves against gram-positive bacteria (S. aureus and Bacillus subtilis), gram-negative bacteria (K. pneumoniae and E. coli), and fungal species (Aspergillus niger and C. albicans) (Esimone et al., 1999). Indeed, our and others’ results are consistent with the fact that Gram-negative bacteria are more resistant to antibodies and antibiotics than Gram-positive bacteria due to the existence of the large impermeable cell wall (Silhavy et al., 2010). K. pneumoniae is known as capsulated bacterium. Although the capsule of thid Gram-negative bacteria forms the major physical barrier between inside and outside of the bacterial cell, the capsule contents (lipopolysaccharide, O-antigen and lipid A) of the bacteria might serve as a target for antimicrobial compounds. For example, cationic antimicrobial peptides can disrupt the electrostatic interactions between lipid A moieties and subsequently disrupt bacterial outer membrane (Doorduijn et al., 2016). Our results showed that ILE is an effective anthelmintic and antibacterial agent. Further studies are required to specifically isolate the active compound(s), test broad range of pathogens and elucidate its mechanism of action.
Funding
The work is supported by the Deanship of Scientific Research at King Saud University for funding this research group project No RG-198.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors would like to thank the Dean of Scientific Research and RSSU at King Saud University for their technical support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel Moneim A.E. Indigofera oblongifolia prevents lead acetate-induced hepatotoxicity, oxidative stress, fibrosis and apoptosis in rats. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajaiyeoba E.O., Onocha P.A., Olarenwaju O.T. In vitro anthelmintic properties of Buchholzia coriaceae and Gynandropsis gynandra extracts. Pharm. Biol. 2001;39:217–220. [Google Scholar]
- Bauer A.W., Kirby W.M.M., Sherris J.C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966;36:493–496. [PubMed] [Google Scholar]
- Bonev B., Hooper J., Parisot J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J. Antimic Chemother. 2008;61:1295–1301. doi: 10.1093/jac/dkn090. [DOI] [PubMed] [Google Scholar]
- Cosgrove S.E., Qi Y., Kaye K.S., Harbarth S., Karchmer A.W., Carmeli Y. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect. Control Hosp. Epidemiol. 2005;26(2):166–174. doi: 10.1086/502522. [DOI] [PubMed] [Google Scholar]
- Dewanto V., Wu X., Liu R.H. Processed sweet corn has higher antioxidant activity. J. Agr. Food Chem. 2002;50:4959–4964. doi: 10.1021/jf0255937. [DOI] [PubMed] [Google Scholar]
- Dkhil M.A., Al-Shaebi E., Al-Quraishy S. Effect of Indigofera oblongifolia on the hepatic oxidative status and expression of inflammatory and apoptotic genes during blood-stage murine malaria. Oxid. Med. Cell. Longev. 2019;2019:8264861. doi: 10.1155/2019/8264861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorduijn D.J., Rooijakkers S.H.M., van Schaik W., Bardoel B.W. Complement resistance mechanisms of Klebsiella pneumoniae. Immunobiology. 2016;6:1102–1109. doi: 10.1016/j.imbio.2016.06.014. [DOI] [PubMed] [Google Scholar]
- Elliott D.E., Summers R.W., Weinstock J.V. Helminths as governors of immune-mediated inflammation. Int. J. Parasitol. 2007;37:457–464. doi: 10.1016/j.ijpara.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Elliott D.E., Weinstock J.V. Helminth-host immunological interactions: prevention and control of immune-mediated diseases. Year Immunol. 2012;1247:83–96. doi: 10.1111/j.1749-6632.2011.06292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esimone C.O., Adikwu M.U., Muko K.N. Antimicrobial properties of Indigofera dendroides leaves. Fitoterapia. 1999;70:517–520. [Google Scholar]
- Kim D.O., Chun O.K., Kim Y.J., Moon H.Y., Lee C.Y. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agr. Food Chem. 2003;51:6509–6515. doi: 10.1021/jf0343074. [DOI] [PubMed] [Google Scholar]
- McSorley H.J., Maizels R.M. Helminth infections and host immune regulation. Clin. Microbiol. Rev. 2012;25:585–608. doi: 10.1128/CMR.05040-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlhorn H. Springer Press; Berlin: 2014. Encyclopedic Reference of Parasitology 6th ed. (V. 1, Ed.) [Google Scholar]
- Molento M.B., Tasca C., Gallo A., Ferreira M., Bononi R., Stecca E. Método Famacha como parâmetro clínico individual de infecção por Haemonchus contortus em pequenos ruminantes. Ciência Rural. 2004;34:1139–1145. [Google Scholar]
- Murugamani V., Raju L., Anand RajV.B., Sarma Kataki M., Sankar G.G. The new method developed for evaluation of anthelmintic activity by housefly worms and compared with conventional earthworm method. ISRN Pharmacol. 2012;2012:709860. doi: 10.5402/2012/709860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renukadevi K.P., Sultana S.S. Determination of antibacterial, antioxidant and cytotoxicity effect of Indigofera tinctoria on lung cancer cell line. Int. J. Pharmacol. 2011;7:356–362. [Google Scholar]
- Rodrigues A.B., Athayde A.C., Rodrigues O.G., Silva W.W., Faria E.B. Evaluation of the efficacy of anthelmintics to control gastrointestinal nematodes in goats raised in the state of Paraiba Pesquisa. Veter. Bras. 2007;27:162–166. [Google Scholar]
- Sharif A., Ahmed E., Malik A., Riaz N., Afza N., Nawaz S.A., Arshad M., Shah M.R., Choudhary M.I. Lipoxygenase inhibitory constituents from Indigofera oblongifolia. Arch. Pharmacol Res. 2005;28:761–764. doi: 10.1007/BF02977339. [DOI] [PubMed] [Google Scholar]
- Silhavy T.J., Kahne D., Walker S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010;2(5):a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duin D., Paterson D.L. Multidrug-resistant bacteria in the community: trends and lessons learned. Inf. Disease Clin. North Am. 2016;30(2):377–390. doi: 10.1016/j.idc.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley S.G., Berkow E.L., Rybak J.M., Nishimoto A.T., Barker K.S., Rogers P.D. Azole antifungal resistance in Candida albicans and emerging non-albicans Candida species. Front. Microbiol. 2017;7:2173. doi: 10.3389/fmicb.2016.02173. [DOI] [PMC free article] [PubMed] [Google Scholar]