Abstract
There is growing evidence that vaping has the potential to cause adverse health effects. Vaping is affecting the younger and healthier population which is a public concern. Mycoplasma pneumoniae pneumonia is a benign condition and is usually underdiagnosed and is managed in an outpatient setting. Here we present a case of fulminant MPP in a young adult probably associated with VAPI.
A 24-year-old woman presented to our hospital for severe hypoxic respiratory failure needing intubation and intensive care unit admission. She had a history for vaping for 2 years prior to presentation. She had fever and an elevated white count. Her Chest X-Ray and CT scan of the chest were consistent with bilateral predominantly lower lobe patchy opacities. She had mildly elevated serum LDH and Urine toxicology screen was positive for THC. Serum IgM Mycoplasma level was positive and her BAL fluid analysis showed lipid-laden macrophages. She was diagnosed as a probable case of VAPI per CDC guidelines with superimposed fulminant MPP.
Vaping is known to increase the risk of viral and bacterial pneumonia by compromising the respiratory local immune response. Vaping also causes lipoid pneumonia where the alveoli are filled with lipid-laden macrophages with surrounding inflammation. We hypothesize that this patient had fulminant MPP in the setting of background VAPI.
The association between vaping and MPP infection has not been established in the literature and this is the first documented report to establish a link between e-cigarettes and fulminant MPP. Further research is needed to confirm this association.
Abbreviations: EC, e-cigarettes; MPP, Mycoplasma Pneumoniae Pneumonia; MP, Mycoplasma Pneumoniae; VAPI, Vaping Associated Pulmonary Injury; CDC, Center for Disease Control; PAFR, Platelet Activating Factor Receptor; CRP, C-Reactive Protein; LDH, Lactate Dehydrogenase; PJP, Pneumocystis Jiroveci Pneumonia
1. Introduction
Mycoplasma pneumoniae pneumonia (MPP) is a common cause of atypical pneumonia and is most commonly seen in younger adults. It is also one of the usual organisms known to cause community acquired pneumonia. Pneumonia caused by Mycoplasma was initially described in textbooks as “Walking Pneumonia” because of its presumed benign nature. It usually causes a very mild form of illness and patients rarely require an admission to the hospital. The overall mortality of MP infection is low, but mortality of up to 30% has been reported in the literature, especially among the elderly [1].
MPP remains largely underdiagnosed because of its benign presentation, lack of diagnostic tests with good sensitivity and specificity, and other diseases or infections that either co-exist or mimic MPP [2,3]. The current evidence suggests that the incidence of MPP is high in children older than 4 years and adolescents, but the true impact on adults remains unclear [4].
Although the definition of “fulminant and severe MPP” has not been established, it has been defined in the published literature as confirmed MPP cases with respiratory failure or fatal cases without respiratory failure. Severe MPP accounts for 0.5–2% of all MPP cases and affects young healthy individuals [5].
Within the past few years, vaping has reached epidemic proportions among younger generations. Marketing companies have advertised e-cigarettes as a modality for tobacco cessation. But, e-cigarettes do not mean “No cigarettes”. E-cigarettes are not entirely safe; they also have their side-effects. It is just a matter of time until we uncover the complications related to vaping. The definition of a definite and probable case of Vaping Associated Pulmonary Injury (VAPI) has recently been illustrated by CDC [6].
Vaping is known to increase the risk of viral and bacterial pneumonia by compromising the respiratory local immune response [7]. Vaping is also known to cause lipoid pneumonia where the alveoli are filled with lipid laden macrophages (also called foamy macrophages) with or without surrounding inflammation [8]. However, many other varieties of distinct pathological manifestations of VAPI have been recently published [9,10]. In this article we present an interesting case of probable VAPI associated with co-infection with MPP.
2. Case synopsis
A 26-year-old African American female presented to the emergency room with worsening productive cough and difficulty breathing for a period of 1 week. She had a past medical history of schizoaffective disorder and was on ziprasidone for 3 years. Prior to presentation, she had experienced 1 month of progressive breathlessness on exertion and daily cough with white productive sputum. She never smoked cigarettes but was vaping tobacco and occasional marijuana for approximately 2 years prior. She worked in a grocery store and did not have any environmental or occupational exposures. There were no significant inhalational exposures such as birds or animals, molds, damp, hot tubs or Jacuzzis, compost, feather or down bedding.
On presentation, her vitals were significant for tachypnea, tachycardia and severe hypoxia with an oxygen saturation of 84% on room air, which improved to 92% on 4L/min of oxygen. She also had a fever of 101° F. Her laboratory studies on presentation were significant for elevated White Blood Cell count of 12.7. Urine toxicology screen was positive for THC (tetra hydro cannabinoids).
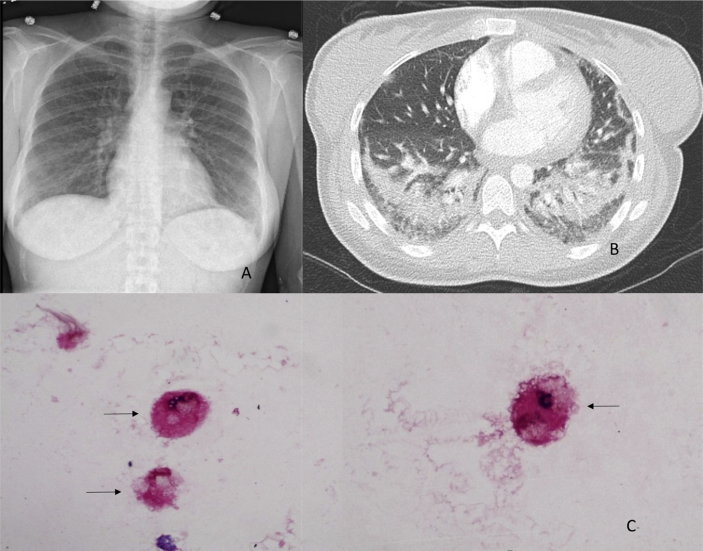
Chest X-ray showed bilateral patchy opacities (Fig. 1 Panel A). A Computed Tomography Pulmonary Embolism protocol scan was performed and did not show any pulmonary embolism but showed bilateral scattered Ground glass opacities (GGO) and dense opacities predominantly in both lower lobes of the lung (Fig. 1 Panel B). Due to worsening respiratory failure, she was eventually intubated. She was also started on broad spectrum antibiotics, vancomycin, pipercillin-tazobactam and azithromycin. She underwent a bronchoscopy procedure with Broncho Alveolar Lavage (BAL), and fluid cell count analysis. BAL was significant for predominantly neutrophils and also showed evidence of lipid-laden macrophages (Fig. 1 Panel C). Follow up laboratory testing was remarkable for elevated IgM antibodies for Mycoplasma with a titer of 1274 units/mL (normal <770), HIV test was negative, CRP 32, LDH 356. Urine legionella antigen was negative, blood and sputum cultures negative, BAL was negative for bacterial, viral, fungal cultures and PJP staining, influenza flu swab was negative.
Fig. 1.
A. Chest X Ray: Bilateral lower lobe patchy infiltrates. B. Chest CT scan - axial view: Bilateral lower lobe infiltrates. C. Lipid Laden Macrophages marked with arrows (“Foamy macrophages”).
Her oxygen requirement and fever curve improved over the course of the hospitalization. She was extubated and her antibiotics were down titrated to fluoroquinolones; after 10 days of being hospitalized, she was discharged home with a total of 14 days of antibiotics. She was not started on steroids as she had documented infection and showed an overall clinical improvement. During her outpatient appointment 3 weeks later, she was feeling better. However, she was still vaping intermittently and was thoroughly counselled to quit vaping.
3. Discussion
In the literature, there has been no documented association between vaping and MPP. A probable case of VAPI per the CDC definition is, “a patient being exposed to vaping in the last 90 days, presence of pulmonary infiltrates, infection identified via culture or PCR, but the clinical team believes this infection is not the sole cause of the underlying lung injury with exclusion of alternative plausible diagnoses like cardiac, rheumatologic and neoplastic” [6]. This patient satisfied the criteria for a probable case of VAPI, as the infection with Mycoplasma is less likely to cause a severe pneumonia and the presence of lipid laden macrophages are seen in VAPI and not seen in pneumonia solely from Mycoplasma.
Mycoplasma usually causes a mild disease but severe and fulminant forms have been described in the literature [5]. There is no clear definition for severe and fulminant MPP and clinical guidelines need to be established for the diagnosis and management of severe and fulminant MPP.
E-Cigarette's (ECs) contain a liquid solution mixture of nicotine, propylene glycol, water, flavoring agent and often vegetable glycerin, which is heated in a cartridge to produce aerosol (‘vapor’) containing nicotine. Vegetable glycerin/glycerol is a sweet-tasting, colorless and odorless polyol that is extracted from palm, soy or coconut oil triglycerides by hydrolysis; when heated, it is responsible in part for the visible ‘smoke’ element of the vapor, also commonly known as the “theatrical smoke” [[11], [12]].
Vaping has been associated with an increase in lipid laden macrophages in the BAL specimen [13]. The only source of lipid in this case would be the vegetable glycerin found in EC's. There are previous case reports of lipoid pneumonia due to vegetable glycerin in ECs [8]. The presence of lipid laden macrophages on BAL specimen to pathologic lipoid pneumonia on biopsy specimens, could be a spectrum of the pathological disease progression.
It is known that among those who used e-cigarettes and got sick, around 80% of them reported the use of both nicotine products and tetrahydrocannabinol (THC) or cannabidiol (CBD) products. It is mostly the acute toxic lung injury from the toxins that causes the symptoms rather than active infection (e-cigarette fluids contaminated with infectious organisms). Mixing of multiple ingredients may result in the production of new agents that may be toxic [9].
Normal lung has multiple inbuilt protecting mechanisms to fight off the exogenous particles, invaders and toxins. These mechanisms are the ciliary apparatus, mucus layer, cough reflex, and lipid layer in the alveoli. The lipid layer collects those invaders and toxins, while binding and preventing them from reaching the air sacs. Macrophages gobble up any invaders that they encounter and also help in recycling the lung lipid lining.
Research has shown that PAFR (Platelet Activating Factor Receptor) levels in the respiratory airway lining cells are increased in people who vape. Pneumococcal pneumoniae easily bind to PAFR receptors; and this suggests that vaping makes the airways more vulnerable to bacteria sticking to airway lining cells and are prone to develop pneumococcal pneumonia. This phenomenon has been tested both in humans and mice [14]. Although only the effects of e-cigarettes leading to increased pneumococcal bacterial infections have been studied, it can be hypothesized that a similar mechanism are involved in patients with infection from other organisms. Mice exposed to chronic e-cigarette vapor aberrantly altered the physiology of lung epithelial cells and resident immune cells which promoted poor response to infectious challenge [15]. Remarkably, these changes in the immune impairment and lipid homeostasis are independent of nicotine, thereby warranting more extensive investigation of the vehicle solvents used in e-cigarettes [7]. From the above observation, it is clearly noticeable that not only do the macrophages become less effective lipid recyclers, they also get sidetracked from their main task of screening out toxins and the invaders that enter the airways.
The limitations of our case are: A trans bronchial biopsy along with the bronchoscopy was not performed, and it could have given information on any evidence of other varied pathologies including lipoid pneumonia. It is also possible that the association of VAPI and co-infection with MP could have been merely a random incidental finding occurring by chance. The presence of THC in urine toxicology and its effect on the lung could have a superimposed effect.
4. Conclusion
Although vaping is advertised as a modality to assist with combustible cigarette smoking cessation, it is not immune from side-effects. There is growing evidence that inhaling vapor has the potential to cause adverse health effects. By contrast, other aids to quit smoking such as patches or gum do not result in airway cells being exposed to high concentrations of potentially toxic compounds. Vaping is affecting the younger healthier population which is a public concern. There exists an epidemic of vaping especially among youth and young adults, and many VAPI cases have been reported and are still under investigation. The association between vaping and MPP infection has not been established in the literature and this is the first documented report to establish link between e-cigarettes and MPP. The effect of adding ingredients such as THC to this mix needs to be investigated. Further research is needed to confirm these associations.
Funding source
None.
Consent for publication
Obtained.
Declaration of competing interest
None.
Acknowledgements
The authors acknowledge that the material of the manuscripts read and approved by all authors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2020.100997.
Contributor Information
Shravan Kooragayalu, Email: skoor@ghvhs.org, knsk86@gmail.com.
Samer El-Zarif, Email: selzarif@ghvhs.org.
Sunit Jariwala, Email: sjariwal@montefiore.org.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Chan E.D., Welsh C.H. Fulminant Mycoplasma pneumoniae pneumonia. West. J. Med. 1995;162(2):133–142. [PMC free article] [PubMed] [Google Scholar]
- 2.Waites K.B., Xiao L., Liu Y., Balish M.F., Atkinson T.P. Mycoplasma pneumoniae from the respiratory tract and beyond. Clin. Microbiol. Rev. 2017;30(3):747–809. doi: 10.1128/CMR.00114-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobs E., Ehrhardt I., Dumke R. New insights in the outbreak pattern of Mycoplasma pneumoniae. Int. J. Med. Microbiol. 2015;305(7):705–708. doi: 10.1016/j.ijmm.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Miyashita N., Ouchi K., Kawasaki K., Oda K., Kawai Y., Shimizu H., Kobashi Y. Mycoplasma pneumoniae pneumonia in the elderly. Med. Sci. Monit. 2008;14(8):387–391. [PubMed] [Google Scholar]
- 5.Izumikawa K. Clinical features of severe or fatal Mycoplasma pneumoniae pneumonia. Front. Microbiol. 2016;7:800. doi: 10.3389/fmicb.2016.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC . Lung Injury Surveillance Case Definition. Center for Disease Control; Sep 2019. [Google Scholar]
- 7.Madison M.C., Landers C.T., Gu B.-H. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J. Clin. Investig. 2019;129(10):4290–4304. doi: 10.1172/JCI128531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viswam D., Trotter S., Sherwood Burge P. Respiratory failure caused by lipoid pneumonia from vaping e-cigarettes. Br. Med. J. Case Rep. 2018 doi: 10.1136/bcr-2018-224350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christiani D.C. Vaping-induced lung injury. N. Engl. J. Med. 2019 doi: 10.1056/NEJMe1912032. [DOI] [PubMed] [Google Scholar]
- 10.Henry T.S., Kanne J.P., Kligerman S.J. Imaging of vaping-associated lung disease. N. Engl. J. Med. 2019;381:1486–1487. doi: 10.1056/NEJMc1911995. [DOI] [PubMed] [Google Scholar]
- 11.McCauley L., Markin C., Hosmer D. Vol. 141. CHEST; 2012. An Unexpected Consequence of Electronic Cigarette Use; pp. 1110–1113. [DOI] [PubMed] [Google Scholar]
- 12.Modi S., Sangani R., Alhajhusain A. 4th. Vol. 148. Chest; 2015. Acute Lipoid Pneumonia Secondary to E-Cigarettes Use: an Unlikely Replacement for Cigarettes; p. 382. [Google Scholar]
- 13.Maddock S.D., Cirulis M.M., Callahan S.J. Pulmonary lipid-laden macrophages and vaping. N. Engl. J. Med. 2019;381:1488–1489. doi: 10.1056/NEJMc1912038. [DOI] [PubMed] [Google Scholar]
- 14.Miyashita L., Suri R., Dearing E. E-cigarette vapour enhances pneumococcal adherence to airway epithelial cells. Eur. Respir. J. 2018;51(2) doi: 10.1183/13993003.01592-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Q., Jiang D., Minor M., Chu H.W. 9th. Vol. 9. PLOS one; 2014. Electronic cigarette liquid increases inflammation and virus infection Primary Human Airway Epithelial Cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.