Abstract
Mycotic/fungal keratitis is a suppurative, generally ulcerative infection of the cornea. The filamentous fungi, Aspergillus spp. are the second leading cause of mycotic keratitis, particularly in India. Aspergillus spp. produce a range of extracellular enzymes that are used to break down complex molecules and used for growth and reproduction, also for survival on/in host organism. The current study was designed with an objective to screen in vitro extracellular enzyme activity of Fusarium and Aspergillus isolates from mycotic keratitis patients and to correlate the same as a putative virulence factor. Extracellular enzymes viz., deoxyribonuclease (DNase), protease, lipase, elastase, keratinase, etc., produced by Aspergillus have key role in keratomycosis and hence their (n = 85) in vitro activities were investigated. It was found that, the majority of the Aspergillus isolates produced protease (n = 75; 88% of 85) followed by lipase (n = 59; 69% of 85), DNase (n = 35; 41% of 85), elastase (n = 26; 31% of 85) and keratinase (n = 13; 15% of 85). The enzyme activity indices (EAI) for DNase, elastase, protease and lipase ranged between 1.01 and 1.98, whereas elastase EAI varied between 1.26 and 1.92. DNase, protease and lipase showed a maximum EAI of 1.98 and lowest EAI value of 1.01, respectively. Extracellular enzymes of Aspergillus spp. may have potential role in the onset and progression of keratitis.
Keywords: Mycotic keratitis, Aspergillus, Extracellular enzyme activity, Virulence, Corneal ulcer, Eye infections
1. Introduction
Mycotic keratitis/keratomycosis is the infection of the cornea due to pathogenic fungi capable of invading the ocular surface (Mravičić et al., 2012). Fusarium, Aspergillus, Curvularia, Bipolaris and yeast fungi such as Candida (Thomas, 2003) are the most common causative agents of keratomycosis. In the Southern part of India, the major etiologic agents of fungal keratitis are Fusarium and Aspergillus (Gopinathan et al., 2002, Manikandan et al., 2013, Srinivasan, 2004). Interestingly, Aspergillus spp. are the second leading etiological agents of mycotic keratitis, invasive aspergillosis and superficial infections (Hedayati et al., 2007). Fungi secrete several extracellular hydrolytic enzymes like keratinases, collagenases, gelatinases, phopholipases, lipases and acid proteinases in culture media (Khan et al., 2010). These enzymes not only have a mail role in the metabolism but also serve as virulence factor by causing potential harm to the host cells to satisfy the nutritional needs of the pathogen. Aspergillus spp. produce a range of extracellular enzymes that are used to break down complex polysaccharides into simple sugars to be assimilated and used for growth and reproduction, also for survival on host organism.
Research on extracellular enzymes production as a virulence factors for Aspergillus isolated from ocular infection remains unexplored (Bouchara et al., 1995, Latgé, 1999, Tomee and Kauffman, 2000). Fungi secrete several extracellular hydrolytic enzymes such as keratinases, collagenases, gelatinases, phospholipases, lipases, and acid proteinases in culture media (Khan et al., 2010). Extracellular proteinases help in the adherence and survival of the pathogen on mucosal surfaces (Borg and Rüchel, 1988), invasion of host tissues (Odds, 1985, Rüchel, 1986) and digestion of immunoglobulins (Rüchel, 1986, Yuan and Cole, 1987) and corneal matrix degradation (Gopinathan et al., 2001). Park et al. (2013) (Park et al., 2013) reported that lipolytic enzymes also have been implicated in fungal virulence and has been extensively studied in Candida species. Khan et al. (2010); (Alp and Arikan, 2008, Khan et al., 2010) stated that lipase of Aspergillus species has a role in tissue damage. Elastase cleaves the peptide bonds in elastin, aiding in the digestibility of this elastic protein. The keratomycosis aided by the extracellular enzymes of Aspergillus thus will add to the severity of the infection. Against this background, the present in vitro analysis was undertaken with the objective of examining the role of the extracellular enzyme activities as putative virulence factors in Aspergillus keratitis.
2. Materials and methods
2.1. Isolation and identification of Aspergillus spp.
Corneal scrapings were collected by an ophthalmologist from the patients with suspected keratomycosis at Aravind Eye Hospital and Postgraduate Institute of Ophthalmology (Coimbatore, Tamilnadu, India) during 2013-2015. The collected material was inoculated directly onto 5% sheep blood agar, Chocolate agar, brain heart infusion broth and potato dextrose agar (PDA) (HiMedia, Mumbai, India) and also spread on a glass slide for direct microscopy after 10% KOH wet mount. The Culture plates were incubated at 37 °C (for bacteria) and 27 °C (for fungi), examined daily, and discarded after 1 week if no growth were present. The fungi that were initially identified based on colony morphology on SDA were further characterized microscopically after lactophenol cotton blue staining (Harris, 2000). Suspected A. flavus isolates were further screened on Aspergillus differentiation agar (ADA) to differentiate other similar morphological species of Aspergillus genera (Rodrigues et al., 2007). All the isolates were stored in screw capped tubes containing 0.85% saline at 4 °C.
2.2. Fungal inoculum preparation
The test isolates Aspergillus were grown on potato dextrose agar slants and incubated at 28 °C for seven days. Sterile saline (0.9% NaCl, 2 mL) was added to the culture slant, and the conidia were harvested after gentle vortexing and the mycelial remnants from the conidial suspension were separated by filtration through sterile cotton-wool. The conidial suspension was used as inoculum.
2.3. Extracellular enzyme assays
DNase test agar, rose Bengal elastin agar, tributyrin agar and skim milk agar were aseptically prepared and autoclaved for assays of DNase, elastase, lipase and protease, respectively. All the chemicals were purchased from HiMedia, Mumbai. For keratinase assay, basal medium (BM) overlaid with keratin azure (Sigma-Aldrich, USA) was used (Scott and Untereiner, 2004). The assay media were inoculated with 30 µl of spore suspension and incubated at 28° C in darkness. After incubation, DNase assay plates were flooded with 1 N hydrochloric acid for clarity in the zone of hydrolysis (Sánchez and Colom, 2010). Elastase (Kothary et al., 1984), lipase (Griebeler et al., 2011) and protease assay plates (J Sharma et al., 2005) were observed for the zone of clearance after fungal mats were removed with the help of a sterile cotton swab (Mythili et al., 2014). Keratinase activity was evaluated visually from the release of azure dye into the colourless lower layer of BM after third day and within three weeks of inoculation (Scott and Untereiner, 2004).
For DNase, elastase, lipase and protease enzymes, enzyme activity index (EAI) was calculated by the following formula (Blanco et al., 2002);
The mean EAI was calculated from three observations and the calculated values were grouped under four classes as high EAI (2.0 to 1.75), medium EAI (1.75 to 1.25), low EAI (1.25 to 1.10) and negligible or no EAI (1.10 to 1.00).
3. Results
A total of 1628 ocular specimens were collected from suspected cases of keratitis, from which 85 isolates of Aspergillus species were obtained. These isolates were further analysed for microscopic and macroscopic morphology so as to identify at the species level. Microscopic characteristics such as stipes colour, surface appearance, vesicle serration, shape of vesicle and conidia surface were observed after staining with LCB and compared with the standard morphological characteristics (Diba et al., 2007) and they were identified as A. flavus (n = 53), A. fumigatus (n = 14), A. terreus (n = 9), A. tamarii (n = 6) and A. niger (n = 3).
The extracellular enzyme assay revealed that all the A. flavus isolates had one or the other enzyme activities and each isolate were found to have different enzyme activity indices (EAIs) ranging from 1 to 1.97 (Table 1). Among the 47 isolates tested for DNase activity, medium EAI was observed for 16 isolates, low EAI value for 30 isolates, negligible or no EAI value for one isolate and none of the isolate showed high EAI value (Fig. 1). The highest (1.59) and lowest (1.08) was exhibited by the isolate AF29 and AF18 respectively. For elastase enzyme 26, 9, 4 and 8 isolates exhibited high, medium, low and negligible or no EAI, respectively. The elastase EAI ranged from 1.0 to 1.97, the highest value of 1.97 (AF05) and the minimum value of 1.0 (AF10, AF11, AF12, AF13, AF17 and AF18). While analyzing lipase activity indices, it was found that 12, 29 and 6 isolates had medium, low and negligible or no EAI respectively. Of 47 isolates tested for protease activity a total of 12, 30 and 5 isolates exhibited medium, low and negligible or no EAI respectively. The highest value for protease activity was found to be 1.55 and the least value of 1.00. A total of five isolates (AF04, AF08, AF14, AF21 and AF27) were positive for keratinase production.
Table 1.
Frequency distribution of EAIs of Aspergillus isolates from keratitis.
| Enzymes | Isolates | Enzyme activity indices (EAIs) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.0–1.10 | 1.11–1.20 | 1.21–1.30 | 1.31–1.40 | 1.41–1.50 | 1.51–1.60 | 1.61–1.70 | 1.71–1.80 | 1.81–1.90 | 1.91–2.0 | ||
| DNase | A. flavus (n = 53) | 1 | 10 | 7 | 1 | 1 | – | – | – | – | – |
| A. fumigatus (n = 14) | – | 2 | – | – | 1 | – | – | – | – | 1 | |
| A. terreus (n = 9) | – | 1 | 1 | 2 | – | – | – | – | – | – | |
| A. tamarii (n = 6) | 1 | 2 | 1 | 1 | – | – | 1 | – | – | – | |
| A. niger (n = 3) | – | – | 1 | – | – | – | – | – | – | – | |
| Elastase | A. flavus (n = 53) | – | – | 1 | 1 | – | 2 | 2 | 3 | 6 | 4 |
| A. fumigatus (n = 14) | – | – | – | – | – | 2 | – | – | – | 1 | |
| A. terreus (n = 9) | – | – | – | – | – | – | 1 | – | 1 | – | |
| A. tamarii (n = 6) | – | – | – | – | 1 | – | – | – | – | – | |
| A. niger (n = 3) | – | – | – | 1 | – | – | – | – | – | – | |
| Protease | A. flavus (n = 53) | 7 | 17 | 17 | 2 | 3 | 1 | – | – | – | – |
| A. fumigatus (n = 14) | 3 | 1 | 2 | 3 | – | – | 2 | – | – | – | |
| A. terreus (n = 9) | 2 | 3 | 1 | 1 | – | – | 1 | – | – | – | |
| A. tamarii (n = 6) | – | 3 | 1 | – | – | – | 1 | – | – | 1 | |
| A. niger (n = 3) | – | 2 | 1 | – | – | – | – | – | – | – | |
| Lipase | A. flavus (n = 53) | 6 | 18 | 3 | 8 | 3 | – | – | – | – | – |
| A. fumigatus (n = 14) | 1 | 2 | – | 2 | – | – | – | 1 | – | – | |
| A. terreus (n = 9) | 2 | – | 2 | 1 | – | 1 | – | – | – | 1 | |
| A. tamarii (n = 6) | – | – | 1 | 2 | – | – | 1 | 1 | – | – | |
| A. niger (n = 3) | 1 | – | 2 | – | – | – | – | – | – | – | |
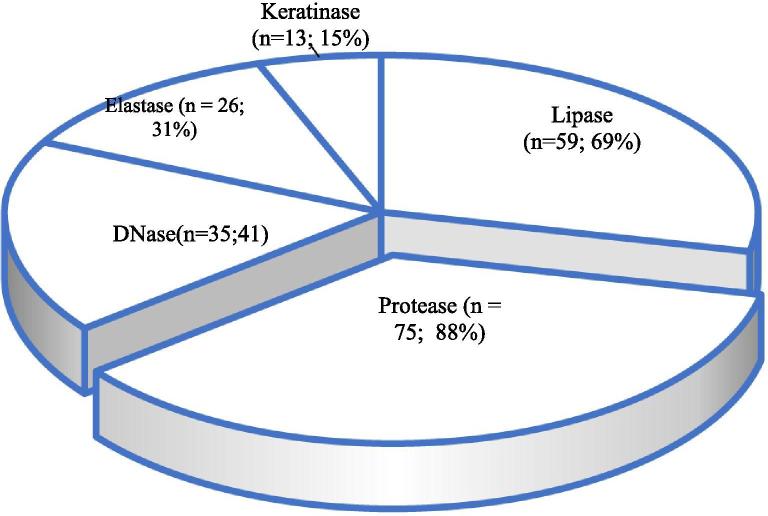
Fig. 1.
Total number (%) of Aspergillus isolates (n = 85) with different extracellular enzyme activity.
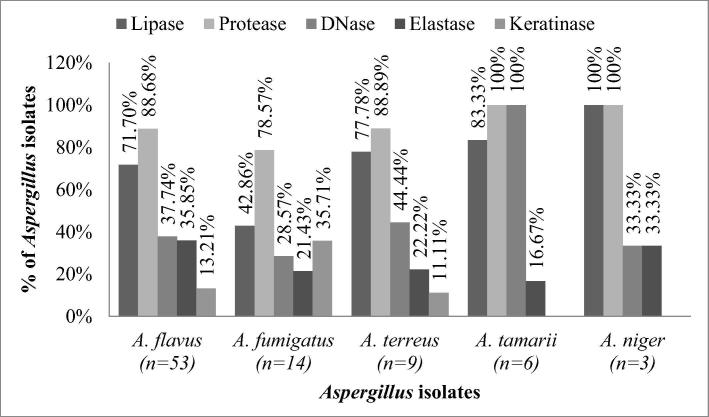
Majority of the Aspergillus isolates produced protease (n = 75; 88% of 85) followed by lipase (n = 59; 69% of 85), DNase (n = 35; 41% of 85), elastase (n = 26; 31% of 85) and keratinase (n = 13; 15% of 85) (Fig. 1, Fig. 2). The EAI for the enzymes DNase, elastase, protease and lipase ranged between 1.01 and 1.98. DNase, protease and lipase showed a maximum EAI of 1.98 and lowest EAI value of 1.01, whereas elastase EAI was found to be varying between 1.26 and 1.92. Of 53 isolates of A. flavus 88.68% (n = 47), 71.7% (n = 38), 37.74% (n = 20), 35.85% (n = 19) and 13.21% (n = 7) had protease, lipase, DNase, elastase and keratinase activities, respectively. All the isolates of A. tamarii (n = 6) and A. niger (n = 3) exhibited protease activity. A total of 7, 5 and one isolates of A. flavus (13.21% of 53), A. fumigatus (35.71% of 14) and A. terreus (11.11% of 9), respectively showed keratinase activity. The extracellular enzyme activity indices of Aspergillus isolates were calculated and tabulated as frequency table (Table 1). On DNase test agar, isolates of Aspergillus exhibited varying magnitude of enzyme activity. One of the isolates of A. flavus showed very low/negligible EAI (1.0 to 1.10). Relatively low EAI (1.11 to 1.30) was exhibited by 17 isolates and medium EAI (1.31 to 1.60) was shown by 2 isolates and none of the isolates of A. flavus showed high EAI (1.61 to 2.0). High DNase EAI (1.61 to 2.0) was exhibited by one isolate of each A. fumigatus and A. tamarii. A. terreus had low to medium EAI (1.11 to 1.40) and A. niger exhibited low EAI (1.21 to 1.30).
Fig. 2.
Percentage distribution of species of Aspergillus showing various extracellular enzyme activities.
4. Discussion
Certain extracellular enzymes such as DNase, elastase, protease, lipase, keratinase, etc., are known to play an important role in the process of infection by pathogenic including fungi (U Gajjar, 2019). In the present study five clinically important enzymes viz., DNase, elastase, protease, lipase and keratinase were screened for their activity in order to evaluate the overall spectrum of extracellular enzyme activities of Aspergillus (n = 85) strains isolated from fungal keratitis.
Most of the isolates (n = 25; 96.15% of 26) of Aspergillus showed medium to high elastase activity. Fifteen isolates of A. flavus showed high elastase activity indices (1.61 to 2.0) and one isolate each of A. tamarii and A. niger showed medium elastase activity indices (1.31 to 1.60). For protease, exactly, 34 (64.15% of 53) isolates of A. flavus showed relatively low EAIs (1.11 to 1.30) and high EAIs were exhibited by A. fumigatus (n = 2), A. tamarii (n = 2) and A. terreus (n = 1). A total of 21 and 11 isolates of A. flavus showed relatively low and medium lipase activity, respectively. Two isolates of A. tamarii and one isolate each of A. fumigatus and A. terreus had high lipase activity.
In this study, the possible role of the extracellular enzymes as a putative virulence factor in Aspergillus keratitis was explored. It was observed that the isolates of Aspergillus were able to produce an array of extracellular enzymes. Aspergillus isolates had lesser activities of DNase (n = 35) and keratinase(n = 15) when compared to protease, elastase and lipase. The DNase enzyme activity was analysed by observing the halo zone around the colony, similar to that of Sanchez and Colom (Sánchez and Colom, 2010) where 85 Cryptococcus isolates were reported to be positive for DNase activity. In the present study, 69% (n = 59) and 88% (n = 75) of Aspergillus isolates produced lipase and protease, respectively. Higher lipase activity in Fusarium isolates was observed by St. Leger et al. (St. Leger et al., 1986). Similar to the present study, Gajjar et al. (U Gajjar, 2019) and Selvam et al. (Selvam et al., 2014) reported 83% of protease activity in Aspergillus isolates. Alp and Arikan (Alp and Arikan, 2008) investigated the production of extracellular elastase, acid proteinase, and phospholipase from the clinical isolates of Aspergillus and found that 84.9, 27.4, and 65.8% of Aspergillus isolates were able to produce elastase, acid proteinase and phospholipase, respectively and also reported that none of the A. niger isolate produced elastase. Whereas, in the present study, 31% of Aspergillus isolates produced elastase and one isolate among 3 isolates of A. niger also produced elastase activity. The production of elastin lytic enzymes by Aspergillus suggests that elastase play a role in pathogenesis (Blanco et al., 2002, Kolattukudy et al., 1993, Kothary et al., 1984, Rhodes et al., 1988). Kothary et al. (Kothary et al., 1984) stated that non-elastase producing environmental Aspergillus isolates were relatively less virulent compared with high elastase producers. Bazan (Bazan, 2005) stated that lipid and lipid mediated compounds play an important role in the recovery of corneal inflammations in a complex manner. These probably answer the critical role of extracellular lipase produced by the test isolates of Aspergillus from keratomycosis patients.
Although the present study detected keratinase activity only among 15% Aspergillus, Friedrich et al. (Friedrich et al., 1999) and Sharma et al. (Sharma et al., 2011) reported keratinase activity in many of these species. Oyeleke et al. (Oyeleke and Auta, 2010) stated that Aspergillus yielded high amount of protease enzymes. In the present study, protease activity was profoundly seen among Aspergillus isolates (88% of 85). Protease is proven to be associated with cornea that showed progressive ulcer along with the presence of dense inflammatory cells (Gopinathan et al., 2001). Burda and Fisher (Burda and Fisher, 1960) and Dudley and Chick (DUDLEY and CHICK, 1964) have demonstrated extracellular protease activity in corneal matrix degradation in mycotic keratitis using rabbit model. The pathogenic role of extracellular protease in keratitis has been reported by various authors (Barletta et al., 1996, Mahmoud et al., 2007, Zhu et al., 1990).
5. Conclusion
The present study clearly indicates the potential role of extracellular enzymes of Aspergillus spp. in the onset and progression of keratitis. However, the number of isolates Aspergillus keratitis cases is low and the problem remains to be further clarified by concordant examination of other potential virulence factors. Further studies on the multifactorial impact on virulence of the isolates causing fungal keratitis are required.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Acknowledgements
The study was supported by Science and Engineering Research Board, Government of India (DST No. File No. YSS/2015/000267). AA and PM would like to thank Deanship of Scientific Research at Majmaah University, Al Majmaah, 11952, Saudi Arabia for supporting this work under the Project Number 1439/75.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alp S., Arikan S. Investigation of extracellular elastase, acid proteinase and phospholipase activities as putative virulence factors in clinical isolates of Aspergillus species. J. Basic Microbiol. 2008 doi: 10.1002/jobm.200700349. [DOI] [PubMed] [Google Scholar]
- Barletta J.P., Angella G., Balch K.C., Dimova H.G., Stern G.A., Moser M.T., Van Setten G.B., Schultz G.S. Inhibition of pseudomonal ulceration in rabbit corneas by a synthetic matrix metalloproteinase inhibitor. Investig. Ophthalmol. Vis. Sci. 1996 [PubMed] [Google Scholar]
- Bazan H.E.P. Cellular and molecular events in corneal wound healing: Significance of lipid signalling. Exp. Eye Res. 2005 doi: 10.1016/j.exer.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Blanco J.L., Hontecillas R., Bouza E., Blanco I., Pelaez T., Muñoz P., Perez Molina J., Garcia M.E. Correlation between the elastase activity index and invasiveness of clinical isolates of Aspergillus fumigatus. J. Clin. Microbiol. 2002 doi: 10.1128/JCM.40.5.1811-1813.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg M., Rüchel R. Expression of extracellular acid proteinase by proteolytic Candida spp. during experimental infection of oral mucosa. Infect. Immun. 1988;56:626–631. doi: 10.1128/iai.56.3.626-631.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchara J.-P., Tronchin G., Larcher G., Chabasse D. The search for virulence determinants in Aspergillus fumigatus. Trends Microbiol. 1995;3:327–330. doi: 10.1016/s0966-842x(00)88965-9. [DOI] [PubMed] [Google Scholar]
- Burda C.D., Fisher E. Corneal destruction by extracts of cephalosporium mycelium*. Am. J. Ophthalmol. 1960;50:926–937. doi: 10.1016/0002-9394(60)90345-7. [DOI] [PubMed] [Google Scholar]
- Diba K., Kordbacheh P., Mirhendi S.H., Rezaie S., Mahmoudi M. Identification of Aspergillus species using morphological characteristics. Pakistan J. Med. Sci. 2007;23:867–872. [Google Scholar]
- Dudley M.A., Chick E.W. Corneal lesions produced in rabbits by an extract of fusarium moniliforme. Arch. Ophthalmol. 1964;72:346–350. doi: 10.1001/archopht.1964.00970020346012. [DOI] [PubMed] [Google Scholar]
- Friedrich J., Gradišar H., Mandin D., Chaumont J.P. Screening fungi for synthesis of keratinolytic enzymes. Lett. Appl. Microbiol. 1999;28:127–130. [Google Scholar]
- Gopinathan U., Garg P., Fernandes M., Sharma S., Athmanathan S., Rao G.N. The epidemiological features and laboratory results of fungal keratitis. Cornea. 2002;21:555–559. doi: 10.1097/00003226-200208000-00004. [DOI] [PubMed] [Google Scholar]
- Gopinathan U., Ramakrishna T., Willcox M., Rao C.M., Balasubramanian D., Kulkarni A., Vemuganti G.K., Rao G.N. Enzymatic, clinical and histologic evaluation of corneal tissues in experimental fungal keratitis in rabbits. Exp. Eye Res. 2001;72:433–442. doi: 10.1006/exer.2000.0971. [DOI] [PubMed] [Google Scholar]
- Griebeler N., Polloni A.E., Remonatto D., Arbter F., Vardanega R., Cechet J.L., Di Luccio M., de Oliveira D., Treichel H., Cansian R.L., Rigo E., Ninow J.L. Isolation and screening of lipase-producing fungi with hydrolytic activity. Food Bioprocess Technol. 2011;4:578–586. [Google Scholar]
- Harris J.L. Letter to the editor: Safe, low-distortion tape touch method for fungal slide mounts. J. Clin. Microbiol. 2000 doi: 10.1128/jcm.38.12.4683-4684.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedayati M.T., Pasqualotto A.C., Warn P.A., Bowyer P., Denning D.W. Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology. 2007;153:1677–1692. doi: 10.1099/mic.0.2007/007641-0. [DOI] [PubMed] [Google Scholar]
- Sharma J., Singh A., Kumar R., Mittal A. Partial purification of an alkaline protease from a new strain of aspergillus oryzae AWT 20 and its enhanced stabilization in entrapped Ca-alginate beads. Internet J. Microbiol. 2005;2 [Google Scholar]
- Khan M.S.A., Ahmad I., Aqil F., Owais M., Shahid M., Musarrat J. Combating Fungal Infections. Springer Berlin Heidelberg; Berlin, Heidelberg: 2010. Virulence and pathogenicity of fungal pathogens with special reference to candida albicans; pp. 21–45. [Google Scholar]
- Kolattukudy P.E., Lee J.D., Rogers L.M., Zimmerman P., Ceselski S., Fox B., Stein B., Copelan E.A. Evidence for possible involvement of an elastolytic serine protease in aspergillosis. Infect. Immun. 1993;61:2357–2368. doi: 10.1128/iai.61.6.2357-2368.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothary M.H., Chase T., MacMillan J.D. Correlation of elastase production by some strains of Aspergillus fumigatus with ability to cause pulmonary invasive aspergillosis in mice. Infect. Immun. 1984 doi: 10.1128/iai.43.1.320-325.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latgé J.P. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud Y.A.-G., Abu El-Souod S.M., El-Shourbagy S.M., El-Badry A.S.M. Characterisation and inhibition effect of cetrimide on collagenase produced byAspergillus flavus, isolated from mycotic ulcers. Ann. Microbiol. 2007;57:109–113. [Google Scholar]
- Manikandan P., Varga J., Kocsubé S., Anita R., Revathi R., Németh T.M., Narendran V., Vágvölgyi C., Panneer Selvam K., Shobana C.S., Babu Singh Y.R., Kredics L. Epidemiology of Aspergillus keratitis at a tertiary care eye hospital in South India and antifungal susceptibilities of the causative agents. Mycoses. 2013;56:26–33. doi: 10.1111/j.1439-0507.2012.02194.x. [DOI] [PubMed] [Google Scholar]
- Mravičić V., Dekaris I., Gabrić N., Romac I., Glavota V., Mlinarić-Missoni E. Srinivasan MKeratitis. InTech; 2012. An overview of fungal keratitis and case report on trichophyton keratitis. [Google Scholar]
- Mythili A., Babu Singh Y.R., Priya R., Shafeeq Hassan A., Manikandan P., Panneerselvam K., Narendran V., Shobana C.S. In vitro and comparative study on the extracellular enzyme activity of molds isolated from keratomycosis and soil. Int. J. Ophthalmol. 2014;7:778–784. doi: 10.3980/j.issn.2222-3959.2014.05.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds F.C. Candida albicans proteinase as a virulence factor in the pathogenesis of Candida infections. Zentralbl. Bakteriol. Mikrobiol. Hyg. A. 1985;260:539–542. doi: 10.1016/s0176-6724(85)80069-9. [DOI] [PubMed] [Google Scholar]
- Oyeleke S.B., Auta H. Screening of Aspergillus flavus and Aspergillus fumigatus strains for extracellular protease enzyme production. J. Microbiol. Antimicrob. 2010;2:83–87. [Google Scholar]
- Park M., Do E., Jung W.H. Lipolytic enzymes involved in the virulence of human pathogenic fungi. Mycobiology. 2013;41:67–72. doi: 10.5941/MYCO.2013.41.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J.C., Bode R.B., McCuan-Kirsch C.M. Elastase production in clinical isolates of Aspergillus. Diagn. Microbiol. Infect. Dis. 1988;10:165–170. doi: 10.1016/0732-8893(88)90036-3. [DOI] [PubMed] [Google Scholar]
- Rodrigues P., Soares C., Kozakiewicz Z., Paterson R.R.M., Lima N., Venâncio A. Identification and characterization of Aspergillus flavus and aflatoxins. Commun. Curr. Res. Educ. Top. Trends Appl. Microbiol. 2007:527–534. [Google Scholar]
- Rüchel R. Cleavage of immunoglobulins by pathogenic yeasts of the genus Candida. Microbiol. Sci. 1986;3:316–319. [PubMed] [Google Scholar]
- Sánchez M., Colom F. Extracellular DNase activity of Cryptococcus neoformans and Cryptococcus gattii. Rev. Iberoam. Micol. 2010;27:10–13. doi: 10.1016/j.riam.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Scott J.A., Untereiner W.A. Determination of keratin degradation by fungi using keratin azure. Med. Mycol. 2004;42:239–246. doi: 10.1080/13693780310001644680. [DOI] [PubMed] [Google Scholar]
- Selvam K.P., Singh Y.R.B., Shobana C.S., Karunakaran N.K., Vágvölgyi C., Kredics L., Al-Baradie R.S., Manikandan P. Extracellular enzymes and mycotoxins as virulence factors in Fusarium and Aspergillus keratitis. Biosci. Biotechnol. Res. Asia. 2014;11 [Google Scholar]
- Sharma Mukesh, Sharma Meenakshi, Rao Vijay Mohan. In vitro biodegradation of keratin by dermatophytes and some soil keratinophiles. African J. Biochem. Res. 2011;5:1–6. [Google Scholar]
- Srinivasan M. Fungal keratitis. Curr. Opin. Ophthalmol. 2004;15:321–327. doi: 10.1097/00055735-200408000-00008. [DOI] [PubMed] [Google Scholar]
- St. Leger R.J., Charnley A.K., Cooper R.M. Cuticle-degrading enzymes of entomopathogenic fungi: Synthesis in culture on cuticle. J. Invertebr. Pathol. 1986;48:85–95. [Google Scholar]
- Thomas P.A. Current perspectives on ophthalmic mycoses. Clin. Microbiol. Rev. 2003 doi: 10.1128/CMR.16.4.730-797.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomee J.F., Kauffman H.F. Putative virulence factors of Aspergillus fumigatus. Clin. Exp. Allergy. 2000;30:476–484. doi: 10.1046/j.1365-2222.2000.00796.x. [DOI] [PubMed] [Google Scholar]
- U Gajjar D. Extracellular proteases from keratitis causing fusarium, aspergillus and dematiaceous Species. Trends Ophthalmol. Open Access J. 2019;2 [Google Scholar]
- Yuan L., Cole G.T. Isolation and characterization of an extracellular proteinase of Coccidioides immitis. Infect. Immun. 1987;55:1970–1978. doi: 10.1128/iai.55.9.1970-1978.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W.-S., Wojdyla K., Donlon K., Thomas P.A., Eberle H.I. Extracellular proteases of Aspergillus flavus: Fungal keratitis, proteases, and pathogenesis. Diagn. Microbiol. Infect. Dis. 1990;13(6):491–497. doi: 10.1016/0732-8893(90)90081-6. [DOI] [PubMed] [Google Scholar]