Abstract
Recent trends in anticancer therapy is to use therapeutic agents which not only kill the cancer cell, but are less toxic to surrounding normal cells/tissue. One approach is to cut the nutrient supply to growing tumor cells, by blocking the formation of new blood vessels around the tumor. As the phytochemicals and botanical crude extracts have proven their efficacy as natural antiangiogenic agents with minimum toxicities, there is need to explore varieties of medicinal plants for novel antiangiogenic compounds.
Rumex vesicarius L. (Humeidh), is an annual herbal plant with proven medicinal values. The antiangiogenic potential, and developmental toxicity of humeidh in experimental animal models has never been studied before. The crude extracts were prepared from the roots, stems, leaves and flowers of Rumex vesicarius L. in methanol, chloroform, ethyl acetate and n-hexane. The developmental toxicity screening in zebrafish embryos, has revealed that Rumex vesicarius was not toxic to zebrafish embryos. The chloroform stem extract showed significant level of antiangiogenic activity in zebrafish angiogenic assay on a dose dependent manner. Thirty five (35) bioactive compounds were identified by gas chromatography mass spectrophotometry (GC–MS) analysis in the stem extract of Rumex vesicarius. Propanoic acid, 2-[(trimethylsilyl)oxy]-, trimethylsilyl ester, Butane, 1,2,3-tris(trimethylsiloxy), and Butanedioic acid, bis(trimethylsilyl) ester were identified as major compound present in the stem of R. vasicarius.
The anticancer activity of roots, stem, leaves and flowers crude extract was evaluated in human breast cancer (MCF7), human colon carcinoma (Lovo, and Caco-2), human hepatocellular carcinoma (HepG2) cell lines. Most of the crude extracts did not show significant level of cytotoxicity in tested cancer cells line, except, chloroform extract of stem which exhibited strong anticancer activity in all tested cancer cells with IC50 values in micro molar range.
Based on these results, it is recommended that formulation prepared from R. vesicarius can further be tested in clinical trials in order to explore its therapeutic potential as an effective and safe natural anticancer product.
Keywords: Rumex vasicarius L., Angiogenesis, Phytochemical screening, Developmental toxicity, Zebrafish embryos
1. Introduction
Solid tumors produce blood vessels to get nourish and to migrate to other organs, a process known as metastasis. The angiogenesis (formation of secondary blood vessels) is a normal process during embryonic development, and during wound healing and the menstrual cycle. However, angiogenesis get activated in pathological condition, such as in cancer. The activation of angiogenesis exclusively by cancer, and quiescence in normal cells makes regulation of angiogenesis as an attractive therapeutic target for anti-tumor drug discovery” (Al-Abd et al., 2017), and hence many small molecules have been synthesized and tried in tumor cells to suppress the angiogenesis (Khalid et al., 2016, Wang et al., 2015), however, majority of these compounds either failed in clinical trials, or discontinued due to having lot of side effects (Cao, 2016, Lu et al., 2019, Medina et al., 2007). One reason for the inefficacy of angiogenesis inhibitors could be due to the fact that, the majority of synthetic anti-angiogenic molecules target only single angiogenic pathway for example, interacting only to “ vascular endothelial growth factor (VEGF)” or its receptors, VEGF is a protein which is responsible for the proliferation of endothelial cells (Abdel-Qadir et al., 2017, Qin et al., 2019). Crude extract or pure compounds derived from traditional medicinal plants act on multiple targets and have shown good anti-angiogenic effects with least toxicities (Erices et al., 2018, Gezici and Sekeroglu, 2019, Song et al., 2019). Hence, there is a need to explore new antiangiogenic natural sources most likely from medicinal plants and herbs by suitable in vivo and in vitro angiogenic assays.
In order to find out novel natural antiangiogenic products, the Saudi medicinal plants were explored in this study. One hundred and fifty (150) medicinal plants were collected from various regions of Saudi Arabia and from folk medicine practitioners (aatar). The crude extracts were prepared in methanol, chloroform, ethyl acetate and hexane. The antiangiogenic activity was evaluated in zebrafish transgenic line which express green florescent protein constitutively in blood vessels (Lawson and Weinstein, 2002).
“Rumex vesicarius” bladder dock (Arabic; Humeidh) has been identified as one of the plant, which had showed significant level of antiangiogenic activity in our pilot study. R. vesicarius is a branched tender perennial herbal plant which belongs to Polygonaceae family and is widely distributed throughout Saudi Arabia (Harley, 1991). R. vesicarius has been used in traditional medicine as flatulence, tonic, digestion enhancer, laxative, Anti- nausea, spleen disorders, antiasthma, bronchitis, analgesic and in some hepatic diseases in Egypt, India, and Saudi Arabia (Vasas et al., 2015).
The antiangiogenic property of R. vesicarius has not been reported before, and hence, the antiangiogenic activity and developmental toxicity of R. vesicarius was explored in zebrafish embryos in this study. The bioactive compounds present in R. vesicarius were identified using Gas chromatography-Mass spectrometry (GC-MS) analysis. In order to find out “target protein” for the major bioactive compounds present in the stem of R. vesicarius an online proteomic web tool “Swiss target prediction” was used. (Gfeller et al., 2013). The anti-cancer activity of the crude extracts of roots, stem, and flowers of R. vesicarius was checked in human breast cancer (MCF7), human colon carcinoma (Lovo, and Caco-2), and human hepatocellular carcinoma (HepG2) cell lines.
2. Material and methods
2.1. Plant collection and preparation of crude extract
The plant was collected in flowering season (February- March) from Riyadh region, Saudi Arabia. The plant was washed thoroughly with running tape water. Roots, stems, leaves and flower were separated and let to dry under shade for several days. The crude extract from the roots, stem, leaves and flower was prepared in methanol, chloroform, hexane, and ethyl acetate essentially same as reported previously (Nasr et al., 2018).
2.2. Cytotoxicity evaluation
The toxicity of roots, stem, leaves, and flowers of R. vesicarius was tested in human breast cancer cell line (MCF7), human colon cancer cell lines (Lovo, Caco-2), and human liver carcinoma cell lines (HepG2). MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) colorimetric cell proliferation assay was used to assess the cytotoxicity of the extracts on cancer cell lines using Vybrant® MTT Cell Proliferation Assay Kit (Cat # V13154 lot # 1,129,031 Invitrogen) following the protocol provided by the manufacturer. The cell culture conditions were same as reported previously (Farooq et al., 2019)
2.3. Zebrafish developmental toxicity and angiogenic assays
The source of animals and maintenance was the same as described previously (Farooq et al., 2018). Thirty (30) embryos were exposed to extracts at gastrulation stage with serial dilution (10–500 µM) of each extract. The effect of extracts on embryonic survival, toxicity was monitored in wild type embryos. The treated embryos were examined at 24, 48, and 72 h post exposure. The developmental toxicity was evaluated based on, 1) Calculating the % lethality in response to serial dilution of the extracts, 2) embryonic abnormalities by observing the defects in otoliths, eyes, somite formation, tail detachment, heartbeat, blood circulation, hatching, and active swimming and % embryonic defects in each condition was calculated by equation
The antiangiogenic activity was evaluated in transgenic zebrafish embryos. The experiments were repeated for three times. The percent antiangiogenic activity in transgenic zebrafish embryos was assessed by the equation as follow.
The blood vessels in control and treated live Tg (fli1:EGFP) were counted by observing the embryos using fluorescent microscope (Zeiss Observer D1; Zeiss, Germany) under FITC filter. At least five embryos in each condition were examined in order to calculate the mean % antiangiogenic activity of all the extracts.
3. Gas chromatography-mass spectrometry analysis
3.1. Extraction using ultra-sonication
Plant samples (1 g) was added to 25 mL of ethyl acetate: hexane solvent mixture (1:1) in a beaker. The ultrasonic probe was placed in the beaker and power was set to 60 W. Ultrasonic temperature was maintained at 45 °C by using a water bath without any external heating. After extraction, the sample was filtered using polypropylene sheet membrane (157 μm thickness and 0.2 μm pore size) was purchased from Membrana (Wuppertal, German). Extract contains both polar and non-polar analytes, hence the extract was derivatized using Bis(trimethylsilyl)trifluoroacetamide (BSTFA). A ratio of extract and BSTFA (1:1) was added and then the 1 µL of extract was injected to GC–MS. Shimadzu (Kyoto, Japan) QP2010 GC–MS instrument equipped with a Shimadzu AOC-20s auto sampler and AOC-20 auto injector and Rxi-5 Sil MS column with thickness of 0.25 μm, length of 30.0 m and diameter of 0.25 mm (Restek, Bellefonte, US). The high purity helium gas was employed as carrier gas at flow rate of 1.01 mL/min. All the samples were injected in spilt mode (1:100 ratio) and GC injection port temperature was kept 250 °C. The opening time of split vent was 1.0 min. The GC–MS interface temperature was 220 °C and ion source temperature was 200 °C. The oven temperature was programmed as follows: initial temperature was 40 °C and held for 1 min; then increased to 100 °C at 10 °C/min and held for 2 min; then increased to 165 °C at 10 °C/min and held for 0 min; then increased to 190 °C at 6 °C/min and held for 3 min; after that it was increased to 220 °C at 3 °C/min and held for 3 min; and finally increased to 240 °C at 2 °C/min and held for 1 min. For qualitative analysis, data acquisition was performed in scan mode to confirm the retention times of target compounds. The identification of the compounds was performed by similarity searches and mass spectra data in the NIST (National Institute for Standard and Technology) MS Library.
3.2. Statistical analysis and calculation of IC50 and LC50
The data of zebrafish mortalities to serial dilution of the extract was put in Excel sheet and mean values, standard deviation were calculated. Similarly the dose response to cancer cells to the extract was obtained. The IC50 values for the cytotoxicity in cancer cell liens, and LD50 values for zebrafish mortality were calculated by probit analysis (R.H.D, 1952).
4. Results
4.1. Developmental toxicity R. vesicarius in zebrafish embryos
The comparative developmental toxicity data at 24, 48 and 72 hpf of chloroform fraction of stem part is shown in Table 1. It is quite evident from these values that chloroform extract of stem part of part R. vesicarius did not induce any significant level of toxicity in zebrafish embryos. Similarly no developmental toxicity was observed in zebrafish embryos treated with crude extract prepared in hexane, methanol, chloroform and water from the stem part of R. vesicarius. Zebrafish embryos treated with crude extracts prepared from root, leaves, and flowers did not show any significant level of toxicity as well.
Table 1.
The developmental toxicioty of Rumex vesicarius in zebrafish embryos.
| Rumex vesicarius stem | Con. µg/mL | Embryo lethality¥ |
Otoliths |
Eyes |
Heart beat |
Blood circulation |
Hatching |
Active swimming |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean* | S.D | Mean | S.D | Mean | S.D | Mean | S.D | Mean | S.D | Mean* | S.D | Mean | S.D | ||
| 24hpf | Control | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | – | – | – | – | – | – | – | – |
| 0.001 | 1 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | – | – | – | – | – | – | – | – | |
| 0.01 | 1 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | – | – | – | – | – | – | – | – | |
| 1 | 1 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | – | – | – | – | – | – | – | – | |
| 10 | 2 | ±0.57 | 0 | ±0.0 | 0 | ±0.0 | – | – | – | – | – | – | – | – | |
| 100 | 2 | ±0.57 | 0 | ±0.0 | 0 | ±0.0 | – | – | – | – | – | – | – | – | |
| 300 | 5 | ±0.47 | 0 | ±0.0 | 0 | ±0.0 | – | – | – | – | – | – | – | – | |
| 48hpf | Control | 0 | ±0 | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 |
| 0.001 | 0 | ±0 | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | |
| 0.01 | 0 | ±0 | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | |
| 1 | 0 | ±0 | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | |
| 10 | 0 | ±0 | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | 1 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | |
| 100 | 0 | ±0 | 0 | ±0.0 | 0 | ±0.0 | 2 | ±0.57 | 5 | ±0.57 | 0 | ±0.0 | 0 | ±0.0 | |
| 300 | 0 | ±0 | 0 | ±0.0 | 0 | ±0.0 | 5 | ±0.81 | 5 | ±0.57 | 1 | ±0.0 | 0 | ±0.0 | |
| 72hpf | Control | 0 | ±0 | 0 | ±0.0 | 0 | ±0.0 | 1 | ±0.57 | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 |
| 0.001 | 0 | ±0 | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | |
| 0.01 | 0 | ±0 | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | 0 | ± 0.0 | 0 | ±0.0 | 0 | ±0.0 | |
| 1 | 0 | ±0 | 0 | ±0.0 | 0 | ±0.0 | 0 | 0 | 0 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | |
| 10 | 0 | ±0 | 0 | ±0.0 | 0 | ±0.0 | 2 | ±0.57 | 11 | ±0.75 | 0 | ±0.0 | 0 | ±0.0 | |
| 100 | 0 | ±0 | 0 | ±0.0 | 0 | ±0.0 | 5 | ±0.57 | 20 | ±0.12 | 0 | ±0.0 | 0 | ±0.0 | |
| 300 | 0 | ±0 | 0 | ±0.0 | 0 | ±0.0 | 20 | ±0.62 | 50 | ±0.0 | 0 | ±0.0 | 0 | ±0.0 | |
¥ Percentage of embryos with developmental defects.* mean values from three biological replicates. S.D; standard deviation.
4.2. Chloroform stem extract of R. vesicarius exhibited antiangiogenic activity in zebrafish embryos.
The percent antiangiogenic activity of crude extract in various solvent of rots, stem, leaves and flowers R. vesicarius is presented in Table 2. The antiangiogenic activity was observed with chloroform crude extract prepared from the stem part of R. vesicarius (Fig. 1). The stem CHCl3 extract obstructed ≥70% of inter-segmental blood vessels (isv) and 100% of sub-intestinal vein (siv) blood vessels formation in treated embryos at 30 µg/ml (Fig. 1B and C). The crude extracts from the stem part prepared in methanol, hexane, chloroform and water showed very weak (≤10%) level of antiangiogenic activity, which was statistically insignificant compared to untreated control embryos. Moreover, the crude extract prepared from other plants parts such as roots, leaves and flowers of R. vesicarius were completely in active in term of antiangiogenic activity in transgenic zebrafish embryos.
Table 2.
Cytotoxicity of various plant parts of Rumex Vesicarius represented as half maximal inhibitory concentration (IC50) in human cancer cell lines evaluated by MTT cell proliferation assay.
| Cell line | Part of the plants used | Cytotoxicity* (IC 50) (µg/mL) |
||||
|---|---|---|---|---|---|---|
| n-hexane | Chloroform | Ethyl acetate | Methanol | Water | ||
| MCF7 | Roots | N.A | N.A | N.A | N.A | N.A |
| Stem | ≥300 | 33.45 ± 0.24 | 64.26 ± 0.33 | ≥300 | N.A | |
| Leaves | ≥300 | ≥500 | ≥500 | N.A | N.A | |
| flower | N.A | N.A | N.A | N.A | N.A | |
| Lovo | Roots | N.A | N.A | N.A | N.A | N.A |
| Stem | ≥300 | 35.90 ± 0.33 | 78.32 ± 0.21 | ≥300 | N.A | |
| Leaves | ≥300 | 164 ± 0.24 | ≥300 | ≥300 | NA | |
| flower | N.A | N.A | N.A | N.A | N.A | |
| Caco-2 | Roots | N.A | N.A | N.A | N.A | N.A |
| Stem | ≥300 | 45.22 ± 0.24 | 86.35 ± 0.01 | N.A | N.A | |
| Leaves | ≥300 | ≥300 | N.A | N.A | N.A | |
| flower | N.A | N.A | N.A | N.A | N.A | |
| HepG2 | Roots | N.A | N.A | N.A | N.A | N.A |
| Stem | ≥500 | 235.56 ± 0.02 | 159 ± 0.32 | ≥500 | N.A | |
| Leaves | ≥500 | ≥500 | ≥500 | N.A | N.A | |
| flower | N.A | N.A | N.A | N.A | N.A | |
The vales are average of triplicate experiments, N.A: Not active.
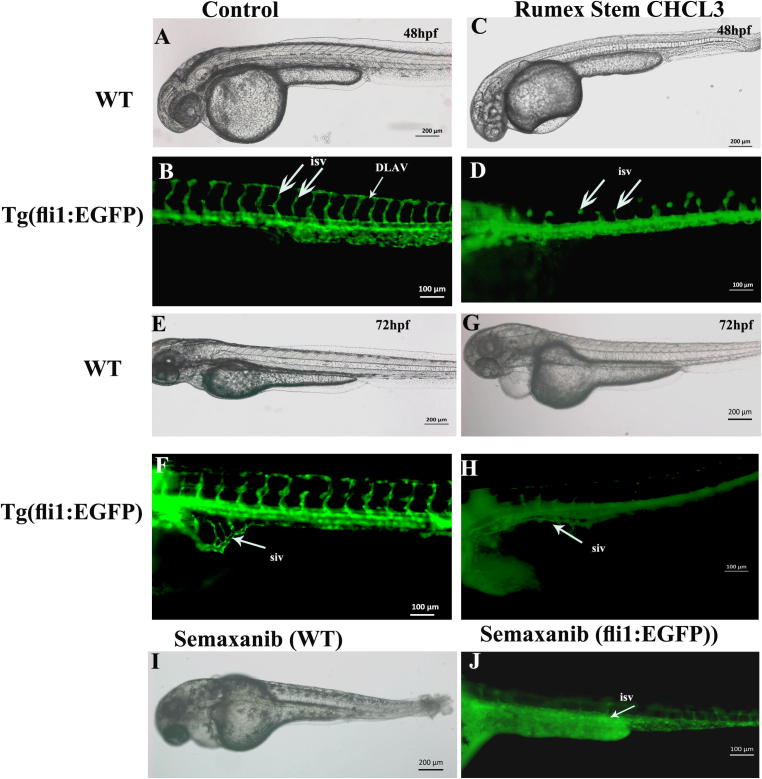
Fig. 1.
Rumex vesicarius inhibited the angiogenic blood vessels formation in Tg (fli1:EGFP) embryos. Representative microphotograph of live zebrafish embryos at 48 and 72hpf of embryonic development. A) Wild type control embryos at 48hpf shows normal embryonic development and growth. B) Control embryos from transgenic zebrafish line Tg (fli1:EGFP) at 48hpf. The green color indicate the vasculature in live zebrafish embryos. The inter-segmental blood vessels (isv) in the trunk of control embryos are shown by thick white arrows, the dorsal longitudinal anastomotic vessels (DLAV) which connect the “ isv” has been shown by thin white arrows. D) Zebrafish embryos treated with 30 µg/mL of the stem chloroform extract. The thick arrows point to the immature inter-segmental blood vessels, The “ isv” are either very thin, and did not grow to connect the DLAV. E) The control WT embryos at 72hpf, grow and developed normally. F) The blood vessels in control embryos also developed and second round of angiogenesis blood vessels which are sub-intestinal vein (siv) indicated by thin white arrow are present as basket of numerous vessels. G) WT zebrafish embryos treated with the chloroform extract of stem also developed to protruding mouth stage but exhibited cardiac edema due to defects in vasculature. H) The vasculature in Tg(fli1:EGFP) embryos treated with stem chloroform extract. It is evident that sub-intestinal vein did not form in these embryos, indicated by thin white arrow. I) WT zebrafish embryos treated with semaxanib (1 µM) at 72hp, exhibited severe malformation. The size of the embryos were much smaller as compared to control at the same developmental stage (E), tail degeneration is evident, severe cardiac edema. J) The vasculature of Tg(fli1:EGFP) embryos treated with semaxanib; inter-segmental blood vessels and sub intestinal vein did not form properly. Anterior is towards the left, scale bar with measurement is present in each image. Abbreviation: isv; (inter-segmental blood vessels), siv (sub-intestinal vein), DLAV (dorsal longitudinal anastomotic vessel), hpf; hours post fertilization.
Semaxanib (SU 5416) was used as positive control. As expected Semaxanib has shown potent antiangiogenic activity in zebrafish embryos. As shown in Fig. 1, the zebrafish embryos treated with Semaxanib (1 µM) exhibited malformation of the inter-segmental and sub-intestinal angiogenic blood vessels. However, Semaxanib treated embryos were smaller in size as compared to untreated control and also to embryos which were treated with various solvents extract of R. vesicarius, were unable to hatch, had blood pooling of blood in truck region, lacked pigmentation, and had abnormal craniofacial structure 1 µM concentration.
4.3. The stem crude extract of R. vesicarius affected the cell survival of human cancer cell lines
Four different human cancer cell lines were used in order to judge the anticancer activity of R. vesicarius. The cytotoxicity of roots, stem, leaves, and flowers of R. vesicarius is expressed by IC50 values which is presented in Table 2. It is quite apparent from the data in Table 2 that the crude extracts from roots, stem and leaves has shown cytotoxicity in tested cancer cells, but flower part was completely inactive. Among all the extracts, the chloroform extract of stem was most active with IC50 values of 33.45 ± 0.24, 35.90 ± 0.33, 45.22 ± 0.24, and 62.56 ± 0.02 µM in MCF7, Lovo, Caco-2 and HepG2 cells respectively. The second level of cytotoxicity was shown by ethyl acetate extract of stem with IC50 values of 64.26 ± 0.33, 78.32 ± 0.21, 86.35 ± 0.01, and 159 ± 0.32 µM in MCF7, Lovo, Caco-2 and HepG2 cells. The IC50 values of the crude extracts prepared from the roots, and leaves were above than 500 µM.
4.4. Identification of potential antiangiogenic molecule in the stem of R. vesicarius
Table 3 shows the GC–MS spectrum and the list of compounds with molecular weight, retention time, and percentage of the compound present in extract with molecule formula. The GC–MS spectrum showed thirty five peaks corresponding to different compounds. Most of the compounds are novel and were identified in the stem of R. vesicarius for the first time in this study. The “Propanoic acid, 2,3-bis[(trimethylsilyl)oxy]-, trimethylsilyl ester, Butanedioic acid, bis(trimethylsilyl) ester and Butane, 1,2,3-tris(trimethylsiloxy) were identified as major compounds.
Table 3.
GC–MS analysis of the stem of Rumex vesicarius.
| No | Retention time | PECENTAGE | Compound name | Formula | structure |
|---|---|---|---|---|---|
| 1 | 6.178 | 4.64 | Nicotinaldehyde thiosemicarbazone tritms | C16H32N4SSi3 | 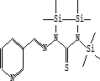 |
| 2 | 6.216 | 3.63 | 2-Oxovaleric acid, tert-butyldimethylsilyl ester | C11H22O3Si | 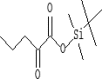 |
| 3 | 6.435 | 9.65 | Propanoic acid, 2-[(trimethylsilyl)oxy]-, trimethylsilyl ester | C9H22O3Si2 | 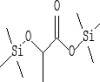 |
| 4 | 6.688 | 2.51 | Acetic acid, [(trimethylsilyl)oxy]-, trimethylsilyl ester | C8H20O3Si2 | 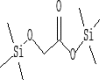 |
| 5 | 6.78 | 1.05 | Sulforidazine | C21H26N2O2S2 | 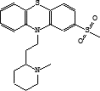 |
| 6 | 6.816 | 5.06 | 2-Cyclopenten-1-one, 2,3-dimethyl- | C7H10O | 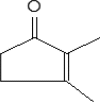 |
| 7 | 6.866 | 3.6 | D-Mannitol | C12H22O6 | 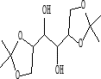 |
| 8 | 7.494 | 2.05 | Trimethylsilyl ether of glycerol | C12H32O3Si3 | 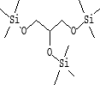 |
| 9 | 7.68 | 0.69 | 3,4-Dihydroxymandelic acid, ethyl ester, tri-TMS | C19H36O5Si3 | 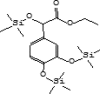 |
| 10 | 7.737 | 2.17 | Oxanilic acid, O,O'-bis(trimethylsilyl) | C14H23NO3Si2 | 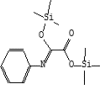 |
| 11 | 8.16 | 1.15 | Pentanoic acid, trimethylsilyl ester | C8H18O2Si | 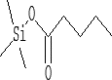 |
| 12 | 8.245 | 7.5 | Butane, 1,2,3-tris(trimethylsiloxy)- | C13H34O3Si3 | 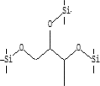 |
| 13 | 8.462 | 1.2 | Propanoic acid, | C10H24O3Si2 | 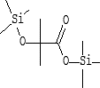 |
| 14 | 8.874 | 2.47 | Butanoic acid, | C13H32O4Si3 | 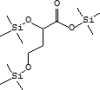 |
| 15 | 9.031 | 0.66 | 1H-Indole-2-carboxylic acid, 5-ethyl-1-(trimethylsilyl)-, trimethylsilyl ester | C17H27NO2Si2 | 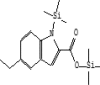 |
| 16 | 9.541 | 4.59 | Trimethylsilyl ether of glycerol | Trimethylsilyl ether of glycerol |  |
| 17 | 9.624 | 0.54 | Monoamidomalonic acid, tris(trimethylsilyl) | C12H29NO3Si3 |  |
| 18 | 10.013 | 1.37 | 3-Octenoic acid, trimethylsilyl ester | 3-Octenoic acid, trimethylsilyl ester |  |
| 19 | 10.06 | 0.71 | Isotridecanol- | C13H28O |  |
| 20 | 10.146 | 7.76 | Butanedioic acid, bis(trimethylsilyl) ester | C10H22O4Si2 | 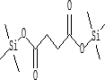 |
| 21 | 10.324 | 3.21 | Propanoic acid, 2,3-bis[(trimethylsilyl)oxy]-, trimethylsilyl ester | C12H30O4Si3 | 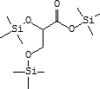 |
| 22 | 10.819 | 0.67 | Nonanoic acid, trimethylsilyl ester | C12H26O2Si | 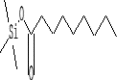 |
| 23 | 10.927 | 0.7 | 2,3-Dimethyl-3-hydroxyglutaric acid, tris(trimethylsilyl) | C16H36O5Si3 | 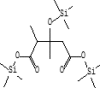 |
| 24 | 11.417 | 0.92 | Tetradecane | C14H30 | 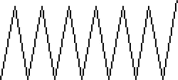 |
| 25 | 12.429 | 4.13 | Malic acid, O-(trimethylsilyl)-, bis(trimethylsilyl)ester | C13H30O5Si3 | 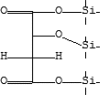 |
| 26 | 13.125 | 1.08 | 3-Octadecanone | C18H36O | 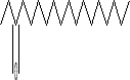 |
| 27 | 13.565 | 0.85 | 2-Hydroxyisocaproic acid, trimethylsilyl ester | C9H20O3Si | 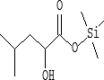 |
| 28 | 14.133 | 1.34 | D-Ribofuranose, 1,2,3,5-tetrakis-O-(trimethylsilyl)- | C17H42O5Si4 | 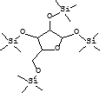 |
| 39 | 15.719 | 1.08 | 2-Deoxy-3,4,5-tris-O-(trimethylsilyl)pentose | C14H34O4Si3 | 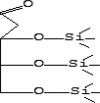 |
| 30 | 18.194 | 5.68 | D-Xylofuranose, | C17H42O5Si4 | 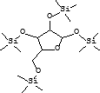 |
| 31 | 18.975 | 3.07 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | C20H40O |  |
| 32 | 19.099 | 5.59 | 2-Monopalmitin trimethylsilyl ether | C25H54O4Si2 |  |
| 33 | 19.22 | 1.47 | D-Galactose, 2,3,4,5,6-pentakis-O-(trimethylsilyl)- | C21H52O6Si5 |  |
| 34 | 23.974 | 4.69 | Hexadecanoic acid, ethyl ester | C18H36O2 |  |
| 35 | 29.919 | 2.52 | 9,12-Octadecadienoic acid, ethyl ester | C20H36O2 |  |
 | |||||
In order to find out the proteins target to which these molecule will bind, “Swiss target prediction” was used. Supplementary Tables 1–3 contain the list of all the possible protein targets for major compounds identified in the stem of R. vesicarius. Among all the proteins, Leucine-rich repeat serine/threonine protein kinase 2 (LRRK2), Adenosine receptors (A1, A2a, A2b, and A3), FK506 binding protein 1A (FKBP1A), Tyrosine-protein kinase JAK3, JAK1, and JAK2, Muscarinic acetylcholine receptor, Glycogen synthase kinase-3 beta and alpha, Vascular Endothelial receptor 2 (KDR). Most of target proteins have role in angiogenesis which has been summarized in Table 4.
Table 4.
Angiogenesis related protein targets as identified by online Swiss target prediction tool for the major compounds from crude stem extract of Rumex vesicarius.
| Identified compound | Protein targets | Common name | Uniport ID | Role in angiogenesis | Reference |
|---|---|---|---|---|---|
| Propanoic acid, 2-[(trimethylsilyl)oxy]-, trimethylsilyl ester | Leucine-rich repeat serine/threonine protein kinase 2 | LRRK2 | Q5S007 | LRRK2 inhibitor (BAY 43–9006) proved substantial activity against (VEGFR)-2, VEGFR-3 | (Wilhelm et al., 2004) |
| Adenosine A receptors | ADORA1 ADORA2A ADORA2B |
P30542 P29274 P29275 |
modulation of angiogenesis by all Adenosine receptors | (Clark et al., 2007) | |
| FK506-binding protein 1A | FKBP1A | P62942 | antiangiogenic activity by FKBPL and its peptide | Yakkundi et al., 2015) | |
| Muscarinic acetylcholine receptor M4 | CHRM4 | P08173 | “expressed by endothelial cells and are target fortherapeutic modulation of angiogenesis” | (Cooke and Ghebremariam, 2008) | |
| Vascular endothelial growth factor receptor 2 | KDR | P35968 | Endothelial cell migration and proliferation. | (Miettinen et al., 2012) | |
| Butanedioic acid, bis(trimethylsilyl) ester | c-Jun N-terminal kinase | MAPK8, MAPK10, MAPK9 | P45983 P53779 P45984 |
activation of MAPK signaling by VEGF | (Song and Finley, 2018) |
| Phosphodiesterase 5A | PDE5A | O76074 | PDE5A inhibitors stimulates angiogenesis | (Zhu et al., 2009) | |
| Serine/threonine protein phosphatase PP1-alpha catalytic subunit | PPP1CA | P62136 | Reverse Genetic Screen identify PP1CA as Novel Angiogenesis Targets | (Kalen et al., 2009) | |
| Butane, 1,2,3-tris(trimethylsiloxy) | Presenilin 1 Presenilin 2 |
PSEN1 PSEN2 | P49768 P49810 | Endothelial progenitor cells growth and differentiation | (Boulton et al., 2008) |
| Vascular endothelial growth factor receptor 2 | KDR | P35968 | Regulates endothelial migration and proliferation. | (Miettinen et al., 2012) | |
| Fibroblast growth factor receptor 1 | FGFR1 | P11362 | Roles in metastasis and angiogenesis of breast cancer. | (Chen et al., 2019) | |
5. Discussion
Angiogenesis intervention is considered as one of the target for anticancer therapy due to the concept that the tumor growth can be control if oxygen and nutrients supply to the tumor site could be reduced (Viallard and Larrivee, 2017).
Saudi Arabian flora is composed of over two thousand species comprising of more than one hundred forty two families which contain not only quite big number of indigenous species, but also have mixture of Asian, African and Mediterranean regions (Rahman et al., 2004). However, the antiangiogenic potential of Saudi medicinal plants is least explored, and hence efforts are needed to screen these plants through suitable antiangiogenic assays to find out novel antiangiogenic phytochemicals.
A bioactivity guided identification of antiangiogenic medicinal plants strategy was adopted in this study. Zebrafish embryos were used as bioactivity guided experimental animal model. Zebrafish transgenic line Tg (fli1:EGFP) which expresses enhanced green fluorescent protein (EGFP) in endothelial cells (blood vessels) provides an excellent in vivo animal model to screen angiogenic compounds (Butler et al., 2017, Raghunath et al., 2009).
The chloroform extract of stem of R. vesicarius suppressed the formation of angiogenic blood vessels in zebrafish embryos. The stem CHCl3 extract obstructed ≥70% of inter-somatic blood vessels (isv) and 100% of sub-intestinal vein (siv) blood vessels formation in treated embryos at 30 µg/ml. the R. vesicarius did not produce any embryonic abnormities or toxicity at this concentration and even at very high tested concentration of 700 µM in zebrafish embryos. This mean that inhibition of blood vessels as seen in zebrafish embryos by R. vesicarius is the primary biological activity and it is not due to the secondary effect by toxicity.
Semaxanib (SU 5416) which is an inhibitor of the vascular endothelial growth factor receptor (Fong et al., 1999, Haspel et al., 2002, Mendel et al., 2000) was used as positive control. However, Semaxanib induced severe abnormalities and toxicity in zebrafish embryos besides inhibiting the angiogenic blood vessels. These abnormalities were shortened bodies, enlarge cardiac edema, cardiac hypertrophy, pooling of blood cells (hemorrhages), and craniofacial cartilage deformities, at ≤1 µM concentration and more than 70% mortalities at 5 µM or more concentration.
The herbs based antiangiogenic phytochemicals include, “Artemisinin“, Viscum album (Harmsma et al., 2004), Curcuma longa (Arbiser et al., 1998), Scutellaria baicalensis (Chinese Skullcap) (Liu et al., 2003), Resveratrol and Proanthocyanidin (Grape Seed Extract) (Cao et al., 2005). As the phytochemicals and botanical crude extracts have proven their efficacy as natural antiangiogenic agents with minimum toxicities (Dhillon et al., 2008, Ishikawa et al., 2006), there is urgent need to explore new medicinal plants in order to identify and isolate novel antiangiogenic compounds.
The chloroform extract of stem part was subjected to phytochemical analysis using GC–MS in order to identify antiangiogenic compounds. The major compounds which were identified were 2-[(trimethylsilyl)oxy]-, trimethylsilyl ester, Butane, 1,2,3-tris(trimethylsiloxy), and Butanedioic acid, bis(trimethylsilyl) ester. The online Swiss target prediction tool was used to find out the protein target for these molecules. Interestingly, the target proteins were all angiogenesis regulator proteins and were reported to be involve in normal or pathological angiogenesis. The predicted proteins target for the major compounds and their relationship to angiogenesis are shown in Table 4. These bioactive molecules need to be isolated in pure form from R. vesicarius and then should be tested in suitable antiangiogenic assays, however, the antiangiogenic activity of stem of R. vesicarius as observed in this study, could be due to the synergetic effect of bioactive molecules which are present in the crude methanol extract of stem.
The MTT cell proliferation assays has revealed that the crude extracts from the roots, leaves, and flowers did not show cytotoxicity towards tested cancer cells lines. Similarly the methanol, hexane extract prepared from the stem part of the plant showed weaker cytotoxicity and with IC50 values were more than 300 µM. The chloroform and ethyl acetate extract from the stem part R. vesicarius has induced significant level of cytotoxicity in MCF7, HepG2, Lovo, and Caco-2 cell lines with IC50 values less than 50 µM. The anticancer activity of R. vesicarius in various human cancer cell lines has also been reported by other studies. The methanol extract prepared from the R. vesicarius inhibited the proliferation of CCRF-CEM (human T lymphoblastoid) cells, with IC50 value of 37.13 μg/mL (Kuete et al., 2013), whole plant methanol extract showed cytotoxicity against HepG2 cells (human hepatocellular carcinoma) with IC 50 values 563.33 ± 0.8. An Ethyl acetate extract of R. vesicarius prepared from leaves has shown cytotoxicity in HT-29 (human colorectal carcinoma cell line) and PC-3 (human prostate cancer) with IC50 values 54.81 ± 0.8405 70.90 ± 1.3080 respectively (Manure, 2017). The anticancer profile of various parts of R. vesicarius in” human breast carcinoma cell line (MDA-MB-231)” have been previously reported by us (Nasr et al., 2018). The IC 50 values of chloroform and ethyl acetate extract prepared from the stem of R. vesicarius in this study, greatly varies with published results. It could be due to the climatic differences from where the plant has been collected as the chemical nature of soil and nutrients greatly affect the biological activity of plants. Moreover, the anticancer activity of this plant has never been reported in MCF7, Caco-2 and lovo. The LC50 values in Caco-2 and Lovo which are derived from human colon cancer are closely related to anticancer activity of R. vesicarius in HT-29 cells which are also derived from human colon cancer. The polarity of the solvent used to prepare the extract also contribute a lot for the variation in biological activity.
Zebrafish screening assays also provided an insight into the development toxicity of R. vesicarius in animal system. The acute and chronic toxicities studies have reported that R. vesicarius was safe to be use in experimental animals (Ganaie et al., 2015, Subramaniyan et al., 2018). However, the effect of R. vesicarius on embryonic development has never been tested before in pregnant animals. The zebrafish screening assays done in this study has revealed that the crude extracts prepared from various parts of R. vesicarius did not induce teratogenicity or toxicity in developing zebrafish embryos even at very high concentration. The effect of R. vesicarius on fetus development need to be tested in mammalian animal models but the zebrafish developmental toxicity screening results from this study suggest, it could be a safer choice of antiangiogenic medication in pregnant cancer patients as well.
6. Conclusion
The crude methanol extract of stem of R. vesicarius has shown significant level of antiangiogenic activity, while being nontoxic to zebrafish embryos. Novel bioactive molecules, which target antiangiogenic regulating proteins have been identified in the stem of R. vesicarius. The chloroform extract of stem was also cytotoxic to human breast, colon and liver carcinoma cells lines.
Based on the results from this study, it is recommended that formulation prepared from Rumex vesicariusm L. could further be tested in clinical trials to evaluate its therapeutic potential as an effective and safe anticancer agent and remedy to treat diabetic retinopathy.
Funding
The authors would like to acknowledge the support of the National Plan for Science, Technology, and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia through Award number 11-BIO1871-02. This support is highly appreciated and acknowledged.
Declaration of Competing Interest
No conflict of interest to be declared.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2019.11.042.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Abdel-Qadir H., Ethier J.L., Lee D.S., Thavendiranathan P., Amir E. Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: a systematic review and meta-analysis. Cancer Treat Rev. 2017:53120–53127. doi: 10.1016/j.ctrv.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Al-Abd A.M., Alamoudi A.J., Abdel-Naim A.B., Neamatallah T.A., Ashour O.M. Anti-angiogenic agents for the treatment of solid tumors: potential pathways, therapy and current strategies – a review. J. Adv. Res. 2017;8(6):591–605. doi: 10.1016/j.jare.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbiser J.L., Klauber N., Rohan R., van Leeuwen R., Huang M.T., Fisher C., Flynn E., Byers H.R. Curcumin is an in vivo inhibitor of angiogenesis. Mol. Med. 1998;4(6):376–383. [PMC free article] [PubMed] [Google Scholar]
- Boulton M.E., Cai J., Grant M.B. Gamma-secretase: a multifaceted regulator of angiogenesis. J. Cell Mol. Med. 2008;12(3):781–795. doi: 10.1111/j.1582-4934.2008.00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler C.T., Reynolds A.L., Tosetto M., Dillon E.T., Guiry P.J., Cagney G., O'Sullivan J., Kennedy B.N. A quininib analogue and cysteinyl leukotriene receptor antagonist inhibits vascular endothelial growth factor (VEGF)-independent angiogenesis and exerts an additive antiangiogenic response with bevacizumab. J. Biol. Chem. 2017;292(9):3552–3567. doi: 10.1074/jbc.M116.747766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y. Future options of anti-angiogenic cancer therapy. Chin. J. Cancer. 2016;3521 doi: 10.1186/s40880-016-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Fu Z.D., Wang F., Liu H.Y., Han R. Anti-angiogenic activity of resveratrol, a natural compound from medicinal plants. J. Asian Nat. Prod. Res. 2005;7(3):205–213. doi: 10.1080/10286020410001690190. [DOI] [PubMed] [Google Scholar]
- Chen Z., Tong L.J., Tang B.Y., Liu H.Y., Wang X., Zhang T., Cao X.W., Chen Y., Li H.L., Qian X.H., Xu Y.F., Xie H., Ding J. C11, a novel fibroblast growth factor receptor 1 (FGFR1) inhibitor, suppresses breast cancer metastasis and angiogenesis. Acta Pharmacol. Sin. 2019;40(6):823–832. doi: 10.1038/s41401-018-0191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A.N., Youkey R., Liu X., Jia L., Blatt R., Day Y.J., Sullivan G.W., Linden J., Tucker A.L. A1 adenosine receptor activation promotes angiogenesis and release of VEGF from monocytes. Circ. Res. 2007;101(11):1130–1138. doi: 10.1161/CIRCRESAHA.107.150110. [DOI] [PubMed] [Google Scholar]
- Cooke J.P., Ghebremariam Y.T. Endothelial nicotinic acetylcholine receptors and angiogenesis. Trends Cardiovasc. Med. 2008;18(7):247–253. doi: 10.1016/j.tcm.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon N., Aggarwal B.B., Newman R.A., Wolff R.A., Kunnumakkara A.B., Abbruzzese J.L., Ng C.S., Badmaev V., Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008;14(14):4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- Erices J.I., Torres A., Niechi I., Bernales I., Quezada C. Current natural therapies in the treatment against glioblastoma. Phytother. Res. 2018;32(11):2191–2201. doi: 10.1002/ptr.6170. [DOI] [PubMed] [Google Scholar]
- Farooq M., Al Marhoon Z.M., Abu Taha N., Baabbad A.A., Al-Wadaan M.A., El-Faham A. Synthesis of novel class of N-alkyl-isatin-3-iminobenzoic acid derivatives and their biological activity in zebrafish embryos and human cancer cell lines. Biol. Pharm. Bull. 2018;41(3):350–359. doi: 10.1248/bpb.b17-00674. [DOI] [PubMed] [Google Scholar]
- Farooq M., Sharma A., Almarhoon Z., Al-Dhfyan A., El-Faham A., Taha N.A., Wadaan M.A.M., Torre B.G., Albericio F. Design and synthesis of mono-and di-pyrazolyl-s-triazine derivatives, their anticancer profile in human cancer cell lines, and in vivo toxicity in zebrafish embryos. Bioorg. Chem. 2019:87457–87464. doi: 10.1016/j.bioorg.2019.03.063. [DOI] [PubMed] [Google Scholar]
- Fong T.A., Shawver L.K., Sun L., Tang C., App H., Powell T.J., Kim Y.H., Schreck R., Wang X., Risau W., Ullrich A., Hirth K.P., McMahon G. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999;59(1):99–106. [PubMed] [Google Scholar]
- Ganaie M.A., Khan T.H., Siddiqui N.A., Ansari M.N. Ameliorative effect of methanol extract of Rumex vesicarius on CCl4-induced liver damage in Wistar albino rats. Pharm. Biol. 2015;53(8):1163–1167. doi: 10.3109/13880209.2014.967782. [DOI] [PubMed] [Google Scholar]
- Gezici S., Sekeroglu N. Current perspectives in the application of medicinal plants against cancer: novel therapeutic agents. Anticancer Agents Med. Chem. 2019;19(1):101–111. doi: 10.2174/1871520619666181224121004. [DOI] [PubMed] [Google Scholar]
- Gfeller D., Michielin O., Zoete V. Shaping the interaction landscape of bioactive molecules. Bioinformatics. 2013;29(23):3073–3079. doi: 10.1093/bioinformatics/btt540. [DOI] [PubMed] [Google Scholar]
- Harley R.M. Flora of Eastern Saudi-Arabia – Mandaville, Jp. Tls-Times Lit. 1991 Suppl (4598) 20–20. [Google Scholar]
- Harmsma M., Gromme M., Ummelen M., Dignef W., Tusenius K.J., Ramaekers F.C. Differential effects of Viscum album extract IscadorQu on cell cycle progression and apoptosis in cancer cells. Int. J. Oncol. 2004;25(6):1521–1529. [PubMed] [Google Scholar]
- Haspel H.C., Scicli G.M., McMahon G., Scicli A.G. Inhibition of vascular endothelial growth factor-associated tyrosine kinase activity with SU5416 blocks sprouting in the microvascular endothelial cell spheroid model of angiogenesis. Microvasc. Res. 2002;63(3):304–315. doi: 10.1006/mvre.2001.2383. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Saeki T., Otani T., Suzuki T., Shimozuma K., Nishino H., Fukuda S., Morimoto K. Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. J. Nutr. 2006;136(3 Suppl):816S–820S. doi: 10.1093/jn/136.3.816S. [DOI] [PubMed] [Google Scholar]
- Manure J.Y., Naikwade N.S. Evaluation of anticancer activity of leaves of Rumex vesicarius Linn and Symplocos racemosa Roxb. by brine shrimp lethality and (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) methods. Int. J. Green Pharm. 2017;11:(4)|S742. [Google Scholar]
- Kalen M., Wallgard E., Asker N., Nasevicius A., Athley E., Billgren E., Larson J.D., Wadman S.A., Norseng E., Clark K.J., He L., Karlsson-Lindahl L., Hager A.K., Weber H., Augustin H., Samuelsson T., Kemmet C.K., Utesch C.M., Essner J.J., Hackett P.B., Hellstrom M. Combination of reverse and chemical genetic screens reveals angiogenesis inhibitors and targets. Chem. Biol. 2009;16(4):432–441. doi: 10.1016/j.chembiol.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid, El Bairi, Meghawry E.L., Kenawy Ayman E.L., Rahman Heshu, Abdelkarim Guaadaoui, Najda Agnieszka. Natural products against cancer angiogenesis. Tumor Biol. 2016;37(11):14513–14536. doi: 10.1007/s13277-016-5364-8. [DOI] [PubMed] [Google Scholar]
- Kuete V., Wiench B., Alsaid M.S., Alyahya M.A., Fankam A.G., Shahat A.A., Efferth T. Cytotoxicity, mode of action and antibacterial activities of selected Saudi Arabian medicinal plants. BMC Complement Altern. Med. 2013 doi: 10.1186/1472-6882-13-354. 13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson N.D., Weinstein B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 2002;248(2):307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- Liu J.J., Huang T.S., Cheng W.F., Lu F.J. Baicalein and baicalin are potent inhibitors of angiogenesis: inhibition of endothelial cell proliferation, migration and differentiation. Int. J. Cancer. 2003;106(4):559–565. doi: 10.1002/ijc.11267. [DOI] [PubMed] [Google Scholar]
- Lu V.M., Ravindran K., Graffeo C.S., Perry A., Van Gompel J.J., Daniels D.J., Link M.J. Efficacy and safety of bevacizumab for vestibular schwannoma in neurofibromatosis type 2: a systematic review and meta-analysis of treatment outcomes. J. Neurooncol. 2019;144(2):239–248. doi: 10.1007/s11060-019-03234-8. [DOI] [PubMed] [Google Scholar]
- Medina M.A., Munoz-Chapuli R., Quesada A.R. Challenges of antiangiogenic cancer therapy: trials and errors, and renewed hope. J. Cell Mol. Med. 2007;11(3):374–382. doi: 10.1111/j.1582-4934.2007.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel D.B., Schreck R.E., West D.C., Li G., Strawn L.M., Tanciongco S.S., Vasile S., Shawver L.K., Cherrington J.M. The angiogenesis inhibitor SU5416 has long-lasting effects on vascular endothelial growth factor receptor phosphorylation and function. Clin. Cancer Res. 2000;6(12):4848–4858. [PubMed] [Google Scholar]
- Miettinen M., Rikala M.S., Rys J., Lasota J., Wang Z.F. Vascular endothelial growth factor receptor 2 as a marker for malignant vascular tumors and mesothelioma: an immunohistochemical study of 262 vascular endothelial and 1640 nonvascular tumors. Am. J. Surg. Pathol. 2012;36(4):629–639. doi: 10.1097/PAS.0b013e318243555b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasr Fa.hd.A., Abutaha Nael, Al-Zahrani Mohammad, Farooq Muhammad, Wadaan Mohammad A. Anticancer potential of plant extracts from riyadh (Saudi Arabia) on Mda-Mb-231 breast cancer cells. Afr. J. Traditional, Complem. Alternat. Med. 2018;15(4):46–53. [Google Scholar]
- Qin S., Li A., Yi M., Yu S., Zhang M., Wu K. Recent advances on anti-angiogenesis receptor tyrosine kinase inhibitors in cancer therapy. J. Hematol. Oncol. 2019;12(1):27. doi: 10.1186/s13045-019-0718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R.H.D, undefined 1952. Probit Analysis. By D. J. Finney, M.A., Sc.D., [2nd ed. Pp. xiv + 318. Cambridge University Press, 1952. 35s.]. J. Inst. Actuaries 78(3), pp. 388–390.
- Raghunath M., Wong Y.S., Farooq M., Ge R. Pharmacologically induced angiogenesis in transgenic zebrafish. Biochem. Biophys. Res. Co. 2009;378(4):766–771. doi: 10.1016/j.bbrc.2008.11.127. [DOI] [PubMed] [Google Scholar]
- Rahman M.A., Mossa J.S., Al-Said M.S., Al-Yahya M.A. Medicinal plant diversity in the flora of Saudi Arabia 1: a report on seven plant families. Fitoterapia. 2004;75(2):149–161. doi: 10.1016/j.fitote.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Song M., Finley S.D. Mechanistic insight into activation of MAPK signaling by pro-angiogenic factors. BMC Syst. Biol. 2018;12(1):145. doi: 10.1186/s12918-018-0668-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Wang H., Pan Y., Liu T. Investigating the multi-target pharmacological mechanism of hedyotis diffusa willd acting on prostate cancer: a network pharmacology approach. Biomolecules. 2019;9(10) doi: 10.3390/biom9100591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniyan V., Shaik S., Bag A., Manavalan G., Chandiran S. Potential action of Rumex vesicarius (L.) against potassium dichromate and gentamicin induced nephrotoxicity in experimental rats. Pak. J. Pharm. Sci. 2018;31(2):509–516. [PubMed] [Google Scholar]
- Vasas A., Orban-Gyapai O., Hohmann J. The genus rumex: review of traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2015:175198–175228. doi: 10.1016/j.jep.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Viallard C., Larrivee B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20(4):409–426. doi: 10.1007/s10456-017-9562-9. [DOI] [PubMed] [Google Scholar]
- Wang Zongwei, Dabrosin Charlotta, Yin Xin, Fuster Mark M., Alexandra Arreola W., Rathmell Kimryn, Generali Daniele, Nagaraju Ganji P., El-Rayes Bassel, Ribatti Domenico, Chen Yi Charlie, Honoki Kanya, Fujii Hiromasa, Georgakilas Alexandros G., Nowsheen Somaira, Amedei Amedeo, Niccolai Elena, Amr Amin S., Ashraf Salman, Helferich Bill, Yang Xujuan, Guha Gunjan, Bhakta Dipita, Ciriolo Maria Rosa, Aquilano Katia, Chen Sophie, Halicka Dorota, Mohammed Sulma I., Azmi Asfar S., Alan Bilsland W., Keith Nicol, Jensen Lasse D. Broad targeting of angiogenesis for cancer prevention and therapy. Seminars Cancer Biol. 2015;35:S224–S243. doi: 10.1016/j.semcancer.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S.M., Carter C., Tang L., Wilkie D., McNabola A., Rong H., Chen C., Zhang X., Vincent P., McHugh M., Cao Y., Shujath J., Gawlak S., Eveleigh D., Rowley B., Liu L., Adnane L., Lynch M., Auclair D., Taylor I., Gedrich R., Voznesensky A., Riedl B., Post L.E., Bollag G., Trail P.A. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- Yakkundi A., Bennett R., Hernandez-Negrete I., Delalande J.M., Hanna M., Lyubomska O., Arthur K., Short A., McKeen H., Nelson L., McCrudden C.M., McNally R., McClements L., McCarthy H.O., Burns A.J., Bicknell R., Kissenpfennig A., Robson T. FKBPL is a critical antiangiogenic regulator of developmental and pathological angiogenesis. Arterioscler Thromb Vasc. Biol. 2015;35(4):845–854. doi: 10.1161/ATVBAHA.114.304539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B., Zhang L., Alexeyev M., Alvarez D.F., Strada S.J., Stevens T. Type 5 phosphodiesterase expression is a critical determinant of the endothelial cell angiogenic phenotype. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;296(2):L220–L228. doi: 10.1152/ajplung.90474.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.