Abstract
Complement is a key first line innate host defense system in the blood of vertebrates. Upon activation, this powerful defense mechanism can elicit inflammatory responses, lyse non-self-cells, or mark them for opsonophagocytic removal. Blood-feeding arthropods thus require the ability to block host complement activation in the bloodmeal to prevent undesired cell or tissue damage during feeding. The soft tick Ornithodoros moubata produces a complement inhibitory protein, OmCI. This protein binds to a mammalian complement protein C5 and blocks further activation of complement cascades, which results in the prevention of complement-mediated bacterial killing through membrane attack complex. Interestingly, the amino acids involved in OmCI binding are highly conserved among mammalian and avian C5, but the ability of this protein to inhibit the complement from birds remains unclear. Here we demonstrated that OmCI is capable of preventing quail complement-mediated erythrocyte lysis, inhibiting the capability of this animal’s complement to eliminate a serum-sensitive Lyme disease bacterial strain. We also found that the ability of OmCI to inhibit quail complement-mediated killing of Lyme disease bacteria can be extended to different domestic and wild birds. Our results illustrate the utility of OmCI to block bird complement. These results provide the foundation for further use of this protein as a tool to study the molecular basis of avian complement and pathogen evasion to such a defense mechanism.
Keywords: Bacterial killing, Lyme borreliae, OmCI, Avian complement
1. Introduction
The complement system is composed of a group of proteins, that function as the first line of host defense in the blood of vertebrates in response to invading pathogens (Meri, 2016; Trouw et al., 2017; Zipfel and Skerka, 2009). Complement is activated on the surface of the pathogen by three pathways: the classical, lectin, and alternative pathways (Meri, 2016; Trouw et al., 2017; Zipfel and Skerka, 2009). Activation of the classical pathway (initiated by antibody-bacterial antigen complexes) and the lectin pathway (initiated by lectin-microbial carbohydrate complexes) results in the formation of C3 convertase, C4b2a. Activation of the alternative pathway (initiated by the interaction of C3b with the microbial surface) triggers the formation of C3 convertase, C3bBb. These C3 convertases recruit the complement protein C3b; addition of a C3b molecule to C3 convertases results in formation of the C5 convertases, C4b2a3b, and C3bBb3b. The C5 convertases then cleave C5 to C5a and C5b to release the anaphylatoxin C5a that attracts neutrophils, monocytes, and mast cells to induce inflammatory responses. C5b assembles with C6, C7, C8, and C9, resulting in the formation of a pore-forming membrane attack complex (MAC or C5b-9) on the pathogen surfaces to cause lysis.
When complement is not properly controlled, those complement complexes can be formed on the surface of host cells, leading to tissue destruction (Sjoberg et al., 2009). Numerous complement inhibitory molecules including synthetic compounds, monoclonal antibodies, and prokaryotic or eukaryotic proteins inhibit different steps of the complement cascades (Morgan and Harris, 2015; Ricklin et al., 2016). Some of these molecules have been used or are under development as therapeutics to treat diseases caused by unregulated complement activation (Morgan and Harris, 2015; Ricklin et al., 2016). However, because of variation of complement proteins across different hosts, most of the complement inhibitory molecules developed to target human complement are human- or primate-specific (Jore et al., 2016; Kai et al., 1983). With the exception of mice, the investigation of complement-associated mechanisms in non-mammalian hosts is very limited.
One such complement inhibitory molecule is OmCI (also known as conversin), a 17-kDa protein that was initially isolated from the saliva of a soft tick species, Ornithodoros moubata (Nunn et al., 2005). This protein is believed to protect tick cells/tissues from destruction by host complement attack during blood feeding and promote survival of the pathogens carried by ticks at the stage of transmission (Fredslund et al., 2008). OmCI binds to C5 and prevents its cleavage by C5 convertases (Fredslund et al., 2008; Hepburn et al., 2007; Jore et al., 2016), which renders tissue damage or pathogen killing by complement of mice, rats, guinea pigs, pigs, and humans (Barratt-Due et al., 2013; Barratt-Due et al., 2011; Garcia et al., 2013; Hepburn et al., 2007; Jore et al., 2016; Nunn et al., 2005). Therefore, OmCI is currently under clinical trials for its use in human complement inhibitory therapy and has been commonly utilized to investigate the C5-mediated complement activation in mammalian hosts (Barratt-Due et al., 2013; Berends et al., 2015; Blom et al., 2014; Fluiter et al., 2014; Garcia et al., 2013; Kuhn et al., 2016; Macpherson et al., 2018; Pischke et al., 2017). Interestingly, the residues in human C5 involved in OmCI binding bear close identity to C5 from avian and mammalian hosts (Jore et al., 2016). This finding raises the possibility that OmCI can inhibit the complement from non-mammalian hosts such as Aves. In this study, we tested the ability of OmCI to inhibit avian complement-mediated hemolysis and have used the Lyme disease bacterium as an example to study the consequences of blocking avian-mediated bacterial killing.
2. Material and Methods
2.1. Birds and bacterial strains
American robins (Turdus migratorius) and Gray catbirds (Dumetella carolinensis) were mist netted on Block Island, RI from June-August, 2016. Serum was collected from 14 birds (n = 6 robins; n = 8 catbirds) using BD Microtainer Capillary Blood Collector tubes (Fisher Scientific, Hampton, NH). The Borrelia burgdorferi sensu stricto (B. burgdorferi), Borrelia duttonii, and Escherichia coli strains used in this study are described in Table S1. The E. coli strain BL21 (DE3) was grown in Luria-Bertani broth or agar (BD Bioscience, Franklin lakes, NJ), supplemented with antibiotics as described previously (Nazarova and Avaeva, 1973). All B. burgdorferi and B. duttonii strains were grown in BSK-II completed medium with no antibiotics (Barbour, 1984).
2.2. The production of OmCI.
The recombinant OmCI protein was purified in a similar manner as previously described (Blom et al., 2014). Briefly, the open reading frames lacking the putative signal sequences of omcI with TEC-His6 sequences were codon optimized and synthetized (gift of Dr. Strömberg, SOBI, Sweden) and cloned into pET16b vector (Novagen, Merck). The resulting plasmid was transformed into E. coli strain BL21 (DE3), which was then induced with IPTG for 3h at 30°C. The harvested cells were then suspended into PBS buffer containing lysozyme and then lysed by sonication. Subsequently, the inclusion bodies were isolated by centrifugation and then solubilized in 6M guanidine hydrochloride in 20 mM Tris-HCl, pH 8.0 and 10 mM reduced glutathione. The protein was then dialyzed overnight against 20 mM Tris-HCl pH 8.0. The refolded OmCI was then applied to Ni-NTA column (Qiagen) and eluted using 20 mM Tris, pH 7 with 700 mM imidazole. The pooled fractions with OmCI were then dialyzed against 50 mM Tris-HCl at pH 8.0 with 150 mM NaCl, and stored at −70 °C.
2.3. Three-dimensional structure modeling of quail C5d
The structure of C5d from Coturnix quail was modelled according to the crystal structure of Human C5d (PDB ID: 5I5K) using the Swiss-Model protein modelling server as described (Arnold et al., 2006). The structure of C5d from Coturnix quail is considered as a high-quality model as the amino acid identity of C5d from human and quail is 55.54 %.
2.4. Hemolytic assays.
The procedure for hemolytic assays was modified from a previously described protocol (Jore et al., 2016). Sheep red blood cells previously sensitized by incubating with anti-sheep red blood cell stroma antibody in GVB2+ buffer (142 mM NaCl, 50 mM Sodium 5,5-diethylbarbiturate, 0.1 % gelatin, 0.15 mM CaCl2, 0.5 mM MgCl2, pH 7.35) were obtained from Diamedix (Miami Lakes, FL). Next, the cell suspension was incubated with 5 % of serum from humans (Complement technology, Inc, Tyler, TX) or Coturnix quail (Canola poultry market, Brooklyn, NY) as well as different concentrations of OmCI (2 × 10−11 to 1.6 × 10−6 M) at 37 °C for 1 h. Subsequently, 100 μl of PBS was added, and the supernatant collected by centrifugation was transferred to a 96-well ELISA plate. Plates were read at 405 nm using a Tecan Sunrise Microplate reader. The supernatant from the cells with GVB2+ buffer instead of serum was included as control (no lysis). The supernatant from the cells suspended in water was a positive control used for normalization (100 % lysis). To determine the concentration of OmCI that inhibits 50 % levels of erythrocyte lysis (IC50) in the presence of serum, the data points were fitted using nonlinear regression methods by GraphPad Prism software (Version 7, La Jolla, CA).
2.5. Serum resistance assays.
Serum resistance of B. burgdorferi strains B313 and B31-5A4 and B. duttonii strains V and LA1 was determined as described previously (Hart et al., 2018; Marcinkiewicz et al., 2019). Briefly, the mid-log phase of B. burgdorferi or B. duttonii strains were cultivated in triplicate. The resulting spirochete culture was diluted to a final concentration of 5×106 bacteria per milliliter into BSKII medium without rabbit serum. The cell suspensions were then mixed with 40 % of the normal serum from human, Coturnix (quail), Gallus gallus (chickens) (Biowest, Riverside, MO), Anser anser (geese) (BioIVT, Hicksville, NY), Meleagris gallopavo (turkey) (BioIVT), Turdus migratorius (American robins) or Dumetella carolinensis (Gray catbirds) in the presence or absence of OmCI. We also included spirochetes mixed with 40 % heat-inactivated (55 °C for 2 h) serum from these animals as negative controls for complement-mediated killing. OmCI was used from 0.07 to 6.66 μM for human and quail serum and at 2 μM for sera from chickens, geese, turkey, American robins, or Gray catbirds. Note that the concentrations of OmCI were greater in this assay, compared to that in hemolytic assays described in section 2.4 because the amount of sera applied to this assay (40 % of sera) is higher than that in the hemolytic assays (5 %). At 0 and 4 h after the incubation with serum, an aliquot was taken from each condition and counted using a Petroff-Hausser counting chamber (Hausser Scientific, Horsham, PA) and a Nikon Eclipse E600 darkfield microscope (Nikon, Melville, NY). We determined the percentage of surviving spirochetes by two independent approaches, motile spirochete measuring and Live/Dead staining. For motile spirochete measuring, the number of motile spirochetes at 0 and 4 h post incubation with sera was counted under microscopy as described (Hart et al., 2018). For Live/Dead staining, spirochetes immediately after mixed with sera or incubated with sera for 4h were treated for 15 min with 1× SYBR Green I (ThermoFisher) and 6 μM of propidium iodide (ThermoFisher) in 0.5 % BSA in PBS as described (Feng et al., 2014; Marcinkiewicz et al., 2019). The live (green) and dead (red) spirochetes were visualized by overlaid FITC and Texas Red filters using an Olympus BX51 fluorescence microscope (Olympus Corporation, Waltham, MA). We then calculated the number of live spirochetes at 4 h post incubation normalized to that immediately after incubation with serum. Additionally, we determined the concentration of OmCI required to inhibit killing by human or quail serum via 50 % (IC50) fitting the data points using nonlinear regression methods from GraphPad Prism 7.0 software (GraphPad Software, Inc., San Diego, CA).
2.6. Statistical analysis.
Significant differences between samples were determined using a one-way ANOVA with post hoc Dunn’s test using GraphPad Prism 7.0 Software. P-values were determined for each sample. A P-value < 0.05 (*) was considered to be significant.
3. Results
3.1. OmCI inhibits the ability of quail complement to lyse red blood cells.
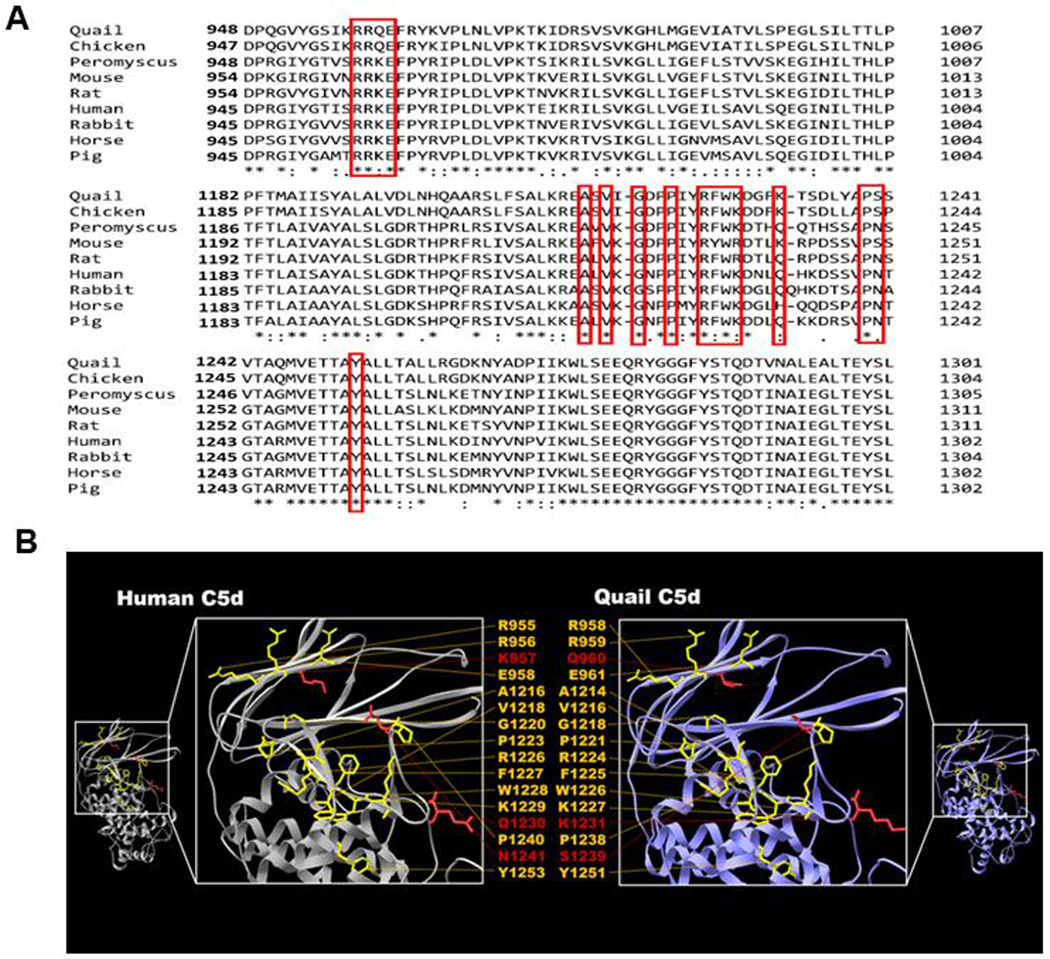
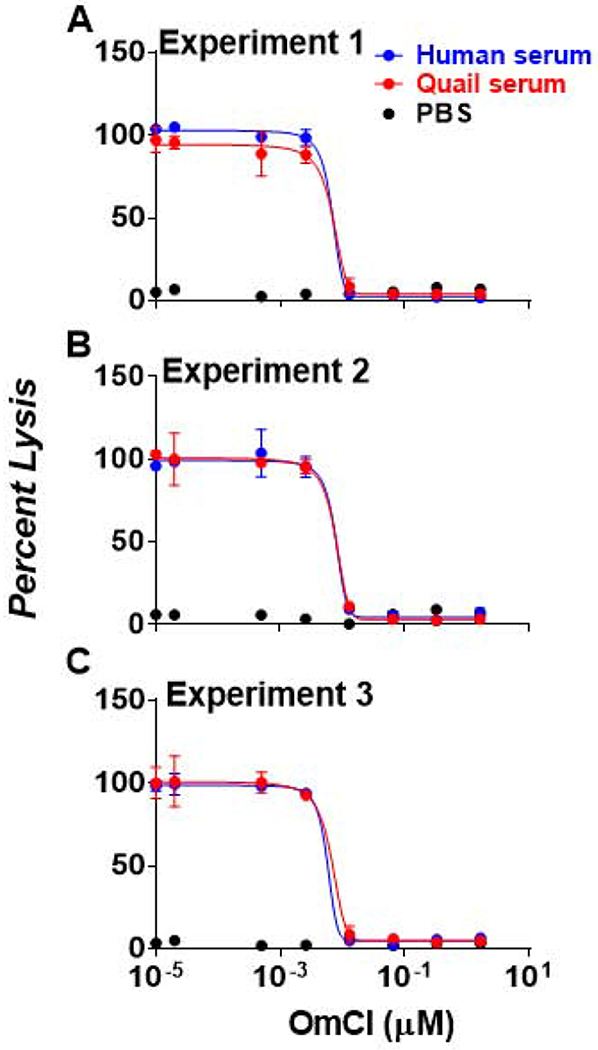
The high resolution structure of OmCI with human C5 indicates the amino acids on C5d fragment involved in the binding to OmCI, and these residues are conserved across different mammalian species (Fig. 1A) (Jore et al., 2016). We observed that these amino acids are also highly conserved in C5 from multiple avian hosts including quail and chicken (Fig. 1A). Similarly, when building the tertiary structure modelling of quail C5d using human C5d as a template, the topography of the amino acids from human C5d critical for OmCI-binding is nearly identical to that of the corresponding residues from quail C5d (Fig. 1B). These results raise the possibility that OmCI may inhibit avian complement. We thus used quail serum as a model to evaluate this protein’s ability to inhibit quail complement-mediated erythrocyte lysis. Antibody-sensitized sheep erythrocytes were incubated with sera from quail (or humans, as a positive control) containing different concentrations of recombinant OmCI. In the absence of OmCI, close to 100 % of the erythrocytes incubated with human serum were lysed whereas red blood cells in PBS buffer (control) displayed undetectable lysis (Fig. 2). The addition of OmCI to serum reduced the percent lysis of those cells in a dose dependent manner (IC50= 6.80 ± 1.07 nM) (Fig. 2), consistent with the ability of OmCI to inhibit human complement (Nunn et al., 2005). Quail serum added to erythrocytes in the absence of OmCI resulted in more than 90 % lysis (Fig. 2). However, OmCI inhibited sheep red blood cell lysis in a dose-dependent manner, suggesting that this protein inhibits quail complement (IC50 = 7.15 ± 0.61 nM) (Fig. 2).
Figure 1. Comparison of primary and tertiary structure of OmCI-binding site on C5 among avian and mammalian hosts.
(A) Shown is the alignment of partial amino acid sequences of C5 from Peromyscus leucopus mouse (white-footed mouse), Mus musculus mouse (house mouse), rat, human, rabbit, horse, pig, chicken, and quail analyzed by ClustalW. The amino acids of human C5 that make contact with OmCI in the crystal structures shown previously (Jore et al., 2016) are highlighted by red squares. (B) The crystal structure of human C5d (5I5K, residues 932-1372 of human C5) is shown in gray ribbons, and the amino acids involved in binding to OmCI are shown and labeled. The modelled structure of Coturnix quail C5d is indicated (residues 930-1370 of quail C5) as purple ribbons. The quail C5d amino acids corresponding with the residues of human C5d that bind to OmCI are shown and labelled. Graphics were generated using Swiss PDB Viewer (Guex and Peitsch, 1997). The amino acids labelled in yellow are conserved between human and quail C5, whereas the amino acids that vary between these animals’ C5 are shown in red.
Figure 2. OmCI inhibits the ability of human and quail complement to initiate antibody-mediated erythrocyte lysis.
Sheep erythrocytes previously treated with anti-erythrocyte antibody were incubated with indicated concentrations of OmCI as well as human or quail serum with a final concentration as 5 % or PBS buffer (control) for 1 h. The levels of erythrocyte lysis were evaluated by measuring the absorbance at 405 nm of the supernatant from the reactions. The absorbance values at 405 nm of each reaction were normalized to that obtained from incubating these erythrocytes with water in the absence of OmCI. The work was performed on three independent experiments; within each experiment, samples were run in triplicate. The result shown here is the experiment (A) 1, (B) 2, and (C) 3 from the average percent lysis ± standard deviation of three replicates in each experiment. The concentration of OmCI to inhibit 50 % of erythrocyte lysis (IC50) in the presence of human (blue) and quail (red) serum was obtained by fitting the data points using nonlinear regression methods (IC50 = 6.80 ± 1.07 nM for human serum, IC50 = 7.15 ± 0.61 nM for quail serum).
3.2. OmCI reduces the capability of the quail serum to eradicate a serum sensitive B. burgdorferi strain.
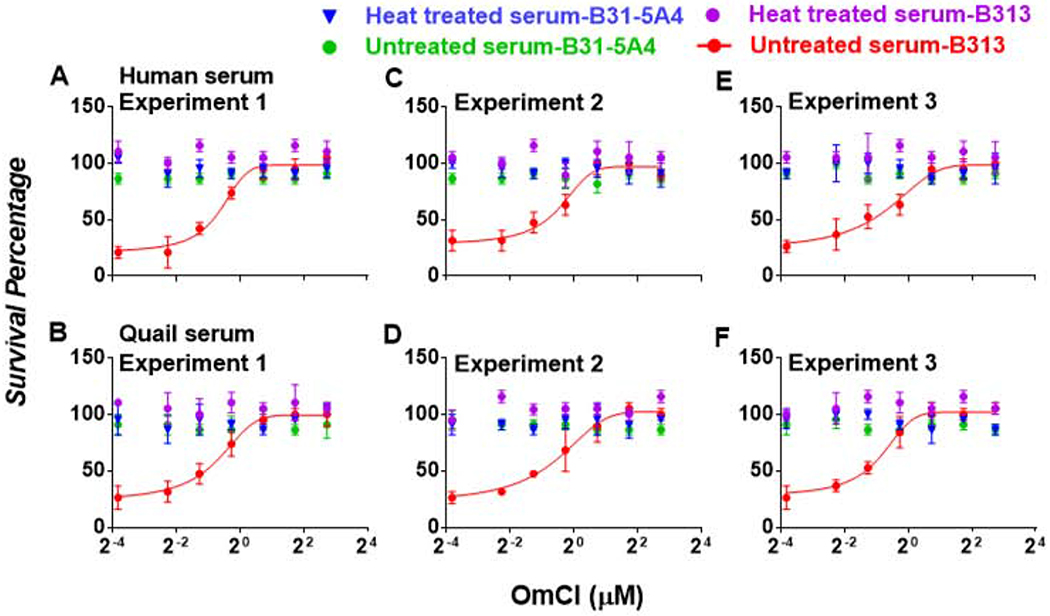
We next verified the ability of OmCI to inhibit human complement-mediated bacterial killing. Calculating the number of motile cells and determining the live cells by Live/Dead staining using dark field microscope are two most common approaches to measure complement’s ability to kill bacteria. We thus initially utilized a Lyme disease causing bacterium, B. burgdorferi, as a model bacterium to verify the ability of OmCI in inhibiting spirochete killing by complement. These sera at a final concentration of 40 % containing different concentrations of OmCI were incubated with a high passage, non-pathogenic, and serum sensitive B. burgdorferi strain B313. We also included a low passage, pathogenic, and serum resistant B. burgdorferi strain B31-5A4 as a control. When measuring spirochete viability by counting the percentage of motile bacteria under microscopy, we found that more than 95 % of strain B31-5A4 survives in untreated or heat-treated human sera, independent on the presence of OmCI (Fig. S1). Similarly, close to 95 % of strain B313 was detected motile in heat-treated human sera, in the presence or absence of OmCI (Fig. S1). Though greater than 95 % of this strain was motile in untreated human sera in the presence OmCI, merely 30 % of strain B313 were motile in untreated human sera in the absence of OmCI (Fig. S1). These results indicate that OmCI inhibits human complement-mediated bacterial killing. Further, there was no statistical difference in percent survival determined by Live/Dead staining, compared to that by calculating motile spirochete using dark field microscopy (Fig. S1). Though there is a caveat that non-motile bacteria may still be alive, our results suggest that the viability and motility of Lyme borreliae in this experimental setup are nearly equivalent. As calculating the percentage of motile spirochete was a state-of-the-art approach to quantitatively determine Lyme borreliae viability (Garcia et al., 2016; Hart et al., 2018; Marcinkiewicz et al., 2017; Marcinkiewicz et al., 2019; Wang et al., 2002), we evaluated spirochete survival in the following work using this approach.
We next compared the efficacy of OmCI in inhibiting bacterial killing mediated by quail and human sera (control) by mixing spirochetes with each of these sera in the presence of different concentrations of OmCI. In the absence of OmCI, more than 90 % of strain B31-5A4 survived in either untreated or heat-treated human serum, consistent with previous studies (Hart et al., 2018; Marcinkiewicz et al., 2019) (Fig. 3A, C, and E). The presence of OmCI in sera did not impact survival (Fig. 3A, C, and E). Similarly, strain B313 showed approximately 98 % survival in heat-treated human serum, which was not altered by the addition of OmCI (Fig. 3A, C, and E). However, less than 20 % of this strain survived in untreated human serum in the absence of OmCI; survival of B313 increased in the presence of increasing concentrations of OmCI (IC50 = 3.78 ± 1.71 μM) (Fig. 3A, C, and E). These results indicate that OmCI can block human serum-mediated killing of a serum sensitive Lyme borreliae strain. Furthermore, close to 95 % of strain B31-5A4 was found to survive in either untreated or heat-treated quail serum in the absence of OmCI, and this survival was independent of the presence of OmCI (Fig. 3B, D, and F). Close to 100 % of strain B313 was viable in the heat-treated quail serum whether or not OmCI was present (Fig. 3B, D, and F). Conversely, only 25 % of this strain remained motile in untreated quail serum in the absence of OmCI, in agreement with previous findings (Hart et al., 2018; Marcinkiewicz et al., 2019) (Fig. 3B, D, and F). The addition of OmCI increased survival of strain B313 in a dose dependent manner. (Fig. 3B, D, and F). These findings indicate that OmCI prevents killing of a serum sensitive Lyme borreliae strain by quail complement.
Figure 3. OmCI inhibits the ability of human and quail serum to kill a serum sensitive B. burgdorferi strain in a dose dependent manner.
A low passage, infectious, and serum resistant B. burgdorferi strain B31-5A4 (“B31-5A4”) or a high passage, non-infectious, and serum sensitive B. burgdorferi strain B313 (“B313”) was incubated for 4h with indicated concentrations of OmCI as well as (A, C, and E) human or (B, D, and F) quail serum with a final concentration of 40 %. Heat-inactivated human or quail serum was included as controls. The number of motile spirochetes was then assessed microscopically. The percentage of survival for those B. burgdorferi strains was calculated using the number of mobile spirochetes at 4 h post incubation normalized to that prior to the incubation with serum. The work was performed on three independent experiments; within each experiment, samples were run in triplicate. The result shown here is the experiment (A and B) 1, (C and D) 2, and (E and F) 3 from the average survival percentage ± standard deviation of three replicates in each experiment. The concentration of OmCI to inhibit 50 % levels of serum killing (IC50) was obtained by fitting the data points using nonlinear regression methods (IC50 = 3.78 ± 1.71 μM for human serum, IC50 = 2.94 ± 0.83 μM for quail serum).
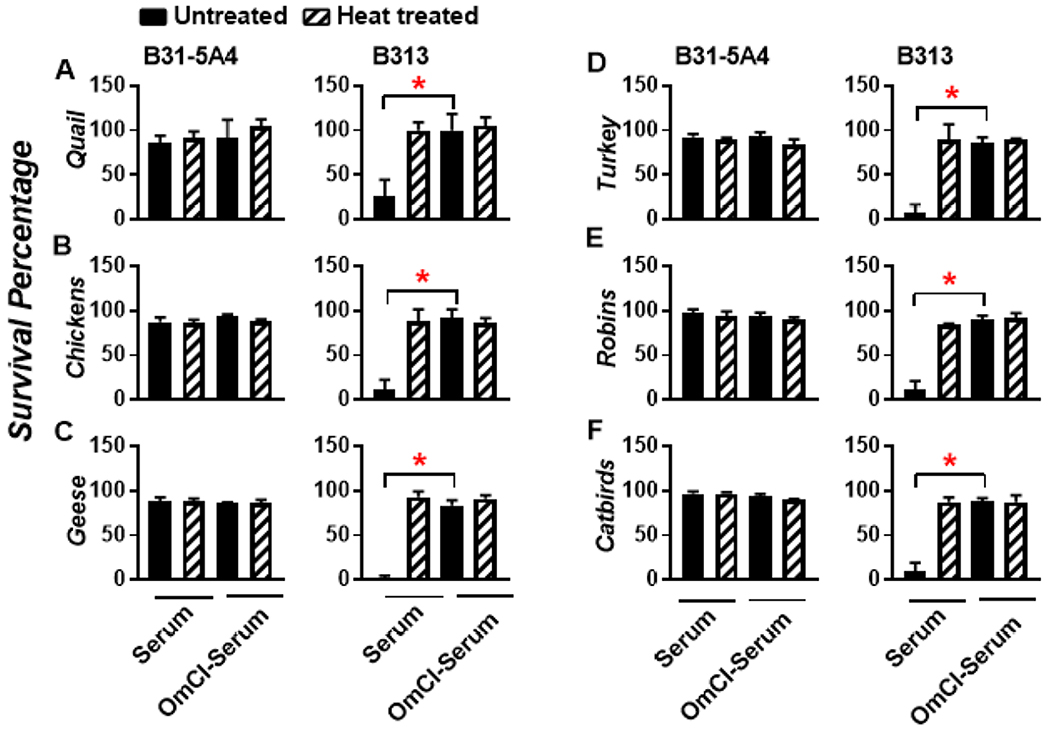
3.3. OmCI inhibits the killing of a serum sensitive B. burgdorferi strain by the sera from different domestic and wild birds.
To examine if OmCI can block complement-mediated bacterial killing of a serum sensitive B. burgdorferi strain by sera from additional avian hosts, we tested sera from domestic birds including chicken, geese, and turkey, and wild Aves including American robins and Gray catbirds. Sera samples from these species were serologically verified for non-infectious status of Lyme disease bacteria as described (data not shown) (Hart et al., 2018; Marcinkiewicz et al., 2019). We then incubated each of these avian sera with B. burgdorferi strains B31-5A4 or B313 in the presence or absence of OmCI. In the absence of OmCI, greater than 90 % of the strain B31-5A4 survived in either untreated or heat-treated serum from all tested avian hosts (Fig. 4A to F, left panel); survival was not altered by the presence of OmCI (Fig. 4A to F, left panel). The results derived from quail sera in Fig. 3 were included as control (Fig. 4A). Though close to 100 % of strain B313 survived in heat-treated avian sera, less than 20 % of this strain was found motile in the untreated sera from each tested avian host. These results suggest the inability of strain B313 to evade avian complement (Fig. 4, right panel). The presence of OmCI in untreated sera permitted greater than 90 % survival of strain B313 (Fig. 4, right panel). As expected, bacteria survived greater than 90 % in heat treated sera and were not affected by the presence of OmCI. Note that the relapsing fever spirochete Borrelia duttonii can be transmitted from Ornithodoros moubata ticks to humans or potentially to chicken (Elbir et al., 2013; McCall et al., 2007). We thus also included two B. duttonii strains in this study and found that both strains survived in human or chicken sera at levels of nearly 100 %, independent of heat or OmCI treatment (Fig. S2). As no serum sensitive B. duttonii strains are currently available, our finding strongly suggests the need of using a serum sensitive Borrelia strain (e.g. B. burgdorferi B313) as a tool to examine OmCI-mediated inhibition of bacterial killing. Taken together, using B. burgdorferi B313 as a model, we showed that OmCI can block complement-dependent bacterial killing in the sera of a variety of Aves.
Figure 4. OmCI reduces the ability of serum from domestic and wild avian hosts to eradicate a serum sensitive B. burgdorferi strain.
A low passage, infectious, and serum resistant B. burgdorferi strain B31-5A4 (“B31-5A4”) or a high passage, a non-infectious, and serum sensitive B. burgdorferi strain B313 (“B313”) was incubated for 4h with the serum from (A) Coturnix quail (“quail”), (B) chicken, (C) geese, (D) turkey, (E) American robins (“robins”), or (F) Gray catbirds (“catbirds”) at a final concentration of 40 % in the presence (“OmCI-serum”) or absence (“serum”) of 2 μM of OmCI. Note that the results from quail were derived from Figure 3. The heat-inactivated serum from the above-mentioned animals was included as a control (“heat-treated”). The number of motile spirochetes was assessed microscopically. The percentage of survival for those B. burgdorferi strains was calculated using the number of mobile spirochetes at 4 h post incubation normalized to that prior to the incubation with serum. The experiments were performed on three independent experiments; within each experiment, samples were run in triplicate, and the survive percentage for each experiment was calculated by averaging the results from triplicate experiments. The result shown here is the average ± standard deviation of the survival percentage from three independent experiments. (*), the significant difference (P < 0.05) of the percent survival of spirochetes between indicated groups was determined using the one-way ANOVA with post hoc Dunn’s test.
4. Discussion
Complement is a pivotal host innate immune defense mechanism in the vertebrates’ blood to eradicate the non-self-cells (Meri, 2016; Trouw et al., 2017; Zipfel and Skerka, 2009). Blood-feeding arthropods such as ticks generate a variety of proteins in their saliva to prevent damage to their own cells caused by attack from host complement in a blood meal (Chmelar et al., 2016a; Chmelar et al., 2016b; Francischetti et al., 2009; Nuttall, 2019; Valenzuela et al., 2000). One of these proteins, OmCI, inhibit complement from diverse mammalian species, which prevents tissue damage caused by complement activation (Fluiter et al., 2014; Pischke et al., 2017; Roversi et al., 2013). Unlike other tick salivary proteins that display mammalian or primate-specific complement inhibition (Jore et al., 2016), we found that OmCI prevents erythrocyte lysis mediated avian complement. OmCI is produced by O. moubata ticks, which feed on humans and domestic and wild swine, as well as poultry such as chickens (Elbir et al., 2013; McCall et al., 2007). Our results thus support the possibility that OmCI prevents potential tissue damage during blood feeding of ticks on avian hosts, illustrating the co-evolution between a tick salivary protein and the hosts that the tick parasitizes. Further, O. moubata can carry and transmit pathogens, including B. duttonii and African Swine Fever viruses (Burrage, 2013; Dixon et al., 2019; Dworkin et al., 2008; Talagrand-Reboul et al., 2018). Thus, there is a possibility that OmCI facilitates the transmission of these pathogens from ticks to vertebrates by inhibiting complement-mediated bactericidal activity (Nunn et al., 2005). However, no serum sensitive and O. moubata-transmitted pathogens were reported, indicating the limitations of using these pathogens to test such a possibility. Furthermore, some of O. moubata-transmitted pathogens including B. duttonii use OmCI-independent strategies to evade complement (e.g. producing complement inhibitory proteins) (Arias et al., 2017; Hovis et al., 2004; Meri et al., 2006; Rossmann et al., 2007). This caveat increases the complexity to delineate the phenotypes of these pathogens’ tick-to-host transmission conferred by OmCI, which warrants further investigations.
Numerous anti-complement proteins have been identified to inhibit mammalian complement, allowing them to be used as tools to tackle the molecular mechanisms of human complement activation or a pathogen’s complement evading strategies (Morgan and Harris, 2015; Ricklin et al., 2016). However, the sequences of complement proteins varying among different vertebrates often render the possibility in using those anti-complement proteins to investigate the functions of non-mammalian complement (Kai et al., 1983; Kai et al., 1985; Mavroidis et al., 1995; Vogel and Muller-Eberhard, 1985). Many microbes such as Lyme borreliae survive in bird sera or can be carried by Aves, highlighting the need to identify a tool to study avian complement evasion of these microorganisms (Kurtenbach et al., 2002; Tufts et al., 2019). We demonstrated that OmCI blocks complement from avian hosts and promotes the survival of a serum sensitive Lyme borreliae strain in the sera from different domestic and wild birds. These findings thus illustrate an innovative use of OmCI and this bacteria strain as a model to perform mechanistic study of avian complement activation and pathogen evasion to that complement.
5. Conclusions
In this study, we demonstrated that a tick salivary protein, OmCI, can inhibit not only human but also avian complement-mediated hemolysis. We further used Lyme disease bacteria as a model to demonstrate that OmCI-mediated avian complement inhibition facilitates bacteria evading the complement-mediated killing. It is noteworthy that B. burgdorferi is transmitted by the hard tick species Ixodes rather than the soft tick O. moubata, from which OmCI was derived (Radolf et al., 2012; Steere et al., 2016). Thus, our observation here does not suggest the role of this protein to facilitate the transmission of Lyme disease bacteria from O. moubcitci ticks to avian hosts. Instead, the information derived from this study provides a useful tool to inhibit avian complement, which could contribute to the novel insights into bird innate immune defense mechanisms and pathogens’ ability to evade bird complement.
Supplementary Material
Supplemental Figure 1. No difference of Lyme borreliae survival in human sera determined by motile spirochete measuring and Live/Dead staining. A low passage, infectious, and serum resistant B. burgdorferi strain B31-5A4 (“B31-5A4”) or a high passage, a non-infectious, and serum sensitive B. burgdorferi strain B313 (“B313”) was incubated for 4h with the human sera at a final concentration of 40 % in the presence (“OmCI-serum”) or absence (“serum”) of 2 μM of OmCI. The heat-inactivated human sera were included as a control (“heat-treated”). The number of motile spirochetes was assessed microscopically (“Motile spirochete measuring”) or using Live/Dead staining. The percentage of survival for those B. burgdorferi strains was calculated using the number of live spirochetes at 4 h post incubation normalized to that prior to the incubation with serum. The work was performed on three independent experiments; within each experiment, samples were run in triplicate, and the survive percentage for each experiment was calculated by averaging the results from triplicate experiments. The result shown here is the average ± standard deviation of the survival percentage from three independent experiments. (*), the significant difference (P < 0.05) of the percent survival of spirochetes between indicated groups was determined using the one-way ANOVA with post hoc Dunn’s test.
Supplemental Figure 2. B. duttonii survives in human and chicken sera independent on heat or OmCI treatment. B. duttonii strains V (“V”) or LA1 (“LA1”) was incubated for 4h with the serum from (A) human or (B) chicken at a final concentration of 40 % in the presence (“OmCI-serum”) or absence (“serum”) of 2 μM of OmCI. The heat-inactivated sera from the above-mentioned animals were included as a control (“heat-treated”). The number of motile spirochetes was assessed microscopically. The percentage of survival for those B. burgdorferi strains was calculated using the number of mobile spirochetes at 4 h post incubation normalized to that prior to the incubation with serum. The work was performed on three independent experiments; within each experiment, samples were run in triplicate, and the survive percentage for each experiment was calculated by averaging the results from triplicate experiments. The result shown here is the average ± standard deviation of the survival percentage from three independent experiments using the one-way ANOVA with post hoc Dunn’s test, no statistical difference (P > 0.05) were observed between groups.
Supplemental Table 1. Bacteria strains used in this study
Acknowledgements
We thank Ashley Marcinkiewicz and Grace Chen for valuable technical advice, Frida Mohlin for expert technical assistance, and Susan Madison-Antenucci for allowing us to use her fluorescence microscope, and Noel Espina for the assistance with that microscope. We also thank George Chaconas and Patricia Rosa for providing B. burgdorferi strain B31-5A4 and B313, respectively.
Funding sources
This work was supported by NM-U01CK000509 (DT and MDW), NSF-IOS1755286 (DT, MDW, AF, TMH, and YL), DoD-TB170111, New York State Department of Health Wadsworth Center Start-Up Grant (AF, TMH, and YL), NIH R01AI121401 (PK), and the LOEWE Center DRUID Novel Drug Targets against Poverty-Related and Neglected Tropical Infectious Diseases, project C3 (PK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- OmCI
Ornithodoros moubata complement inhibitor
- B31-5A4
Borrelia burgdorferi strain B31-5A4
- B313
Borrelia burgdorferi strain B313
- MAC
Membrane attack complex
- IC50
50 % levels of lysis or bacterial killing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics statement
The experiments involved in collecting serum from wild birds (American robins and catbirds) were performed in strict accordance with all provisions of the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and the PHS Policy on Humane Care and Use of Laboratory Animals. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Columbia University (Protocol number AC-AAAS5402). The bleeding and banding permits were approved by US Fish and Wildlife (Permit number MB122969-1). All efforts were made to minimize animal suffering.
Declarations of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Arias M, de la Torre A, Dixon L, Gallardo C, Jori F, Laddomada A, Martins C, Parkhouse RM, Revilla Y, Rodriguez FAJ, Sanchez V, 2017. Approaches and Perspectives for Development of African Swine Fever Virus Vaccines. Vaccines (Basel) 5, E35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold K, Bordoli L, Kopp J, Schwede T, 2006. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22, 195–201. [DOI] [PubMed] [Google Scholar]
- Barbour AG, 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med 57, 521–525. [PMC free article] [PubMed] [Google Scholar]
- Barratt-Due A, Thorgersen EB, Egge K, Pischke S, Sokolov A, Hellerud BC, Lindstad JK, Pharo A, Bongoni AK, Rieben R, Nunn M, Scott H, Mollnes TE, 2013. Combined inhibition of complement C5 and CD14 markedly attenuates inflammation, thrombogenicity, and hemodynamic changes in porcine sepsis. J Immunol 191, 819–827. [DOI] [PubMed] [Google Scholar]
- Barratt-Due A, Thorgersen EB, Lindstad JK, Pharo A, Lissina O, Lambris JD, Nunn MA, Mollnes TE, 2011. Ornithodoros moubata complement inhibitor is an equally effective C5 inhibitor in pigs and humans. J Immunol 187, 4913–4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berends ET, Mohan S, Miellet WR, Ruyken M, Rooijakkers SH, 2015. Contribution of the complement Membrane Attack Complex to the bactericidal activity of human serum. Mol Immunol 65, 328–335. [DOI] [PubMed] [Google Scholar]
- Blom AM, Volokhina EB, Fransson V, Stromberg P, Berghard L, Viktorelius M, Mollnes TE, Lopez-Trascasa M, van den Heuvel LP, Goodship TH, Marchbank KJ, Okroj M, 2014. A novel method for direct measurement of complement convertases activity in human serum. Clin Exp Immunol 178, 142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrage TG, 2013. African swine fever virus infection in Ornithodoros ticks. Virus Res 173, 131–139. [DOI] [PubMed] [Google Scholar]
- Chmelar J, Kotal J, Karim S, Kopacek P, Francischetti IMB, Pedra JHF Kotsyfakis M, 2016a. Sialomes and Mialomes: A Systems-Biology View of Tick Tissues and Tick-Host Interactions. Trends Parasitol 32, 242–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmelar J, Kotal J, Kopecky J, Pedra JHF, Kotsyfakis M, 2016b. All For One and One For All on the Tick-Host Battlefield. Trends Parasitol 32, 368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler SJ, Akintunde CO, Moss J, Fukunaga M, Kurtenbach K, Talbert A, Zhang H, Wright DJ, Warrell DA, 1999. Successful in vitro cultivation of Borrelia duttonii and its comparison with Borrelia recurrentis. Int J Syst Bacteriol 49 Pt 4, 1793–1799. [DOI] [PubMed] [Google Scholar]
- Dixon LK, Sun H, Roberts H, 2019. African swine fever. Antiviral Res 165, 34–41. [DOI] [PubMed] [Google Scholar]
- Dworkin MS, Schwan TG, Anderson DE Jr., Borchardt SM, 2008. Tick-borne relapsing fever. Infect Dis Clin North Am 22, 449–468, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbir H, Raoult D, Drancourt M, 2013. Relapsing fever borreliae in Africa. Am J Trop Med Hyg 89, 288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Wang T, Zhang S, Shi W, Zhang Y, 2014. An optimized SYBR Green I/PI assay for rapid viability assessment and antibiotic susceptibility testing for Borrelia burgdorferi. PLoS One 9, e111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluiter K, Opperhuizen AL, Morgan BP, Baas F, Ramaglia V, 2014. Inhibition of the membrane attack complex of the complement system reduces secondary neuroaxonal loss and promotes neurologic recovery after traumatic brain injury in mice. J Immunol 192, 2339–2348. [DOI] [PubMed] [Google Scholar]
- Francischetti IM, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JM, 2009. The role of saliva in tick feeding. Front Biosci (Landmark Ed) 14, 2051–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredslund F, Laursen NS, Roversi P, Jenner L, Oliveira CL, Pedersen JS, Nunn MA, Lea SM, Discipio R, Sottrup-Jensen L, Andersen GR, 2008. Structure of and influence of a tick complement inhibitor on human complement component 5. Nat Immunol 9, 753–760. [DOI] [PubMed] [Google Scholar]
- Garcia BL, Zhi H, Wager B, Hook M, Skare JT, 2016. Borrelia burgdorferi BBK32 Inhibits the Classical Pathway by Blocking Activation of the C1 Complement Complex. PLoS Pathog 12, e1005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia CC, Weston-Davies W, Russo RC, Tavares LP, Rachid MA, Alves-Filho JC, Machado AV, Ryffel B, Nunn MA, Teixeira MM, 2013. Complement C5 activation during influenza A infection in mice contributes to neutrophil recruitment and lung injury. PLoS One 8, e64443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guex N, Peitsch MC, 1997. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18, 2714–2723. [DOI] [PubMed] [Google Scholar]
- Hart T, Nguyen NTT, Nowak NA, Zhang F, Linhardt RJ, Diuk-Wasser M, Ram S, Kraiczy P, Lin YP, 2018. Polymorphic factor H-binding activity of CspA protects Lyme borreliae from the host complement in feeding ticks to facilitate tick-to-host transmission. PLoS Pathog 14, e1007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepburn NJ, Williams AS, Nunn MA, Chamberlain-Banoub JC, Hamer J, Morgan BP, Harris CL, 2007. In vivo characterization and therapeutic efficacy of a C5-specific inhibitor from the soft tick Ornithodoros moubata. J Biol Chem 282, 8292–8299. [DOI] [PubMed] [Google Scholar]
- Hovis KM, McDowell JV, Griffin L, Marconi RT, 2004. Identification and characterization of a linear-plasmid-encoded factor H-binding protein (FhbA) of the relapsing fever spirochete Borrelia hermsii. J Bacteriol 186, 2612–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jore MM, Johnson S, Sheppard D, Barber NM, Li YI, Nunn MA, Elmlund H, Lea SM, 2016. Structural basis for therapeutic inhibition of complement C5. Nat Struct Mol Biol 23, 378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai C, Yoshikawa Y, Yamanouchi K, Okada H, 1983. Isolation and identification of the third component of complement of Japanese quails. J Immunol 130, 2814–2820. [PubMed] [Google Scholar]
- Kai C, Yoshikawa Y, Yamanouchi K, Okada H, Morikawa S, 1985. Ontogeny of the third component of complement of Japanese quails. Immunology 54, 463–470. [PMC free article] [PubMed] [Google Scholar]
- Kuhn N, Schmidt CQ, Schlapschy M, Skerra A, 2016. PASylated Coversin, a C5-Specific Complement Inhibitor with Extended Pharmacokinetics, Shows Enhanced Anti-Hemolytic Activity in Vitro. Bioconjug Chem 27, 2359–2371. [DOI] [PubMed] [Google Scholar]
- Kurtenbach K, De Michelis S, Etti S, Schafer SM, Sewell HS, Brade V, Kraiczy P, 2002. Host association of Borrelia burgdorferi sensu lato--the key role of host complement. Trends Microbiol 10, 74–79. [DOI] [PubMed] [Google Scholar]
- Macpherson A, Liu X, Dedi N, Kennedy J, Carrington B, Durrant O, Heywood S, van den Elsen J, Lawson ADG, 2018. The rational design of affinity-attenuated OmCI for the purification of complement C5. J Biol Chem 293, 14112–14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewicz A, Kraiczy P, Lin Y-P, 2017. There is a method to the madness: Strategies to study host complement evasion by Lyme disease and relapsing fever spirochetes. Frontiers in Microbiology 8, 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewicz AL, Dupuis AP 2nd, Zamba-Campero M, Nowak N, Kraiczy P, Ram S, Kramer LD, Lin YP, 2019. Blood treatment of Lyme borreliae demonstrates the mechanism of CspZ-mediated complement evasion to promote systemic infection in vertebrate hosts. Cell Microbiol 21, e12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavroidis M, Sunyer JO, Lambris JD, 1995. Isolation, primary structure, and evolution of the third component of chicken complement and evidence for a new member of the alpha 2-macroglobulin family. J Immunol 154, 2164–2174. [PubMed] [Google Scholar]
- McCall PJ, Hume JC, Motshegwa K, Pignatelli P, Talbert A, Kisinza W, 2007. Does tick-borne relapsing fever have an animal reservoir in East Africa? Vector Borne Zoonotic Dis 7, 659–666. [DOI] [PubMed] [Google Scholar]
- Meri S, 2016. Self-nonself discrimination by the complement system. FEBS Lett 590, 2418–2434. [DOI] [PubMed] [Google Scholar]
- Meri T, Cutler SJ, Blom AM, Meri S, Jokiranta TS, 2006. Relapsing fever spirochetes Borrelia recurrentis and B. duttonii acquire complement regulators C4b-binding protein and factor H. Infect Immun 74, 4157–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan BP, Harris CL, 2015. Complement, a target for therapy in inflammatory and degenerative diseases. Nat Rev Drug Discov 14, 857–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarova TI, Avaeva SM, 1973. [Reaction of yeast inorganic pyrophosphatase with radioactive inorganic phosphate and pyrophosphate]. Biokhimiia 38, 169–173. [PubMed] [Google Scholar]
- Nunn MA, Sharma A, Paesen GC, Adamson S, Lissina O, Willis AC, Nuttall PA, 2005. Complement inhibitor of C5 activation from the soft tick Ornithodoros moubata. J Immunol 174, 2084–2091. [DOI] [PubMed] [Google Scholar]
- Nuttall PA, 2019. Wonders of tick saliva. Ticks Tick Borne Dis 10, 470–481. [DOI] [PubMed] [Google Scholar]
- Pischke SE, Gustavsen A, Orrem HL, Egge KH, Courivaud F, Fontenelle H, Despont A, Bongoni AK, Rieben R, Tonnessen TI, Nunn MA, Scott H, Skulstad H, Barratt-Due A, Mollnes TE, 2017. Complement factor 5 blockade reduces porcine myocardial infarction size and improves immediate cardiac function. Basic Res Cardiol 112, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purser JE, Norris SJ, 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci U S A 97, 13865–13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Caimano MJ, Stevenson B, Hu LT, 2012. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol 10, 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D, Reis ES, Lambris JD, 2016. Complement in disease: a defence system turning offensive. Nat Rev Nephrol 12, 383–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann E, Kraiczy P, Herzberger P, Skerka C, Kirschfink M, Simon MM, Zipfel PF, Wallich R, 2007. Dual binding specificity of a Borrelia hermsii-associated complement regulator-acquiring surface protein for factor H and plasminogen discloses a putative virulence factor of relapsing fever spirochetes. J Immunol 178, 7292–7301. [DOI] [PubMed] [Google Scholar]
- Roversi P, Ryffel B, Togbe D, Maillet I, Teixeira M, Ahmat N, Paesen GC, Lissina O, Boland W, Ploss K, Caesar JJ, Leonhartsberger S, Lea SM, Nunn MA, 2013. Bifunctional lipocalin ameliorates murine immune complex-induced acute lung injury. J Biol Chem 288, 18789–18802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadziene A, Wilske B, Ferdows MS, Barbour AG, 1993. The cryptic ospC gene of Borrelia burgdorferi B31 is located on a circular plasmid. Infect Immun 61, 2192–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöberg AP, Trouw LA, Blom AM, 2009. Complement activation and inhibition: a delicate balance. Trends Immunol 30, 83–90. [DOI] [PubMed] [Google Scholar]
- Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW, Li X, Mead PS, 2016. Lyme borreliosis. Nat Rev Dis Primers 2, 16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talagrand-Reboul E, Boyer PH, Bergstrom S, Vial L, Boulanger N, 2018. Relapsing Fevers: Neglected Tick-Borne Diseases. Front Cell Infect Microbiol 8, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouw LA, Pickering MC, Blom AM, 2017. The complement system as a potential therapeutic target in rheumatic disease. Nat Rev Rheumatol 13, 538–547. [DOI] [PubMed] [Google Scholar]
- Tufts DM, Hart TM, Chen GF, Kolokotronis SO, Diuk-Wasser MA, Lin YP, 2019. Outer surface protein polymorphisms linked to host-spirochete association in Lyme borreliae. Mol Microbiol. 111, 868–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela JG, Charlab R, Mather TN, Ribeiro JM, 2000. Purification, cloning, and expression of a novel salivary anticomplement protein from the tick, Ixodes scapularis. J Biol Chem 275, 18717–18723. [DOI] [PubMed] [Google Scholar]
- Vogel CW, Muller-Eberhard HJ, 1985. The cobra complement system: I. The alternative pathway of activation. Dev Comp Immunol 9, 311–325. [DOI] [PubMed] [Google Scholar]
- Wang G, Ojaimi C, Wu H, Saksenberg V, Iyer R, Liveris D, McClain SA, Wormser GP, Schwartz I, 2002. Disease severity in a murine model of lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strain. J Infect Dis 186, 782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel PF, Skerka C, 2009. Complement regulators and inhibitory proteins. Nat Rev Immunol 9, 729–740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. No difference of Lyme borreliae survival in human sera determined by motile spirochete measuring and Live/Dead staining. A low passage, infectious, and serum resistant B. burgdorferi strain B31-5A4 (“B31-5A4”) or a high passage, a non-infectious, and serum sensitive B. burgdorferi strain B313 (“B313”) was incubated for 4h with the human sera at a final concentration of 40 % in the presence (“OmCI-serum”) or absence (“serum”) of 2 μM of OmCI. The heat-inactivated human sera were included as a control (“heat-treated”). The number of motile spirochetes was assessed microscopically (“Motile spirochete measuring”) or using Live/Dead staining. The percentage of survival for those B. burgdorferi strains was calculated using the number of live spirochetes at 4 h post incubation normalized to that prior to the incubation with serum. The work was performed on three independent experiments; within each experiment, samples were run in triplicate, and the survive percentage for each experiment was calculated by averaging the results from triplicate experiments. The result shown here is the average ± standard deviation of the survival percentage from three independent experiments. (*), the significant difference (P < 0.05) of the percent survival of spirochetes between indicated groups was determined using the one-way ANOVA with post hoc Dunn’s test.
Supplemental Figure 2. B. duttonii survives in human and chicken sera independent on heat or OmCI treatment. B. duttonii strains V (“V”) or LA1 (“LA1”) was incubated for 4h with the serum from (A) human or (B) chicken at a final concentration of 40 % in the presence (“OmCI-serum”) or absence (“serum”) of 2 μM of OmCI. The heat-inactivated sera from the above-mentioned animals were included as a control (“heat-treated”). The number of motile spirochetes was assessed microscopically. The percentage of survival for those B. burgdorferi strains was calculated using the number of mobile spirochetes at 4 h post incubation normalized to that prior to the incubation with serum. The work was performed on three independent experiments; within each experiment, samples were run in triplicate, and the survive percentage for each experiment was calculated by averaging the results from triplicate experiments. The result shown here is the average ± standard deviation of the survival percentage from three independent experiments using the one-way ANOVA with post hoc Dunn’s test, no statistical difference (P > 0.05) were observed between groups.
Supplemental Table 1. Bacteria strains used in this study