Abstract
Colibactins are genotoxic secondary metabolites whose biosynthesis is encoded in the clb gene cluster harbored by certain strains of gut commensal Escherichia coli. Using synthetic colibactin analogues, we previously provided evidence that colibactins alkylate DNA by addition of a nucleotide to an electrophilic cyclopropane intermediate. However, natural colibactin–nucleobase adducts have not been identified, to the best of our knowledge. Here we present the first identification of such adducts, derived from treatment of pUC19 DNA with clb+ E. coli. Previous biosynthetic studies established cysteine and methionine as building blocks in colibactin biosynthesis; accordingly, we used cysteine (ΔcysE) and methionine (ΔmetA) auxotrophic strains cultured in media supplemented with l-[U-13C]Cys or l-[U-13C]Met to facilitate the identification of nucleobases bound to colibactins. Using MS2 and MS3 analysis, in conjunction with the known oxidative instability of colibactin cyclopropane-opened products, we were able to characterize adenine adducts derived from cyclopropane ring opening. This study provides the first reported detection of nucleobase adducts derived from clb+ E. coli and lends support to our earlier model suggesting DNA alkylation by addition of a nucleotide to an electrophilic cyclopropane.
Colibactins are secondary metabolites encoded in a hybrid nonribosomal peptide synthetase–polyketide synthase (NRPS–PKS) gene cluster, termed clb, that is harbored by certain strains of gut commensal Escherichia coli.1–5
The presence of the clb cluster is epidemiologically correlated to colorectal cancer formation in humans, and studies suggest colibactins are genotoxic and cause tumors in mouse models.
We previously provided evidence that colibactin genotoxicity derives in part from addition of a nucleotide to an electrophilic cyclopropane,6 a mechanism of DNA damage established for several classes of natural products.7,8 Because natural colibactins have eluded isolation, this conclusion was based on studies of synthetic colibactin analogues such as 1–3 (Scheme 1A). First, linearized pBR322 plasmid DNA was extensively degraded following treatment with nanomolar concentrations of synthetic colibactin analogue 1, which bears the putative electrophilic cyclopropane residue. Second, and consistent with the cyclopropane behaving as a DNA electrophile, dimer 2 was found to form DNA interstrand cross-links. Finally, construct 3, which is identical to 1 save for conversion of the cyclopropane to a geminal-dimethyl substituent, did not lead to detectable levels of DNA damage (≤500 μM concentration), as expected if the cyclopropane were a DNA-reactive functionality.
Scheme 1.
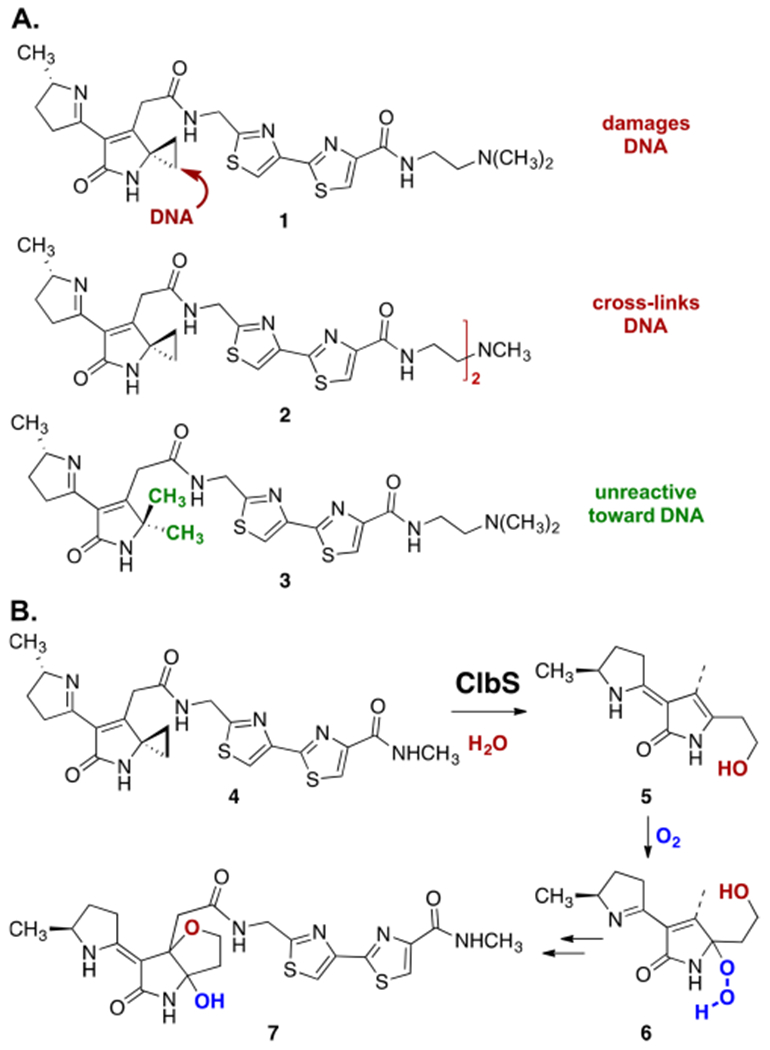
(A) Structures of Synthetic Colibactins 1–3 and Their Reactivity toward DNA and (B) Pathway for ClbS-Mediated Conversion of 4 to Hydroxyfuran 7
Perhaps the strongest evidence implicating the cyclopropane as a reactive locus underlying the genotoxicity of clb+ E. coli was obtained through studies of the resistance enzyme ClbS. ClbS is encoded in the clb cluster and was shown to be essential for bacterial viability.9 We demonstrated that purified ClbS cleaved the cyclopropane residue in synthetic colibactin 4, ultimately resulting in formation of 3-hydroxytetrahydrofuran 7 (Scheme 1B).10 The formation of 7 was shown to proceed by ClbS-catalyzed cyclopropane hydrolysis (4 → 5), aerobic oxidation (5 → 6), and cyclization with concomitant reduction of the alkyl hydrogen peroxide (6 → 7). This study established that the bacteria evolved a mechanism to eliminate self-toxicity deriving from the reactivity of the cyclopropane.
Despite these advances, the identification of natural colibactin–nucleobase adducts has not been described, to the best of our knowledge. In a recent study, clb+ E. coli were demonstrated to cross-link exogenous DNA.11 This suggested to us the possibility of characterizing natural colibactin–nucleobase adducts directly from bacterial cultures. To achieve this, here we conducted tandem mass spectrometry (MS) analysis of the products formed after incubation of linearized pUC19 plasmid DNA with clb+ E. coli BW25113. We conducted parallel assays using a cysteine auxotroph (ΔcysE) and a methionine auxotroph (ΔmetA)12 cultured in media supplemented with l-[U-13C]Cys or l-[U-13C]Met, respectively. Biochemical studies have established that the thiazole and aminocyclopropane residues of colibactins are derived from cysteine13 and methionine via SAM,13–15 respectively. Thus, products derived from clb metabolites were expected to be mass-shifted by 3 units for each cysteine or 4 units for each methionine in these auxotrophs, facilitating their identification. clb− E. coli were used as a negative control.
Linearized pUC19 DNA and bacteria were incubated in M9 medium for 4.5 h at 37 °C. The bacteria were separated by centrifugation, and the DNA was isolated and analyzed by denaturing gel electrophoresis. As shown in Figure 1, DNA was cross-linked upon being exposed to clb+, ΔcysE, or ΔmetA E. coli but not upon being exposed to clb− E. coli, as expected. The cross-linked DNA was digested with the Nucleoside Digestion Mix (New England Biolabs) and analyzed by liquid chromatography and tandem MS.
Figure 1.
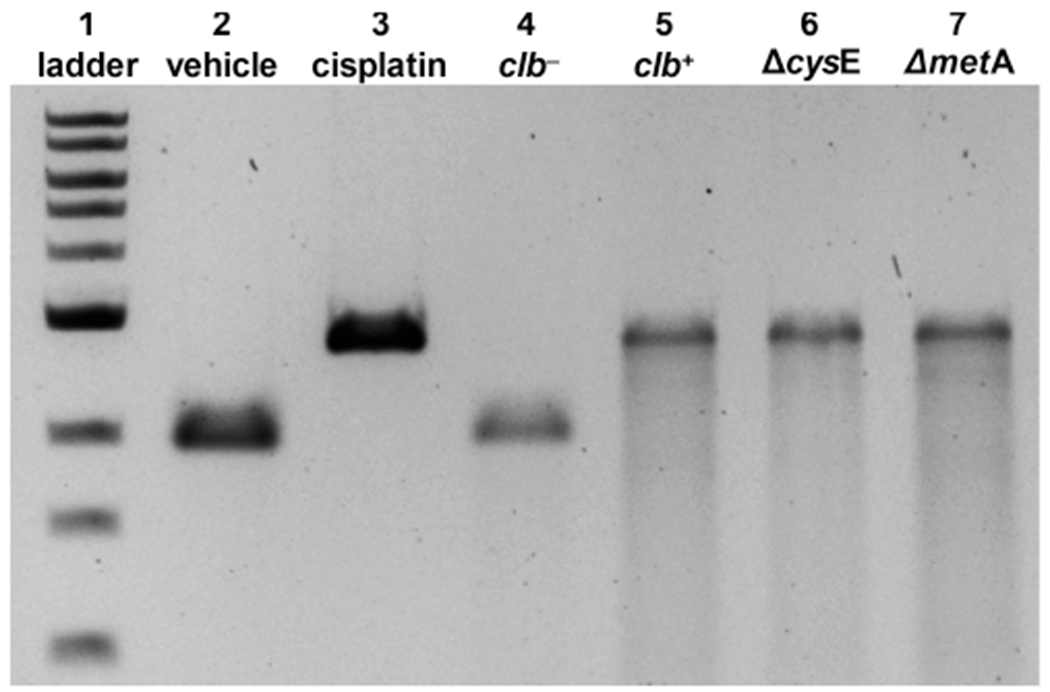
DNA cross-linking assays employing linearized pUC19 DNA and E. coli variants. Cisplatin was used as a positive control: DNA ladder (lane 1), no treatment (lane 2), cisplatin (100 μM, lane 3), clb− BW25113 E. coli (lane 4), clb+ BW25113 E. coli (lane 5), ΔcysE clb+ BW25113 E. coli (lane 6), and ΔmetA clb+ BW25113 E. coli (lane 7). Conditions for lanes 2 and 3: linearized pUC19 DNA (31 μM in base pairs), pH 5 sodium citrate buffer (10 mM), 4.5 h, 37 °C. Conditions for lanes 4 and 5: linearized pUC19 DNA, M9 medium, 4.5 h, 37 °C. Conditions for lanes 6 and 7: linearized pUC19 DNA, modified M9 medium (containing l-[U-13C]Cys or l-[U-13C]Met for Cys and Met auxotrophs, respectively), 4.5 h, 37 °C. DNA was isolated and analyzed by denaturing agarose gel electrophoresis (90 V, 1.5 h).
Prominent peaks at m/z 522.1668, 538.1618, 540.1775, and 556.1722 (z = 1) and m/z 261.5871, 269.5844, 270.5925, and 278.5899 (z = 2) were identified in DNA treated with clb+ E. coli. These peaks were mass-shifted by +3 or +4 units (z = 1) or +1.5 or +2 units (z = 2) in the ΔcysE/l-[U-13C]Cys or ΔmetA/l-[U-13C]Met cultures, respectively. The +3 or +1.5 mass shift in the products derived from the ΔcysE culture indicates the presence of only one thiazole ring. These observed masses fit the proposed ion structures 8–11 within 1.5 ppm of error (Figure 2 and Table 1).
Figure 2.
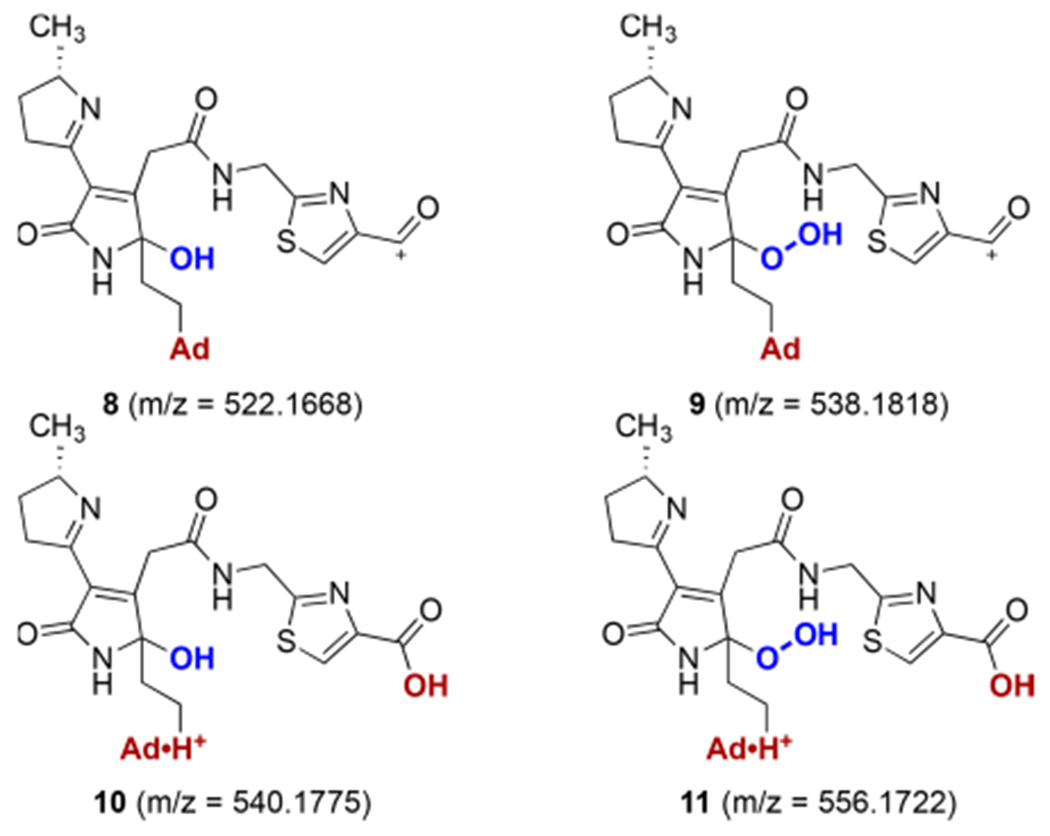
Proposed structures of colibactin–adenine adducts derived from incubation of pUC19 DNA with clb+ E. coli. Ad = adenine.
Table 1.
High-Resolution Mass Spectrometry Data of Colibactin–Nucleobase Adducts
| strain | ion | z | exp | theo | error (ppm) |
|---|---|---|---|---|---|
| clb+ | 8 | 1 | 522.1668 | 522.1666 | 0.38 |
| 9 | 1 | 538.1618 | 538.1616 | 0.37 | |
| 10 | 1 | 540.1775 | 540.1772 | 0.56 | |
| 11 | 1 | 556.1722 | 556.1721 | 0.18 | |
| 8 | 2 | 261.5871 | 261.5870 | 0.38 | |
| 9 | 2 | 269.5844 | 269.5844 | 0.00 | |
| 10 | 2 | 270.5925 | 270.5922 | 1.11 | |
| 11 | 2 | 278.5899 | 278.5897 | 0.72 | |
| clb+ΔcysE | [13C3]-8 | 1 | 525.1765 | 525.1767 | 0.29 |
| [13C3]-9 | 1 | 541.1714 | 541.1717 | 0.46 | |
| [13C3]-10 | 1 | 543.1874 | 543.1873 | 0.28 | |
| [13C3]-11 | 1 | 559.1822 | 559.1822 | 0.09 | |
| [13C3]-8 | 2 | 263.0920 | 263.0920 | 0.10 | |
| [13C3]-9 | 2 | 271.0897 | 271.0894 | 1.01 | |
| [13C3]-10 | 2 | 272.0976 | 272.0972 | 1.38 | |
| [13C3]-11 | 2 | 280.0947 | 280.0947 | 0.09 | |
| clb+ΔmetA | [13C4]-8 | 1 | 526.1804 | 526.1800 | 0.76 |
| [13C4]-9 | 1 | 542.1745 | 542.1750 | 0.92 | |
| [13C4]-10 | 1 | 544.1907 | 544.1906 | 0.18 | |
| [13C4]-11 | 1 | 560.1860 | 560.1855 | 0.89 | |
| [13C4]-8 | 2 | 263.5938 | 263.5937 | 0.38 | |
| [13C4]-9 | 2 | 271.5914 | 271.5911 | 1.10 | |
| [13C4]-10 | 2 | 272.5992 | 272.5989 | 1.10 | |
| [13C4]-11 | 2 | 280.5965 | 280.5964 | 0.36 |
The connectivity of 8–11 (in particular, the location of the adenine base) was established by extensive MS2 and MS3 analysis in conjunction with the mass shifts in the auxotrophic strains anticipated on the basis of the known label origins of the thiazole and cyclopropane residues.13,14
Thus, daughter ions 15 and 19 were observed in the MS spectra of 10 (Scheme 2). Ion 14 was observed as a daughter ion in the MS and MS2 spectra of 10, while ions 12 and 13 were observed only in the MS2 spectrum of 10. Thiazole 14 was mass-shifted by +3 units in the ΔcysE culture but did not change in the ΔmetA culture, consistent with the known biosynthesis of the thiazole residues from cysteine. Ions 15 and 19 were mass-shifted by +4 units in the ΔmetA culture but did not shift in the ΔcysE culture, consistent with the derivation of the cyclopropane from labeled methionine. The masses of adenine·H+ adducts were detected in the MS2 spectrum of unlabeled 10 and its Cys and Met-labeled isotopologs (error of <3 ppm). Indeed, the exact masses of 15 and 19 support incorporation of adenine, and their attendant +4 mass shift in the ΔmetA auxotroph allows us to associate the adenine residue with the region that contained the cyclopropane. Ion 12 further fragmented to 16 and 17, and consistent with their structures, all five of the ions (10, 12, 13, 16, and 17) were mass-shifted by +3 and +4 units in the Cys and Met auxotrophs, respectively. The fragmentation of 17 to 16 results in a loss of 43 mass units. This difference is consistent with loss of a fragment containing a nitrogen atom and supports the location of the hydroxyl group in 17 and in its parent ions. Future studies will focus on determining the site of formation of the bond to adenine (e.g., N7, C6–NH2).
Scheme 2.
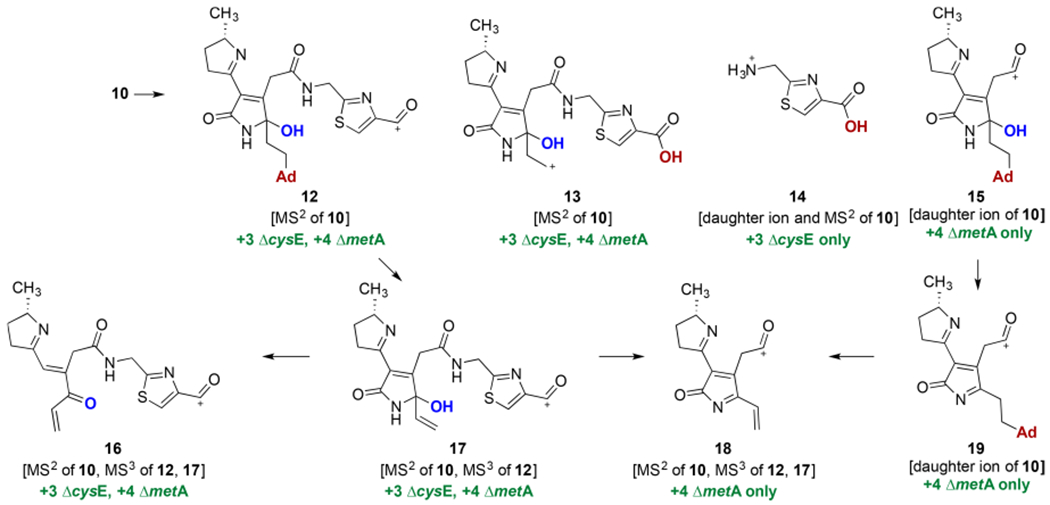
Selected Fragmentation and Proposed Tandem MS Products Derived from 10
Previously, it has been shown that advanced colibactins contain a two-carbon spacer between the thiazole rings.16 This two-carbon spacer is derived from an α-aminomalonate residue and has been shown to be essential for genotoxic effects.17,18 The identification of colibactin–nucleobase monoadducts 8–11 derived from the cross-linked precursor product suggests a potential role for this two-carbon spacer in cross-linking and genotoxicity, as the spacer and one of the thiazole rings are lacking in the detectable nuclease digestion products. Additionally, the cellular role of the electrophilicity of the lactam of metabolites resembling cyclopropane ring-opened 6 remains unknown. Further studies will be required to fully elucidate these points.
In summary, we have described the first structural evidence of the production of natural colibactin–nucleobase adducts. This study lends further support to our earlier work suggesting the cyclopropane as a DNA-reactive locus. Further studies will focus on elucidating the second site of reactivity leading to formation of DNA cross-links.
Supplementary Material
Acknowledgments
Funding
Financial support from the National Institutes of Health (1R01CA215553; SIG Grants 1S10OD019967 and 1S10ODOD018034) is gratefully acknowledged.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.biochem.8b01023.
Figures S1–S13, Tables S1–S3, detailed experimental procedures, and characterization data for all new compounds (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Trautman EP, and Crawford JM (2016) Linking biosynthetic gene clusters to their metabolites via pathway-targeted molecular networking. Curr. Top. Med. Chem 16, 1705–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Balskus EP (2015) Colibactin: Understanding an elusive gut bacterial genotoxin. Nat. Prod. Rep 32, 1534–1540. [DOI] [PubMed] [Google Scholar]
- (3).Taieb F, Petit C, Nougayrede JP, and Oswald E (2016) The enterobacterial genotoxins: Cytolethal distending toxin and colibactin. EcoSal Plus 7, n/a DOI: 10.1128/ecosalplus.ESP-0008-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Healy AR, and Herzon SB (2017) Molecular basis of gut microbiome-associated colorectal cancer: A synthetic perspective. J. Am. Chem. Soc 139, 14817–14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Faïs T, Delmas J, Barnich N, Bonnet R, and Dalmasso G (2018) Colibactin: More than a new bacterial toxin. Toxins 10, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Healy AR, Nikolayevskiy H, Patel JR, Crawford JM, and Herzon SB (2016) A mechanistic model for colibactin-induced genotoxicity. J. Am. Chem. Soc 138, 15563–15570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Boger DL, and Garbaccio RM (1999) Shape-dependent catalysis: Insights into the source of catalysis for the CC-1065 and duocarmycin DNA alkylation reaction. Acc. Chem. Res 32, 1043–1052. [Google Scholar]
- (8).Tichenor MS, and Boger DL (2008) Yatakemycin: Total synthesis, DNA alkylation, and biological properties. Nat. Prod. Rep 25, 220–226. [DOI] [PubMed] [Google Scholar]
- (9).Bossuet-Greif N, Dubois D, Petit C, Tronnet S, Martin P, Bonnet R, Oswald E, and Nougayrede JP (2016) Escherichia coli ClbS is a colibactin resistance protein. Mol. Microbiol 99, 897–908. [DOI] [PubMed] [Google Scholar]
- (10).Tripathi P, Shine EE, Healy AR, Kim CS, Herzon SB, Bruner SD, and Crawford JM (2017) ClbS is a cyclopropane hydrolase that confers colibactin resistance. J. Am. Chem. Soc 139, 17719–17722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Bossuet-Greif N, Vignard J, Taieb F, Mirey G, Dubois D, Petit C, Oswald E, and Nougayrede JP (2018) The colibactin genotoxin generates DNA interstrand cross-links in infected cells. mBio 9, e02393–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, and Mori H (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol 2, 2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Vizcaino MI, and Crawford JM (2015) The colibactin warhead crosslinks DNA. Nat. Chem 7, 411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Bian X, Plaza A, Zhang Y, and Müller R (2015) Two more pieces of the colibactin genotoxin puzzle from Escherichia coli show incorporation of an unusual 1-aminocyclopropanecarboxylic acid moiety. Chem. Sci 6, 3154–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Zha L, Jiang Y, Henke MT, Wilson MR, Wang JX, Kelleher NL, and Balskus EP (2017) Colibactin assembly line enzymes use S-adenosylmethionine to build a cyclopropane ring. Nat. Chem. Biol 13, 1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Li ZR, Li J, Gu JP, Lai JY, Duggan BM, Zhang WP, Li ZL, Li YX, Tong RB, Xu Y, Lin DH, Moore BS, and Qian PY (2016) Divergent biosynthesis yields a cytotoxic aminomalonate-containing precolibactin. Nat. Chem. Biol 12, 773–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Nougayrède J-P, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, Buchrieser C, Hacker J, Dobrindt U, and Oswald E (2006) Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 313, 848–851. [DOI] [PubMed] [Google Scholar]
- (18).Brachmann AO, Garcie C, Wu V, Martin P, Ueoka R, Oswald E, and Piel J (2015) Colibactin biosynthesis and biological activity depend on the rare aminomalonyl polyketide precursor. Chem. Commun 51, 13138–13141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


