Abstract
Nucleophosmin (NPM1) is an abundant nucleolar protein that is implicated in a variety of biological processes and in the pathogenesis of several human malignancies. For hematologic malignancies, approximately one-third of anaplastic large-cell non-Hodgkin’s lymphomas were found to express a fusion between NPM1 and the catalytic domain of anaplastic lymphoma receptor tyrosine kinase. About 50–60% of acute myeloid leukemia patients with normal karyotype carry NPM1 mutations, which are characterized by cytoplasmic dislocation of the NPM1 protein. Nevertheless, NPM1 is overexpressed in various hematologic and solid tumor malignancies. NPM1 overexpression is considered a prognostic marker of recurrence and progression of cancer. Thus, NPM1 abnormalities play a critical role in several types of hematologic malignancies. This has led to intense interest in the development of an NPM1 targeting strategy for cancer therapy. The aim of this review is to summarize present knowledge on NPM1 origin, pathogenesis, and therapeutic interventions in hematologic malignancies.
Keywords: hematologic malignancy, mutation, Nucleophosmin1, overexpression, therapy
Introduction
Nucleophosmin (NPM1), also known as B23 protein, resides primarily in the nucleus, but shuttles continuously between the nucleus and cytoplasm.1,2 The NPM1 protein contains a numbers of motifs that act to mediate interactions with binding partners and affect its cellular localization.1,3 Intracellular NPM1 is predominantly oligomeric and binds to other proteins, including tumor suppressor proteins.3 NPM1 is also a multifunctional phosphoprotein that plays multiple roles in ribosome biogenesis, mRNA processing, chromatin remodeling, and embryogenesis.4 For human hematologic malignancies, NPM1 mutations are significantly implicated in newly diagnosed de novo acute myeloid leukemia (AML) cases,1,5–7 which account for approximately one-third of all AML patients and have distinct genetic, pathologic, immunophenotypic, and clinical features.1,8,9 Notably, mutated NPM1 is a reliable marker of AML status in the majority of patients.10 NPM1 mutations can be detected in AML at relapse, even many years after the initial diagnosis.11–14 Because of its distinct biological and clinical characteristics, NPM1-mutated AML has been defined as a distinct molecular leukemia entity in the recently updated World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia.15–18 Nevertheless, NPM1 abnormality has an enormous impact on the biological diagnosis, prognostic stratification, and monitoring of minimal residual disease (MRD) in hematologic malignancies. The discovery of NPM1 gene alterations represents a rational basis for the development of molecular targeted drugs for leukemia and lymphoma.19–22 The aim of this present review is to update our knowledge of the discoveries of NPM1 and its alternations in different hematologic malignancies, as well as to deepen our understanding of recent findings concerning NPM1 therapeutic targeting.
NPM1 structure and biological function
The human NPM1 gene, located on chromosome 5q35, contains 12 exons ranging in size from 58 to 358 bp.23–25 NPM1, a multifunctional phosphoprotein, is found localized primarily to the granular regions of the nucleolus. The protein can shuttle between the nucleus, the nucleoplasm, and the cytoplasm during the cell cycle,2 and is involved in several biological processes, such as ribosome biogenesis, tumor suppression, nucleolar stress response, and cell apoptosis.3 In the majority of patient cases, NPM1 mutations are heterozygous, and localize to exon 12 of the gene. Around 50 different types of mutations have been found, all creating the cytoplasm-dislocated mutant NPM1 (NPM1c+) protein.8,9 The NPM1c+ protein in AML is critical to its oncogenicity. All NPM1 mutations act to maximize the export of the mutant to the cytoplasm, including rare mutations found outside of exon 12.26 The loss of NPM1c from the cytoplasm, either through nuclear relocalization or targeted degradation, results in immediate downregulation of homeobox (HOX) genes, and is followed by AML differentiation.27 The cellular distribution of wild type (wt) and mutant NPM1 is shown in Figure 1. Normally, NPM1 molecules contain distinct domains that account for multiple biological functions. The N-terminal hydrophobic regions of NPM1 are responsible for the self-oligomerization and chaperone activities of the molecule.28 The C-terminus of NPM1 accounts for the ribonuclease activity of the protein. The C-terminus also contains a short aromatic stretch with two tryptophan residues, which are crucial for NPM1 binding to the nucleolus.29
Figure 1.
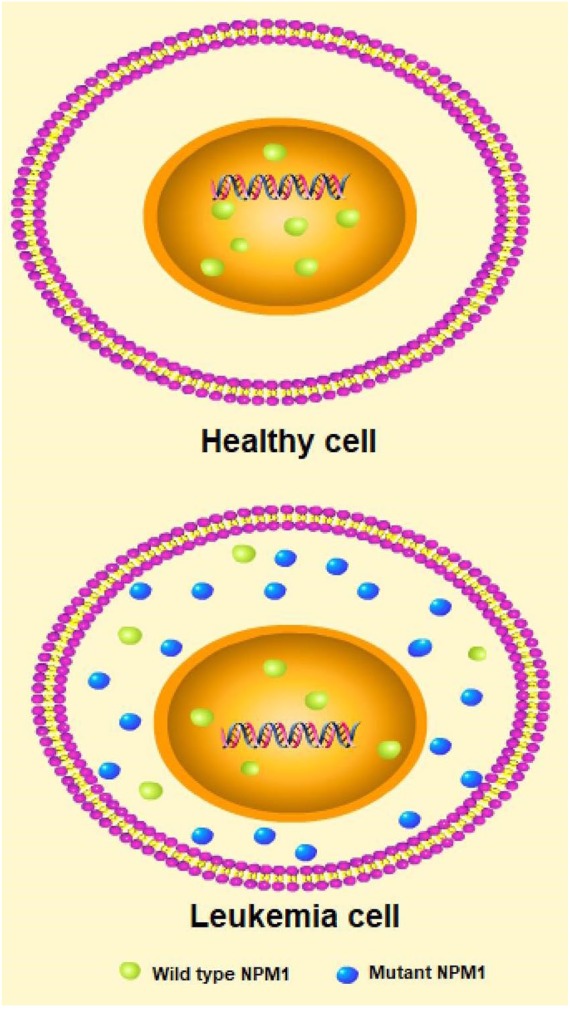
The cellular distribution of wild type and mutant NPM1 in a healthy cell and a leukemia cell.
NPM1, Nucleophosmin.
The expression of NPM1 is higher in proliferating cells than in quiescent cells,30 which may modulate cell cycle progression and centrosome duplication.31,32 Multifunctional characteristics of NPM1 also appear to regulate the various post-translational modifications of NPM1, such as acetylation, phosphorylation, polyubiquitination, and sumoylation.33–35 When NPM1 expression aberrantly increases, the protein acts as an oncogene via promoting abnormal cell survival.36 Conversely, NPM1 may play a critical role in modulating the growth-suppressive pathway due to its decreased expression, inhibition of NPM1 shuttling, or colocalization with other oncosuppressors, such as the ADP-ribosylation factor (ARF).24,37 In general, NPM1 involvement in cell proliferation is probably the result of several activities, which include modulation of ribosome biogenesis as well as interactions with histone oncosuppressor proteins.
Anti-NPM1 antibodies for the diagnosis of hematologic malignancies
In recent years, several studies have explored the utility of anti-NPM1 antibodies for monitoring therapeutic outcomes, or as indicators of cancer prognosis after treatment. Of those, the serum anti-NPM1 autoantibody has been shown to potentially function as a biomarker for the immunodiagnosis and prognosis of prostate cancer.38 For diagnostic purposes, three different types of antibodies directed against fixative-resistant epitopes of NPM1 have potential utility for immunohistochemistry in hematologic malignancies: those recognizing wt and mutant NPM1 proteins, and those specifically directed against either the mutant or the wt NPM1 protein. Monoclonal antibodies that recognize both wt and mutant NPM1 are the most reliable reagents for immunohistochemical diagnosis of AML with mutated NPM11,39,40; they label leukemic cells in cytoplasm (which contains mutant and wt NPM1) and the nucleus (which contains only wt NPM1).24,41 Polyclonal antibodies that recognize mutant but not wt NPM1 label only the cytoplasm of leukemic cells, providing more evidence that mutant NPM1 is completely dislocated in the cytoplasm.24,42,43 If a monoclonal antibody recognizing only wt NPM1 stains leukemia cells in the nucleus and cytoplasm, then this is an indication of AML with mutated NPM1, since the mutant recruits wt NPM1 into the cytoplasm. In this case, the best control for specificity of aberrant cytoplasmic expression of NPM1 is immunostaining with an antibody against nucleolin (NCL), which is another abundant shuttling nucleolar protein; in AML with mutated NPM1, the protein will be located only in the nucleus.40
It has been reported that a monoclonal antibody (T26) that recognizes 10 of the 21 known NPM1 mutants in AML cells did not cross react with wt NPM1 or unrelated cellular proteins when assessed by immunofluorescence and flow cytometry analysis. These data indicate that T26 may become a helpful tool for rapid molecular diagnosis of AML.44 The value of anti-NPM1 antibody-based immunohistochemistry in bone marrow biopsies and molecular analysis for the detection of NPM1 mutations was further evaluated by Woolthuis and colleagues from the University of Groningen.45 They observed a high percentage of concordance between the two methods of mutation detection. A small subgroup of patients showed discordant results from using the two methods, which could be caused by fixation and histotechnical factors as found in previously published studies.1,41,45–48 Moreover, cases with mutated NPM1 do not always show overt cytoplasmic staining of NPM1 on bone marrow biopsies with formalin fixation. Cytoplasmic NPM1 localization is not always caused by a conventional NPM1 mutation, and the authors suggest that, for the screening of NPM1 abnormalities, more information will be obtained via combining immunohistochemistry with molecular analysis.45
NPM1 mutations in human hematologic malignancies
NPM1 mutations in AML
Mutations in the NPM1 gene are the most frequent genetic abnormalities of AML, and are highly specific to de novo AML.1,5,7,44 NPM1 mutations usually cause a frameshift in the region encoding the C-terminus of the protein. The altered reading frame results in the disruption of a nucleolar localization signal and the introduction of an additional nuclear export signal; this generally results in aberrant expression of the mutated NPM1c+ protein.26,27,40,49,50 Mutations within NPM1 are a founder genetic alteration in AML, and the presence of NPM1c+ is critical for disease maintenance.
Clinically, NPM1 mutations have an important prognostic significance. Mutations in the NPM1 gene have been associated with a favorable prognosis in the absence of concomitant internal tandem duplications (ITD) of the fms-related tyrosine kinase 3 (FLT3) gene in cytogenetically normal acute myeloid leukemia (CN-AML).1,51–53 CN-AML is the largest and most heterogeneous cytogenetic AML subgroup. NPM1 is the most frequently encountered mutation in CN-AML.1 In the European Leukemia Net and other prognostic classifications, NPM1-mutated CN-AML in the absence of FLT3-ITD (NPM1mut/FLT3-ITD-negative) is considered part of the favorable genetic group.54,55 The comparative value of postremission treatment in patients with CN-AML subclassified by NPM1 and FLT3-ITD allelic ratio has been debated in a recent study. Among 521 patients with CN-AML in first complete remission (CR1), favorable overall survival (OS) was found for patients with mutated NPM1 without FLT3-ITD (71 ± 4%). The outcome in patients with a high FLT3-ITD allelic ratio appeared to be very poor, with OS and relapse-free survival (RFS) of 23 ± 8% and 12 ± 6%, respectively.56 A favorable outcome is further identified in older AML patients with NPM1mut/FLT3-ITD-negative who underwent allogeneic hematopoietic cell transplantation in CR1. These data offer encouraging possibilities compared with results from historical nontransplant approaches.57
Several studies have provided definitive evidence demonstrating that de novo AML with mutated NPM1 is frequently associated with a normal karyotype and frequent comutations in DNMT3A, IDH1/IDH2, and TET2, as well as, notably, in FLT3-ITD.5,51,52,57–59 It has been reported that IDH1 mutations may adversely impact the favorable prognosis associated with the NPM1-mutated/FLT3-ITD-negative (NPM1mut/FLT3-ITDneg) genotype, indicating that IDH1 mutation analysis might serve to refine prognostic stratification in NPM1-mutated AML cases without FLT3-ITD.60,61 Recently, a large study evaluated the potential prognostic impact of karyotype in 2426 intensively treated patients with NPM1mut/FLT3-ITDneg/low AML in a pooled analysis of individual patient data from nine international cohorts. Of these, 2000 patients (82.4%) had a normal karyotype and 426 (17.6%) had an abnormal karyotype. A total of 1845 patients with NPM1wt/FLT3-ITDneg/low and adverse-risk cytogenetics were identified in this international collaborative study. Of note, AML patients with NPM1 mutations harboring the FLT3-ITDneg/low genotype and adverse-risk cytogenetics were found to share the same unfavorable prognosis as their counterparts with wt NPM1.62 Interestingly, the investigators from the Wellcome Trust Sanger Institute in Cambridge found that the combination of NPM1c+ and Flt3-ITD had an early profound effect on gene expression and hematopoiesis. Also, both types of comutations drove AML in the majority of mice, and the leukemias in NPM1c Flt3-ITD mice were more aggressive and undifferentiated. Their data demonstrate that molecular synergy between NPM1c+ and Flt3-ITD underpins the co-occurrence patterns, phenotype, and prognosis of NPM1-mutant AML.63 More studies further revealed that the co-occurrence of DNMT3A, NPM1, and FLT3-ITD mutations represents a distinct entity with a very poor AML outcome.64–66 A recent study identified Hepatic Leukemia Factor (HLF) as a novel leukemic stem cell regulator in DNMT3A, NPM1, and FLT3-ITD triple-mutated AML, which is also genetically defined as a high-risk subgroup in AML.64 Furthermore, there are still some mutations that rarely co-occur with NPM1 mutations, such as partial tandem duplication in the mixed lineage leukemia (MLL) gene, and mutations in RUNX1, CEBPA, and TP53 genes.67
To characterize the genetic composition of NPM1-mutated AML, a study showed recently that NPM1c+ leukemia cells displayed increased transcription of stem-cell-associated genes, including HOXA, HOXB, and MEIS1.27 Relocalization of NPM1c+ to the nucleus resulted in a downregulation of the HOX/MEIS1 gene signature and subsequent differentiation of AML cells. These results demonstrate the potential therapeutic benefit of inducing nuclear relocalization of NPM1. Interestingly, when the mutation status of five recurrently mutated oncogenes in 129 paired NPM1 mutated samples obtained at diagnosis and relapse was assessed,21 the authors found a mild shift in the genetic pattern from diagnosis to relapse including the loss of NPM1 mutations. At the time of relapse, NPM1 mutation loss patients feature distinct mutational patterns that share almost no somatic mutation with the corresponding diagnosis sample, and affect different signaling pathways. By contrast, profiles of patients with the persistence of the NPM1 mutation are demonstrated to have a high overlap of mutations between diagnosis and relapse. A recent study further showed that upregulation of the HOXA5, HOXB5, HOXA10, PBX3, and MEIS1 genes was associated with AML cells harboring NPM1 gene mutations, which was also correlated with a worse prognosis in AML. The in vitro data in this latter study suggests that a complex involving the HOX genes with PBX3 and MEIS1 cofactors may behave as an advanced therapeutic target in NPM1-mutated AML patients.68 These results were consistent with previous findings showing that the gene expression profile of NPM1c+ AML is characterized by upregulation of genes involved in stem cell maintenance.53,69
Moreover, Brodska and colleagues described several fusion products with other genes resulting from chromosomal translocations in hematological malignancies in particular.70 The fusion proteins contain the NPM1 N-terminal domain, which serves mostly as an oligomerization interface promoting the oncogenic potential of the fusion partner. Of note, NPM-RAR, NPM-MLF1, or NPM-ALK fusions can be found specifically in acute promyelocytic leukemia (APL, AML-M3), myelodysplastic syndrome (MDS), or non-Hodgkin’s lymphoma (NHL), respectively. Recently, a unique NPM1–RARG–NPM1 chimeric fusion was discovered in an older male subject, who presented with morphological and immunophenotypical features of hypergranular APL, but lacked response to all-trans retinoic acid (ATRA) and arsenic trioxide [ATO (As2O3)] therapies. Further study demonstrated that the NPM1–RARG–NPM1 fusion leads to both impairment of the NPM1 protein and abnormal RARG, which contributes to impaired differentiation and leukogenesis.71
To investigate the correlation of NPM1 mutation with clinical features and biological characteristics, the NPM1 mutation was analyzed in bone marrow cells from 173 consecutive patients with de novo AML.12 The results revealed a remarkable difference in the incidence of NPM1 mutation between adult and pediatric patients. Children had a significantly lower incidence of NPM1 mutations than adults. Of note, NPM1 mutation presented at diagnosis and disappeared at complete remission (CR), and the same mutation reappeared at relapse, suggesting that the NPM1 mutation is probably an early event in the development of AML but may play little role in the progression of the disease. Another group found that cytoplasmic localization of the promyelocytic leukemia gene (PML) could be mediated by interacting with mutant NPM1, which could stabilize PML through inhibiting proteasome-mediated degradation, and further enhance autophagic activity and cell survival in AML. Their results indicate conclusively that pharmacological inhibitors of PML or autophagy are potential therapeutics for NPM1-mutated AML therapy.72
NPM1 mutations in chronic myelomonocytic leukemia
Chronic myelomonocytic leukemia (CMML) is a clonal hematopoietic stem cell disorder, characterized by overlapping features of both a myeloproliferative neoplasm and a myelodysplastic syndrome.18 It is a rare hematological malignancy that occurs in 0.3–0.52/100,000 patients.73 NPM1 mutations in the context of CMML are extremely infrequent. Zuo and colleagues reported that, of 152 patients with CMML who were tested, 8 (5%) were positive for the NPM1 mutation.74 Investigators from the Mayo Clinic identified 8 (2%) patients with NPM1 mutation in a total of 373 WHO-defined CMML patients.75 Similar results were analyzed in primary marrow samples from 150 patients with various chronic myeloid disorders. Of those, NPM1 mutations were detected in three (2%) patients, all of whom had CMML and less than 1-year survival.76 Notably, the previous findings show that patients with CMML and NPM1 mutations have a more aggressive clinical course and a higher probability of AML progression.
In comparison with those harboring the wt counterpart, NPM1-mutant CMML patients were more likely to be anemic, have a ‘dysplastic CMML subtype’, have an increased frequency of DNMT3A and FLT3-ITD, and a lower incidence of TET2 and ASXL1 mutations.75 To better understand the molecular events following acquisition of the NPM1 mutation in CMML patient, Bolli and colleagues performed exome sequencing of bone marrow DNA at CMML diagnosis, AML diagnosis, and first CR. They found that DNMT3A and TET2 mutations were acquired first, followed by NPM1 and CEBPA mutations. All four mutations had high variant allele frequencies (VAF) in CMML, which is consistent with a clonal sweep after acquisition of the last mutation (CEBPA or NPM1) and prior to AML transformation. Mutations affecting NRAS and FLT3 were almost undetectable in CMML, but present at high VAF in AML samples.77
NPM1 mutations in MDS
MDS is a heterogeneous group of chronic myeloid neoplasms in which progression to secondary AML (sAML) is common.78–81 To elucidate differential roles of mutations in MDS, the investigators analyzed clonal dynamics using whole-exome or targeted sequencing based on 699 patient samples. Of those, 122 samples were analyzed longitudinally. In comparison with high-risk MDS, FLT3, PTPN11, WT1, IDH1, NPM1, IDH2, and NRAS mutations tended to be newly acquired, and were associated with faster sAML progression and a shorter OS time.78 Similar to the incidence in CMML, NPM1 mutations are very rare in MDS or MDS/myeloproliferative neoplasm (MPN). Only 31 (1.6%) of 1900 patients with newly diagnosed MDS or MDS/MPN had NPM1 mutations. The authors found those NPM1-mutated patients had distinct clinical features, were younger, and likely to be female; they also had lower hemoglobin, higher median bone marrow blast percentage at diagnosis, and a higher frequency of normal karyotype compared with wt NPM1 patients. In this regard, these data were in agreement with recent findings, which suggest that patients with NPM1-mutant MDS or MDS/MPN who are candidates for intensive therapeutic strategies and allogeneic stem cell transplantation may have improved outcomes and may benefit the most from chemotherapy, rather than MDS-based treatment approaches.82
NPM1 translocations in lymphomas
The NPM1 gene is translocated in CD30+ anaplastic large-cell lymphoma (ALCL) and in rare variants of AML.83 Due to the loss of one functional allele of the NPM1 gene, cells from these tumors contain an oncogenic fusion protein (NPM1–ALK, NPM–RARα or NPM1–MLF1) and a reduced level of wt NPM1. Because of ALK gene translocations, about 60% of ALCL express the ALK protein. ALK+ T cell lymphoma is an aggressive neoplasm, and around 85% of ALK+ ALCL carry the t(2;5)(p23;q35) chromosome translocation,83,84 in which the ALK gene on chromosome 2 is fused with the NPM1 gene on chromosome 5.85 The chimeric gene encodes a fusion protein comprising the amino-terminal portion of NPM1 and the entire cytoplasmic region of ALK. Lymphomas with this condition characteristically express the ALK protein both in the cytoplasm and, ectopically, in the nucleus. The NPM1 oligomerization domain promotes NPM1–ALK heterodimer formation with wt NPM1, which, in turn, via shuttling, imports NPM1-ALK into the nucleoli. Meanwhile, due to the presence of the NPM1–ALK fusion protein, ALCL cells also show aberrant NPM1 cytoplasmic expression.86
More than 80% of ALK+ T cell lymphoma cases harbor the NPM1–ALK oncogene; NPM1–ALK functions as a constitutively activated cytoplasmic tyrosine kinase that is capable of translocating to the nucleus.87 The activation of NPM1-ALK induces activation of several downstream signaling pathways, such as Janus kinases/signal transducers and activators of transcription (JAK/STAT) and Ras/mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK), all of which play crucial roles in cell survival and proliferation in ALK+ NHLs.88–92 A previous study indicated that NPM acts through its heterodimerization domain to bind sequences located in the transactivation domain (TAD) of the Forkhead Box M1(FOXM1), an oncogenic transcription factor.93 A recent study showed that FOXM1 is highly expressed in ALK+ lymphoma and contributes to its oncogenesis. The authors found that the NPM1 portion in the NPM1-ALK fusion protein was crucial for binding to FOXM1, as the ALK portion alone cannot efficiently interact with FOXM1. This study provided evidence of the important pathogenetic role of NPM1 in ALK+ lymphoma. Disruption of the binding between FOXM1 and NPM1 in heterodimers may serve as a highly specific anticancer therapeutic approach for NPM1-ALK+ lymphoma.94
NPM1 overexpression in solid tumors and hematologic malignancies
NPM1 overexpression has been found in numerous human solid tumors, and has been extensively implicated as a biomarker of poor prognosis.36,95–98 When NPM1 expression is significantly increased, it may function as an oncogene by promoting aberrant cell growth through enhancement of ribosome machinery.36 A previous study showed the upregulation of NPM1 transcripts in colorectal cancer (CRC) cell lines, and increasing NPM1 protein expression with progression from normal colon to adenoma to CRC in a tissue microarray. Modulation of NPM1 expression occurred within CRC cells, affecting cellular viability, p53-dependent senescence, and cell-cycle progression, indicating that NPM1 may play a fundamental role in colorectal carcinogenesis.97 The high expression of NPM1 has also been reported to link to gradient drug resistance in bladder cancer,99,100 lung cancer,15 hepatoma carcinoma,101 and breast carcinoma.102 The downregulation of NPM1 expression markedly reversed the effects of multidrug resistance (MDR) in human hepatoma cells via inhibition of P-glycoprotein expression.101
To date, relatively little is known about the role of NPM1 overexpression in hematologic malignancies. A recent study identified mutated chronic lymphocytic leukemia cells that were characterized by a MYC-related overexpression of NPM1 and ribosome-associated components.103 In our previously reported work, we first validated that overexpression of NPM1 and NCL may be involved in drug resistance, and might be an important indicator for prognosis evaluation in AML and in acute lymphoblastic leukemia (ALL).104 We also reported that knockdown of NPM1 by RNA interference may reverse MDR in resistant leukemic cells.105 Further investigation by our group found that knockdown of NPM1 reversed drug resistance by downregulating P-gp and the Akt/mTOR signal pathway, indicating that NPM1 may serve as a potential modulator for drug resistance.106 Our findings were in agreement with a previous report in human hepatoma cells.101 Moreover, we recently found that high expression of NCL, another important nucleocytoplasmic multifunctional protein, promotes drug resistance in Burkitt’s lymphoma, which may be related to the stabilization of Bcl-2 mRNA and the decreased induction of apoptosis.107 Currently, ongoing studies in our group are aimed at better understanding the interacting systems of NPM1 and NCL in hematologic malignancies. Meanwhile, we are screening and assessing new therapeutic strategies and agents that we expect to facilitate the targeting NPM1 and NCL and to improve the outcome of patients with hematologic malignancies.
Mutated NPM1 as a biomarker for assessment of residual disease in AML
Mutated NPM1 is a reliable biomarker for assessment of disease status in AML. A previous study in 173 patients of de novo AML demonstrated that NPM1 mutations occur in an age-dependent fashion. The NPM1 mutation disappeared with CR, but the same mutation reappeared at relapse.12 A study involving a large cohort of intensively treated AML patients further showed that the NPM1 mutation is an excellent marker for prediction of residual disease. The persistence of NPM1-mutated transcripts in blood after the second chemotherapy cycle was associated with a greater risk of relapse after 3 years of follow-up. NPM1 mutations were detected in 69 of 70 AML patients at the time of relapse. In this study, the authors addressed that the presence of MRD, as determined by quantification of NPM1-mutated transcripts, and found that MRD might provide valuable prognostic information independent of other risk factors.10 Moreover, another study recently found that mutated NPM1 is of particular importance for monitoring disease dynamics in AML patients. A good initial response is essential to reach lower NPM1 levels after treatment. A good initial mutated NPM1 clearing cannot prevent relapse, but postpones it. However, this postponement positively correlates with OS. This study emphasized that the most informative time point for the determination of the minimal NPM1 measurement as a predictor for survival and relapse risk is 9 months after therapy start.108 These findings might impact the design of further studies and guide novel MRD-guided therapeutic strategies in AML.
Targeting NPM1 mutation therapy in AML
NPM1 acts as a hub protein and is involved in trafficking other proteins to the nucleolus. Consequently, it is essential for the formation and maintenance of a functional nucleolus. NPM1 mutations act deterministically to promote nuclear export of the mutants, and aberrant cytoplasmic delocalization of NPM1 is thought to be critical for leukaemogenesis.6,26,50 However, the manner in which the NPM1 mutation disrupts cell control and induces leukemia remains unclear. Thus, the consequences of targeting NPM1, either wt or mutant, remain worth exploring. To date, the potential approaches for targeting NPM1-mutated AML include the following22: interfering with the aberrant transport of the NPM1 mutant protein; interfering with wt NPM1 functions; demethylating agents; immunotherapy with monoclonal antibodies; ATRA and arsenic trioxide. Promising molecules for the therapeutic targeting of mutant NPM1 in AML are described in the following and summarized in Table 1.
Table 1.
Promising molecules for therapeutic targeting of mutant NPM1 in AML.
| Molecules | Target cells | Consequences in cellular processes | Study |
|---|---|---|---|
| ATRA/ATO | OCI-AML3 and IMS-M2 (NPM1 mutated cell lines) | Proteasome-dependent degradation of NPM1c protein | Martelli109 |
| AML patient primary cells with NPM1 mutation | Oxidative stress induction/cell apoptosis | ||
| EAPB0503 | OCI-AML3 | Proteasome-mediated degradation of NPM1c protein | Nabbouh110 |
| OCI-AML3 xenograft mice | Restore wt-NPM1 nucleolar localization/growth arrest/apoptosis | ||
| NSC348884 | OCI-AML3 | Interference with the oligomerization of NPM1 | Balusu111 |
| AML patient primary cells with NPM1 mutation | Cell apoptosis/sensitize NPM1c+ AML cells to ATRA | ||
| MI-2-2 | OCI-AML3 | Inhibition of menin-MLL1 and DOT1L | Kuhn112 |
| EPZ4777 | AML patient primary cells with NPM1 mutation | Suppression of HOX and FLT3 expression | |
| MI-503 | OCI-AML3 xenograft mice | Cell differentiation/inhibition NPM1mut leukemia initiation |
AML, acute myeloid leukemia; ATO, arsenic trioxide; ATRA, all-trans retinoic acid; EAPB0503, 1-(3-methoxyphenyl)-N-methylimidazoquinoxalin-4-amine; FLT3, fms-related tyrosine kinase 3; HOX, homeobox; NPM1, nucleophosmin; wt, wild type.
NPM1-mutated leukemia cells display increased transcription of stem cell-associated genes such as the clustered HOX genes. Aberrant HOX expression is found in almost all AML cells that harbor a mutated NPM1 gene, and FLT3 is concomitantly mutated in about 60% of these cases.52,68,113 Little is known about how mutant NPM1 cells maintain aberrant gene expression. A recent study demonstrated that the chromatin regulators MLL1 and DOT1L control HOX and FLT3 expression in mutated NPM1 AML. These genes are therapeutically targetable via the pharmaceutical inhibition of menin-MLL1 and DOT1L. Small-molecule inhibition of these two histone modifiers results in the differentiation of mutated NPM1 AML cells in vitro and in vivo. This study indicated that both menin-MLL1 and DOT1L inhibitors, as single agents or in combination, represent novel therapeutic opportunities for NPM1-mutated AML.112
Imiquimod is a toll-like receptor 7 immunomodulator. It has been reported that the imiquimod analog 1-(3-methoxyphenyl)-N-methylimidazoquinoxalin-4-amine (EAPB0503) has promising antitumor activity, which could selectively induce NPM1c+ proteasomal degradation in NPM1c+ AML cells and lead to their apoptosis. Nevertheless, EAPB0503 treatment restores wt NPM1 nucleolar localization in vitro and in ex vivo treated blasts, and it selectively reduces the leukemia burden in NPM1c+ AML xenograft mice.110 These findings reinforce the idea of targeting the NPM1c+ oncoprotein to eradicate leukemic cells using this promising drug.
NPM1 is translocated or mutated in various lymphomas and leukemias, forming fusion proteins or NPM1-mutant products.86,87 Several studies have shown that small molecule inhibitors may function as anticancer and antileukemic agents via disrupting NPM1 dimer/oligomer formation, which can result in growth inhibition and apoptosis in cancer cells.111,114,115 Among those inhibitors, NSC348884 has been shown to be lethal for various cancer cell-types with mutant NPM1.111,116,117 For leukemia, NSC348884 induced apoptosis and sensitized cultured and primary AML cells with NPM1 mutation to ATRA, but did not impact those AML cells coexpressing FLT3-ITD or normal CD34+ progenitor cells expressing wt NPM1.111 Currently, these molecules have not been approved for AML patients with NPM1 mutations. However, there is growing interest in developing novel molecules aimed at targeting NPM1 mutations, which might provide a more theoretical basis and scientific evidence for the anti-NPM1 agents in future preclinical in vivo studies.
ATRA or ATO are highly effective molecular targeted therapies in APL with promyelocytic PMLRARα gene rearrangement.118 The NPM1 mutation is frequent and represents a founder genetic lesion in AML. Interestingly, data from a previous study showed that elderly patients with NPM1-mutant AML appeared to benefit from ATRA treatment.119 Moreover, it was reported that AML cells carrying NPM1 mutations are more sensitive to ATO. The expression of NPM1-mutant protein with an acquired C-terminal cysteine-288 may enhance the sensitivity of AML cells to oxidative stress induced by ATO.120 The mechanisms underlying this action were independently reported by two studies. The NPM1-mutant oncoprotein can be a target of ATO/ATRA, which induces proteasome-dependent degradation of NPM1 leukemic protein and apoptosis in NPM1-mutated AML cell lines and the cells of primary patients. PML intracellular distribution is altered in NPM1-mutated AML cells, and reverted by ATO via oxidative stress induction.109 The combination of ATO and ATRA significantly reduced leukemic blasts in the bone marrow of 3 NPM1-mutant AML patients, and restored nucleolar localization of NPM1 and PML both in vitro and in vivo.121 Collectively, these findings provide evidence that the ATO/ATRA strategy may represent a viable option in NPM1-mutant AML.
Other therapeutic strategies associated with NPM1 mutation in AML
The precise mechanism of action of the NPM1 mutant in AML is not well known, and other contributing events and therapeutic strategies still need to be investigated. Several studies have evidenced that NPM1-mutated AML cells strongly express CD33.122–124 However, in the same study, the authors also found no correlation between FLT3 gene mutations and CD33 expression in NPM1-mutated AML cells.123 These results establish a rational basis for the therapeutic use of the anti-CD33 antibody in NPM1-mutated AML cells. A previous study reported that Dactinomycin may induce nucleolar stress by influencing ribosome biogenesis through the inhibition of RNA polymerase I,125 which is active in Wilms’ tumor and other cancers. Similar therapeutic effects of targeting NPM1 mediated by Dactinomycin in AML cells were also presented by Falini and colleages.20 They hypothesized that the nucleolus of NPM1-mutated AML cells might be vulnerable to Dactinomycin, and trigger a nucleolar stress response. They observed that one patient with NPM1-mutated AML without FLT3 internal tandem duplication mutations achieved morphologic and immunohistochemical CR after two cycles of Dactinomycin therapy.20 Moreover, Actinomycin D (actD), another chemotherapy drug used widely in various cancer types, has been found to induce cell death in all studied leukemic cell lines via an increase in nucleolar stress leading to a redistribution of mutated NPM1 in the nucleoplasm.126
More recent work from the University of Nottingham showed that DNA damage caused by drugs may induce a switch in the aberrant cytoplasmic localization of NPM1c+ to a predominantly nucleolar localization in NPM1-mutated AML cells. Their results showed that the exploitation of nucleolar NPM1-replete cells to treat nucleolar stress would be effective only in the absence of DNA damage.19 Going forward, immune responses may contribute to clinical outcomes via lysis of residual leukemic cells through specific T cells after chemotherapy. NPM1 mutations are one of the most frequent molecular alterations in AML. Algorithm-based CD4+ and CD8+ T-cell epitopes derived from mutated NPM1 were reported to feasibly elicit a coordinative immune response against NPM1-mutated AML cells, suggesting that NPM1 mutations might constitute an ideal target structure for individualized immunotherapeutic approaches.127 Recently, an unusual case of CMML harboring an NPM1 mutation associated with extensive myeloid sarcomas was reported.128 The patient had rapid resolution of lymphadenopathy, and attained remission after being administered idarubicin and cytarabine induction chemotherapy, in addition to a matched unrelated donor allogeneic bone marrow transplant. This study highlights that the NPM1 chemosensitivity noted in AML might be applicable to other hematological conditions. Thus, other potential multiple therapeutic interventions are certainly worthy of further investigation.
Closing remarks
The answers to several questions regarding the role of NPM1 abnormality in the development of hematopoietic malignancies remain unclear. More studies are required for a better understanding of the underlying mechanisms associated with the development of drug resistance caused by NPM1 overexpression. Moreover, NPM1 gene alternation status is not a single parameter in predicting clinical significance in hematopoietic malignancies. Many studies are currently ongoing with the aim of elucidating how NPM1 abnormalities contribute to oncogenesis, and to develop strategies to take advantage of specific characteristics for the improvement of therapy. In this review, we provide a summary of current knowledge in this field. Importantly, present investigations may eventually lead to the development of more specific antihematopoietic malignancy strategies in the future.
Footnotes
Author contributions: Writing original draft: Y.C.; Writing, reviewing, and editing: Y.C., J.H.; Funding acquisition: Y.C, J.H.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: Y.C. and J.H. were supported by Joint Funds for the Innovation of Science and Technology in Fujian province(2016Y9029, 2016Y9032), and National Natural Science Foundation of China (81500158, 81470326); Y.C. was the recipient of grants from Fujian Provincial Natural Science Foundation(2015J05152), and the Program of New Century Excellent Talents in Fujian Province University(2016B032); J.H. was the recipient of funding from the Cooperation Project of University and Industry (2017Y4005), Backbone Talent Training Project of Fujian Provincial Health Commission (2019-ZQN-42) Backbone Talent Training Project of Fujian Provincial Health Commission (2019-ZQN-42), and the Program of New Century; This work was also supported by the Construction Project of Fujian Medical Center of Hematology(Min201704).
Conflict of interest statement: The author(s) declare no conflicts of interest in preparing this article.
ORCID iD: Yingyu Chen  https://orcid.org/0000-0003-1243-3607
https://orcid.org/0000-0003-1243-3607
Contributor Information
Yingyu Chen, Department of Hematology, Fujian Institute of Hematology, Fujian Medical University Union Hospital, No.29 Xinquan Road, Fuzhou, Fujian 350001, China.
Jianda Hu, Department of Hematology, Fujian Institute of Hematology, Fujian Medical University Union Hospital, Fuzhou, Fujian, China.
References
- 1. Falini B, Mecucci C, Tiacci E, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med 2005; 352: 254–266. [DOI] [PubMed] [Google Scholar]
- 2. Borer RA, Lehner CF, Eppenberger HM, et al. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell 1989; 56: 379–390. [DOI] [PubMed] [Google Scholar]
- 3. Colombo E, Alcalay M, Pelicci PG. Nucleophosmin and its complex network: a possible therapeutic target in hematological diseases. Oncogene 2011; 30: 2595–2609. [DOI] [PubMed] [Google Scholar]
- 4. Yung BY, Busch H, Chan PK. Translocation of nucleolar phosphoprotein B23 (37 kDa/pI 5.1) induced by selective inhibitors of ribosome synthesis. Biochim Biophys Acta 1985; 826: 167–173. [DOI] [PubMed] [Google Scholar]
- 5. Papaemmanuil E, Dohner H, Campbell PJ. Genomic classification in acute myeloid leukemia. N Engl J Med 2016; 375: 900–901. [DOI] [PubMed] [Google Scholar]
- 6. Liso A, Bogliolo A, Freschi V, et al. In human genome, generation of a nuclear export signal through duplication appears unique to nucleophosmin (NPM1) mutations and is restricted to AML. Leukemia 2008; 22: 1285–1289. [DOI] [PubMed] [Google Scholar]
- 7. Kandoth C, McLellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature 2013; 502: 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Falini B, Nicoletti I, Martelli MF, et al. Acute myeloid leukemia carrying cytoplasmic/mutated nucleophosmin (NPMc+ AML): biologic and clinical features. Blood 2007; 109: 874–885. [DOI] [PubMed] [Google Scholar]
- 9. Falini B, Sportoletti P, Martelli MP. Acute myeloid leukemia with mutated NPM1: diagnosis, prognosis and therapeutic perspectives. Curr Opin Oncol 2009; 21: 573–581. [DOI] [PubMed] [Google Scholar]
- 10. Ivey A, Hills RK, Simpson MA, et al. Assessment of minimal residual disease in standard-risk AML. N Engl J Med 2016; 374: 422–433. [DOI] [PubMed] [Google Scholar]
- 11. Falini B, Martelli MP, Bolli N, et al. Acute myeloid leukemia with mutated nucleophosmin (NPM1): is it a distinct entity? Blood 2011; 117: 1109–1120. [DOI] [PubMed] [Google Scholar]
- 12. Chou WC, Tang JL, Lin LI, et al. Nucleophosmin mutations in de novo acute myeloid leukemia: the age-dependent incidences and the stability during disease evolution. Cancer Res 2006; 66: 3310–3316. [DOI] [PubMed] [Google Scholar]
- 13. Falini B, Martelli MP, Mecucci C, et al. Cytoplasmic mutated nucleophosmin is stable in primary leukemic cells and in a xenotransplant model of NPMc+ acute myeloid leukemia in SCID mice. Haematologica 2008; 93: 775–779. [DOI] [PubMed] [Google Scholar]
- 14. Meloni G, Mancini M, Gianfelici V, et al. Late relapse of acute myeloid leukemia with mutated NPM1 after eight years: evidence of NPM1 mutation stability. Haematologica 2009; 94: 298–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim CK, Nguyen TL, Lee SB, et al. Akt2 and nucleophosmin/B23 function as an oncogenic unit in human lung cancer cells. Exp Cell Res 2011; 317: 966–975. [DOI] [PubMed] [Google Scholar]
- 16. Martelli MP, Sportoletti P, Tiacci E, et al. Mutational landscape of AML with normal cytogenetics: biological and clinical implications. Blood Rev 2013; 27: 13–22. [DOI] [PubMed] [Google Scholar]
- 17. Sportoletti P, Varasano E, Rossi R, et al. Mouse models of NPM1-mutated acute myeloid leukemia: biological and clinical implications. Leukemia 2015; 29: 269–278. [DOI] [PubMed] [Google Scholar]
- 18. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the world health organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127: 2391–2405. [DOI] [PubMed] [Google Scholar]
- 19. Bailey GD, Qutob HMH, Akhtar A, et al. DNA damage corrects the aberrant cytoplasmic localisation of nucleophosmin in NPM1 mutated acute myeloid leukaemia. Br J Haematol 2019; 186: 343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Falini B, Brunetti L, Martelli MP. Dactinomycin in NPM1-mutated acute myeloid leukemia. N Engl J Med 2015; 373: 1180–1182. [DOI] [PubMed] [Google Scholar]
- 21. Cocciardi S, Dolnik A, Kapp-Schwoerer S, et al. Clonal evolution patterns in acute myeloid leukemia with NPM1 mutation. Nat Commun 2019; 10: 2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Falini B, Sportoletti P, Brunetti L, et al. Perspectives for therapeutic targeting of gene mutations in acute myeloid leukaemia with normal cytogenetics. Br J Haematol 2015; 170: 305–322. [DOI] [PubMed] [Google Scholar]
- 23. Chan WY, Liu QR, Borjigin J, et al. Characterization of the cDNA encoding human nucleophosmin and studies of its role in normal and abnormal growth. Biochemistry 1989; 28: 1033–1039. [DOI] [PubMed] [Google Scholar]
- 24. Falini B. Acute myeloid leukemia with mutated nucleophosmin (NPM1): molecular, pathological, and clinical features. Cancer Treat Res 2010; 145: 149–168. [DOI] [PubMed] [Google Scholar]
- 25. Kunchala P, Kuravi S, Jensen R, et al. When the good go bad: mutant NPM1 in acute myeloid leukemia. Blood Rev 2018; 32: 167–183. [DOI] [PubMed] [Google Scholar]
- 26. Falini B, Bolli N, Liso A, et al. Altered nucleophosmin transport in acute myeloid leukaemia with mutated NPM1: molecular basis and clinical implications. Leukemia 2009; 23: 1731–1743. [DOI] [PubMed] [Google Scholar]
- 27. Brunetti L, Gundry MC, Sorcini D, et al. Mutant NPM1 maintains the leukemic state through HOX expression. Cancer Cell 2018; 34: 499–512. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Herrera JE, Correia JJ, Jones AE, et al. Sedimentation analyses of the salt- and divalent metal ion-induced oligomerization of nucleolar protein B23. Biochemistry 1996; 35: 2668–2673. [DOI] [PubMed] [Google Scholar]
- 29. Nishimura Y, Ohkubo T, Furuichi Y, et al. Tryptophans 286 and 288 in the C-terminal region of protein B23.1 are important for its nucleolar localization. Biosci Biotechnol Biochem 2002; 66: 2239–2242. [DOI] [PubMed] [Google Scholar]
- 30. Dergunova N, Bulycheva TI, Artemenko EG, et al. A major nucleolar protein B23 as a marker of proliferation activity of human peripheral lymphocytes. Immunol Lett 2002; 83: 67–72. [DOI] [PubMed] [Google Scholar]
- 31. Okuda M, Horn HF, Tarapore P, et al. Nucleophosmin/B23 is a target of CDK2/cyclin E in centrosome duplication. Cell 2000; 103: 127–140. [DOI] [PubMed] [Google Scholar]
- 32. Okuda M. The role of nucleophosmin in centrosome duplication. Oncogene 2002; 21: 6170–6174. [DOI] [PubMed] [Google Scholar]
- 33. Tokuyama Y, Horn HF, Kawamura K, et al. Specific phosphorylation of nucleophosmin on Thr199 by cyclin-dependent kinase 2-cyclin E and its role in centrosome duplication. J Biol Chem 2001; 276: 21529–21537. [DOI] [PubMed] [Google Scholar]
- 34. Liu X, Liu Z, Jang SW, et al. Sumoylation of nucleophosmin/B23 regulates its subcellular localization, mediating cell proliferation and survival. Proc Natl Acad Sci USA 2007; 104: 9679–9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shandilya J, Swaminathan V, Gadad SS, et al. Acetylated NPM1 localizes in the nucleoplasm and regulates transcriptional activation of genes implicated in oral cancer manifestation. Mol Cell Biol 2009; 29: 5115–5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ruggero D, Pandolfi PP. Does the ribosome translate cancer? Nat Rev Cancer 2003; 3: 179–192. [DOI] [PubMed] [Google Scholar]
- 37. Yu Y, Maggi LB, Jr., Brady SN, et al. Nucleophosmin is essential for ribosomal protein L5 nuclear export. Mol Cell Biol 2006; 26: 3798–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dai L, Li J, Xing M, et al. Using serological proteome analysis to identify serum anti-nucleophosmin 1 autoantibody as a potential biomarker in European-American and African-American patients with prostate cancer. Prostate 2016; 76: 1375–1386. [DOI] [PubMed] [Google Scholar]
- 39. Cordell JL, Pulford KA, Bigerna B, et al. Detection of normal and chimeric nucleophosmin in human cells. Blood 1999; 93: 632–642. [PubMed] [Google Scholar]
- 40. Falini B, Bolli N, Shan J, et al. Both carboxy-terminus NES motif and mutated tryptophan(s) are crucial for aberrant nuclear export of nucleophosmin leukemic mutants in NPMc+ AML. Blood 2006; 107: 4514–4523. [DOI] [PubMed] [Google Scholar]
- 41. Falini B, Martelli MP, Bolli N, et al. Immunohistochemistry predicts nucleophosmin (NPM) mutations in acute myeloid leukemia. Blood 2006; 108: 1999–2005. [DOI] [PubMed] [Google Scholar]
- 42. Pasqualucci L, Liso A, Martelli MP, et al. Mutated nucleophosmin detects clonal multilineage involvement in acute myeloid leukemia: Impact on WHO classification. Blood 2006; 108: 4146–4155. [DOI] [PubMed] [Google Scholar]
- 43. Quentmeier H, Martelli MP, Dirks WG, et al. Cell line OCI/AML3 bears exon-12 NPM gene mutation-A and cytoplasmic expression of nucleophosmin. Leukemia 2005; 19: 1760–1767. [DOI] [PubMed] [Google Scholar]
- 44. Gruszka AM, Lavorgna S, Consalvo MI, et al. A monoclonal antibody against mutated nucleophosmin 1 for the molecular diagnosis of acute myeloid leukemias. Blood 2010; 116: 2096–2102. [DOI] [PubMed] [Google Scholar]
- 45. Woolthuis CM, Mulder AB, Verkaik-Schakel RN, et al. A single center analysis of nucleophosmin in acute myeloid leukemia: value of combining immunohistochemistry with molecular mutation analysis. Haematologica 2013; 98: 1532–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Luo J, Qi C, Xu W, et al. Cytoplasmic expression of nucleophosmin accurately predicts mutation in the nucleophosmin gene in patients with acute myeloid leukemia and normal karyotype. Am J Clin Pathol 2010; 133: 34–40. [DOI] [PubMed] [Google Scholar]
- 47. Konoplev S, Huang X, Drabkin HA, et al. Cytoplasmic localization of nucleophosmin in bone marrow blasts of acute myeloid leukemia patients is not completely concordant with NPM1 mutation and is not predictive of prognosis. Cancer 2009; 115: 4737–4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Falini B, Martelli MP, Pileri SA, et al. Molecular and alternative methods for diagnosis of acute myeloid leukemia with mutated NPM1: flexibility may help. Haematologica 2010; 95: 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Grummitt CG, Townsley FM, Johnson CM, et al. Structural consequences of nucleophosmin mutations in acute myeloid leukemia. J Biol Chem 2008; 283: 23326–23332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bolli N, Nicoletti I, De Marco MF, et al. Born to be exported: COOH-terminal nuclear export signals of different strength ensure cytoplasmic accumulation of nucleophosmin leukemic mutants. Cancer Res 2007; 67: 6230–6237. [DOI] [PubMed] [Google Scholar]
- 51. Dohner K, Schlenk RF, Habdank M, et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood 2005; 106: 3740–3746. [DOI] [PubMed] [Google Scholar]
- 52. Schnittger S, Schoch C, Kern W, et al. Nucleophosmin gene mutations are predictors of favorable prognosis in acute myelogenous leukemia with a normal karyotype. Blood 2005; 106: 3733–3739. [DOI] [PubMed] [Google Scholar]
- 53. Verhaak RG, Goudswaard CS, van Putten W, et al. Mutations in nucleophosmin (NPM1) in acute myeloid leukemia (AML): association with other gene abnormalities and previously established gene expression signatures and their favorable prognostic significance. Blood 2005; 106: 3747–3754. [DOI] [PubMed] [Google Scholar]
- 54. Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017; 129: 424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010; 115: 453–474. [DOI] [PubMed] [Google Scholar]
- 56. Versluis J, In ‘t, Hout FE, Devillier R, et al. Comparative value of post-remission treatment in cytogenetically normal AML subclassified by NPM1 and FLT3-ITD allelic ratio. Leukemia 2017; 31: 26–33. [DOI] [PubMed] [Google Scholar]
- 57. Aldoss I, Nakamura R, Yang D, et al. Favorable outcomes for allogeneic hematopoietic cell transplantation in elderly patients with NPM1-mutated and FLT3-ITD-negative acute myeloid leukemia. Bone Marrow Transplant. Epub ahead of print 14 May 2019. DOI: 10.1038/s41409-019-0553-x. [DOI] [PubMed] [Google Scholar]
- 58. Patel SS, Kuo FC, Gibson CJ, et al. High NPM1-mutant allele burden at diagnosis predicts unfavorable outcomes in de novo AML. Blood 2018; 131: 2816–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Patel SS, Ho C, Ptashkin RN, et al. Clinicopathologic and genetic characterization of nonacute NPM1-mutated myeloid neoplasms. Blood Adv 2019; 3: 1540–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Paschka P, Schlenk RF, Gaidzik VI, et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol 2010; 28: 3636–3643. [DOI] [PubMed] [Google Scholar]
- 61. Marcucci G, Maharry K, Wu YZ, et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a cancer and leukemia group B study. J Clin Oncol 2010; 28: 2348–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Angenendt L, Rollig C, Montesinos P, et al. Chromosomal abnormalities and prognosis in NPM1-mutated acute myeloid leukemia: a pooled analysis of individual patient data from nine international cohorts. J Clin Oncol 2019; 37: 2632–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dovey OM, Cooper JL, Mupo A, et al. Molecular synergy underlies the co-occurrence patterns and phenotype of NPM1-mutant acute myeloid leukemia. Blood 2017; 130: 1911–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Garg S, Reyes-Palomares A, He L, et al. Hepatic leukemia factor is a novel leukemic stem cell regulator in DNMT3A, NPM1, and FLT3-ITD triple-mutated AML. Blood 2019; 134: 263–276. [DOI] [PubMed] [Google Scholar]
- 65. Loghavi S, Zuo Z, Ravandi F, et al. Clinical features of de novo acute myeloid leukemia with concurrent DNMT3A, FLT3 and NPM1 mutations. J Hematol Oncol 2014; 7: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Guryanova OA, Shank K, Spitzer B, et al. DNMT3A mutations promote anthracycline resistance in acute myeloid leukemia via impaired nucleosome remodeling. Nat Med 2016; 22: 1488–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cancer Genome Atlas Research Network, Ley TJ, Miller C, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 2013; 368: 2059–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nagy A, Osz A, Budczies J, et al. Elevated HOX gene expression in acute myeloid leukemia is associated with NPM1 mutations and poor survival. J Adv Res 2019; 20: 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Alcalay M, Tiacci E, Bergomas R, et al. Acute myeloid leukemia bearing cytoplasmic nucleophosmin (NPMc+ AML) shows a distinct gene expression profile characterized by up-regulation of genes involved in stem-cell maintenance. Blood 2005; 106: 899–902. [DOI] [PubMed] [Google Scholar]
- 70. Brodska B, Sasinkova M, Kuzelova K. Nucleophosmin in leukemia: consequences of anchor loss. Int J Biochem Cell Biol 2019; 111: 52–62. [DOI] [PubMed] [Google Scholar]
- 71. Chen X, Wang F, Zhang Y, et al. A novel NPM1-RARG-NPM1 chimeric fusion in acute myeloid leukaemia resembling acute promyelocytic leukaemia but resistant to all-trans retinoic acid and arsenic trioxide. Br J Cancer 2019; 120: 1023–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zou Q, Tan S, Yang Z, et al. NPM1 Mutant Mediated PML delocalization and stabilization enhances autophagy and cell survival in leukemic cells. Theranostics 2017; 7: 2289–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Guru Murthy GS, Dhakal I, Mehta P. Incidence and survival outcomes of chronic myelomonocytic leukemia in the United States. Leuk Lymphoma 2017; 58: 1648–1654. [DOI] [PubMed] [Google Scholar]
- 74. Peng J, Zuo Z, Fu B, et al. Chronic myelomonocytic leukemia with nucleophosmin (NPM1) mutation. Eur J Haematol 2016; 96: 65–71. [DOI] [PubMed] [Google Scholar]
- 75. Vallapureddy R, Lasho TL, Hoversten K, et al. Nucleophosmin 1 (NPM1) mutations in chronic myelomonocytic leukemia and their prognostic relevance. Am J Hematol 2017; 92: E614–E618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Caudill JS, Sternberg AJ, Li CY, et al. C-terminal nucleophosmin mutations are uncommon in chronic myeloid disorders. Br J Haematol 2006; 133: 638–641. [DOI] [PubMed] [Google Scholar]
- 77. Grove CS, Bolli N, Manes N, et al. Rapid parallel acquisition of somatic mutations after NPM1 in acute myeloid leukaemia evolution. Br J Haematol 2017; 176: 825–829. [DOI] [PubMed] [Google Scholar]
- 78. Makishima H, Yoshizato T, Yoshida K, et al. Dynamics of clonal evolution in myelodysplastic syndromes. Nat Genet 2017; 49: 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Harris NL, Jaffe ES, Diebold J, et al. World health organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the clinical advisory committee meeting-airlie house, Virginia, November 1997. J Clin Oncol 1999; 17: 3835–3849. [DOI] [PubMed] [Google Scholar]
- 80. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the world health organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009; 114: 937–951. [DOI] [PubMed] [Google Scholar]
- 81. Malcovati L, Porta MG, Pascutto C, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol 2005; 23: 7594–7603. [DOI] [PubMed] [Google Scholar]
- 82. Montalban-Bravo G, Kanagal-Shamanna R, Sasaki K, et al. NPM1 mutations define a specific subgroup of MDS and MDS/MPN patients with favorable outcomes with intensive chemotherapy. Blood Adv 2019; 3: 922–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Falini B, Mason DY. Proteins encoded by genes involved in chromosomal alterations in lymphoma and leukemia: clinical value of their detection by immunocytochemistry. Blood 2002; 99: 409–426. [DOI] [PubMed] [Google Scholar]
- 84. Falini B. Anaplastic large cell lymphoma: pathological, molecular and clinical features. Br J Haematol 2001; 114: 741–760. [DOI] [PubMed] [Google Scholar]
- 85. Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science 1994; 263: 1281–1284. [DOI] [PubMed] [Google Scholar]
- 86. Falini B, Nicoletti I, Bolli N, et al. Translocations and mutations involving the nucleophosmin (NPM1) gene in lymphomas and leukemias. Haematologica 2007; 92: 519–532. [DOI] [PubMed] [Google Scholar]
- 87. Bischof D, Pulford K, Mason DY, et al. Role of the nucleophosmin (NPM) portion of the non-Hodgkin’s lymphoma-associated NPM-anaplastic lymphoma kinase fusion protein in oncogenesis. Mol Cell Biol 1997; 17: 2312–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Amin HM, McDonnell TJ, Ma Y, et al. Selective inhibition of STAT3 induces apoptosis and G1 cell cycle arrest in ALK-positive anaplastic large cell lymphoma. Oncogene 2004; 23: 5426–5434. [DOI] [PubMed] [Google Scholar]
- 89. Shi W, George SK, George B, et al. TrkA is a binding partner of NPM-ALK that promotes the survival of ALK+ T-cell lymphoma. Mol Oncol 2017; 11: 1189–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Marzec M, Kasprzycka M, Liu X, et al. Oncogenic tyrosine kinase NPM/ALK induces activation of the rapamycin-sensitive mTOR signaling pathway. Oncogene 2007; 26: 5606–5614. [DOI] [PubMed] [Google Scholar]
- 91. Amin HM, Lai R. Pathobiology of ALK+ anaplastic large-cell lymphoma. Blood 2007; 110: 2259–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhang Q, Wang HY, Liu X, et al. STAT5A is epigenetically silenced by the tyrosine kinase NPM1-ALK and acts as a tumor suppressor by reciprocally inhibiting NPM1-ALK expression. Nat Med 2007; 13: 1341–1348. [DOI] [PubMed] [Google Scholar]
- 93. Bhat UG, Jagadeeswaran R, Halasi M, et al. Nucleophosmin interacts with FOXM1 and modulates the level and localization of FOXM1 in human cancer cells. J Biol Chem 2011; 286: 41425–41433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Haque M, Li J, Huang YH, et al. NPM-ALK is a key regulator of the oncoprotein FOXM1 in ALK-positive anaplastic large cell lymphoma. Cancers (Basel) 2019; 11: pii: E1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Nozawa Y, Van Belzen N, Van der Made AC, et al. Expression of nucleophosmin/B23 in normal and neoplastic colorectal mucosa. J Pathol 1996; 178: 48–52. [DOI] [PubMed] [Google Scholar]
- 96. Tsui KH, Cheng AJ, Chang P, et al. Association of nucleophosmin/B23 mRNA expression with clinical outcome in patients with bladder carcinoma. Urology 2004; 64: 839–844. [DOI] [PubMed] [Google Scholar]
- 97. Wong JC, Hasan MR, Rahman M, et al. Nucleophosmin1, upregulated in adenomas and cancers of the colon, inhibits p53-mediated cellular senescence. Int J Cancer 2013; 133: 1567–1577. [DOI] [PubMed] [Google Scholar]
- 98. Poletto M, Malfatti MC, Dorjsuren D, et al. Inhibitors of the apurinic/apyrimidinic endonuclease 1 (APE1)/nucleophosmin (NPM1) interaction that display anti-tumor properties. Mol Carcinog 2016; 55: 688–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Meng Q, Lei T, Zhang M, et al. Identification of proteins differentially expressed in adriamycin-resistant (pumc-91/ADM) and parental (pumc-91) human bladder cancer cell lines by proteome analysis. J Cancer Res Clin Oncol 2013; 139: 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hu H, Meng Q, Lei T, et al. Nucleophosmin1 associated with drug resistance and recurrence of bladder cancer. Clin Exp Med 2015; 15: 361–369. [DOI] [PubMed] [Google Scholar]
- 101. Luo F, Li H, Liang J, et al. Downregulation of NPM reverses multidrug resistance in human hepatoma cells via inhibition of P-glycoprotein expression. Mol Med Rep 2017; 15: 2360–2368. [DOI] [PubMed] [Google Scholar]
- 102. Chen S, Meng T, Zheng X, et al. Contribution of nucleophosmin overexpression to multidrug resistance in breast carcinoma. J Drug Target 2018; 26: 27–35. [DOI] [PubMed] [Google Scholar]
- 103. Pozzo F, Bittolo T, Vendramini E, et al. NOTCH1-mutated chronic lymphocytic leukemia cells are characterized by a MYC-related overexpression of nucleophosmin 1 and ribosome-associated components. Leukemia 2017; 31: 2407–2415. [DOI] [PubMed] [Google Scholar]
- 104. Hu J, Lin M, Liu T, et al. DIGE-based proteomic analysis identifies nucleophosmin/B23 and nucleolin C23 as over-expressed proteins in relapsed/refractory acute leukemia. Leuk Res 2011; 35: 1087–1092. [DOI] [PubMed] [Google Scholar]
- 105. Lin M, Hu J, Liu T, et al. Knockdown of nucleophosmin by RNA interference reverses multidrug resistance in resistant leukemic HL-60 cells. Immunobiology 2013; 218: 1147–1154. [DOI] [PubMed] [Google Scholar]
- 106. Wang L, Chen B, Lin M, et al. Decreased expression of nucleophosmin/B23 increases drug sensitivity of adriamycin-resistant Molt-4 leukemia cells through mdr-1 regulation and Akt/mTOR signaling. Immunobiology 2015; 220: 331–340. [DOI] [PubMed] [Google Scholar]
- 107. Mei X, Chen Y, Gan D, et al. Effect of nucleolin on adriamycin resistance via the regulation of B-cell lymphoma 2 expression in Burkitt’s lymphoma cells. J Cell Physiol 2019; 234: 22666–22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hoffmann H, Thiede C, Glauche I, et al. The prognostic potential of monitoring disease dynamics in NPM1-positive acute myeloid leukemia. Leukemia 2019; 33: 1531–1534. [DOI] [PubMed] [Google Scholar]
- 109. Martelli MP, Gionfriddo I, Mezzasoma F, et al. Arsenic trioxide and all-trans retinoic acid target NPM1 mutant oncoprotein levels and induce apoptosis in NPM1-mutated AML cells. Blood 2015; 125: 3455–3465. [DOI] [PubMed] [Google Scholar]
- 110. Nabbouh AI, Hleihel RS, Saliba JL, et al. Imidazoquinoxaline derivative EAPB0503: A promising drug targeting mutant nucleophosmin 1 in acute myeloid leukemia. Cancer 2017; 123: 1662–1673. [DOI] [PubMed] [Google Scholar]
- 111. Balusu R, Fiskus W, Rao R, et al. Targeting levels or oligomerization of nucleophosmin 1 induces differentiation and loss of survival of human AML cells with mutant NPM1. Blood 2011; 118: 3096–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kuhn MW, Song E, Feng Z, et al. Targeting chromatin regulators inhibits leukemogenic gene expression in NPM1 mutant leukemia. Cancer Discov 2016; 6: 1166–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Spencer DH, Young MA, Lamprecht TL, et al. Epigenomic analysis of the HOX gene loci reveals mechanisms that may control canonical expression patterns in AML and normal hematopoietic cells. Leukemia 2015; 29: 1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Okuwaki M. The structure and functions of NPM1/nucleophsmin/B23, a multifunctional nucleolar acidic protein. J Biochem 2008; 143: 441–448. [DOI] [PubMed] [Google Scholar]
- 115. Penthala NR, Ketkar A, Sekhar KR, et al. 1-Benzyl-2-methyl-3-indolylmethylene barbituric acid derivatives: anti-cancer agents that target nucleophosmin 1 (NPM1). Bioorg Med Chem 2015; 23: 7226–7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Qi W, Shakalya K, Stejskal A, et al. NSC348884, a nucleophosmin inhibitor disrupts oligomer formation and induces apoptosis in human cancer cells. Oncogene 2008; 27: 4210–4220. [DOI] [PubMed] [Google Scholar]
- 117. Liu GY, Shi JX, Shi SL, et al. Nucleophosmin regulates intracellular oxidative stress homeostasis via antioxidant PRDX6. J Cell Biochem 2017; 118: 4697–4707. [DOI] [PubMed] [Google Scholar]
- 118. Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med 2013; 369: 111–121. [DOI] [PubMed] [Google Scholar]
- 119. Schlenk RF, Dohner K, Kneba M, et al. Gene mutations and response to treatment with all-trans retinoic acid in elderly patients with acute myeloid leukemia. Results from the AMLSG trial AML HD98B. Haematologica 2009; 94: 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Huang M, Thomas D, Li MX, et al. Role of cysteine 288 in nucleophosmin cytoplasmic mutations: sensitization to toxicity induced by arsenic trioxide and bortezomib. Leukemia 2013; 27: 1970–1980. [DOI] [PubMed] [Google Scholar]
- 121. El Hajj H, Dassouki Z, Berthier C, et al. Retinoic acid and arsenic trioxide trigger degradation of mutated NPM1, resulting in apoptosis of AML cells. Blood 2015; 125: 3447–3454. [DOI] [PubMed] [Google Scholar]
- 122. Ehninger A, Kramer M, Rollig C, et al. Distribution and levels of cell surface expression of CD33 and CD123 in acute myeloid leukemia. Blood Cancer J 2014; 4: e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. De Propris MS, Raponi S, Diverio D, et al. High CD33 expression levels in acute myeloid leukemia cells carrying the nucleophosmin (NPM1) mutation. Haematologica 2011; 96: 1548–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Liu YR, Zhu HH, Ruan GR, et al. NPM1-mutated acute myeloid leukemia of monocytic or myeloid origin exhibit distinct immunophenotypes. Leuk Res 2013; 37: 737–741. [DOI] [PubMed] [Google Scholar]
- 125. Burger K, Muhl B, Harasim T, et al. Chemotherapeutic drugs inhibit ribosome biogenesis at various levels. J Biol Chem 2010; 285: 12416–12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Brodska B, Holoubek A, Otevrelova P, et al. Low-dose actinomycin-D induces redistribution of wild-type and mutated nucleophosmin followed by cell death in leukemic cells. J Cell Biochem 2016; 117: 1319–1329. [DOI] [PubMed] [Google Scholar]
- 127. Greiner J, Ono Y, Hofmann S, et al. Mutated regions of nucleophosmin 1 elicit both CD4+ and CD8+ T-cell responses in patients with acute myeloid leukemia. Blood 2012; 120: 1282–1289. [DOI] [PubMed] [Google Scholar]
- 128. Matanes F, AbdelAzeem BMA, Shah G, et al. Chronic myelomonocytic leukemia associated with myeloid sarcomas and NPM1 mutation: a case report and literature review. Ther Adv Hematol 2019; 10: 2040620719854596. [DOI] [PMC free article] [PubMed] [Google Scholar]


