Short abstract
Aims
The purpose of this study was to assess the effectiveness of a smartphone app (MyHealthyGut) in helping adults self-manage celiac disease or gluten intolerance and improve their gut health.
Methods
Adults diagnosed with celiac disease or gluten intolerance (N = 115) were randomized into two groups: experimental group 1 (had access to the app for a one-month period) or wait list control (WLC). After one month, WLC participants were given one-month access to the app (experimental group 2). An online questionnaire was administered to assess (a) user satisfaction with the app and (b) changes in the following patient-reported outcomes: adherence to a gluten-free diet, quality of life (QoL), self-regulatory efficacy, and feelings of depression and anxiety. Generalized estimating equations were used to assess changes in the outcome variables over time between the groups.
Results
Participants reported high levels of app usability, were satisfied with features of the app, and felt that the app was best suited for people newly diagnosed with celiac disease. Participants in the experimental groups reported improvements in adherence, gastrointestinal symptoms (experimental group 1 only), QoL, self-regulatory efficacy (experimental group 2 only), anxiety (experimental group 1 only), and depression (experimental group 2 only). Experimental group 1 and the WLC group reported significantly worse adherence after using the app based on the Celiac Dietary Adherence Test, which was in contrast to the accidental and purposeful measures of gluten consumption and symptoms for experimental group 1 but consistent with reports of accidental and purposeful gluten consumption and symptoms for the WLC group.
Conclusions
Based on feedback from the participants, the app may be best suited for individuals newly diagnosed or struggling with celiac disease or gluten intolerance. After using the MyHealthyGut app for a one-month period, adults with celiac disease reported improvements in psychosocial outcomes. Further iterations of the app are needed to meet the needs of this population better. MyHealthyGut is the first evidence-based app designed to help people with celiac disease or gluten intolerance.
Keywords: Theory-based app, self-regulation, gut health, celiac disease, gluten intolerance, mHealth
Introduction
Advancements in technology and connectivity have the potential to play an increasingly important role in health care.1 The term “mobile health” (“mHealth”) describes the use of mobile and wireless technologies (e.g., smartphones) for achieving various health goals.2 As the global burden of chronic disease increases,3 there is a need for alternative strategies health-care providers can recommend to patients to help manage health, particularly because these patients spend the majority of their time outside of the clinical setting.4–6 mHealth technology can help patients adhere and comply with treatment, manage symptoms and medication, and monitor disease activity.7
Findings from multiple studies have revealed that participants report more self-monitoring and better adherence to a weight management and/or dietary intervention when delivered via a smartphone app or personal digital assistant compared to a website or paper diary.8–11 Wang et al.6 reported that patients who used smartphone apps felt secure knowing that their illness was closely monitored and were able to participate in their health management, and it gave them the feeling that they were well taken care of outside of the clinical setting and not forgotten by their doctors. A population-based survey reported that smartphone apps that involved the characteristics of feedback and/or monitoring improved participants’ adherence to a doctor’s advice.12 Additional research indicates that the attractions of using apps as health-care tools include convenience, time efficiency, low cost, and the potential to reach a high percentage of the population.4,13,14
There is an opportunity for smartphone apps to aid in the management of gastrointestinal diseases because these patients often spend most of their time outside of a health-care facility. Indeed, patient behaviors at home, self-monitoring of symptoms, and compliance with treatment protocols are all essential in improving clinical outcomes and reducing the frequency of flare-ups.15 A recent systematic assessment of mobile apps specifically for self-management of inflammatory bowel disease (IBD) concluded that although apps may be a useful adjunct to IBD management, there is room for improvement in apps, specifically more focus on the inclusion of evidence-based guidelines.16
Building on the previous systematic review on IBD, Helsel et al.7 conducted a systematic review of mHealth interventions (mobile phones, computers, and web-based tools) that included patients with IBD, ulcerative colitis, Crohn’s disease, irritable bowel syndrome, and colorectal cancer. They looked at a variety of outcomes, and the results were promising, with patient compliance (measured using factors such as medical and medication adherence, completed symptom diaries, and questionnaires) ranging from 25% to 100% among the various trials. Notably, participant satisfaction with the mHealth tool was between 74% and 100%. Findings from numerous studies show disease improvement as measured by symptom severity scales and physiological biomarkers, and in some, general and disease-specific quality of life (QoL) improved with mHealth interventions in as little as 12 weeks.7
mHealth and management of celiac disease
Celiac disease is a genetically based autoimmune condition that affects approximately 1% of the general population worldwide and is characterized by a range of physical (i.e., gastrointestinal, weight loss, abdominal distention, fatigue, migraines, and body pain) and psychological (e.g., depression, anxiety) symptoms.17 Social issues should also be counted in the burden of illness, as many patients expressed concern regarding the impact of celiac disease on socialization and having to abstain from important things in life.18 The only available treatment for celiac disease is strict adherence to a gluten-free diet (GFD), which can alleviate both short- and long-term consequences (e.g., infertility, intestinal cancers, and osteoporosis).17 Unfortunately, many patients find following such a strict diet to be difficult; there is evidence that long-term adherence can vary, and this is predictably associated with diminished QoL.19,20 Additionally, although a GFD often immediately improves symptoms and QoL,19 some patients continue to experience gastrointestinal symptoms, despite reported strict adherence.21 As such, there is a need for more effective strategies to help people follow a GFD, manage symptoms, cope with celiac disease, and heal their gut—collectively, health behavior change.
In terms of mobile apps and health behavior change, weight loss, physical activity, and dietary choices are all common targets. Results from a systematic review provide modest evidence (70% of analyzed studies showed improvements) that smartphone interventions can alter diet, physical activity, and sedentary behavior, although there is greater support for multicomponent interventions that also include another type of intervention strategy.22 Another review revealed similar results, in that 74% of studies reported significant positive effects in the targeted behavior change.13 In a small pilot study of 30 healthy adults, those who used the popular diet tracking app MyFitnessPal reported greater reductions in sodium intake and greater satisfaction with their method of tracking compared to a control group that used a paper journal.11 Chin et al.23 analyzed data from 35,921 participants using the Noom Coach app, and 77.9% reported a decrease in body weight while using the app; more frequent use was also associated with sustained long-term weight loss. Looking specifically at smartphone apps for the management of gastrointestinal disease, in 2017, an interventional trial was conducted to assess the effects of a smartphone/tablet app (MyIBDcoach) on the management of IBD. The app included self-monitoring, outpatient visit modules, e-learning modules, a personal care plan, and an administrator page (for health-care providers). The researchers found that the use of the app reduced the number of outpatient visits, telephone consultations and hospital admissions and increased medication adherence compared to standard care. Furthermore, it was deemed to be safe and patient-reported quality of health care was high.24
Despite the prevalence of celiac disease, there have been no smartphone applications available for this population. Recently, Dowd et al.25 developed and pilot tested the MyHealthyGut app to help people manage celiac disease and gluten intolerance and promote overall gut health. Key features of the MyHealthyGut app include: educational content about celiac disease, gut health, and potential causes of gastrointestinal distress; a list of evidence-based gut health–promoting foods and recipes; diet and corresponding symptom-tracking functionality; food lists; and the ability to share this information with health-care providers.25
Objectives
The purpose of this study was to assess (a) user satisfaction with the app and (b) the effectiveness of the MyHealthyGut app at helping adults with celiac disease or gluten intolerance to self-manage their condition. In this randomized controlled trial, we examined user satisfaction with the app and the impact of using the app for a one-month period on end users’ adherence to a GFD, gastrointestinal symptoms, QoL, self-regulatory efficacy, and psychopathologies (depression and anxiety). We hypothesized that (a) participants would report positive satisfaction with the app and (b) participants in the experimental groups would report significant improvements in all outcomes after using the app for a one-month period, whereas no significant changes would be reported for participants in the wait list control (WLC) group.
Method
Participants
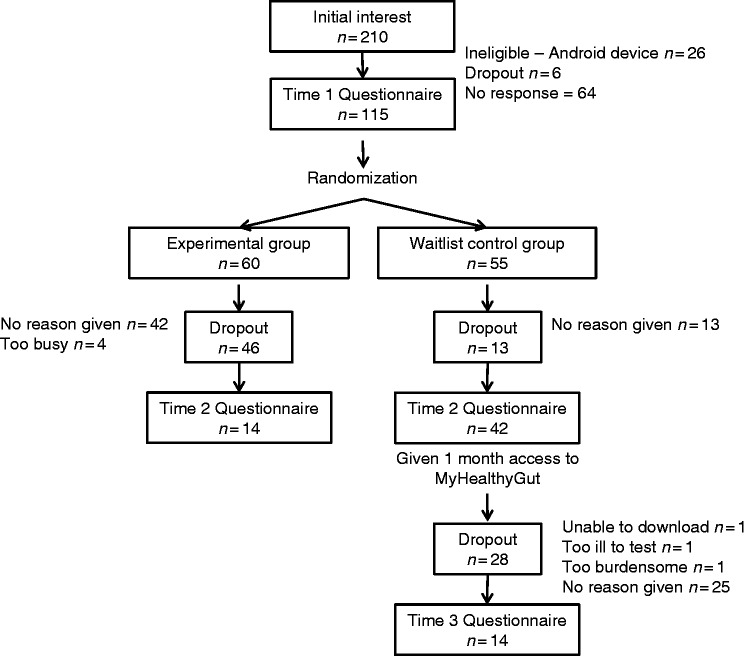
Institutional ethical approval was obtained from the University of Calgary (REB15-2979) before data were collected. Individuals were recruited via the Canadian Celiac Association (CCA) webpage, CCA regional chapters’ emails, Facebook pages, and Twitter postings. To be eligible, participants were required to have a confirmed diagnosis of celiac disease (either through blood test and/or endoscopy, or a health professional) or gluten intolerance, own an Apple mobile device (iOS interface—iPad, iPhone, or iPod), speak English, have an access to computers, be older than 18 years of age, and live in the Calgary area. Initially, 210 participants indicated interest in the study, and 115 participants (Mage = 42.72 years, 96% female, Myears diagnosed = 7.33) were eligible and completed time 1 questionnaires at baseline. Please see Figure 1 for participant flow through the MyHealthyGut app evaluation study, and see Table 1 for further information on participant demographics.
Figure 1.
Participant flow through the MyHealthyGut App evaluation study.
Table 1.
Descriptive statistics for study participants at Time 1.
| Variable |
Time 1, M (SD) |
||||
|---|---|---|---|---|---|
| All participants, N = 115 | Experimental, N = 60 (all) | Experimental (completers), N = 14 | WLC, N = 55 | WLC (completers), N = 42 | |
| Age (years) | 42.72 (13.39) | 41.02 (14.96) | 46.93 (16.75) | 44.58 (11.28) | 45.62 (10.92) |
| Sex | 110 female; 5 male | 56 female; 4 male | 14 female | 54 female; 1 male | 41 female; 1 male |
| BMI | 25.03 (5.52) | 25.13 (6.05) | 24.55 (6.33) | 24.92 (4.93) | 25.06 (5.20) |
| Diagnosis | |||||
| Intestinal biopsy | 83 | 46 | 11 | 37 | 30 |
| Blood test | 88 | 49 | 9 | 39 | 31 |
| Skin biopsy | 3 | 1 | 0 | 2 | 2 |
| Not formally diagnosed | 7 | 1 | 0 | 6 | 4 |
| Years since diagnosis | 7.33 (7.52) | 7.03 (7.00) | 10.61 (7.35) | 7.60 (8.23) | 7.53 (7.84) |
| Accidental GC | 79 = No (67%); 36 = Yes | 40 = No (67%); 20 = Yes | 9 = No (64%); 5 = Yes | 39 = No (71%); 16 = Yes | 31 = No (74%); 11 = Yes |
| Purposeful GC | 110 = No (96%); 5 = Yes | 57 = No (95%); 3 = Yes | 12 = No (86%); 2 = Yes | 53 = No (96%); 2 = Yes | 40 = No (95%); 2 = Yes |
| Negative GI symptoms | 37 = No (32%); 78 = Yes | 21 = No (32%); 39 = Yes | 0 = No (0%); 14 = Yes | 16 = No (29%); 39 = Yes | 3 = No (4.5%); 39 = Yes |
Accidental and purposeful gluten consumption indicates whether the person consumed gluten in/unintentionally over the past week. Negative gastrointestinal symptoms refer to whether the participant experienced gastrointestinal pain over the previous week. There were no significant differences between conditions on basic demographic variables.
SD: standard deviation; WLC: wait list controls; BMI: body mass index; GC: gluten consumption; GI: gastrointestinal.
Procedure
After individuals consented to participate in the study, participants were randomly assigned to one of two different groups: experimental or WLC. Those in experimental group 1 were asked to use the MyHealthyGut app for a one-month period, while those randomized to the WLC group had to wait one month before being given access to the app. After one month, those in the WLC group were able to download the app and became experimental group 2. Participants completed online questionnaires to assess outcomes at time 1 (baseline; experimental group 1 and WLC), time 2 (one month later; experimental group 1 and WLC), and time 3 (one month later; experimental group 2). All participants were entered into a draw to win one of four $25 gift cards.
Measures
All participant outcomes (adherence to a GFD, gastrointestinal symptoms, celiac-specific QoL, self-regulatory efficacy, depression, anxiety, and satisfaction and engagement with the app) were assessed via an online questionnaire through the Drupal 7 online portal at time 1 (baseline) and time 2 (one month later), and participants in experimental group 2 also completed the online questionnaire at time 3 (after using the app for a one-month period).
Demographics
Participants were asked to report basic information on demographics, including age, sex, ethnicity, means of diagnosis (blood test and/or biopsy), and time since diagnosis.
App usability and satisfaction
Participants were asked to respond to the following questions. (1) How easy is the MyHealthyGut app to use on a scale ranging from 1 (“extremely difficult”) to 5 (“extremely easy”)? (2) How likely are you to use the MyHealthyGut app to help you manage celiac disease or gluten intolerance in the future on a scale ranging from 1 (“not at all likely”) to 5 (“very likely”)? (3) How satisfied are you with each of the following functions of the MyHealthyGut app: (a) diet tracking, (b) symptom journaling, (c) seven-day meal-plan content, (d) meal planning, (e) education, (f) supplements, (g) 50 nutritious GF food list, and (h) general content on a scale ranging from 1 (“very unsatisfied”) to 5 (“very satisfied”)? (4) How much do you agree with the statement “The MyHealthyGut app will improve my health” on a scale ranging from 1 (“strongly disagree”) to 5 (“strongly agree”)? (5) What was your first reaction to the MyHealthyGut app on a scale ranging from 1 (“very negative”) to 5 (“very positive”)? (6) How would you rate the quality of the MyHealthyGut app overall on a scale ranging from 1 (“very low quality”) to 5 (“very high quality”)? (7) How likely would you be to purchase the MyHealthyGut app on a scale ranging from 1 (“not at all likely”) to 5 (“very likely”)? (8) Did anything about the MyHealthyGut app confuse you (yes/no)? (9) How likely is it that you would recommend MyHealthyGut to a friend or colleague on a scale ranging from 1 (“not at all likely”) to 5 (“very likely”)? (10) What was your favorite part of the MyHealthyGut app? These items were used to assess user engagement and satisfaction with the app in the initial development study as reported in Dowd et al.25
Celiac Dietary Adherence Test
The Celiac Dietary Adherence Test (CDAT), a seven-item measure developed by Leffler et al.,26 assessed four different aspects of adherence to a GFD: celiac symptoms, self-efficacy, reasons to follow a GFD, and perceived adherence to a GFD. An example item is “I am able to follow a GFD when dining outside my home” assessed on a scale ranging from 1 (“strongly agree”) to 5 (“strongly disagree”). Responses to items are summed for a total score, with lower scores indicating stricter adherence to a GFD. Data derived from the CDAT provides evidence of face validity and test–retest reliability (Pearson’s r = 0.82) as reported by Leffler et al.26 One-month test–retest reliability in this study was Pearson’s r = 0.53.
Accidental and purposeful consumption of gluten
Adherence to a GFD was also assessed through two questions with the options of answering “never” or indicating a specific number of times over the past week. Questions included how many times participants had eaten foods containing gluten by accident, and how many times they had eaten foods containing gluten on purpose. Data derived from these items have demonstrated evidence of test–retest reliability in data collected previously by Dowd et al.27 (Pearson’s raccidental = 0.39, p < 0.01; Pearson rpurposeful = 0.70, p < 0.01). Test–retest reliability for data collected in this study were Pearson raccidental = 0.69, p < 0.01, and Pearson rpurposeful = 0.60, p < 0.01.
Gastrointestinal symptoms
A one-item measure was used to assess the frequency in which participants experienced negative gastrointestinal symptoms over the past week. Test–retest reliability for data collected in this study was Pearson’s r = 0.69, p < 0.01.
Celiac disease QoL
Overall, QoL was assessed by a 20-item celiac disease–specific QoL questionnaire on a scale ranging from 1 (“not at all”) to 5 (“a great deal”).28 An example item is “I feel limited by celiac disease.” Items are reverse scored, and the sum of all 20 items is computed. Celiac disease–specific QoL overall is then computed with the following formula: [(Total_overall–20)/80)] × 100, where higher scores indicate better QoL. Data derived from this instrument have shown acceptable divergent validity from the irritable bowel syndrome questionnaire (r = 0.62), and convergent validity with psychological distress and abdominal pain (r2 = 0.35–0.65).29 Cronbach’s alphas in this study were ≥ 0.92.
Self-regulatory efficacy
Self-regulatory efficacy was assessed using a revised version of the eight-item measure developed by Strahan and Brawley.30 Participants responded to items regarding their confidence to self-regulate their behavior to consume a GFD on a standard 0% (“not at all confident”) to 100% (“completely confident”) self-efficacy scale.31 An example item is “How confident are you that you can use safe, effective cooking techniques to ensure your meals are gluten-free over the next month?” The mean score across all items is calculated, with a higher score indicating greater self-regulatory efficacy. Dowd et al.27,32 have previously demonstrated acceptable reliability of this instrument in assessing self-regulatory efficacy in adults with celiac disease (Cronbach’s α ≥ 0.87). Cronbach’s alphas in this study were ≥ 0.71.
Depression
Depression was assessed using an adapted 20-item scale33 where participants indicate how much of the time each statement describes how they have been feeling during the past several days (“a little of the time,” “some of the time,” “a good part of the time,” or “most of the time”). An example item is “I feel down-hearted and blue.” The Zung Self-Rating Depression Scale was confirmed by Biggs et al.34 to be a reliable, sensitive, and valid instrument that can differentiate between different diagnostic groups. Cronbach’s alphas in this study were ≥ 0.83.
Anxiety
The State–Trait Anxiety Inventory35 was used to assess trait anxiety, which is anxiety that can be useful for identifying variances in anxiety proneness. Participants were asked to respond on a scale ranging from 1 (“almost never”) to 4 (“almost always”) of how they generally feel to 20 different items. An example item is “I worry too much over something that really doesn’t matter.” Data from previous studies using this measure provide evidence of internal consistency (Cronbach’s α ≥0.86), and test–retest reliability ranged from 0.65 to 0.75 over a two-month period.36 Cronbach’s alphas in this study were ≥0.91.
Data analysis
All data were analyzed using IBM SPSS Statistics for Windows v25.0 (IBM Corp., Armonk, NY). Means, totals, and/or frequencies were calculated for all demographic variables. A generalized estimating equation (GEE; i.e., GEE under GENLIN procedure in SPSS v25) was employed to examine between group changes in the outcome variables over time. This type of analysis is appropriate for longitudinal data in which the responses are correlated and unbalanced across time points. Alpha was set at 0.05, as such, any computed p-values of <0.05 were considered statistically significant. Bonferroni corrections were utilized to account for multiple tests in the GEE.
Results
Participants
There were no significant differences between groups on any of the basic demographic variables (p > 0.05). A large proportion of participants were lost at follow-up (76% experimental group 1; 24% WLC group; 67% experimental group 2). Dropouts were more likely to be have been diagnosed with celiac disease or gluten intolerance more recently (Mparticipants = 10.10, SD = 8.05; Mdropouts = 6.40, SD = 7.14), t(113) = 2.34, p = 0.02, and to report poorer QoL at baseline (Mparticipants = 3.35, SD = 0.81; Mdropouts = 3.05, SD = 0.67), t(113) = 1.98, p = 0.05.
Satisfaction with app
There was a range of positive and negative responses regarding the perceived usability and satisfaction with the MyHealthyGut app (see Table 2 for full summary of findings). Participants felt the MyHealthyGut app was relatively easy to use (M = 3.53, SD = 1.06), were generally satisfied with the features (M ≥ 3.50, SD ≤ 1.14), felt the app would help improve their health (M = 3.57, SD = 0.82), was of satisfactory quality (M = 3.55, SD = 0.75), had an overall positive first reaction to the app (M = 3.65, SD = 0.93), and were likely to refer the app to a friend (M = 4.31, SD = 1.05). Participants reported less than the neutral (i.e., value of 3 on a 1–5 scale) response to the following items: likelihood of using the app to manage celiac disease in the future (M = 2.27, SD = 1.21), likelihood of purchasing the app (M = 2.43, SD = 1.65). A few participants were confused about the “back” buttons in the app (n = 3/14). Explanations regarding participants’ intended future use of the app indicated that they felt the app was best suited for people newly diagnosed with celiac disease.
Table 2.
MyHealthyGut app evaluation—participant responses for experimental groups 1 and 2.
| Question | Response (N = 28) | Most frequent comments |
|---|---|---|
| How easy is the MyHealthyGut app to use? | M = 3.53, SD = 1.06 | 1 (“extremely difficult”)– 5 (“extremely easy”) |
| How likely are you to use the MyHealthyGut app to help you manage your celiac disease or gluten intolerance in the future? | M = 2.27, SD = 1.21 | 1 (“very unlikely”)– 5 (“very likely”)n = 7 felt app more useful for newly diagnosed (vs. them)n = 5 felt app was easy to use, provided helpful information, and assisted in diet or supplement routinen = 3 unable to download the full app |
| How satisfied are you with the following features? | 1 (“very unsatisfied”)– 5 (“very satisfied”)n = 5 found educational content very interesting, helpful, and/or good balance of Western + alternative medicine | |
| • Diet tracking | M = 3.50, SD = 1.05 | |
| • Symptom journaling | M = 3.60, SD = 1.14 | |
| • 7 day meal plan content | M = 4.00, SD = 1.00 | |
| • Meal planning | M = 4.00, SD = 0.00 | |
| • Education | M = 4.40, SD = 0.52 | |
| • Supplements | M = 4.42, SD = 0.5 | |
| • 50 recommended foods | M = 4.20, SD = 0.63 | |
| • General content | M = 4.00, SD = 0.82 | |
| How much do you agree with: the MyHealthyGut app will improve my health? | M = 3.57, SD = 0.82 | |
| What was your first reaction to the MyHealthyGut app? | M = 3.65, SD = 0.93 | 1 (“very negative”)– 5 (“excellent”) |
| How would you rate the MyHealthyGut app overall? | M = 3.55, SD = 0.75 | 1 (“poor”)– 5 (“excellent”) |
| How likely would you be to purchase the MyHealthyGut app? | M = 2.43, SD = 1.65 | 1 (“very unlikely”)– 5 (“very likely”) |
| Did anything about the MyHealthyGut app confuse you? Content, layout, design, etc. | Yes = 3No = 11 | Yes/No |
| How likely is it that you would recommend MyHealthyGut to a friend or colleague? | M = 4.31, SD = 1.05 | 1 (“not at all likely”)– 5 (“very likely”) |
| What was your favorite part of the MyHealthyGut app? | n = 6 recipes + top foodsn = 6 diet + symptom tracking n = 6 educationn = 5 functionality/layoutn = 2 reminders n = 1 food search | |
Primary outcomes
Adherence to a GFD (CDAT)
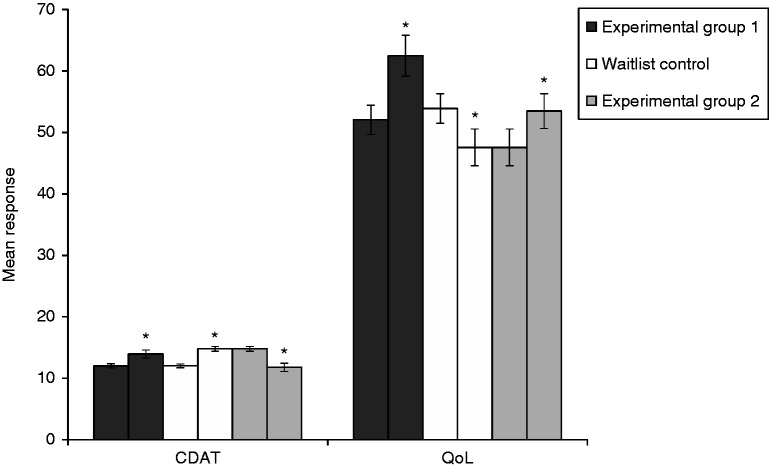
GEE analysis revealed there was no significant group–time interaction effect (χ2(1) = 1.016, p = 0.313), no significant group effect (χ2(1) = 0.705, p = 0.401), and a significant main effect for time for responses on the CDAT (χ2(2) = 37.85, p < 0.001). Pairwise comparisons indicated that compared to time 1 (EMM = 12.03; SE = 0.23), participants in both experimental group 1 and the WLC group reported significantly worse adherence to a GFD based on the CDAT at time 2 (EMM = 14.37; SE = 0.38; Mdifference = –2.34 (SE = 0.39), p < 0.001). After waiting one month for experimental group 1, participants in experimental group 2 (i.e., those randomized to the WLC group and given delayed access to use the app for a one-month period) reported significant improvements in adherence based on the CDAT (EMM = 11.78; SE = 0.68; Mdifference = 2.59 (SE = 0.77), p < 0.001). These findings are displayed in Tables 3 and 4 and Figure 2.
Table 3.
Estimated marginal means or totals for outcome variables at each time point for study participants (N = 115).
| Variable |
Time 1, M or total (SE or % n) |
Time 2, M (SE or % n) |
Time 3, M (SE or % n) |
||
|---|---|---|---|---|---|
| Experimental 1, N = 14 | WLC, N = 42 | Experimental 1, N = 14 | WLC/Experimental 2, N = 42 | Experimental 2, N = 14 | |
| CDAT | 12.01 (0.35) | 12.04 (0.30) | 13.96 (0.65)* | 14.78 (0.40)* | 11.78 (0.68)* |
| Accidental | 9 = No (64%); 5 = Yes | 31 = No (74%); 11 = Yes | 11 = No (79%); 3 = Yes | 28 = No (67%); 14 = Yes* | 14 = No (100%); 0 = Yes |
| Purposeful | 12 = No (86%); 2 = Yes | 40 = No (95%); 2 = Yes | 13 = No (93%); 1 = Yes* | 40 = No (95%); 2 = Yes | 14 = No (100%); 0 = Yes |
| Negative GI symptoms | 6 = No (43%); 8 = Yes | 12 = No (29%); 30 = Yes | 8 = No (57%); 6 = Yes* | 7 = No (17%); 35 = Yes* | — |
| CD-QoL | 52.05 (2.38) | 53.90 (2.40) | 62.48 (3.34)* | 47.56 (2.99) | 53.48 (2.84) |
| SRE | 96.90 (0.59) | 96.71 (0.63) | 96.07 (1.47) | 96.18 (0.87) | 97.62 (0.52)* |
| Depression | 50.02 (1.36) | 51.63 (1.10) | 44.88 (2.87) | 52.44 (1.56) | 48.96 (1.68)* |
| Anxiety | 48.53 (1.67) | 47.90 (1.29) | 39.69 (3.39)* | 49.25 (1.52) | 47.16 (2.11) |
Accidental and purposeful consumption of gluten are reported in total number of participants in each group that reported consuming gluten that way. Time 3 refers to responses from participants in the wait list control (WLC) group after having the opportunity to use the MyHealthyGut app for a one-month period. See Figure 1 for participant flow through the study for more information.
*Denotes a significant change between the two time points.
— indicates that data for this outcome were missing at time 3 due to a technological error.
CDAT: celiac dietary adherence test; CD-QoL: Celiac disease quality of life; SRE: self-regulatory efficacy.
Table 4.
Generalized estimating equation coefficients and estimates.
|
Group effect |
Time effect |
Group–time effect |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE | χ2 | p | B | SE | χ2 | p | B | SE | χ2 | p |
| CDAT | –0.81 | 0.77 | 0.71 | 0.401 | 0.263.00 | 0.600.80 | 37.85 | 0.000 | 0.79 | 0.78 | 1.02 | 0.313 |
| Accidental | –0.54 | 0.73 | 0.02 | 0.898 | –0.02 | 0.38 | 0.38 | 0.536 | 0.93 | 0.80 | 1.37 | 0.242 |
| Purposeful | 0.48 | 1.27 | 0.70 | 0.403 | –0.01 | 0.74 | 0.53 | 0.467 | 0.77 | 1.06 | 0.53 | 0.467 |
| Negative GI symptoms | –1.95 | 0.68 | 4.91 | 0.027 | –0.68 | 0.41 | 0.04 | 0.845 | 1.26 | 0.56 | 5.12 | 0.024 |
| CD-QoL | 14.92 | 4.35 | 3.88 | 0.049 | 0.42–5.92 | 2.272.89 | 2.80 | 0.247 | –16.77 | 4.11 | 16.64 | 0.000 |
| SRE | –0.06 | 1.70 | 0.01 | 0.950 | –0.91–1.49 | 0.440.39 | 21.42 | 0.000 | 0.252 | 1.57 | 0.025 | 0.874 |
| Depression | –7.56 | 3.33 | 4.51 | 0.034 | 2.673.48 | 1.321.23 | 8.22 | 0.016 | 5.95 | 3.24 | 3.38 | 0.066 |
| Anxiety | –9.56 | 3.73 | 3.62 | 0.057 | 0.742.09 | 1.631.67 | 4.18 | 0.124 | 10.19 | 3.89 | 6.86 | 0.009 |
B and SE values under time effect correspond to time 1 and time 2 (upper and lower values, respectively).
Figure 2.
Changes in main outcome variables after using the MyHealthyGut App for a one-month period (experimental groups 1 and 2) and the wait list control (WLC). Experimental group 2 refers to responses from participants in the WLC group after having the opportunity to use the MyHealthyGut app for a one-month period. *Denotes a significant change between the two time points.
Accidental consumption of gluten
GEE analysis revealed that there were no statistically significant group, time, or group–time interaction effects for accidental consumption of gluten (χ2 (1) <1.37, p > 0.242). These findings are displayed in Tables 3 and 4.
Purposeful consumption of gluten
GEE analysis revealed that there were no statistically significant group, time, or group–time interaction effects for purposeful consumption of gluten (χ2 (1) <0.70, p = 0.403). These findings are displayed in Tables 3 and 4.
QoL
GEE analysis revealed a statistically significant group–time interaction effect for responses on the celiac disease–specific QoL questionnaire (χ2(1) = 16.64, p < 0.001), which means that the group effect varies with time or vice versa. Pairwise comparisons indicated that compared to time 1 (EMM = 52.05; SE = 2.38), participants in experimental group 1 reported significant improvements in QoL at time 2 (EMM = 62.48; SE = 3.34; Mdifference = 10.43 (SE = 3.18), p < 0.001). Participants in the WLC group reported significant reductions in QoL at time 2 (EMM = 47.56; SE = 2.99) compared to time 1 (EMM = 53.90; SE = 2.40; Mdifference = –6.34 (SE = 2.57), p < 0.01). Participants in experimental group 2 (i.e., those given delayed access to the app) reported significant improvements in QoL at time 3 (EMM = 53.48; SE = 2.84; Mdifference = 5.92 (SE = 2.89), p < 0.05) compared to time 2. At baseline, QoL was not significantly different between experimental group 1 (M = 52.05) and the WLC group (M = 53.90; p = 0.586). However, there was a statistically significant difference between the groups at time 2 (Mexperimental = 62.48; MWLC = 47.56; p = 0.001). These findings are displayed in Tables 3 and 4 and Figure 2.
Secondary outcomes
Gastrointestinal symptoms
GEE analysis revealed a statistically significant group–time interaction effect for gastrointestinal symptoms (χ2(1) = 5.12, p = 0.024), which means that the group effect varies with time or vice versa. Pairwise comparisons indicated that at time 1, there were no significant differences between experimental group 1 (EMM = 0.57; SE = 0.13) and the WLC group (EMM = 0.73; SE = 0.07) on the occurrence of gastrointestinal symptoms (Mdifference = –0.16, SE = 0.15, p = 0.293). At time 2, participants in experimental group 1 (EMM = 0.43; SE = 0.13) were significantly less likely to have gastrointestinal symptoms than participants in the WLC group were (EMM = 0.84; SE = 0.06; Mdifference = –0.41 (SE = 0.14), p = 0.004). These findings are displayed in Tables 3 and 4.
Self-regulatory efficacy
GEE analysis revealed that there was no statistically significant group–time interaction effect (χ2(1) = 0.025, p = 0.874), no statistically significant group effect (χ2(1) = 0.004, p = 0.950), and a statistically significant main effect for time for self-regulatory efficacy (χ2(2) = 21.42, p < 0.001). Pairwise comparisons indicated no significant changes in self-regulatory efficacy from time 1 (EMM = 96.81; SE = 0.42) to time 2 (EMM = 96.10; SE = 0.86; Mdifference = –0.71 (SE = 0.81), p>0.05) for participants in experimental group 1 and the WLC group. Compared to time 2, participants (i.e., only those in experimental Group 2) reported significant improvements in self-regulatory efficacy at time 3 (EMM = 97.62; SE = 0.52; Mdifference = 1.52 (SE = 0.68), p < 0.05). These findings are displayed in Tables 3 and 4.
Depression
GEE analysis revealed statistically significant main effects for group (χ2(1) = 4.51, p = 0.034) and time (χ2(2) = 8.22, p = 0.016) for depression. The group–time interaction was not statistically significant (χ2(1) = 3.38, p = 0.066). Pairwise comparisons indicated that compared to time 1 (EMM = 50.02; SE = 1.36), improvements in depression at time 2 (EMM = 44.88; SE = 2.87) were not statistically significant for participants in experimental group 1 (Mdifference = –5.14 (SE = 3.11), p = 0.10). No significant change in depression was reported among participants in the WLC group between time 1 (EMM = 51.63; SE = 1.10) and time 2 (EMM = 52.44; SE = 1.56; Mdifference = 0.81 (SE = 1.01), p = 0.42). Participants in experimental group 2 (i.e., those given delayed access to the app) reported significant improvements in depression at time 3 (EMM = 48.96; SE = 1.68; Mdifference = –3.48 (SE = 1.23), p < 0.01) compared to time 2. These findings are displayed in Tables 3 and 4.
Anxiety
GEE analysis revealed a statistically significant group–time interaction effect (χ2(1) = 6.86, p = 0.009). Pairwise comparisons indicated that compared to time 1 (EMM = 48.53; SE = 1.67), experimental group 1 reported significant improvements in anxiety at time 2 (EMM = 39.69; SE = 3.39; Mdifference = –8.84 (SE = 3.81), p < 0.05). No significant change in anxiety was reported among participants in the WLC group between time 1 (EMM = 47.90; SE = 1.29) and time 2 (EMM = 49.25; SE = 1.52; Mdifference = –1.35 (SE = 0.88), p = 0.13). Participants in experimental group 2 (i.e., those given delayed access to the app) did not report significant improvements in anxiety at time 3 (EMM = 47.16; SE = 2.11; Mdifference = –2.09 (SE = 1.67), p < 0.01) compared to time 2. These findings are displayed in Tables 3 and 4.
Discussion
MyHealthyGut is the first evidence-based smartphone app specifically designed to help people with celiac disease or gluten intolerance. In this randomized controlled trial, we assessed user satisfaction with the app and the effects of using the MyHealthyGut app for one month on adherence to a GFD, gastrointestinal symptoms, QoL, self-regulatory efficacy to follow a strict GFD, and key mental-health outcomes (depression and anxiety) among adults with celiac disease or gluten intolerance. This study builds on the initial development and user evaluation of the app25 and expands our understanding of the effects of an evidence-based smartphone app on behavioral and psychosocial outcomes among a chronic disease population.
Participants provided feedback regarding usability of and satisfaction with content, features, and functionality of the app. Consistent with our hypothesis, overall, participants reported that the app was easy to use, and they were satisfied with the features and content of the app. Interestingly, participants felt that the app would be best suited for someone newly diagnosed with celiac disease or gluten intolerance versus someone who had been living with the disease for a while. Participants were likely to recommend a friend or colleague use to app to help them manage digestive health issues and reported a variety of favorite parts of the app. However, a minority of the participants intended to use the app in the future. Based on these preliminary positive findings, the first version of the app was released to the public for download. The app will continue to be revised in an iterative process as we continue to receive feedback regarding the usability and functionality of the app.
After using the MyHealthyGut app, participants reported improvements in psychological outcomes. Consistent with our hypothesis, after using the MyHealthyGut app for one month, participants in experimental group 1 were less likely to report gastrointestinal symptoms and reported significant improvements in QoL and anxiety. Improvements (i.e., 64% at time 1 to 79% at time 2 did not consume gluten inadvertently) in instances of accidental consumption of gluten and depression (Mtime 1 = 50.02; Mtime 2 = 44.88) were not statistically significant. Interestingly, and in contrast to our hypothesis and the items pertaining to accidental and purposeful consumption of gluten, gastrointestinal symptoms, and QoL, experimental group 1 participants also reported significant declines in adherence based on the CDAT. No significant changes were reported for self-regulatory efficacy among participants in experimental group 1. Consistent with our hypothesis, participants in experimental group 2 (those who were assigned to the WLC group for the first month and then given access to the app) reported significant improvements in adherence based on the CDAT, significantly enhanced QoL and self-regulatory efficacy, and less depression. As there were no instances of accidental or purposeful consumption of gluten at time 3, we were unable to analyze this categorical variable statistically. However, visual inspection of the data suggests participants in experimental group 2 reported improvements in these variables from before (time 2 = 67% did not consume gluten by accident and 95% did not consume gluten on purpose; time 3 = 100% did not consume gluten by accident and 100% did not consume gluten on purpose) to after using the app. Participants in the WLC group reported significant reductions in adherence based on the CDAT and instances of accidental gluten consumption, worse QoL, and more negative gastrointestinal symptoms during the one-month waiting period.
The main features of the app that pertain to behavior change (i.e., chronic disease management) are the education (e.g., understand your gut, celiac disease and gluten intolerance, how to be gluten free) and diet and symptom reporting (i.e., user-friendly food diary and symptom log). Knowledge of the GFD is predictive of adherence behavior among people with celiac disease,32 and self-monitoring (diet and symptom reporting) is a key part of self-regulation and disease management.12 As such, we hypothesized that as participants used the app, adherence and self-regulatory efficacy would improve. Baseline values of adherence (M ≤ 12.01) and self-regulatory efficacy (M ≥ 96.71) indicated that overall, participants were highly compliant26 and confident in following a strict GFD (100% = maximum score). These findings are in line with the mean length of time since diagnosis among participants (M = 10.10 years), as there is a general association between better adherence and the longer people are diagnosed and managing their condition.37 Indeed, failing to find a change in self-regulatory efficacy is likely due to ceiling effects in the population studied.
With regard to adherence as assessed by the CDAT, findings were contrary to our hypothesis for experimental group 1, and while we did not specifically hypothesize that we would see reductions in adherence among the WLC group, it is interesting to note that adherence in both of these groups worsened over the course of the one-month period (experimental group 1 using the app and the WLC group waiting to use the app). Findings could simply be due to participants being more aware of behaviors as a result of participation in the study.38 Further, participants in experimental group 1 may have also paid more attention to adherence (specifically the items as assessed on the CDAT such as “Have you been bothered by low energy or headaches over the past four weeks?” or “How important to your health are accidental gluten exposures?”) as a result of using the app—either via the education and/or tracking features of the app. Of note, experimental group 1 did report fewer gastrointestinal symptoms after using the app. However, we cannot draw strong conclusions on the effects of the app on adherence behavior due to the contradictory findings for experimental group 1. Experimental group 2 did report significant improvements in adherence after using the app based on the CDAT, indicating that using the MyHealthyGut app may help adults with adherence to the strict GFD. Future research should explore the effects of using the MyHealthyGut app on adherence and self-regulatory efficacy among people newly diagnosed and/or struggling with celiac disease or gluten intolerance who are more likely to start off with worse adherence and lower confidence to follow a GFD.
Improvements in QoL, depression (not significant for experimental group 1), and anxiety (only experimental group 1) were reported among participants in the experimental groups after using the app for a one-month period. It is promising that apps can help in self-management of this chronic gastrointestinal disease, given the high perceived burden of the GFD18,39 and high levels of mental-health concerns in this population, along with the preliminary evidence that using this app may help participants feel better able to cope with the disease (in terms of improved celiac-specific QoL and reductions in depression and anxiety). These findings are consistent with results from previous mHealth studies. Medication and medical adherence have been found to improve following mHealth interventions in patients with digestive diseases such as IBD.7,24 Adherence is especially important in digestive disease populations because these patients often have higher non-adherence rates compared to other chronic conditions,7 and compliance with disease management protocols is an important predictor of positive long-term outcomes (i.e., reduced frequency of disease relapse).24
It follows that increases in QoL scores often parallel successful disease management. One such example comes from an interventional trial for cardiac rehabilitation.40 Participants using a smartphone app had significantly higher adherence and completion rates (compared to those in the traditional rehabilitation group), and both groups experienced improvements in emotional state and QoL alongside other improved indexes.40 Likewise, in a systematic review conducted by Helsel et al.,7 researchers noted that both generic and disease-specific QoL for patients with IBS improved as result of an mHealth intervention.
Several strengths and weaknesses of the study should be acknowledged. In terms of strengths, the randomized controlled design of the study enabled comparison of changes reported in the experimental groups to the WLC group. Improvements in behavioral and psychological outcomes were noted in participants after using the MyHealthyGut app versus those in the WLC group. This study also explored the effects of a novel theory-based app, and as such, changes observed in behaviors and cognitions may be attributable to specific theory-driven components of the app—namely, the education and self-regulation components (diet and symptom tracking). Unfortunately, the high dropout rates in the experimental groups, while consistent with other online mHealth evaluations,41,42 potentially reduced the ability to assess changes in outcomes. In addition, dropouts were more recently diagnosed with celiac disease and reported poorer QoL. Taken together, it is important to acknowledge, given the population that dropped out, the remaining participants in this study had been coping with celiac disease or gluten intolerance for more than seven years on average, and thus were all quite confident in managing the disease and following a strict GFD. As such, participants may have been a more responsive and/or made up a more favorable sample. Positive findings in this study should be considered in conjunction with the participant demographics. Although it is speculative, newly diagnosed participants may be more likely to drop out because of feeling overwhelmed or getting information from another source. As such, future research should look to identify factors that make the app more amenable to helping those newly diagnosed or particularly struggling with QoL.
Smartphones in particular have earned recognition for being an effective and efficient means to facilitate behavior change12,13,22,43 and assist in the management and improvement of many health conditions and chronic diseases.1,4–6,13,44–46 Researchers and app developers (and/or IT specialists) should continue to work together to ensure that smartphone apps are created based on current evidence to educate users and to promote health behavior change effectively with the ultimate goal of optimizing chronic disease management.
Acknowledgements
Special thanks to KORE Digital Health Therapeutics, Darlene Higbee Clarkin, and Stephen Drozdik for developing the app, Desiree Nielsen for writing the content of the app, and Terry Walters for creating the meal plans.
Contributorship
Principal responsibility for the study design and conduct was assumed by A.J.D. and S.N.C.R. T.F. and K.T.Y.T conducted the data analysis. A.J.D. and C.B.W. drafted the manuscript. All authors read and commented on drafts and approved the final manuscript.
Conflict of interests
A.J.D. is one of the founders of the MyHealthyGut app.
Ethical approval
The University of Calgary Behavioural Research Ethics Board approved this study.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Calgary, Vancouver, and Kamloops Chapters of the Canadian Celiac Association, Mitacs Accelerate, The Calgary Foundation.
Guarantor
A.J.D.
ORCID iD
A Justine Dowd https://orcid.org/0000-0002-0577-3809
Peer review
This manuscript was reviewed by reviewers who have chosen to remain anonymous.
References
- 1.Steinhubl SR, Muse ED, Topol EJ. The emerging field of mobile health. Sci Transl Med 2015; 7: 283rv3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. mHealth: New horizons for health through mobile technologies: based on the findings of the secondary global survey on eHealth. Global Observatory for eHealth Series – Volume 3 Geneva: World Health Organization, 2011. [Google Scholar]
- 3.World Health Organization. Noncommunicable diseases country profiles 2014. Geneva: World Health Organization, 2014. [Google Scholar]
- 4.Whitehead L, Seaton P. The effectiveness of self-management mobile phone and tablet apps in long-term condition management: a systematic review. J Med Internet Res 2016; 18: e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merck SF. Chronic disease and mobile technology: an innovative tool for clinicians. Nurs Forum 2017; 52: 298–305. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Wang Y, Wei Cet al. Smartphone interventions for long-term health management of chronic diseases: an integrative review. Telemed J E Health 2014; 20: 570–583. [DOI] [PubMed] [Google Scholar]
- 7.Helsel BC, Williams JE, Lawson Ket al. Telemedicine and mobile health technology are effective in the management of digestive diseases: a systematic review. Dig Dis Sci 2018; 63: 1392–1408. [DOI] [PubMed] [Google Scholar]
- 8.Carter MC, Burley VJ, Nykjaer Cet al. Adherence to a smartphone application for weight loss compared to website and paper diary: pilot randomized controlled trial. J Med Internet Res 2013; 15: e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke LE, Styn MA, Sereika SMet al. Using mHealth technology to enhance self-monitoring for weight loss: a randomized trial. Am J Prev Med 2012; 43: 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lieffers JR, Hanning RM. Dietary assessment and self-monitoring with nutrition applications for mobile devices. Can J Diet Pract Res 2012; 73: e253–260. [DOI] [PubMed] [Google Scholar]
- 11.Ipjian ML, Johnston CS. Smartphone technology facilitates dietary change in healthy adults. Nutrition 2017; 33: 343–347. [DOI] [PubMed] [Google Scholar]
- 12.Ernsting C, Dombrowski SU, Oedekoven Met al. Using smartphones and health apps to change and manage health behaviors: a Population-based survey. J Med Internet Res 2017; 19: e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao J, Freeman B, Li M. Can mobile phone apps influence people’s health behavior change? An evidence review. J Med Internet Res 2016; 18: e287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mertz L. mHealth to the rescue: growing use of wireless and mobile technologies improves community health, even in rural areas. IEEE Pulse 2016; 7: 16–24. [DOI] [PubMed] [Google Scholar]
- 15.Riaz MS, Atreja A. Personalized technologies in chronic gastrointestinal disorders: self-monitoring and remote sensor technologies. Clin Gastroenterol Hepatol 2016; 14: 1697–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Con D, De Cruz P. Mobile phone apps for inflammatory bowel disease self-management: a systematic assessment of content and tools. JMIR Mhealth Uhealth 2016; 4: e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guandalini S, Assiri A. Celiac disease: a review. JAMA Pediatr 2014; 168: 272–278. [DOI] [PubMed] [Google Scholar]
- 18.Hallert C, Grännö C, Hultén Set al. Living with coeliac disease: controlled study of the burden of illness. Scand J Gastroenterol 2002; 37: 39–42. [DOI] [PubMed] [Google Scholar]
- 19.Nachman F, Del Campo MP, González Aet al. Long-term deterioration of quality of life in adult patients with celiac disease is associated with treatment noncompliance. Dig Liver Dis 2010; 42: 685–691. [DOI] [PubMed] [Google Scholar]
- 20.Casellas F, Rodrigo L, Lucendo AJet al. Benefit on health-related quality of life of adherence to gluten-free diet in adult patients with celiac disease. Rev Esp Enferm Dig 2015; 107: 196–201. [PubMed] [Google Scholar]
- 21.El-Salhy M, Hatlebakk JG, Gilja OHet al. The relation between celiac disease, nonceliac gluten sensitivity and irritable bowel syndrome. Nutrition 2015; 14: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoeppe S, Alley S, Van Lippevelde Wet al. Efficacy of interventions that use apps to improve diet, physical activity and sedentary behaviour: a systematic review. Int J Behav Nutr Phys Act 2016; 13: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chin SO, Keum C, Woo Jet al. Successful weight reduction and maintenance by using a smartphone application in those with overweight and obesity. Sci Rep 2016; 6: 34563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Jong MJ, Van Der Meulen-De Jong AE, Romberg-Camps MJet al. Telemedicine for management of inflammatory bowel disease (myIBDcoach): a pragmatic, multicentre, randomised controlled trial. Lancet 2017; 390: 959–968. [DOI] [PubMed] [Google Scholar]
- 25.Dowd AJ, Jackson C, Tang KTYet al. MyHealthyGut: development of a theory-based self-regulatory app to effectively manage celiac disease. Mhealth 2018; 4: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leffler DA, Dennis M, Edwards George Jet al. A validated disease-specific symptom index for adults with celiac disease. Clin Gastroenterol Hepatol 2009; 7: 1328–1334, 1334.e1–3. [DOI] [PubMed] [Google Scholar]
- 27.Dowd AJ, Jung ME. Self-compassion directly and indirectly predicts dietary adherence and quality of life among adults with celiac disease. Appetite 2017; 113: 293–300. [DOI] [PubMed] [Google Scholar]
- 28.Dorn SD, Hernandez L, Minaya MTet al. The development and validation of a new coeliac disease quality of life survey (CD-QOL). Aliment Pharmacol Ther 2010; 31: 666–675. [DOI] [PubMed] [Google Scholar]
- 29.Dorn S, Hernandez L, Minaya Met al. The development and validation of a new coeliac disease quality of life survey (CD-QOL). Aliment Pharmacol Ther 2010; 31: 666–675. [DOI] [PubMed] [Google Scholar]
- 30.Strachan SM, Brawley LR. Reactions to a perceived challenge to identity: a focus on exercise and healthy eating. J Health Psychol 2008; 13: 575–588. [DOI] [PubMed] [Google Scholar]
- 31.McAuley E, Mihalko SL. Measuring exercise-related self-efficacy In: Duda JL. (ed) Advances in sport and exercise psychology measurement. Morgantown: Fitness Information Technology, 1998, pp.371–381. [Google Scholar]
- 32.Dowd AJ, Chen MY, Jung MEet al. Prediction of adherence to a gluten-free diet using protection motivation theory among adults with coeliac disease. J Hum Nutr Diet 2016; 29: 391–398. [DOI] [PubMed] [Google Scholar]
- 33.Zung WW. A self-rating depression scale. Arch Gen Psychiatry 1965; 12: 63–70. [DOI] [PubMed] [Google Scholar]
- 34.Biggs JT, Wylie LT, Ziegler VE. Validity of the Zung self-rating depression scale. Br J Psychiatry 1978; 132: 381–385. [DOI] [PubMed] [Google Scholar]
- 35.Spielberger CD, Gorsuch RL, Lushene Ret al. Manual for the State–Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press, 1983. [Google Scholar]
- 36.Spielberger CD. State–Trait Anxiety Inventory: bibliography. 2nd ed. Palo Alto: Consulting Psychologists Press, 1989. [Google Scholar]
- 37.Zarkadas M, Dubois S, MacIsaac Ket al. Living with coeliac disease and a gluten-free diet: a Canadian perspective. J Hum Nutr Diet 2013; 26: 10–23. [DOI] [PubMed] [Google Scholar]
- 38.McCambridge J, Kypri K, Elbourne D. In randomization we trust? There are overlooked problems in experimenting with people in behavioral intervention trials. J Clin Epidemiol 2014; 67: 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah S, Akbari M, Vanga Ret al. Patient perception of treatment burden is high in celiac disease compared with other common conditions. Am J Gastroenterol 2014; 109: 1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neubeck L, Lowres N, Benjamin EJet al. The mobile revolution – using smartphone apps to prevent cardiovascular disease. Nat Rev Cardiol 2015; 12: 350–360. [DOI] [PubMed] [Google Scholar]
- 41.Kannisto KA, Korhonen J, Adams CEet al. Factors associated with dropout during recruitment and follow-up periods of a mHealth-based randomized controlled trial for mobile.net to encourage treatment adherence for people with serious mental health problems. J Med Internet Res 2017; 19: e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lie SS, Karlsen B, Oord ERet al. Dropout from an eHealth intervention for adults with type 2 diabetes: a qualitative study. J Med Internet Res 2017; 19: e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Higgins JP. Smartphone applications for patients’ health and fitness. Am J Med 2016; 129: 11–19. [DOI] [PubMed] [Google Scholar]
- 44.Carroll JK, Moorhead A, Bond Ret al. Who uses mobile phone health apps and does use matter? A secondary data analytics approach. J Med Internet Res 2017; 19: e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cafazzo JA, Casselman M, Hamming Net al. Design of an mHealth app for the self-management of adolescent type 1 diabetes: a pilot study. J Med Internet Res 2012; 14: e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hood M, Wilson R, Corsica Jet al. What do we know about mobile applications for diabetes self-management? A review of reviews. J Behav Med 2016; 39: 981–994. [DOI] [PubMed] [Google Scholar]