Abstract
Objective
The purpose of the study was to investigate the treatments and outcomes of patients with traumatic carotid-cavernous sinus fistula (TCCF).
Methods
All patients diagnosed with TCCF at our institution from January 2013 to December 2018 and meeting the inclusion/exclusion criteria were included in the study.
Results
A total of 24 patients were included in this study. Of them, 21 (87.5%) were treated with detachable balloon embolization, 1 (4%) with coil embolization, 1 (4%) with balloon-assisted coil embolization, and 1 (4%) with balloon-assisted coil and glue embolization. Among the 21 patients treated with detachable balloon embolization, 10 underwent double-balloon technique embolization including double-detachable balloon embolization (n = 6) and balloon-assisted detachable balloon embolization (n = 4). The fistulas in 17 patients (17/21, 81%) were successfully occluded after the first attempt of detachable balloon embolization, while those in the remaining 4 patients were occluded after a second surgery due to TCCF recurrence or pseudoaneurysm development. Preservation of the internal carotid artery (ICA) was observed in 19 cases after the first treatment by detachable balloon embolization (19/21, 90.4%). ICA was occluded in the remaining two patients, as revealed by a complete angiographic evaluation of the circle of Willis. All patients achieved complete resolution of ocular and orbital manifestations as well as pulsatile bruit, except for three patients whose oculomotorius and/or abducens remained paralyzed during the follow-up period.
Conclusion
Although several endovascular treatment options are available for TCCF, the detachable balloon embolization is still the preferred method of TCCF, as evidenced in our study. Furthermore, double balloon technique, an improvement upon the conventional detachable balloon embolization, is extremely safe and can effectively treat patients with refractory TCCF.
Keywords: Traumatic carotid-cavernous sinus fistula, interventional embolization, detachable balloon, double balloon
Traumatic carotid-cavernous fistula (TCCF) is a direct shunt between the cavernous portion of the internal carotid artery (ICA) and the cavernous sinus (CS), which can result from traumatic laceration at the cavernous segment of ICA.1–3 Prior to 1970s, the treatment option for TCCF was limited to ICA obliteration due to the lack of understanding of CS anatomy. In 1974, the first case of endovascular occlusion of CCF using detachable balloon was reported by Serbinenko.4 Since then, endovascular treatment has become a safe and effective option for treating CCF. Further, with the development of interventional techniques and materials, the endovascular management of CCF using multiple devices has been widely accepted.5,6 At our institution, we typically perform detachable balloon embolization for the treatment of TCCF. In addition, we have improved TCCF embolization by using double balloon technique involving double-detachable balloon embolization and balloon-assisted detachable balloon embolization, which are considered to be very safe procedures with rare complications.
Methods
The study protocol was approved by the Institutional Review Board of our hospital. We retrospectively reviewed the patient charts from January 2013 to December 2018, in order to identify potential TCCF cases during this period. The inclusion criteria of this study were: (1) positively confirmed CCF, (2) solid evidence of head trauma, (3) no history of spontaneous CCF, and (4) no intervention performed on the patients before being admitted to our hospital.
Patient clinical data, such as the first and second treatments, first and second angiography results, parent artery preservation, complications and long-term outcome, were collected (Table 1). Long-term outcome was determined by the clinical symptoms at the last follow-up visit. Recurrence, pseudoaneurysm and ICA occlusion as the complications of endovascular embolization of TCCF have attracted much attention; therefore, we integrated “second treatment”, “second angiography result,” and “parent artery preservation” into the clinical data. First and second angiography results were defined as the results of immediate angiography after the first and second embolization.
Table 1.
Patient clinical treatments and outcomes.
| Patient no. | First treatment | First angiography result | Second treatment | Second angiography result | Parent artery preservation | Complication | Last follow-up angiography and result | Long-term outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | Balloon-assisted Coil | Fistula occlusion | None | None | Yes | None | MRA (3D-TOF/fistula occlusion | Cure |
| 2 | Double-Detachable Balloon | Fistula occlusion | None | None | Yes | None | CTA/fistula occlusion | Cure |
| 3 | Balloon alone | Fistula occlusion | Balloon-assisted coil | Fistula occlusion and ICA occlusion | No | Recurrence | CTA/fistula occlusion and ICA occlusion | Cure |
| 4 | Balloon alone | Fistula occlusion | None | None | Yes | None | CTA/fistula occlusion | Cure |
| 5 | Balloon alone | Fistula occlusion | None | None | Yes | None | DSA/fistula occlusion | Cure |
| 6 | Double-detachable balloon | Fistula occlusion | None | None | Yes | None | CTA/fistula occlusion | Cure |
| 7 | Balloon alone | Fistula occlusion | None | None | Yes | None | MRI (3D-TOF)/ fistula occlusion | Oculomotorius paralysis |
| 8 | Coil | Fistula occlusion | None | None | Yes | Thrombosis | CTA/fistula occlusion | Cure |
| 9 | Balloon alone | Fistula occlusion and ICA occlusion | None | None | No | None | CTA/fistula occlusion and ICA occlusion | Oculomotorius and abducens paralysis |
| 10 | Balloon alone | Fistula occlusion | None | None | Yes | None | DSA/fistula occlusion | Cure |
| 11 | Double-Detachable Balloon | Fistula occlusion | Balloon-assisted Detachable Balloon | Fistula occlusion | Yes | Recurrence | CTA/fistula occlusion | Cure (Third Treatment of balloon-assisted coil and glue embolization due to second recurrence) |
| 12 | Balloon-assisted Detachable Balloon | Fistula occlusion | None | None | Yes | None | CTA/fistula occlusion | Cure |
| 13 | Balloon alone | Fistula occlusion | None | None | Yes | None | CTA/fistula occlusion | Cure |
| 14 | Balloon alone | Fistula occlusion | Balloon-assisted Coil + Glue | aneurysm occlusion | Yes | Pseudoaneurysm | DSA/fistula occlusion and aneurysm occlusion | Cure |
| 15 | Balloon-assisted Coil + Glue | Fistula occlusion | None | None | Yes | None | CTA/fistula occlusion | Cure |
| 16 | Balloon alone | Fistula occlusion and ICA occlusion | None | None | No | None | CTA/fistula occlusion and ICA occlusion | Cure |
| 17 | Balloon-assisted Detachable Balloon | Fistula occlusion | None | None | Yes | None | CTA/fistula occlusion | Cure |
| 18 | Double-Detachable Balloon | Fistula occlusion | None | None | Yes | None | CTA/fistula occlusion | Cure |
| 19 | Double-Detachable Balloon | Fistula occlusion | None | None | Yes | None | CTA/fistula occlusion | Cure |
| 20 | Double-Detachable Balloon | Fistula occlusion | None | None | Yes | None | CTA/fistula occlusion | Cure |
| 21 | Balloon-assisted Detachable Balloon | Fistula occlusion | None | None | Yes | None | CTA/fistula occlusion | Cure |
| 22 | Balloon alone | Fistula occlusion | None | None | Yes | None | CTA/fistula occlusion | Abducens paralysis |
| 23 | Balloon alone | Residual fistula | Balloon | Fistula occlusion | Yes | Recurrence | DSA/fistula occlusion | Cure |
| 24 | Balloon-assisted Detachable Balloon | Fistula occlusion | None | None | Yes | None | CTA/fistula occlusion | Cure |
ICA: internal carotid artery; CTA: computed tomographic angiography; DSA: digital subtraction angiography; MRA 3D-TOF: three-dimensional time-of-flight magnetic resonance angiography.
Results
Clinical presentation
During the five-year period from January 2013 to December 2018, a total of 24 patients with TCCF were treated in our department. The major clinical manifestations of TCCF included proptosis, chemosis, orbital bruits, oculomotor paralysis, and abducens paralysis. A complete cerebral vascular angiography involving bilateral ICA, external carotid artery, and vertebral artery was carried out prior to TCCF intervention. Angiography confirmed a major drainage toward anterior via superior ophthalmic veins (SOV), toward an inferior via inferior petrosal sinus and toward a posterior via superior petrosal sinus, as well as the minor drainages including cortex vein and intercavernous sinuses.
Treatment
Among the 24 patients, 21 (87.5%) were treated with detachable balloon embolization, 1 (4%) with coil embolization, 1 (4%) with balloon-assisted coil embolization, and 1 (4%) with balloon-assisted coil and glue embolization. In 6 of the 21 patients treated with detachable balloon (GOLDBAL2, Balt Extrusion, Montmorency, France) embolization, a double-detachable balloon technique was performed when TCCF can not be completely occluded using one balloon with repeated adjustment, and when multiple inflated balloons are needed to completely occlude the orifice due to a large fistulous ostium and rapid blood flow. Under the first circumstance, the blood flow and volume of CS were significantly reduced by the first balloon. It was anticipated that the second balloon could not pass into the CS. To ensure that the second balloon could negotiated into the CS, the first balloon was deflated and remained nondetached at the fistula. When the second balloon was placed into the fistula, there were two deflated balloons in the CS with their respective microcatheter. The position and volume of the two balloons were adjusted coordinately until the fistula was occluded with unobstructed ICA. The two balloons were then detached sequentially after confirming the fistula occlusion by angiography. Under the second circumstance, multiple balloons were employed. One balloon was detached and the next one was deflated to ensure that the third one could successfully pass into the CS. Before the complete occlusion of fistula, two detachable balloons with their respective microcatheter were simultaneously placed into the CS and the aforementioned embolization steps were followed. In 4 of the 21 patients treated with detachable balloon embolization, another double-balloon (bracing balloon-assisted detachable balloon) technique was performed. This technique could be considered a valid option under the following three circumstances. (1) When the exact location of the fistula orifice was blurry, the detachable balloon might not be able to pass through the fistula. The bracing balloon (Scepter C, MicroVention, California, USA) was placed in the ICA distal to the fistula orifice followed by inflation to occlude the blood flow. Then, the detachable balloon was directed to the venous side through the fistula by blood flow. (2) When the detachable balloon might be retracted to the ICA after inflation, the bracing balloon was placed into the ICA overlying the detachable balloon. Then, the bracing balloon was inflated to an appropriate size in order to facilitate the detachable balloon to locate the fistula orifice and prevent the occlusion of ICA. (3) When the detachable balloon might be retracted to the ICA after detachment, the bracing balloon was inflated overlying the orifice. Then, the detachable balloon was detached and fixed in the fistula.
Outcome
Among the 24 patients with TCCF, 20 (83.3%) achieved fistula occlusion after the first endovascular embolization, 17 of whom were treated with detachable balloons. The remaining four (16.7%) patients were eventually occluded by a second surgery due to TCCF recurrence (3) or pseudoaneurysm (1). Among the three patients with TCCF recurrence, the second embolization procedures were implemented with detachable balloon, detachable balloon-assisted coil, balloon-assisted detachable balloon, and balloon-assisted coil and glue, respectively, and thus achieving TCCF occlusion. One patient suffered from pseudoaneurysm progression after the first treatment and underwent a second surgery for aneurysm occlusion by balloon-assisted coil and glue embolization. Preservation of ICA was achieved in 21 out of 24 cases (87.5%). In two patients, ICA was occluded by the detachable balloon after a complete angiographic evaluation of the circle of Willis. Meanwhile, another patient suffered from TCCF recurrence, and then ICA was occluded during the second procedure, as the detachable balloon could not navigate into the CS through the fistula into the CS. Among the three patients treated with coil or glue embolization, two did not experience any surgery-related morbidity or mortality, while the other one suffered intraoperative thrombosis. For this patient, thrombolysis and thrombectomy were performed immediately, followed by fistula occlusion with temporal and occipital lobe infarction. Follow-up angiography including computed tomographic angiography (CTA), digital subtraction angiography (DSA) and three-dimensional time-of-flight magnetic resonance angiography (MRA 3D-TOF) was performed in all patients after a mean follow-up period of five months (range: 1–24 months). Last follow-up images showed that all fistula were occluded with no recurrent or pseudoaneurysm. Complete resolution of ocular and orbital symptoms and pulsatile bruit were observed in all cases after clinical follow-up. However, oculomotor and/or abducens paralysis occurred in three patients after detachable balloon embolization.
Illustrative cases
Case 1
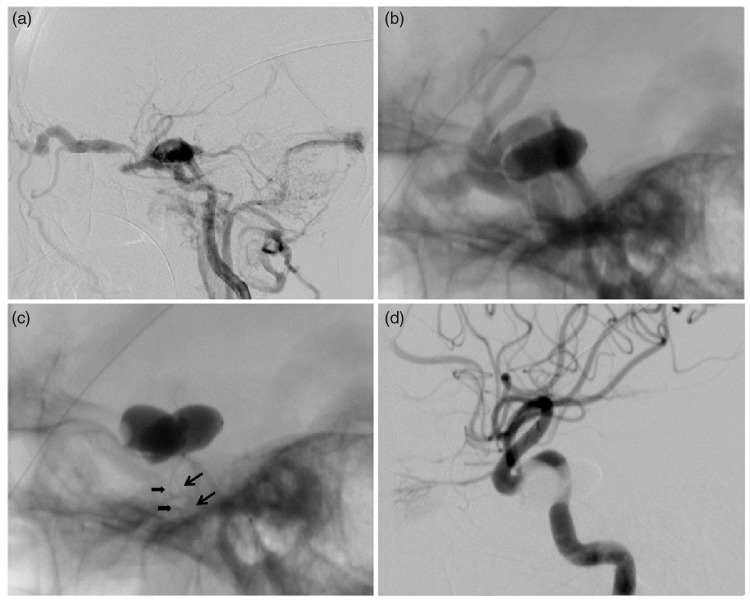
A 68-year-old female patient suffered from proptosis, chemosis, and orbital bruits on day 30 after a traffic accident. Cerebral angiography showed CCF with high-flow shunts and severe steal (Figure 1(a)). The first detachable balloon was inflated at highest volume to alleviate the shunt; however, the fistula remained visible (Figure 1(b)). Subsequently, the second balloon was placed into the CS, while the first one was temporarily deflated but remained nondetached at the fistula (Figure 1(c)). The position and volume of the two balloons were then adjusted repeatedly and coordinately. When angiography confirmed disappearance of fistula and preservation of ICA (Figure 1(d)), the second balloon was detached, followed by the first one.
Figure 1.
Illustrative case 1. (a) Lateral angiogram of the ICA showing the CCF with large high-flow shunt and severe steal with a little filling of intracranial arteries. (b) Native angiographic image showing that the first detachable balloon could not completely occlude the CCF. (c) The two detachable balloons were simultaneously placed in the CS with their respective balloon catheter (arrow and arrowhead). (d) Immediate angiogram after the procedure showing resolution of the CCF with parent artery preservation.
Case 2
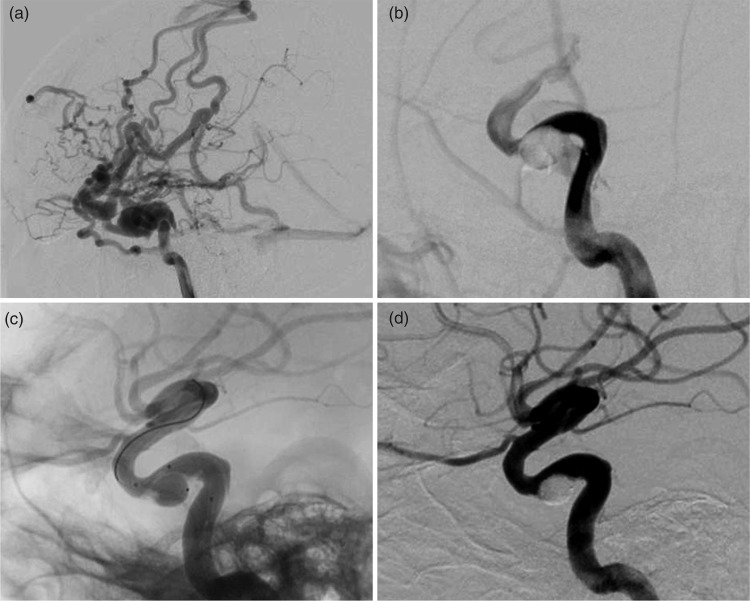
A 34-year-old male patient was admitted to our hospital with orbital bruits and diplopia after a motor vehicle accident. Cerebral angiography revealed a direct CCF (Figure 2(a)). The detachable balloon was successfully placed into the fistula, but might be retracted to the ICA after inflation (Figure 2(b)). Thus, a bracing balloon was placed into the ICA, overlying the detachable balloon. Then, the bracing balloon was inflated to an appropriate size in order to facilitate the detachable balloon to locate the fistula orifice and to prevent ICA stenosis (Figure 2(c)). Once the size and position of the detachable balloon were appropriately adjusted and finalized, it was detached first. Then, the bracing balloon was deflated and retreated from ICA. Post-procedure angiography showed a complete resolution of the fistula while preserving the ICA (Figure 2(d)).
Figure 2.
Illustrative case 2. (a) Lateral ICA angiographic image showing a direct CCF and the major drainage toward CV and SOV. (b) The detachable balloon retracted to the ICA after inflation due to its shallow space in the CS. (c) The double-lumen balloon was placed overlying the orifice of the fistula, and assisted the detachable balloon to adapt a shallow space in the CS and remain inside the CS. (d) Angiography showing the disappearance of the CCF and the preserved ICA.
Case 3
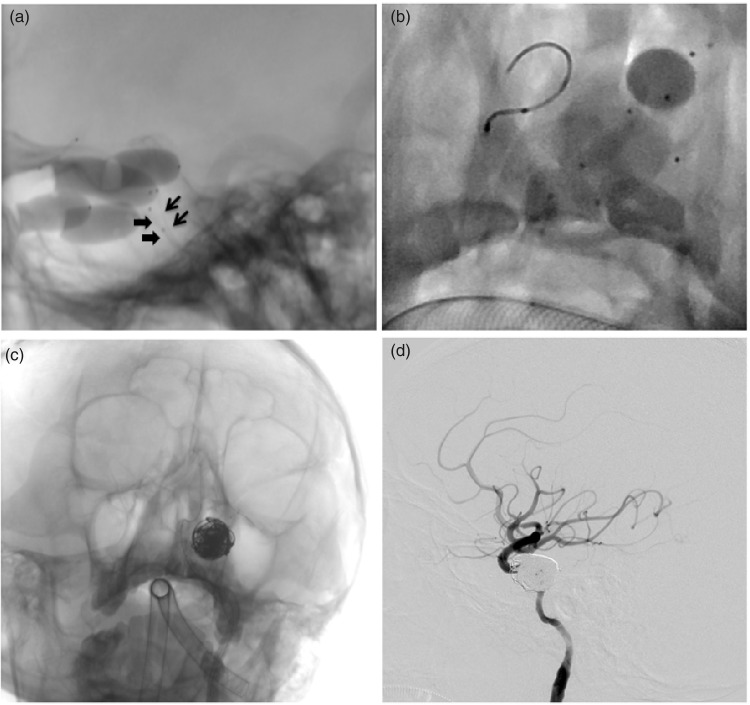
A 32-year-old male patient suffered from proptosis and diplopia for eight months after a traumatic brain injury. Cerebral angiography revealed a direct CCF. TCCF was occluded by double-detachable balloon technique (Figure 3(a)) with complete resolution of ocular and orbital manifestations. However, this patient developed TCCF recurrence on day 34 post surgery. Balloon-assisted detachable balloon technique was employed again for the fistula occlusion (Figure 3(b)), and immediate angiography showed the disappearance of CCF and preservation of ICA. At five-month follow-up visit, CTA showed a recurrence of TCCF. Balloon-assisted coil and glue embolization was selected for fistula occlusion in this patient (Figure 3(c)). Post-procedure angiography revealed both fistula occlusion and ICA preservation (Figure 3(d)).
Figure 3.
Illustrative case 3. (a) Lateral unsubtracted projection demonstrating the TCCF occlusion by double-detachable balloon technique, and two detachable balloons were simultaneously placed in the CS with their respective balloon catheter (arrow and arrowhead). (b) Native angiographic image showing implementation of the balloon-assisted detachable balloon embolization since the TCCF recurred on day 34 post the first surgery. (c) The balloon-assisted coil and glue embolization was ultimately adopted for the TCCF occlusion since the recurrence developed at five months of follow-up. (d) Post-procedure angiography showing the TCCF occlusion with coil and glue.
Discussion
TCCF is an abnormal vascular shunt that allows blood to flow from the ICA directly to the CS, as a consequence of traumatic event. Patients with TCCF commonly manifest with symptoms of proptosis, chemosis, cranial nerve palsies and orbital bruits, due to the engorgement of draining veins and orbital venous congestion. Therefore, the goal of treatment is to occlude the abnormal shunt between the ICA and the CS, in order to reduce venous congestion and improve cerebral perfusion. In 1809, the surgical treatment of TCCF began with Travers' ligation procedure and external carotid compression by a wooden appliance.7 In 1974, a TCCF case was successfully treated with endovascular occlusion using detachable balloon.4 Since then, detachable balloon embolization has become a novel treatment option with well-established safety records and less complications. Later on, metallic coils or liquid embolic agents have been used to replace balloon for the treatment of CCF, in order to avoid unexpected events associated with balloons, including early detachment, premature deflation, and puncture of fractured fragments.8,9 Disadvantages of this option include high cost, potential occlusion of main venous drainage, and occurrence of cranial nerve palsy.10 Recently, covered stent has become an attractive alternative treatment for CCF.11–14 However, one concern with stent is that anticoagulation and/or antiplatelet agents may prevent thrombus formation around the fistula, thus increasing the risk of recurrence during the perioperative period.15 In addition, covered stent could not be easily navigated through the tortuous intracranial vessels.16
Transarterial detachable balloon embolization has been a routine treatment for most TCCF patients in our institution, but treatment outcomes of the procedure depend largely on the size of CS and blood flow through fistula. We would consider embolization with detachable balloon alone if the CCF meets all these three criteria: (1) size of the fistula is smaller than that of an inflated balloon, but large enough to allow the passage of a deflated or partially inflated balloon. (2) Blood flow through the fistula is sufficient for the passage of the balloon. (3) CS is large enough to house the balloon for the complete occlusion of the fistula. In our cohort, only six TCCF patients met the above criteria and were successfully occluded by detachable balloon alone without any complication. However, TCCF recurrence was observed in one patient (No. 23) due to the presence of residual fistula. In this patient, CS was too large, in which the foregoing detachable balloon could not completely occlude the CCF orifice. The blood flow through fistula and volume of CS were reduced by the inflated balloon; as a result, the next balloon was unable to pass into the CS. In two other patients (Nos. 9, 16), the location of the orifice was blurry, and the blood flow was insufficient to drive the detachable balloon into the CS. Eventually, ICA was occluded after complete angiographic evaluation of the circle of Willis. Collectively, these findings suggest that embolization with detachable balloon alone may possess some limitations, mostly due to the size of CS. Therefore, we developed a novel “double-balloon” technique, which was modified upon the conventional detachable balloon embolization techniques. Two approaches of double balloon techniques were developed as follows: double-detachable balloons and balloon-assisted detachable balloon. For the first approach, double detachable balloons are slowly and sequentially negotiated into the fistula with large CS. Both balloons with their attached microcatheters are simultaneously placed in the CS and are adjusted alternately. For another approach, a bracing balloon is used to assist the detachable balloon to pass into the CS, fixed in the CS and detached safely from the fistula.
We have adopted the approach of double-detachable balloons for the first time in 2012. This approach is remarkably useful when blood flow and CS volume are significantly reduced by the first balloon and the next balloon could not be negotiated into the fistula for complete CCF occlusion, and when several balloons are required to embolize a large CCF. Among 24 patients with TCCF, 6 were treated by this approach with successful fistula obliteration and ICA preservation. Only one patient (No. 11) suffered from TCCF recurrence due to the migration of balloon after surgery. During this procedure, two non-inflated balloons were placed simultaneously in the CS with their respective microcatheter, in which both position and volume were adjusted synchronously to effectively occlude the fistula. TCCF recurrence and pseudoaneurysm development were significantly reduced after treating fistula with large CS by using this approach. Despite the numerous advantages of this technique, attention should be drawn to the risk associated with the simultaneous placement of two balloon catheters in the same vessel. Therefore, we highlighted the following technical tips based on our experience. (1) There might be resistance when placing two balloon catheters simultaneously under a single guiding catheter, which could increase the risk of entwined catheters and premature balloon detachment. Therefore, bilateral femoral artery puncture and double-guiding catheters should be used. (2) Before placing the second balloon into the CS, the first balloon should be deflated in order to ensure sufficient blood flow and space in the CS for the successful passing of the second balloon. (3) After the second balloon is localized, both balloon catheters should not be much advanced in order to avoid entwining in the ICA. (4) After angiograms confirming no residual fistula and no ICA stenosis, the latter passing balloon should be detached first, and then the former one.
As another approach of double balloon technique, the bracing balloon-assisted detachable balloon has been published for the first time in 2000 by Teng et al.17 According to their experience, this approach is particularly beneficial when the CS is too small or blood flow is too slow for the passage of a deflated or slightly inflated balloon, and when the width of the CS is smaller than that of the fistula orifice. Under the first circumstance, the bracing balloon temporarily occluded the ICA distal to the fistula orifice and then blocked the blood flow in ICA, resulting in the increased blood flow into CS and facilitated the passage of detachable balloon into the CS through the fistula. By using this double balloon approach, we successfully navigated the detachable balloon into the small CS and obliterated the fistula in one patient (No. 12). Based on our experience, this approach could make passing of the balloon through the orifice of the fistula easier when the location of the orifice was blurry, and thus reduced the risk of premature balloon detachment. In addition, ICA occluded by bracing balloon could effectively prevent the distal vessel blockage caused by premature detachment of the detachable balloon. However, this approach failed in one patient whose fistula was extremely small (No. 8); thus, coil embolization was implemented. Under another circumstance, the detachable balloon might be retracted to the ICA after inflation due to the small size of the CS. Therefore, the bracing balloon was placed overlying the fistula orifice in the ICA, which could facilitate the detachable balloon to adapt a shallow space in the CS and stay inside the CS. In this study, two patients (Nos. 17 and 21) were treated by this double balloon approach with ICA preservation, and this approach was used in another patient (No. 24) to assist the detachment of detachable balloon. After detachment, the detachable balloon might retract to the ICA. The bracing balloon could be inflated overlying the orifice to retain the detachable balloon in the CS. Furthermore, unlike the technique described by Teng et al.,17 we used a double-lumen balloon catheter, in which a guiding wire was used to navigate the bracing balloon into the distal of ICA through the tortuous intracranial vessels. The high compliance of the bracing balloon makes it extremely suitable for facilitating the placement of detachable balloon. There were many benefits of the bracing balloon-assisted detachable balloon technique for treating TCCF patients. However, we should be very cautious during these procedures due to the following circumstances. (1) Similar to the above-described double detachable balloon approach, bilateral femoral artery puncture, and double-guiding catheter are highly recommended. (2) During balloon adjustment, the extended period of balloon occlusion within ICA can worsen the hypo-perfusion status, thus leading to acute infarction. Therefore, the blocking time should be limited to less than 5 min. (3) The bracing balloon should not be overinflated in order to avoid fistula expansion. The balloon should be inflated just enough to block blood flow.
According to previous literature, the conventional detachable balloon embolization techniques are indeed achieve a high occlusion rate, but the rate of ICA preservation may not be ideal.5,18,19 For instance, Debrun et al.18 have reported a successful balloon embolization rate of 98.1% among CCF patients. However, the blood flow in the ICA is only preserved in 59% of the patients. In another study, fistula is completely occluded in 86 patients (86/88, 97.7%),5 while ICA blood flow is preserved in only 66 patients (66/88, 75%). In the present study, 21 patients were initially treated with detachable balloon embolization, and the developed complications were as follows: residual fistula (1, 4.7%), ICA occlusion (2, 9.5%), recurrence (3, 14.3%), and pseudoaneurysm (1, 4.7%). The rate of successful occlusion after the first balloon embolization reached 81% (17/21), and the rate of ICA preservation achieved 90.4% (19/21). Notably, among the 10 patients treated with double balloon technique, fistulas were successfully occluded in 9 (90%) patients, and ICA was preserved in all 10 fistulas (100%). Only one patient (No. 11) suffered from TCCF recurrence without pseudoaneurysm. Based on our findings, after transitioning from the conventional detachable balloon technique to double balloon technique, the rates of fistulas occlusion and ICA preservation are increased, while the rates of TCCF recurrence and pseudoaneurysm development are reduced. In the present study, we did not include small pseudoaneurysm as a complication because they present spontaneous regression by the clinical follow-up, and the benign behavior of these lesions did not indicate the necessity of an aggressive approach.20 TCCF recurrence and pseudoaneurysm development were two common complications of detachable balloon embolization, which can be easily remedied. With the development of interventional techniques and materials, covered stent implantation and balloon-assisted glue embolization have been used for the treatment of patients with recurrent TCCF and pseudoaneurysm.21–23 As could be seen in illustrative case 3, balloon-assisted coil and glue embolization was adopted for fistula occlusion in order to treat TCCF recurrence.
Conclusion
Endovascular embolization with detachable balloon is relatively simple and minimally invasive, and thus serves as an effective treatment for TCCF. The occlusion rate and safety profile of this routine method can be greatly enhanced by double balloon technique, thereby conferring a fresh new vitality. Although several endovascular treatment options are available for TCCF, detachable balloon embolization still represents the first choice of method for treating TCCF.
Acknowledgments
The authors thank all patients enrolled in the study.
Authors’ contribution
All authors have contributed equally to the study design, data collection and article writing.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Barrow DL, Spector RH, Braun IF, et al. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg 1985; 62: 248–256. [DOI] [PubMed] [Google Scholar]
- 2.Van Rooij WJ, Sluzewski M, Beute GN. Ruptured cavernous sinus aneurysms causing carotid cavernous fistula: incidence, clinical presentation, treatment, and outcome. AJNR Am J Neuroradiol 2006; 27: 185–189. [PMC free article] [PubMed] [Google Scholar]
- 3.Kupersmith MJ, Stiebel-Kalish H, Huna-Baron R, et al. Cavernous carotid aneurysms rarely cause subarachnoid hemorrhage or major neurologic morbidity. J Stroke Cerebrovasc Dis 2002; 11: 9–14. [DOI] [PubMed] [Google Scholar]
- 4.Serbinenko FA. Balloon catheterization and occlusion of major cerebral vessels. J Neurosurg 1974; 41: 125–145. [DOI] [PubMed] [Google Scholar]
- 5.Lewis AI, Tomsick TA, Tew JM. Management of 100 consecutive direct carotid-cavernous fistulas: results of treatment with detachable balloons. Neurosurgery 1995; 36: 239–244. [DOI] [PubMed] [Google Scholar]
- 6.Gupta AK, Purkayastha S, Krishnamoorthy T, et al. Endovascular treatment of direct carotid cavernous fistulae: a pictorial review. Neuroradiology 2006; 48: 831–839. [DOI] [PubMed] [Google Scholar]
- 7.Locke CE: Intracranial arterio-venous aneurism or pulsating exophthalmos. Ann Surg 1924; 80: 272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo CB, Teng MM, Chang FC, et al. Transarterial detachable coil embolization of direct carotid-cavernous fistula: immediate and long-term outcomes. J Chin Med Assoc JCMA 2013; 76: 31–36. [DOI] [PubMed] [Google Scholar]
- 9.Wang ZG, Ding X, Zhang JQ, et al. HydroCoil occlusion for treatment of traumatic carotid-cavernous fistula: preliminary experience. Eur J Radiol 2009; 71: 456–460. [DOI] [PubMed] [Google Scholar]
- 10.Briganti F, Tortora F, Marseglia M, et al. Covered stent implantation for the treatment of direct carotid-cavernous fistula and its mid-term follow-up. Interv Neuroradiol 2009; 15: 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber W, Henkes H, Berg-Dammer E, et al. Cure of a direct carotid cavernous fistula by endovascular stent deployment. Cerebrovasc Dis 2001; 12: 272–275. [DOI] [PubMed] [Google Scholar]
- 12.Archondakis E, Pero G, Valvassori L, et al. Angiographic follow-up of traumatic carotid cavernous fistulas treated with endovascular stent graft placement. AJNR Am J Neuroradiol 2007; 28: 342–347. [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez F, Escobar W, Gomez AM, et al. Treatment of carotid cavernous fistulas using covered stents: midterm results in seven patients. AJNR Am J Neuroradiol 2007; 28: 1762–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi BJ, Lee TH, Kim CW, et al. Endovascular graftstent placement for treatment of traumatic carotid cavernous fistulas. J Korean Neurosurg Soc 2009; 46: 572–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moron FE, Klucznik RP, Mawad ME, et al. Endovascular treatment of high-flow carotid cavernous fistulas by stent-assisted coil placement. AJNR Am J Neuroradiol 2005; 26: 1399–1404. [PMC free article] [PubMed] [Google Scholar]
- 16.Lang M, Habboub G, Mullin JP, et al. A brief history of carotid-cavernous fistula. J Neurosurg 2017; 126: 1995–2001. [DOI] [PubMed] [Google Scholar]
- 17.Teng MM, Chang CY, Chiang JH, et al. Double-balloon technique for embolization of carotid cavernous fistulas. Am J Neuroradiol 2000; 21: 1753–1756. [PMC free article] [PubMed] [Google Scholar]
- 18.Debrun G, Lacour P, Vinuela F, et al. Treatment of 54 traumatic carotid-cavernous fistulas. J Neurosurg 1981; 55: 678–692. [DOI] [PubMed] [Google Scholar]
- 19.Higashida RT, Halbach VV, Tsai FY, et al. Interventional neurovascular treatment of carotid cavernous fistula with detachable balloon. Am J Roentgenol 1989; 153: 577–582. [DOI] [PubMed] [Google Scholar]
- 20.Marques MC, Caldas JG, Nalli DR, et al. Follow-up of endovascular treatment of direct carotid-cavernous fistulas. Neuroradiology 2010; 52: 1127–1133. [DOI] [PubMed] [Google Scholar]
- 21.Kalia JS, Niu T, Zaidat OO. The use of a covered stent graft for obliteration of high-flow carotid cavernous fistula presenting with life-threatening epistaxis. J Neurointerv Surg 2009; 1: 142–145. [DOI] [PubMed] [Google Scholar]
- 22.He XH, Li WT, Peng WJ, et al. Endovascular treatment of posttraumatic carotid-cavernous fistulas and pseudoaneurysms with covered stents. J Neuroimaging 2014; 24: 287–291. [DOI] [PubMed] [Google Scholar]
- 23.Luo CB, Teng MM, Chang FC, et al. Transarterial balloon-assisted n-butyl-2-cyanoacrylate embolization of direct carotid cavernous fistulas. AJNR Am J Neuroradiol 2006; 27: 1535–1540. [PMC free article] [PubMed] [Google Scholar]