Abstract
Deviations from normal embryologic development can manifest in different anatomical variants of the ophthalmic artery. We present a case of an infant treated for a high-flow dural arteriovenous fistula of the superior sagittal sinus, in whom an arterial circle involving the ophthalmic artery, the middle meningeal artery, the inferolateral trunk and a hypoplastic segment of the internal carotid artery was found. The embryologic development is briefly reviewed with emphasis on the possible genesis of this interesting constellation.
Keywords: Anatomy, dural AV fistula, embryology, ophthalmic artery, stapedial artery
Introduction
The embryonic development of the ophthalmic artery (OA) is complex and involves different embryologic precursors of its ocular and orbital branches. The stapedial artery is an embryonic vessel that represents a common precursor of the future middle meningeal artery (MMA) and of the orbital branches of the OA, and deviations from its expected development explain some frequently encountered anomalies involving the OA and MMA.1 In this report, we present a case with anomalous origin of the MMA from the inferolateral trunk (ILT) completing an arterial circle around a segmental hypoplasia of the internal carotid artery (ICA) involving also the OA, the ILT and their anastomoses, found in a pediatric patient with a high-flow dural arteriovenous fistula (dAVF) of the superior sagittal sinus (SSS).
Case presentation
An eight-month-old female baby was referred to our service for the work-up of a potential high-flow cranial vascular malformation that was suspected due to marked hypertrophy of the left MMA on her cranial MRI (Figure 1). The baby was suffering from recurrent episodes of refractory coughing that led to the diagnosis of marked cardiomegaly on chest imaging. Further work-up included the aforementioned cranial MRI.
Figure 1.
Maximal intensity projection (a) and axial source image (b) from the time of flight (TOF) MRI performed for the work-up of cardiomegaly in an eight-month-old baby showing marked hypertrophy of the frontal and parietal branches of the left middle meningeal artery (arrows in (a) and (b)). Note also the hypertrophied callosomarginal branch of the right anterior cerebral artery (arrow in (c)) and the extensive network of small vessels at the posterior falx (circle in (c)).
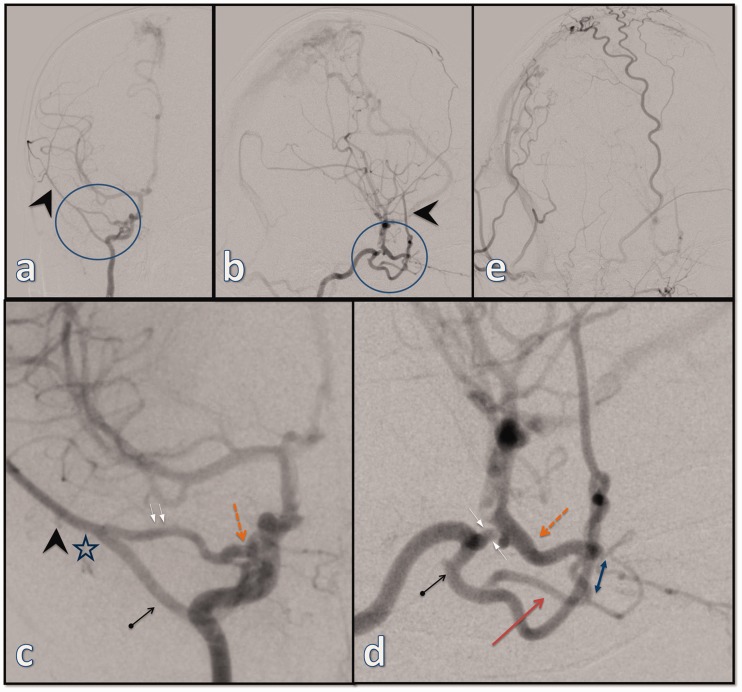
The baby then underwent a diagnostic catheter angiography at our institution, which confirmed a high-flow dAVF of the SSS (Figure 2) supplied mainly through the markedly hypertrophied parietal and frontal branches of the left MMA with less important arterial supply from the right MMA, from both superficial temporal arteries and from both occipital arteries. The fistula also showed marked pial recruitment from the parietal branches of the hypertrophied right anterior cerebral artery (ACA).
Figure 2.
The initial digital subtraction angiography (a: lateral projection and b: frontal projection of selective left external carotid artery (ECA) injection; c: lateral projection of right ECA injection; d: lateral projection of selective left ICA injection) showing an extensive dural arteriovenous fistula of superior sagittal sinus with extensive supply from the frontal and parietal branches of the left middle meningeal artery (MMA) (arrows in (a) and (b)) as well as from the right occipital artery (thick arrow in (c)) and the right superficial temporal artery (thin arrow in (c)) with significant recruitment of pial feeders from the right anterior cerebral artery (thick arrow in (d), better seen via the left ICA injection). Note also minor supply from the tentorial branch of the left ICA (thin arrow in (d)).
The injection of the right external carotid artery (ECA) showed no opacification of the MMA, but interestingly, the selective injection of the right ICA revealed that the right MMA originates from a markedly hypertrophied ILT and participates in the supply of the dAVF (Figure 3). The injection of the right ICA showed two distinct vessels entering the orbit and anastomosing around the optic nerve at the orbital apex: an intradural, proximally hypertrophied OA originating from the right ICA and entering the orbit through the optic canal and an extradural vessel originating from the ILT and entering the orbit through the superior orbital fissure. There was also a marked hypertrophy of the recurrent meningeal branch of the lacrimal artery connecting to the MMA at the lesser sphenoid wing and completing an arterial circle between the ILT and the OA. To make things rather more interesting, the ICA segment between the ILT and the OA was hypoplastic in comparison to the remainder of the ICA with a smaller caliber than either the ILT or the OA.
Figure 3.
Selective injection of the right ICA (a: frontal projection; b: lateral projection; c and d: magnified views) demonstrating an arterial circle (circle in (a) and (b)) formed between the inferolateral trunk (arrow with dot in (d)) and the ophthalmic artery (dashed arrow in (c) and (d)). The ICA segment in-between is hypoplastic and with a smaller caliber than either the OA or the ILT (opposing arrows in (d)). Note also the dilution of the contrast due to the inflow at the level of the posterior communicating artery (d). There is hypertrophied deep recurrent OA branching off the ILT and entering the orbit (arrow in (d)) to connect to the second part of the OA (double arrow in (d)). The right middle meningeal artery (arrowheads in (a), (b) and (c)) originates from the ILT. The hypertrophied recurrent meningeal branch of the lacrimal artery (two parallel arrows in (c)) joins the right MMA at the level of the lesser sphenoid wing (star in (d)). The selective injection of the ipsilateral external carotid artery (e) shows no opacification of the right middle meningeal artery.
The dAVF was treated through endovascular transarterial embolisation, resulting in complete obliteration of the dural arterial supply (Figure 4). The remnant of the fistula supplied by the pial feeders was then surgically disconnected. Interestingly, although the previously hypertrophied dural feeders – including the right MMA – decreased significantly in size as expected after the treatment, the arterial ring involving the OA, ILT and proximal segment of the MMA remained relatively unchanged (Figure 5), which led us to presume that the aforementioned ring serves as a collateral route around the segmental hypoplasia of the ICA between the ILT and OA.
Figure 4.
A follow-up angiography (a: frontal left vertebral injection; b: lateral left ICA injection; c: frontal right ECA injection; d; frontal left ECA injection; e: frontal right ICA injection) performed after the endovascular treatment of the fistula demonstrating the complete obliteration of the dural supply. Note that the normalization of the right MMA after its supply to the dAVF has been disconnected (arrowhead in (e)). A small remnant of the fistula supplied by the pial feeders from the left posterior cerebral artery (arrow in (a)) and the right anterior cerebral artery (dashed arrow in (b)) was subsequently treated surgically.
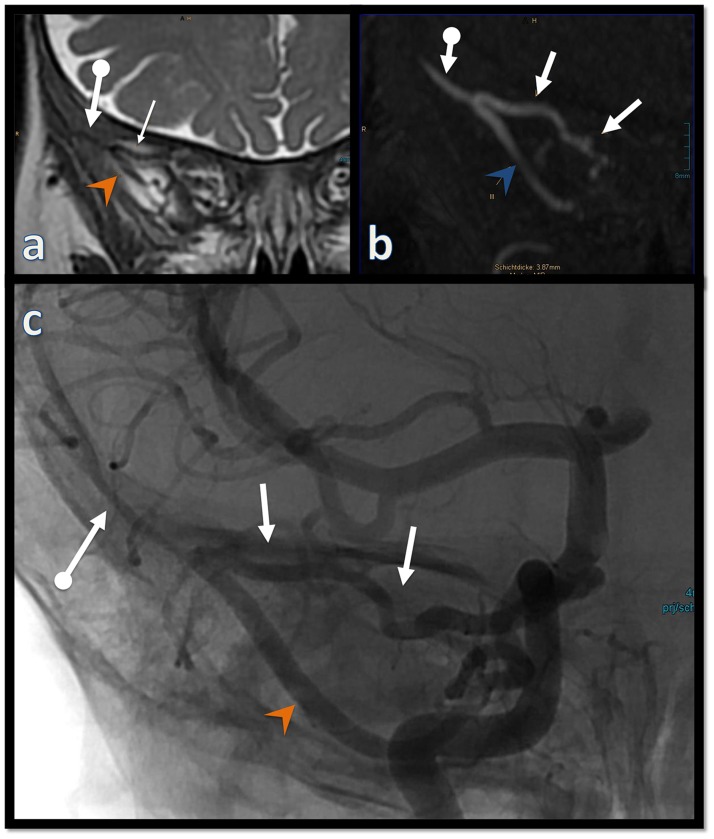
Figure 5.
Coronal T2w (a), coronal reformation of the TOF MRA (b) prior to the treatment; frontal unsubstracted view from the right ICA injection (c) after the treatment: the arterial segment (arrows with dot) of the right middle meningeal artery distal to the junction of the recurrent meningeal branch of the lacrimal artery (arrows) decreased in size after the treatment, whereas the MMA segment proximal to it (arrowheads) as well as the recurrent meningeal branch remained largely unchanged.
Discussion
The presented case demonstrates an anomalous origin of the MMA from the ILT and a well-developed arterial circle around a hypoplastic segment of the ICA just before it becomes intradural. The circle consists of the ILT, the deep recurrent OA, the OA proper, the MMA and the recurrent meningeal branch of the lacrimal artery, all of which are hypertrophied. There are two arteries with distinct origins converging around the optic nerve inside the orbit: a proximal, extradural artery branches off the ILT from the cavernous ICA and enters the orbit through the superior orbital fissure and the OA proper originating from the anteromedial wall of the ICA and entering the orbit through the optic canal (Figure 6). The branches of the OA distal to the connection of these two arteries are of normal caliber (Figure 7). This constellation of anatomic variants seems confusing at first glance and underscores the importance of understanding embryologic development for correct interpretation and proper planning of the treatment strategy. Although a comprehensive review of the anatomy and embryology of the OA and MMA is beyond the scope of this report, we summarize some key points that are relevant to the presented case.
Figure 6.
Sagittal oblique (a) and axial oblique (b) reformations of the TOF MRA illustrating the origin of the hypertrophied inferolateral trunk (arrow with dot in (a) and (b)) from the right ICA (dashed arrow in (a)). The hypertrophied deep recurrent OA (arrow in (a) and (b)) passes through the superior orbital fissure (double arrow in (b)) and connects with the OA. A magnified view of the T2w axial images at the level of the orbit (c) shows the ophthalmic arteries entering the orbit through the optic canal. Note the marked difference in caliber between the ICAs at this level (arrows in (c)).
Figure 7.
Magnified lateral views of a selective injection of the right internal carotid artery (a: substracted, b: unsubstracted) demonstrating the hypertrophied deep recurrent OA (arrow) connecting the hypertrophied inferolateral trunk (arrow with dot) with the ophthalmic artery. The nasal septum branches (arrowheads) and the choroidal blush (ovoid) are highlighted. Note also the anastomosis (double arrow) between the ILT and the internal maxillary artery (dashed arrow) in the pterygopalatine fossa.
The adult-type OA is usually the first intradural branch of the ICA arising from its anteromedial or superomedial aspect.2 It enters the orbit through the optic canal, where it runs inferolateral to the optic nerve and terminates at the superomedial orbital angle by dividing into the dorsal nasal and supratrochlear arteries with a wide variation in branching patterns.3 The branches of the OA are broadly divided into ocular and orbital based on their supply to the bulbar and non-bulbar structures, respectively. This distinction is also based on embryological considerations due to the different origins of these vessels, as the primitive OA develops from the primitive dorsal ophthalmic artery (PrDOA) and primitive ventral ophthalmic artery (PrVOA), whereas the primitive orbital artery develops from a different embryonic vessel named the stapedial artery.4–8
The stapedial artery develops around the fifth week from the hyoid artery, which in turn is a remnant of the second aortic arch. It passes between the crura of the stapes (hence the name), and then divides into a supraorbital division and a maxillofacial division.9 The maxillofacial division exits the cranium via the foramen spinosum and becomes annexed by the ventral pharyngeal artery – the precursor of the ECA – and thus forms the proximal segment of the MMA.10 The intracranial segment of the MMA develops from the supraorbital division of the stapedial artery. Around the 20 mm embryonic stage, the branches of the supraorbital division of the stapedial artery begin to anastomose with and later become assimilated by the developing primitive OA to supply the orbital non-bulbar structures.7,11 The proximal segment of this division then regresses and becomes the recurrent meningeal branch of the lacrimal artery, an anastomosis between the OA and the MMA.6 Being a common precursor of both the MMA and the orbital branches of the OA, variations in the development of this embryonic vessels explain the not uncommon origin of the MMA from the OA or vice versa.
There are controversies regarding the definition of PrDOA and PrVOA and the developmental steps that lead to the formation of the adult-type OA. Older reports describe the PrDOA artery as a branch of the cavernous segment of the ICA that enters the orbit via the superior orbital fissure and consider the ILT to be a remnant of the PrDOA after its regression.12 The PrVOA, on the other hand, originates from the ACA and enters the orbit through the optic canal.13 Recent reports, however, disprove this interpretation and consider both PrDOA and PrVOA to be intradural branches of ICA that enters the orbit through the optic canal and related the cavernous origin of the OA to the deep recurrent OA, a branch of the ILT.5–7,11,14
A key finding in the presented case is the segmental hypoplasia of the ICA between the origins of ILT and OA. Lasjaunias and Santoyo-Vazquez divided the ICA into six independent segments, each corresponding to the origin of a specific embryonic artery.15 Each segment has a specific course, and in case of agenesis, the arterial supply would be redirected to bypass the corresponding segment. Accordingly, the segmental hypoplasia of the ICA segment between the ILT and the OA might has been the initial event in the presented case with the arterial circle representing a collateral rout to the terminal segment of the ICA. The presence of the dAVF might have accentuated this collateral circle due to the sump effect exerted on the right MMA and ACA. Considering the aforementioned developmental steps, the anastomosis between the OA and MMA (the recurrent meningeal branch of the lacrimal artery) represents a remnant of the stapedial artery. The orbital branch of the ILT connecting with the OA represents a markedly hypertrophied deep recurrent OA, whereas the ICA branch entering the optic canal represents the true OA. The MMA origin from the ILT is most likely related to a failed annexation of the maxillofacial branch of the stapedial artery by the developing ECA.
Limitations
Our interpretation of the case above is based on standard frontal and lateral DSA runs and reconstructions of the initially performed MRI. Additional projection with higher frame rates and rotational angiography with multiplanar reconstructions would have aided greatly in better understanding of the anatomy and the relationship between the vascular and bony structure. Such additional images were, however, not performed due to the young age of the patient and the site of the fistula being distant from the region of the presented variants.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical adherence
The ethics committee responsible (Ethik-Kommission der Landesärztekammer Baden-Württemberg) issued a waiver in written form for case reports; the mother of the patient signed an informed consent document allowing the anonymous publication of this case report.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Hakim A, Gralla J, Rozeik C, et al. Anomalies and Normal variants of the cerebral arterial supply: a comprehensive pictorial review with a proposed workflow for classification and significance: cerebral arterial supply variants: classification and significance. J Neuroimaging 2018; 28: 14–35. [DOI] [PubMed] [Google Scholar]
- 2.Hayreh SS, Dass R. The ophthalmic artery. I. Origin and intra-cranial and intra-canalicular course. Br J Ophthalmol 1962; 46: 65–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayreh SS. Orbital vascular anatomy. Eye 2006; 20: 1130–1144. [DOI] [PubMed] [Google Scholar]
- 4.Padget DH. The development of cranial arteries in the human embryo. Contrib Embryol 1948; 32: 205–261. [Google Scholar]
- 5.Komiyama M. Embryology of the ophthalmic artery: a revived concept. Interv Neuroradiol 2009; 15: 363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gailloud P, Gregg L, Ruiz DSM. Developmental anatomy, angiography, and clinical implications of orbital arterial variations involving the stapedial artery. Neuroimaging Clin N Am 2009; 19: 169–179. [DOI] [PubMed] [Google Scholar]
- 7.Gregg L, San Millán D, Orru’ E, et al. Ventral and dorsal persistent primitive ophthalmic arteries. Oper Neurosurg (Hagerstown) 2016; 12: 141–152. [DOI] [PubMed] [Google Scholar]
- 8.Toma N. Anatomy of the ophthalmic artery: embryological consideration. Neurol Med Chir(Tokyo) 2016; 56: 585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silbergleit R, Quint DJ, Mehta BA, et al. The persistent stapedial artery. AJNR Am J Neuroradiol 2000; 21: 572–577. [PMC free article] [PubMed] [Google Scholar]
- 10.Dilenge D, Ascherl GF. Variations of the ophthalmic and middle meningeal arteries: relation to the embryonic stapedial artery. AJNR Am J Neuroradiol 1980; 1: 45–54. [PMC free article] [PubMed] [Google Scholar]
- 11.Komiyama M. Embryology of the ophthalmic artery. Interv Neuroradiol. Epub ahead of print 20 April 2019. DOI:10.1177/1591019919845511. [DOI] [PMC free article] [PubMed]
- 12.Lasjaunias P, Moret J, Mink J. The anatomy of the inferolateral trunk (ILT) of the internal carotid artery. Neuroradiology 1977; 13: 215–220. [DOI] [PubMed] [Google Scholar]
- 13.Kam CK, Alvarez H, Lasjaunias P. Double internal carotid origin of the ophthalmic artery with ruptured aneurysm of the posterior communicating artery: a case report. Intervent Neurorad 2003; 9: 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertelli E, Regoli M, Bracco S. An update on the variations of the orbital blood supply and hemodynamic. Surg Radiol Anat 2017; 39: 485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lasjaunias P, Santoyo-Vazquez A. Segmental agenesis of the internal carotid artery: angiographic aspects with embryological discussion. Anat Clin 1984; 6: 133–141. [DOI] [PubMed] [Google Scholar]