Abstract
Background and purpose
Flow-diverter stents are well-established for the treatment of cerebral aneurysms. Flow Redirection Endoluminal Device differs from other flow-diverter stents by its dual-layer design and has proved equality to other devices in numerous short-term surveys. However, follow-up data covering substantially more than one year are still limited for this device. We present our long-term experience with Flow Redirection Endoluminal Device.
Materials and methods
Seventy-eight patients harboring distal internal carotid artery (91%) or vertebrobasilar (9%) cerebral aneurysms treated with Flow Redirection Endoluminal Device with or without adjunctive coiling met the inclusion criteria. All cases were evaluated for aneurysm occlusion (according to Modified Raymond Roy Classification, MRRC), for flow-diverter stents patency and configuration and for procedure- and device-related morbidity and mortality.
Results
Mean follow-up interval was 36.9 ± 9.5 months (<30 months: n = 18; 31–42 months: n = 31; >42 months: n = 24). Total and subtotal aneurysm occlusion after six months was assessed in 92.0% (MRRC1 = 77.3%, MRRC2 = 14.7%, MRRC3a =2.7%, MRRC3b = 4.1%) and increased to 95.9% (MRRC1 = 90.5%, MRRC2 = 5.4%, MRRC3a = 2.7%). There was one case of aneurysm growth requiring early re-treatment. Procedure-related morbidity was observed in three cases (3.8%; one transient hemiparesis, one suspected foreign-body reaction, and one micro-wire perforation). There was no procedure- or device-related mortality. In-stent stenosis due to intimal hyperplasia was observed in two cases and fish-mouthing in three cases.
Conclusions
Our long-term data covering two to five years after flow diversion confirm that Flow Redirection Endoluminal Device is a safe and effective device for the treatment of cerebral aneurysms with progressive high aneurysm occlusion rates; recurrence rates were very low. Overall device-related morbidity was low and was not observed later than six months after intervention.
Keywords: Cerebral aneurysm, flow diversion, Flow Redirection Endoluminal Device
Introduction
Flow-diverter stents (FDSs) have significantly extended the treatment options for intracranial aneurysms. Several devices are on the market with slightly different structural properties of the flow-diverting mesh. Yet, they share the same working principle and proved high occlusion rates—whereas most data are available on Pipeline Embolization Device (PED).
The efficacy of FDS in wide-neck aneurysms has been shown in numerous publications through the past decade.1–5 Short-term occlusion rates had already been promising with over 80%, especially if treatment is supported by additional coiling.6–9 More than 10 years after release of the first FDS, long-term occlusion rates are available, e.g., for the PED and SILK FDS and show progressive aneurysm occlusion in up to 97% with few late-term adverse events.
FRED (Flow Redirection Endoluminal Device; MicroVention, Tustin, CA) was released in 2013. Whereas other FDSs have a single layer of narrow-meshed threads, FRED differs from that by a dual-layer design. The outer layer is a self-expanding and open-pored stent, and the inner layer is the actual flow diverter.10 It is approximately 3 mm shorter than the outer layer on both the proximal and the distal end.
The dual-layer construction is supposed to facilitate the deployment procedure. On the other hand, this causes FRED to not significantly expand over its nominal diameter, which can be an issue in certain anatomical settings. This has to be kept in mind in treatment planning as well as the fact that the actual flow-diverting layer is shorter than the overall FDS.
At short-term follow-up, FRED has proved initial occlusion rates up to 87% with a high safety profile.11,12 Recent data available are from a multi-center study (EuFRED) published in 2018.13 EuFRED reports on 531 patients treated with FRED with and without adjunctive coiling. Aneurysm occlusion increased from 82.5% after six months to 95.3% after a time span of more than one year. Again, the data show high procedural safety with an overall morbidity of 4.0% and mortality of 1.5%.
Whereas long-term studies with a defined several years coverage are available for other FDSs, comparable long-term follow-up of efficacy and safety of FRED is still rare.
Here, we present our single-center experience with FRED covering a follow-up of 36.9 ± 9.5 (24–59) months after the endovascular treatment of cerebral aneurysms in a consecutive group of patients.
Material and methods
Patient inclusion
All patients with cerebral aneurysms treated in our center with either FRED-only or FRED and adjunctive coiling were screened. Patients were included if follow-up imaging was available for at least 24 months, or if morbidity or mortality were recorded within this period interfering with the demanded follow-up.
History of subarachnoid hemorrhage (SAH), pre-treatment of the targeted aneurysm and aneurysm location (anterior vs. posterior circulation) had no influence on patient inclusion.
Medication
Based on our institutional standards, all patients were prepared with 75 mg of clopidogrel and 100 mg of aspirin seven days before the intervention. Drug response was verified by Multiplate analyzer (Roche, Basel, Switzerland) performed one day prior to the intervention. Upon FDS deployment, 5000 IU of heparin were applied.
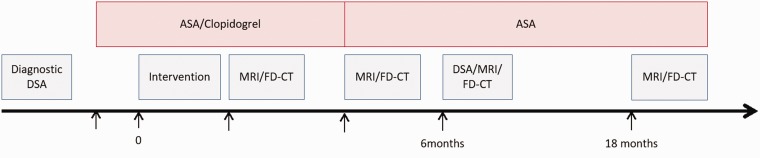
Depending on follow-up, clopidogrel was stopped routinely after 3 months, whereas aspirin was continued until at least 1.5 years after intervention. See Figure 1 for an overview of medication and follow-up imaging.
Figure 1.
Medication and follow-up scheme used in our center for FDS treatment. Further follow-up is scheduled for 30 and 54 months with MRI. ASA: acetylsalicylic acid; DSA: digital subtraction angiography; MRI: magnetic resonance imaging; FD-CT: fully diagnostic computed tomography.
Materials
Depending on the vessel status, a 6-French guiding catheter (Cordis Envoy MPD or Envoy XB DA MPD) with or without long introducer sheath was placed in the sub-petrous or petrous segment of the internal carotid artery (ICA) or in the proximal V3 segment of the vertebral artery.
FRED is delivered via a 0.027″ microcatheter (Headway 27; MicroVention, Tustin, CA; distal: 2.6F/proximal: 3.1F). A FRED diameter is recommended slightly wider than the parent vessel maximum diameter to avoid endoleaks.
In our study group, FRED FDSs were used from 3.5 × 13 × 7 mm to 5.0 × 36 × 29 mm.
In case of additional coiling, bioactive (Codman Neuro; Raynham, MA) or Hydrogel coils (MicroVention) were used according to the aneurysm size. If applicable, the microcatheter was placed in the aneurysm before deployment of the FDS (“jailing technique”).
Imaging
Prior to intervention, every patient underwent a diagnostic catheter angiography for treatment planning.
Interventions were performed on a biplane flat detector angiographic system (Artis zee Biplane System, Siemens AG, Healthineers, Forchheim, Germany).
Routine magnetic resonance imaging (MRI; diffusion and perfusion-weighted imaging, time-of-flight and contrast-enhanced angiography, T2w FLAIR, T2*, and contrast-enhanced T1w sequences) and flat detector CT with intravenous contrast14,15 (FD-CT; 10 s DSA protocol: 10 s mask run, bolus watching, 10 s fill run; evaluation of multiplane and volume rendering technique reconstructions) were acquired two weeks and three months after intervention to monitor brain parenchyma (e.g., silent ischemia), FDS configuration, and FDS lumen. As current criterion standard DSA was performed six months after treatment. If no pathologic findings occurred, a further follow-up with MRI and FD-CT is scheduled after 18 months and 30+ months. Again, see Figure 1 for an overview of medication and follow-up imaging.
Imaging follow-up
The Modified Raymond-Roy occlusion classification (MRRC1: complete occlusion; MRRC2: neck remnant (“dog ear”); MRRC3a: remnant aneurysm, central; MRRC3b: remnant aneurysm, sidewall) was applied for occlusion assessment in DSA and FD-CT. The O’Kelly Marotta occlusion scale, which would have been even more appropriate for FDS assessment, requires dynamic angiography and thus could not be used for assessment of steady-state examinations such as FD-CT or MRA.
Aneurysm occlusion, FDS wall adaption, configuration (e.g., fish-mouthing, foreshortening), endothelial thickening, and in-stent thrombosis were evaluated in DSA and FD-CT. MRI was evaluated for indirect signs of FDS malfunction (e.g., time-to-peak delay in perfusion-weighted imaging).
MRI was also analyzed for asymptomatic pathologic changes of brain tissue (e.g., post-ischemic lesions, inflammation) and hemorrhage.
Clinical follow-up
Patients were examined by a neurologist at admission, after the intervention, and at discharge. Assessment criteria were procedure- or device-related neurologic deficits or death.
Furthermore, patients were assessed clinically at the follow-up visits after 1, 3, 6, and 18 months.
Results
Patient inclusion
Ninety-two patients could be identified treated with a FRED at least 24 months prior to data acquisition. Moreover, 14 patients had not yet obtained a follow-up longer than 24 months after intervention due to our standard-of-care follow-up regime. Three patients died within the follow-up period until six months after intervention (severe craniocerebral injury; breast cancer; sequelae from SAH). One patient with aneurysm growth went for retreatment (stent-in-stent FDS) and did not meet the criteria for follow-up. One patient refused to have further follow-up after a six-month DSA was non-remarkable. This results in 78 patients being included and 73 patients being available for long-term follow-up (<30 months: n = 18; 31–42 months: n = 31; > 42 months: n = 24). In addition, 71 patients (91%) were treated for a distal ICA aneurysm, whereas 7 patients (9%) had a vertebrobasilar aneurysm. See Table 1 for patient demographics and aneurysm anatomy and supplementary table for more detailed information on the treated aneurysms.
Table 1.
Patient demographics and anatomical data of the aneurysms initially treated.
| Demography (n = 78 treated patients) | ||
|---|---|---|
| Age | 56.1 ± 12.1 (24–77) years | |
| Sex (F/M) | 62/16 | |
| Aneurysm location | 71 (91%) (ICA including Pcomm and AChoA origin aneurysms) | 7 (9%) posterior circulation (V4 segments and Basilar artery) |
| History of SAH | 4 (5.1%) from targeted aneurysm (not acute, pre-treatment) 6 (7.7%) from different aneurysm | |
| Pretreatment | 18 (23.1%) Coiling: 7; stent: 5; stent-assisted coiling: 2; FDS (other than FRED): 3; clipping: 1 | |
| Aneurysm size | Overall: 2.0–18.5 mm (5.1 ± 3.9 mm) FRED (n = 46): 2.0–13.0 mm (3.4 ± 2.4 mm) FRED & coils (n = 32): 2.5–18.5 mm (7.4 ± 4.4 mm) | |
FRED: Flow Redirection Endoluminal Device; FDS: flow-diverter stent; SAH: subarachnoid hemorrhage; ICA: internal carotid artery; AChoA: anterior choroidal artery; Pcomm: posterior communicating artery.
Medication
All patients received medication according to the routine protocol. There was no non-responder to aspirin or clopidogrel. In all 10 cases with a history of SAH, there was a time interval of at least three months between hemorrhage and FRED treatment.
Technical success
A total of 83 FREDs were used. In four cases without adjunctive coiling, two FREDs were implanted (stent-in-stent technique) in fusiform ICA aneurysms. In one case, the FRED initially chosen turned out to be too short during deployment and thus was re-captured and replaced by the next size in length. Moreover, 46 patients (59%) were treated exclusively with one or two FDSs; 32 patients (41%) were treated with FRED and adjunctive coiling.
Imaging and clinical follow-up
After the procedure, one patient had a slight, yet transient contralateral hemiparesis because of punctuate DWI lesions located in the central region. Another one patient presented with conspicuous headache three days after intervention and showed punctual ipsilateral contrast-enhancing lesions in post-procedural MRI. This is a rare condition described for all kinds of endovascular treatments and is thought to be caused by an immune response to scraped-off hydrophilic coating of microcatheters.16 The patient was treated with glucocorticoids for two weeks until the symptoms had completely resolved. Later than three months, no symptomatic events were observed. There was no procedure- or device-related mortality.
Between the six months and long-term follow-ups, complete occlusion (MRRC1) increased in the overall study population from 77.3% (6 months) to 90.58% (long-term). Cases with neck remnants (MRRC2) and residual aneurysms (MRRC3a/b) decreased from 14.7% to 5.4% and from 6.7% to 2.7%, respectively. There was one case of aneurysm growth during follow-up, leading to retreatment.
In two of the patients, the ICA aneurysm was located at the origin of the ophthalmic artery, producing a continuous pressure gradient over the aneurysm wall. Both patients were treated with a FRED-only in order not to compromise the ophthalmic artery. Yet, at long-term follow-up, each one of the patients was rated as MRRC1 and MRRC2.
By the end of each procedure, all stents were open and completely wall-adapted. Within the first six months, we noticed two cases of fish-mouthing and two cases of intimal hyperplasia, each with low-grade narrowing of the FDS lumen. Under continuation of dual-platelet inhibition, endothelial hyperplasia remained stable over the observed period in both cases. Fish-mouthing was constant throughout the follow-up. Another case of fish-mouthing occurred within the first 18-month interval with only minor change of the FDS lumen. Later than 18 months, no further changes in FDS configuration were observed. Images demonstrating pathological findings and device visibility in FD-CT and MRA are provided in a supplementary collage.
Flow diversion versus flow diversion and adjunctive coiling
The subgroup analysis between FDS and FDS/coiling cases was not statistically significant regarding short-term (p = 0.263) or long-term (p = 0.440) occlusion rates. Further breakdown to aneurysms up to a size of 5 mm compared to those larger than 5 mm again was not significant (p = 0.077) in favor of additional coiling in larger aneurysms regarding sufficient long-term occlusion.
Table 2 provides detailed information on occlusion rates of the overall study population and the FRED-only and FRED & coils subgroups as well as on morbidity and device-related abnormalities.
Table 2.
Procedure related events und follow-up results regarding occlusion rate, morbidity and device-related findings.
| Procedure-related events and follow-up results | ||||||||
|---|---|---|---|---|---|---|---|---|
| Procedure-related events | Not device related 1 suspected foreign body reaction 1 wire-related aneurysm perforation—not related to FRED | Device related 2 FREDs not deployable. Deployment of 2nd FRED successful in both cases | ||||||
| Follow-up | Short term (n = 74) | Long term (n = 73) | ||||||
| Time interval | 6 months | 36.9 ± 9.5 (24–59) months 24–30 months: n = 18; 30–42 months: n = 31; >42 months: n = 24 | ||||||
| Dropouts | 1 early retreatment (aneurysm growth) 3 non-procedure-related deaths | 1 refusal of further follow-up | ||||||
| Occlusion rate | FRED (n = 43) | FRED & Coilsa (n = 31) | Overalla (n = 74) | FRED (n = 43) | FRED & Coilsa (n = 30) | Overalla (n = 73) | ||
| MRRC1 | 32 (74.4%) | 26 (81.3%) | 58 (77.3%) | 38 (88.4%) | 29 (93.5%) | 67 (90.5%) | ||
| MRRC2 | 7 (16.2%) | 4 (12.5%) | 11 (14.7%) | 4 (9.3%) | 0 | 4 (5.4%) | ||
| MRRC3a | 2 (4.7%) | 0 | 2 (2.7%) | 1 (2.3%) | 1 (3.2%) | 2 (2.7%) | ||
| MRRC3b | 2 (4.7%) | 1 (3.1%) | 3 (4.0%) | 0 | 0 | 0 | ||
| New symptomatic infarct Progressive aneurysm remnant | 1 (1.4%) 1 (1.4%) → retreatment | None None | ||||||
| New device-related findings | 2 fish-mouthing 2 endothelial hyperplasia | 1 fish-mouthing | ||||||
FRED: Flow Redirection Endoluminal Device; MRRC: Modified Raymond Roy Classification. afor statistical accuracy occlusion rates are calculated for including the one patient who went for early retreatment.
Discussion
Occlusion rates and patient outcome
Data available for FRED published by Möhlenbruch et al. and Killer-Oberpfalzer et al. (EuFRED) cover follow-up intervals of up to six months and over one year, respectively. Both studies report similar results with a rate of sufficient aneurysm occlusion after six months of 90.0% and 82.5%. Moreover, EuFRED observed progressive aneurysm occlusion up to 95.3% after more than one year. Möhlenbruch reported 3% disabling and 6.8% transient morbidity with no mortality. EuFRED observed transient and permanent morbidity in 3.2% and 0.8%, respectively, whereas mortality was 1.5%.
Concordantly to these data, our results show a progressive occlusion rate of 97.3% at long-term follow-up. Also the procedure-/device-related morbidity of 2.6% in our series is comparable to the results of the FRED studies mentioned.
Due to its release already in 2007, follow-up data for PED with regard to occlusion rates as well as ischemic and hemorrhagic complications are available for many years: Becske et al.17 prospectively analyzed treatment results of 109 aneurysms in 107 subjects for a five-year follow-up and found progressive occlusion up to 96.8% (95.2% MRRC1; 1.6% MRRC2). Nameable ischemic stroke events occurred in only 3.5% between one and three years. Briganti et al.18 reported on 60 patients harboring 69 aneurysms and found complete aneurysm occlusion in 91% (O’Kelly-Marotta (OKM) grade D, according to MRRC1) and 6% subtotal occlusion (OKMC, according to MRRC2) after seven years. No ischemic or hemorrhagic events were noticed later than two years after intervention. Chiu et al.19 examined data of 98 patients with 119 aneurysms and found complete occlusion in 93.2% after more than two years, not considering neck remnants (MRRC2). Device-related morbidity (including transient ischemic attack, minor stroke, and major stroke) and mortality in their cohort were 7.6% and 0.8%, respectively—all within six months post procedure. A very recent Italian registry study involving 166 patients in 30 Italian centers20 reported a three- to six-month complete or almost complete occlusion in 94% of cases, increasing to 96% at 12 - to 24-month follow-up. As expected from the FRED data already available as well as from several PED studies,21,22 we observed an increasing rate of total and subtotal occlusion from 77.3% and 14.7% to 90.5% and 5.4% in the overall group between six months and the late follow-up. In particular, no MRRC3b remnants were recorded, which are carrying the highest risk of (re-)hemorrhage. MRI acquired later than six months was unremarkable in all patients concerning device-/procedure-related changes of brain parenchyma.
Since FRED was released not before 2013, our follow-up can only cover a mean interval of three years (24–59 months). Yet, after three years, occlusion rates as well as the low rate of complications in our study population are comparable to results after PED treatment. Furthermore, it is noteworthy that only one case (with adjunctive coiling) was observed with a progress of inflow into the aneurysm. This, concomitantly, was the largest aneurysm in this study group with a mean size of 18.5 mm.
Like other authors, we did observe changes in configuration in a few FRED FDSs. This phenomenon usually is referred to as “fish-mouthing” and describes tapering of one or both ends of an FDS.23 The finding can be visualized best in either DSA or FD-CT. In our survey, we found fish-mouthing in three cases (6.9%). Cohen et al. report fish-mouthing in 2 out of 16 patients (13%) treated with a SILK FDS, with no case in their group of patients treated with a PED.24 Whereas changes in configuration are mostly reported for the first months after FDS implantation, we found one case in the interval between 6 and 18 months. Interestingly, the two cases with early fish-mouthing had a mild delay in time-to-peak map of perfusion-weighted imaging, whereas there was no hemodynamic abnormality in the late case. As a consequence, clopidogrel was re-administered in the early cases until the next follow-up and then stopped again in favor of aspirin only, since no increase in fish-mouthing was seen. No action was taken in the late case. The cause for the fish-mouthing phenomenon is still not understood. Incorrect sizing, especially oversizing, of stents is discussed. Kocer et al. recently found a statistically significant association to a history of contact allergy to imitational jewelry indicating a possible allergic reaction to non-noble metals25 that are part of all FDSs alloying. Mild endothelial hyperplasia within the first six months was observed in only one case with no consequences in medicinal management.
Flow diversion versus flow diversion and adjunctive coiling
Although there is no cut-off value for this situation in our department, we tend to use additional coiling in aneurysms above a size of 4–5 mm, if technically feasible. The subgroup analysis between FRED and FRED & coils groups showed no statistically significant difference regarding occlusion rates, although mean aneurysm size in the FRED & coils group was significantly larger than in the FRED-only group (7.4 vs. 3.4 mm, p < 0.0001). Also further differentiation with regard to aneurysm size showed no significance (p = 0.077) toward higher total and subtotal long-term occlusion after additional coiling in aneurysms with a size of 5 mm or above. Although this can at most be interpreted as a tendency, we pursue our strategy to go for additional coiling in larger aneurysms. Data published by Park et al.9 state a significantly higher procedure time for interventions with additional coiling (p < 0.0001). Yet, no significantly higher morbidity could be observed (p = 0.13). It is our experience that the stated difference in procedure duration (135.8 ± 63.9 vs. 96.7 ± 46.2 min) is overvalued and can easily be reduced by optimized preparation (eligible projections, choice of suitable guiding catheter, valves, etc.) prior to intervention.
Conclusion
Our long-term data cover a two- to five-year period after endovascular aneurysm treatment with the FRED flow diverter. We observed progressive aneurysm occlusion up to 95.9%. Procedural and clinical safety was favorable with very low device-related morbidity not recorded later than six months. We observed no device-related mortality.
Supplemental Material
Supplemental material, INE878551 Supplemental Material1 for Two- to five-year follow-up of 78 patients after treatment with the Flow Redirection Endoluminal Device by Hannes Luecking, Arnd Doerfler, Philipp Goelitz, Philip Hoelter, Tobias Engelhorn and Stefan Lang in Interventional Neuroradiology
Supplemental material, INE878551 Supplemental Material2 for Two- to five-year follow-up of 78 patients after treatment with the Flow Redirection Endoluminal Device by Hannes Luecking, Arnd Doerfler, Philipp Goelitz, Philip Hoelter, Tobias Engelhorn and Stefan Lang in Interventional Neuroradiology
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Supplemental material
Supplemental material for this article is available online.
References
- 1.Briganti F, Leone G, Marseglia M, et al. Endovascular treatment of cerebral aneurysms using flow-diverter devices: a systematic review. Neuroradiol J 2015; 28: 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lylyk P, Miranda C, Ceratto R, et al. Curative endovascular reconstruction of cerebral aneurysms with the Pipeline embolization device: the Buenos Aires experience. Neurosurgery 2009; 64: 632–642. [DOI] [PubMed] [Google Scholar]
- 3.Becske T, Kallmes DF, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology 2013; 267: 858–868. [DOI] [PubMed] [Google Scholar]
- 4.Kallmes DF, Brinjikji W, Boccardi E, et al. Aneurysm study of pipeline in an observational registry (ASPIRe). Interv Neurol 2016; 5: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walcott BP, Stapleton CJ, Choudhri O, et al. Flow diversion for the treatment of intracranial aneurysms. JAMA Neurol 2016; 73: 1002–1008. [DOI] [PubMed] [Google Scholar]
- 6.Lin N, Brouillard AM, Krishna C, et al. Use of coils in conjunction with the Pipeline embolization device for treatment of intracranial aneurysms. Neurosurgery 2015; 76: 142–149. [DOI] [PubMed] [Google Scholar]
- 7.Nossek E, Chalif DJ, Chakraborty S, et al. Concurrent use of the Pipeline embolization device and coils for intracranial aneurysms: technique, safety, and efficacy. J Neurosurg 2015; 122: 904–911. [DOI] [PubMed] [Google Scholar]
- 8.Park MS, Nanaszko M, Sanborn MR, et al. Re-treatment rates after treatment with the Pipeline embolization device alone versus Pipeline and coil embolization of cerebral aneurysms: a single-center experience. J Neurosurg 2016; 125: 137–144. [DOI] [PubMed] [Google Scholar]
- 9.Park MS, Kilburg C, Taussky P, et al. Pipeline embolization device with or without adjunctive coil embolization: analysis of complications from the IntrePED registry. Am J Neuroradiol 2016; 37: 1127–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alherz AI, Tanweer O, Flamini V. A numerical framework for the mechanical analysis of dual-layer stents in intracranial aneurysm treatment. J Biomech 2016; 49: 2420–2427. [DOI] [PubMed] [Google Scholar]
- 11.Möhlenbruch MA, Herweh C, Jestaedt L, et al. The FRED flow-diverter stent for intracranial aneurysms: clinical study to assess safety and efficacy. AJNR Am J Neuroradiol 2015; 36: 1155–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luecking H, Engelhorn T, Lang S, et al. FRED flow diverter: a study on safety and efficacy in a consecutive group of 50 patients. AJNR Am J Neuroradiol 2017; 38: 596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Killer-Oberpfalzer M, Kocer N, Griessenauer CJ, et al. European multicenter study for the evaluation of a dual-layer flow-diverting stent for treatment of wide-neck intracranial aneurysms: the European flow-redirection intraluminal device study. Am J Neuroradiol 2018; 39: 841–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richter G, Engelhorn T, Struffert T, et al. Flat panel detector angiographic CT for stent-assisted coil embolization of broad-based cerebral aneurysms. AJNR Am J Neuroradiol 2007; 28: 1902–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Struffert T, Saake M, Ott S, et al. Intravenous flat detector CT angiography for non-invasive visualisation of intracranial flow diverter: technical feasibility. Eur Radiol 2011; 21: 1797–1801. [DOI] [PubMed] [Google Scholar]
- 16.Cruz JP, Marotta T, O’Kelly C, et al. Enhancing brain lesions after endovascular treatment of aneurysms. AJNR Am J Neuroradiol 2014; 35: 1954–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becske T, Brinjikji W, Potts MB, et al. Long-term clinical and angiographic outcomes following pipeline embolization device treatment of complex internal carotid artery aneurysms: five-year results of the pipeline for uncoilable or failed aneurysms trial. Neurosurgery 2017; 80: 40–48. [DOI] [PubMed] [Google Scholar]
- 18.Briganti F, Leone G, Cirillo L, et al. Postprocedural, midterm, and long-term results of cerebral aneurysms treated with flow-diverter devices: 7-year experience at a single center. Neurosurg Focus 2017; 42: E3. [DOI] [PubMed] [Google Scholar]
- 19.Chiu AH, Cheung AK, Wenderoth JD, et al. Long-term follow-up results following elective treatment of unruptured intracranial aneurysms with the pipeline embolization device. AJNR Am J Neuroradiol 2015; 36: 1728–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piano M, Valvassori L, Lozupone E, et al. FRED Italian Registry: a multicenter experience with the flow re-direction endoluminal device for intracranial aneurysms. J Neurosurg 2019; 1: 1–8. [DOI] [PubMed] [Google Scholar]
- 21.Yaltirik Bilgin E, Önal B, Emmez H, et al. Endovascular treatment of intracranial anterior circulation aneurysms with flow diverters: a single centre experience with mid and long-term results. Turk Neurosurg. Epub ahead of print 11 July 2017. DOI: 10.5137/1019-5149.JTN.20279-17.2. [DOI] [PubMed] [Google Scholar]
- 22.John S, Bain MD, Hussain MS, et al. Long-term effect of flow diversion on large and giant aneurysms: MRI-DSA clinical correlation study. World Neurosurg 2016; 93: 60–66. [DOI] [PubMed] [Google Scholar]
- 23.Jou LD, Mitchell BD, Shaltoni HM, et al. Effect of structural remodeling (retraction and recoil) of the pipeline embolization device on aneurysm occlusion rate. AJNR Am J Neuroradiol 2014; 35: 1772–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen JE, Gomori JM, Moscovici S. Delayed complications after flow-diverter stenting: reactive in-stent stenosis and creeping stents. J Clin Neurosci 2014; 21: 1116–1122. [DOI] [PubMed] [Google Scholar]
- 25.Kocer N, Mondel PK, Yamac E, et al. Is there an association between flow diverter fish mouthing and delayed-type hypersensitivity to metals? A case-control study. Neuroradiology 2017; 59: 1171–1178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, INE878551 Supplemental Material1 for Two- to five-year follow-up of 78 patients after treatment with the Flow Redirection Endoluminal Device by Hannes Luecking, Arnd Doerfler, Philipp Goelitz, Philip Hoelter, Tobias Engelhorn and Stefan Lang in Interventional Neuroradiology
Supplemental material, INE878551 Supplemental Material2 for Two- to five-year follow-up of 78 patients after treatment with the Flow Redirection Endoluminal Device by Hannes Luecking, Arnd Doerfler, Philipp Goelitz, Philip Hoelter, Tobias Engelhorn and Stefan Lang in Interventional Neuroradiology