Abstract
Purpose
To investigate the mid- and long-term effects of parent artery occlusion on the carotid cavernous fistula and on the quality of life of patients.
Materials and methods
One hundred and twenty-six patients with high-flow direct carotid cavernous fistulas were enrolled. The modified Rankin scale scores, the headache impact test and the short form health survey scores were used to evaluate the patients' clinical status.
Results
Fifty-two patients had parent artery occlusion, while the rest of the 74 patients had embolization of carotid cavernous fistulas with parent artery preservation. No periprocedural complications occurred. Eighteen patients in the parent artery occlusion group had low perfusion symptoms within two weeks following embolization, and three patients had Horner's syndrome on the ipsilateral side. At two months' follow-up, the patients with parent artery occlusion had a significantly (P < 0.05) greater proportion of headache than patients with parent artery preservation. At 12 months, no significant (P > 0.05) difference existed in the headache impact test scores in both groups. At 36 months' follow-up, the patients with parent artery occlusion had decreased SF-30 scores in all the eight health domains compared with patients treated with parent artery preservation, with a significant (P < 0.05) lower score in general health, vitality and bodily pain in the parent artery occlusion compared with the parent artery preservation group. No recurrence was shown in patients with parent artery occlusion, but nine (12.2%) patients were recurrent in patients with parent artery preservation.
Conclusion
Parent artery occlusion may affect the quality of life of patients with carotid cavernous fistulas despite being an effective treatment option for high-flow direct fistulas.
Keywords: Carotid cavernous fistula, endovascular embolization, parent artery occlusion, quality of life, headache
Introduction
Abnormal communications between the internal carotid artery and the cavernous sinus are called carotid cavernous fistulas (CCFs).1 Barrow et al.2 categorized the CCF into four distinct types according to the arterial supply, with type A having direct high-flow communications between the internal carotid artery and the cavernous sinus, while types B–D being indirect dural arteriovenous fistulas fed by the meningeal arteries of the internal carotid artery, external carotid artery or both. Clinical symptoms of CCF is a direct consequence of increase in intracavernous pressure and altered flow patterns caused by highly pressurized arterial blood transmitted directly into the cavernous sinus and draining veins, leading to venous hypertension and various symptoms including chemosis, proptosis, cephalic bruit, diplopia, pain, trigeminal nerve dysfunction, increased intraocular pressure, and visual loss.3 Modalities for managing CCF include medications, manual compression, surgery, stereotactic radiosurgery and endovascular repair.1 Currently, endovascular therapy has evolved as the major therapeutic option for CCF, including detachable balloon occlusion, embolization with coils or other materials, covered stent graft deployment, and parent artery occlusion (PAO). Parent artery sacrifice may be a life-saving emergency therapy when endovascular embolization of a direct CCF with concurrent preservation of the internal artery is not feasible because of extensive traumatic vessel wall injury, active bleeding or a rapidly enlarged hematoma in the soft tissues. Endovascular occlusion of the parent artery can be performed immediately following angiographic diagnosis and allows monitoring of the neurological status,4,5 and balloon occlusion test has to be performed to ensure distal perfusion from collateral circulation before parent occlusion.3,6,7 However, the quality of life in patients with direct CCFs which were treated with PAO has never been reported in the literature. We hypothesized that PAO may affect the quality of life, and this study was to investigate the long-term effects of internal carotid artery occlusion on the quality of life in patients with direct CCFs treated in 10 years in our center.
Materials and methods
This study was approved by the ethics committee of our hospital with signed informed consent from all patients with direct CCFs. Between September 2001 and February 2015, a total of 126 patients were enrolled in this study, including 82 male and 44 female patients with an age range of 23–54 years (mean 34 ± 9 years). All patients had high-flow direct CCFs caused by trauma. The symptoms included orbital bruit in 95 patients, conjunctival hyperemia and chemosis in 88, proptosis in 65, decrease visual acuity in 56, ptosis in 35 and ocular motor palsy in 34.
An intravenous bolus of heparin was injected based on the patients weight (0.6 mg/kg), and continuous infusion of heparinized normal saline was maintained for keeping the activated clotting time at about twice the baseline value. Endovascular procedures were performed under local anesthesia through transfemoral access after cerebral angiography. A temporary balloon occlusion test was performed for obtaining precise information about the fistula including the flow pattern, venous drainage, and collateral circulation. A guiding catheter was initially placed in the internal carotid artery before a balloon-mounted microcatheter (Balt, Somerset, NJ, France) was sent slowly into the fistula under flow guidance for occlusion of the fistula. A covered stent (Jostent, Abbott, USA) may also be used for occluding the fistula. For patients with residual fistula after embolization, coils were used for embolizing the cavernous sinus in case of bad collateral circulation, and balloon occlusion of the parent artery was performed in case of good collateral circulation. Before PAO, a balloon occlusion test was performed by sending a balloon catheter into the ipsilateral internal carotid artery with the balloon being inflated for 30 min. If the patient tolerated well with no neurological symptoms and the ipsilateral cerebral hemisphere had excellent collateral flow from the contralateral internal carotid artery via the anterior communicating artery and from the posterior cerebral artery via the posterior communicating artery, PAO would be considered safe and subsequently performed. Occlusion of the internal carotid artery was usually performed by use of a Balt balloon (Balt, Somerset, NJ, France) to occlude the cavernous segment of the internal carotid artery. Then, one or two balloons would be placed proximal to the cavernous segment for prevention of early leakage or rupture of the balloon. After embolization, the patients were usually followed up clinically and angiographically for evaluation of the conditions of the CCFs. The modified Rankin score (mRS), the headache impact test (HIT-6) and the quality of life scores (short form health survey, SF-36) were evaluated in all patients.
Statistical analysis
The statistical analysis was performed using the SPSS 16.0 software package (SPSS Inc., Chicago, IL, USA). Continuous data were expressed as mean ± standard deviation, and independent sample test was performed. Categorical data were tested with Chi-square test. The significance was set at P < 0.05.
Results
Among 126 patients with CCFs, 52 (41.3%) patients had PAO (Figure 1) of the internal carotid artery, while the rest of the 74 patients had embolization of the CCFs with parent artery preservation (Figure 2). No significant (P > 0.05) difference existed in the clinical data of patients with PAO and parent artery preservation before treatment. Among 52 patients with PAO, 49 were occluded by a detachable balloon, whereas the remaining three patients were initially treated with a covered stent resulting in partial occlusion of the fistula and were retreated with PAO. No periprocedural complications occurred. Eighteen patients in the PAO group had low perfusion symptoms (postural dizziness which disappeared after rest) within two weeks following embolization, and three patients had Horner's syndrome on the ipsilateral side with shrunk pupils and small ocular fission which were usually concealed by ocular symptoms. At two months following the procedure, the patients with PAO had a significantly (P < 0.05) greater proportion of headache than patients with parent artery preservation, while no significant (P > 0.05) differences existed in the mRS between the two groups (Table 1). In patients treated with PAO, the mRS was ≤ 2 in 43 patients but > 2 in 9, whereas in patients with preservation of the parent artery, the mRS was ≤ 2 in 64 but > 2 in 10.
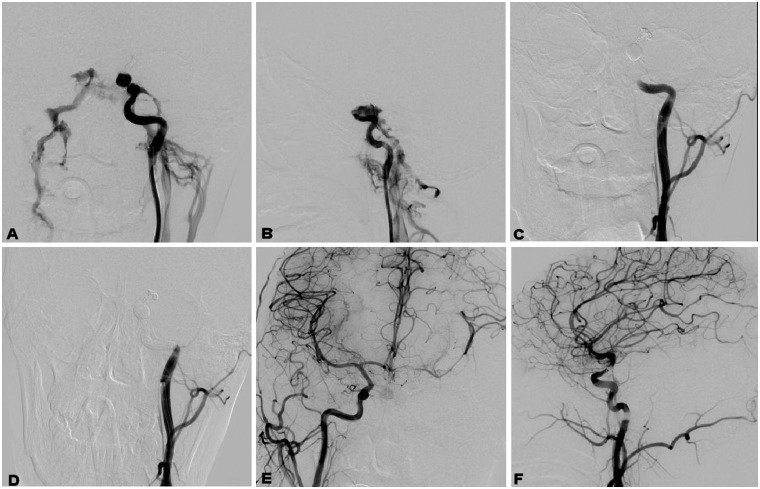
Figure 1.
A 36-year-old patient had head trauma. (a) and (b) A left carotid cavernous fistula (CCF) was revealed. (c) A balloon was sent and occluded the internal carotid artery at the level of the cavernous sinus. (d) A second balloon was deployed proximal to the first balloon for further occlusion of the internal carotid artery. (e) and (f) After occlusion of the internal carotid artery, angiography through the right internal carotid artery revealed good circulation of blood.
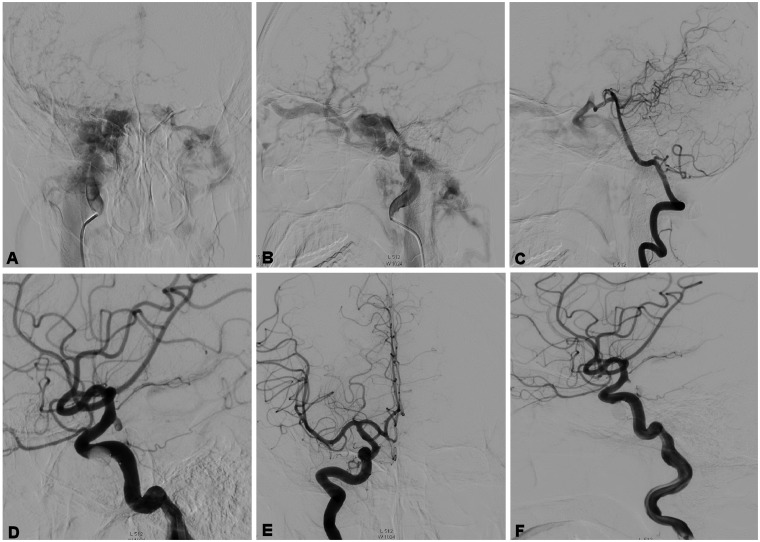
Figure 2.
A 45-year-old man had a head trauma. (a) and (b) A left carotid cavernous fistula (CCF) was demonstrated and was drained through the ophthalmic veins and intracavernous sinuses. (c) The posterior communicating artery was opened to supply blood. (d) A balloon was sent into the fistula without detachment. (e) and (f) The balloon was detached and the fistula was totally occluded.
Table 1.
Demography of the patients with carotid cavernous fistulas.
| Groups | No. | Sex |
2 month follow-up |
||||
|---|---|---|---|---|---|---|---|
| M | F | Headache | Dizziness | mRS ≤ 2 | mRS > 2 | ||
| PAO | 52 | 31 | 21 | 38 | 18 | 43 | 9 |
| PAP | 74 | 51 | 23 | 43 | 34 | 64 | 10 |
| P | 0.53 | 0.03 | 0.66 | 0.82 | |||
PAO: parent artery occlusion; PAP: parent artery preservation; mRS: modified Rankin score.
One hundred and ten patients had follow-up at 12 months following embolization. Of 43 patients treated with PAO, the sequela affecting daily life included headache in 23 patients (53.5%), dizziness in 25 (58.1%), memory deterioration in 12 (27.9%) and visual damage in 4 (9.3%). Among 67 patients with parent artery preservation, the sequela included headache in 35 (52.2%), dizziness in 31 (46.3%), memory deterioration in 16 (23.9%) and visual damage in 6 (9.0%). The HIT-6 score was over 50 in 11 patients (16.4%) in the parent artery preservation group but 12 patients (27.9%) in the PAO group, with no significant (P > 0.05) difference (Table 2). Seventy-five patients had 36 months' follow-up including 33 patients with PAO (Table 3). The patients with PAO had decreased scores in all the eight health domains compared with patients treated with the parent artery preservation, and in particular, a significant (P < 0.05) lower score existed in the domains of general health, vitality and bodily pain in the PAO group compared with the group of parent artery preservation (Figure 3). No recurrence was shown in patients with PAO in computed tomography angiography; however, nine (12.2%) patients were recurrent among 74 patients with embolization and preservation of the internal carotid artery.
Table 2.
12-Month follow-up data.
| Groups | No. | Headache | Dizziness | Memory deterioration | Visual damage | HIT-6 score |
|
|---|---|---|---|---|---|---|---|
| ≥50 | <50 | ||||||
| PAO | 43 | 23 | 25 | 12 | 4 | 8 | 35 |
| PAP | 67 | 35 | 31 | 16 | 6 | 7 | 60 |
| P | 0.90 | 0.13 | 0.69 | 0.76 | 0.16 | ||
PAO: parent artery occlusion; PAP: parent artery preservation; HIT: headache impact test.
Table 3.
SF-36 scores at 36-month follow-up.
| Variables | PAO | PAP | P |
|---|---|---|---|
| No. | 33 | 42 | |
| Physical functioning | 70.7 ± 18.1 | 74.1 ± 16.6 | 0.06 |
| Physical health | 80.4 ± 19.7 | 81.5 ± 20.2 | 1.10 |
| Bodily pain | 73.6 ± 18.4 | 78.0 ± 16.1 | 0.02 |
| General health | 55.2 ± 18.0 | 59.3 ± 16.5 | 0.04 |
| Vitality | 49.1 ± 18.7 | 52.8 ± 19.5 | 0.04 |
| Social functioning | 80.0 ± 16.4 | 80.4 ± 15.4 | 0.06 |
| Emotional problems | 77.6 ± 19.0 | 79.5 ± 21.7 | 1.00 |
| Mental health | 56.8 ± 20.6 | 58.1 ± 21.0 | 0.86 |
PAO: parent artery occlusion; PAP: parent artery preservation; SF-36: short form health survey.
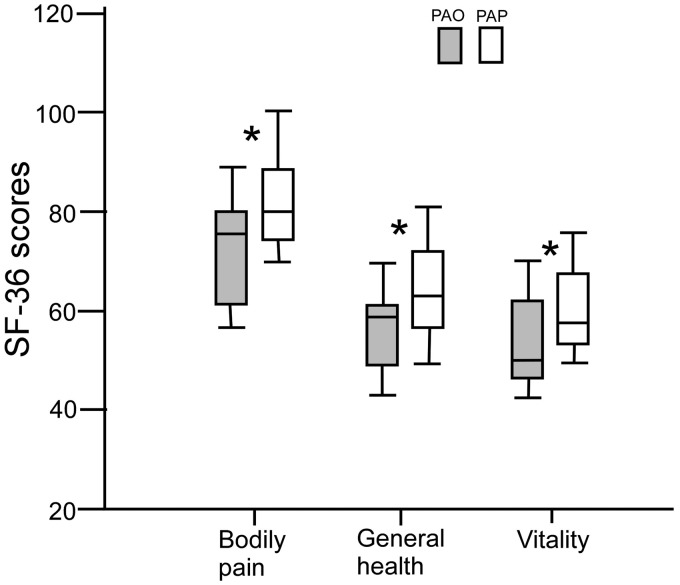
Figure 3.
Boxplot graph shows that a significant (P < 0.05) difference existed in the SF-36 scores between patients with PAO and PAP in the items of bodily pain, general health and vitality. SF-36: short form health survey; PAO: parent artery occlusion; PAP: parent artery preservation.
Discussion
Traumatic CCFs are rare and occur in 0.2%–0.3% of craniomaxillofacial injuries.8 The general goal in managing CCFs is to close the abnormal communications between the internal carotid artery and the cavernous sinus while preserving the parent artery.9 The CCFs can currently be treated with various embolization materials like detachable balloons, coils, liquid adhesive and covered stents. Microcoils are currently more commonly used in the management of CCFs, especially in smaller fistulas with a diameter of 2–3 mm.8–10 But in case of a larger cavernous sinus, many coils would be needed to completely occlude the sinus, consequently making the procedure more difficult and increasing the probability of cranial nerve compression symptoms. Balloon-assisted or stent-assisted liquid adhesive embolization with or without coils may be an effective choice of treatment because the balloons and coils can slow the flow within the cavernous sinus while serving as a scaffold for polymerization of the liquid adhesive and forming a physical barrier for prevention of further movement of the liquid adhesive.11,12 Covered stent grafts have recently been considered as a promising option for CCFs, especially for recurrent, residual and multiple fistulas as well as fistulas combined with pseudoaneurysms or dissections.8,11,13 However, the shortcomings of current covered stents included stiffness, difficulty of navigation and possibly unified perioperative medication.
Even though the detachable balloons were removed from the U.S. market in 2004 and coil embolization has emerged as the first-line treatment strategy in the U.S,14 detachable balloons are still an important treatment option to choose in other countries because of their efficacy, safety, procedural simplicity and cost-effectiveness. However, detachable balloons also have some disadvantages because the size of the fistulas crucially affects success of embolization and smaller fistulas will not allow passage of a larger balloon. A larger cavernous sinus will affect embolization of the CCFs. In these conditions, the internal carotid artery may have to be sacrificed. In this study, PAO was performed in 52 (41.3%) patients, and the PAO rate was markedly greater than other studies.4,15,16 The reasons for this may include technique limitations before 2008 when the reliable embolization materials were mostly detachable balloons which could not enter smaller fistulas. Moreover, some fistulas were irregular and could not be occluded completely, leaving a residual fistula. The residual fistuals could not be managed with covered stents which were not routinely used or with coils for embolization of the cavernous sinus due to expense issue. Recurrence is another disadvantage with detachable balloon embolization of CCFs, and the recurrence rate has been reported as 12.1%.17 In our study, nine patients in the parent artery preservation group had recurrence, resulting in a recurrence rate of 12.2%. However, no recurrence was found during follow-up in patients with PAO.
Successful therapy may resolve the ocular symptoms rapidly following embolization, but patients may become temporarily more symptomatic because of propagation of thrombus throughout the cavernous sinus and superior ophthalmic vein. This kind of clinical deterioration is called the paradoxical worsening phenomenon and can be observed in patients following transarterial treatment and gamma knife radiosurgery. These symptoms are disconcerting to the patient and can usually resolve spontaneously over time or with treatment of corticosteroids to reduce inflammation related to sinus thrombosis.1,18,19 The most obvious symptoms are headache following embolization, especially dull pain around the ipsilateral eye socket and frontotemporal bone, which is probably caused by balloon compression or coil stimulation of the duramater and can be relieved by pain killers. In our study, the patients with low perfusion symptoms following embolization had good collateral circulation before treatment. The reason for the Horner's syndrome was injury of the sympathetic plexus surrounding the internal carotid artery by balloon expansion and acute occlusion of the internal carotid artery.
The long-term effects of PAO on the quality of life of the patients are not clear. Even though most patients with PAO as well as with parent artery preservation had an mRS score less than two, some relevant symptoms still existed including headache, dizziness and memory deterioration. These patients had traditional “cure” but did not get “real health recovery”. The evaluation of the two groups with HIT-6 demonstrated a high proportion of severe headache (HIT-6 score greater than 50) in patients with PAO, but no significance (P > 0.05) was reached probably because of a small cohort of patients. The HIT-6 is a brief tool for evaluating the impact of headache in clinical research and practice.20 The development and validation study suggested that the HIT-6 possessed good psychometric properties among headache sufferers.21 Evaluation of the quality of life with SF-36 score showed lower scores in the PAO group than in the parent artery preservation group especially in the bodily pain, general health and vitality (P < 0.05). The SF-36 is a brief and easily administered measure of health-associated quality of life with 36 multiple-choice items evaluating eight health domains: physical functioning, physical health, emotional problems, vitality, social functioning, general health, mental health and bodily pain.22 The score for each domain ranges from 0 to 100, with 100 indicating an excellent health status and no reported symptoms.
Severe headache occurred in both PAO and parent artery preservation patients as demonstrated by the greater HIT-6 scores (≥50). Headache may be caused by persistent compression, stimulation and traction of the duramater by balloons or coils after embolization. In patients with parent artery preservation, severe headache may be associated with cerebral hyperfusion after occlusion of the high-flow direct CCF. However, in patients with PAO, cerebral hyperfusion may not exist because of occlusion of one internal carotid artery. Low perfusion syndrome may cause postural dizziness which will disappear after rest or change of body position. However, the exact mechanism of severe headache is not certain in this preliminary study and will need further investigation.
In the literature, few studies were found to investigate the cerebral blood flow using transcranial Doppler ultrasonography (TCD) or single-photon emission computerized tomography (SPECT) in direct CCFs to say nothing of positron emission computed tomography (PET).23–26 In a 27-year-old man with a right traumatic direct CCF, SPECT scan showed diffusely decreased perfusion in the right cerebral hemisphere before occlusion of the right internal carotid artery for treating the CCF.23 Two weeks after occlusion of the parent artery of the right internal carotid artery, SPECT scan revealed increased perfusion in the entire right cerebral hemisphere. Giller et al.24 studied TCD together with SPECT in predicting tolerance to carotid artery occlusion in patients undergoing possible carotid artery occlusion, and during the balloon test occlusion of one carotid artery, 15 out of 22 patients showed a fall in the flow velocity of no more than 65% in the middle cerebral artery with 14 of these patients clinically tolerating the balloon occlusion test. The remaining seven patients demonstrated a fall in the flow velocity of over 65% with six of these patients failing the balloon occlusion test and developing a focal deficit before completion of the 45-min observation period. In this study,24 a linear correlation existed between the SPECT index and the percentage decrease in the TCD flow velocity in the middle cerebral artery. In 10 patients with direct CCFs studied by Watanabe et al.,25 the regional cerebral blood flow decreased in the region with cortical venous drainage in six patients, which proved the usefulness of SPECT in assessing the cerebral circulation in cases of CCF.
In conclusion, PAO may affect the quality of life of patients with CCFs even though it may be an effective treatment option for high-flow direct fistulas.
Footnotes
These authors contributed equally to this work.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Korkmazer B, Kocak B, Tureci E, et al. Endovascular treatment of carotid cavernous sinus fistula: a systematic review. World J Radiol 2013; 5: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrow DL, Spector RH, Braun IF, et al. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg 1985; 62: 248–256. [DOI] [PubMed] [Google Scholar]
- 3.Ringer AJ, Salud L, Tomsick TA. Carotid cavernous fistulas: anatomy, classification, and treatment. Neurosurg Clin N Am 2005; 16: 279–295. viii. [DOI] [PubMed] [Google Scholar]
- 4.Higashida RT, Halbach VV, Tsai FY, et al. Interventional neurovascular treatment of traumatic carotid and vertebral artery lesions: results in 234 cases. AJR Am J Roentgenol 1989; 153: 577–582. [DOI] [PubMed] [Google Scholar]
- 5.Kocer N, Kizilkilic O, Albayram S, et al. Treatment of iatrogenic internal carotid artery laceration and carotid cavernous fistula with endovascular stent-graft placement. AJNR Am J Neuroradiol 2002; 23: 442–446. [PMC free article] [PubMed] [Google Scholar]
- 6.Gemmete JJ, Ansari SA, Gandhi DM. Endovascular techniques for treatment of carotid-cavernous fistula. J Neuroophthalmol 2009; 29: 62–71. [DOI] [PubMed] [Google Scholar]
- 7.Ross IB, Buciuc R. The vascular plug: a new device for parent artery occlusion. AJNR Am J Neuroradiol 2007; 28: 385–386. [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W, Li YD, Li MH, et al. Endovascular treatment of post-traumatic direct carotid-cavernous fistulas: a single-center experience. J Clin Neurosci 2011; 18: 24–28. [DOI] [PubMed] [Google Scholar]
- 9.Gemmete JJ, Chaudhary N, Pandey A, et al. Treatment of carotid cavernous fistulas. Curr Treat Options Neurol 2010; 12: 43–53. [DOI] [PubMed] [Google Scholar]
- 10.Bavinzski G, Killer M, Gruber A, et al. Treatment of post-traumatic carotico-cavernous fistulae using electrolytically detachable coils: technical aspects and preliminary experience. Neuroradiology 1997; 39: 81–85. [DOI] [PubMed] [Google Scholar]
- 11.Li MH, Li YD, Tan HQ, et al. Treatment of distal internal carotid artery aneurysm with the Willis covered stent: a prospective pilot study. Radiology 2009; 253: 470–477. [DOI] [PubMed] [Google Scholar]
- 12.Luo CB, Teng MM, Chang FC, et al. Transarterial balloon-assisted n-butyl-2-cyanoacrylate embolization of direct carotid cavernous fistulas. AJNR Am J Neuroradiol 2006; 27: 1535–1540. [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Lan ZG, Xie XD, et al. Traumatic carotid-cavernous fistulas treated with covered stents: experience of 12 cases. World Neurosurg 2010; 73: 514–519. [DOI] [PubMed] [Google Scholar]
- 14.Ducruet AF, Albuquerque FC, Crowley RW, et al. The evolution of endovascular treatment of carotid cavernous fistulas: a single-center experience. World Neurosurg 2013; 80: 538–548. [DOI] [PubMed] [Google Scholar]
- 15.Gupta AK, Purkayastha S, Krishnamoorthy T, et al. Endovascular treatment of direct carotid cavernous fistulae: a pictorial review. Neuroradiology 2006; 48: 831–839. [DOI] [PubMed] [Google Scholar]
- 16.Luo CB, Teng MM, Yen DH, et al. Endovascular embolization of recurrent traumatic carotid-cavernous fistulas managed previously with detachable balloons. J Trauma 2004; 56: 1214–1220. [DOI] [PubMed] [Google Scholar]
- 17.Xu XQ, Liu S, Zu QQ, et al. Follow-up of 58 traumatic carotid-cavernous fistulas after endovascular detachable-balloon embolization at a single center. J Clin Neurol 2013; 9: 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komiyama M, Nakajima H, Nishikawa M, et al. Brachial plexus and supraclavicular nerve injury caused by manual carotid compression for spontaneous carotid-cavernous sinus fistula. Surg Neurol 1999; 52: 306–309. [DOI] [PubMed] [Google Scholar]
- 19.Meyers PM, Halbach VV, Dowd CF, et al. Dural carotid cavernous fistula: definitive endovascular management and long-term follow-up. Am J Ophthalmol 2002; 134: 85–92. [DOI] [PubMed] [Google Scholar]
- 20.Rendas-Baum R, Yang M, Varon SF, et al. Validation of the headache impact test (HIT-6) in patients with chronic migraine. Health Qual Life Outcomes 2014; 12: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosinski M, Bayliss MS, Bjorner JB, et al. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res 2003; 12: 963–974. [DOI] [PubMed] [Google Scholar]
- 22.Kelly A, Rush J, Shafonsky E, et al. Detecting short-term change and variation in health-related quality of life: within- and between-person factor structure of the SF-36 health survey. Health Qual Life Outcomes 2015; 13: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung TS, Lee JD, Suh JH, et al. Increased cerebral perfusion after detachable balloon embolization of carotid cavernous fistula on technetium-99m-HMPAO brain SPECT. J Nucl Med 1993; 34: 1987–1989. [PubMed] [Google Scholar]
- 24.Giller CA, Mathews D, Walker B, et al. Prediction of tolerance to carotid artery occlusion using transcranial Doppler ultrasound. J Neurosurg 1994; 81: 15–19. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe A, Ishii R, Suzuki Y, et al. The cerebral circulation in cases of carotid cavernous fistula. Findings of single photon emission computed tomography. Neuroradiology 1990; 32: 108–113. [DOI] [PubMed] [Google Scholar]
- 26.Yoon WK, Kim YW, Kim SR, et al. Transarterial coil embolization of a carotid-cavernous fistula which occurred during stent angioplasty. Acta Neurochir 2009; 151: 849–853. discussion 53–54. [DOI] [PubMed] [Google Scholar]