Abstract
PURPOSE
Survival after a breast cancer diagnosis is poorer in First Nations women with a preexisting comorbidity compared with comorbidity-free First Nations women in Ontario, Canada. Given the high prevalence of diabetes in this population, it is important to determine whether preexisting diabetes is related to poorer survival after a breast cancer diagnosis.
METHODS
All First Nations women were identified from a cohort of First Nations people diagnosed with breast cancer in diagnostic periods—1995 to 1999 and 2000 to 2004—and seen at a regional cancer program (RCP) in Ontario. Preexisting diabetes status and other factors, such as age at diagnosis, body mass index, and stage at diagnosis, were collected from medical charts at the regional cancer programs. The association between preexisting diabetes and First Nations status was examined by each of the demographic, personal, tumor, and treatment factors using logistic regression models. Survival was compared between First Nations women with (n = 67) and without (n = 215) preexisting diabetes, adjusted by significant study factors using a Cox proportional hazards regression model.
RESULTS
The 5-year survival rate among First Nations women with diabetes was 59.8% versus 78.7% among those without diabetes (P < .01). Preexisting diabetes significantly increased the risk of death among First Nations women with breast cancer (hazard ratio, 1.87; 95% CI, 1.12 to 3.13) after adjustment for age group, period of diagnosis, body mass index, other comorbidities at diagnosis, and stage.
CONCLUSION
This study recommends awareness of this survival discrepancy among the treatment team for First Nations patients with breast cancer with preexisting diabetes.
CONTEXT
Key Objective
Never before has survival after a breast cancer diagnosis been estimated among an Indigenous cohort of those with and without a previous diagnosis of diabetes.
Knowledge Generated
Our findings show significantly poorer survival among First Nations women with preexisting diabetes compared with other First Nations women without that comorbidity.
Relevance
Since rates of breast cancer are increasing among First Nations women in Ontario, and a high proportion of them have had a diagnosis of diabetes, it is important for cancer treatment teams to be aware of the potential impact of this comorbidity.
INTRODUCTION
Incidence rates of both breast cancer and type 2 diabetes are increasing in Canada’s First Nations populations.1-4 Type 2 diabetes has become widespread, with an age standardized prevalence of up to 26% in First Nations communities5 and the onset of diabetes occurring at younger ages in this population.6 In addition, there is a greater than five-fold risk of death from diabetes for First Nations people living on reserve communities compared with non–First Nations people.7 Concurrently, the incidence of breast cancer, which was lower in First Nations women compared with non–First Nations women in the province of Ontario before 2010, has recently caught up.4,8 Our earlier study found breast cancer prognosis in First Nations women in Ontario to be worse than that in non–First Nations women as they are more likely to be diagnosed at a later stage of breast cancer, have ever smoked, have higher body mass index (BMI), and have comorbidities at breast cancer diagnosis.9
According to the 2016 Canadian census, there are approximately 375,000 people with Indigenous identity in the province of Ontario, including more than 236,000 First Nations, 120,000 Métis, and 3,800 Inuit.10 Because of historic and methodologic restrictions, however, census values underestimate the true size of these populations.11 Risk factor data for First Nations people in the province highlights higher rates of tobacco smoking and obesity, and vegetable and fruit consumption was low.12,13 Furthermore, Indigenous peoples are more likely to have a lack of trust in the health care system because of the impacts of colonialism and implicit bias and racialized health disparities.14-16
Studies have evaluated the association between preexisting diabetes and survival after a breast cancer diagnosis. A meta-analysis showed that diabetes was associated with an increased mortality across all cancer types, with greater risk of death for some cancers, including breast cancer.17 Consistently elsewhere, the association remained significant after adjustment for age, race, smoking status, and/or BMI.18-20 Only two studies have examined the influence of diabetes on cancer survival among an Indigenous population.21,22 Poorer survival was found among an Australian Indigenous cohort that included all cancer sites, with only a small sample of patients with breast cancer (n = 81), of which 20% had preexisting diabetes, resulting in low statistical power.21 The other study, in which diabetes was not a significant predictor of breast cancer survival, was among 5 ethnic groups, including a Native Hawaiian cohort.22
Using a comprehensive data set of prognostic characteristics of Ontario First Nations women from their breast cancer diagnosis linked to provincial mortality data gives us a unique opportunity to investigate the influence of preexisting diabetes on survival in a larger Indigenous sample.23
METHODS
Study Population
This study used the Ontario Cancer Registry (OCR) database and a cohort of Ontario First Nations people housed at the provincial cancer agency to identify eligible women with breast cancer. The OCR is a population-based cancer registry that includes tumor details, such as body site, morphology, and date at diagnosis; demographic details, such as date of birth, sex, and postal code of residence at diagnosis; and date and cause of death. It does not consistently include clinical, treatment, or stage of disease data. The OCR does not record race or ethnicity. As a result, it is not possible to routinely estimate cancer mortality rates for any ethnic population directly from OCR data; therefore, to capture First Nations status, a cohort of Ontario First Nations people was identified composed of all those with Registered Indian Status, as defined by the federal government, at any time between 1968 and 1991—approximately 141,000 people.24 These individuals were then linked to both the OCR (through 2004) and the mortality database (through 2007).9 Analyses of the completion of OCR have suggested a high level of completeness in case ascertainment for breast cancer.25 Information on deaths was obtained from mortality data collected for all Ontario residents by the Office of the Registrar General. As registration of death is a legal process in Ontario, this ensures that all deaths are registered.26
Study Design
All provincial First Nations women identified from the cohort of First Nations people who were diagnosed with a primary invasive breast cancer—International Classification of Diseases, Ninth Revision, code 174—from 1995 to 2004 and seen at one of Ontario’s specialized cancer centers, known as regional cancer programs (RCPs), were eligible for this study. RCPs deliver all cancer radiotherapy in the province and are often involved in the diagnostic workup and treatment planning aspects for breast cancer. RCPs retain patient records, generally encompassing diagnostic and treatment details for additional care received at other institutions. Since more than 90% of First Nations women with a breast cancer diagnosis were seen at an RCP—similar to the proportion of non–First Nations women with breast cancer23 —we did not expect to introduce any bias as a result of restricting our study to these women.
Definition of Diabetes Status
Study abstractors collected data on preexisting health conditions from RCP charts included in the Charlson index.27 This comorbidity index was created to capture conditions that are likely to influence the probably of mortality in patients with breast cancer. Both categories of diabetes—having or not having chronic complications—were grouped together to create a variable on diabetes status (yes or no). There was no differentiation between type 1 and type 2 diabetes.
Definition of Demographic Factors
Age at diagnosis was captured from OCR data. Analyses were carried out using age as both a continuous and a categorical (binary) variable; however, results are similar and so are reported here only using the binary age variable (< 50 v ≥ 50 years) to facilitate interpretation of age group as a proxy for menopausal status. Period of diagnosis was defined as 1995 to 1999 and 2000 to 2004 to capture possible differences in breast cancer diagnosis and treatment over the 10-year period—for instance, testing for the genes BRCA1 and BRCA2. While there were eight RCP locations visited for data collection, these were dichotomized for analytic purposes as northern (two rural centers) versus southern (seven urban centers). Distance from residence at diagnosis (postal code) to the RCP was computed by calculating the straight-line distance (to the nearest kilometer),28 then categorizing as close, moderate, and far with cutoff points defined by the distance tertiles of the women. Tertile cut points are: close: for northern women, less than 118 km and for southern women, less than 30 km; moderate: for northern women, 118 to less than 267 km and for southern women, 30 to less than 81 km; and far: for northern women, 267 km or greater and for southern women, 81 km or greater.
Definition of Personal Factors
BMI was calculated as weight in kilograms divided by height in square meters—measured by a health professional at diagnosis—and was categorized as: normal weight: BMI less than 25 kg/m2; overweight: BMI 25 or greater to less than 30 kg/m2; obese: BMI 30 kg/m2 or greater.29 Because of the effect of current and/or former active cigarette smoking on breast cancer survival,30 we grouped all the ever smokers together for the smoking status variable. Other comorbidity was defined as having any of the concurrent health conditions captured in the Charlson index—other than diabetes—at the time of diagnosis.27 Having a family history included women with a first-degree relative with breast cancer and/or ovarian cancer.
Definition of Tumor Factors
In Ontario, routine breast cancer screening is recommended to begin at age 50 years for women at average risk of developing breast cancer. Method of detection was grouped as screen detected for women whose breast cancer was detected through participation in routine mammography. Nonscreen detected included women whose breast cancer was detected by themselves or by a physician or other health professional. Histologic grade was captured as grade 1 (well differentiated), grade 2 (moderately differentiated), and grade 3 (poorly differentiated). We used the American Joint Committee on Cancer TNM classification scheme for staging breast cancers.31 Stage at diagnosis was categorized as stage I, stage II, and stages III to IV. Status of hormonal receptors estrogen and progesterone receptors was defined as being either positive or neither positive.
Definition of Treatment Factors
A variable was created to capture the most extensive surgery that a woman received. All potential procedures and surgeries were grouped into the following categories: less extensive, which included no surgery, fine-needle aspiration, or partial and simple mastectomy; and more extensive, which included those whose most extensive surgery was a modified radical or radical mastectomy. Radiotherapy, chemotherapy, and hormonal therapy were each defined as yes if the therapies were received within 12 months of diagnosis. Otherwise, these variables were coded as no. A representative variable for stage- and age-appropriate treatment using data that captured receipt of radiotherapy, chemotherapy, and surgeries—yes or no for each—and age at diagnosis was created on the basis of guidelines developed by the Physician’s Data Query.32
Statistical Analysis
Unadjusted odds ratios (ORs) and their corresponding 95% CIs were estimated to compare the odds of having preexisting diabetes by each of the study factors: age at diagnosis, period of diagnosis, RCP location, distance to RCP, BMI, smoking status, other comorbidities, family history, method of detection, histologic grade, stage at diagnosis, estrogen/progesterone receptor status, surgery, radiotherapy, chemotherapy, hormonal therapy, and stage- and age-appropriate treatment. Factors that demonstrated a significant increase or decrease in the odds of having preexisting diabetes at P < .05 were included in the survival analysis as long as the proportional hazards assumption of parallel lines held for each factor. In addition, stage at diagnosis was added to the survival analysis as its influence on survival time was established in our previous study.23
Survival time was calculated for each woman, beginning at the date of breast cancer diagnosis until the date of death or censoring at the end of the study (December 31, 2007), whichever occurred first. Deaths not only from breast cancer, but also from all causes were used in our analyses because data on the fact of death were available up to the end of 2007, whereas data on specific causes of death were only available up to December 31, 2005. We estimated overall survival using the Kaplan-Meier method and compared by diabetes status with a log-rank test. A Cox proportional hazards regression model was generated to examine the effect of diabetes status on the risk of death (our base model). Subsequently, each of the factors of interest were individually and cumulatively added to the base model to assess whether the hazard ratio (HR) for preexisting diabetes status remained significant after adjustment. All analyses were conducted using SAS,33 and all reported P values were for two-sided alternatives.
Ethical approval of this research was obtained from the Human Subjects Ethics Review Committee of the University of Toronto. In addition, ethics approval for chart abstractions was obtained from each of the RCPs where women were treated for breast cancer.
RESULTS
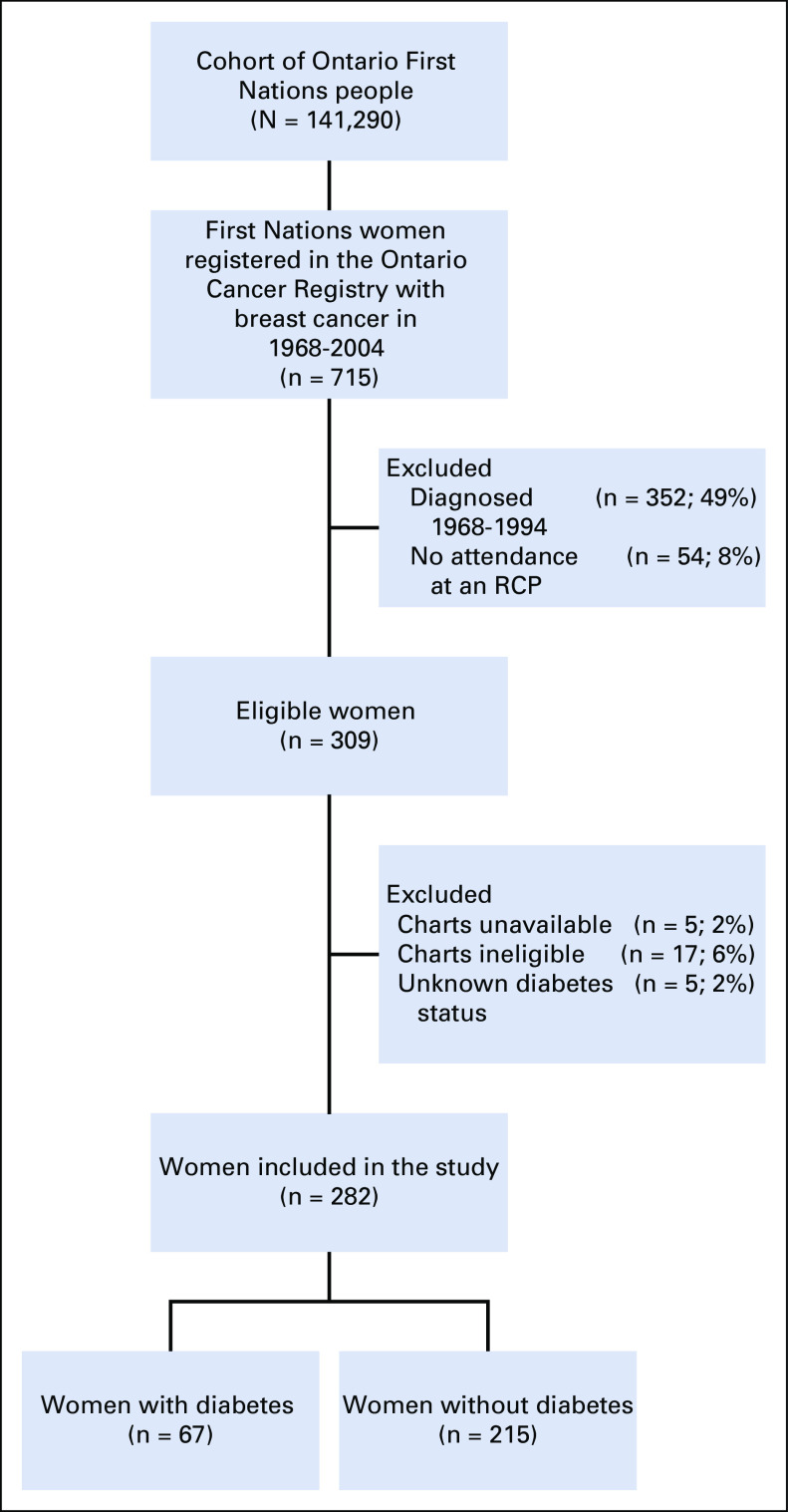
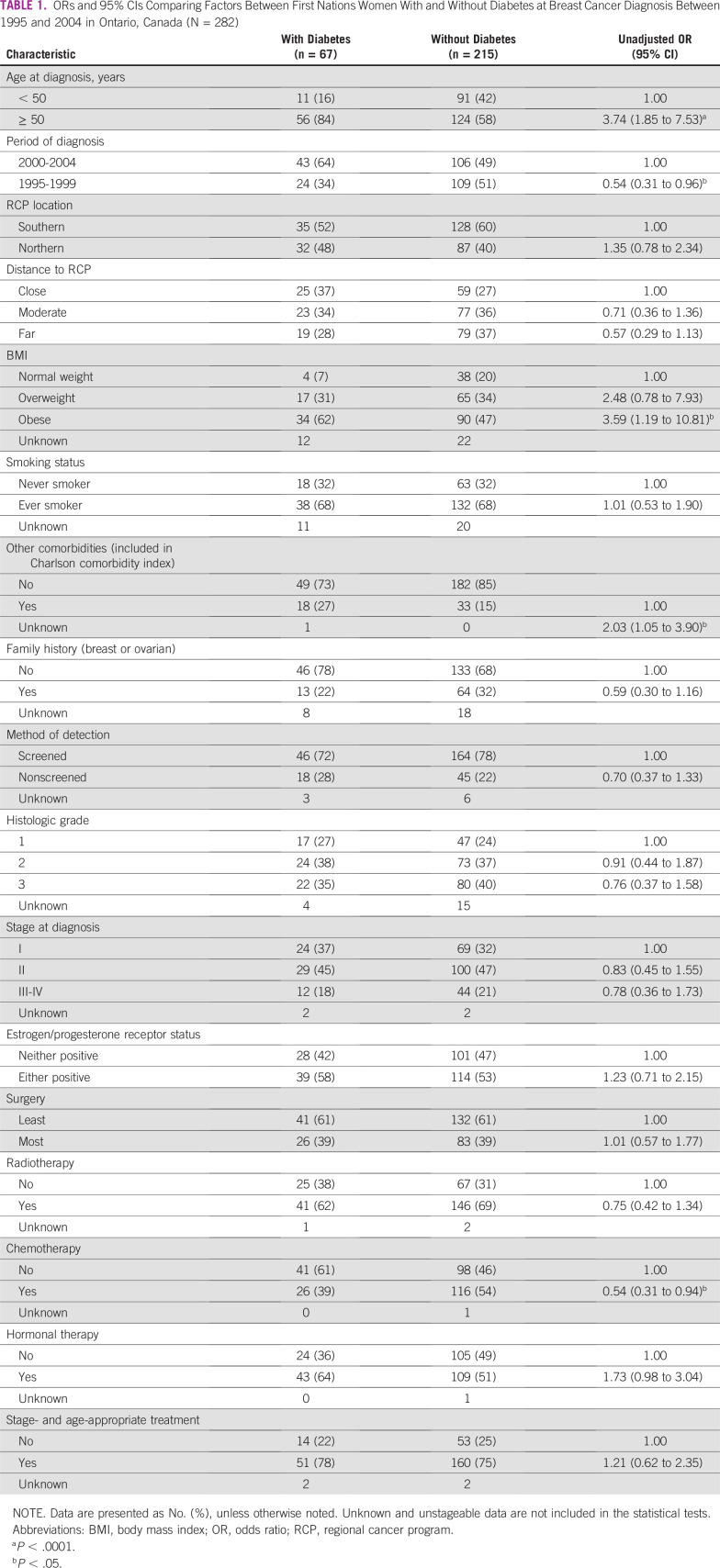
Of the 309 eligible First Nations women who were diagnosed with breast cancer between 1995 and 2004 in Ontario, 282 (91%) remained in the study. Of this group, 67 (24%) had been diagnosed with diabetes (Fig 1). Those with diabetes were significantly more likely to be older (OR, 3.74; 95% CI, 1.85 to 7.53), less likely to be diagnosed in the earlier period of diagnosis (OR, 0.54; 95% CI, 0.31 to 0.96); three and a half times more likely to be obese (OR, 3.59; 95% CI, 1.19 to 10.81), twice as likely to have another comorbidity at breast cancer diagnosis (OR, 2.03; 95% CI, 1.05 to 3.90); and half as likely to have received chemotherapy (OR, 0.54; 95% CI, 0.31 to 0.94) compared with First Nations women without preexisting diabetes (Table 1). The distribution of smoking status was the same between the two groups, 10% more of those without diabetes at diagnosis had a documented family history of breast or ovarian cancer, and rates of negative receptor status were high in both groups. The proportional hazards assumption for parallel lines held true for each of the significant factors above with the exception of chemotherapy.
FIG 1.
Flowchart of eligibility criteria for First Nations women. RCP, regional cancer program.
TABLE 1.
ORs and 95% CIs Comparing Factors Between First Nations Women With and Without Diabetes at Breast Cancer Diagnosis Between 1995 and 2004 in Ontario, Canada (N = 282)
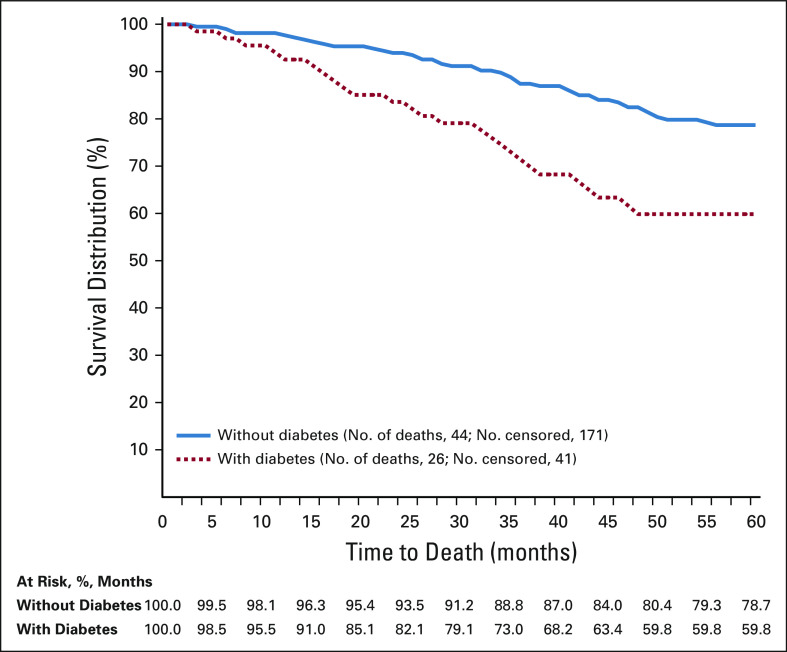
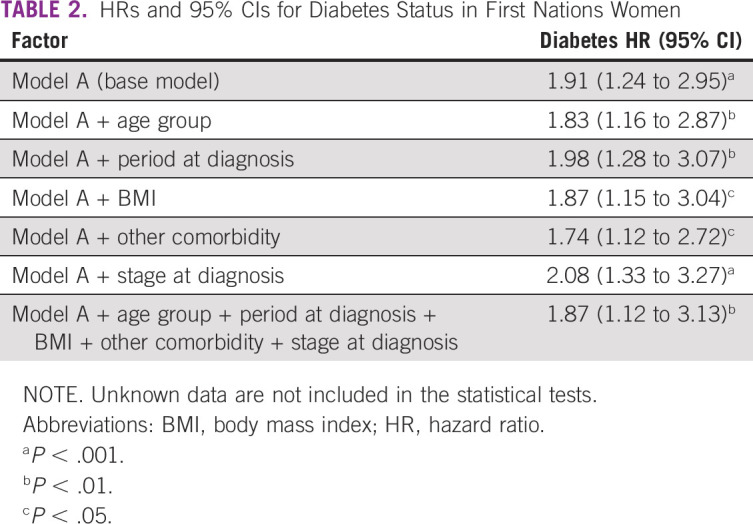
After 5 years of observation, the overall survival rate among First Nations women with diabetes was 59.8% compared with 78.7% among First Nations women without diabetes (P < .01; Fig 2). For the base model, unadjusted diabetes HR was significant (HR, 1.91; 95% CI, 1.24 to 2.95). After adjustment for age, period of diagnosis, BMI, other comorbidity, and stage at diagnosis, HR for diabetes was unchanged (HR, 1.87; 95% CI, 1.12 to 3.13), which suggests that First Nations women with a preexisting diabetes diagnosis are almost twice as likely to die compared with First Nations women without the condition at their breast cancer diagnosis (Table 2).
FIG 2.
Five-year survival distribution stratified by diabetes status at breast cancer diagnosis (n = 282).
TABLE 2.
HRs and 95% CIs for Diabetes Status in First Nations Women
DISCUSSION
Our study found that diabetes significantly increased the risk of death among First Nations women with breast cancer. This finding enhances the growing literature in this field and corresponds with what has been published with non-Indigenous populations.17-20
Canadian literature draws attention to the increasing rates of obesity in First Nations people, especially among women.3 Furthermore, obesity in this population is predominately centrally distributed, characterized by a high waist-to-hip ratio, which is associated with an increased risk of diabetes3 and increased risk of breast cancer mortality, independent of the effect of diabetes.34 Research has demonstrated an association between central obesity and higher levels of insulin, a marker of insulin resistance.35 In addition, an association between high levels of fasting insulin and distant recurrence and death among women without diabetes diagnosed with early-stage breast cancer has been found.36 Therefore, as First Nations women without diabetes in this study also have a high prevalence of obesity, the survival disadvantage observed among women with diabetes may have been underestimated since a portion of the patients in the reference population may also be at increased risk for recurrence and death compared with a population with a lower prevalence of obesity. Moreover, in light of what is known about the prevalence of other cardiometabolic cluster conditions in Indigenous populations,37,38 it is conceivable that these conditions were prevalent in the reference population—that is, First Nations women without diabetes—again potentially minimizing the difference in the risk of death between groups.
A consequence of the presence of comorbidities is that physicians are less likely or able to prescribe stage-specific treatment because of potential contraindications,39 which may influence survival. In line with the literature,21 receipt of stage- and age-appropriate treatment did not vary by diabetes status in our study; however, treatment completion was not incorporated into our analyses and may contribute to explaining our findings. Some of the complications related to diabetes, such as renal impairment, influence imaging modalities and may affect the dose of chemotherapy that can be prescribed.21 Patients with breast cancer and diabetes are in fact at increased risk of chemotherapy-related toxicities compared with patients with breast cancer without diabetes,40 which potentially explains our finding that those with diabetes received less chemotherapy compared with those without diabetes. A limitation of our analyses was the omission of human epidermal growth factor receptor 2/neu status as it is a major prognostic factor and can vary by race.41
As we evaluated all-cause mortality, and not breast cancer–specific mortality, it is conceivable that at least some of the increased risk of death observed among those with preexisting diabetes may be attributable to diabetes itself. For instance, diabetes is an established risk factor for cardiovascular disease and its outcomes in First Nations adults.42 The accumulating burden of these conditions over time been has been explored in a Canadian Indigenous context and the data reveal interactions between diabetes and cardiovascular disease over one’s life course.43 However, among our data, the preexisting prevalence of acute myocardial infarction, congestive heart failure, peripheral vascular disease, and cerebrovascular disease were too low to evaluate (n = 7, n = 10, n = 4, and n = 2, respectively).
The accuracy of identifying First Nations women from a database linkage—and therefore the reliable representation—and generalizability of the findings is a strength of this study. In addition, to our knowledge, this is the first analysis to have the statistical power to examine the influence of diabetes and survival after a breast cancer diagnosis in an Indigenous population. The comprehensive data set of prognostic characteristics further strengthens the investigation. One of the study limitations, however, is that we did not differentiate between type 1 and type 2 diabetes. Nonetheless, in other research in which diabetes type was ascertained within an Indigenous sample, 95% of diabetes cases were diagnosed as type 2.21 Another limitation was our method of ascertaining diabetes status. While the information was collected from the medical charts of women seen at an RCP under physician or nurse interview, charts did not include specific diagnostic information. Definitive diabetes tests were not performed at breast cancer diagnosis; therefore, it is possible that a proportion of women not known to have diabetes in fact had glucose levels that were in the diabetes range. The implication of this potential misclassification may minimize the difference in the risk of death between groups. Although we did not have data on metabolic control, the literature shows that First Nations people with diabetes experience a high prevalence of elevated A1C, potentially as a result of suboptimal glucose and blood pressure control.44,45
There was an overall higher proportion of hormone receptor–negative women than usually observed in a breast cancer population cohort.46 As other studies have demonstrated an increased proportion of negative hormone receptor status by race and ethnicity,47,48 our finding suggests that additional examination is warranted.
Additional survival analyses should consider the effect of socioeconomic status. An individual’s socioeconomic status can influence nutritional choices, access to transportation, and physical activity. These may each independently and/or synergistically influence treatment and ultimately survival time. Socioeconomic stressors, such as lack of employment and low wages, which have been shown to disproportionately affect First Nations people, may supersede health concerns, which has been demonstrated to further affect the psychological impact of a breast cancer diagnosis.49,50
In conclusion, First Nation women with breast cancer are at a two-fold increased risk of dying if also diagnosed with diabetes compared with other First Nations women without preexisting diabetes. These findings recommend awareness of this survival discrepancy among the treatment team for First Nations patients with breast cancer.
ACKNOWLEDGMENT
The authors thank the study staff: Lindsay Stewart, Sarah Ahmad, Lucia Mirea, and Tanya Cecic; and Alethea Kewayosh, Director of the Indigenous Cancer Care Unit, Cancer Care Ontario, for support. The authors also thank the participants of the Aboriginal Breast Cancer Care Workshop who shared their relevant experiences and contributed their interpretations of the study findings.51 The authors acknowledge their esteemed colleague Diane Nishri, whose commitment to ensuring the accuracy of population data is unmatched. Furthermore, the authors appreciate and value her foundational role with this project.
Footnotes
Presented at the Spirit of Eagles: Changing Patterns of Cancer in Native Communities Conference, Niagara Falls, NY, September 21-24, 2017.
AUTHOR CONTRIBUTIONS
Conception and design: Amanda J. Sheppard, Anna M. Chiarelli, Loraine D. Marrett
Collection and assembly of data: Amanda J. Sheppard, Anna M. Chiarelli
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jgo/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.Mazereeuw MV, Withrow DR, Diane Nishri E, et al. Cancer incidence among First Nations adults in Canada: Follow-up of the 1991 Census Mortality Cohort (1992-2009) Can J Public Health. 2018;109:700–709. doi: 10.17269/s41997-018-0091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGahan CE, Linn K, Guno P, et al. Cancer in First Nations people living in British Columbia, Canada: An analysis of incidence and survival from 1993 to 2010. Cancer Causes Control. 2017;28:1105–1116. doi: 10.1007/s10552-017-0950-7. [DOI] [PubMed] [Google Scholar]
- 3.Young TK, Reading J, Elias B, et al. Type 2 diabetes mellitus in Canada’s first nations: Status of an epidemic in progress. CMAJ. 2000;163:561–566. [PMC free article] [PubMed] [Google Scholar]
- 4.Cancer Care Ontario . Cancer in First Nations People in Ontario: Incidence, Mortality, Survival and Prevalence. Toronto, ON, Canada: Cancer Care Ontario; 2017. [Google Scholar]
- 5.Harris SB, Gittelsohn J, Hanley A, et al. The prevalence of NIDDM and associated risk factors in native Canadians. Diabetes Care. 1997;20:185–187. doi: 10.2337/diacare.20.2.185. [DOI] [PubMed] [Google Scholar]
- 6.Dyck R, Osgood N, Lin TH, et al. Epidemiology of diabetes mellitus among First Nations and non-First Nations adults. CMAJ. 2010;182:249–256. doi: 10.1503/cmaj.090846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao Y, Moloughney BW, Semenciw RM, et al. Indian Reserve and registered Indian mortality in Canada. Can J Public Health. 1992;83:350–353. [PubMed] [Google Scholar]
- 8.Canadian Cancer Society’s Steering Committee . Canadian cancer statistics 2009. Toronto, ON, Canada: Canadian Cancer Society; 2009. [Google Scholar]
- 9.Sheppard AJ, Chiarelli AM, Marrett LD, et al. Detection of later stage breast cancer in First Nations women in Ontario, Canada. Can J Public Health. 2010;101:101–105. doi: 10.1007/BF03405573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Statistics Canada Aboriginal population profile. https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/abpopprof/index.cfm?Lang=E
- 11.Smylie J, Firestone M. Back to the basics: Identifying and addressing underlying challenges in achieving high quality and relevant health statistics for indigenous populations in Canada. Stat J IAOS. 2015;31:67–87. doi: 10.3233/SJI-150864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Statistics Canada Projections of the Aboriginal populations, Canada, provinces and territories, 2001 to 2017. https://www150.statcan.gc.ca/n1/pub/89-645-x/2010001/c-g/c-g013-eng.htm
- 13.Chiefs of Ontario. Cancer Care Ontario . Cancer in First Nations in Ontario: Risk factors and screening. Toronto, ON, Canada: Cancer Care Ontario; 2016. [Google Scholar]
- 14.Allan B, Smylie J. First Peoples, second class treatment: The role of racism in the health and well-being of Indigenous peoples in Canada. Toronto, ON, Canada: The Wellesley Institute; 2015. [Google Scholar]
- 15.Jull J, Mazereeuw M, Sheppard A, et al. Tailoring and field-testing the use of a knowledge translation peer support shared decision making strategy with First Nations, Inuit and Métis people making decisions about their cancer care: A study protocol. Res Involv Engagem. 2018;4:6. doi: 10.1186/s40900-018-0085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenwood M, de Leeuw S, Lindsay MN.(eds)Determinants of Indigenous Peoples' Health, Second Edition; Beyond the Social Toronto, ON, Canada: Canadian Scholars’ Press; 2018 [Google Scholar]
- 17.Barone BB, Yeh HC, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: A systematic review and meta-analysis. JAMA. 2008;300:2754–2764. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coughlin SS, Calle EE, Teras LR, et al. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159:1160–1167. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 19.Verlato G, Zoppini G, Bonora E, et al. Mortality from site-specific malignancies in type 2 diabetic patients from Verona. Diabetes Care. 2003;26:1047–1051. doi: 10.2337/diacare.26.4.1047. [DOI] [PubMed] [Google Scholar]
- 20.Giordano SH, Jiralerspong S, Lopez A, et al. Diabetes, obesity, and survival in a large cohort of early-stage breast cancer patients. J Clin Oncol. 2010;28(suppl; abstr 1503) doi: 10.1093/annonc/mdt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin JH, Coory MD, Valery PC, et al. Association of diabetes with survival among cohorts of indigenous and non-Indigenous Australians with cancer. Cancer Causes Control. 2009;20:355–360. doi: 10.1007/s10552-008-9249-z. [DOI] [PubMed] [Google Scholar]
- 22.Maskarinec G, Shvetsov YB, Conroy SM, et al. Type 2 diabetes as a predictor of survival among breast cancer patients: The multiethnic cohort. Breast Cancer Res Treat. 2019;173:637–645. doi: 10.1007/s10549-018-5025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheppard AJ, Chiarelli AM, Marrett LD, et al. Stage at diagnosis and comorbidity influence breast cancer survival in First Nations women in Ontario, Canada. Cancer Epidemiol Biomarkers Prev. 2011;20:2160–2167. doi: 10.1158/1055-9965.EPI-11-0459. [DOI] [PubMed] [Google Scholar]
- 24.Marrett LD, Chaudhry M. Cancer incidence and mortality in Ontario First Nations, 1968-1991 (Canada) Cancer Causes Control. 2003;14:259–268. doi: 10.1023/a:1023632518568. [DOI] [PubMed] [Google Scholar]
- 25.Holowaty EJ, Marrett LD, Fehringer G. Methods Cancer Incidence in Ontario: Trends and Regional Variations in the 1980s. Toronto, ON, Canada: Publications Ontario; 1995. [Google Scholar]
- 26.Marrett LD, Swift M, Reynolds DL, et al. Geographic distribution of cancer in OntarioinAtlas of Cancer Mortality Vol. 1Toronto, ON, Canada: Ontario Cancer Treatment and Research Foundation; 1995. pp1976–1985. [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Groome PA, Schulze KM, Keller S, et al. Demographic differences between cancer survivors and those who die quickly of their disease. Clin Oncol (R Coll Radiol) 2008;20:647–656. doi: 10.1016/j.clon.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 29. National Institutes of Health: Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults–The Evidence Report. Bethesda, MD, National Heart, Lung, and Blood Institute, NIH publication 98-4083, 1998.
- 30.Sagiv SK, Gaudet MM, Eng SM, et al. Active and passive cigarette smoke and breast cancer survival. Ann Epidemiol. 2007;17:385–393. doi: 10.1016/j.annepidem.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 31.American Joint Committee on Cancer . Cancer Staging Manual. ed 6. New York, NY: American Joint Committee on Cancer; 2002. [Google Scholar]
- 32.Maskarinec G, Pagano IS, Yamashiro G, et al. Influences of ethnicity, treatment, and comorbidity on breast cancer survival in Hawaii. J Clin Epidemiol. 2003;56:678–685. doi: 10.1016/s0895-4356(03)00079-9. [DOI] [PubMed] [Google Scholar]
- 33.SAS Institute . Statistical Analysis Software. ed 9.1. Cary, NC: SAS Institute; 2004. [Google Scholar]
- 34.Kumar NB, Cantor A, Allen K, et al. Android obesity at diagnosis and breast carcinoma survival: Evaluation of the effects of anthropometric variables at diagnosis, including body composition and body fat distribution and weight gain during life span, and survival from breast carcinoma. Cancer. 2000;88:2751–2757. doi: 10.1002/1097-0142(20000615)88:12<2751::aid-cncr13>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 35.Scheen AJ, Lefèbvre PJ. Insulin action in man. Diabetes Metab. 1996;22:105–110. [PubMed] [Google Scholar]
- 36.Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer: Results of a prospective cohort study. J Clin Oncol. 2002;20:42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 37.Resnick HE, Jones K, Ruotolo G, et al. Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease in nondiabetic American Indians: The Strong Heart Study. Diabetes Care. 2003;26:861–867. doi: 10.2337/diacare.26.3.861. [DOI] [PubMed] [Google Scholar]
- 38.Ley SH, Harris SB, Mamakeesick M, et al. Metabolic syndrome and its components as predictors of incident type 2 diabetes mellitus in an Aboriginal community. CMAJ. 2009;180:617–624. doi: 10.1503/cmaj.080972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris EER, Hwang WT, Urtishak SL, et al. The impact of comorbidities on outcomes for elderly women treated with breast-conservation treatment for early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2008;70:1453–1459. doi: 10.1016/j.ijrobp.2007.08.059. [DOI] [PubMed] [Google Scholar]
- 40.Srokowski TP, Fang S, Hortobagyi GN, et al. Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J Clin Oncol. 2009;27:2170–2176. doi: 10.1200/JCO.2008.17.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stark AT, Claud S, Kapke A, et al. Race modifies the association between breast carcinoma pathologic prognostic indicators and the positive status for HER-2/neu. Cancer. 2005;104:2189–2196. doi: 10.1002/cncr.21463. [DOI] [PubMed] [Google Scholar]
- 42.Harris SB, Zinman B, Hanley A, et al. The impact of diabetes on cardiovascular risk factors and outcomes in a native Canadian population. Diabetes Res Clin Pract. 2002;55:165–173. doi: 10.1016/s0168-8227(01)00316-3. [DOI] [PubMed] [Google Scholar]
- 43.Kmetic A, Reading J, Estey E. Taking a life course perspective on cardiovascular disease and diabetes in First Nations peoples. Can J Nurs Res. 2008;40:58–78. [PubMed] [Google Scholar]
- 44.Hanley AJ, Harris SB, Mamakeesick M, et al. Complications of type 2 diabetes among Aboriginal Canadians: Prevalence and associated risk factors. Diabetes Care. 2005;28:2054–2057. doi: 10.2337/diacare.28.8.2054. [DOI] [PubMed] [Google Scholar]
- 45.Harris SB, Naqshbandi M, Bhattacharyya O, et al. Major gaps in diabetes clinical care among Canada’s First Nations: Results of the CIRCLE study. Diabetes Res Clin Pract. 2011;92:272–279. doi: 10.1016/j.diabres.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: A prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9:R6. doi: 10.1186/bcr1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li CI, Malone KE, Daling JR. Differences in breast cancer hormone receptor status and histology by race and ethnicity among women 50 years of age and older. Cancer Epidemiol Biomarkers Prev. 2002;11:601–607. [PubMed] [Google Scholar]
- 48.Jack RH, Davies EA, Renshaw C, et al. Differences in breast cancer hormone receptor status in ethnic groups: A London population. Eur J Cancer. 2013;49:696–702. doi: 10.1016/j.ejca.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 49.Simon AE, Wardle J. Socioeconomic disparities in psychosocial wellbeing in cancer patients. Eur J Cancer. 2008;44:572–578. doi: 10.1016/j.ejca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 50.Anand SS, Yusuf S, Jacobs R, et al. Risk factors, atherosclerosis, and cardiovascular disease among Aboriginal people in Canada: The Study of Health Assessment and Risk Evaluation in Aboriginal Peoples (SHARE-AP) Lancet. 2001;358:1147–1153. doi: 10.1016/s0140-6736(01)06255-9. [DOI] [PubMed] [Google Scholar]
- 51.Sheppard AJ, Chiarelli AM, Stewart L, et al. Aboriginal breast cancer care workshop report: Strategies to improve First Nations cancer care in Ontario. Circumpolar Health Suppl. 2010;7:168–173. [Google Scholar]