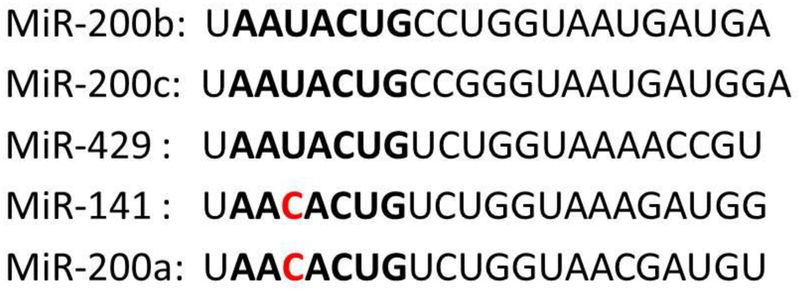Abstract
MicroRNA’s are small non-coding RNAs that regulate the expression of genes by targeting the 3’ UTR’s of mRNA. Studies reveal that miRNAs play a pivotal role in normal musculoskeletal function such as mesenchymal stem cell differentiation, survivability and apoptosis, osteogenesis, and chondrogenesis. Changes in normal miRNA expression have been linked to a number of pathological disease processes. Additionally, with aging, it is noted that there is dysregulation in the normal function of stem cell differentiation, bone formation/degradation, chondrocyte function, and muscle degeneration. Due to the change in expression of miRNA in degenerative musculoskeletal pathology, it is believed that these molecules may be at least partially responsible for cellular dysfunction. A number of miRNAs have already been identified to play a role in osteoarthritis, osteoporosis and sarcopenia. One miRNA that has become of interest recently is miRNA 141. The purpose of this article is to review the current literature available on miRNA 141 and how it could play a role in osteoporosis, osteoarthritis and musculoskeletal pathology overall.
Keywords: MicroRNA-141, MiR-200 family, aging, musculoskeletal
1. Introduction
Aging is one of the important contributing factors in musculoskeletal pathophysiology. As it occurs, there is degeneration of various musculoskeletal tissue including muscle, bone, and cartilage, which leads to pathological states such as sarcopenia, osteopenia/osteoporosis and osteoarthritis (Dawson & Dennison, 2016; Leveille et al, 2004). Osteoporosis alone is estimated to affect 200 million women worldwide with increasing incidence with age (Sozen, Ozisik, & Basaran, 2017). One in three women and one in five men over age 50 will experience a fracture secondary to osteoporosis at least once in their lifetime (Sozen et al., 2017). In addition, patients on long-term glucocorticoid therapy have been shown to induce fractures in 30–50% of the patients and osteonecrosis in 9– 40% (Weinstein., 2012). This creates a greater dilemma for older patients that may be using steroids for treatment of other diseases, accelerating possible osteopenia. Sarcopenia, another important age related musculoskeletal disease affect millions of people worldwide and associated with higher health cost. Janssen et al (2004) estimated that in year 2000, the direct cost of sarcopenia in unites state was $18.5 billion (Janssen et al., 2004).The pathophysiology of these degenerative conditions is widely accepted to be complex and not completely understood. It is well known that with aging, the gene expression of cells changes in a way that results in phenotypic aging (Bennett et al., 2015; Fushan et al., 2015; Ryan et al., 2015). Changes in gene expression are regulated by a number of signaling mechanisms, one of them being epigenetic factors. Epigenetics is defined as the mechanism by which changes are made to the genome without affecting the DNA sequence (Coolen et al., 2011). This is done through a number of different mechanisms, which include DNA methylation, histone modification and changes to coding/noncoding RNA (Brunet & Berger, 2014). Recent studies have been directed at these epigenetic factors that influence aging and musculoskeletal degeneration (Brown & Goljanek-Whysall, 2015; Grebennikova et al., 2015; Kim, Kang, Cho, & Kim, 2015). One such factor is noncoding, microRNA (miRNA). MiRNA’s are a group of naturally occurring, short, noncoding RNA’s which regulate the expression of other genes by targeting the 3’ UTR of messenger RNA, either inhibiting their translation or signaling them for degradation (Hammond et al., 2015). MicroRNAs are first transcribed from DNA sequences into immature pri-miRNA molecules. These pri-miRNA are subsequently cleaved by RNAses both inside the nucleus, producing a pre-miRNA and in the cytoplasm before it’s considered a mature miRNA (Filipowicz, Jaskiewicz, Kolb, & Pillai, 2005; Wahid, Shehzad, Khan, & Kim, 2010). Once the miRNA is unwound, one strand is usually degraded and the other can go on to bind with Argonuate and subsequently, a miRNA-induced silencing complex or miRISC (B. Zhang, Wang, & Pan, 2007). This miRNA-miRISC complex can target mRNA for degradation or inhibition, depending on the degree of complementarity with the 3’ UTR of its target mRNA and the 5’ seed sequence of the miRNA. It is by this ability to alter gene expression that miRNA can play a pivotal role in cell processes such as survivability, differentiation, stress and inflammatory responses, and cell death.
MiRNA’s have been shown to play a role in several cellular functions which include musculoskeletal development and pathophysiology. In fact, there is growing evidence that miRNA’s can regulate bone homeostasis and osteogenesis, which could make them potential therapeutic targets in degenerative bone disease and fracture healing (Pi, Li, Zhou, & Gao, 2015). As more and more of these molecules are being discovered, their functions in biological processes are being given greater attention. One miRNA that has been of interest is miRNA-141, a member of the miRNA 200 family. Scientific literature has shown that miRNA 141 targets several genes known to be critical for bone mesenchymal stem cell (BMSC) migration, differentiation, and survival. The goal of this article is to summarize the potential role of miRNA 141 in degenerative musculoskeletal pathology and include the relevant data from other members of the miRNA 200 family. We mainly focused on the miRNAs with validated targets (Table 1) and their involvement in musculoskeletal pathophysiology. Furthermore, we also identified novel potential targets for miRNA 141 using bioinformatics tools (such as TargetScan) and identified several important stem cell differentiation and proliferation, myogenic, chondrogenic and bone related genes. These genes would be good topics for future research in miRNA 141 and how it affects the musculoskeletal system.
Table 1:
Verified targets of microRNA-141 and their molecular function(s).
| Table. 1 | Target Gene (s) | Molecular Function of genes | References |
|---|---|---|---|
| 1 | SVCT2 | Stem cell differentiation, Oxidative stress | Sangani et al., 2014 |
| 2 | ZMPSTE24 | Phenotypic aging, BMSC biology | Yu et al., 2013 |
| 3 | Wnt, CDC25A | MSC proliferation and osteoblast differentiation | Qiu & Kassem, 2014 |
| 4 | Dlx5 | Bone formation | Itoh et al., 2009 |
| 5 | BMI1 | stem cells and senescence regulation | Dimri et al., 2013 |
| 6 | TGFb1 | Growth Factor, Muscle biology | Zhou & Yu, 2017 |
| 7 | ICAM-1 | Inflammation, Oxidative stress, Osteogenic differentiation | R. R. Liu et al., 2015 |
| 8 | PTEN | mitochondrial | Ji et al., 2015 |
| 9 | PAPP-A | osteoblast proliferation,bone formation | Zhang et al., 2015 |
| 10 | SDF1 | Bone biology, Cell migration | Sudharsan Periyasamy-Thandavan et al., 2018 |
2. MiRNA-141/200 family
Micro RNA-141 is a part of miRNA-200 family which consists of miRNA’s 141, 200a, 200b, 200c and 429. MiRNA 200 family is categorized based on their chromosomal locations and seed sequences. The miRNA 200 family is clustered into two polycistronic pri-miRNA transcripts located on human chromosomes 1 and 12 (Chen & Zhang, 2017). Chromosome 1 contains the miRNA-200a-200b-429 loci while 12 contains the miRNA-200c-141 loci. In addition to their locations on the human chromosomes, the miRNA 200 family can also be categorized by the similarity in seed sequences. The seed sequence typically lies between the 2nd to 8th nucleotide sequences on the 5’ end of the miRNA. MiRNA’s 200b, 200c, and 429 share a seed sequence of AAUACUG while miRNA’s 141 and 200a share a sequence of AACACUG. Apart from a single nucleotide difference, these 5 miRNA’s are identical (Fig. 1). This creates a strong possibility that the targets of these miRNA’s may be similar or can cross over. These targets can be predicted via the use of a bioinformatics tool, which can match the seed sequences of the miRNA with the 3’ UTR’s of mRNA. Genes involved in bone metabolism can then be focused on to assess the physiological role of miRNA in bone. The microRNA-200 family is known for their role in oncogenesis and tumor suppression; however recent studies showed they play an important role in stem cell biology (Itoh, Nozawa, & Akao, 2009; Sangani et al., 2015). The miRNA 200 family’s relationship with bone formation has also been explored and they share a common pathway with miRNA 141 in regards to musculoskeletal pathology and verified targets of this miRNA are listed in (Table 1) (Qiu & Kassem, 2014; Sangani et al., 2015).
Figure.1:
The sequences of miRNA-200 family members were aligned, and bolded letters are the seed sequence of miRNAs. The red letters indicate change in single nucleotide change in seed sequence.
3. MIRNA-141/200a in Stem cell biology
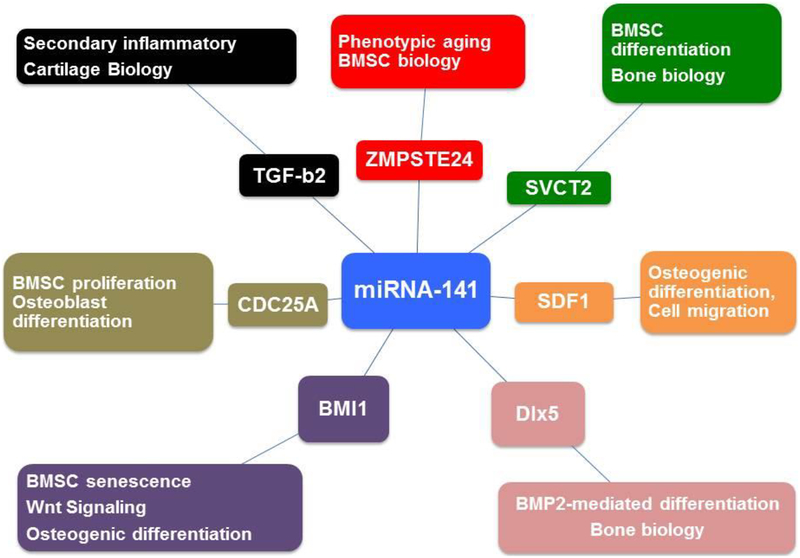
Mesenchymal stem cells are central to musculoskeletal development due to their ability to differentiate into adipocytes, chondrocytes, myocytes, osteoblasts and subsequently osteocytes (Cook & Genever, 2013). A number of microRNAs are known to play an important role in differentiation of stem cells to other cell types, including members of the miRNA 200 family. Recently, our group reported that mouse BMSCs expressed miRNA-141 and miRNA-200a, which subsequently repressed SVCT2 expression at the functional level by targeting the 3-untranslated region of mRNA. We found that miRNA-141 and miRNA-200a reduce vitamin C uptake in BMSCs. Furthermore, we reported that miRNA-141 and miRNA-200a decreased osteogenic differentiation by targeting SVCT2 transporter. Previously, we demonstrated that sodium-coupled vitamin C transporter 2 (SVCT2) plays a vital role in bone marrow stromal cell differentiation to osteogenesis (Fulzele et al., 2013) (Fig.2).
Figure.2:
Involvement of miRNA-141-3p in musculoskeletal system based on published data.
Our group also demonstrated that miRNA-141 targets stromal derived factor-1 SDF1, also known as CXCL12 (Sudharsan Periyasamy-Thandavan et al, 2018). SDF1 is a chemokine who’s effects are mediated mainly by binding to its primary receptor, C-X-C chemokine receptor type 4 (CXCR4). This interaction has become a focal point of several studies, which revealed its key role in BMSC differentiation and osteogenesis (Hwang et al., 2015; Kawakami et al., 2015; X. Liu et al., 2013). We discovered that miRNA 141 inhibits expression of SDF1; it therefore has the potential to negatively impact bone integrity. Our lab is the first to report that SDF1 is the target of miRNA-141 at functional level using luciferase assay. We also reported that transfection of miRNA-141 to BMSCs inhibit osteogenesis differentiation by targeting SDF1 (Sudharsan Periyasamy-Thandavan et al., 2018). Previously, it has been shown by our group that SDF1 is an integral part of osteogenic differentiation of BMSCs (Herberg et al., 2013). Additionally, Cao et al revealed the importance of these miRNAs in the differentiation of adult epithelial stem cells to ameloblasts, which generate tooth enamel (Cao et al., 2013). Similarly, Itoh et al also reported miRNA’s 141 and 200a ability to affect pre-osteoblast MC3T3-E1 cells differentiation by targeting distal-less homeobox 5 (Dlx5) (Itoh et al., 2009). The same group discovered that BMP2 elevate the expression of RUNX2, osteorix (Osx) and Dlx5 and down-regulate the miRNA-141 and 200a expression. RUNX2, osteorix (Osx) and Dlx5 are all transcription factors that push stem cells towards osteogenic differentiation (Farshdousti Hagh et al., 2015). These studies suggest that miRNA-141 and 200a play central roles in BMP2 mediated differentiation of mouse pre-osteoblasts in part by targeting Dlx5 (Fig.2).
MiRNA-141 was also determined to regulate the expression level of Intercellular Adhesion Molecule 1 (ICAM-1), also known as CD54. Liu et al performed a study on endothelial cells revealing that high levels of miRNA-141 inhibited ICAM-1 expression and diminished leukocyte adhesion to endothelial cells (R. R. Liu et al., 2015). Furthermore, Xu et al showed that ICAM-1 expression was negatively correlated with osteogenesis but enhanced BMSC proliferation. They further revealed that by inhibiting the extracellular signal-regulated kinase/mitogen activated protein kinase (ERK/MAPK) and nuclear factor kappa-light chain-enhancer of activated B cells (NF-kB) resulted in partial rescue of osteogenic activity. They also showed inhibition of p38/MAPK and PI3K/AKT pathway caused a worsening of osteogenic suppression. This creates an interesting balance between miRNA 141 mediated inhibition of osteogenesis via the other mechanisms discussed in this article vs. the targeting of ICAM-1, which could cause osteogenic suppression (F. F. Xu et al., 2014).
Wnt/β-catenin signaling plays important roles in musculoskeletal development and stem cell engineering (Ahmadzadeh, Norozi, Shahrabi, Shahjahani, & Saki, 2016; Majidinia, Aghazadeh, Jahanban-Esfahlani, & Yousefi, 2018). Wnt/β-catenin signaling pathways is known to affect BMSC differentiation, specifically towards osteogenesis. Qui et al reported that miRNA-141 targets Wnt and cell division cycle 25A (CDC25A) genes and acts as a negative regulator of human MSC proliferation and osteoblast differentiation (Qiu & Kassem, 2014). CDC25A a gene required for the progression from G1 to the S phase of the cell cycle. Qui et al 2014 also reported that knocking down CDC25A inhibits osteogenesis by reducing expression of RUNX2 and COL1A1 without affecting ALP. Interestingly, a study by Dimri et al demonstrated that miRNA-141 also targets BMI1 gene expression and regulated the Wnt signaling cascade. This suggests that miRNA-141 regulates Wnt by directly targeting multiple genes in the Wnt/ β-catenin signaling pathway (Dimri et al., 2013).
Zhang et al (2015) reported that pregnancy-associated plasma protein A (PAPP-A), is a target of miRNA 141 (Y. Zhang et al., 2015). In a separate study performed by Clifton and Conover group (2015) showed the anabolic effects PAPP-A in parathyroid hormone dependent bone formation in a mouse model (Clifton & Conover, 2015). Due to the ability of miRNA’s to target expression of multiple genes, it’s possible that there are a number of other genes in these key pathways, apart from the above mentioned, that could contribute to the effects of miRNA 141 on osteogenic differentiation.
4. Role of miRNA-141 in aging and the aging musculoskeletal phenotype
Advancing age is one of the largest risk factors for musculoskeletal degeneration such as osteoporosis, and osteoarthritis, however the mechanisms by which the degeneration occurs are still mostly unknown. In females, post menopause estrogen deficiency is one of the important contributing factor for musculoskeletal related pathophysiology (Sapir-Koren et al., 2017). With aging, bone marrow stem cells (progenitor cells of bone) lose the capacity to proliferate and differentiate to osteogenic and chondrogenic lineage, and undergo premature senescence (Yang et al, 2018, Zhang et al, 2008). Cellular chronic stress such as inflammation and oxidative stress activates tumor suppressor pathways to initiate senescence (Khosla et al., 2018). The senescence cells secrete various extracellular cytokines and proteases also called as senescence-associated secretory phenotype (SASP) which affect normal cellular health (Khosla et al., 2018). There are evidence that miRNAs are differentially expressed in mesenchymal stem cells (MSCs) with aging and senescence (Caravia & Lopez-Otin, 2015, Yu et al., 2013). Yu et al (2013) reported that miRNA-141 was up-regulated in human MSC’s during senescence (Yu et al., 2013). Moreover they reported that miRNA-141 directly targets ZMPSTE24 and dysregulates its expression. ZMPSTE24 is a zinc metalloproteinase involved in the post-translational proteolytic cleavage of carboxy terminal residues of prelamin A (Pacheco et al., 2014; Yu et al., 2013, Bergo et al, 2002). Studies performed by Yu et al demonstrated that elevated level of miR-141 down-regulate ZMPSTE24 expression which reduces post-translational proteolytic cleavage of prelamin A. Excessive accumulation of prelamin A in human MSC’s resulted in accelerated cellular senescence and cellular dysfunction (Pacheco et al., 2014; Yu et al., 2013).
Importantly, ZMPSTE24 −/− knockout mice showed phenotypic changes such as abnormal gait, muscle weakness, kyphosis, alopecia, and spontaneous bone fractures (Bergo et al., 2002). Of particular note, the fractures healed poorly and were infiltrated with fibrous tissue. Additionally ALP activity, an early osteogenic marker, was decreased, however calcium levels, osteoclast number and urine deoxypyridinoline, a bone breakdown product, remained normal compared to the wild type. This suggests that ZMPSTE24 not only plays a role in cellular aging, but it also affects bone formation. Furthermore, there are reports that ZMPSTE24 alter metabolic activity, autophagy process and dysregulate mitochondrial functions in differentiated cells (Peinado et al, 2011, Sieprath et al, 2015, Marino, et al 2008). As a target of miRNA-141, it suggests one of the potential mechanisms or pathways through which miRNA-141 may play a role in musculoskeletal aging and dysfunction. This also supports the need for further studies to determine how the miRNA-200 family affects osteogenesis through ZMPSTE24 in combination with other mechanisms.
A study performed by Dimri et al has shown that miRNA-141 and miRNA-200c can also play a role in cellular senescence by targeting one of the polycomb group proteins, BMI1 (Dimri, Carroll, Cho, & Dimri, 2013). It is known that BMI1 is expressed in several types of human cancer, but it also plays roles in self-renewal of stem cells and cellular senescence with aging. Dimri et al reported that overexpression of miRNA-141 slowed cellular proliferation in human diploid fibroblast cells through BMI1 down-regulation. This study, along with others, shows several mechanisms by which the microRNA 200 family could exacerbate age related pathophysiological changes in the musculoskeletal system. Therapy could perhaps be directed towards anti-miRNA 141 agents to restore BMI1 expression and stem cell function in age related musculoskeletal problems.
5. MiRNA-141 regulates age-related stimuli (Inflammatory and Oxidative stress)
It is well know that elevated level of oxidative stress in musculoskeletal cells (e.g. bone, muscle, and chondrocytes) leads to increase cell apoptosis and senescence of progenitor cells and mature cells that causes degeneration and loss of tissues (Domazetovic et al 2017, Siu et al 2009, Davalli et la 2016, Meng et al 2010, Yang et al 2014). The miRNA family also appears to play a role in the downstream effects of reactive oxygen species (ROS) that result in senescence and apoptosis. Magenta et al (2011) showed that exposure of human umbilical vein endothelial cells (HUVECs) to oxidative stress (H2O2) elevated all of the miRNAs in the miR-200 family. Specifically, miR-141 and miR-200c were highly up-regulated in presence of H2O2 compared to miR-200a, miR-200b and miR-429. Magenta et al (2011) also reported that overexpression of miR-200c or H2O2 exposure induced HUVEC growth arrest, apoptosis and senescence. Their study revealed partial rescue of these cells through anti-miRNA 200c treatment (Magenta et al., 2011). They proposed that the zinc finger E-box binding homeobox 1 (ZEB1) mRNA, a transcription factor that is a target of miRNA 200c, was the mechanism by which miRNA’s played a role in ROS-induced senescence.
In addition, Shi et al (2016) revealed that miRNA-200a activates the NF-κB signaling pathway through targeting IκBα, which is a negative regulator of NF-kB. Their particular study was limited to nasopharyngeal carcinoma cells (Shi et al., 2016). It has been previously reported that NF-kB transcription activity was elevated in a number of tissues with aging and that inhibition of NF-kB leads to a delayed age-related pathogenesis {Tilstra et al, 2012 }. In addition, Lam et al showed miRNA-141 targeting TGF-β2, a molecule that can act as both an immunosuppressant and pro-inflammatory messenger (Lam et al., 2013).
MiRNA-429, another member of the miRNA 200 family, has been shown to regulate cell death and apoptosis in human osteoblastic cell lines through targeting the catalytic subunit of protein phosphatase 2A (PP2A) (Guo, Chen, Ji, Mao, & Xie, 2017). Guo et al (2017) also showed that miRNA-429 targets PP2A and indirectly activates AMP-activated protein kinase (AMPK) thereby protecting human osteoblastic cells from dexamethasone dependent oxidative stress. Previously, Jeyabalan et al (2012) showed AMPK also playing a pivotal role in bone metabolism through regulation of the PI3K/AKT/mTOR pathway, which also plays a role in osteogenic differentiation (Jeyabalan, Shah, Viollet, & Chenu, 2012). Additionally, Li et al (2017) demonstrated that miR-429 inhibition promotes fibroblast apoptosis and prevent epidural fibrosis in rat models by targeting RhoE (Li et al., 2017). This is significant because the lack of normal fibroblast function would be detrimental to hematoma and callous formation in secondary fracture healing and development of heterotopic ossification.
Recently, Ji et al (2015) reported that elevated expression of miRNA-141 in obese mice leads to mitochondrial dysfunction. They discovered that phosphatase and tensin homolog (PTEN) was the target of mitochondrial related miRNA 141 that resulted in mitochondrial dysfunction (Ji et al., 2015). Of note, Bluml et al also discovered the loss of PTEN led to enhanced receptor activation of NF-κB ligand (RANKL) in mice myeloid cells (Bluml et al., 2015). RANKL is a key factor driving the differentiation of monocytes into osteoclasts. PTEN also acts to inhibit the PI3-Kinase pathway, so it would be reasonable to hypothesize that miRNA 141 plays a role in osteoporosis by targeting PTEN, up-regulating the PI3-kianse pathway and leading to enhanced osteoclastogenesis. This is a pathway further linking the miRNA-200 family as an important mediator of age-related mechanisms that lead to musculoskeletal tissue degeneration. Further studies would be necessary to completely understand the miRNA-200 family’s relationship with inflammation, oxidative stress senescence and apoptosis in aging models.
6. Role of miRNA 200 family in muscle biology
Musculoskeletal health not only depends on maintaining bone homeostasis but also on muscle homeostasis. Reduced muscle mass (sarcopenia) and poor bone quality (osteoporosis) together increase the incidence of falls and bone fractures (Scott et al 2018, Tarantino et al., 2015, Cederholm et al., 2013). MicroRNAs are known to involve in skeletal muscle development, skeletal muscle cell proliferation, differentiation, and age-related pathophysiology (Jung et al., 2018). Recently, D’Agostino et al (2018) and his group analyzed expression of miR-200c expression in mdx mice muscles and in differentiated human myoblasts of Duchenne muscular dystrophy. They found elevated level of miR-200c expression in both mice and human (D’Agostino et al., 2018). Additionally, they showed that overexpression of miR-200c in culture myoblast decrease skeletal muscle differentiation and anti-miR-200c treatment ameliorates myogenic differentiation. D’Agostino et al (2018) also reported that miR-200c overexpression induces p66Shc phosphorylation in Ser-36 and affect myogenic differentiation (D’Agostino et al, 2018). There is a strong possibility that the p66Shc gene and miR-200c interaction may play a role in physiologic muscle wasting as well.
Zhou et al (2017) performed a study demonstrating the importance of the miR-141/TGF-β1 axis in myocardial fibrosis of diabetic mice models (Zhou & Yu, 2017). They demonstrated that TGF-β1 is the target of miRNA-141 and plays vital role in myocardial fibrosis (Zhou & Yu, 2017). TGF-β1 is the important cytokine involved in satellite cell activation and muscle repair (Delaney et al., 2017). MicroRNA 200b, another member of the family, also appears to have a relationship with cardiac myocytes. Yao et al performed a study revealing GATA-4 is a target of 200b (Yao et al., 2013). GATA-4 has been found to play a role in several developmental processes of the heart, including myocyte proliferation, differentiation, and survival. It is reasonable to think there may be some amount of crossover in the relationship with these miRNA and age-related muscle pathophysiology.
Fuzimaki et al (2014) demonstrated that Wnts play a critical role in exercise dependent myogenesis (Fuzimaki et al., 2014). MiR-141 is known for targeting Wnt signaling and affects human MSC proliferation and osteoblast differentiation (Qiu & Kassem., 2014). It may play an additional role in sarcopenia through this same pathway. Wnt/B-catenin pathway has been shown to modulate the conversion of satellite cells to the activated state, which would assist in muscle formation and regeneration (Fuzimaki et al., 2014). As mention above, ZMPSTE24 plays an important role in the aging phenotype and a target of miR-141. Song et al (2013) studies on the ZMPSTE24 knockout mice showed limited muscle regeneration, accelerating typical changes associated with aging, including muscle wasting (Song et al., 2013). Kowalski et al (2016) reported that SDF-1 lone and in combination granulocyte-colony stimulating factor treatment stimulate regeneration of skeletal muscle. Our laboratory recently demonstrated that MiR-141 target 3’ UTR of SDF1 and prevent its expression in both human and murine cells and affect osteogenic differentiation (Sudharsan Periyasamy-Thandavan et al., 2018). It appears that SDF1 and miR-141 might plays role in muscle loss during aging. It will be interesting to investigate miR-141 role in muscle biology. As per our knowledge, no studies have specifically been performed directly elucidating the relationship between muscle loss and the miRNA 200 family. Future detail studies are needed to identify the cross-talk between miRNA-200 family members and age-related muscle pathophysiology.
7. In silico predication of miRNA-141 targets genes of musculoskeletal importance
A single miRNA can target a number of genes for degradation. For practical reasons it isn’t possible to list every target, but bionfirmatics analysis can predict which genes could be potential targets based on the degree of complementation between the 3’ UTR of the mRNA and the 5’seed sequence of the miRNA. We used TargetScan bioinformatics tool to predict the targets of importance of stem cell biology, osteogenic, chondrogenic, and myogenic genes in silico (Table 2). HOXC13, HOXA11, and HOXB5 are all 3 genes that are particularly intriguing targets of miRNA 141 due to their potential function in stem cell differentiation. Future studies should be directed toward some of these target genes to see how their degradation could modulate stem cell function. MiRNA 141 can also affect osteoblast/osteocyte and osteoclast functions via a number of targets, which include SPOCK2 and calcitonin receptor (CALCR). SPOCK2 is a gene that encodes a protein with calcium and glycosaminoglycan binding domains to form part of the extracellular matrix. CALCR modulates the physiological effect of calcitonin, which normally lowers serum Ca2+ levels through a number of mechanisms, which include inhibition of osteoclasts. MiRNA-141 also potentially targets a number of important chondrogenic genes such as RUNX1 and HS2ST1 (LeBlanc et al., 2015). RUNX1 is a transcription factor expressed by chondrocytes and BMSC’s differentiation towards chondrogenesis, particularly during cartilage repair, while HS2ST1 codes for a heparin sulfate biosynthetic enzyme, which may play a role in chondrogenic differentiation (J. Wang et al., 2013; Zhao, Wang, Chen, & Chen, 2015). Further research on these genes could reveal an additional role by which miRNA 141 could contribute to osteoarthritis and osteoporosis. Finally, miRNA-141 also target genes of myogenic importance such as, phosphofructokinase (PFKN), a molecule which regulates and takes part in glycolysis in skeletal muscle cells, and ATP2A2, a gene responsible for coding striated muscle specific Ca2+ ATPase (Wills & Mansour, 1990; Toth et al., 2015). Details of the targets of this miRNA are found in Table 2.
Table 2:
In silico prediction of miRNA-141 targets genes of musculoskeletal importance
| Stem cells: | HOXC13 | HOXA11 | HOXB5 | WNT5A | SOX5 | SOX6 | SOX11 | SOX17 | GDF6 | SYNPO2 |
| Osteogenic: | SPOCK2 | STAT5A | STAT5B | FGF13 | ZDHHC7 | ATF2 | CALCR | FKBP5 | CSF3 | CAMSAP1 |
| Chondrogenic: | FGFR1OP | TNIK | EGR2 | SERPINC1 | HDAC4 | RUNX1 | HS2ST1 | DSEL | PAPLN | CHRDL1 |
| Myogenic: | MTPN | PFKM | ATP2A2 | MBNL1 | MBNL3 | CFL2 | TCF23 | PAX3 | CDK13 | CACNA2D1 |
8. Conclusion
MiRNA-141 and 200a have shown some promise as mediators/effectors of normal and abnormal musculoskeletal biology and, therefore, merit further research along with the rest of the miRNA 200 family. While there has been extensive research on its role in cancer, its role in musculoskeletal pathophysiology is limited. The mechanisms summarized in this article suggest that this miRNA could have a significant negative impact on bone, cartilage and muscle health (Fig.2). Future studies need to identify in vivo regulation of musculoskeletal metabolism to further identify the miRNA-200 family’s relationship with these disease states. Antagonists of these molecules could potentially be developed as therapeutic agents to address age-related musculoskeletal diseases. There are still a number of other potential targets that have yet to be explored and may be of interest. These genes have been identified as potential targets of miRNA-141 according to the 5’seed sequence. These could even reveal possible therapeutic targets for prevention and treatment of these degenerative diseases.
Highlights.
MIRNA-141 plays a vital role in stem cell senescence.
MIRNA-141 negatively regulated stem cell differentiation.
Oxidative and inflammatory stress factors regulate MiRNA-141.
MiRNA-141 dysregulated with aging and the aging musculoskeletal phenotype.
Antagonists of miR-141 could potentially be therapeutic agents to address age-related musculoskeletal diseases.
Acknowledgements:
This publication is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development Program (VA Merit Award 1I01CX000930 01, WDH) and the National Institutes of Health (NIA-AG036675 SF, MH, CS, WDH). The contents of this publication do not represent the views of the Department of Veterans Affairs, or the United States Government. The above mentioned funding did not lead to any conflict of interests regarding the publication of this manuscript. The authors also declare that there is no other conflict of interest regarding the publication of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no conflicts of interest.
References:
- Ahmadzadeh A, Norozi F, Shahrabi S, Shahjahani M, & Saki N (2016). Wnt/beta-catenin signaling in bone marrow niche. Cell and Tissue Research, 363(2), 321–335. doi: 10.1007/s00441-015-2300-y [DOI] [PubMed] [Google Scholar]
- Bennett JA, Singh KP, Unnisa Z, Welle SL, & Gasiewicz TA (2015). Deficiency in Aryl Hydrocarbon Receptor (AHR) Expression throughout Aging Alters Gene Expression Profiles in Murine Long-Term Hematopoietic Stem Cells. PloS One, 10(7), e0133791. doi: 10.1371/journal.pone.0133791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergo MO, Gavino B, Ross J, Schmidt WK, Hong C, Kendall LV, … Young SG (2002). Zmpste24 deficiency in mice causes spontaneous bone fractures, muscle weakness, and a prelamin A processing defect. Proceedings of the National Academy of Sciences of the United States of America, 99(20), 13049–13054. doi: 10.1073/pnas.192460799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluml S, Friedrich M, Lohmeyer T, Sahin E, Saferding V, Brunner J, … Redlich K (2015). Loss of phosphatase and tensin homolog (PTEN) in myeloid cells controls inflammatory bone destruction by regulating the osteoclastogenic potential of myeloid cells. Annals of the Rheumatic Diseases, 74(1), 227–233. doi: 10.1136/annrheumdis-2013-203486 [DOI] [PubMed] [Google Scholar]
- Brown DM, & Goljanek-Whysall K (2015). microRNAs: Modulators of the underlying pathophysiology of sarcopenia? Ageing Res Rev, 24(Pt B), 263–273. doi: 10.1016/j.arr.2015.08.007 [DOI] [PubMed] [Google Scholar]
- Brunet A, & Berger SL (2014). Epigenetics of aging and aging-related disease. Journals of Gerontology. Series A: Biological Sciences and Medical Sciences, 69 Suppl 1, S17–20. doi: 10.1093/gerona/glu042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Jheon A, Li X, Sun Z, Wang J, Florez S, … Amendt BA (2013). The Pitx2:miR-200c/141:noggin pathway regulates Bmp signaling and ameloblast differentiation. Development, 140(16), 3348–3359. doi: 10.1242/dev.089193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravia XM, & Lopez-Otin C (2015). Regulatory Roles of miRNAs in Aging. Advances in Experimental Medicine and Biology, 887, 213–230. doi: 10.1007/978-3-319-22380-3_11 [DOI] [PubMed] [Google Scholar]
- Cederholm T, Cruz-Jentoft AJ, Maggi S. Sarcopenia and fragility fractures. Eur J Phys Rehabil Med. 2013. February;49(1):111–7. Review. [PubMed] [Google Scholar]
- Chen Y, & Zhang L (2017). Members of the microRNA-200 family are promising therapeutic targets in cancer. Experimental and Therapeutic Medicine, 14(1), 10–17. doi: 10.3892/etm.2017.4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton KB, & Conover CA (2015). Pregnancy-associated plasma protein-A modulates the anabolic effects of parathyroid hormone in mouse bone. Bone, 81, 413–416. doi: 10.1016/j.bone.2015.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D, & Genever P (2013). Regulation of mesenchymal stem cell differentiation. Advances in Experimental Medicine and Biology, 786, 213–229. doi: 10.1007/978-94-007-6621-1_12 [DOI] [PubMed] [Google Scholar]
- Coolen MW, Statham AL, Qu W, Campbell MJ, Henders AK, Montgomery GW, … Clark SJ (2011). Impact of the genome on the epigenome is manifested in DNA methylation patterns of imprinted regions in monozygotic and dizygotic twins. PloS One, 6(10), e25590. doi: 10.1371/journal.pone.0025590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino M, Torcinaro A, Madaro L, Marchetti L, Sileno S, Beji S, Salis C, Proietti D, Imeneo G, C Capogrossi M, De Santa F, Magenta A. Role of miR-200c in Myogenic Differentiation Impairment via p66Shc: Implication in Skeletal Muscle Regeneration of Dystrophic mdx Mice. Oxid Med Cell Longev. 2018. February 13;2018:4814696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalli P, Mitic T, Caporali A, Lauriola A, D’Arca D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid Med Cell Longev. 2016;2016:3565127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A, & Dennison E (2016). Measuring the musculoskeletal aging phenotype. Maturitas, 93, 13–17. doi: 10.1016/j.maturitas.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney K, Kasprzycka P, Ciemerych MA, Zimowska M. The role of TGF-β1 during skeletal muscle regeneration. Cell Biol Int. 2017. July;41(7):706–715. doi: 10.1002/cbin.10725. Epub 2017 Jan 19. Review. [DOI] [PubMed] [Google Scholar]
- Domazetovic V, Marcucci G, Iantomasi T, Brandi ML, Vincenzini MT. Oxidative stress in bone remodeling: role of antioxidants. Clin Cases Miner Bone Metab. 2017. May-Aug;14(2):209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri M, Carroll JD, Cho JH, & Dimri GP (2013). microRNA-141 regulates BMI1 expression and induces senescence in human diploid fibroblasts. Cell Cycle, 12(22), 3537–3546. doi: 10.4161/cc.26592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farshdousti Hagh M, Noruzinia M, Mortazavi Y, Soleimani M, Kaviani S, Abroun S, … Mahmoodinia M (2015). Different Methylation Patterns of RUNX2, OSX, DLX5 and BSP in Osteoblastic Differentiation of Mesenchymal Stem Cells. Cell J, 17(1), 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipowicz W, Jaskiewicz L, Kolb FA, & Pillai RS (2005). Post-transcriptional gene silencing by siRNAs and miRNAs. Current Opinion in Structural Biology, 15(3), 331–341. doi: 10.1016/j.sbi.2005.05.006 [DOI] [PubMed] [Google Scholar]
- Fujimaki S, Hidaka R, Asashima M, Takemasa T, Kuwabara T. Wnt protein-mediated satellite cell conversion in adult and aged mice following voluntary wheel running. J Biol Chem. 2014. March 14;289(11):7399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulzele S, Chothe P, Sangani R, Chutkan N, Hamrick M, Bhattacharyya M, … Ganapathy V (2013). Sodium-dependent vitamin C transporter SVCT2: expression and function in bone marrow stromal cells and in osteogenesis. Stem Cell Res, 10(1), 36–47. doi: 10.1016/j.scr.2012.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fushan AA, Turanov AA, Lee SG, Kim EB, Lobanov AV, Yim SH, … Gladyshev VN (2015). Gene expression defines natural changes in mammalian lifespan. Aging Cell, 14(3), 352–365. doi: 10.1111/acel.12283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebennikova TA, Belaya Zh E, Rozhinskaya LY, Mel’nichenko GA, & Dedov II. (2015). [Epigenetic Aspects of Osteoporosis]. Vestn Ross Akad Med Nauk(5), 541–548. [DOI] [PubMed] [Google Scholar]
- Guo S, Chen C, Ji F, Mao L, & Xie Y (2017). PP2A catalytic subunit silence by microRNA-429 activates AMPK and protects osteoblastic cells from dexamethasone. Biochemical and Biophysical Research Communications, 487(3), 660–665. doi: 10.1016/j.bbrc.2017.04.111 [DOI] [PubMed] [Google Scholar]
- Hammond SM (2015). An overview of microRNAs. Adv Drug Deliv Rev, 87, 3–14. doi: 10.1016/j.addr.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Tong J, He F, Yu X, Fan L, Hu J, … Chen Z (2015). miR-141 regulates TGF-beta1-induced epithelial-mesenchymal transition through repression of HIPK2 expression in renal tubular epithelial cells. International Journal of Molecular Medicine, 35(2), 311–318. doi: 10.3892/ijmm.2014.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Shi T, Zhou Q, Shi S, Zhao R, Shi H, … Zhang J (2014). miR-141 Regulates colonic leukocytic trafficking by targeting CXCL12beta during murine colitis and human Crohn’s disease. Gut, 63(8), 1247–1257. doi: 10.1136/gutjnl-2012-304213 [DOI] [PubMed] [Google Scholar]
- Hwang HD, Lee JT, Koh JT, Jung HM, Lee HJ, & Kwon TG (2015). Sequential Treatment with SDF-1 and BMP-2 Potentiates Bone Formation in Calvarial Defects. Tissue Eng Part A, 21(13–14), 2125–2135. doi: 10.1089/ten.TEA.2014.0571 [DOI] [PubMed] [Google Scholar]
- Itoh T, Nozawa Y, & Akao Y (2009). MicroRNA-141 and −200a are involved in bone morphogenetic protein-2-induced mouse pre-osteoblast differentiation by targeting distal-less homeobox 5. Journal of Biological Chemistry, 284(29), 19272–19279. doi: 10.1074/jbc.M109.014001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Shepard DS, Katzmarzyk PT, & Roubenoff R (2004). The healthcare costs of sarcopenia in the United States. Journal of the American Geriatrics Society, 52(1), 80–85. [DOI] [PubMed] [Google Scholar]
- Jeyabalan J, Shah M, Viollet B, & Chenu C (2012). AMP-activated protein kinase pathway and bone metabolism. Journal of Endocrinology, 212(3), 277–290. doi: 10.1530/joe-11-0306 [DOI] [PubMed] [Google Scholar]
- Ji J, Qin Y, Ren J, Lu C, Wang R, Dai X, … Wang X (2015). Mitochondria-related miR-141–3p contributes to mitochondrial dysfunction in HFD-induced obesity by inhibiting PTEN. Scientific Reports, 5, 16262. doi: 10.1038/srep16262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HJ, Lee KP, Kwon KS, Suh Y. MicroRNAs in skeletal muscle aging: Current issues and perspectives. J Gerontol A Biol Sci Med Sci. 2018. September 12. doi: 10.1093/gerona/gly207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y, Ii M, Matsumoto T, Kuroda R, Kuroda T, Kwon SM, … Asahara T (2015). SDF-1/CXCR4 axis in Tie2-lineage cells including endothelial progenitor cells contributes to bone fracture healing. Journal of Bone and Mineral Research, 30(1), 95–105. doi: 10.1002/jbmr.2318 [DOI] [PubMed] [Google Scholar]
- Khosla S, Farr JN, Kirkland JL. Inhibiting Cellular Senescence: A New Therapeutic Paradigm for Age-Related Osteoporosis. J Clin Endocrinol Metab. 2018. April 1;103(4):1282–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Kang D, Cho Y, & Kim JH (2015). Epigenetic Regulation of Chondrocyte Catabolism and Anabolism in Osteoarthritis. Molecules and Cells, 38(8), 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski K, Archacki R, Archacka K, Stremińska W, Paciorek A, Gołąbek M, Ciemerych MA, Brzoska E. Stromal derived factor-1 and granulocyte-colony stimulating factor treatment improves regeneration of Pax7−/− mice skeletal muscles. J Cachexia Sarcopenia Muscle. 2016. September;7(4):483–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam WY, Yeung AC, Ngai KL, Li MS, To KF, Tsui SK, & Chan PK (2013). Effect of avian influenza A H5N1 infection on the expression of microRNA-141 in human respiratory epithelial cells. BMC Microbiology, 13, 104. doi: 10.1186/1471-2180-13-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc KT, Walcott ME, Gaur T, O’Connell SL, Basil K, Tadiri CP, … Fanning PJ (2015). Runx1 Activities in Superficial Zone Chondrocytes, Osteoarthritic Chondrocyte Clones and Response to Mechanical Loading. Journal of Cellular Physiology, 230(2), 440–448. doi: 10.1002/jcp.24727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveille SG (2004). Musculoskeletal aging. Current Opinion in Rheumatology, 16(2), 114–118. [DOI] [PubMed] [Google Scholar]
- Li X, Chen H, Wang S, Dai J, Yan L, Wang J, & Sun Y (2017). Tacrolimus induces fibroblasts apoptosis and reduces epidural fibrosis by regulating miR-429 and its target of RhoE. Biochemical and Biophysical Research Communications, 490(4), 1197–1204. doi: 10.1016/j.bbrc.2017.06.181 [DOI] [PubMed] [Google Scholar]
- Liu RR, Li J, Gong JY, Kuang F, Liu JY, Zhang YS, … Chen LH (2015). MicroRNA-141 regulates the expression level of ICAM-1 on endothelium to decrease myocardial ischemiareperfusion injury. American Journal of Physiology: Heart and Circulatory Physiology, 309(8), H1303–1313. doi: 10.1152/ajpheart.00290.2015 [DOI] [PubMed] [Google Scholar]
- Liu X, Zhou C, Li Y, Ji Y, Xu G, Wang X, & Yan J (2013). SDF-1 promotes endochondral bone repair during fracture healing at the traumatic brain injury condition. PloS One, 8(1), e54077. doi: 10.1371/journal.pone.0054077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magenta A, Cencioni C, Fasanaro P, Zaccagnini G, Greco S, Sarra-Ferraris G, … Capogrossi MC (2011). miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death and Differentiation, 18(10), 1628–1639. doi: 10.1038/cdd.2011.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majidinia M, Aghazadeh J, Jahanban-Esfahlani R, & Yousefi B (2018). The roles of Wnt/beta-catenin pathway in tissue development and regenerative medicine. Journal of Cellular Physiology, 233(8), 5598–5612. doi: 10.1002/jcp.26265 [DOI] [PubMed] [Google Scholar]
- Mariño G, Ugalde AP, Salvador-Montoliu N, Varela I, Quirós PM, Cadiñanos J, van der Pluijm I, Freije JM, López-Otín C. Premature aging in mice activates a systemic metabolic response involving autophagy induction. Hum Mol Genet. 2008. July 15;17(14):2196–211. [DOI] [PubMed] [Google Scholar]
- Meng SJ, Yu LJ. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci. 2010. April 12;11(4):1509–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy L, & Helmick CG (2012). The impact of osteoarthritis in the United States: a population-health perspective: A population-based review of the fourth most common cause of hospitalization in U.S. adults. Orthopaedic Nursing, 31(2), 85–91. doi: 10.1097/NOR.0b013e31824fcd42 [DOI] [PubMed] [Google Scholar]
- Pacheco LM, Gomez LA, Dias J, Ziebarth NM, Howard GA, & Schiller PC (2014). Progerin expression disrupts critical adult stem cell functions involved in tissue repair. Aging (Albany NY), 6(12), 1049–1063. doi: 10.18632/aging.100709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado JR, Quirós PM, Pulido MR, Mariño G, Martínez-Chantar ML, Vázquez-Martínez R, Freije JM, López-Otín C, Malagón MM. Proteomic profiling of adipose tissue from Zmpste24−/− mice, a model of lipodystrophy and premature aging, reveals major changes in mitochondrial function and vimentin processing. Mol Cell Proteomics. 2011. November;10(11):M111.008094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi C, Li YP, Zhou X, & Gao B (2015). The expression and function of microRNAs in bone homeostasis. Front Biosci (Landmark Ed), 20, 119–138. [DOI] [PubMed] [Google Scholar]
- Qiu W, & Kassem M (2014). miR-141–3p inhibits human stromal (mesenchymal) stem cell proliferation and differentiation. Biochimica et Biophysica Acta, 1843(9), 2114–2121. doi: 10.1016/j.bbamcr.2014.06.004 [DOI] [PubMed] [Google Scholar]
- Ryan MM, Guevremont D, Luxmanan C, Abraham WC, & Williams JM (2015). Aging alters long-term potentiation--related gene networks and impairs synaptic protein synthesis in the rat hippocampus. Neurobiology of Aging, 36(5), 1868–1880. doi: 10.1016/j.neurobiolaging.2015.01.012 [DOI] [PubMed] [Google Scholar]
- Sangani R, Pandya CD, Bhattacharyya MH, Periyasamy-Thandavan S, Chutkan N, Markand S, … Fulzele S (2014). Knockdown of SVCT2 impairs in-vitro cell attachment, migration and wound healing in bone marrow stromal cells. Stem Cell Res, 12(2), 354–363. doi: 10.1016/j.scr.2013.11.002 [DOI] [PubMed] [Google Scholar]
- Sangani R, Periyasamy-Thandavan S, Kolhe R, Bhattacharyya MH, Chutkan N, Hunter M, … Fulzele S (2015). MicroRNAs-141 and 200a regulate the SVCT2 transporter in bone marrow stromal cells. Molecular and Cellular Endocrinology, 410, 19–26. doi: 10.1016/j.mce.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir-Koren R, Livshits G. Postmenopausal osteoporosis in rheumatoid arthritis: The estrogen deficiency-immune mechanisms link. Bone. 2017. October;103:102–115. doi: 10.1016/j.bone.2017.06.020. [DOI] [PubMed] [Google Scholar]
- Scott D, Seibel M, Cumming R, Naganathan V, Blyth F, Le Couteur DG, Handelsman DJ, Waite LM, Hirani V. Does combined osteopenia/osteoporosis and sarcopenia confer greater risk of falls and fracture than either condition alone in older men? The Concord Health and Ageing in Men Project. J Gerontol A Biol Sci Med Sci. 2018. July 18. doi: 10.1093/gerona/gly162. [DOI] [PubMed] [Google Scholar]
- Shi Z, Hu Z, Chen D, Huang J, Fan J, Zhou S, … Huang F (2016). MicroRNA-200a mediates nasopharyngeal carcinoma cell proliferation through the activation of nuclear factor-kappaB. Mol Med Rep, 13(2), 1732–1738. doi: 10.3892/mmr.2015.4738 [DOI] [PubMed] [Google Scholar]
- Sieprath T, Corne TD, Nooteboom M, Grootaert C, Rajkovic A, Buysschaert B, Robijns J, Broers JL, Ramaekers FC, Koopman WJ, Willems PH, De Vos WH. Sustained accumulation of prelamin A and depletion of lamin A/C both cause oxidative stress and mitochondrial dysfunction but induce different cell fates. Nucleus. 2015;6(3):236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu PM, Wang Y, Alway SE. Apoptotic signaling induced by H2O2-mediated oxidative stress in differentiated C2C12 myotubes. Life Sci. 2009. March 27;84(13–14):468–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Lavasani M, Thompson SD, Lu A, Ahani B, Huard J. Muscle-derived stem/progenitor cell dysfunction in Zmpste24-deficient progeroid mice limits muscle regeneration. Stem Cell Res Ther. 2013. March 25;4(2):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozen T, Ozisik L, & Basaran NC (2017). An overview and management of osteoporosis. Eur J Rheumatol, 4(1), 46–56. doi: 10.5152/eurjrheum.2016.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periyasamy-Thandavan Sudharsan, Burke John, Mendhe Bharati, Kondrikova Galina, Kolhe Ravindra, Hunter Monte, Isales Carlos M, Hamrick Mark W, Hill William D, Fulzele Sadanand; MicroRNA-141–3p Negatively Modulates SDF-1 Expression in Age-Dependent Pathophysiology of Human and Murine Bone Marrow Stromal Cells, The Journals of Gerontology: Series A, , gly186, 10.1093/gerona/gly186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantino U, Piccirilli E, Fantini M, Baldi J, Gasbarra E, Bei R. Sarcopenia and fragility fractures: molecular and clinical evidence of the bone-muscle interaction. J Bone Joint Surg Am. 2015. March 4;97(5):429–37. doi: 10.2106/JBJS.N.00648. Review. [DOI] [PubMed] [Google Scholar]
- Wahid F, Shehzad A, Khan T, & Kim YY (2010). MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochimica et Biophysica Acta, 1803(11), 1231–1243. doi: 10.1016/j.bbamcr.2010.06.013 [DOI] [PubMed] [Google Scholar]
- Wang J, Wang X, Holz JD, Rutkowski T, Wang Y, Zhu Z, & Dong Y (2013). Runx1 is critical for PTH-induced onset of mesenchymal progenitor cell chondrogenic differentiation. PloS One, 8(9), e74255. doi: 10.1371/journal.pone.0074255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SX, Ganguli AX, Bodhani A, Medema JK, Reichmann WM, & Macaulay D (2017). Healthcare resource utilization and costs by age and joint location among osteoarthritis patients in a privately insured population. Journal of Medical Economics, 1–27. doi: 10.1080/13696998.2017.1377717 [DOI] [PubMed] [Google Scholar]
- Weinstein RS. Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinol Metab Clin North Am. 2012. September;41(3):595–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Tian B, Qu X, Liu F, Tang T, Qin A, … Dai K (2014). MicroRNAs play a role in chondrogenesis and osteoarthritis (review). International Journal of Molecular Medicine, 34(1), 13–23. doi: 10.3892/ijmm.2014.1743 [DOI] [PubMed] [Google Scholar]
- Xu FF, Zhu H, Li XM, Yang F, Chen JD, Tang B, … Zhang Y (2014). Intercellular adhesion molecule-1 inhibits osteogenic differentiation of mesenchymal stem cells and impairs bio-scaffold-mediated bone regeneration in vivo. Tissue Eng Part A, 20(19–20), 2768–2782. doi: 10.1089/ten.TEA.2014.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Mei Q, Xiong C, & Zhao J (2014). Tumor-suppressing effects of miR-141 in human osteosarcoma. Cell Biochemistry and Biophysics, 69(2), 319–325. doi: 10.1007/s12013-013-9801-7 [DOI] [PubMed] [Google Scholar]
- Yang Y, Zheng X, Li B, Jiang S, Jiang L. Increased activity of osteocyte autophagy in ovariectomized rats and its correlation with oxidative stress status and bone loss. Biochem Biophys Res Commun. 2014. August 15;451(1):86–92. [DOI] [PubMed] [Google Scholar]
- Yang YK, Ogando CR, Wang See C, Chang TY, Barabino GA. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res Ther. 2018. May 11;9(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao CX, Wei QX, Zhang YY, Wang WP, Xue LX, Yang F, Zang MX (2013). miR-200b targets GATA-4 during cell growth and differentiation. RNA Biology, 10(4), 465–480. doi: 10.4161/rna.24370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu KR, Lee S, Jung JW, Hong IS, Kim HS, Seo Y, … Kang KS (2013). MicroRNA-141–3p plays a role in human mesenchymal stem cell aging by directly targeting ZMPSTE24. Journal of Cell Science, 126(Pt 23), 5422–5431. doi: 10.1242/jcs.133314 [DOI] [PubMed] [Google Scholar]
- Zhang B, Wang Q, & Pan X (2007). MicroRNAs and their regulatory roles in animals and plants. Journal of Cellular Physiology, 210(2), 279–289. doi: 10.1002/jcp.20869 [DOI] [PubMed] [Google Scholar]
- Zhang W, Ou G, Hamrick M, Hill W, Borke J, Wenger K, Chutkan N, Yu J, Mi QS, Isales CM, Shi XM. Age-related changes in the osteogenic differentiation potential of mouse bone marrow stromal cells. J Bone Miner Res. 2008. July;23(7):1118–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen B, Ming L, Qin H, Zheng L, Yue Z, … Ji B (2015). MicroRNA-141 inhibits vascular smooth muscle cell proliferation through targeting PAPP-A. International Journal of Clinical and Experimental Pathology, 8(11), 14401–14408. [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Wang Z, Chen J, & Chen J (2015). Preparation of heparan sulfate-like polysaccharide and application in stem cell chondrogenic differentiation. Carbohydrate Research, 401, 32–38. doi: 10.1016/j.carres.2014.10.019 [DOI] [PubMed] [Google Scholar]
- Zhou B, & Yu JW (2017). A novel identified circular RNA, circRNA_010567, promotes myocardial fibrosis via suppressing miR-141 by targeting TGF-beta1. Biochemical and Biophysical Research Communications, 487(4), 769–775. doi: 10.1016/j.bbrc.2017.04.044 [DOI] [PubMed] [Google Scholar]