Abstract
Background: Growing evidence suggests that pediatric palliative care (PPC) teams influence the care received by children and young adults with chronic, life-limiting illnesses. Little is known about how PPC involvement affects advance care planning (ACP) and circumstances of death in pediatric populations with a wide range of diagnoses.
Objective: To determine the relationship between PPC involvement, ACP, and circumstances of death for pediatric patients.
Design: A retrospective chart review of 558 pediatric patients who died between January 1, 2012 and December 31, 2016 was conducted. Descriptive statistics were used to characterize the sample. A multivariable logistic regression was used to obtain associations between PPC involvement and ACP.
Setting: Large, multidisciplinary tertiary care center in a rural state.
Measurements: Data abstracted for each patient included the following: demographic information, diagnosis, location of primary unit, hospice involvement, goals of care (GOC), code status, Physician Orders for Life-Sustaining Treatment (POLST) completion, and location of death.
Results: Patients with PPC involvement were more likely to have had ACP addressed before death. After adjusting for covariates in the model, patients with PPC were more likely to have their GOC documented (odds ratio [OR] = 96.93), completion of POLST (OR = 24.06), do-not-resuscitate code status (OR = 7.71), and hospice involvement at the time of death (OR = 11.70) compared with those who did not receive PPC.
Conclusions: Pediatric patients are more likely to have ACP addressed if they have PPC involvement. Patients with chronic complex conditions are most likely to receive palliative care.
Keywords: advance care planning, death location, pediatric palliative care, Physician Orders for Life-Sustaining Treatment
Introduction
Pediatric palliative care (PPC) strives to ease distress and pain associated with serious illnesses. For patients and their families, PPC services can include management of distressing symptoms; support for emotional, spiritual, and psychological needs; and advance care planning (ACP), including discussing goals of care (GOC).1,2 GOC discussions often include preferences regarding resuscitation, the degree of medical intervention desired, and the location of care delivery. Previous research has demonstrated that patients receiving palliative care often receive less aggressive treatment at end of life, which can reduce the burden of care that is no longer beneficial in achieving patient goals.3 For example, oncology patients who have PPC involvement may receive fewer cure-directed treatments such as high-dose chemotherapy during the last month of life, when cure is no longer possible.4
Although hospital-based PPC programs have increased over the past 15 years,5 in part because of the National Academy of Sciences report released in 2003,6 the majority of PPC studies have focused on specific populations. These studies suggest that PPC involvement leads to fewer aggressive, life-prolonging interventions at the end of life in patients with malignant solid tumors,2 stem cell transplants,7–9 or cardiac disease.10 Little is known about how PPC involvement affects the care received by children with other chronic or life-limiting conditions. PPC teams care for children of all ages with a wide variety of diagnoses and who receive treatment across different settings (e.g., intensive care unit, pediatric medical-surgical units and skilled nursing facilities, home).11 It is unknown if there are differences in the impact that PPC has across these varying pediatric populations and places of care. There have been descriptive studies of end-of-life care received by children with a variety of complex chronic conditions; however, these previous studies either have not addressed ACP or have done so in a more limited manner.12–14
The University of Iowa Stead Family Children's Hospital (UISFCH) is the largest children's hospital in Iowa, staffed with pediatric specialists who provide care for many children and young adults with complex chronic conditions. The PPC service at the UISFCH was instituted in 2009 and has had a hospice and palliative medicine board certified physician since 2012. The PPC team consisted of a physician, nurse practitioner, and nurse clinician, all of whom had certification in palliative care and collaborated with spiritual services, child life specialists, social services, and other providers in either the inpatient or outpatient setting. Because of the rural landscape of the state of Iowa, patients often live in remote areas that can be up to several hours of travel time away from a major medical center. These circumstances present unique challenges to the delivery of highly specialized health care, as transportation of medically fragile patients is difficult. This study was conducted to identify differences in ACP preferences and outcomes at the time of death between patients who received PPC consultation and those who did not, with an interest in rural disparities.
Methods
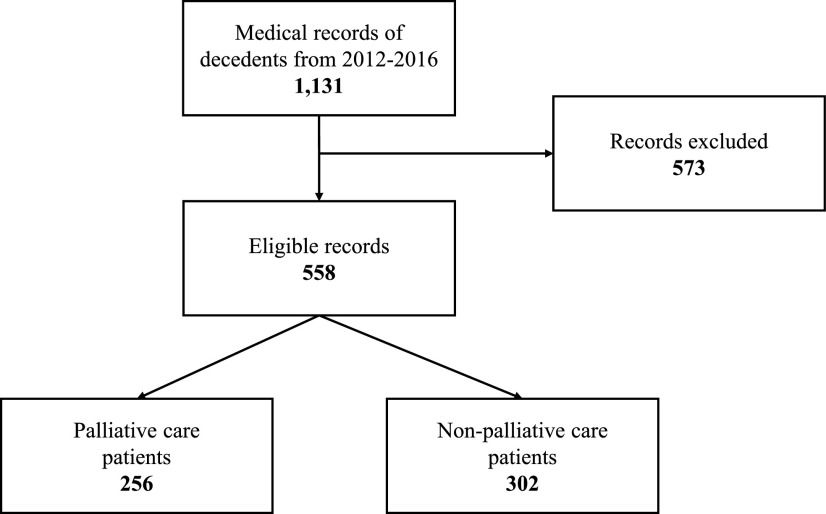
A retrospective, single-center study was performed using the electronic medical record to identify pediatric patients who died between January 1, 2012 and December 31, 2016. Patients who had a palliative care consult were identified using the International Classification of Diseases, Ninth or Tenth Revision codes and were considered to be cared for by PPC for the purposes of this study.15 Exclusion criteria included records of stillborn infants, terminated pregnancies, miscarriages, phone consultations, records of patients seen by only adult services, and external medical examiner cases. The initial sample of 1131 records was reduced to 558 after 573 patients were excluded based on the criteria outlined previously (Fig. 1). Data were abstracted by a member of the research team, including: primary diagnosis, date of birth, sex, race, zip code, religious preference documentation, location of primary unit for care, hospice involvement, documentation of GOC, code status, Physician Orders for Life-Sustaining Treatment (POLST) completion, and location of death. Institutional Review Board approval was obtained before beginning this study.
FIG. 1.
Flowchart of study sample.a aExclusion criteria included records of stillborn infants, terminated pregnancies, miscarriages, phone consultations, records of patients seen by only adult providers, and external medical examiner cases.
The patient's primary diagnosis that led to death was used to select the primary diagnosis category including acquired cardiac conditions, congenital heart disease, congenital malformation and genetic (including metabolic conditions), infection, malignancy, neurologic (including stroke, chronic severe neuroimpairment, muscular dystrophy), complications of prematurity, respiratory conditions, trauma (including accident and suicide), and other (including endocrine, gastrointestinal, genitourinary, hematologic, multiorgan system failure, renal, and unknown). The categories selected were derived from those used in previous studies.14,16 A physician member of the research team determined the primary diagnosis category using the aforementioned disease categories; if any questions arose, they were discussed with the other members of the team. Age was indicated by a categorical variable to capture differences across the pediatric lifespan based on developmental milestones and was divided into categories of 0–30 days, 31–364 days, 1–4 years, 5–9 years, 10–14 years, and 15 years of age and older. The patient's primary place of care was determined based on where the patient received the majority of their care, and was divided into six categories: inpatient (all nonintensive care inpatient medical-surgical units), outpatient, neonatal intensive care unit (NICU), pediatric intensive care unit (PICU), and other (included emergency department, labor and delivery, newborn nursery, home, or unknown). Documentation of ACP was derived from reviewing each patient's medical record for relevant information, including documentation of GOC, POLST completion, code status, hospice involvement, and location of death, and data were entered into a spreadsheet using a standardized method of data collection for each patient.
The patient's zip code was utilized to incorporate a measure of rurality using Rural Urban Commuting Area (RUCA) codes, which are zip code and Census tract-based classification method that is standardized using population density, daily commuting, and urbanization.17 RUCA codes classify areas as metropolitan (or urban), micropolitan (or large rural), small town (or small rural), and rural (or isolated).17
Statistical analysis
Analyses were conducted using Stata 15 software.18 Patient demographics, clinical characteristics, and GOC and ACP preferences were explained using descriptive statistics. To understand differences in demographic and clinical characteristics between patients who received PPC consultation and those who did not, Pearson's chi-square tests were performed. Multivariable logistic regression models were developed to examine associations using adjusted odds ratios (ORs) between the outcomes of interest with patient demographic and clinical characteristics, including palliative care status, age at death, diagnosis, primary unit, documentation of religion, RUCA code, and year of death. The outcomes were separated into two categories: ACP (including GOC and POLST) and circumstances of death (including do-not-resuscitate [DNR] code status, hospice involvement, and location of death) as binary measures. A significance level of p < 0.05 was used for all models.
Results
Clinical and demographic characteristics
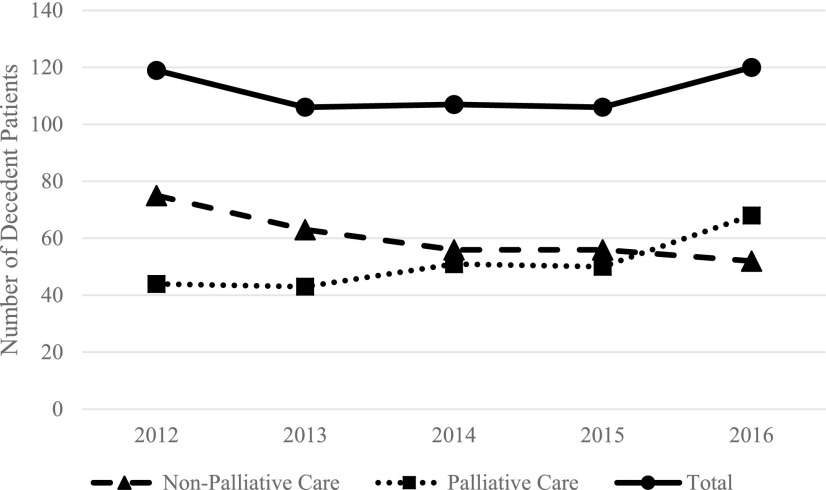
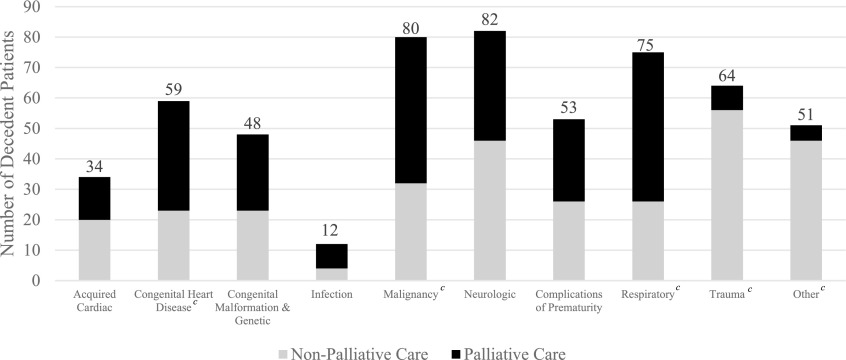
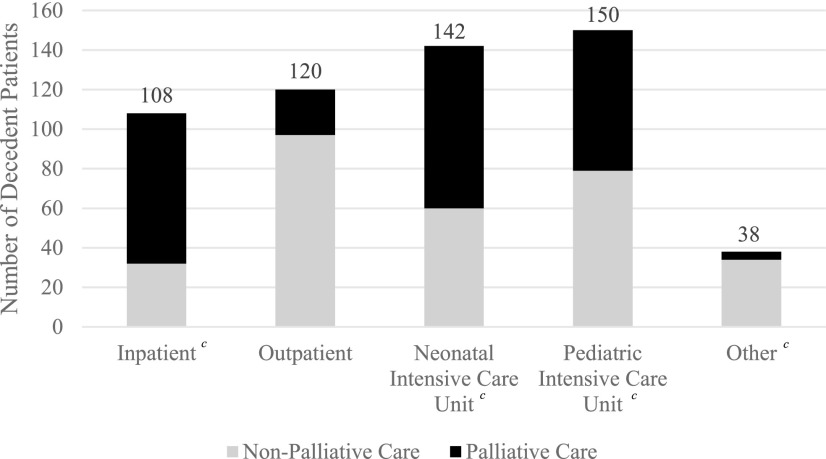
From 2012 to 2016, 256 of the 558 pediatric deaths (45.9%) received PPC, the remaining 306 (54.1%) did not (Table 1). There was a significant relationship between PPC and non-PPC over time throughout the study period (p = 0.028). In 2012, PPC was involved in 37% of the deaths that occurred that year, which increased to 57% in 2016 (Fig. 2), which was also the first year that PPC was involved in caring for most of the decedents. When examined by clinical and demographic variables, the largest number of deaths occurred among those 0–30 days old, and PPC involvement was highest among this age group. The most common diagnoses in children who did not receive palliative care were trauma (n = 56) and “other” diagnoses (n = 46) (Table 1 and Fig. 3). Conversely, deaths from malignancy and respiratory diseases had the highest PPC utilization. The most common places of care before death were the PICU (n = 150) and NICU (n = 142). Patients whose primary place of care was outpatient or “other” experienced more deaths without PPC, whereas patients whose primary place of care was inpatient had more deaths with PPC involvement (Table 1 and Fig. 4). Overall, 62.2% (n = 347) of patients were from urban areas, 13.3% (n = 74) were from large rural areas, another 13.3% (n = 74) were from small rural areas, and 11.3% (n = 63) were from isolated areas (Table 1). Given hereunder are the results of the multivariable models by each outcome.
Table 1.
Clinical and Demographic Characteristics and Outcomes of Interest by Palliative Care Status (N = 558)
| Type | Variable | Category | Palliative care services provided | Overall sample, N = 558 | Unadjusted chi-square p-value | |
|---|---|---|---|---|---|---|
| No | Yes | |||||
| n = 302 | n = 256 | |||||
| Clinical and demographic characteristics | Age category | 0–30 days | 58 (19.2%) | 78 (30.5%) | 136 (24.4%) | 0.003 |
| 31–364 days | 55 (18.2%) | 48 (18.8%) | 103 (18.5%) | |||
| 1–4 years | 48 (15.9%) | 48 (18.8%) | 96 (17.2%) | |||
| 5–9 years | 39 (12.9%) | 15 (5.9%) | 54 (9.7%) | |||
| 10–14 years | 42 (13.9%) | 24 (9.4%) | 66 (11.8%) | |||
| 15+ years | 60 (19.9%) | 43 (16.8%) | 103 (18.5%) | |||
| Diagnosis category | Acquired cardiac condition | 20 (6.6%) | 14 (5.5%) | 34 (6.1%) | <0.001 | |
| Congenital heart disease | 23 (7.6%) | 36 (14.1%) | 59 (10.6%) | |||
| Congenital malformation and genetic | 23 (7.6%) | 25 (9.8%) | 48 (8.6%) | |||
| Infection | 4 (1.3%) | 8 (3.1%) | 12 (2.2%) | |||
| Malignancy | 32 (10.6%) | 48 (18.8%) | 80 (14.3%) | |||
| Neurologic | 46 (15.2%) | 36 (14.1%) | 82 (14.7%) | |||
| Complications of prematurity | 26 (8.6%) | 27 (10.5%) | 53 (9.5%) | |||
| Respiratory | 26 (8.6%) | 49 (19.1%) | 75 (13.4%) | |||
| Trauma | 56 (18.5%) | 8 (3.1%) | 64 (11.5%) | |||
| Other | 46 (15.2%) | 5 (2.0%) | 51 (9.1%) | |||
| Race | White | 199 (65.9%) | 184 (71.9%) | 383 (68.6%) | 0.13 | |
| Nonwhite | 103 (34.1%) | 72 (28.1%) | 175 (31.4%) | |||
| Sex | Male | 171 (56.6%) | 142 (55.5%) | 313 (56.1%) | 0.78 | |
| Female | 131 (43.4%) | 114 (44.5%) | 245 (43.9%) | |||
| Primary place of care | Neonatal intensive care unit | 60 (19.9%) | 82 (32.0%) | 142 (25.4%) | <0.001 | |
| Pediatric intensive care unit | 79 (26.2%) | 71 (27.7%) | 150 (26.9%) | |||
| Inpatient | 32 (10.6%) | 76 (29.7%) | 108 (19.4%) | |||
| Outpatient | 97 (32.1%) | 23 (9.0%) | 120 (21.5%) | |||
| Other | 34 (11.3%) | 4 (1.6%) | 38 (6.8%) | |||
| Religion documented | Yes | 137 (45.4%) | 139 (54.3%) | 276 (49.5%) | 0.035 | |
| No | 165 (54.6%) | 117 (45.7%) | 282 (50.5%) | |||
| Rural Urban Commuting Area code | Urban | 189 (62.6%) | 158 (61.7%) | 347 (62.2%) | 0.33 | |
| Large rural | 36 (11.9%) | 38 (14.8%) | 74 (13.3%) | |||
| Small rural | 46 (15.2%) | 28 (10.9%) | 74 (13.3%) | |||
| Isolated | 31 (10.3%) | 32 (12.5%) | 63 (11.3%) | |||
| Year of deatha | 2012 | 75 (63.03%) | 44 (36.97%) | 119 (21.3%) | 0.028 | |
| 2013 | 63 (59.43%) | 43 (40.57%) | 106 (19.0%) | |||
| 2014 | 56 (52.34%) | 51 (47.66%) | 107 (19.2%) | |||
| 2015 | 56 (52.83%) | 50 (47.17%) | 106 (19.0%) | |||
| 2016 | 52 (43.33%) | 68 (56.67%) | 120 (21.5%) | |||
| Advance care planning | Goals of care documented | Yes | 28 (9.3%) | 230 (89.8%) | 258 (46.2%) | <0.001 |
| No | 274 (90.7%) | 26 (10.2%) | 300 (53.8%) | |||
| Physician Orders for Life-Sustaining Treatment form | Yes | 5 (1.7%) | 46 (18.0%) | 51 (9.1%) | <0.001 | |
| No | 297 (98.3%) | 210 (82.0%) | 507 (90.9%) | |||
| Circumstances of death | Hospice | Yes | 13 (4.3%) | 56 (21.9%) | 69 (12.4%) | <0.001 |
| No | 289 (95.7%) | 200 (78.1%) | 489 (87.6%) | |||
| Code status | Do not resuscitate | 67 (22.2%) | 181 (70.7%) | 248 (44.4%) | <0.001 | |
| Limited | 5 (1.7%) | 12 (4.7%) | 17 (3.0%) | |||
| Full Code | 230 (76.2%) | 63 (24.6%) | 293 (52.5%) | |||
| Location of death | Emergency department | 24 (7.9%) | 7 (2.7%) | 31 (5.6%) | <0.001 | |
| Home | 38 (12.6%) | 42 (16.4%) | 80 (14.3%) | |||
| Inpatient | 25 (8.3%) | 19 (7.4%) | 44 (7.9%) | |||
| Neonatal intensive care unit | 52 (17.2%) | 94 (36.7%) | 146 (26.2%) | |||
| Pediatric intensive care unit | 81 (26.8%) | 79 (30.9%) | 160 (28.7%) | |||
| Other | 82 (27.2%) | 15 (5.9%) | 97 (17.4%) | |||
Frequencies are displayed for the overall sample and by palliative care status; row percentages are included in parentheses.
Column percentages are displayed for year of death.
FIG. 2.
Decedent patients by year of death from 2012 to 2016 by palliative care status and total.a aNumber of decedent patients by year of death from 2012 to 2016; patients were divided into groups based on palliative care versus nonpalliative care status.
FIG. 3.
Patient primary diagnosis by palliative care status.a,b,c aPatients were categorized by primary diagnosis and subdivided into palliative care and nonpalliative care status. bCategory “Other” includes endocrine, gastrointestinal, genitourinary, hematologic, multiorgan system failure, renal complications, and unknown diagnoses. cStatistically significant at p < 0.05; reference value of neurologic diagnosis.
FIG. 4.
Primary place of care by palliative care status.a,b,c aPrimary place of care was identified for all patients; each patient was subdivided into palliative care and nonpalliative care status. bCategory “Other” includes emergency department, labor and delivery, home, and unknown primary unit. cStatistically significant at p < 0.05; reference value of outpatient unit.
Goals of care
Patients with PPC involvement were significantly associated with having their GOC documented (OR = 96.93), after adjusting for age at death, diagnosis, unit, religion, RUCA, and year of death (Table 2). By age category, those who were 5–14 years of age were less likely to have their GOC documented after adjusting for the remaining variables in the model (5–9 years, OR = 0.21; 10–14 years, OR = 0.27). Patients with cardiac conditions (OR = 0.26), infection (OR = 0.15), and those who experienced trauma (OR = 0.17) as their primary cause of death were also less likely to have their GOC documented.
Table 2.
Adjusted Odds of Advance Care Planning Preferences (N = 558)
| Variable | Category | Advance care planning | Circumstances of death | |||
|---|---|---|---|---|---|---|
| Goals of care documented | Physician Orders for Life-Sustaining Treatment | Code status: do not resuscitate | Hospice involvement | Location of death at home | ||
| Primary variable of interest | Palliative care | 96.93*** | 24.06*** | 7.71*** | 11.70*** | 2.23* |
| Nonpalliative care | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | |
| Age | 0–30 days | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) |
| 31–364 days | 0.56 | 3.52 | 0.33** | 1.54 | 4.85* | |
| 1–4 years | 0.62 | 2.32 | 0.37* | 0.78 | 1.42 | |
| 5–9 years | 0.21 | 1.26 | 0.16** | 1.15 | 0.93 | |
| 10–14 years | 0.27 | 1.74 | 0.25** | 0.57 | 0.65 | |
| 15+ years | 0.62 | 1.79 | 0.19*** | 0.85 | 1.39 | |
| Diagnosis | Cardiac | 0.26 | 0.09* | 0.14** | 1.00 | 0.24 |
| Congenital heart disease | 0.45 | 0.82 | 0.15*** | 0.81 | 0.51 | |
| Congenital malformation and genetic | 0.67 | 1.05 | 0.76 | 1.1 | 0.58 | |
| Infection | 0.15 | 1.00 | 0.13* | 1.00 | 1.00 | |
| Malignancy | 1.39 | 0.38 | 1.94 | 2.48 | 1.80 | |
| Neurologic | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | |
| Complications of prematurity | 0.95 | 0.59 | 0.47 | 1.00 | 0.28 | |
| Respiratory | 1.03 | 0.21 | 0.90 | 0.13* | 0.18* | |
| Trauma | 0.17* | 0.18 | 0.63 | 1.00 | 0.43 | |
| Other | 0.36 | 1.00 | 0.21* | 1.00 | 0.14** | |
| Primary place of care | Neonatal intensive care unit | 0.33 | 0.22 | 0.94 | 0.93 | 3.71 |
| Pediatric intensive care unit | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | |
| Inpatient | 1.50 | 13.95*** | 0.83 | 7.67** | 20.61*** | |
| Outpatient | 0.71 | 16.32*** | 0.26** | 16.36*** | 60.33*** | |
| Other | 0.10* | 6.77 | 0.24* | 5.71 | 25.93*** | |
| Religion | Documented | 0.48* | 1.31 | 1.13 | 1.01 | 1.52 |
| Not documented | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | |
| Rural Urban Commuting Area | Urban | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) |
| Large rural | 0.54 | 0.73 | 0.98 | 0.47 | 0.32* | |
| Small rural | 1.18 | 1.56 | 2.09* | 2.07 | 1.89 | |
| Isolated | 1.12 | 0.25 | 0.99 | 0.53 | 0.72 | |
| Year of death | 2012 | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) |
| 2013 | 1.62 | 4.32 | 0.96 | 1.74 | 1.99 | |
| 2014 | 2.43 | 6.80* | 0.55 | 1.50 | 0.86 | |
| 2015 | 3.26* | 7.61** | 2.06* | 0.94 | 0.49 | |
| 2016 | 2.52 | 8.77** | 1.07 | 0.97 | 1.17 | |
Coefficients presented above are odds ratios of each advance care planning model (goals documented, POLST completion, code status, hospice, preferred place of death at home, and actual location of death at home).Reference values were selected based on the largest sample size across the distribution; an exception was made for year, where the first year of the study was selected. *p < 0.05, **p < 0.01, ***p < 0.001.
POLST, Physician Orders for Life-Sustaining Treatment.
POLST and code status
The likelihood of having a POLST documented at the time of death increased over the study period. Patients receiving PPC were more likely to have a POLST form (OR = 24.06) or inpatient DNR status (OR = 7.71). In the multivariate model, patients receiving PPC in the nonintensive care inpatient environment (OR = 13.95) and outpatient environment (OR = 16.32) were more likely to have a completed POLST at time of death compared with patients receiving their care in the NICU or PICU (Table 2). Patients with cardiac disease (OR = 0.14), congenital heart disease (OR = 0.15), and infection (OR = 0.13) were less likely to have DNR code status at the time of death compared with patients with neurologic diseases in the multivariate model (Table 2). All patients in the sample who were >30 days old at the time of death were less likely (OR = 0.16–0.37) to have DNR as their code status compared with infants who were <30 days old.
Hospice involvement
Patients who received PPC were more likely to have had hospice care at the time of death (OR = 11.7). Patients who received care in an inpatient (OR = 7.67) or outpatient (OR = 16.36) setting were more likely to have been under the care of hospice at time of death compared with patients receiving their care in the PICU (Table 2). Those treated for malignancy were more likely to have hospice involved with their care (OR = 2.48) compared with those with neurologic conditions. However, patients with respiratory conditions were less likely to have hospice involved with their care before death (OR = 0.13).
Location of death
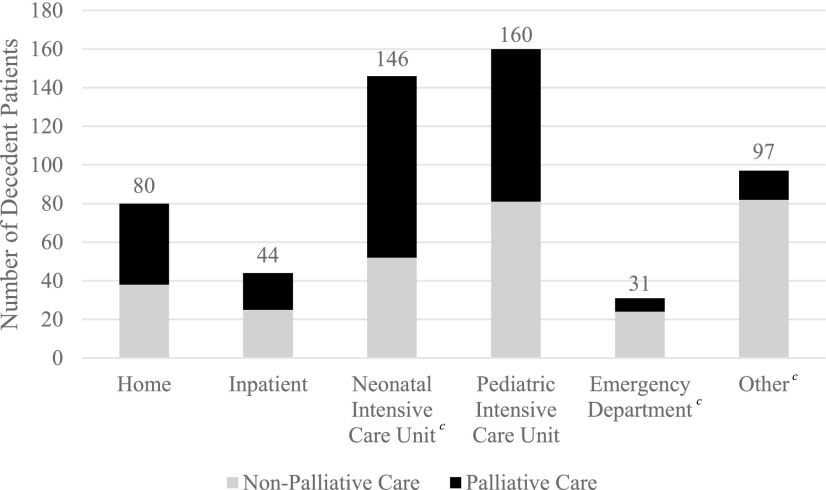
After adjusting for the clinical and demographic covariates in the model, palliative care patients were more likely (OR = 2.23) to die at home (Table 2). Those patients who died at home were more likely to have had their primary place of care in an inpatient setting (OR = 20.61), outpatient setting (OR = 60.33), or other (OR = 25.93) compared with their counterparts seen in the PICU. Results presented in Figure 5.
FIG. 5.
Location of death by palliative care status.a,b,c aLocation of death was identified for all patients; each patient was subdivided into palliative care and nonpalliative care status. bCategory “Other” includes inpatient hospice, accident scene, long-term care setting, and unknown location of death. cStatistically significant at p < 0.05; reference value of pediatric intensive care unit.
Discussion
PPC emphasizes relief of suffering, maximizing quality of life, and assisting with ACP to help patients with serious illness and their families live and die as well as possible.1 This study is among a small group of others to describe the characteristics of pediatric patients receiving care at a tertiary care center in a rural state and to evaluate the impact of PPC on ACP and circumstances of death across the full range of pediatric conditions who received palliative care. Significant differences in ACP were found between patients who received PPC and those who did not. These differences may be the result of many factors.
Palliative care involvement
Although there is variation across the United States regarding the role of the PPC provider on the care team, timing of consultation, services provided, and credentials and training of providers, agreement has been reached within the context of comprehensive pediatric care that this specialty fills a gap in care and provides much-needed support to patients and their families.1 Variation in the role of PPC on the clinical care team of pediatric patients has been discussed in the literature.2–4,19–21 In this study, PPC was provided by a dedicated team of physicians, nurses, and advance practice providers with certification in palliative care, in collaboration with hospital-based services providing spiritual services, social work and care coordination support, music therapy, pharmacy, and child life services. The PPC team was established 2 years before the start of the study; however, there was growth of the team during the study period, which might partially explain the increased proportion of PPC involvement over time.
Deaths from malignancy and respiratory issues had the highest utilization of PPC, possibly because of the nature of those diseases, which are life threatening and whose trajectories have natural opportunities for PPC to be consulted. In contrast, patients with trauma diagnoses were less likely to have PPC involvement, which may be expected because of the acute nature of those conditions. This is also likely the case for the “other” category, which were often either poorly understood, highly uncertain, or rapidly deteriorating, making it difficult for the primary teams involved to decide if and when to consult palliative care. The diversity in disease types represented in the study emphasizes the benefit of early integration of PPC for children with serious illness to avoid “unexpected” deaths of patients, with underlying medical conditions, who could have benefited from PPC support but did not receive it.22 Many deaths from diagnoses other than trauma would be consistent with what Feudtner et al.16 refer to as chronic complex conditions, where early involvement of PPC allows for support of quality of life and ACP that can impact the circumstances at death.
Advance care planning
Facilitating ACP is one of the important tasks of palliative care. Discussing GOC is an early step that helps align the treatment plan with patient and family preferences and priorities. We found that GOC were much more likely to be documented in patients followed by PPC, as it is part of every consult. As may be expected, patients who experienced trauma were less likely to have their GOC documented before death, likely because the goal remained full disease-directed treatment because of the acute nature of their clinical needs. Patients with acute cardiac conditions less frequently had completed POLST documentation compared with those with neurologic diseases, which may also be expected because of the acute versus chronic trajectory of illness. Furthermore, those who received the bulk of their care in an inpatient (non-ICU) or outpatient setting were more likely to have a completed POLST form. These findings are similar to studies by Marcus et al.10 and Morell et al.,23 and perhaps are illustrative of efforts by the PPC team to offer POLST documentation to patients with serious illness before discharge or in the outpatient setting.
Circumstances of death
Patients <31 days of age were more likely than all other age groups to have a code status of DNR at death, after adjusting for the other variables in the model. This may be because of perinatal counseling that allowed some families to choose comfort care for their infants with life-threatening illness, and a change in code status if treatment was initially focused on life prolongation but later redirected to a comfort focus.
Patients with PPC involvement were significantly more likely to have hospice involved and their location of death at home compared with those without PPC involved in their care. Patients with PPC involvement were significantly more likely to die at home after adjusting for the remaining covariates in the model. Of note, those treated in non-ICU settings (i.e., inpatient, outpatient, and other) were significantly more likely to die at home after adjusting for PPC and the other covariates. This may be because of logistical challenges in discharging a patient from the ICU in a rural state (e.g., setting up oxygen for transport and use at home). In addition, some of the deaths at home could have been considered “unexpected” even if there was a life-threatening illness present. Furthermore, patients who did not receive PPC may have had hospice support at home or in the hospital before death at the referral of their primary care provider or specialty team. This discrepancy in hospice enrollment and PPC involvement highlights that hospice care does not necessarily equal palliative care, and vice versa. Pediatric patients can, and often do, receive palliative care alongside curative therapies.
Rural disparities
There is little research that has examined PPC delivery and its effect on outcomes, particularly ACP, in rural areas. Although the adult literature shows that rural hospice patients rate their care very highly,24 it is unknown if the same is true for pediatric patients. The location of a pediatric patient's death has been examined in two studies, with conflicting findings. The first study by Feudtner et al. (2006), reported on patients in Washington state who died between 1989–2002. It indicated that patients who lived far away from a tertiary care center were more likely to die in the hospital.25 Conversely, in 2015, Jamorabo et al. found that a patient's distance away from the hospital has little, if any, bearing on where they die or the care they receive.26 Because of these conflicting study results in combination with our findings, additional study of rural disparities for children is needed to understand the rural differential for PPC and how to use the most precise mechanism to measure or define rural areas (e.g., zip code or Census tract level measurements instead of county-based measurement).
The rural nature of a patient's home did influence their location of death. In the multivariable model, patients residing in a large rural (or micropolitan) area were less likely to die at home than their urban counterparts. Given the previous work showing that pediatric patients with chronic disease and life-limiting conditions are shifting where they die,27–29 these data suggest that further work needs to be carried out to ascertain why patients from urban areas are more likely to die at home than their large rural (or micropolitan) counterparts. This study underscores the importance of future research to address rural disparities through effective policy change.
Limitations
This study is not without limitations. First, this retrospective chart review relies on accuracy and completeness of the medical record; however, documentation may be variable from provider to provider. It is possible that comprehensive discussions of GOC may have occurred but were not documented. Second, because there is no uniform place in the medical record to document GOC conversations, such data can be challenging to extract. The electronic medical record is not necessarily designed or intended with research in mind, and thus the meaning of available data might be unclear.30 The research team member abstracting data from the medical record was not blinded and this could have biased our results.30 However, to address potential bias in determining the primary diagnosis category, clarification of the primary diagnosis category was discussed if needed.
Conclusion
PPC support seems to contribute to important ACP opportunities and end-of-life outcomes for pediatric patients and their families. Specifically, in patients for whom PPC was involved, there was an increased likelihood of documentation of GOC, use of POLST forms, and hospice support. In addition, this study provides a compelling glimpse into how a patient's rurality may affect their location of death. Future work in this area, incorporating data across multiple rural states, is needed to truly understand the implications of PPC involvement for pediatric patients and their families.
Acknowledgments
The authors thank Kenneth Hacker, for assistance in abstracting data from EPIC and Dan Shane, for assistance with study design and methods.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Feudtner C, Friebert S, Jewell J: Policy statement: Pediatric palliative care and hospice care commitments, guidelines, and recommendations. Pediatrics 2013;132:966–972 [DOI] [PubMed] [Google Scholar]
- 2. Vern-Gross TZ, Lam CG, Graff Z, et al. : Patterns of end-of-life care in children with advanced solid tumor malignancies enrolled on a palliative care service. J Pain Symptom Manage 2015;50:305–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Widger K, Sutradhar R, Rapoport A, et al. : Predictors of specialized pediatric palliative care involvement and impact on patterns of end-of-life care in children with cancer. J Clin Oncol 2018;36:801–807 [DOI] [PubMed] [Google Scholar]
- 4. Ullrich CK, Lehmann L, London WB, et al. : End-of-life care patterns associated with pediatric palliative care among children who underwent hematopoietic stem cell transplant. Biol Blood Marrow Transplant 2016;22:1049–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feudtner C, Womer J, Augustin R, et al. : Pediatric palliative care programs in children's hospitals: A cross-sectional national survey. Pediatrics 2013;132:1063–1070 [DOI] [PubMed] [Google Scholar]
- 6. Committee on Palliative and End-of-Life Care for Children and Their Families: Board on Health Sciences Policy: When Children Die: Improving Palliative and End-of-Life Care for Children and Their Families. Washington, D.C.: National Academies Press, 2003 [Google Scholar]
- 7. Gao W, Verne J, Peacock J, et al. : Place of death in children and young people with cancer and implications for end of life care: A population-based study in England, 1993–2014. BMC Cancer 2016;16:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thienprayoon R, LeBlanc T: Early integration of palliative care into the care of patients with cancer. Hematology Am Soc Hematol Educ Program 2015;2015:479–483 [DOI] [PubMed] [Google Scholar]
- 9. Thienprayoon R, Lee SC, Leonard D, et al. : Hospice care for children with cancer: Where do these children die? J Pediatr Hematol Oncol 2015;37:373–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marcus KL, Balkin EM, Al-Sayegh H, et al. : Patterns and outcomes of care in children with advanced heart disease receiving palliative care consultation. J Pain Symptom Manage 2018;55:351–358 [DOI] [PubMed] [Google Scholar]
- 11. Feudtner C, Kang TI, Hexem KR, et al. : Pediatric palliative care patients: A prospective multicenter cohort study. Pediatrics 2011;127:1094–1101 [DOI] [PubMed] [Google Scholar]
- 12. Lindley L, Lyon M: A profile of children with complex chronic conditions at the end of life among Medicaid beneficiaries: Implications for health care reform. J Palliat Med 2013;16:1388–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feudtner C, DiGiuseppe DL, Neff JMJBM: Hospital care for children and young adults in the last year of life: A population-based study. BMC Med 2003;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stutz M, Kao RL, Huard L, et al. : Associations between pediatric palliative care consultation and end-of-life preparation at an Academic Medical Center: A retrospective EHR analysis. Hosp Pediatr 2018;8:162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization, United States Center for Disease Control and Prevention, United States National Center for Health Statistics: International Classification of Diseases and Related Health Problems—Clinical Modifications, ninth and tenth revisions (ICD-9-CM and ICD-10-CM). World Health Organization, United States Center for Disease Control and Prevention, and the United States National Center for Health Statistics, 2007. and2012 [Google Scholar]
- 16. Feudtner C, Christakis DA, Connell FA: Pediatric deaths attributable to complex chronic conditions: A population-based study of Washington State, 1980–1997. Pediatrics 2000;106:205–209 [PubMed] [Google Scholar]
- 17. Larson E, Skillman S: Rural Urban Commuting Area Code. Seattle, WA: WWAMI Rural Health Research Center, University of Washington, 2005 [Google Scholar]
- 18. StataCorp: Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC, 2017 [Google Scholar]
- 19. Kaye EC, Friebert S, Baker JN: Early integration of palliative care for children with high-risk cancer and their families. Pediatr Blood Cancer 2016;63:593–597 [DOI] [PubMed] [Google Scholar]
- 20. Kaye EC, Gushue CA, DeMarsh S, et al. : Illness and end-of-life experiences of children with cancer who receive palliative care. Pediatr Blood Cancer 2018;65:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mack JW, Wolfe J: Early integration of pediatric palliative care: For some children, palliative care starts at diagnosis. Curr Opin Pediatr 2006;18:10–14 [DOI] [PubMed] [Google Scholar]
- 22. DeCourcey DD, Silverman M, Oladunjoye A, et al. : Patterns of care at the end of life for children and young adults with life-threatening complex chronic conditions. J Pediatr 2018;139:196–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morell E, Wolfe J, Scheurer M, et al. : Patterns of care at end of life in children with advanced heart disease. Arch Pediatr Adolesc Med 2012;166:745–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baernholdt M, Campbell CL, Hinton ID, et al. : Quality of hospice care: Comparison between rural and urban residents. J Nurs Care Qual 2015;30:247–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feudtner C, Silveira MJ, Shabbout M, et al. : Distance from home when death occurs: A population-based study of Washington State, 1989–2002. Pediatrics 2006;117:e932–e939 [DOI] [PubMed] [Google Scholar]
- 26. Jamorabo DS, Belani CP, Martin EW: Complex chronic conditions in Rhode Island's pediatric populace: Implications for palliative and hospice services, 2000–2012. J Palliat Med 2015;18:350–357 [DOI] [PubMed] [Google Scholar]
- 27. Feudtner C, Feinstein JA, Satchell M, et al. : Shifting place of death among children with complex chronic conditions in the United States, 1989–2003. JAMA 2007;297:2725–2732 [DOI] [PubMed] [Google Scholar]
- 28. Fraser LK, Fleming S, Parslow R: Changing place of death in children who died after discharge from paediatric intensive care units: A national, data linkage study. Palliat Med 2018;32:337–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fraser LK, Miller M, Draper ES, et al. : Place of death and palliative care following discharge from paediatric intensive care units. Arch Dis Child 2011;96:1195–1198 [DOI] [PubMed] [Google Scholar]
- 30. Brookhart M, Stürmer T, Glynn R, et al. : Confounding control in healthcare database research: Challenges and potential approaches. Med Care 2010;48:S114–S120 [DOI] [PMC free article] [PubMed] [Google Scholar]