Abstract
The ultimate challenge of tissue engineering research is the translation of experimental knowledge into clinical application. In the preclinical testing phase of any new therapy, animal models remain the gold standard. Therefore, the methodological choice of a suitable model is critical to meet the requirements for a safe clinical application of the developed treatment. For instance, we have shown in rats that the application of calcium phosphate cement (CPC)/propylene glycol alginate (PGA) with bone morphogenetic protein (BMP)-2 or fibroblast growth factor (FGF)-2 resulted in the regeneration of periodontal defects. However, it is debated whether using small models form a predictive method for translation to larger species. At the same time, the 3R framework is encouraged as guiding principles of the ethical use of animal testing. Therefore, based on the successful rat study, the objective of this study was to further investigate the periodontal regenerative efficacy of the CPC/BMP and PGA/FGF system in a periodontal defect model with a low number of nonhuman primates (NHPs). Three Macaca fascicularis—overstocked from breeding for other purposes—were used (reuse of animals and appropriateness of the experimental animal species according to 3R framework). Three-wall periodontal defects were surgically created in the mandible. In total, 10 defects were created and distributed over two groups: (1) control group: PGA+CPC (n = 5) and (2) experimental group: PGA/FGF+CPC/BMP (n = 5). After 3 months, tissue regeneration was evaluated by histomorphometry and radiographic measurements. Data showed that epithelial downgrowth, cementum, and ligament regeneration were significantly enhanced in the experimental group compared with the control group (n = 5; p = 0.013, p = 0.028, and p = 0.018, respectively). However, the amount of newly formed bone did not differ (p = 0.146). Overall, as a translational proof-of-principle study, the hybrid periodontal regenerative method of CPC/BMP+PGA/FGF promoted periodontal regeneration in NHPs. This study warrants the application of CPC/BMP/PGA/FGF in clinical trials.
Impact Statement
This study validated an earlier successful periodontal regeneration strategy from a rat model into a few spare nonhuman primates (NHPs). The hybrid periodontal regenerative method of calcium phosphate cement (CPC)/bone morphogenetic protein (BMP)-2/propylene glycol alginate (PGA)/fibroblast growth factor (FGF)-2 promoted periodontal regeneration in NHPs, which corroborated the previous rat results. This translational approach was a very practical option and thus reduced the number and species of experimental animals in translational research. These results found in NHPs indicate a consistent conclusion with the earlier findings in the rat model. It further warrants the application of CPC/BMP-2+PGA/FGF-2 in human clinical trials.
Keywords: calcium phosphate cement, bone morphogenetic protein-2, fibroblast growth factor-2, periodontal regeneration, primates, animal models, methods
Introduction
Periodontitis is an inflammatory disease affecting the supporting tissues surrounding teeth, that is, the periodontal ligament (PDL), cementum, and alveolar bone. Severe periodontitis leads to the destruction of tooth-supporting tissues and eventually causes tooth loss. Clinical reviews indicate that ∼46% of individuals at 30 years of age and older in the United States have periodontitis.1 Numerous approaches have been investigated to achieve regeneration of periodontal tissues, mainly by applying grafting materials carried with or without bioactive factors into bone defects.2
For example, in one of our previous studies, the periodontal regeneration capacity of a biodegradable calcium phosphate cement (CPC) in combination with recombinant human bone morphogenetic protein (BMP)-2 or fibroblast growth factor (FGF)-2 was tested in a periodontal defect model in rat. For this purpose, either growth factor was dissolved in propylene glycol alginate (PGA) and then applied on the root of the tooth, comparable to commercial periodontal regeneration products like Emdogain®. Subsequently, the defect was filled with the CPC, in which poly(d,l-lactic-co-glycolic) acid (PLGA) was loaded as a porogen to increase the CPC degradation and to support the bone ingrowth. The results demonstrated favorable responses for bone as well as PDL healing. The regenerative approach using CPC/BMP-2 resulted in a significant 2.4-fold increase of bone, compared to applying CPC alone (p < 0.05), whereas CPC/FGF-2 increased 1.9-fold. In addition, CPC/FGF-2 showed superior PDL tissue regeneration with a 2.6-fold increase compared to CPC.3 Some studies have demonstrated that the combination of BMP-2 and FGF-2 resulted in higher periodontal regenerative efficacy than either one alone.4–6
Therefore, based on our own rat results and other preclinical progress with BMP/FGF, we proposed a hybrid implantation method with FGF-2 loaded in gel coated onto the root surface for ligament regeneration and BMP-2 loaded in CPC to promote periodontal regeneration with spatial order.
The methodological choice of a suitable model is critical to test a hypothesis, which in this case depends on the similarity of the periodontium and the nature of the disease to that of humans. The commonly used animal models for studying periodontal regeneration include rats, rabbits, dogs, and nonhuman primates (NHPs). It has been noted that periodontal disease in rodents is not closely related to the human varieties because rodents have different dental physiology than humans, for example, their teeth show continuous eruption and drift throughout life. Still, rats have been extensively used in periodontal studies.7 The low breeding and housing costs and easy handling make it possible to carry out studies with enough numbers of animals for accurate statistical analysis.
However, it is debatable if small animal models form a predictive method for the translational proof-of-principle and whether large animals should be employed instead. The latter show greater similarity with humans concerning dental anatomy, healing characteristics, higher reproducibility of surgery, and experimentally induced chronic defects that rarely spontaneously regenerate.8 At the same time, researchers are encouraged to design their studies according to the 3R framework (replacement, reduction, and refinement) as the guiding principles of the ethical use of animals for testing. Therefore, the objective of this study was to validate and extend upon previous rat results and to further investigate the periodontal regenerative capacity of the CPC/BMP-2 and PGA/FGF-2 system in a periodontal defect model with a low number of NHPs.
Three Macaca fascicularis—extras from breeding for another study—were used. This use of animals to assess the appropriateness of the experimental animal species allowed us to meet the 3R principle, as the animals were spare and would otherwise have been culled. Adult M. fascicularis have a body status, which is described as equivalent to that of middle-aged people (i.e., the major group suffering from periodontitis).9
Materials and Methods
PGA gel containing FGF-2
PGA was chosen as the FGF-2 carrier in this experiment due to its wide use as a root conditioning gel to deliver growth factors, such as enamel matrix derivative.10 The preparation of FGF/PGA gel was done as described previously.3 Briefly, 125 μg of FGF-2 (R&D Systems, Minneapolis, MI) was reconstituted in 500 μL phosphate-buffered saline (PBS; Gibco, Paisley, United Kingdom) containing 0.1% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO), and divided into 10 separate Eppendorf tubes. Next, 20 μL PGA solution (6.5% weight/weight) was added. After thorough mixing, the gel was stored at −20°C until further use. Before the surgery, the FGF/PGA gel was defrosted, 20 μL of sterilized MilliQ™ was added, and the mixture was applied onto the root surface. As control, PGA gel was prepared in the same manner, but without the addition of FGF-2.
Preparation of BMP-2/PLGA/CPC cement composite
CPC consisted of a mixture of 59.1 wt% alpha-tricalcium phosphate (α-TCP; CAM Bioceramics BV, Leiden, the Netherlands), 1.5 wt% carboxymethylcellulose (CMC; CAM Bioceramics, Leiden, the Netherlands), and 39.4 wt% cryoground PLGA particles (<200 μm) with a 50:50 molar ratio of lactic to glycolic acid (PURASOB 5002A; Purac, Gorinchem, the Netherlands).11 Recombinant human BMP-2 (R&D Systems) was added to the powder through resuspension into the setting solution.11 Briefly, BMP-2 was first resuspended in 4 mM HCl containing 0.1% bovine serum albumin. For delivery to the defect, a 2-mL syringe (Sherwood Medical, Den Bosch, the Netherlands) was closed at the tip with a plastic stopper and filled with the CPC powder, and then sterilized by gamma radiation at 25 kGy (Synergy Health BV, Ede, the Netherlands). Preoperatively, 32 μg of BMP-2 was mixed with the setting solution and added to the syringe. For 160 mg of CPC powders, 90 μL of 4% NaH2PO4·2H2O was used as the setting solution. Finally, all components were mixed vigorously for 30 s with a dental shaker machine (Silamat®; Vivadent, Schaan, Liechtenstein). As control, CPC powders were prepared exactly in the same way, but without the addition of BMP-2.
Experimental animals
Three adult M. fascicularis NHPs, each weighing ∼6 kg with ages between 12 and 15 years, were used in this study. These animals were spared from a breeding program not related to this study and had previously been designated to be culled. All procedures were in accordance with the national guidelines for the care and use of laboratory animals. The Institutional Animal Care and Use Committee (IACUC) of SingHealth approved the study protocol (2016/SHS/1160). The research performed was carried out in the SingHealth Experimental Medicine Centre, accredited by the International Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). All three animals presented with full adult dentition and were in a healthy condition at the baseline checkup. All animals were housed in individual cages. Solid food was provided to each animal daily and water was available ad libitum, but withheld overnight preoperatively. Soft food was given for a week postsurgery (teeth extraction, creation of periodontal defects, and regenerative surgery) to ensure uninterrupted wound healing. Meanwhile, animals were routinely checked for general health, body weight, and normal behavior.
Creation of experimental defects and experimental periodontitis
Before surgical procedures, the animals were tranquilized with an intramuscular (IM) injection of ketamine (15 mg/kg) and atropine 0.05 mg/kg subcutaneously (SC). General anesthesia was performed by a qualified veterinarian using isoflurane 2% intubation, followed by local anesthesia (1 mL Scandonest/2% adrenaline 1:100,000 given through inferior alveolar nerve block, buccal nerve block, and infiltration at the surgical sites). Antibiotics (ampicillin/cloxacillin, 6–8 mg/kg, IM) and analgesics (keterolac, 15–30 mg/kg, IM) were routinely given at the end of the surgery. The operation was performed using sterile routines. As a pretreatment, the first premolars and first molars at both sides of the mandible were extracted 2 months before the surgical preparation of defects.12
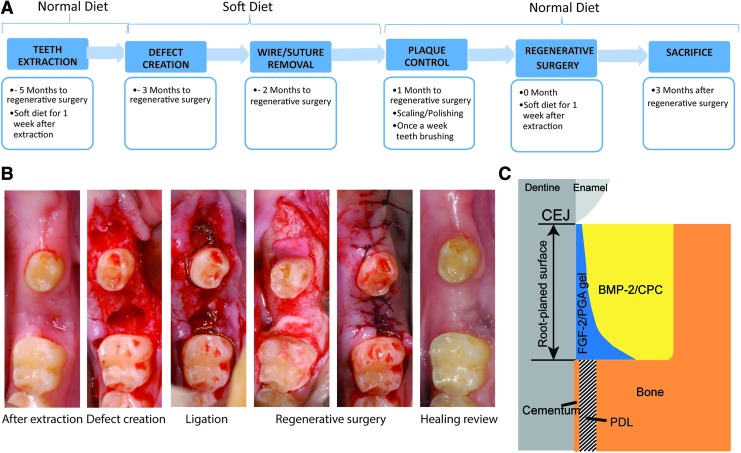
The time schedule and surgical procedures are depicted in Figure 1. Following general and local anesthesia, an intracrevicular incision was made around the second premolar and second molar on both sides of the mandible. Then, its buccal and lingual full-thickness flaps were raised. Three-wall intrabony defects were created mesially of the second premolar and second molar, using a piezotome (Varosurg Piezo-tome, NSK, United Kingdom). Stainless steel wires (Ø 0.4 mm) were twisted around the neck of the experimental teeth and the end of the knots were extended to the base of the bony defects. Because of root fracture during tooth extraction and removal of buccal bone for the root retrieval, to avoid baseline variation at the defect sites, these two positions were exempt from defect creation. Therefore, in total, 10 defects were produced in the mandible.
FIG. 1.
Overview of the experimental procedure. (A) Timeline of the whole experiment. (B) Representative surgical and healing images of the procedure. (C) Descriptive scheme of reconstructive surgery. Experimental group: FGF/PGA was applied on the root surface (blue area) and BMP/CPC was applied into the bone defect (yellow area). Control group: PGA was applied on the root surface (blue area) and CPC was applied in the bone defect (yellow area). BMP, bone morphogenetic protein; CPC, calcium phosphate cement; FGF, fibroblast growth factor; PGA, propylene glycol alginate. Color images are available online.
The flaps were then repositioned and sutured. After the defect creation, no oral hygiene measures were performed, and the animals were fed with soft diets to encourage plaque attachment around the experimental teeth. Wires were removed 1 month after the defect creation and a plaque control procedure consisting of tooth brushing, scaling, polishing, and irrigation with 0.2% chlorhexidine solution was carried out once a week for 1 month before the regenerative surgery. During the healing period, the clinical assessment, including plaque index, probing depth, bleeding on probing, and gingival inflammation index, was recorded monthly.
Periodontal regenerative surgery
The regenerative surgery was performed 3 months after defect creation. Buccal and lingual full-thickness flaps were raised, and the granulation tissue was removed using scalers. The bone defects were further standardized using a piezotome to the final dimensions (mesiodistal width × depth × buccal to the lingual line angle = 3 × 4 × 5 mm). The root surface was scaled and planed by an ultrasonic scaler and a hand scaler. Defects and root surfaces were then rinsed with sterile saline and dried using sterile gauze. Immediately after, the root surface was coated with an injection of PGA and the bone defect was filled with CPC (control group, n = 5) or with root modification gel PGA/FGF-2 (12.5 μg/site) followed by CPC/BMP-2 (9 μg/site) (experimental group, n = 5). The experimental materials were rotated between defect sites in subsequent animals. Finally, the defects were closed using resorbable sutures (Vicryl 5–0 resorbable suture) (Fig. 1). After the regenerative surgery, the animals received antibiotics and analgesics similar to that used during preparation of experimental defects. Remaining sutures were removed after 14 days of healing and postoperative plaque control was maintained once a week till the end of the study, as previously described. Meanwhile, the same clinical assessment mentioned above was recorded monthly. During the surgery, the materials were prepared by a different operator and then given to the surgeons. The surgeons and caretakers were blinded to the study groups.
Radiographic and histological processing
Animals were closely monitored postoperatively. General demeanor and behavior pattern of the animals were assessed, including changes in posture and gait, vocalization, and daily activity. Three months after the reconstructive surgery, the animals were first tranquilized with an IM injection of ketamine (15 mg/kg) and atropine 0.05 mg/kg (SC). Subsequently, they were euthanized by an IV injection of pentobarbital 100 mg/kg. Animal vital signs were closely monitored by an experienced veterinarian and the death of the animals was verified by assessing vital signs, and manually confirming apnea using a stethoscope and heartbeat palpation. All defects with the surrounding soft and hard tissues were dissected and immediately fixed in 10% buffered formalin (pH 7.4). After fixation, specimens were scanned using ex vivo computed tomography (CT; Inveon Micro-CT/PET, Siemens Medical Solution, Knoxville, TN). Specimens were placed into a spongy support and scanned along the coronal plane. Images were recorded with an acquisition time of 6 min with 30 μm spatial resolution, 80 kV tungsten anode, and exposure time of 1000 ms.
After scanning, specimens were decalcified for 3 weeks under a low voltage current in a TDE™30 bath (Sakura Finetek, Zoeterwoude, the Netherlands) and subsequently dehydrated in a graded series of ethanol (70–100%). The mesiodistal axis of the teeth and the defect margins were marked by pen for discerning the central portion of the defects to obtain optimal histologic sections. The samples were split mesiodistally in the central portion into two halves and then embedded in Paraplast® paraffin (Klinipath, Duiven, the Netherlands). Sections with a thickness of 5 μm were cut in a mesiodistal direction and routinely stained with hematoxylin and eosin (H&E)13 and trichrome (Goldner's Masson trichrome).3
Histomorphometric and radiographic analysis
All histological sections were scanned with a Panoramic 250 Flash series digital scanner (3DHISTECH) equipped with a computerized image system (cellSens; Olympus Corp., The Digital Pathology Company, Budapest, Hungary). For histomorphometric analysis, three sections ∼90 μm apart were selected from the central area of each defect.
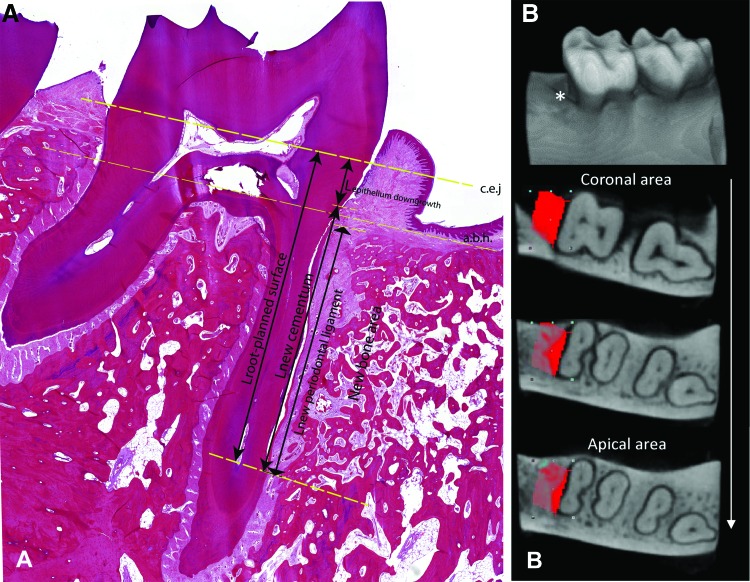
A qualitative histological analysis was done to obtain an overall description of the regenerative response. Therefore, the contour of the defect area was first identified, forming roughly a rectangle (Fig. 2): (1) the basis of the defect was defined by the end of the instrumentation artifacts on the surface of the root apex, and (2) in the posterior-anterior direction and at the alveolar bone crest, the defect was outlined by the interface of the original and new bone.
FIG. 2.
Representative micrograph indicating the scoring systems: (A) histological measurement and (B) CT measurement. (A) The landmarks for the histomorphometric measurements, the a.b.h. and c.e.j., are marked with straight dashed lines. For the principal measurements, length of root planed surface (Lroot-planed surface), length of epithelial downgrowth (Lepithelium downgrowth), length of regenerated cementum (Lnew cementum), and length of regenerated PDL (Lnew periodontal ligament), are represented with arrows. (B) The three-dimensional reconstruction of one sample with representative two-dimensional cross-sections of the reconstructed CT images from corona to apex of the defect area. *The defect area. The red area, the region of interest (defect area). a.b.h., alveolar bone height; c.e.j., cementum-enamel junction; PDL, periodontal ligament. Color images are available online.
Subsequently, the quantitative histomorphometric analysis was done according to a histological scoring system. Histomorphometry was performed for three sections of each specimen and for both stains (Fig. 2).3,14,15 The linear measurements were performed with ImageJ (v1.51s; NIH, Bethesda, MD). The mean value of each histomorphometric parameter was calculated for each site. First, the root-planed length (RPL) was assessed as the distance between cementum-enamel junction (CEJ) and apical extent of root planing. Thereafter, the following parameters were investigated (Fig. 2A): (1) epithelium downgrowth (%): distance between CEJ and apical margin of junctional epithelium/RPL × 100; (2) cementum regeneration (%): distance between apical end of root-planed surface and coronal extent of cementum regeneration or cementum-like deposit on root/RPL × 100; and (3) PDL regeneration (%): distance between apical end of new PDL and coronal extent of ligament regeneration/RPL × 100. The new PDL formation was defined as the perpendicularly oriented fibers inserted into the newly formed cementum and bone.
Bone regeneration was assessed based on CT acquisitions, as these provide three-dimensional (3D) information of the complete defect. CTan (v1.11; SkyScan, Kontich, Belgium) was used for quantification of all reconstructed CT images (Fig. 2B).16 Bone regeneration was detected in a cube surrounding the root with a dimension of 3 × 5 × 4 mm region of interest (ROI), which started from the apical end of the root planed surface. Total defect ROI volume was identified as tissue volume (TV). In two-dimensional cross-sections of the reconstructed CT images, a standardized threshold of the gray values for bone within the total data was set. Thus, the percentage of regenerated bone volume (BV) was calculated. The result is shown as BV/TV × 100%. Furthermore, 3D reconstruction of the samples was done by ImageJ.
All measurements were made by the same blinded examiner. Intraexaminer reproducibility was ensured by reading all sections by the same examiner and repeating the same scoring procedure 3 days later.
Statistical analysis
All data are given as mean ± standard deviation. The observations in each group were normally distributed. Independent-samples t-test combined with Levene's test was performed using SPSS version 20.0 (IBM Corp., Armonk, NY). Based on previous knowledge that BMP-2/FGF-2 can be applied to improve periodontal regeneration,3 investigating for such an enhanced effect was our only hypothesis. Thus, to maximize the power of detection, a one-tailed independent-sample t-test was used. Differences were considered statistically significant at p < 0.05.
Results
Clinical observations
All surgical procedures were tolerated well by the animals. One surgical site in the experimental groups was excluded from the study because the pulp was exposed due to curvature of the root during defect creation instrumentation. After defect creation, periodontitis was successfully induced, indicated by increased plaque index, inflammation index, and probing depth in both groups (data not shown). After plaque control, inflammation almost returned to the baseline level. Thereafter, regenerative surgery evoked a mild inflammatory response during the first 2 months, which disappeared in the third month. At the end of the study, healing was complete for all surgical sites.
Descriptive histology
The contour of the surgically created alveolar defects could be identified by visual inspection of the H&E-stained slides. Remnants of the CPC were seen only in one sample from the experimental group (1/10). No ankylosis or root resorption was observed in any sample.
Control group
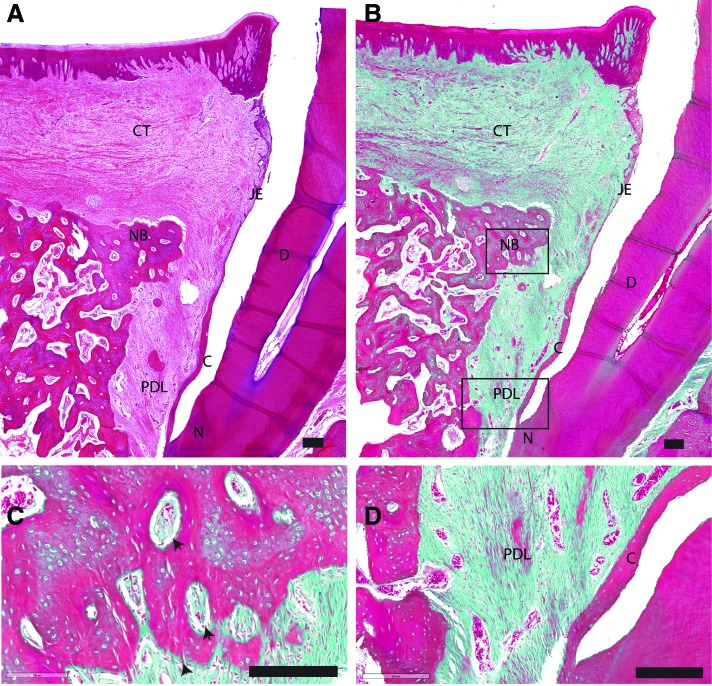
Figure 3 presents a histological sample from the control group stained with H&E and trichrome staining. In the control group, the apical extension of the junctional epithelium varied for the different sections, ranging from a long junctional epithelium to limited epithelium downgrowth. On the root surface, a freshly deposited layer of cementum was present, in which collagen fibers were inserted. In almost all histological samples, the regenerated cementum appeared detached from the underlying circum-pulpal dentine, while the native cementum was still in contact with the underlying root surface. Newly formed bone was observed in the defect site. The vertical height of the bone filling varied between the different samples. Osteoids were found on and in between the new bone (Fig. 3C). In all sections of the control group, compared with the nonoperated site of the tooth, a wide gap was observed between the newly deposited bone and the cement layer. In the apical part of the gap, obliquely oriented PDL connected the cementum layer with the bone (Fig. 3D).
FIG. 3.
Representative overview of histological sections, H&E/trichrome stained, of the control group. Scale bar is 200 μm. (A) Lower magnification of the defect area stained in H&E. (B) Lower magnification of the defect area stained in Masson's trichrome. (C) Higher magnification of the framed area (NB) in (B). (D) Higher magnification of the framed area (PDL) in (B). black arrow: osteoids with osteoblasts. C, cementum; CT, connective tissue; D, root dentin; H&E, hematoxylin and eosin; JE, junctional epithelium; N, notch of planed surface; NB, new bone; R, gingival epithelium. Color images are available online.
Experimental group
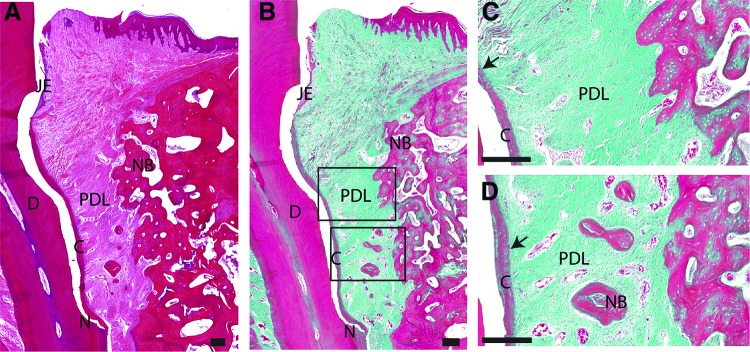
In the experimental group (Fig. 4), junctional epithelium demonstrated limited apical downgrowth compared with the control group. Thicker and longer layer of regenerated cementum with obliquely inserted collagen fibers was consistently observed (Fig. 4A, B). Extensive bone formation was noticed in the defect area, but still no complete regeneration was seen. Also, a wide gap existed between the bone and cement layers. Isolated osteoids, surrounded by an area of densely packed osteoblasts, were commonly seen distributed among the connective fibers (Fig. 4D). The newly formed PDL was highly vascularized and confined to the area between the newly deposited cementum and new bone (Fig. 4C, D).
FIG. 4.
Representative overview of histological sections, H&E/trichrome stained, of the experimental group. Scale bar is 200 μm. (A) Lower magnification of the defect area stained in H&E. (B) Lower magnification of the defect area stained in Masson's trichrome. (C) High magnification of the framed area (upper) in (B). (D) High magnification of the framed area (lower) in (B). black arrow: PDL inserted into new formed cementum. Color images are available online.
Histomorphometric and radiographic results
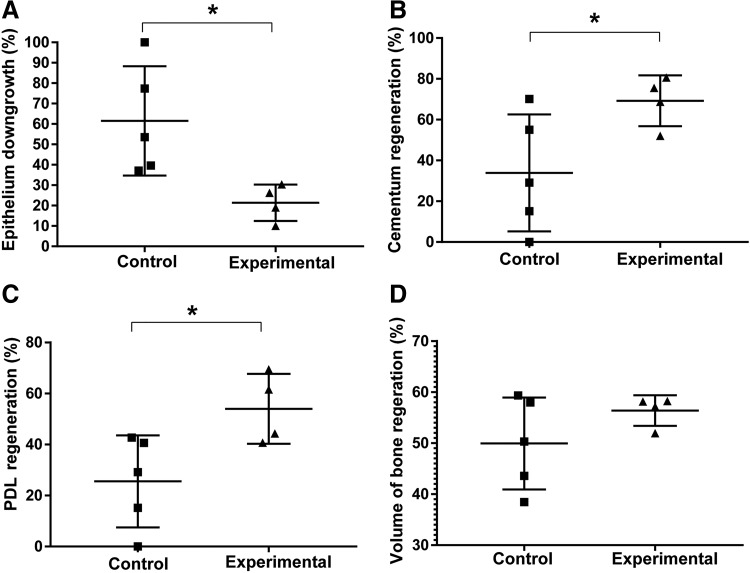
The results of the histomorphometrical and radiographic analysis are summarized in Figure 5, including epithelial downgrowth, and cementum, bone, and PDL regeneration. Intraexaminer reproducibility calibration was above the 90% level. The experimental group showed significantly less epithelial downgrowth compared with the control group (p = 0.013). Statistical analysis also demonstrated that significantly more cementum (p = 0.028) and PDL (p = 0.018) tissues were regenerated in the experimental group. Specifically, 30% more cementum and PDL tissues was formed in the experimental group. The amount of newly formed bone did not differ between the experimental and control group (p = 0.146), that is, both groups showed 50–60% of new bone formation on average.
FIG. 5.
Histomorphometric (A–C) and CT (D) results: (A) Relative epithelial downgrowth. (B) Relative cementum regeneration. (C) Relative PDL regeneration. (D) Relative alveolar bone regeneration. Statistical analysis, *p < 0.05.
Discussion and Conclusions
Preclinical animal models remain a critical component in the development of new clinical treatment approaches. In vivo models provide distinct advantages for a better understanding of aspects of the molecular, cellular, tissue, and anatomical processes that occur in response to surgical interventions. From a regulatory point of view, in vivo animal experiments are required before a new device/treatment can be applied in humans. Due to natural differences between animal species, caution is required in the interpretation of animal experimental results for translational application. Many preclinical studies fail to be accurately corroborated in translational clinical trials.17 In view of this, the use of small animal data for clinical trials is controversial, while large animal data are considered to be more predictive. Critical considerations on the advantages and disadvantages of different species might help to achieve more reliable results by consuming fewer animals. For instance, rat studies usually cost less time and expense with very accurate statistical results, while large animals consume far more time and money. Therefore, using a few large animals to corroborate or extend the results from small animals might be an acceptable choice, which can improve their acceptance and validation for translational ability. Based on the successful results of the rat study,3 this experiment takes a step forward in comparing the periodontal healing efficacy of hybrid usage of FGF-2/PGA gel on the root surface and BMP-2/CPC as a bone filler in standardized osseous defects with periodontitis after a healing period of 3 months.
There are some technical considerations pertaining to our study. The intrabony defect could be easily created by surgery and is commonly used in periodontal models. Compared with small animals such as rats, a more realistic disease model can be established in large animals. In this study, clinically relevant dimensions of the defect were created, which were easier for standardization and reproducibility. To reduce spontaneous regeneration, a metal wire was positioned in the defect to provoke chronic inflammation. However, chronic defects may lead to different bone resorption at baseline, which could result in bias on the final regenerative results, especially with such a small sample size. Therefore, the periodontal defect was eventually further standardized to form a comparable surgical defect among groups, which was similar to the rat study, to extract valuable regenerative information. It is worth mentioning that comparison of the extent of regeneration across species, however, would be misleading, because of many confounding factors, for example, defect size, local physiological environment, individual variance, and healing ability. Although periodontal tissues are generally considered one of the most difficult tissues to regenerate, scientific evidence has shown that rats have strong spontaneous healing ability, while primates are considered to have partial spontaneous healing in periodontal disease models.18 Therefore, the extent of quantitative regeneration is not comparable between rats and NHPs. However, the consistency of the regenerative results from rats to primates could still be seen. In primates, histological parameters with supplementary clinical examination provide us with more detailed and reliable results. For example, easier identification of the newly regenerative cementum, noninvasive clinically symptomatic examination, that is, probing depth, gingival inflammation, and plaque index, could be inspected as done in clinics. However, it has to be emphasized that the defect was still artificial. As a consequence, the microbiological and inflammatory condition created do not resemble “natural” periodontitis.
Cementum, bone, and PDL are the three pillars in periodontal regeneration. The experimental group showed significant improvements in epithelial downgrowth, cementum, and ligament regeneration when compared to the control. However, periodontal regeneration was also found in the control groups, which might be ascribed to the dual effect from partial spontaneous healing and the CPC. CPC has been reported to enhance the periodontal regeneration.19,20 Both our rat and the NHP studies displayed better regeneration in the experimental group in terms of PDL healing and epithelial downgrowth. Although FGF-2 only improved 30% of PDL regeneration in NHPs, such a gain is relevant from a clinical point of view, especially considering the restricted follow-up time. Regenerative cementum in our rat study could hardly be distinguished from the new dentin, but this study results showed more obvious cementum formation in the experimental group. Alveolar bone regeneration was not significantly different between groups, which is inconsistent with results from the rat study. The defect size in the NHP differed from the rat, and the dose of incorporated growth factors might have contributed to these differences. To compare the translational consistency from rats to NHPs, the concentration of growth factors was kept consistent with the rat study. Therefore, in this study, the applied concentration of BMP-2 (0.15 μg/mm3) is considered a relatively low dosage for large animals, even though a similar low concentration can be found in the literature.21 However, the efficient concentration of growth factors has been an issue of debate for years.22 It is also worth mentioning that the FGF/PGA gel cannot attach firmly on the surface of the roots due to its flowable viscosity. Thus, besides a thin layer of gel presented on the root surface, FGF was always partially mixed with BMP at the bottom of the defect. This is a common phenomenon when using root conditioning gels. A proper double layer application requires improvement of the viscoelasticity of the hydrogel. Finally, there is stronger individual variability in the regenerative process in NHPs,23 which of course is also known for humans. Nevertheless, in terms of periodontal regeneration, the hybrid periodontal regenerative method of CPC/BMP-2/PGA/FGF-2 promoted periodontal regeneration in the NHP model.
In conclusion, the 3R principle of replacement, reduction, and refinement is of great importance when deciding on the ethical dilemmas presented by animal experimentation, in which the potential benefits for humans stand against the costs borne by the animals.24 Based on this, the use of spare animals from other studies was indeed found to be a very practical option to validate and extend previous results and thus reduce the number and species of experimental animals. The results of this study showed that the hybrid periodontal regenerative method of CPC/BMP-2/PGA/FGF-2 promoted periodontal regeneration in NHPs, which corroborated the extended assumption from our previous rat study. This study warrants the application of CPC/BMP-2+PGA/FGF-2 in human clinical trials.
Acknowledgments
The authors thank Mr. Too Jianhui for his help with surgical work; Ms. Natasja van Dijk and Ms. Pia Helmich for their help with the histology; Mr. Rene van Rheden and Mr. Rob van de Loo for their help with slide scanning; Dr. Vincent M.J.I. Cuijpers for the professional instruction of CT analysis; Mr. Ewald Bronkhorst for the statistical help; and Ms. Safiyya Mohamed Ali for editorial assistance.
Disclosure Statement
No competing financial interests exist.
Funding Information
This work was funded by the National Medical Research Council Singapore (Transition Award NMRC/TA.0045/2016) and the China Scholarship Council (No. 201509370020).
References
- 1. Papapanou P.N., and Susin C.. Periodontitis epidemiology: is periodontitis under-recognized, over-diagnosed, or both? Periodontology 2000 75, 45, 2017 [DOI] [PubMed] [Google Scholar]
- 2. Bartold P.M., Mcculloch C.A., Narayanan A.S., and Pitaru S.. Tissue engineering: a new paradigm for periodontal regeneration based on molecular and cell biology. Periodontology 2000 24, 253, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Oortgiesen D.A., Walboomers X.F., Bronckers A.L., Meijer G.J., and Jansen J.A.. Periodontal regeneration using an injectable bone cement combined with BMP-2 or FGF-2. J Tissue Eng Regen Med 8, 202, 2014 [DOI] [PubMed] [Google Scholar]
- 4. Wang L., Zou D., Zhang S., Zhao J., Pan K., and Huang Y.. Repair of bone defects around dental implants with bone morphogenetic protein/fibroblast growth factor-loaded porous calcium phosphate cement: a pilot study in a canine model. Clin Oral Implants Res 22, 173, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Tanaka E., Ishino Y., Sasaki A., et al. . Fibroblast growth factor-2 augments recombinant human bone morphogenetic protein-2-induced osteoinductive activity. Ann Biomed Eng 34, 717, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Lan J., Wang Z., Wang Y., Wang J., and Cheng X.. The effect of combination of recombinant human bone morphogenetic protein-2 and basic fibroblast growth factor or insulin-like growth factor-I on dental implant osseointegration by confocal laser scanning microscopy. J Periodontol 77, 357, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Kantarci A., Hasturk H., and Van Dyke T.E.. Animal models for periodontal regeneration and peri-implant responses. Periodontology 2000 68, 66, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weinberg M.A., and Bral M.. Laboratory animal models in periodontology. J Clin Periodontol 26, 335, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Tigges J., Gordon T.P., McClure H.M., Hall E.C., and Peters A.. Survival rate and life span of rhesus monkeys at the Yerkes Regional Primate Research Center. Am J Primatol 15, 263, 1988 [DOI] [PubMed] [Google Scholar]
- 10. Heitz-Mayfield L.J., Schätzle M., Löe H., et al. . Clinical course of chronic periodontitis. J Clin Periodontol 30, 902, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Mastrogiacomo S., Güvener N., Dou W., et al. . A theranostic dental pulp capping agent with improved MRI and CT contrast and biological properties. Acta Biomater 62, 340, 2017 [DOI] [PubMed] [Google Scholar]
- 12. Gottlow J., Nyman S., Karring T., and Lindhe J.. New attachment formation as the result of controlled tissue regeneration. J Clin Periodontol 11, 494, 1984 [DOI] [PubMed] [Google Scholar]
- 13. Shao J., Wang B., Bartels C.J., et al. . Chitosan-based sleeves loaded with silver and chlorhexidine in a percutaneous rabbit tibia model with a repeated bacterial challenge. Acta Biomater 82, 102, 2018 [DOI] [PubMed] [Google Scholar]
- 14. Saito E., Saito A., Kato H., et al. . A novel regenerative technique combining bone morphogenetic protein-2 with fibroblast growth factor-2 for circumferential defects in dog incisors. J Periodontol 87, 1067, 2016 [DOI] [PubMed] [Google Scholar]
- 15. Babo P.S., Cai X., Plachokova A.S., et al. . Evaluation of a platelet lysate bilayered system for periodontal regeneration in a rat intrabony three-wall periodontal defect. J Tissue Eng Regen Med 12, e1277, 2018 [DOI] [PubMed] [Google Scholar]
- 16. Cuijpers V.M., Jaroszewicz J., Anil S., Al Farraj Aldosari A., Walboomers X.F., and Jansen J.A.. Resolution, sensitivity, and in vivo application of high-resolution computed tomography for titanium-coated polymethyl methacrylate (PMMA) dental implants. Clin Oral Implants Res 25, 359, 2014 [DOI] [PubMed] [Google Scholar]
- 17. Pellegrini G., Seol Y., Gruber R., and Giannobile W.. Pre-clinical models for oral and periodontal reconstructive therapies. J Dent Res 88, 1065, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rios H.F., and Giannobile W.V.. Preclinical protocols for periodontal regeneration. In: Giannobile W.V. and Nevins M., eds. Osteology Guidelines for Oral and Maxillofacial Regeneration Preclinical Models for Translational Research. New Malden, London, United Kingdom: Quintessence Publishing Co., Ltd, 2011, pp. 77–104 [Google Scholar]
- 19. Shirakata Y., Yoshimoto T., Goto H., et al. . Favorable periodontal healing of 1-wall infrabony defects after application of calcium phosphate cement wall alone or in combination with enamel matrix derivative: a pilot study with canine mandibles. J Periodontol 78, 889, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Hayashi C., Kinoshita A., Oda S., Mizutani K., Shirakata Y., and Ishikawa I.. Injectable calcium phosphate bone cement provides favorable space and a scaffold for periodontal regeneration in dogs. J Periodontol 77, 940, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Boden S.D., Kang J., Sandhu H., and Heller J.G.. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial 2002 volvo award in clinical studies. Spine 27, 2662, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Kokubo S., Fujimoto R., Yokota S., et al. . Bone regeneration by recombinant human bone morphogenetic protein-2 and a novel biodegradable carrier in a rabbit ulnar defect model. Biomaterials 24, 1643, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Aspenberg P., and Turek T.. BMP-2 for intramuscular bone induction: effect in squirrel monkeys is dependent on implantation site. Acta Orthop Scand 67, 3, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Franco N.H., and Olsson I.A.S.. Scientists and the 3Rs: attitudes to animal use in biomedical research and the effect of mandatory training in laboratory animal science. Lab Anim 48, 50, 2014 [DOI] [PubMed] [Google Scholar]