Abstract
Aims.
To study the systematic assessment of need for care and clinical parameters for use in treatment plans in patients diagnosed with severe mental illness.
Methods.
The Cumulative Needs for Care Monitor (CNCM) includes various validated instruments, such as the Camberwell Assessment of Need. A Markov-type cost-effectiveness model (health care perspective, 5-year time horizon) was used to compare CNCM with care as usual (CAU). Two studies were used to determine model parameters: a before–after study (n = 2155) and a matched-control study (n = 937).
Results.
The CNCM may lead to a gain in psychiatric functioning according to the models. CNCM patients remain in (outpatient) care, while CAU patients drop out more frequently. There is only a small difference in inpatient care. As a result, average costs per patient in the CNCM group are between €2809 (before–after model) and €5251 (matched-control model) higher. The iCER was between €45 127 and €57 839 per life year without psychiatric dysfunction gained.
Conclusions.
CNCM may be only cost-effective when willingness to pay for a life year without psychiatric dysfunction is higher than €45 000. However, this result is highly sensitive to the level of psychiatric dysfunctioning in patients who do not receive care.
Key words: Assessments of health care needs, cost-effectiveness, functioning, psychotic disorders
Introduction
In general, the use of needs assessments to plan treatment has been shown to impact on health care use of patients diagnosed with severe mental illness (SMI) (Slade et al. 2006; Robert et al. 2007; Van Os &Triffaux, 2008). However, the question is whether these changes are cost-effective.
The use of a simple patient-reported questionnaire on 20 perceived need areas in treatment plans was associated with higher satisfaction with care at 12-month follow-up, and treatment change was more likely in patients with more reported needs (Van Os et al. 2004; Robert et al. 2007). Another study similarly showed that needs that came to light in an assessment were addressed before the next assessment, albeit not in all areas (Drukker et al. 2008). DIALOG, a tool to discuss 11 domains of clinical need, has been associated with improvement in quality of life and unmet needs for care after 12 months, although symptoms did not change (Priebe et al. 2007). Furthermore, in subjects in which a monthly Routine Outcome Assessment tool was used, inpatient health care use was lower, but there were no differences in subjective outcomes (Slade et al. 2006).
SMI is defined as all patients from ‘integrated care’ services treating patients with severe and enduring psychiatric illnesses (Drukker et al. 2010b). Approximately, 75% of SMI patients are diagnosed with schizophrenia, other psychotic disorder or bipolar disorder (Drukker et al. 2010a). Recently, cost-effectiveness of evidence-based treatment has been identified as a knowledge gap in schizophrenia (Nasrallah et al. 2011). The subgroup of SMI is often target group in research and treatment because it is a relatively homogeneous subgroup at the most severe end of the spectrum of mental disorders (Aagaar & Nielsen, 2004). Therefore, this knowledge gap in cost-effective treatment can be extrapolated to the total SMI group.
In South-Limburg, the Netherlands (population 660 000), the Cumulative Needs for Care Monitor (CNCM) regularly assesses, among others, need for care in SMI patients (Drukker et al. 2010a). Using these data, systematic feedback on individual patient outcomes is provided to professional carers, with a view to induce tailored, needs-based treatment for individual patients (Drukker et al. 2008; Drukker et al. 2010b). Mental health professionals (nurses, social workers, psychiatrists and psychologists) are trained to administer CNCM forms to all patients diagnosed with SMI, who are either outpatients or patients admitted to a general or psychiatric hospital. All patients receiving mental health care, both inpatients and outpatients, are assessed yearly and with every major change in treatment or setting (e.g. hospitalization, start of new treatment and discharge) by their professional carer. Of the yearly evaluations, 63% are performed within 15 months of the previous assessment and 25% between 15 months and 2 years. The main instrument is the Camberwell Assessment of Needs (CAN) (Drukker et al. 2010a). The CAN combines the ratings from both patient and interviewer using a priori decision rules. Quality of life and quality of care are scored by the patient, while the Global Assessment of Functioning (GAF) and other instruments are scored by the interviewer (Drukker et al. 2010a).
Previously, it has been shown that use of the CNCM was associated with an increase in functioning and outpatient care consumption as well as a small decrease in inpatient care consumption (Drukker et al. 2010b, 2011a). However, policy makers need an estimation of the costs of improved functioning to decide on the use of the CNCM. Health technology assessment (HTA) methods have been developed to assess cost-effectiveness. Recently, these methods have been implemented in mental health research throughout Europe (Evers et al. 2007).
The present paper used decision-analytic modelling to evaluate the costs, effects and cost-effectiveness of the CNCM as compared with care as usual (CAU) in patients diagnosed with SMI. The main outcome is life years without psychiatric dysfunction (GAF).
Methods
Psychiatric Case Registers and population characteristics
Psychiatric Case Registers (PCR) are anonymous cumulative registers of admissions (including duration), outpatient contacts and days in day care (e.g. from psychiatric hospital, community mental health centre and psychiatric department of university hospital). The PCR South Limburg includes the CNCM region.
In the CNCM region, approximately 1.6% of the adult population is diagnosed with SMI (Drukker et al. 2010a). Prevalence of SMI has been constant over the years. Yearly incidence figures are around 2–3 per 10 000 inhabitants (McGrath, 2006).
Treatment arms
In the present paper, addition of the CNCM to CAU was studied. Thus, treatment arms were CNCM and CAU. CNCM is described above. CAU primarily consists of hospital-based crisis intervention, sheltered living and follow-up case management after discharge. Some ambulatory patients went regularly to the outpatient clinic. Services were split between ‘cure’ and ‘care’. Long-term care services were almost entirely ‘care’ based and almost all the modern evident-based practices were not available. Medication is provided to prevent relapse and medication regimes are reviewed approximately once a year (if no crisis occurs).
Model description
A Markov model was constructed to simulate the course of events in CNCM and CAU in a hypothetical cohort of 1000 SMI patients. The cohort had the age (mean: 42 years) distribution as observed in the CNCM data.
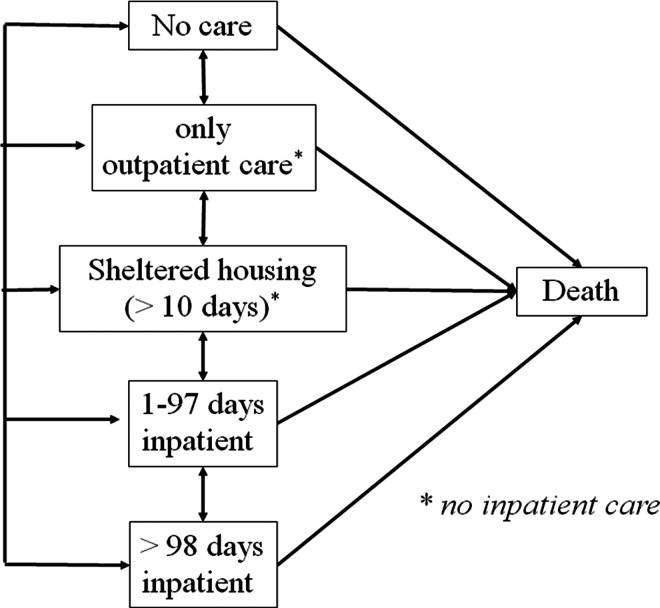
Health states were defined based on mental health care use, as a proxy for severity of illness. Health states included in the models were: no care (NOCA), outpatient care only (OU), sheltered housing (SHEL), short-term inpatient care (STIN; ≤97 days per year), and long-term inpatient care (LTIN >97 days per year). Figure 1 is a graphical presentation of the model structure.
Fig. 1.
Graphical representation of the model structure. A patient can transit from health state-to-health state (including remaining in the same health state). Death is the absorbing state.
The effectiveness of CNCM was determined based on data from two separate studies: a before–after study and a matched control study. This resulted in two model analyses: a before–after model and a matched-control model. The time-horizon was set at 5 year for both model analyses. The before–after model uses data between a maximum of 5 years before and a maximum of 5 years after the CNCM assessment (2155 subjects), and the matched-control model uses data between one year before and one year after the (hypothetical) assessment (n = 937). Extrapolation of the matched-control data to five years was thought to be plausible because figures are relatively constant over the years in an adult SMI population. Results of extrapolation to a life-time time horizon were deemed unrealistic. The cycle length was set at one year, because CNCM is assessed yearly in stable SMI patients. Future costs and effects were discounted to their present value by a rate of 4 and 1.5%, respectively, according to Dutch guidelines (College voor zorgverzekeringen, 2006; Council for Public Health and Health Care, 2006). Analyses to calculate the model inputs were performed in Stata version 11 (StataCorp., 2009). Models were built in Excel.
Transition probabilities
Mental health care use was obtained from the PCRs. Data were available until December 31st, 2008. CNCM and PCR data were matched anonymously at the level of individual patients using an encrypted identification code (Drukker et al. 2010a). For the before–after model, of each CNCM patient the first assessment after January 2007 (CNCM) as well as the first assessment ever (CAU) were matched with the PCR, to obtain mental health care use in the five year before and the five year after this specific CNCM date (as far as available).
For the matched-control model, CNCM patients were matched with control patients from the North of the Netherlands (population 1.7 million), using propensity score matching, as has been reported in a previous paper (Drukker et al. 2011a). Mental health care use in the year before and in the year after this date was obtained from the PCRs.
Transition probabilities were obtained from a cross-tabulation of patients in a health state in one year by the health state in the next year, stratified by CNCM and CAU. In Table 1, these numbers are presented in the column ‘uncalibrated’. The numbers after calibration are presented in the column optimal GOF (goodness of fit). Distribution of the cohort over the health states in year 1 was based on the percentages in the control region (NOCA = 50; OU = 600; SHEL = 50; STIN = 150; LTIN = 150).
Table 1.
Transition probabilities in model 1 (before–after) and model 2 (matched-control)
| Health states | Cumulative monitor of needs for care | Care as usual | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Numbers of patients | Numbers of patients | ||||||||
| From | To | Uncalibrated | Optimal GOF | Total | Transition probability (optimal GOF) (%) | Uncalibrated | Optimal GOF | Total | Transition probability (optimal GOF) (%) |
| Model 1 | |||||||||
| No care | 237 | 710 | |||||||
| Outpatient | 82 | 82 | 35 | 152 | 152 | 21 | |||
| Sheltered housing | 12 | 12 | 5 | 13 | 13 | 2 | |||
| Short-term inpatient | 30 | 26.70 | 11 | 90 | 90 | 13 | |||
| Long-term inpatient | 19 | 16.91 | 7 | 38 | 38 | 5 | |||
| Outpatient | 822 | 745 | |||||||
| No care | 15 | 15 | 2 | 52 | 52 | 7 | |||
| Sheltered housing | 54 | 54 | 7 | 44 | 44 | 6 | |||
| Short-term inpatient | 110 | 97.90 | 12 | 130 | 130 | 17 | |||
| Long-term inpatient | 56 | 49.84 | 6 | 51 | 51 | 7 | |||
| Sheltered housing | 624 | 247 | |||||||
| No care | 3 | 3 | 0.5 | 1 | 1 | 0.4 | |||
| Outpatient | 17 | 17 | 3 | 5 | 5 | 2 | |||
| Short-term inpatient | 42 | 37.38 | 6 | 11 | 11 | 4 | |||
| Long-term inpatient | 12 | 10.68 | 2 | 3 | 3 | 1 | |||
| Short-term inpatient | 367 | 227 | |||||||
| No care | 7 | 7 | 2 | 16 | 16 | 7 | |||
| Outpatient | 96 | 96 | 26 | 76 | 76 | 31 | |||
| Sheltered housing | 50 | 50 | 14 | 20 | 20 | 8 | |||
| Long-term inpatient | 85 | 75.65 | 21 | 52 | 52 | 21 | |||
| Long-term inpatient | 611 | 342 | |||||||
| No care | 0 | 0 | 0 | 3 | 3 | 1 | |||
| Outpatient | 32 | 32 | 5 | 20 | 20 | 6 | |||
| Sheltered housing | 36 | 36 | 6 | 16 | 16 | 5 | |||
| Short term inpatient | 63 | 63 | 10 | 47 | 47 | 14 | |||
| Model 2 | |||||||||
| No care | 237 | 46 | |||||||
| Outpatient | 17 | 17 | 37 | 17 | 17 | 37 | |||
| Sheltered housing | 0 | 0 | 1 | 0 | 0 | 1 | |||
| Short-term inpatient | 8 | 2.80 | 6 | 8 | 8 | 17 | |||
| Long-term inpatient | 1 | 0.35 | 0.7 | 1 | 1 | 2 | |||
| Outpatient | 822 | 94 | |||||||
| No care | 0 | 0 | 0.5 | 49 | 49 | 12 | |||
| Sheltered housing | 2 | 2 | 2 | 0 | 0 | 0.1 | |||
| Short-term inpatient | 8 | 2.80 | 3 | 31 | 31 | 8 | |||
| Long-term inpatient | 2 | 0.70 | 0.7 | 4 | 4 | 1 | |||
| Sheltered housing | 624 | 47 | |||||||
| No care | 0 | 0 | 1 | 3 | 3 | 7 | |||
| Outpatient | 1 | 1 | 2 | 3 | 3 | 7 | |||
| Short-term inpatient | 2 | 0.70 | 1 | 4 | 4 | 9 | |||
| Long-term inpatient | 1 | 0.35 | 0.7 | 1 | 1 | 2 | |||
| Short-term inpatient | 367 | 39 | |||||||
| No care | 2 | 2 | 5 | 12 | 12 | 12 | |||
| Outpatient | 10 | 10 | 26 | 45 | 45 | 44 | |||
| Sheltered housing | 3 | 3 | 8 | 3 | 3 | 3 | |||
| Long-term inpatient | 4 | 1.40 | 4 | 9 | 9 | 9 | |||
| Long-term inpatient | 611 | 55 | |||||||
| No care | 0 | 0 | 0 | 1 | 1 | 1 | |||
| Outpatient | 2 | 2 | 4 | 9 | 9 | 9 | |||
| Sheltered housing | 0 | 0 | 1 | 2 | 2 | 2 | |||
| Short-term inpatient | 4 | 4 | 7 | 3 | 3 | 3 | |||
CAN, Camberwell Assessment of Needs
CAU, Care as Usual
CEA, Costs Effectiveness Analysis
CEAC, Costs Effectiveness Acceptibility Curve
CNCM, Cumulative Monitor of Needs for Care
FACT, Flexible Assertive Community Treatment
GAF, Global Assessment of Functioning
GOF, Goodness of Fit
HTA, Health Technology Assessment
ICER, incremental cost-effectiveness ratio
LTIN, Health state: Long-term inpatient care
NOCA, Health state: No care
OU, Health state: Outpatient care
PCR, Psychiatric Case Register
QALY, Quality adjusted life year
RCT, Randomised Controlled Trail
SHEL, health state: Sheltered housing
SMI, Severe mental illness
STIN, Health state: short term inpatient care
Mortality figures in SMI patients were obtained from Wijnand Laan (PCR Middle Netherlands, using PCR data as well as data from Statistics Netherlands, unpublished results).
Health effects
Outcome was psychiatric functioning as measured with the GAF (fifth axis of the DSM IV). Scores range from 0 (poor) to 100 (very good) (Ramirez et al. 2008). The GAF used in the CNCM is divided into its Impairment component and its Psychopathological symptoms component (Jones et al. 1995). Thus, ‘life year without psychiatric impairment’ and ‘life year without psychiatric symptoms’ were the two outcomes in the present analyses. These outcomes represent a better alternative than QALYs, because instruments needed to calculate QALYs are invalid in psychiatry (Chisholm et al. 1997; Roick et al. 2004; Van de Willige et al. 2005; Konnopka et al. 2006), while the life year without psychiatric dysfunction score approximates valid QALYs. GAF scores were only available from the CNCM database. These data were used to estimate average functioning per health state (Table 2). GAF-scores were transformed because scores between 85 and 100 indicate optimal functioning. Thus, all scores of 85 and higher were recoded into 1 and all scores below 85 were multiplied by 100/8500, resulting in transformed scales ranging 0–1. These scales were used as weights to calculate life years adjusted for psychiatric functioning (transformed scale *year = adjusted life year).
Table 2.
Functioning per health state
| Mean | Standard error | n | Notes | |
|---|---|---|---|---|
| Functioning impairment | ||||
| No care | 0.40 | 0.078 | 3 | Small n |
| Outpatient only | 0.61 | 0.014 | 97 | |
| Sheltered housing | 0.53 | 0.015 | 81 | |
| Short-term inpatient care | 0.53 | 0.030 | 38 | |
| Long-term inpatient care | 0.46 | 0.021 | 56 | |
| Functioning symptoms | ||||
| No care | 0.44 | 0.090 | 3 | Small n |
| Outpatient only | 0.63 | 0.015 | 100 | |
| Sheltered housing | 0.54 | 0.018 | 81 | |
| Short-term inpatient care | 0.50 | 0.030 | 38 | |
| Long-term inpatient care | 0.46 | 0.027 | 57 |
n, number of patients.
Costs
Mental health care use per health state is presented in Table 3. Mental health care costs were costs per unit X actual mental health care use. Costs per unit (year = 2003) were obtained from the Dutch costs manual (Oostenbrink et al. 2004) and were calculated to their 2007 value using price index figures from Statistics Netherlands (CBS, 2009) (outpatient contact: €93.12; sheltered housing €105.82). Additionally, the administration of the largest institution in the CNCM-region supplied unit costs for short-term and long-term inpatients (personal communication, 07-12-2009, Richard Janssen, Petra Soeters; short-term days indexed to 2007: €250.72; long-term days indexed: €207.33).
Table 3.
Health care use per health state in model 1 (before–after) and model 2 (matched-control)
| Model 1 (CAU = baseline) | Model 2 (CAU = NN) | |||
|---|---|---|---|---|
| Mean | Standard error | Mean | Standard error | |
| Inpatient days per year | ||||
| No care | 0 | 0 | ||
| Outpatient only | 0 | 0 | ||
| Sheltered housing | 0 | 0 | ||
| Short-term inpatient care | 33.3 | 0.75 | 22.0 | 1.79 |
| Long-term inpatient care | 301.2 | 2.84 | 323.8 | 5.80 |
| Outpatient contacts per year | ||||
| No care | 0 | 0 | ||
| Outpatient only | 29.3 | 0.72 | 13.4 | 0.61 |
| Sheltered housing | 18.7 | 1.31 | 10.0 | 1.63 |
| Short-term inpatient care | 43.4 | 1.12 | 18.7 | 1.25 |
| Long-term inpatient care | 36.8 | 1.25 | 3.7 | 0.91 |
| Sheltered housing (days per year) | ||||
| No care | 0 | 0 | ||
| Outpatient only | 0.2 | 0.03 | 0 | |
| Sheltered housing | 286.2 | 5.14 | 325.9 | 7.44 |
| Short-term inpatient care | 57.4 | 4.51 | 26.5 | 5.29 |
| Long-term inpatient care | 23.8 | 2.69 | 6.5 | 2.09 |
CAU, care as usual; NN, North of the Netherlands.
The local academic hospital serving Maastricht and surrounding areas (no other somatic hospitals in this region) provided hospital administration data on somatic health care consumption (procedures, admissions, laboratory, diagnostic techniques) during 5 years before and 5 years after the CNCM date, for all patients that were both in the CNCM and in the hospital database (329 CNCM patients). These data were matched using the same encrypted identification code as used above. Unit costs for hospital care from the year 2007 were used to calculate the somatic costs. This way, somatic costs per health state could be estimated in the subsample of patients in the catchment area of this hospital. This subsample can be assumed to be representative for the total sample.
Model assumptions, validation and calibration
In order to validate model outcomes, health care use as predicted by the model was compared to the observed data. In case the model predictions differ substantially from the data, the models should be calibrated so that output is more similar to reality (Vanni et al. 2010). This was done by improving goodness of fit (GOF), based on inpatient health care use, outpatient health care use and the two GAF outcomes, using a multiplication factor between 0 and 1 to calibrate transition rates (see results and appendix).
Analyses
Incremental cost-effectiveness ratios (iCERs) were calculated. Parameter uncertainty surrounding the iCERs was handled probabilistically (Weinstein, 2006). Measures of variance were retrieved from the data as described above. Functioning was assigned a beta-distribution, mental health care consumption was assigned a gamma-distribution and transition rates were assigned a Dirichlet distribution. Parameter values were drawn at random from the assigned distributions, using Monte Carlo simulation with 5000 iterations. Whether CNCM is deemed efficient depends on how much society is willing to pay for a life year in perfect functioning, which is referred to as ceiling ratio (Briggs et al. 2007). For further reading on the theory of willingness to pay for health gain we refer to the literature (Drummond, 2005; Briggs et al. 2007). To illustrate the results of the simulation, cost-effectiveness acceptability curves (CEACs) were calculated (Van Hout et al. 1994; Fenwick et al. 2001). For different ceiling ratios, the net monetary benefit was calculated for each strategy by subtracting the costs from the effects, multiplied by the ceiling ratio.
As data were obtained from the CNCM data base, assessment of functioning in no-care patients was problematic; data from only 3 patients could be included. Therefore, one-way sensitivity analyses were performed to test consistency of the results. The level of functioning in this group was varied (0.1 and 0.2 higher than observed and 0.1 and 0.2 lower than observed).
Results
Without calibration, the number of inpatient days in CNCM was higher than in CAU, while the regression results showed a decrease in inpatient days. Therefore, all transition rates to health states with more inpatient care were multiplied by a multiplication factor, to obtain the best possible GOF (see appendix). Multiplication factor before–after model: 0.89, matched-control model: 0.35.
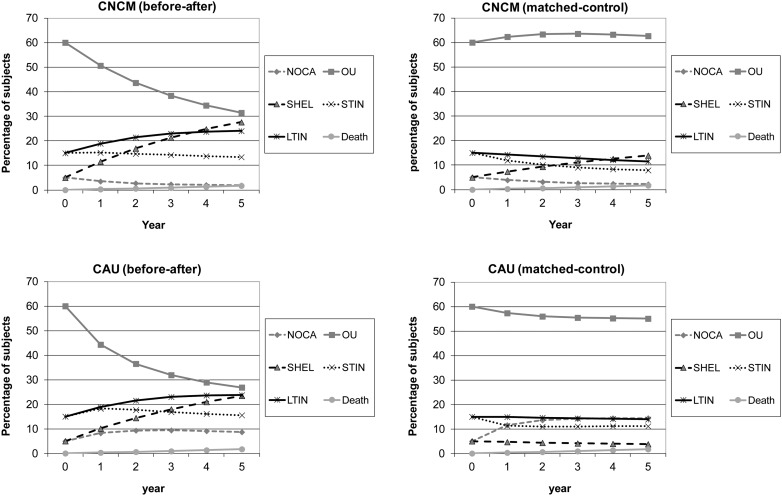
Figure 2 presents expected results based on transition rates. The lines represent the migration of a group of 1000 patients over the health states over 5 years in CNCM group and CAU group. Both models show an increase in patients in the no-care group in CAU and an increase in patients in sheltered housing in CNCM. The average costs per patient are higher in the CNCM strategy (before–after model difference: €2809; matched-control model difference: €5251).
Fig. 2.
Cohort migration. Model 1, before–after and model 2, matched-control. NOCA, no care; OU, outpatient care only; SHEL, sheltered housing; STIN, short-term inpatient care (≤97 days per year); LTIN, long-term inpatient care (>97 days per year).
Cost per life year without psychiatric impairment
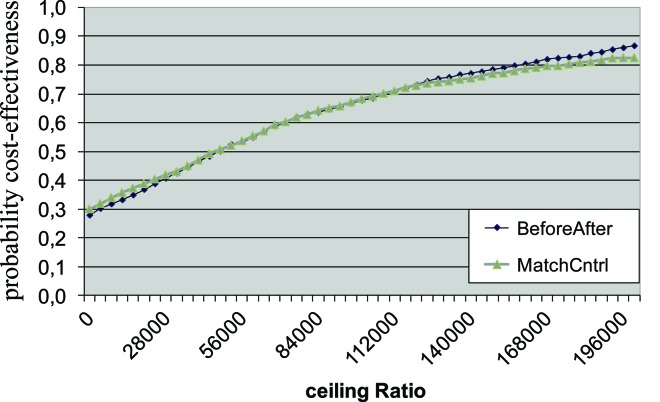
Patients in CNCM gain life years without psychiatric impairment as compared to patients in CAU (before–after model: 0.06, matched-control model: 0.10). This leads to iCERs of €45 127 and €52 991 per life year without psychiatric impairment gained. The bootstrap replicates in the iCER planes were mainly located in the North-East quadrant, indicating health gains and more costs. The uncertainty is considerable, as reflected in the CEAC curves presented in Fig. 3. When willingness to pay is €100 000, the probability that CNCM is cost-effective is 68% (in both before–after and matched-control model). The cost-effectiveness probability of CNCM asymptotically approaches approximately 90%.
Fig. 3.
Cost-effectiveness acceptability curve (impairment). BeforeAfter, before–after model; MatchCntrl, matched-control model.
Costs per life year without psychiatric symptoms
In the CNCM strategy, patients gain life years without psychiatric symptoms as compared with CAU (before–after model: 0.06, matched-control model: 0.09). ICERs were €45 944 and €57 839 per year, respectively. The bootstrap replicates in the iCER planes were again mainly located in the North-East quadrant. When willingness to pay is €100 000, the probability that CNCM is cost-effective is 65 and 62% (before–after and matched-control, respectively).
Sensitivity analyses
Only when patients in the NOCA health state function 0.2 points (or more) better than in the base case analysis (impairment = 0.40; symptoms = 0.44), functioning is no longer better in CNCM than in CAU (Table 4).
Table 4.
Sensitivity analyses: how do outcomes change when functioning in the no-care group varies
| Change in GAF Parameters in NOCA | Input parameters | Outcomes | ||||
|---|---|---|---|---|---|---|
| Impairment (imp) | Symptoms (sym) | GAF (imp) | GAF (sym) | iCER (imp) (1000 euro) | iCER (sym) (1000 euro) | |
| 0.2 < | 0.20 | 0.24 | 0.12 | 0.12 | 22.6 | 22.9 |
| 0.1 < | 0.30 | 0.34 | 0.09 | 0.09 | 30.2 | 30.6 |
| base case | 0.40 | 0.44 | 0.06 | 0.06 | 45.1 | 45.9 |
| 0.1 > | 0.50 | 0.54 | 0.03 | 0.03 | 89.5 | 93.5 |
| 0.2 > | 0.60 | 0.64 | 0 | 0 | not interpretable | |
NOCA, no care health state; imp, impairment; sym, symptoms
Discussion
When need for care and other assessments are used in treatment plans, patients remain in care longer and have improved functioning. The small decrease in inpatient days does, however, not make up for the extra costs of the increase in outpatient contacts. Thus, although the intervention is relatively cheap (one hour salary of a professional carer per year per patient) the iCER is between €45 127 and €57 839 per functioning adjusted life year gained. Thus, CNCM is only cost-effective when willingness to pay for a life year without psychiatric impairment or symptoms exceeds €45 000 per life year without psychiatric dysfunctioning gained. The results are surrounded by considerable uncertainty.
The increase in outpatient costs in combination with a decrease in inpatients costs could be expected because tailored treatment would in theory lead to an immediate increase in extensive outpatient care consumption and, inducing, as a consequence prevention of future deterioration and admissions (Drukker et al. submitted for publication).
A recent paper on crisis resolution teams also showed an increase in non-inpatient costs, also including GP-costs. This increase in non-inpatient costs co-occurred with a decrease in inpatient costs (McCrone et al. 2009). In CNCM, the increase in outpatient care without a drop in inpatient care reduces cost-effectiveness. On the other hand, the long-term use of outpatient care theoretically prevents relapse. Thus, despite the fact that the present results are marginally positive, the increase in outpatient care potentially has much stronger positive results in the long run. This scenario could not be shown in this relatively short-term analysis. A randomized controlled trial (RCT) studying the use of Routine Outcome Monitoring showed a decrease in costs as a consequence of a decrease in inpatient health care use (Slade et al. 2006). The decrease in inpatient care is in line with the present study, but the decrease in total costs is not. This may be a consequence of the patient group; the RCT also included non-SMI patients. Because in the RCT, monitoring was not effective, it was also not cost-effective (Slade et al. 2006).
Systematic assessment of needs in SMI patients, as studied in the present paper, has been advocated (Van Os et al. 2004; Priebe et al. 2007; Robert et al. 2007; Van Os & Triffaux, 2008). Except for the above-mentioned RCT, previous cost-effectiveness research on systematic needs assessment, however, has only been performed in depressive patients (Gilbody et al. 2003). ICERs in depressive patients were between €8649 and €15 234 ($11 341 and $19 976; Pyne et al. 2003), which is lower than iCERs in the present paper. However, the depressed patient group is less severely ill than the SMI patients and iCERs in the present paper were not based on QALYs (see methodological issues).
An economic evaluation of an intervention aimed to decrease drop-out has the limitation that dropped-out care avoiders per definition do not use inpatient or outpatient health care, resulting in health care costs that are close to zero during the period of drop-out. The hypothesized increase in health care use and other costs after deterioration (crisis admission because of risk for self or others) or relapse could not be assessed and, therefore, was not in our models. Although continuity of care is generally accepted as beneficial, models show low cost-effectiveness of the intervention. Models are a simplification of reality. It is almost impossible to assess long-term health care use after the intervention, unless the intervention is old. We feel that continuity of care is beneficial in the long run although our models could not prove this. In addition, parameters of model 1 and 2 differed substantially and reasons for these differences were difficult to allocate. However, both models lead to the same conclusions.
Methodological issues
The PCR uses data that all mental health care institutions also provide to insurers to claim treatment expenses. Thus, PCR data are accurate. In the years before and after the assessment, patients who lived and used mental health care outside the PCR region at that time were erroneously included in the no-care health state. This could have led to a slight overestimation of transition rates to NOCA. However, it is unlikely that results are substantially biased, because this overestimation is similar in both treatment arms.
When model output deviates from empirical analysis results, the model parameters need to be adapted (Vanni et al. 2010), because it seems illogical to choose the model output over real data. Methods from engineering have been proposed to efficiently search the best fit (Kong et al. 2009). However, these methods are still in their infancy and guidelines on calibration methods are lacking. Therefore, in the present paper, transition rates were adapted by adding a multiplication factor (manually) to obtain levels of inpatient care that were more similar to empirical results. Unfortunately, this multiplication factor was only 0.35 in the matched-control model. Although GOF after calibration was similar to GOF in the calibrated before–after model, the subset of transition rates were more different from the original data than in the before–after model.
In part of the CNCM region, a special version of assertive community treatment, flexible assertive community treatment (FACT) was in place (Van Veldhuizen, 2007; Drukker et al. 2011b). CNCM is an ingredient of FACT and, therefore, CNCM and FACT cannot be disentangled. FACT is partly responsible for the pattern of psychiatric care use in the CNCM region. However, similar increases in care consumption were found in the subregion where FACT was introduced later (Drukker et al. 2011a). A post hoc sensitivity analysis was performed excluding the FACT region (before–after model). In this model, ICERs were considerably lower: €25 700 (impairment) and €30 250 (symptoms). Thus, costs may be lower if CNCM is studied in non-FACT regions, but the conclusion remains that CNCM may be only cost-effective when willingness to pay for a life year without psychiatric dysfunction is relatively high.
The present paper has several limitations. First, in the NOCA group, functioning was estimated using data of only three patients, who are likely not representative for all NOCA patients. NOCA patients are not in contact with any mental health service and only patients in care are interviewed. NOCA patients do not necessarily function better than in-care patients, because a substantial part of the NOCA patients are drop-outs, who do need care (Schout et al. 2010). A previous paper showed that non-attenders (within the subgroup of patients being longer in psychiatric care with more serious mental illness) had more severe symptoms (Manchester scale score) and impairment (social adjustment scale score) than attenders (Killaspy et al. 2000). Furthermore, 12 of the 13 drop-outs of standard care were still severely ill with the risk of further deterioration and admission (Sytema et al. 2007).The present data showed that patients who dropped out of care in the next year had lower levels of depression and anxiety while other psychopathology and functioning did not differ (unpublished results). Thus, we had reason to believe that patients in the NOCA health state were as severely ill as inpatients. Using the three patients in NOCA, we found functioning levels similar to the patients using short- or long-term inpatient care (STIN and LTIN). Because this is what we expected, we used these estimates in the model and we conducted sensitivity analyses with higher and lower levels of functioning in the NOCA health state. These analyses show that the results are sensitive to changes in the level of functioning in the NOCA health state. Future research on characteristics and severity of symptoms in drop-outs is crucial, despite the difficulty to motivate these drop-outs to join a study.
Second, the EuroQol-5D is one of few instruments that can be used to calculate utilities (QALYs) for health outcomes for use in cost-utility analyses (EuroQol Group, 1990; Chisholm et al. 1997; Sculpher, 2008). However, its validity in psychiatric populations has been questioned (Chisholm et al. 1997; Roick et al. 2004; Van de Willige et al. 2005; Konnopka et al. 2006). Therefore, the present paper presents cost-effectiveness analyses with GAF as the outcome, rather than cost-utility analyses. The GAF is recognized as a valid and comprehensive measure of psychiatric functioning when used in routine clinical practice (Jones et al. 1995; Tungstrom et al. 2005) and in research (Jones et al. 1995; Startup et al. 2002). Precision of GAF scores at the group level is sufficient (Soderberg et al. 2005).
Third, the present data sets do not include medication use. Most SMI patients use medication on a daily basis. Thus, costs are relatively high. Because each individual patient responds differently, psychiatric medication costs for HTA models can only be assessed by studying large patient cohorts (College voor zorgverzekeringen, 2011). However, amount and type of medication use in patient with severe mental illness do not vary primarily as a function of health states and symptom severity, in the Netherlands (as opposed to, e.g. the United States). In the present data, there are no differences between the health states (OU, SHEL, STIN and LTIN) in use (yes/no) of psychiatric medication (total χ2 = 1.3, df = 3, p = 0.7; antipsychotics χ2 = 1.6, df = 3, p = 0.7; antidepressants χ2 = 1.0, df = 3, p = 0.8 and anxiolitica χ2 = 6.1, df = 3, p = 0.11). Thus, virtually all patients are prescribed one or more maintenance medications and changes in health state/severity is accompanied by changes in medication not impacting on net medication costs. In addition, it is known that the medication factor most impacting on health states indicating severity of symptoms is not medication type and quantity, but medication compliance (Priebe et al. 2010). If medication costs would be lower in the better health states, the iCER of CNCM v. CAU would decrease.
Furthermore, health states were based on mental health care use, as a proxy of severity, because this was the only indicator of the course of disease severity available in CAU data. In the before–after study, functioning was only assessed as part of the CNCM and thus not available in the ‘before’ dataset; functioning similarly was not measured in the control region. Although mental health care use is associated with severity of illness (Van Os et al. 1999), other factors are involved as well. In the future, longitudinal data on the study outcome need to be collected in all treatment arms. Furthermore, if future research benefits from the availability of comparative effectiveness data, health states can be based on indicators such as functioning, quality of life or severity of symptoms.
Finally, the present analyses were performed from the health care perspective, while a societal perspective would have been preferable. Owing to a lack of data on societal consequences in PCR and CNCM, this was not feasible. We chose not to collect data to assess the impact of the CNCM intervention, because this can be regarded as a minimal intervention and could have contaminated the effect. This study is one of the first economic evaluations in the field of SMI. Although not ideal, the fact that the study is based on the use of large sets of real world data adds to the practical relevance of the results. Nevertheless, in addition to real-life data as in the current study, complementary data from randomized controlled trials are also needed. As far as we know, this is the first study in SMI using the GAF to calculate life years without psychiatric impairment or symptoms. As a result, there is no benchmark for the maximum costs per ‘life year without psychiatric impairment or symptoms’ saved.
Conclusion
Although the use of CNCM impacts on mental health care consumption, the models used in the present paper suggest that CNCM is only cost-effective when willingness to pay for relief of 1 year without psychiatric symptoms or 1 year without impairment in functioning exceeds €45 100 or even 57 800.
Acknowledgements
The authors are grateful to all mental health care workers in the region, who administered CNCM forms for use in the individual treatment of patients. We thank Richard Janssen and Petra Soeters (Mondriaan) for giving an indication of inpatient costs for treatment and long stay separately.
Declaration of Interest
We gratefully acknowledge the financial support by ZonMW, the Netherlands Organisation for Health Research and Development (grant number 94507727). The authors declare that they have no competing interests.
References
- Aagaar J, Nielsen JA (2004). Experience from the first ACT programme in Denmark. II. Severe mental illness. A register diagnosis. Nord Journal of Psychiatry 58, 171–174. [DOI] [PubMed] [Google Scholar]
- Briggs A, Claxton K, Sculpher M (2007). Decision Modelling for Health Economic Evaluations. Oxford University Press: Oxford. [Google Scholar]
- CBS (Statistics Netherlands) (2009). StatLine (http://statline.cbs.nl/). Accessed 2 October 2 2009.
- Chisholm D, Healey A, Knapp M (1997). QALYs and mental health care. Social Psychiatry and Psychiatric Epidemiology 32, 68–75. [DOI] [PubMed] [Google Scholar]
- College voor Zorgverzekeringen (2006). Guidelines for pharmacoeconomic research, updated version. Richtlijnen voor farmaco-economisch onderzoek, geactualiseerde versie. Diemen.
- College voor Zorgverzekeringen (2011). Medicijnkosten (http://www.medicijnkosten.nl). Accessed May 2011.
- Council for Public Health and Health Care (2006). Sensible and Sustainable Care. Council for Public Health and Health Care: Zoetermeer. [Google Scholar]
- Drukker M, Van Dillen K, Bak M, Mengelers R, Van Os J, Delespaul P (2008). The use of the Camberwell Assessment of Need in treatment: what unmet needs can be met? Social Psychiatry and Psychiatric Epidemiology 43, 410–417. [DOI] [PubMed] [Google Scholar]
- Drukker M, Bak M, Campo J, Driessen G, Van Os J, Delespaul PA (2010a). The Cumulative Needs for Care Monitor (CNCM), a unique monitoring system in the South of The Netherlands. Social Psychiatry and Psychiatric Epidemiology 45, 475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drukker M, Van Os J, Bak M, Campo J, Delespaul P (2010b). Systematic monitoring of needs for care and global outcomes in patients with severe mental illness. BMC Psychiatry 10, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drukker M, Van Os J, Dietvorst M, Sytema S, Driessen G, Delespaul P (2011a). Does monitoring need for care in patients diagnosed with severe mental illness impact on psychiatric service use? Comparison of monitored patients with matched controls. BMC Psychiatry 11, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drukker M, Van Os J, Sytema S, Driessen G, Visser E, Delespaul P (2011b). Function Assertive Community Treatment (FACT) and psychiatric service use in patients diagnosed with severe mental illness. Epidemiology and Psychiatric Sciences 20, 273–278. [DOI] [PubMed] [Google Scholar]
- Drukker M, Visser E, Sytema S, Van Os J (submitted). Flexible Assertive Community Treatment, severity of symptoms and psychiatric health service use. [DOI] [PMC free article] [PubMed]
- Drummond MF (2005). Methods for the Economic Evaluation of Health Care Programmes. Oxford University Press: Oxford. [Google Scholar]
- EuroQol Group (1990). EuroQol-a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy 16, 199–208. [DOI] [PubMed] [Google Scholar]
- Evers S, Salvador-Carulla L, Halsteinli V, Mcdaid D, Mheen Group (2007). Implementing mental health economic evaluation evidence: building a bridge between theory and practice. Journal of Mental Health 16, 223–241. [Google Scholar]
- Fenwick E, Claxton K, Sculpher M (2001). Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Economics 10, 779–787. [DOI] [PubMed] [Google Scholar]
- Gilbody SM, House AO, Sheldon TA (2003). Outcome measures and needs assessment tools for schizophrenia and related disorders. Cochrane Database of Systrmatic Reviews, 10.1002/14651858.CD003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SH, Thornicroft G, Coffey M, Dunn G (1995). A brief mental health outcome scale-reliability and validity of the Global Assessment of Functioning (GAF). British Journal of Psychiatry 166, 654–659. [DOI] [PubMed] [Google Scholar]
- Killaspy H, Banerjee S, King M, Lloyd M (2000). Prospective controlled study of psychiatric out-patient non-attendance. Characteristics and outcome. British Journal of Psychiatry 176, 160–165. [DOI] [PubMed] [Google Scholar]
- Kong CY, McMahon PM, Gazelle GS (2009). Calibration of disease simulation model using an engineering approach. Value Health 12, 521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnopka A, Gunther OH, Angermeyer MC, Konig HH (2006). [Discriminative ability, construct validity and sensitivity to change of the EQ-5D quality of life questionnaire in paranoid schizophrenia] Diskriminationsvermögen, Konstruktvalidität und Veränderungssensitivität des EQ − 5D Lebensqualitäts − fragebogens bei paranoider Schizophrenie. Psychiatrische Praxis 33, 330–336. [DOI] [PubMed] [Google Scholar]
- McCrone P, Johnson S, Nolan F, Pilling S, Sandor A, Hoult J, Mckenzie N, Thompson M, Bebbington P (2009). Economic evaluation of a crisis resolution service: a randomised controlled trial. Epidemiologia e Psichiatria Sociale 18, 54–58. [DOI] [PubMed] [Google Scholar]
- McGrath JJ (2006). Variations in the incidence of schizophrenia: data versus dogma. Schizophrenia Bulletin 32, 195–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah H, Tandon R, Keshavan M (2011). Beyond the facts in schizophrenia: closing the gaps in diagnosis, pathophysiology, and treatment. Epidemiology and Psychiatric Sciences 20, 317–327. [DOI] [PubMed] [Google Scholar]
- Oostenbrink J, Bouwmans C, Koopmanschap M, Rutten F (2004). [Manual for costs research] Handleiding voor kostenonderzoek; methoden en standaard kostprijzen voor economische evaluaties in de gezondheidszorg. Geactualiseerde versie 2004. College voor zorgverzekeringen: Diemen. [Google Scholar]
- Priebe S, Mccabe R, Bullenkamp J, Hansson L, Lauber C, Martinez-Leal R, Rossler W, Salize H, Svensson B, Torres-Gonzales F, Van Den Brink R, Wiersma D, Wright DJ (2007). Structured patient-clinician communication and 1-year outcome in community mental healthcare: cluster randomised controlled trial. British Journal of Psychiatry 191, 420–426. [DOI] [PubMed] [Google Scholar]
- Priebe S, Sinclair J, Burton A, Marougka S, Larsen J, Firn M, Ashcroft R (2010). Acceptability of offering financial incentives to achieve medication adherence in patients with severe mental illness: a focus group study. Journal of Medical Ethics 36, 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne JM, Rost Km, Zhang M, Williams DK, Smith J, Fortney J (2003). Cost-effectiveness of a primary care depression intervention. Journal of General Internal Medicine 18, 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez A, Ekselius L, Ramklint M (2008). Axis V-Global Assessment of Functioning scale (GAF), further evaluation of the self-report version. European Psychiatry 23, 575–579. [DOI] [PubMed] [Google Scholar]
- Robert PH, Michel E, Van Os J, Altamura AC, Bobes J, Gerlach J, Hellewell Js, Kasper S, Nabel D (2007). [2-COM: presentation of an instrument facilitating communication between physicians and carers in daily practice] 2-COM: presentation d'un instrument permettant de faciliter la communication entre medecin et soignants en pratique quotidienne. Encephale 33, 60–64. [DOI] [PubMed] [Google Scholar]
- Roick C, Thierfelder K, Heider D, Klemm T, Paschke R, Angermeyer MC (2004). [Quality of life instruments and health state preferences to assess effects of medical interventions for mentally and medically ill patients] Untersuchung der Aussagefähigkeit psychometrischer und präferenzbasierter Lebensqualitätindizes bei psychisch und somatisch Kranken. Psychiatrische Praxis 31, 128–137. [DOI] [PubMed] [Google Scholar]
- Schout G, De Jong G, Zeelen J (2010). Establishing contact and gaining trust: an exploratory study of care avoidance. Journal of Advanced Nursing 66, 324–333. [DOI] [PubMed] [Google Scholar]
- Sculpher M (2008). NICE's 2008 methods guide: sensible consolidation or opportunities missed? Pharmacoeconomics 26, 721–724. [DOI] [PubMed] [Google Scholar]
- Slade M, McCrone P, Kuipers E, Leese M, Cahill S, Parabiaghi A, Priebe S, Thornicroft G (2006). Use of standardised outcome measures in adult mental health services: randomised controlled trial. British Journal of Psychiatry 189, 330–336. [DOI] [PubMed] [Google Scholar]
- Soderberg P, Tungstrom S, Armelius BA (2005). Reliability of global assessment of functioning ratings made by clinical psychiatric staff. Psychiatric Services 56, 434–438. [DOI] [PubMed] [Google Scholar]
- Startup M, Jackson MC, Bendix S (2002). The concurrent validity of the Global Assessment of Functioning (GAF). British Journal of Clinical Psychology 41, 417–422. [DOI] [PubMed] [Google Scholar]
- StataCorp (2009). Stata Statistical Software, 11th edn. Stata Corporation: College Station, TX. [Google Scholar]
- Sytema S, Wunderink L, Bloemers W, Roorda L, Wiersma D (2007). Assertive community treatment in the Netherlands; a randomized controlled trial. Acta Psychiatrica Scandinavica 116, 105–112. [DOI] [PubMed] [Google Scholar]
- Tungstrom S, Soderberg P, Armelius BA (2005). Relationship between the Global Assessment of Functioning and other DSM axes in routine clinical work. Psychiatric Services 56, 439–443. [DOI] [PubMed] [Google Scholar]
- Van De Willige G, Wiersma D, Nienhuis FJ, Enner JA (2005). Changes in quality of life in chronic psychiatric patients: a comparison between EuroQol (EQ-5D) and WHOQoL. Quality of Life Research 14, 441–451. [DOI] [PubMed] [Google Scholar]
- Van Hout BA, Al MJ, Gordon GS, Rutten FF (1994). Costs, effects and C/E-ratios alongside a clinical trial. Health Economics 3, 309–319. [DOI] [PubMed] [Google Scholar]
- Van Os J, Triffaux JM (2008). Evidence that the two-way communication checklist identifies patient-doctor needs discordance resulting in better 6-month outcome. Acta Psychiatrica Scandinavica 118, 322–326. [DOI] [PubMed] [Google Scholar]
- Van Os J, Gilvarry C, Bale R, Van Horn E, Tattan T, White I, Murray R (1999). To what extent does symptomatic improvement result in better outcome in psychotic illness? UK700 Group. Psychological Medicine 29, 1183–1195. [DOI] [PubMed] [Google Scholar]
- Van Os J, Altamura AC, Bobes J, Gerlach J, Hellewell JS, Kasper S, Naber D, Robert P (2004). Evaluation of the two-way communication checklist as a clinical intervention. Results of a multinational, randomised controlled trial. British Journal of Psychiatry 184, 79–83. [DOI] [PubMed] [Google Scholar]
- Van Veldhuizen JR (2007). FACT: a Dutch version of ACT. Community Mental Health Journal 43, 421–433. [DOI] [PubMed] [Google Scholar]
- Vanni T, Legood R, White RG (2010). Calibration of disease simulation model using an engineering approach. Value Health 13, 157. [DOI] [PubMed] [Google Scholar]
- Weinstein MC (2006). Recent developments in decision-analytic modelling for economic evaluation. Pharmacoeconomics 24, 1043–1053. [DOI] [PubMed] [Google Scholar]