Abstract
Aims.
For people with schizophrenia, non-adherence to antipsychotic medications may result in high use of health and other services. The objective of our research was to examine the economic consequences of non-adherence in patients with schizophrenia taking antipsychotic medication.
Methods.
Data were taken from QUATRO, a randomized controlled trial that drew a sample of adults with schizophrenia receiving psychiatric services in four European cities: Amsterdam, Leipzig, London and Verona. Trial inclusion criteria were a clinical diagnosis of schizophrenia, requiring on-going antipsychotic medication for at least 1-year following baseline assessment, and exhibiting evidence of clinical instability in the year prior to baseline. The patient-completed Medication Adherence Questionnaire (MAQ) was used to calculate the 5-point Morisky index of adherence. Generalized linear models (GLM) were developed to determine the effect of adherence on (i) health and social care and (ii) societal costs before and after treatment, taking into account other potential cost-influencing factors.
Results.
The effect of non-adherence on costs was mixed. For different groups of services, and according to treatment group assignment, non-adherence was both negatively and positively associated with costs.
Conclusions.
The impact of non-adherence on costs varies across the types of services used by individuals with schizophrenia.
Key words: Costs, Europe, non-adherence, schizophrenia
Introduction
Most people with schizophrenia use antipsychotic medication, which may be required to be taken indefinitely. A relatively high prevalence of non-adherence is observed among those prescribed antipsychotics. A review by Lacro et al. (2002) found rates of non-adherence to antipsychotic medication ranged from 4 to 72% with a mean of 41%. Many patients experience unwanted side effects, which are associated with non-adherence and low quality of life. Systematic reviews have consistently observed several other factors to be associated with non-adherence, such as drug and alcohol misuse, a lack of insight, a poor therapeutic alliance and the severity of symptoms (Kampman & Lehtinen, 1999; Lacro et al. 2002; Nosè et al. 2003).
People with schizophrenia often need and use a range of health and other services, particularly in periods of psychosis and low functioning, which may be related to non-adherence to antipsychotic medications and may result in high costs. For example, Weiden et al. (2004b) found a significant association between measures of partial adherence and the probability of rehospitalization in a sample of patients with schizophrenia. We used data collected in a European study of people with schizophrenia to examine the economic consequences of non-adherence in patients with schizophrenia taking antipsychotic medication. The aim of this study was to determine the impact of non-adherence to medication on service use costs attributable to schizophrenia.
Methods
QUATRO study
We conducted secondary analyses of data from QUATRO, a randomized controlled trial that sampled adults with schizophrenia receiving psychiatric services in four cities: Amsterdam (The Netherlands), Leipzig (Germany), London (United Kingdom) and Verona (Italy). Each sample was recruited from patient records at local in-patient and community settings in 2002/3 (Gray et al. 2006).
Patients included in QUATRO had a clinical diagnosis of schizophrenia (confirmed by the Item Group Checklist of the Schedule for Clinical Assessment in Neuropsychiatry), exhibited evidence of clinical instability in the year prior to baseline (that is, one or more of the following occurred in the previous 12 months: one or more hospital admissions on mental health grounds, a change in type of dose of antipsychotic medication, planned or actual increased frequency of contact with mental health services and indications of clinical instability reported by relatives, carers or the clinical team), and required on-going antipsychotic medication for at least 1 year following baseline assessment (Gray et al. 2006). Written, informed consent was obtained from all participants. All sites gained full approval for the study from local research ethics committees (institutional review board).
QUATRO was a two-arm randomized trial of the effectiveness and cost-effectiveness of adherence therapy (referred to hereafter as ‘treatment’) compared with standard health education in improving health-related quality of life for people with schizophrenia (Gray et al. 2006; Patel et al. submitted for publication). Interviews at baseline and after 12 months were conducted by researchers blinded to trial allocation. Patients were not blinded, but were not told which intervention was regarded by investigators as experimental.
Adherence to medication was based on patient responses to the Medication Adherence Questionnaire (MAQ), summed to obtain the 5-point Morisky score, ranging from 0 (poor adherence) to 4 (good adherence) (Morisky et al. 1986). This scale is widely used to assess adherence (Shalansky, 2004; Day et al. 2005). For the purpose of our new analyses, values 0–2 were interpreted as non-adherence, as per the classification used by the QUATRO team (Gray et al. 2006). Other clinical measures were the mental component summary score on the Medical Outcome Study (MOS) 36-Item Short Form Health Survey (SF-36) (Ware & Sherbourn, 1992) and the Brief Psychiatric Rating Scale-Expanded (BPRS-E) (Lukoff et al. 1986; Ventura et al. 1993).
The Client Service Receipt Inventory (CSRI) (Beecham & Knapp, 1992) collected service use data for a 3-month retrospective period in face-to-face interviews with study participants. That is, service use data for the 3 months prior to baseline and for the 3 months prior to the follow-up visit (12 months after baseline). Other data collected in the interview were demographic characteristics, accommodation, living situation, employment and roles of informal carers. Local-language, validated versions were available from the EPSILON study (Chisholm et al. 2000).
We are primarily interested in direct health and social care costs (medications, special (non-hospital) accommodation, inpatient stays, outpatient visits, community-based day services and community-based professional contacts), and societal costs (health and social care plus criminal justice, informal care and cost of lost employment) (McCrone, 2011). Unit costs for services were estimated at 2003 price levels (the most recent study year for which financial information was expected to be available at the time of the trial) in each study site (Patel, 2006). National average wage levels were used to value lost employment and informal care cost. As study centres were across four countries, unit costs were converted to a common basis using purchasing power parity (PPP)-adjusted Euros (Patel, 2006). Previous work demonstrated the importance of indirect costs in overall economic impact of schizophrenia (Knapp et al. 2004b). Service use frequency was multiplied by the unit cost of each service to estimate service use costs for each individual in the study.
Statistical methods
We examined the effect of adherence on costs before and after treatment, taking into account other potential cost-influencing factors. An indicator variable for adherence status was included, as was its interaction with treatment. We assumed no interaction between time and adherence. That is, we assumed that the relationship between non-adherence and costs would not differ between baseline and at the 12-month follow-up.
The analyses tested models where costs were thought to be affected by demographic characteristics, whether or not the study participant was randomized to receive the treatment or standard health education, whether or not the individual was adherent to medication, the interaction of treatment assignment and adherence status and random error. A value of zero was assigned for treatment effect for all individuals at baseline as at this point no actual treatment was received. The model can be expressed algebraically as follows:
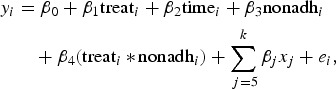 |
where nonadhi = 1 if the patient is non-adherent or nonadhi = 0 if the patient is adherent.
As the outcome was modelled with logistic regression, the interaction term required careful interpretation (Menard, 2002). If the interaction between adherence and treatment was significant, the effect of non-adherence on costs was β3 (the coefficient of the non-adherence variable) for those who did not receive treatment and β3 + β4 (the sum of the coefficients of the non-adherence variable and the treatment by non-adherence interaction) for those who did. Standard errors (s.e.) are clustered by individuals to account for the non-independence between the two time points for each individual.
Patient-specific characteristics, identified from the previous literature as potentially relevant to understanding cost variations, were examined: age, sex, severity of illness (BPRS-E, SF-36 mental component score), whether or not living alone, education level (whether or not completed further or tertiary education), ethnicity (white European or other ethnic background), familiarity with medication (measured by years on medication) and study site (Weiden et al. 2004b; Becker et al. 2007). As the clinical measures were correlated, only the score with the greater significance level was retained in each analysis. This reduced the potential for bias due to omitted variables.
The primary analysis examined the association between non-adherence and health and social care and societal costs. Some previous studies looked at the association between non-adherence and the cost of in-patient stays and community-based care (Svarstad et al. 2001; Knapp et al. 2004a; Weiden et al. 2004b). To allow for comparison with these studies, we conducted secondary analyses examining the association between non-adherence and the costs associated with in-patient stays and community-based day services. Included in community-based day services were community mental health centre visits, day care, group therapy and use of sheltered workshops and specialist education.
Data on two covariates were missing in 4 patients and data on one variable only were missing in 51 patients. Length of time on medication was the variable with the most missing data. These data were not provided by 43 individuals. Other variables with missing data were the Morisky self-assessed adherence score that was missing for 13 individuals, level of education (not reported by two individuals) and whether or not they lived alone (missing for one individual).
Excluding the variable on length of time on medication would reduce the number of observations in the dataset by 10%. This variable was assumed to be missing at random, i.e., a patient would be no more or less likely to report this information based on the length of time they were on medication. Similarly, there would be no reasons to not report this information based on their service use. Making this assumption allowed us to impute missing values using multiple imputations. With respect to the other variables with missing data, we concluded that there are reasons why a patient would not want to disclose that they had a low level of educational qualifications, lived alone or had not taken their medication. For this reason, and the fact that very few observations would be lost by excluding observations where one of these three variables was missing, we did not impute missing values on these variables.
Due to skewness in the cost distribution, generalized linear models (GLM) were estimated. The Park test was employed to determine the appropriate distribution and link functions (Mullahy, 1998). In the secondary analyses of in-patient and community-based day service use costs, two-part modelling (Mullahy, 1998) was needed because some patients did not use particular subsets of services (zero costs). First we ran a logistic regression on whether or not costs were incurred, and then we ran a GLM on costs for the subsample that used services.
Analysis was undertaken using STATA 10.1 (STATA, 2001). Robust s.e. were estimated to account for heteroscedasticity. Significance values below 0.05 were deemed statistically significant. Significance values between 0.1 and 0.05 were identified to indicate associations that approached statistical significance.
Results
Sample characteristics
Four hundred and nine adults were recruited across the four sites (Table 1). Approximately 30% of respondents had Morisky scale scores reflecting non-adherence to their medication. Of the 409 patients interviewed at baseline, 357 were also interviewed at 12 months. There were no significant differences in characteristics between individuals who did or did not complete a follow-up interview (Table 1).
Table 1.
Characteristics of QUATRO study sample at baseline: overall, completers and non-completers
| Characteristic | Completers (n = 357) | Non-completers (n = 52) | Overall (n = 409) |
|---|---|---|---|
| Centre: N (%) | |||
| Amsterdam | 87 (24.4) | 13 (25.0) | 100 (24.5) |
| Leipzig | 81 (22.7) | 16 (30.8) | 97 (23.7) |
| London | 80 (22.4) | 12 (23.1) | 92 (22.5) |
| Verona | 109 (30.5) | 11 (21.2) | 120 (29.3) |
| Age: mean (s.d.) | 41.7 (11.5) | 40.3 (11.4) | 41.5 (11.5) |
| Sex: % male | 59.9 | 59.6 | 59.9 |
| Ethnicity: % White European | 75.1 | 80.8 | 75.8 |
| Education: % with further/tertiary qualifications | 32.4 | 32.7 | 32.4 |
| Years using antipsychotics: mean (s.d.) | 13.9 (9.9) | 11.6 (9.8) | 13.7 (9.9) |
| Living situation: % living alone | 40.2 | 42.3 | 40.4 |
| Morisky scale total score: mean (s.d.) | 3.0 (1.2) | 2.9 (1.2) | 2.9 (1.2) |
| Percent non-adherent to medication at baseline* | 30.5 | 28.2 | 30.3 |
| SF-36 mental component score: mean (s.d.) | 39.1 (11.9) | 40.1 (10.7) | 39.2 (11.7) |
| BPRS-E total score: mean (s.d.) | 45.0 (13.0) | 46.1 (13.5) | 45.2 (13.0) |
*Based on Morisky total scores of 0, 1 or 2 indicating non-adherence.
Health and social care costs
Our first model estimated the significance of non-adherence and other factors on health and social care costs, based on 770 observations across the two time points. A single GLM model was estimated as all individuals with complete data across the independent variables had non-zero health and social care costs. The effect of non-adherence was not statistically significantly associated with health and social care costs (Table 2; p = 0.137). The treatment–adherence interaction variable approaches statistical significance (p = 0.055), suggesting that the effect of non-adherence on costs may differ by the treatment group. For non-adherent patients in the control group, health and social care costs were, on average, 20% lower than for patients who adhered to their medication. Within the treatment group, these costs were, on average, 50% higher. These differences, while potentially important, were not statistically significant because of relatively large s.e. in the estimates.
Table 2.
GLM of factors associated with health and social care and societal costs (n = 770 observations)
| Health and social care costs | Societal costs | |
|---|---|---|
| Potentially associated factors | Coefficient (p-value) | Coefficient (p-value) |
| Treatment… …relative to no treatment |
−0.15 (0.316) | −0.10 (0.493) |
| Time 1(follow-up)… …relative to Time 0 (baseline) |
−0.30 (0.004) | −0.33 (0.001) |
| Non-adherent… …relative to adherent |
−0.20 (0.137) | −0.23 (0.049) |
| Intervention × non-adherence interaction | 0.65 (0.055) | 0.53 (0.065) |
| Age – 5 year increment | 0.030 (0.351) | −0.017 (0.523) |
| Females… …relative to males |
−0.26 (0.036) | −0.029 (0.788) |
| Severity of illness (BPRS-E score) | 0.021 (0.001) | 0.021 (0.001) |
| Lives alone… …relative to lives with others |
−0.36 (0.005) | −0.33 (0.004) |
| Amsterdam (The Netherlands)… | 0.94 (0.001) | 0.67 (0.001) |
| Leipzig (Germany)… | 0.38 (0.073) | 0.088 (0.613) |
| Verona (Italy)… …relative to London (UK) |
−0.26 (0.164) | −0.32 (0.038) |
| Education – further or tertiary……relative to primary, secondary or general | 0.057 (0.641) | 0.13 (0.214) |
| Not White European……relative to White-European | −0.22 (0.137) | −0.38 (0.004) |
| Number of years on medication | −0.0018 (0.789) | 0.00036 (0.951) |
| Constant | 8.94 (0.001) | 9.63 (0.001) |
| Link function | Log | |
| Distributional family | Gamma | |
Of the remaining factors considered, higher costs were incurred for people who lived with others (compared to living alone), and men had higher health and social care costs than women. There was also a positive association between severity of symptoms and health and social care costs.
Societal costs
For societal costs, a two-part model was again unnecessary as every patient had non-zero costs. Patients who reported non-adherence had significantly lower societal costs than those reporting adherence (Table 2; p = 0.049). The interaction of treatment and adherence approached statistical significance (p = 0.065). Non-adherence is associated with significantly lower costs among those who did not receive treatment, but is not among those who did. Societal costs were, on average, over 20% lower among non-adherent patients who did not receive treatment. Individuals who lived with others had significantly higher societal costs compared with those who lived alone, and White Europeans had significantly higher costs compared with respondents of other ethnicities. There was also a positive association between symptom severity and societal costs.
Component costs
Analyses of in-patient stay costs and community-based day service costs required two-part models because, for each, a significant number of individuals in the sample did not use this service.
In-patient stays
Logistic regression (the first part of the two-part process) did not find a statistically significant association between non-adherence and in-patient stays (Table 3). Individuals who lived alone were less likely to have had an in-patient stay as compared with those who lived with others. Greater severity of symptoms (measured by BPRS-E) was associated with a greater probability of an inpatient stay. The model correctly predicted whether or not in-patient stays had occurred for 65% of cases.
Table 3.
Two-part model of factors associated with (i) use of inpatient services and (ii) inpatient costs among those who used inpatient services
| Logistic regression of inpatient services (n = 765 observations) | GLM of inpatient costs (n = 288 observations) | |
|---|---|---|
| Potentially associated factors | Odds ratio (p-value) | Coefficient (p-value) |
| Treatment… …relative to no treatment |
0.91 (0.714) | −0.070 (0.742) |
| Time 1(follow-up)… …relative to Time 0 (baseline) |
0.47 (0.001) | −0.21 (0.239) |
| Non-adherent… …relative to adherent |
1.34 (0.185) | −0.096 (0.630) |
| Intervention × non-adherence interaction | 0.99 (0.992) | 1.00 (0.081) |
| Age (5 year increase in age) | 1.00 (0.981) | 0.0050 (0.902) |
| Females… …relative to males |
0.97 (0.885) | −0.17 (0.239) |
| Severity of illness (BPRS-E score) | 1.02 (0.007) | 0.0077 (0.186) |
| Lives alone… …relative to lives with others |
0.69 (0.032) | −0.13 (0.418) |
| Amsterdam (The Netherlands)… | 1.95 (0.012) | 1.13 (0.001) |
| Leipzig (Germany)… | 4.66 (0.001) | −0.29 (0.269) |
| Verona (Italy)… …relative to London (UK) |
0.84 (0.524) | −0.48 (0.136) |
| Education – further or tertiary… …relative to primary, secondary or general |
1.03 (0.880) | 0.16 (0.375) |
| Not White European… …relative to White European |
1.03 (0.893) | −0.40 (0.045) |
| Number of years on medication | 0.99 (0.208) | 0.0011 (0.911) |
| Constant | 9.60 (0.001) | |
| Link function | Log | |
| Distributional family | Gamma | |
| Link test p-value | 0.4186 | |
| Pearson's chi-squared test p-value | 0.2774 | |
| Hosmer–Lemeshow chi-squared test p-value |
0.2809 | |
| Likelihood ratio chi-squared p-value | 0.0001 | |
| Per cent correctly classified | 65.26 |
The second part of the analysis (GLM) found in-patient costs were not significantly associated with any of the factors considered (Table 3). The interaction of treatment and non-adherence approached statistical significance (p = 0.081), however, suggesting again that the direction of the effect of non-adherence on costs differed according to treatment group assignment. Among patients who received treatment there was a trend towards in-patient costs being significantly higher for non-adherent patients compared with adherent patients (p = 0.086). Indeed, on average, inpatient costs were over twice as great in the non-adherent group. Ethnic minority respondents had significantly lower inpatient costs as compared with White European respondents (p = 0.045).
Community-based day services
In a logistic regression model of whether or not community-based day services were used, living alone was significant (Table 4). Individuals who lived alone were twice as likely to have used community-based day services compared with those who lived with others. There was also a trend (p = 0.069) towards longer time on medication being associated with greater probability of having used community-based day services. This model correctly predicted 61% of cases.
Table 4.
Two-part model of factors associated with (i) use of community-based day services and (ii) community-based day service use costs among those who used community-based day services
| Logistic regression of community-based day services (n = 765 observations) | GLM of community-based day service use costs (n = 281 observations) | |
|---|---|---|
| Potentially associated factors | Odds ratio (p-value) | Coefficient (p-value) |
| Treatment… …relative to no treatment |
1.36 (0.202) | −0.89 (0.010) |
| Time 1 (follow-up)… …relative to Time 0 (baseline) |
1.00 (0.984) | −0.028 (0.924) |
| Non-adherent… …relative to adherent |
0.85 (0.433) | −0.66 (0.059) |
| Intervention × non-adherence interaction | 0.41 (0.075) | 1.05 (0.091) |
| Age – in 5 year increments | 1.04 (0.351) | 0.13 (0.145) |
| Females… …relative to males |
1.04 (0.806) | −0.70 (0.013) |
| Severity of illness (BPRS-E score) | 1.01 (0.443) | −0.0093 (0.483) |
| Lives alone… …relative to lives with others |
2.09 (0.001) | 0.19 (0.530) |
| Amsterdam (The Netherlands)… | 0.82 (0.424) | 0.39 (0.327) |
| Leipzig (Germany)… | 0.93 (0.781) | 0.55 (0.396) |
| Verona (Italy)… …relative to London (UK) |
1.35 (0.230) | −0.36 (0.348) |
| Education – further or tertiary… …relative to primary, secondary or general |
0.87 (0.429) | 0.78 (0.013) |
| Not White European… …relative to White European |
1.18 (0.487) | −0.042 (0.900) |
| Number of years on medication | 1.02 (0.069) | −0.019 (0.256) |
| Constant | 8.15 (0.001) | |
| Link function | Log | |
| Distributional family | Gamma | |
| Link test p-value | 0.3144 | |
| Pearson's chi-squared test p-value | 0.3273 | |
| Hosmer–Lemeshow chi-squared test p-value |
0.5392 | |
| Likelihood ratio chi-squared p-value | 0.0001 | |
| Per cent correctly classified | 61.23 |
The interaction between treatment and non-adherence approached statistical significance (p = 0.075). Among those receiving treatment, the odds of using community-based day services were significantly lower for those who were non-adherent compared with those who were adherent (odds ratio = 0.35, p = 0.021).
Among those who used community-based day services, the GLM model found a trend (p = 0.059) towards non-adherence being associated with lower community-based day service costs compared with those who were adherent (Table 4). On average, costs for community-based day services were 50% lower among non-adherent patients compared with those who adhered.
Sensitivity analyses
We examined the impact of choice of threshold in the Morisky score used to determine non-adherence. Shalansky (2004) suggested using other thresholds to trade-off sensitivity and positive predictive value of the scale in detecting ‘true’ non-adherent patients. Analyses were conducted to determine if using a threshold score of 3 on the Morisky score to define non-adherence would have an impact on the results.
Using the lower threshold level suggested that 52.8% of the sample were non-adherent at baseline (whereas at the higher threshold the figure was 30%). For all cost measures, the effect of non-adherence was statistically non-significant.
Discussion
Main findings
Our results suggest that non-adherence is not significantly associated with total health and social care costs. Costs from a societal perspective were in fact lower among those who did not adhere to their medication.
Limitations
One limitation of the study was the relatively small sample, so results may not be generalizable to wider populations. The effect of ethnicity may not be consistent across minority ethnic groups, but the limited sample size made it infeasible to test for differences between specific minority ethnic groups.
The sample size calculation for the QUATRO trail was based on the SF-36 mental component summary score (Gray et al. 2006). Retrospective estimation of the power based on service use (and, by extension, costs) was difficult as service use typically has high variability, leading to underestimation of power (Gray et al. 1997). Based on the s.e. observed in the analysis of health and social care costs, the study sample size was sufficient to observe a 44% difference in costs with 80% power. With respect to societal costs, the study sample was sufficient to observe a 38% difference in costs with 80% power. These estimates do not, however, account for there being four distinct study sites within which we would expect a degree of similarity in service provision.
Relying on a multi-centre, cross-country sample has advantages, but service systems differ between sites, and unit costs are not always available (Patel, 2006). However, Heider et al. (2009), following samples of people with schizophrenia in France, Germany and the UK for 2 years, found differences in costs for individual services, but that total adjusted costs of health services varied rather less. The QUATRO study did not collect information on some potentially relevant dimensions, including general health status, whether co-morbidity was present, use of illegal drugs or alcohol abuse.
As the Morisky scale relies on self-report, it runs the risk of underestimating non-adherence. The prevalence of non-adherence in studies where self-reporting is used will tend to be underestimated as some patients are unaware of mistakes they are making in the medication regime or will choose to not report non-adherence (Byerly et al. 2005; Velligan et al. 2006). The observed rate of non-adherence was relatively low when compared across studies of patients taking antipsychotic medication (Lacro et al. 2002). The advantage of using self-reported information is that it is more efficient and less costly than other methods (Thompson et al. 2000).
A further limitation is that the QUATRO study duration was short relative to a lifetime of schizophrenia and the (probable) long-term impacts of non-adherence. Some of the negative impacts of non-adherence and effects on service use might not have been observed within the study period.
Despite these limitations, our analyses offer useful new information on the relationship between non-adherence and costs. Unlike previous studies that have predominantly examined this association using cross-sectional data, the 12-month design allowed for examination of change over time in the associations assessed. The study also included a valid and reliable scale for assessing adherence and detailed service use data.
Findings from other studies
Our findings are similar to those of Gilmer et al. (2004) who found that the impact of non-adherence on health care costs varied by the source of costs. In their study, hospital costs were significantly higher for the non-adherent group but total health care expenditure was significantly lower. Similar results regarding the association between non-adherence and inpatient costs were observed in other studies. Svarstad et al. (2001) found non-adherers (patients with a 3-month gap in medication claims) were more likely to have been rehospitalized and incurred significantly higher inpatient costs. Weiden et al. (2004a) observed that gaps in medication therapy, based on prescription claims, were positively correlated with risk of hospitalization. Knapp et al. (2004a) observed a trend towards a significant association between in-patient visits and non-adherence.
In order to shed light on the finding that non-adherence was associated with significantly lower societal costs, we looked at each of the cost categories to determine for which services this relationship was strongest. The data show that outpatient and community services went down over time for the sample as a whole, but the decrease in use of these services was greater among non-adherent patients. This was also true of informal care.
As in Knapp et al. (2004a), non-adherence was also associated with lower community-based day service costs among individuals in the intervention group (when these services were used). These patterns of association may occur if non-adherent patients put off seeking help when their symptoms return. Or by using fewer community services and receiving less informal care, the risk increases that they become non-adherent. This will also depend on the way services are organized and the ease with which they can access services. In addition, the relevance to the findings of the nature of the sample must be considered. The criteria for inclusion in the study that individuals’ treatment in the year before baseline must have been clinically unstable may suggest an underlying difficulty in engaging with non-acute services as opposed to a difficulty with medication taking only. Interventions that develop relationships between patients and therapeutic staff have been found to be effective in improving compliance (Kuipers, 1996).
The results from the QUATRO trial did not find an association between adherence therapy and adherence to medication at follow-up (Gray et al. 2006). The findings from the present study suggest that while the intervention did not impact non-adherence, it did impact on the association between non-adherence and costs. That is, among those receiving adherence therapy there was a trend towards lower inpatient costs and higher odds of using community-based services among those who report adherence to medication as compared with those who did not. This finding is difficult to interpret, but may in part be suggesting that for some individuals, the intervention encouraged engagement with services.
We observed lower health and social care and societal costs among patients living alone, suggesting that when individuals with schizophrenia live with family and/or friends, the latter may play a role in encouraging patients to access services. However, living alone was also significantly associated with a higher probability of using community-based day services, so informal care may act as a substitute for community-based day services but as a complement to other health and social care services. Alternatively, patients living alone may have better functioning and therefore have the skills to live alone. The modelling corrected for severity of illness but may not have accounted for all aspects of functioning.
Our results suggest that the impact of non-adherence varies by the type of services used by people with schizophrenia: adherence interventions have the potential to reduce some costs. Attempts to improve engagement with community-based services and adherence to medication, while potentially increasing costs, may benefit individuals with schizophrenia.
Acknowledgements
The QUATRO study is a multi-centre collaboration between the Health Services Research Department, Institute of Psychiatry, King's College London, London, UK; the Department of Medicine and Public Health, Section of Psychiatry and Clinical Psychology, University of Verona, Italy; the Department of Psychiatry, Leipzig University, and the Department of Psychiatry II, Ulm University, Germany; and the Department of Psychiatry, Academic Medical Center, University of Amsterdam, Netherlands. The study was funded by a grant from the Quality of Life and Management of Living Resources Programme of the European Union (QLG4-CT-2001-01734). The views expressed in this paper are my own and not necessarily those of the funder or collaborators. I wish to acknowledge the contributions of the patients, carers and staff who have taken part in this study and the contributions of the following colleagues to the overall QUATRO study: Amsterdam (Aart Schene, Annemarie Fouwels, Martijn Kikkert, Maarten Koeter, Karin Meijer); Leipzig/Ulm (Thomas Becker, Matthias Angermeyer, Anja Born, Anne Gießler, Hedda Helm, Bernd Puschner); London (Jonathan Bindman, Jayne Camara, Anthony David, Richard Gray, Martin Knapp, Morven Leese, Paul McCrone, Mauricio Moreno, Anita Patel, Debbie Robson, Graham Thornicroft, Ian White); Verona (Michele Tansella, Francesco Amaddeo, Corrado Barbui, Lorenzo Burti, Daniela Celani, Doriana Cristofalo, Claudia Goss, Antonio Lasalvia, Giovanna Marrella, Mariangela Mazzi, Michela Nosè, Mirella Ruggeri, Marta Solfa).
Conflict of Interest
The QUATRO study was funded by a grant from the Quality of Life and Management of Living Resources Programme of the European Union (QLG4-CT-2001-01734). There are no conflicts of interest relevant to the contents of this article for any authors.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Ethical Standard
The authors assert that all procedure contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- Becker M, Young M, Ochshorn E, Diamond R (2007). The relationship of antipsychotic medication class and adherence with treatment outcomes and costs for Florida Medicaid beneficiaries with schizophrenia. Administration and Policy in Mental Health and Mental Health Services Research 34, 307–314. [DOI] [PubMed] [Google Scholar]
- Beecham J, Knapp M (1992). Costing psychiatric interventions In Measuring Mental Health Needs (ed. Thormicroft G., Brewin C. and Wing J.), pp. 163–183. Gaskell: London. [Google Scholar]
- Byerly M, Fisher R, Whatley K, Holland R, Varghese F, Carmody T, Magouirk B, Rush A (2005). A comparison of electronic monitoring vs. clinical rating of antipsychotic adherence in outpatients with schizophrenia. Psychiatry Research 133, 129–133. [DOI] [PubMed] [Google Scholar]
- Chisholm D, Knapp M, Knudsen H, Amaddeo F, Gaite L, van Wijngaarden B (2000). Client socio-demographic and service receipt inventory – European version: development of an instrument for international research. EPSILON Study 5. European psychiatric services: inputs linked to outcome domains and needs. British Journal of Psychiatry 177, S28–S33. [DOI] [PubMed] [Google Scholar]
- Day J, Bentall R, Roberts C, Randall F, Rogers A, Cattell D, Healy D, Rae P, Power C (2005). Attitudes towards antipsychotic medication: the impact of clinical variables and relationships with health professionals. Archives of General Psychiatry 62, 717–724. [DOI] [PubMed] [Google Scholar]
- Gilmer T, Dolder C, Lacro J, Folsom D, Lindamer L, Garcia P, Jeste D (2004). Adherence to treatment with antipsychotic medication and health care costs among medicaid beneficiaries with schizophrenia. American Journal of Psychiatry 161, 692–699. [DOI] [PubMed] [Google Scholar]
- Gray R, Leese M, Bindman J, Becker T, Burti L, David A, Gournay K, Kikkert M, Koeter M, Puschner B, Schene A, Thormicroft G, Tansella M (2006). Adherence therapy for people with schizophrenia – European multicentre randomised controlled trial. British Journal of Psychiatry 189, 508–514. [DOI] [PubMed] [Google Scholar]
- Gray R, Marshall M, Lockwood A, Morris J (1997). Problems in conducting economic evaluations alongside clinical trials – lessons from a study of case management for people with mental disorders. British Journal of Psychiatry 170, 47–52. [DOI] [PubMed] [Google Scholar]
- Heider D, Bernert S, Konig H-H, Matschinger H, Hogh T, Brugha T, Bebbington P, Azorin M, Angermeyer M, Toumi M (2009). Direct medical mental health care costs of schizophrenia in France, Germany and the United Kingdom – findings from the European Schizophrenia Cohort (EuroSC). European Psychiatry 24, 216–224. [DOI] [PubMed] [Google Scholar]
- Kampman O, Lehtinen K (1999). Compliance in psychoses. Acta Psychiatrica Scandinavica 100, 167–175. [DOI] [PubMed] [Google Scholar]
- Knapp M, King D, Pugner K, Lapuerta P (2004a). Non-adherence to antipsychotic medication regimens: associations with resource use and costs. British Journal of Psychiatry 184, 509–516. [DOI] [PubMed] [Google Scholar]
- Knapp M, Mangalore R, Simon J (2004b). The global cost of schizophrenia. Schizophrenia Bulletin 30, 279–293. [DOI] [PubMed] [Google Scholar]
- Kuipers E (1996). The management of difficult to treat patients with schizophrenia, using non-drug therapies. British Journal of Psychiatry 169, 41–51. [PubMed] [Google Scholar]
- Lacro J, Dunn L, Dolder C, Leckband R, Jeste D (2002). Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. Journal of Clinical Psychiatry 63, 892–909. [DOI] [PubMed] [Google Scholar]
- Lukoff D, Liberman R, Neuchterlein K (1986). Symptoms monitoring in the rehabilitation of schizophrenic patients. Schizophrenia Bulletin 12, 578–602. [DOI] [PubMed] [Google Scholar]
- McCrone P (2011). Mental health economics: current methodological issues. Epidemiology and Psychiatric Sciences 20, 239–243. [DOI] [PubMed] [Google Scholar]
- Menard S (2002). Applied Logistic Regression Analysis. Sage University Press: Thousand Oaks, CA. [Google Scholar]
- Morisky D, Green L, Levine D (1986). Concurrent and predictive validity of a self-reported measure of medication adherence. Medical Care 24, 67–74. [DOI] [PubMed] [Google Scholar]
- Mullahy J (1998). Much ado about two: reconsidering retransformation and the two-part model in health economics. Journal of Health Economics 17, 247–281. [DOI] [PubMed] [Google Scholar]
- Nosè M, Barbui C, Tansella M (2003). How often do patients with psychosis fail to adhere to treatment programmes? A systematic review. Psychological Medicine 33, 1149–1160. [DOI] [PubMed] [Google Scholar]
- Patel A (2006). Issues in Multi-national Health Economic Evaluation. Institute of Psychiatry, King's College London, University of London: London. [Google Scholar]
- Patel A, McCrone P, Knapp M, Leese M, Amaddeo F, Tansella M, Kilian R, Angermeyer M, Kikkert M, Schene A. Economic evaluation of adherence therapy compared to a health education intervention for people with schizophrenia. Cost-Effectiveness and Resource Allocation (Forthcoming). [DOI] [PMC free article] [PubMed]
- Shalansky S (2004). Self-reported Morisky score for identifying nonadherence with cardiovascular medications. Annals of Pharmacotherapy 38, 1363–1368. [DOI] [PubMed] [Google Scholar]
- STATA (2001). STATA 10.1. Stata Corporation: College Station, TX.
- Svarstad B, Shireman T, Sweeney J (2001). Using drug claims data to assess the relationship of medication adherence with hospitalization and costs. Psychiatric Services 52, 805–811. [DOI] [PubMed] [Google Scholar]
- Thompson K, Kulkarni J, Sergejew A (2000). Reliability and validity of a new Medication Adherence Rating Scale (MARS) for the psychoses. Schizophrenia Research 42, 241–247. [DOI] [PubMed] [Google Scholar]
- Velligan D, Lam Y-W, Glahn D, Barrett J, Maples N, Ereshefsky L, Miller A (2006). Defining and assessing adherence to oral antipsychotics: a review of the literature. Schizophrenia Bulletin 32, 724–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Green M, Shaner A, Liberman R (1993). Training and quality assurance with the Brief Psychiatric Rating Scale. The ‘drift busters’. International Journal of Methods in Psychiatric Research 3, 221–244. [Google Scholar]
- Ware J, Sherbourn C (1992). The MOS, 36 item Short-Form Health Survey (SF-36). I, Conceptual framework and item selection. Medical Care 30, 473–483. [PubMed] [Google Scholar]
- Weiden P, Kozma C, Grogg A, Locklear J (2004a). Partial compliance and risk of rehospitalization among California medicaid patients with schizophrenia. Psychiatric Services 55, 886–891. [DOI] [PubMed] [Google Scholar]
- Weiden P, Mackell J, McDonnell D (2004b). Obesity as a risk factor for antipsychotic noncompliance. Schizophrenia Research 66, 51–57. [DOI] [PubMed] [Google Scholar]


