Abstract
Aims.
Heterogeneity of schizophrenia is known to be reflected in neuropsychological functioning of patients, but its expression in relatives is understudied. This study aims at exploring relationship between executive functioning and clinical profiles of first-degree relatives of patients who are classified as having or not having the deficit subtype of schizophrenia (DSRELs v. non-DSRELs), with the prediction of greater executive impairment in DSRELs.
Methods.
DSRELs (n = 15) and non-DSRELs (n = 40) were compared with community controls (CCs, n = 55) on executive functioning measured by the Wisconsin Card Sorting Test (WCST) and the phonemic verbal fluency (PVF), and clinical measures. Effects of psychopathology and intelligence quotient (IQ) measures were investigated to determine their association with executive performance.
Results.
DSRELs showed more executive dysfunction on WCST and poorer social functioning than CCs and more severe negative symptoms than non-DSRELs. Differences on WCST-categories achieved (WCST-CA) remained significant after adjustment for clinical confounders and IQ. WCST-CA was associated with apathy and paranoid ideation only within the DSREL subgroup.
Conclusions.
Executive functioning and negative symptoms are severely impaired in first-degree relatives of deficit syndrome patients, thus suggesting that some neurocognitive deficits in patients may be transmitted within families according to the pathophysiology of the probands.
Key words: executive function, negative symptoms, relatives, schizophrenia
Background
A large number of studies report that non-psychotic first-degree relatives of patients with schizophrenia show neurocognitive dysfunctions similar (but to a milder degree) to those displayed by patients with the illness (e.g. Snitz et al. 2006). However, these studies describe heterogeneous patterns of neurocognitive dysfunctions, involving different domains such as memory, attention and executive functions (Conklin et al. 2005; Gur et al. 2007; Skelley et al. 2008). In contrast, some studies did not find any difference in neuropsychological functioning between first-degree relatives of patients with schizophrenia and control subjects (Faraone et al. 1996; Barrantes-Vidal et al. 2007; Erol et al. 2012). Moreover, in any sample of relatives of people with schizophrenia, only a subgroup of relatives is likely to be neuropsychologically impaired (Faraone et al. 1995). In a previous study of first-degree relatives of patients with schizophrenia, we found an impairment of executive functions as compared with controls (Scala et al. 2012). It is possible that the impairment affects only a sub-group of relatives, while the remaining part of the sample might show essentially normal performance. It is also possible that the variability of neuropsychological functioning in relatives could be a reflection of the heterogeneity within patients, perhaps corresponding to pathophysiological features.
Furthermore, a recent systematic review (Dominguez et al. 2009) suggests that different underlying pathophysiological processes associated with different intermediary phenotypes may substantially account for psychopathological heterogeneity in non-affective psychosis. This study specifically found that negative symptoms were moderately associated with impairment in neurocognitive functions, such as verbal fluency and Wisconsin card sorting test (WCST) measures.
Schizophrenia is well known to be a heterogeneous disorder (Lin et al. 2012). Over the years, a number of psychopathological categorizations have been proposed (e.g. Andreasen & Olsen, 1982; Crow, 1985) to describe clinical profiles that may reflect its underlying pathophysiology. Among these categorizations, the deficit v. non-deficit syndrome has gained increasing attention, since it proved to be a promising model from both a heuristic and clinical perspective (Carpenter et al. 1988; Kirkpatrick et al. 1989). The ‘Deficit’ form is characterized by a more frequent familial history of schizophrenia, insidious onset, intellectual deterioration, prominent negative symptoms, poor response to antipsychotic medication and structural brain abnormalities (i.e. enlarged ventricles) (Kirkpatrick & Galderisi, 2008). On the other hand, the ‘non-deficit’ form typically presents acute onset, prominent positive symptoms, relatively normal intellectual functions, less significant brain abnormalities and a better clinical outcome (Carpenter & Kirkpatrick, 1988; Fenton & McGlashan, 1994; Strauss et al. 2010). Underlying neuropathological abnormalities are likely to be more linked to the prominent negative symptoms of the deficit syndrome (Tamminga et al. 1992; Turetsky et al. 1995; Lahti et al. 2001; Cascella et al. 2010). Multidisciplinary studies confirmed that the deficit syndrome as a stable form of schizophrenia is typically more associated with structural brain abnormalities, than the non-deficit form (Buchanan et al. 1990, 1994; Heckers et al. 1999; Amador et al. 1999; Tek et al. 2001).
Moreover, deficit schizophrenia has been found to be associated with a more severe cognitive impairment than the non-deficit syndrome (Pogue-Geile & Harrow, 1985; Cohen et al. 2007; Réthelyi et al. 2012). Recent converging evidence support the notion that executive functions are typically impaired in deficit schizophrenia (Bryson et al. 2001; Wang et al. 2008; Polgár et al. 2010). Dysfunction of the prefrontal cortex has been suggested as a presumed neural substrate associated with deficit schizophrenia (Delamillieure et al. 2000, 2004; Gonul et al. 2003). In particular, dysfunction of prefrontal cortex is associated with both negative symptoms and executive dysfunctions within this subtype of patients (Buchanan et al. 1997; Stolar et al. 1994). Although the conclusions drawn from this literature are still controversial (Goghari, 2011; Benoit et al. 2012), most studies report that dorsal prefrontal cortex functioning is associated with the negative dimensions of apathy and poor ‘volition’ (Taylor et al. 2004; Kimhy et al. 2006; Barch & Dowd, 2010). Moreover, high familial load may play an important role in deficit schizophrenia, since some studies showed that biological relatives of patients affected by the deficit syndrome show a higher risk of developing psychosis than relatives of non-deficit patients (Castle et al. 1994; Dollfus et al. 1996).
Within this framework, the present paper aims to test the hypothesis that first-degree relatives of patients with deficit schizophrenia are more severely impaired in both clinical and neuropsychological measures than relatives of patients with a non-deficit syndrome. Based on the existing literature, it is expected that first-degree relatives of patients with deficit syndrome would display more severe impairment in executive functions. Moreover, it is hypothesized that executive impairment shown by relatives of deficit patients is associated with negative symptoms, such as apathy and lack of interest. As a pilot study, this work might provide a good opportunity to assess the feasibility of future large full-scale studies, enhancing its likelihood of success and potentially avoiding unnecessary expensive research (Thabane et al. 2010).
Methods
Subjects
This is a cross-sectional study conducted on a convenience sample of 55 adult non-psychotic first-degree relatives (age 18–60 years) of patients with diagnosis of schizophrenia receiving care in the South-Verona Community Mental Health Service. Diagnosis was established by using the local Psychiatric Case Register (PCR) (Tansella et al. 2006) and was made by senior professionals using ICD-10 criteria. The reliability of this diagnostic procedure is known to be satisfactory (Amaddeo et al. 1997). The sampling and ascertainment procedures were described previously (Scala et al. 2012). Families of people affected by other major psychoses were excluded. Only parents, children and siblings without a current or lifetime history of psychotic disorder were included in the study. Other exclusion criteria included: (a) current substance or alcohol abuse (time frame: past 6 months); (b) history of head injury with loss of consciousness (>5 min.); (c) evidence of neurological diseases or electroshock history; (d) any major medical illness that could affect neurocognitive function. For each person participating in the study, direct interviews were conducted using the M.I.N.I. (Sheehan et al. 1998) to ascertain the absence of DSM-IV Axis I psychiatric diagnosis, including non-psychotic bipolar and major depressive disorders. Relatives were also assessed for schizotypal personality disorder with the Structured Clinical Interview for Personality Disorders (SCID-II; Spitzer et al. 1990).
The first-degree relatives of patients with schizophrenia were compared with a demographically matched (age, gender and educational level) community control (CC) group of individuals recruited from both hospital staff and the general population selected with the same exclusion criteria as those for relatives, with the addition of any family history of schizophrenia. At the time of recruitment, none of the participants were taking medications that could potentially affect cognitive functions; in particular, none took psychotropic medicines. No information on smoking was collected. However, neither relatives nor controls asked to take any smoke breaks during the assessment. The Ethics Committee of the Verona University Hospital approved the study procedures. Each participant was individually briefed about the nature of the study and provided written informed consent prior to participation.
Deficit v. non-deficit categorization
Participants were dichotomized into deficit v. non-deficit schizophrenia relatives based on assessment of their respective relatives with schizophrenia. Patients were assigned to deficit or non-deficit schizophrenia by using a chart review of ‘deficit syndrome criteria’ (Carpenter et al. 1988; Kirkpatrick et al. 1989), consisting of: (a) occurrence of primary enduring negative symptoms that are not secondary to anxiety, depression, drug or psychotropic effect or environmental deprivation and which persist during chronic period states (observation period: at least 1 year); (b) the concurrent presence of a minimum of two negative symptoms, including any of the following: restricted affect, diminished emotional range, poverty of speech with curbing of interest and decrease in curiosity, diminished sense of purpose, diminished social drive; and (c) at least moderate severity of the negative symptoms, as rated in the middle range between normality and severe occurrence.
Assignment of patients to the deficit v. non-deficit category was performed by two of the authors (S.S and A.L.) on the basis of all available clinical information (e.g. case records) pertaining to the patients (including an electronic search of the South-Verona PCR) and after having interviewed their respective treating psychiatrists. Where both authors agreed on the assignment, this was taken as consensus. Where there was disagreement (only for 1 patient), a consensus decision was made following the suggestion of the psychiatrist who treated the patient for a longer period. Information collected was: diagnosis (specific ICD-10 subtype of schizophrenia), life events (number and type), age of onset, gender, length of illness, number and type of hospitalizations, symptoms (positive, negative and total positive and negative syndrome scale (PANSS) score), global assessment of functioning score (GAF score) [PANSS and GAF scores were averaged at different assessment times during the course of the illness].
Neuropsychological testing
Trained neuropsychologists individually assessed relatives with two of the most commonly employed tests to explore executive functioning in SCZ-RELs (Szoke et al. 2005): (i) WCST (Heaton, 1981) evaluating concept formation, abstraction and set shifting [outcome variables were the number of categories achieved (CA) and perseverative errors (PE)] and the (ii) Verbal phonemic fluency test (VPF test; Milner, 1975), which assesses switching, lexical retrieval and generative verbal ability based on phonemic category [the number of appropriate words generated was the outcome variable]. Vocabulary and Block design subtests (WAIS-R) were also employed for the intelligence quotient (IQ) estimate (Brooker & Cyr, 1986). Neuropsychological assessment was carried out by one of the authors (S. S.) blind to clinical ratings and patients' classification. Subjects were allowed to take breaks as needed. Standard instructions and scoring methods were used.
Clinical assessments
Relatives were assessed with the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1983) blind to the patient's subdiagnosis. For some analyses individual subscales were combined into two factors, ‘Diminished Emotional Expression’ and ‘Apathy-Lack of Interest’, to separately identify ‘negative affect’ and ‘negative volition’, respectively. Self-perceived psychopathology was rated by the Symptom Checklist 90-Revised (SCL-90-R; Derogatis, 1993) which is composed of the following subscales: somatization, obsessive-compulsive, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, sleep disorders and the global severity index. Participants were also assessed using a set of other clinical measures, such as the Disability Assessment Schedule (DAS) – Section of Social Roles (WHO, 1988), and the GAF scale (APA, 1994).
Statistical Analyses
Owing to non-normality of scores, comparisons among groups were tested by non-parametric procedures. Specifically, the Mann–Whitney U test (2 groups) and the Kruskal–Wallis test (>2 groups) were used for continuous variables and the Chi-square test or the Fisher's exact test (if cell frequencies <5) for categorical variables. All tests were bilateral at p < 0.05. Effect sizes were calculated with Cohen's d (Cohen, 1988). After the application of the Kruskal–Wallis test, post-hoc comparisons were made by the Mann–Whitney U test with an adjustment of the a priori alpha level to p < 0.017 (0.05/3) only when a significant main effect had been found. Spearman's rho was used to explore the correlations between continuous variables (cognition and psychopathology and executive functions and IQ). Analysis of covariance (ANCOVA) models were used to test differences in executive functioning after controlling for each of the following variables considered one at a time: all the SANS subscales (including ‘Diminished Emotional Affect’ and ‘Apathy-Lack of Interest’), SCL-90 paranoid ideation and estimated IQ. All analyses were performed with SPSS 17.0 for Windows.
Results
Socio-demographic and clinical characteristics of patients
Of patients included in the study, 27.5% (n = 11) were classified as having deficit syndrome schizophrenia, and 72.5% (n = 29) as non-deficit. As shown in Table 1, deficit and non-deficit patients did not differ in terms of socio-demographic and clinical characteristics. It should however be noted that the P-value for the negative symptoms score showed a trend to statistical significance (p = 0.07), with deficit patients showing higher negative symptom scores than non-deficit patients. The lack of significance could be possibly accounted for the relatively small sample size, as the effect size was in the medium–large range (d = 0.71).
Table 1.
Demographics and clinical characteristics of patients classified as deficit v. non-deficit schizophrenia (n = 40)
| Deficit syndrome (n = 11) | Non-deficit syndrome (n = 29) | p | |
|---|---|---|---|
| Males (%)* | 90.9 | 72.4 | 0.40 |
| Age at the onset, mean (s.d.)† | 23.9 (6.4) | 25.0 (6.8) | 0.69 |
| Illness duration, mean (s.d.)† | 17.7 (6.8) | 16.3 (9.5) | 0.48 |
| Lifetime psychiatric hospitalizations, mean (s.d.)† | 9.0 (11.0) | 7.6 (13.0) | 0.81 |
| Lifetime compulsory admissions, mean (s.d.)† | 1.18 (2.1) | 0.5 (1.5) | 0.20 |
| Alcohol abusers (%)* | 9.1 | 13.8 | 1.0 |
| Drugs abusers (%)* | 18.2 | 24.1 | 1.0 |
| F20.0 diagnosis (%)* | 63.6 | 75.9 | 0.45 |
| Family history of psychosis (%)* | 9.1 | 24.1 | 0.41 |
| GAF, mean (s.d.)† | 43.8 (10.6) | 49.1 (7.9) | 0.16 |
| PANSS Total score, mean (s.d.)† | 76.8 (18.6) | 68.5 (9.4) | 0.11 |
| PANSS Positive symptoms, mean (s.d.)† | 3.2 (1.4) | 2.9 (0.6) | 0.08 |
| PANSS Negative symptoms, mean (s.d.)† | 2.6 (1.2) | 2.1 (0.4) | 0.07 |
| PANSS Disorganization, mean (s.d.)† | 1.9 (0.5) | 2.0 (0.4) | 0.39 |
| PANSS Agitation/aggressivity mean (s.d.)† | 2.3 (0.7) | 2.3 (0.8) | 0.74 |
| PANSS Anxiety/depression mean (s.d.)† | 2.4 (0.5) | 2.3 (0.7) | 0.44 |
*Exact Fisher test was used for categorical variables.
†Mann–Whitney test was used for continuous variables.
PANSS (positive and negative syndrome scale; Kay et al. 1987).
Socio-demographic characteristics of relatives
The DS relatives group consisted of 14 siblings (93.3%) and 1 offspring (6.7%), while the non-DS relatives group consisted of 28 siblings (70%), 5 offspring (12.5%) and 7 parents (17.5%). The DSRELs group comprises one (46.7%) or two (53.3%) relatives for each patient; non-DSRELs comprises one (55%), two (15%) or three (5%) relatives for each patient.
DSRELs (n = 15) were compared with non-DSRELs (n = 40) and CCs (n = 55) with regard to demographic variables. No significant differences in gender, age or education were found (see Table 2).
Table 2.
Demographics of the first-degree relatives of patients with DSRELs and non-DSRELs schizophrenia and CCs
| DSRELs (n = 15) | Non-DSRELs (n = 40) | CCs (n = 55) | P | |
|---|---|---|---|---|
| Gender (males), %* | 33.3 | 42.5 | 40.0 | 0.826 |
| Age (years), mean, s.d.† | 38.0 (6.4) | 39.7 (10.9) | 39.2 (10.4) | 0.941 |
| Education (years), mean, s.d.† | 9.9 (3.0) | 11.3 (3.1) | 10.8 (3.1) | 0.136 |
| Estimated IQ, mean, s.d.† | 94.7 (14.3) | 104.9 (13.7) | 101.7 (11.9) | 0.066 |
*X2 test was used for categorical variables.
†Mann–Whitney test was used for continuous variables.
Executive functioning in relatives
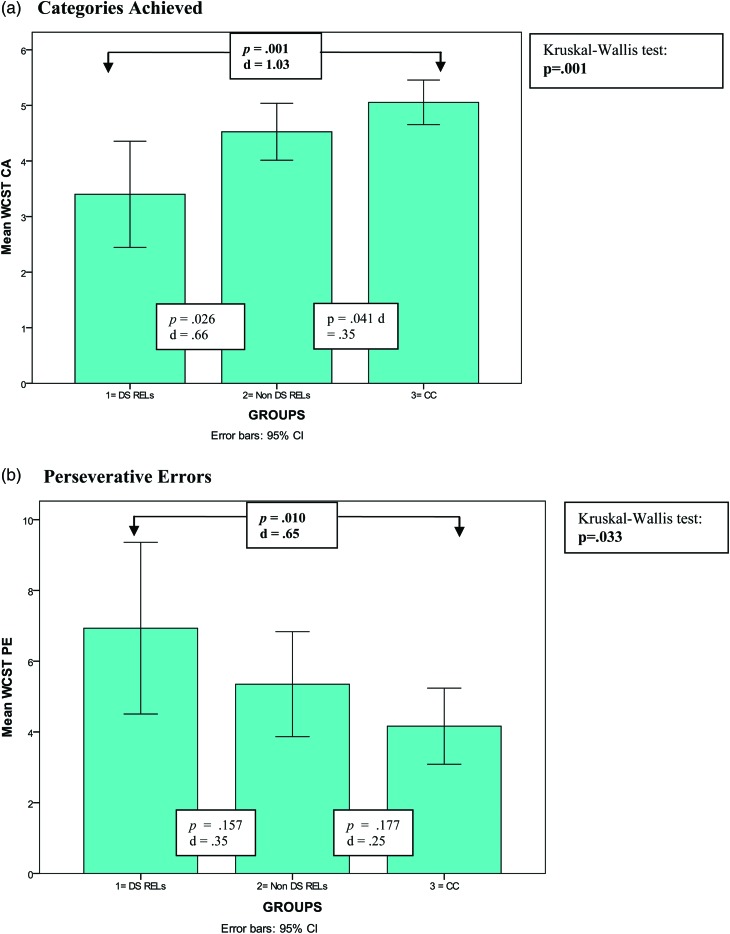
Both WCST-CA and WCST-PE significantly differed among groups (Table 3 and Fig. 1).
Table 3.
Executive functioning in first-degree relatives of patients with DSRELs and non-DSRELs schizophrenia and CCs
| Post hoc | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DS RELs (n = 15) | Non-DSRELs (n = 40) | CCs (n = 55) | p | 1 v. 3 | 1 v. 2 | 2 v. 3 | ||||
| Mean (s.d.) | Mean (s.d.) | Mean (s.d.) | p | D | p | d | p | d | ||
| WCST-CA | 3.4 (1.7) | 4.5 (1.6) | 5.1 (1.5) | 0.001 | 0.001 | 1.03 | 0.026 | 0.66 | 0.041 | 0.35 |
| WCST-PE | 6.9 (4.4) | 5.3 (4.6) | 4.2 (3.9) | 0.033 | 0.010 | 0.65 | 0.157 | 0.35 | 0.177 | 0.25 |
| PVF | 33.4 (9.7) | 36.1 (8.6) | 39.2 (9.5) | 0.102 | – | – | – | – | – | – |
WCST-CA, Wisconsin Card Sorting Test Categories Achieved; WCST-PE, Wisconsin Card Sorting Test Perseverative Errors; PVF, phonemic verbal fluency.
p values based on Kruskal–Wallis test (p < 0.05).
Post-hoc statistics: Mann–Whitney (p < 0.017).
d = effect size (Cohen, 1988).
Bolded p values: significant results.
Fig. 1.
Executive functioning as measured by the Wisconsin Card Sorting Test (WCST) in relatives of deficit patients (DSRELs; n = 15), non-deficit patients (non-DSRELs; n = 40) and CC (n = 55), pairwise post-hoc comparisons: Mann–Whitney at p < 0.017); (a). Categories achieved. (b). Perseverative errors.
DSRELs performed more poorly than non-DSRELs and control subjects on executive functioning as measured by WCST-CA and WCST-PE. Pairwise post-hoc comparisons revealed significant differences between DSRELs and controls on both WCST measures, while no significant differences were detected by comparing with non-DSRELs. No significant effects among the groups were observed on the phonemic verbal fluency (PVF) measure.
Estimated IQ was weakly correlated with both WCST measures, but this association did not reach statistical significance (WCST-CA, rho = 0.24, p = 0.39; WCST-PE, rho = −0.12, p = 0.67).
Clinical measures in relatives
DSRELs were compared with non-DSRELs and CCs on negative symptoms, global functioning and social disability (see Table 4).
Table 4.
Comparison of clinical measures in first-degree relatives of patients with DSRELs and non-DSRELs schizophrenia and CCs
| DSRELs (n = 15) | Non-DSRELs (n = 40) | CCs (n = 55) | Pairwise post-hoc comparisons Mann–Whitney | ||||
|---|---|---|---|---|---|---|---|
| SANS* | Mean (s.d.) | Mean (s.d.) | Mean (s.d.) | P | (1) v. (3) | (1) v. (2) | (2) v. (3) |
| Affective flattening/blunting | 0.91 (0.7) | 0.44 (.6) | 0.21 (0.3) | 0.001* | 0.000** | 0.024 | 0.084 |
| Alogy | 0.82 (0.8) | 0.33 (0.5) | 0.26 (0.3) | 0.043* | 0.015** | 0.027 | 0.843 |
| Diminished emotional expression | 0.86 (1.2) | 0.38 (0.5) | 0.23 (0.2) | 0.010* | 0.002** | 0.037 | 0.411 |
| Avolition/apathy | 1.10 (0.9) | 0.51 (0.5) | 0.35 (0.4) | 0.005* | 0.001** | 0.024 | 0.159 |
| Anhedony/asociality | 1.20 (0.9) | 0.90 (0.8) | 0.56 (0.5) | 0.008* | 0.004** | 0.131 | 0.055 |
| Apathy-lack of interest | 1.20 (0.8) | 0.70 (0.6) | 0.45 (0.4) | 0.004* | 0.002** | 0.060 | 0.066 |
| GAF total mean score† | 67.3 (9.9) | 73.1 (8.6) | 79.7 (6.5) | 0.000* | 0.000** | 0.024 | 0.000** |
| DAS total mean score‡ | 0.60 (0.7) | .26 (0.4) | 0.09 (0.1) | 0.004* | 0.001** | 0.075 | 0.063 |
*Scale for the Assessment of Negative Symptoms (SANS); possible scores: ‘0’ = No symptoms, ‘1’ = Questionable, ‘2’ = Mild, ‘3’ = Moderate; 4 = ‘Marked’, 5 = ‘Severe’.
Diminished emotional expression dimension: Affective flattening/blunting + Alogy.
Apathy-lack of interest dimension: Avolition/Apathy + Anhedony/asociality.
†Disability Assessment Schedule (DAS); possible scores: ‘0’ = No disability; ‘1’ = , minimum; ‘2’ = manifest; ‘3’ = , severe; ‘4’ = very severe; ‘5’ = maximum.
‡Global assessment of functioning (GAF) scale; scores range from ‘1’ = extremely compromised functioning to ‘100’ = superior functioning.
Kruskal–Wallis tests *p < 0.05.
Pairwise post-hoc comparisons: Mann–Whitney (**p < 0.017).
Significant differences among groups were found on the SANS subscales, GAF and DAS (p < 0.05). Pairwise post-hoc comparisons suggested that the scores on the SANS items, GAF and DAS followed a ‘dose-response’ trend among the three groups, with the highest dysfunction in DSRELs and the lowest in the control group. All groups were also compared with self-rated psychopathology for all the SCL-90-R subscales, but no significant differences were found; only ‘paranoid ideation’ approximated to a non-significant trend to statistical significance (p = 0.09), with DSREL (0.83 ± 0.5) showing higher symptoms than both non-DSRELs (0.52 ± 0.4) and CCs (0.56 ± 0.5).
Relationship between cognition and clinical psychopathology
ANCOVA was performed to rule out the effect of negative symptoms, self-rated paranoid ideation and estimated IQ (each considered in a separate model) on executive functions among groups.
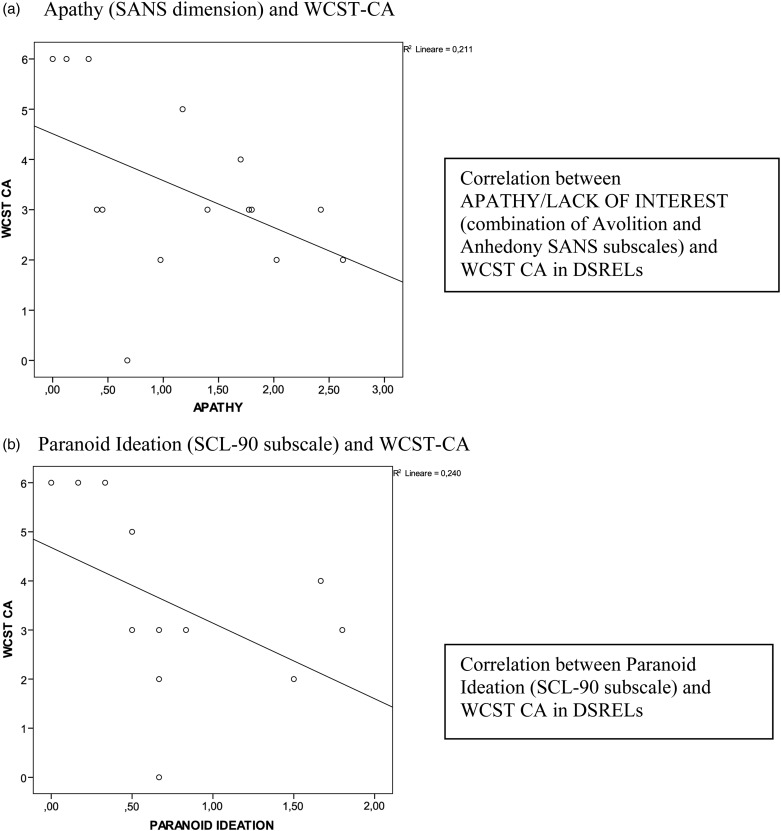
Table 5 shows that differences on WCST-CA were not explained by the clinical measures, since differences in the ‘categories achieved’ still survived after having taken into account all the covariates. On the other hand, differences on WCST-PE lost significance after controlling for the clinical measures. As shown in Fig. 2a, a significant negative correlation between ‘Apathy/lack of interest’ and WCST-CA was found only in the DSRELs group (rho = −0.54, p = 0.03).
Table 5.
Executive functioning measures adjusted for SCL-90-R paranoid ideation subscale, SANS subscales and estimated IQ in first-degree relatives of patients with DSRELs and non-DSRELs schizophrenia and CCs
| DSRELs (n = 15) | Non-DSRELs (n = 40) | CCs (n = 55) | Adjusted statistics (ANCOVA) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Paranoid ideation | Aff.flattening/blunting | Dimin. emotional expression | Alogy | Avolition/apathy | Apathy-lack of interest | Anhedony/asociality | IQ | ||||
| Mean (s.d.) | Mean (s.d.) | Mean (s.d.) | F (p) | F (p) | F (p) | F (p) | F (p) | F (p) | F (p) | F (p) | |
| WCST CA | 3.4 (1.7) | 4.5 (1.6) | 5.0 (1.5) | 5.9 (0.004) | 3.3 (0.039) | 3.1 (0.049) | 3.9 (0.024) | 3.6 (0.031) | 3.2 (0.044) | 3.9 (0.021) | 5.8 (0.004) |
| WCST PE | 6.9 (4.4) | 5.3 (4.6) | 4.2 (3.9) | 2.5 (0.089) | 2.2 (0.113) | 2.0 (0.133) | 2.1 (0.127) | 1.2 (0.295) | 0.99 (0.373) | 1.3 (0.281) | 2.3 (0.102) |
WCST-CA, Wisconsin Card Sorting Test, Categories Achieved; WCST-PE, Wisconsin Card Sorting Test, Perseverative Errors.
p-values on ANCOVA.
Bolded p values: significant result.
Fig. 2.
Correlations for WCST-CA in the DSREL group (n = 15). (a) Apathy (SANS dimension) and WCST-CA. (b) Paranoid ideation (SCL-90 subscale) and WCST-CA.
No significant correlations were found in non-DSRELs group between ‘Apathy/lack of interest’ and WCST-CA (rho = −0.29, p = 0.07), nor between WCST-PE and ‘paranoid ideation’ and ‘apathy/lack of interest’ in either DSRELs or non-DSRELs group. No significant correlations were found between WCST-CA and the ‘Diminished Emotional Expression’ in both DSREL and non-DSREL groups.
WCST-CA was significantly correlated with paranoid ideation in DSRELs (rho = −0.53; p = 0.04) (see Fig. 2b), rather than in non-DSREL group (rho = −0.04; p = 0.80). Significant negative associations between the ‘apathy/lack of interest’ and the paranoid ideation were observed in the DSREL group (rho = −0.68; p = 0.006).
Discussion
We investigated whether clinical heterogeneity in patients with schizophrenia may be reflected in both clinical and executive functioning of their non-psychotic first-degree relatives. The originality of our study consists of examining clinical and cognitive function of unaffected biological families of patients with schizophrenia, using the model of deficit v. non-deficit schizophrenia syndrome to identify possible sources of heterogeneity in the sample of relatives. In fact, previous investigations on the first-degree relatives of patients with DS/non-DS schizophrenia were mainly focused on the heightened risk of psychosis (Dollfus et al. 1996, 1998) or on mild-deficit like features (Kirkpatrick et al. 2000), and not on sub-groups. The size of our subgroup of patients that were classified as having deficit schizophrenia was consistent with the 25–30% rate previously reported in studies on chronic patients (e.g. Wang et al. 2008).
Consistent with Cohen et al. (2010), our sample of patients with deficit schizophrenia was similar to the non-deficit schizophrenia in several clinical aspects, thus, supporting the idea that the deficit syndrome categorization may be able to detect a separate but not a globally more severe disorder (Ross et al., 2000). Furthermore, we did not find a different level of familial psychiatric load or substance/alcohol abuse in the patients meeting the DS schizophrenia criteria v. the non-DS criteria. This finding is consistent with the original concept of the deficit syndrome, according to which the presence or absence of prominent negative symptoms is thought to be independent from the presence of psychotic symptoms (Kirkpatrick et al. 1989).
Based on such dichotomization of patients, we identified two subsamples of first-degree relatives hypothesized to reflect different psychopathological constructs. Overall, our data suggest that DSRELs display a more impaired profile than both non-DSRELs and CCs.
Executive functioning performance
The pattern of executive impairment, including the PVF and the WCST tasks, is consistent with the idea of an increasing severity in the DS familial vulnerability profile: both relative groups performed worse than controls, with DSRELs performing worse than non-DSRELs. Although the profile of severity of the WCST and the PVF differences was not entirely supported by a significant statistical difference, both measures follow a similar linear trend. It is important to highlight that the PVF is also considered as a measure of speed of processing, involving more simple cognitive components such as motor and perceptual functioning (Nuechterlein et al. 2004), that may be less affected in the DSRELs than the conceptual component (Bellani et al. 2009).
Regarding the WCST, we decided to evaluate PE and CA to be consistent with the most recent meta-analytic literature review on executive tests of first-degree relatives of probands, which reports that those are the two WCST outcomes most often used to assess the executive functioning (Szoke et al. 2005). These measures assess conceptual and executive control aspects of problem-solving such as efficient cognitive adjustment and output monitoring, which may be most essential to the deficit syndrome.
The poor WCST performance of DSRELs seems to confirm the hypothesis of a greater vulnerability load for distinct schizophrenia subtypes. Since correlations between the WCST and IQ were not significant, our finding suggests that the executive deficit is independent of generalized intellectual deficit (Byrne et al. 1999).
Negative symptoms and executive functions
Relatives of patients with DS showed higher levels of negative symptoms than both non-DSRELs and controls, even though only the DSRELS v. CC reached significance. A complex relationship between symptoms and executive functions emerged. In particular, DSRELs show higher levels of both executive dysfunction and negative symptoms than relatives of non-DS patients.
DS and non-DS relatives performed worse on WCST-CA than controls, thus revealing a dimensional distribution of impairment in performing accurately after error feedback. The difference was not significant after the clinical adjustment.
On the other hand, the WCST-CA difference among groups persists after taking into account the effect of symptoms, suggesting that differences in complex cognitive processing, such as generating rules, maintaining an internal goal and updating a previously held rule, are at least partially independent of the level of symptoms.
Moreover, DS-RELs reported a correlation between impairment in WCST-CA and the ‘volition’ negative factor supporting the hypothesis that apathy and diminished emotional expression reflect distinct underlying processes associated with the illness. This is consistent with the hypothesis that the WCST perseverative factor (involving both CA and PE) may be related to negative schizophrenia (Cuesta et al. 1995; Cannon et al. 2005). Furthermore, the association between WCST-CA and apathy negative factor found in our study may suggest a more symptomatic profile of vulnerability for deficit schizophrenia. This is also consistent with data regarding patients, showing that impaired monitoring performance is associated with negative symptoms in schizophrenia (Bates & Malhotra, 2002). Associations between high levels of social anhedonia and WCST-CA impairment were also found in individuals characterized by a measure of schizophrenia proneness (Tallent & Gooding, 1999). In particular, the ‘apathy/lack of interest’ dimension is reported to be related to the DLPFC (Nakaya & Ohmori 2008), whereas a ‘limited, subjective emotional experience’ is thought to be related to the inferior parietal cortex (Ross et al. 2001).
An association between lower prefrontal brain activity and a decrease of emotional-volition may be hypothesized (Compton et al. 2003; Milham et al. 2003; Ochsner & Gross, 2005). This is consistent with the recent finding (Kravariti et al. 2012) of a non-linear association between negative symptoms and cognition in patients, supporting the idea that this symptom dimension is not a unitary concept and that different underlying mechanisms may be involved in this relationship. Since we have not directly tested the integrity of prefrontal functioning in this study, we can only relate these results to previous work indicating that relatives show functional (Seidman et al. 2006) and structural alterations of prefrontal cortex (Rosso et al. 2010).
Moreover, the negative correlation between paranoid ideation and the WCST-CA by DSRELs suggests that mental flexibility is to some extent associated with a sub-clinical, psychotic-like ideation. The knowledge store in DSRELs may be partially inaccessible, which would prevent information retrieval during paranoid ideas processing (Vollema & Postma, 2002). Furthermore, the associations between the ‘apathy-volitional’ dimension and paranoid ideation, both related to the impaired categories achieving, would suggest a more impaired vulnerability profile of the DSRELs.
This suggests that inflexible behavioural patterns can be related to an average familial liability for schizophrenia, while difficulties in category-learning may be more typical of the DS subtype of psychosis vulnerability.
Limitations
The main limitation of the current study is the small sample size of the DS subgroup of patients, which has several consequences. The first one is that demographic and psychopathological differences between DS and non-DS patients do not reach statistical significance although some of these variables showed a trend that could result in significance in a larger sample of patients. Another consequence is the small sample size of the DSRELs, which may influence the statistical power increasing the possibility of a type II error. A further consequence of the small number of test subjects, regarding the fact that we did not apply a multilevel approach to data analysis that would have been the appropriate method with a larger sample size.
An additional study limitation is that diagnosis of DS was not made according to the gold standard, such as the scale for the deficit syndrome (SDS; Kirkpatrick et al. 1989). However, the literature suggests (Kirkpatrick et al. 1993; Goetz et al. 2007) that other proxy tools may be used as adequate alternatives to the SDS, when the clinical assessment may not be feasible. In our case, since the assignment of patients to deficit or non-deficit category was made retrospectively on the basis of all available clinical information and of case description provided by treating clinicians, the adoption of a proxy measure for identifying the DS was the only possible option.
Conclusion
Our study indicates a possible strategy to reduce the heterogeneity of schizophrenia by identifying subgroups of first-degree relatives of patients with a more impaired profile of neurocognitive vulnerability. The ‘deficit syndrome’ categorization, whose validity has been supported by independent studies (e.g. Heckers et al. 1999), allowed us to control for the effect of potential sources of heterogeneity in patients with schizophrenia and, in turn, in their unaffected first-degree relatives. Our findings provide evidence that DSRELs exhibit more negative symptoms and more executive dysfunctions than relatives of non-DS patients. Although these impaired dimensions follow a continuous distribution among the subgroups of relatives, associations between executive functions and negative symptoms were found only in the deficit subtype of relatives.
Overall, our finding points to both a dimensional and a categorical distribution of the familial load for the illness, across different subtypes of schizophrenia. Our investigation seems therefore support the adoption of DS as an indicator of a more severely impaired subgroup of relatives with higher vulnerability to schizophrenia.
Acknowledgements
The authors are grateful to patients attending the South-Verona Community Mental Health Service and their relatives for participation in the study.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.
References
- APA (1994). Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). American Psychiatric Association, Washington, DC. [Google Scholar]
- Agnew-Blais J, Seidman LJ (2012). Neurocognition in youth and young adults under age 30 at familial risk for schizophrenia: a quantitative and qualitative review. Cognitive Neuropsychiatry, 1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaddeo F, Beecham J, Bonizzato P, Fenyo A, Knapp M, Tansella M (1997). The use of a case register to evaluate the costs of psychiatric care. Acta Psychiatrica Scandinavica 95, 189–198. [DOI] [PubMed] [Google Scholar]
- Amador XF, Kirkpatrick B, Buchanan RW, Carpenter WT, Marcinko L, Yale SA (1999). Stability of the diagnosis of deficit syndrome in schizophrenia. American Journal of Psychiatry 156, 637–639. [DOI] [PubMed] [Google Scholar]
- Andreasen NC (1983). The Scale of the Assessment of Negative Symptoms (SANS). University of Iowa: Iowa City. [Google Scholar]
- Andreasen NC, Olsen SA (1982). Negative vs. Positive schizophrenia: definition and validation. Archives of General Psychiatry 39, 789–794. [DOI] [PubMed] [Google Scholar]
- Barch DM, Dowd EC (2010). Goal representations and motivational drive in schizophrenia: the role of prefrontal–striatal interactions. Schizophrenia Bulletin 36, 919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrantes-Vidal N, Aguilera M, Campanera S, Fatjó-Vilas M, Guitart M, Miret S, Valero S, Fañanás L (2007). Working memory in siblings of schizophrenia patients. Schizophrenia Research 95, 70–75. [DOI] [PubMed] [Google Scholar]
- Bates JA, Malhotra AK (2002). Genetic factors and neurocognitive traits. CNS Spectrums 7, 274–280, 283–284. [DOI] [PubMed] [Google Scholar]
- Bellani M, Perlini C, Brambilla P (2009). Language disturbances in schizophrenia. Epidemiology and Psychiatric Sciences 18, 314–317. [PubMed] [Google Scholar]
- Benoit A, Bodnar M, Malla AK, Joober R, Lepage M (2012). The structural neural substrates of persistent negative symptoms in first-episode of non-affective psychosis: a voxel-based morphometry study. Frontiers in Psychiatry 3, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker BH, Cyr JJ (1986). Tables for clinicians to use to convert WAIS-R short forms. Journal of Clinical Psychology 42, 982–986. [Google Scholar]
- Bryson G, Whelahan HA, Bell M (2001). Memory and executive function impairments in deficit syndrome schizophrenia. Psychiatry Research 102, 29–37. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Kirkpatrick B, Heinrichs DW, Carpenter WT Jr (1990). Clinical correlates of the deficit syndrome of schizophrenia. American Journal of Psychiatry 147, 290–294. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Strauss ME, Kirkpatrick B, Holstein C, Breier A, Carpenter WT Jr (1994). Neuropsychological impairments in deficit vs. nondeficit forms of schizophrenia. Archives of General Psychiatry 51, 804–811. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Strauss ME, Breier A, Kirkpatrick B, Carpenter WT Jr. (1997). Attentional impairments in deficit and nondeficit forms of schizophrenia. American Journal of Psychiatry 154, 363–370. [DOI] [PubMed] [Google Scholar]
- Byrne M, Hodges A, Grant E, Owens DC, Johnstone EC (1999). Neuropsychological assessment of young people at high genetic risk for developing schizophrenia compared with controls: preliminary findings of the Edinburgh High Risk Study (EHRS). Psychological Medicine 29, 1161–1173. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Glahn DC, Kim J, Van Erp TG, Karlsgodt K, Cohen MS, Nuechterlein KH, Bava S, Shirinyan D (2005). Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Archives of General Psychiatry 62, 1071–1080. [DOI] [PubMed] [Google Scholar]
- Carpenter WT Jr., Kirkpatrick B (1988). The heterogeneity of the long-term course of schizophrenia. Schizophrenia Bulletin 14, 645–652. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Heirrichs DW, Wagner AMI (1988). Deficit and nondeficit forms of schizophrenia: the concept. American Journal of Psychiatry 145, 578–583. [DOI] [PubMed] [Google Scholar]
- Cascella NG, Fieldstone SC, Rao VA, Pearlson GD, Sawa A, Schretlen DJ (2010). Gray-matter abnormalities in deficit schizophrenia. Schizophrenia Research 120, 63–70. [DOI] [PubMed] [Google Scholar]
- Castle DJ, Sham PC, Wessely S, Murray RM (1994). The subtyping of schizophrenia in men and women: a latent class analysis. Psychological Medicine 24, 41–51. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Brown LA, Minor KS (2010). The psychiatric symptomatology of deficit schizophrenia: a meta-analysis. Schizophrenia Research 118, 122–7. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Saperstein AM, Gold JM, Kirkpatrick B, Carpenter WT Jr., Buchanan RW (2007). Neuropsychology of the deficit syndrome: new data and meta-analysis of findings to date. Schizophrenia Bulletin 33, 1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Lawrence Earlbaum Associates, Hillsdale, NJ. [Google Scholar]
- Compton RJ, Banich MT, Mohanty A, Milham MP, Herrington J, Miller GA, Scalf PE, Webb A, Heller W (2003). Paying attention to emotion: an fMRI investigation of cognitive and emotional Stroop tasks. Cognitive, Affective and Behavioral Neurosciece 3, 81–96. [DOI] [PubMed] [Google Scholar]
- Conklin HM, Curtis CE, Calkins ME, Iacono WG (2005). Working memory functioning in schizophrenia patients and their first-degree relatives: cognitive functioning shedding light on etiology. Neuropsychologia 43, 930–942. [DOI] [PubMed] [Google Scholar]
- Crow TJ (1985). The two-syndrome concept: origins and current status. Schizophrenia Bulletin 11, 471–786. [DOI] [PubMed] [Google Scholar]
- Cuesta MJ, Peralta V, Caro F, de Leon J (1995). Schizophrenic syndrome and Wisconsin Card Sorting Test dimensions. Psychiatry Research 58, 45–51. [DOI] [PubMed] [Google Scholar]
- Delamillieure P, Fernandez J, Constans JM, Brazo P, Benali K, Abadie P, Vasse T, Thibaut F, Courtheoux P, Petit M, Dollfus S (2000). Proton magnetic resonance spectroscopy of the medial prefrontal cortex in patients with deficit schizophrenia: preliminary report. American Journal of Psychiatry 157, 641–643. [DOI] [PubMed] [Google Scholar]
- Delamillieure P, Constans JM, Fernandez J, Brazo P, Dollfus S (2004). Relationship between performance on the Stroop test and N-acetylaspartate in the medial prefrontal cortex in deficit and nondeficit schizophrenia: preliminary results. Psychiatry Research: Neuroimaging 132, 87–89. [DOI] [PubMed] [Google Scholar]
- Derogatis LR (1993). Symptom Checklist-90-R (SCL-90). Computer Systems: Minneapolis. [Google Scholar]
- Dollfus S, Ribeyre JM, Petit M (1996). Family history and deficit form in schizophrenia. European Psychiatry 11, 260–262. [DOI] [PubMed] [Google Scholar]
- Dollfus S, Germain-Robin S, Chabot B, Brazo P, Delamillieure P, Langlois S, van der Eist A, Campion D, Petit M (1998). Family history and obstetric complications in deficit and non-deficit schizophrenia: preliminary results. European Psychiatry 13, 270–272. [DOI] [PubMed] [Google Scholar]
- Dominguez MG, Viechtbauer W, Simons CJ, van Os J, Krabbendam L (2009). Are psychotic psychopathology and neurocognition orthogonal? A systematic review of their associations. Psychological Bulletin 135, 157–171. [DOI] [PubMed] [Google Scholar]
- Erol A, Bayram S, Kosger F, Mete L (2012). Executive functions in patients with familial versus sporadic schizophrenia and their parents. Neuropsychobiology 17, 93–99. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Seidman LJ, Kremen WS, Pepple JR, Lyons MJ, Tsuang MT (1995). Neuropsychological functioning among the nonpsychotic relatives of schizophrenic patients: a diagnostic efficiency analysis. Journal of Abnormal Psychology 104, 286–304. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Seidman LJ, Kremen WS, Toomey R, Lyons MJ, Tsuang MT (1996). Neuropsychological functioning among the elderly nonpsychotic relatives of schizophrenic patients. Schizophrenia Research 20, 27–31. [DOI] [PubMed] [Google Scholar]
- Fenton WS, McGlashan TH (1994). Antecedents, symptom progression, and long-term outcome of the deficit syndrome in schizophrenia. American Journal of Psychiatry 151, 351–356. [DOI] [PubMed] [Google Scholar]
- Goetz RR, Corcoran C, Yale S, Stanford AD, Kimhy D, Amador X, Malaspina D (2007). Validity of a 'proxy' for the deficit syndrome derived from the positive and negative syndrome scale (PANSS). Schizophrenia Research 93, 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goghari VM (2011). Executive functioning related brain abnormalities associated with the genetic liability for schizophrenia: an activation likelihood estimation meta-analysis. Psychological Medicine 41, 1239–1252. [DOI] [PubMed] [Google Scholar]
- Gonul AS, Kula M, Esel E, Tutus A, Sofuoglu S (2003). A Tc-99 m HMPAO SPECT study of regional cerebral blood flow in drug-free schizophrenic patients with deficit and non-deficit syndrome. Psychiatry Research: Neuroimaging 123, 199–205. [DOI] [PubMed] [Google Scholar]
- Gur RE, Nimgaonkar VL, Almasy L, Calkins ME, Ragland JD, Pogue-Geile MF, Kanes S, Blangero J, Gur RC (2007). Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. American Journal of Psychiatry 164, 813–819. [DOI] [PubMed] [Google Scholar]
- Heaton RK (1981). Wisconsin Card Sorting Test. Psychological Assessment Resources: Odessa. [Google Scholar]
- Heckers S, Goff D, Schacter DL, Savage CR, Fischman AJ, Alpert NM, Rauch SL (1999). Functional imaging of memory retrieval in deficit vs. nondeficit schizophrenia. Archives of General Psychiatry 56, 1117–1123. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin 13, 261–76. [DOI] [PubMed] [Google Scholar]
- Kimhy D, Yale S, Goetz RR, Marcinko McFarr L, Malaspina D (2006). The factorial structure of the schedule for the deficit syndrome in schizophrenia. Schizophrenia Bulletin 32, 274–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Galderisi S (2008). Deficit schizophrenia: an update. World Psychiatry 7, 143–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT Jr. (1989). The schedule for the deficit syndrome: an instrument for research in schizophrenia. Psychiatry Research 30, 119–123. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Buchanan RW, Breier A, Carpenter WT Jr. (1993). Case identification and stability of the deficit syndrome of schizophrenia. Psychiatry Research 47, 47–56. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Ross DE, Walsh D, Karkowski L, Kendler KS (2000). Family characteristics of deficit and nondeficit schizophrenia in the Roscommon family study. Schizophrenia Research 45, 57–64. [DOI] [PubMed] [Google Scholar]
- Kravariti E, Russo M, Vassos E, Morgan K, Fearon P, Zanelli JW, Demjaha A, Lappin JM, Tsakanikos E, Dazzan P, Morgan C, Doody GA, Harrison G, Jones PB, Murray RM, Reichenberg A (2012). Linear and non-linear associations of symptom dimensions and cognitive function in first-onset psychosis. Schizophrenia Research 140, 221–231. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Holcomb HH, Medoff DR, Weiler MA, Tamminga CA, Carpenter WT Jr. (2001). Abnormal patterns of regional cerebral blood flow in schizophrenia with primary negative symptoms during an effortful auditory recognition task. American Journal of Psychiatry 158, 1797–1808. [DOI] [PubMed] [Google Scholar]
- Lin A, Nelson B, Yung AR (2012). 'At-risk' for psychosis research: where are we heading? Epidemiology and Psychiatric Sciences 30, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Barad V (2003). Competition for priority in processing increases prefrontal cortex's involvement in top-down control: an event-related fMRI study of the Stroop task. Cognitive Brain Research 17, 212–222. [DOI] [PubMed] [Google Scholar]
- Milner B (1975). Psychological aspects of focal epilepsy and its neurosurgical management. Advances in Neurology 8, 299–321. [PubMed] [Google Scholar]
- Nakaya M, Ohmori K (2008). A two-factor structure for the Deficit Syndrome in schizophrenia. Psychiatry Research 158, 256–259. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK (2004). Identification of separable cognitive factors in schizophrenia. Schizophrenia Research 72, 29–39. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ (2005). The cognitive control of emotion. Trends in Cognitive Sciences 9, 242–249. [DOI] [PubMed] [Google Scholar]
- Pogue-Geile MF, Harrow M (1985). Negative symptoms in schizophrenia: their longitudinal course and prognostic importance. Schizophrenia Bulletin 11, 427–439. [DOI] [PubMed] [Google Scholar]
- Polgár P, Réthelyi JM, Bálint S, Komlósi S, Czobor P, Bitter I (2010). Executive function in deficit schizophrenia: what do the dimensions of the Wisconsin Card Sorting Test tell us? Schizophrenia Research 122, 85–93. [DOI] [PubMed] [Google Scholar]
- Réthelyi JM, Czobor P, Polgár P, Mersich B, Bálint S, Jekkel E, Magyar K, Mészáros A, Fábián A, Bitter I (2012). General and domain-specific neurocognitive impairments in deficit and non-deficit schizophrenia. European Archives of Psychiatry and Clinical Neuroscience 262, 107–115. [DOI] [PubMed] [Google Scholar]
- Ross DE, Kirkpatrick B, Karkowski LM, Straub RE, MacLean CJ, O'Neill FA, Compton AD, Murphy B, Walsh D, Kendler KS (2000). Sibling correlation of deficit syndrome in the Irish study of high-density schizophrenia families. American Journal of Psychiatry 157, 1071–1076. [DOI] [PubMed] [Google Scholar]
- Ross E, Orbelo D, Cartwright J, Hansel S, Burgard M, Testa J, Buck R (2001). Affective-prosodic deficits in schizophrenia: profiles of patients with brain damage and comparison with relation to schizophrenic symptoms. Journal of Neurology, Neurosurgery and Psychiatry 70, 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso IM, Makris N, Thermenos HW, Hodge SM, Brown A, Kennedy D, Caviness VS, Faraone SV, Tsuang MT, Seidman LJ (2010). Regional prefrontal cortex gray matter volumes in youth at familial risk for schizophrenia from the Harvard Adolescent High Risk Study. Schizophrenia Research 123, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala S, Lasalvia A, Cristofalo D, Bonetto C, Ruggeri M (2012). Neurocognitive profile and its association with psychopathology in first-degree relatives of patients with schizophrenia. A case-control study. Psychiatry Research 200, 137–143. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Thermenos HW, Poldrack RA, Peace NK, Koch JK, Faraone SV, Tsuang MT (2006). Altered brain activation in dorsolateral prefrontal cortex in adolescents and young adults at genetic risk for schizophrenia: An fMRI study of working memory. Schizophrenia Research 85, 58–72. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry 59, 22–33. [PubMed] [Google Scholar]
- Skelley SL, Goldberg TE, Egan MF, Weinberger DR, Gold JM (2008). Verbal and visual memory: characterizing the clinical and intermediate phenotype in schizophrenia. Schizophrenia Research 105, 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, Macdonald AW, Carter CS (2006). Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophrenia Bulletin 32, 179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer R, Williams JBV, Gibbon M, First MB (1990). Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II). American Psychiatric Press, Washington, DC. (Italian Version: Fava M, Guaraldi GB, Mazzi F, Rigatelli M, 1993). [Google Scholar]
- Stolar N, Berenbaum H, Banich MT, Barch D (1994). Neuropsychological correlates of alogia and affective flattening in schizophrenia. Biological Psychiatry 35, 164–172. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Harrow M, Grossman LS, Rosen C (2010). Periods of recovery in deficit syndrome schizophrenia: a 20-year multi-follow-up longitudinal study. Schizophrenia Bulletin 36, 788–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szoke A, Schurhoff F, Mathieu F, Meary A, Ionescu S, Leboyer M (2005). Tests of executive functions in first-degree relatives of schizophrenic patients: a meta-analysis. Psychological Medicine 35, 771–782. [DOI] [PubMed] [Google Scholar]
- Tallent KA, Gooding DC (1999). Working memory and Wisconsin Card Sorting Test performance in schizotypic individuals: a replication and extension. Psychiatry Research 89, 161–170. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Thaker OK, Buchanan R, Kirkpatrick B, Alphs LD, Chase TN, Carpenter WT (1992). Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Archives of General Psychiatry 49, 522–530. [DOI] [PubMed] [Google Scholar]
- Tansella M, Amaddeo F, Burti L, Lasalvia A, Ruggeri M (2006). Evaluating a community-based mental health service focusing on severe mental illness. The Verona experience. Acta Psychiatrica Scandinavica 429, 90–94. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Welsh RC, Wager TD, Phan KL, Fitzgerald KD, Gehring WJ (2004). Functional neuroimaging study of motivation and executive function. Neuroimage 21, 1045–1054. [DOI] [PubMed] [Google Scholar]
- Tek C, Kirkpatrick B, Buchanan RW (2001). A five-year follow-up study of deficit and nondeficit schizophrenia. Schizophrenia Research 49, 253–260. [DOI] [PubMed] [Google Scholar]
- Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, Robson R, Thabane M, Giangregorio L, Goldsmith CH (2010). A tutorial on pilot studies: the what, why and how. BMC Medical Research Methodology 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky B, Cowell PE, Gur RC, Grossman RI, Shtasel DL, Gur RE (1995). Frontal and temporal lobe brain volumes in schizophrenia. Relationship to symptoms and clinical subtype. Volumetric measure of the frontal and temporal lobe regions in schizophrenia: relationship to negative symptoms. Archives of General Psychiatry 52, 1061–1070. [DOI] [PubMed] [Google Scholar]
- Vollema MG, Postma B (2002). Neurocognitive correlates of schizotypy in first degree relatives of schizophrenia patients. Schizophrenia Bulletin 28, 367–377. [DOI] [PubMed] [Google Scholar]
- Wang XS, Yao S, Kirkpatrick B, Shi C, Yi J (2008). Psychopathology and neuropsychological impairments in deficit and nondeficit schizophrenia of Chinese origin. Psychiatry Research 158, 195–205. [DOI] [PubMed] [Google Scholar]
- World Health Organization (1988). Disability Assessment Schedule-II (DAS). World Health Organization: Geneva. [Google Scholar]